Submitted:
28 September 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Technical Lignin as a Secondary Raw Material
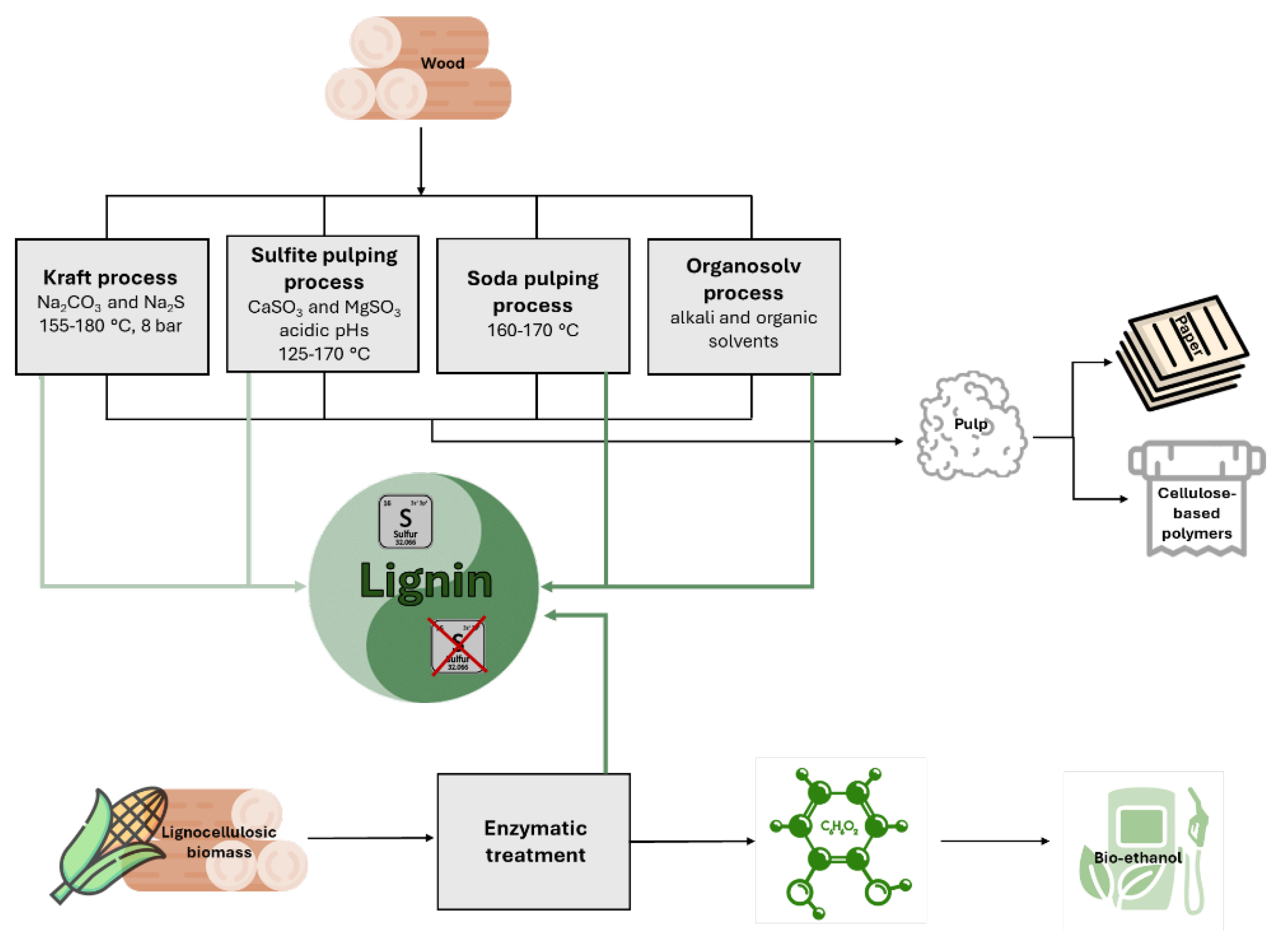
2.1. Lignins from Pulp, Paper, and Cellulose-Based Polymers Manufacturing
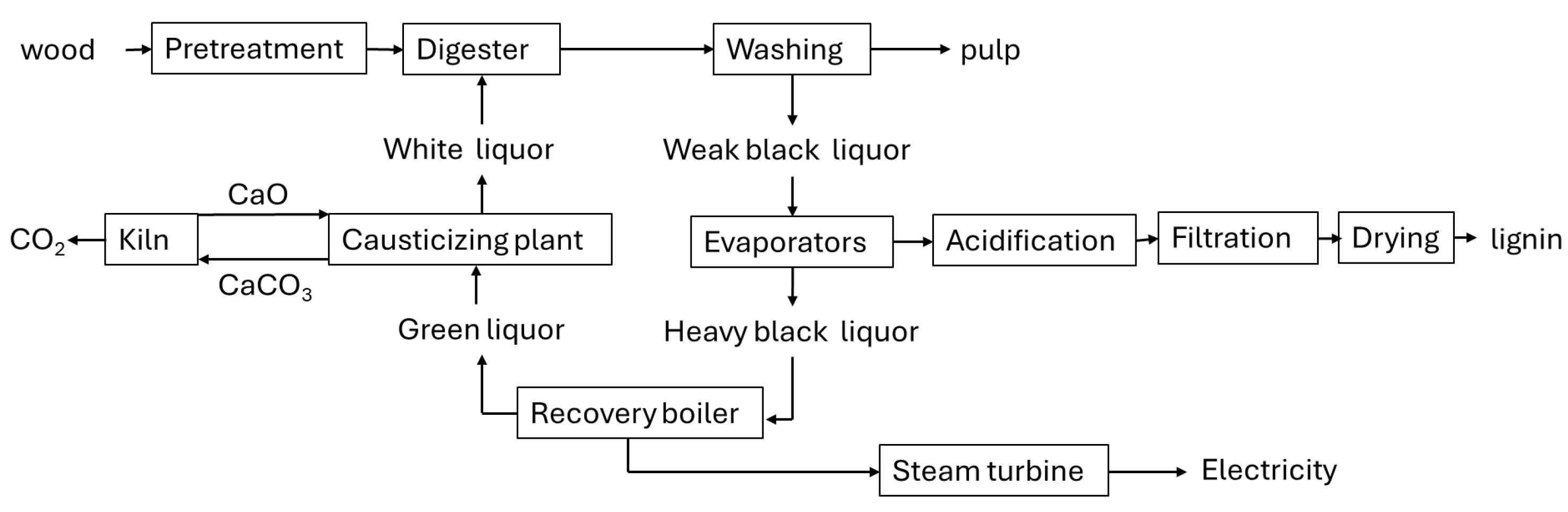
2.2. Lignins from Lignocellulosic Bioethanol Production Processes
3. Lignin Pyrolysis Technologies and Their Products
3.1. Biochar Manufacturing
3.2. Lignin Pyrolysis Bio-Oils
3.3. Lignin Pyrolysis Gas
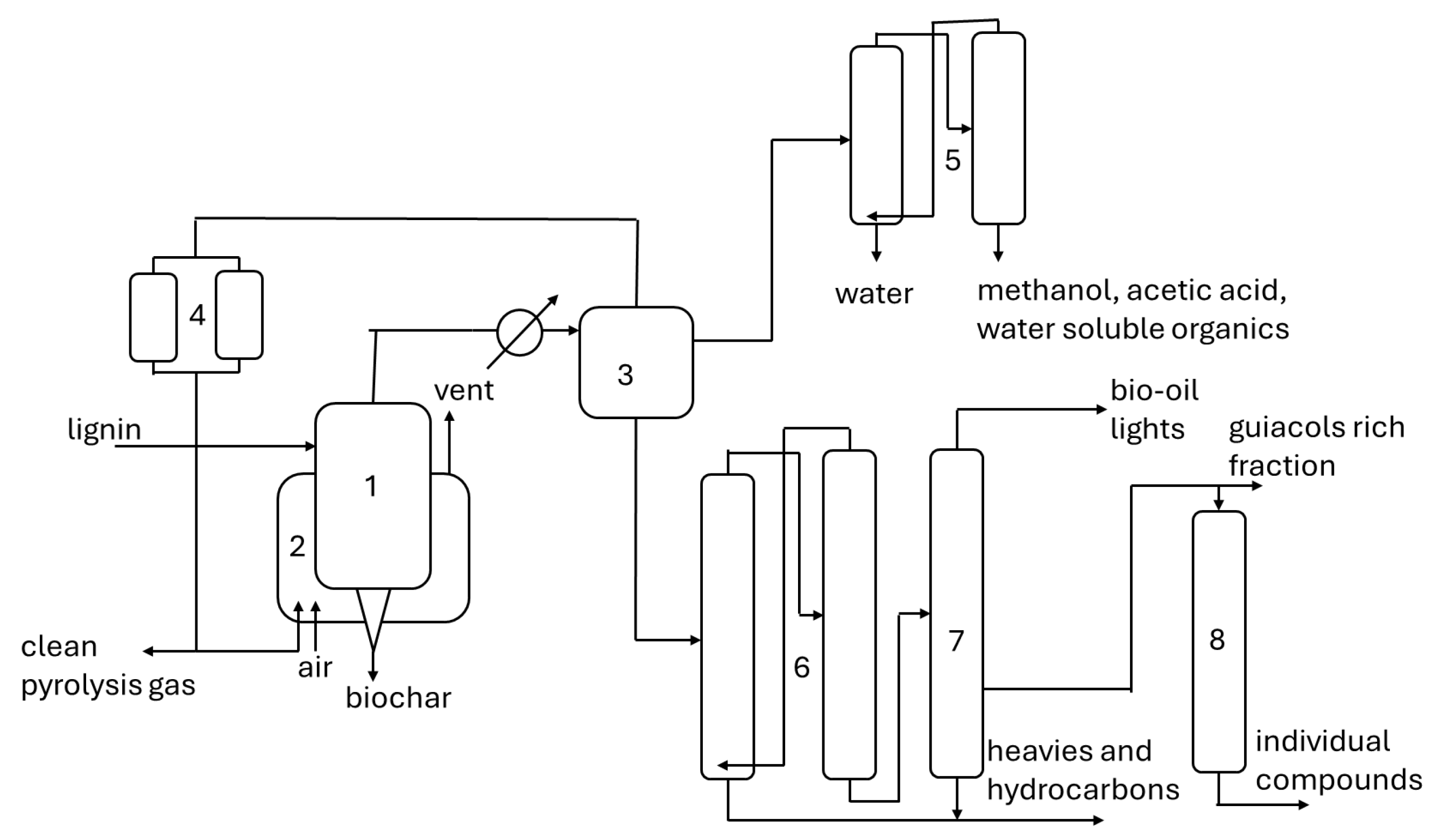
4. Product Separation from Slow Pyrolysis Bio-Oils
5. Exploitation of Individual Components of Biomass Slow Pyrolysis Oils
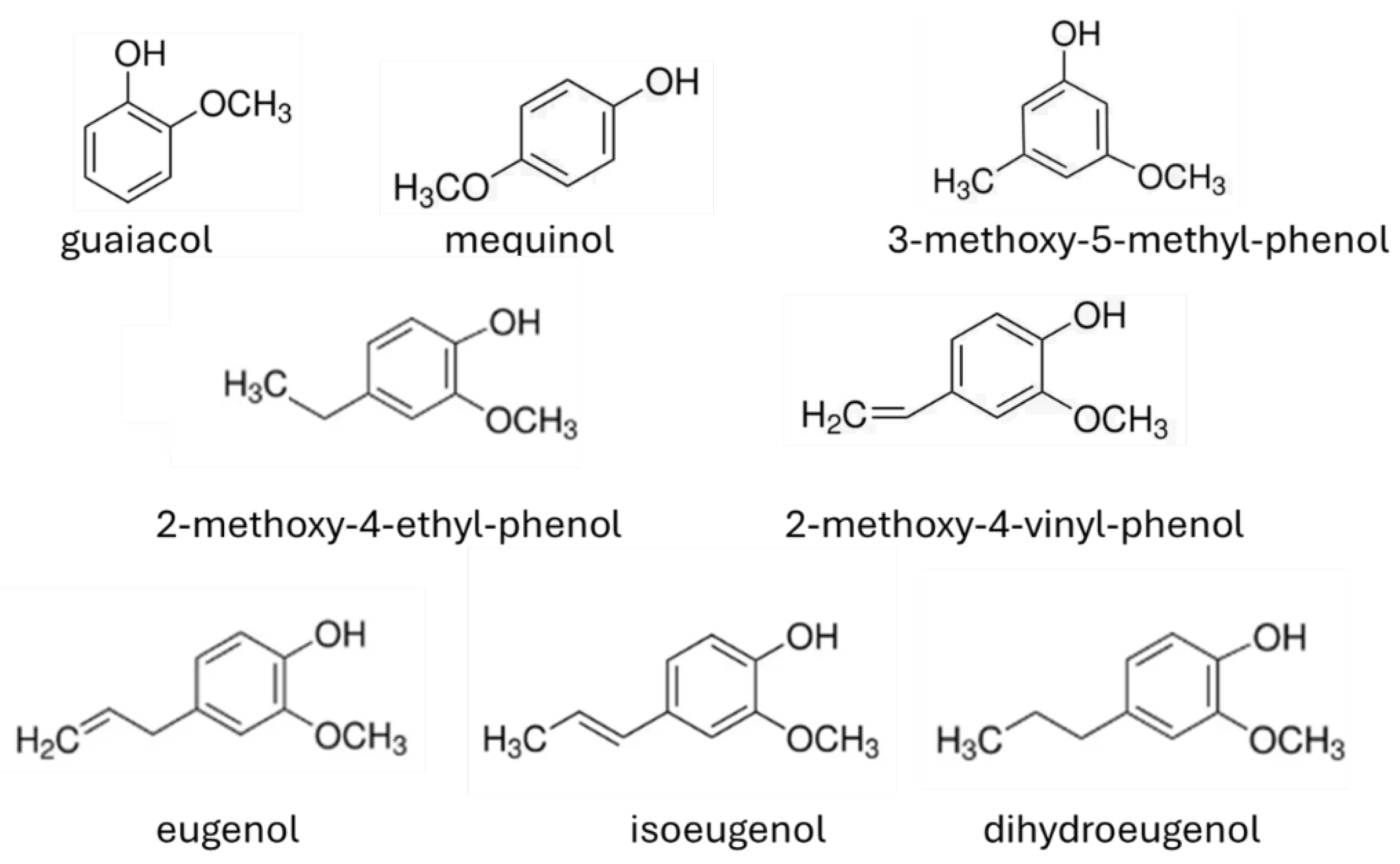
5.1. Guaiacol
5.2. Mequinol
5.3. Other Bio-Oil Components
6. Application of Lignin Pyrolysis Oil Fraction as a Whole
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Busca, G. Critical aspects of energetic transition technologies and the roles of materials chemistry and engineering, Energies, 2024, 17, 3565.
- Arapova, O.V.; Chistyakova, A.V.; Tsodikova, M.V.; Moiseeva, I.I. Lignin as a Renewable Resource of Hydrocarbon Products and Energy Carriers (A Review). Petrol. Chem., 2020, 60, 227–243.
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-carbohydrate complexes: properties, applications, analyses, and methods of extraction, Biotechnol. Biofeuls 2018, 11, 269. [Google Scholar]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach, J. En. Inst. 2020, 93, 235-271.
- Alzate-Gaviria, L.; Domín guez-Maldonado, J.; Chablé-Villacís, R.; Olguin-Maciel, E.; Leal-Bautista,R.M.; Canché-Escamilla, G.; CaballeroVázquez, A.; Hernández-Zepeda, C.; Barredo-Pool, F.A.; Tapia-Tussell, R. Presence of Polyphenols Complex Aromatic “Lignin” in Sargassum spp. From Mexican Caribbean. J. Mar. Sci. Eng. 2021, 9, 6.
- FAO. 2019. Forest Products Annual Market Review, 2018-2019. United Nations Economic Commission for Europe (UNECE). Geneva, Switzerland. [CrossRef]
- Chung, H.; Washburn, N.R. Chemistry of lignin-based materials, Green Mat. 2012, 1, 137-160.
- Yang, S.; Zhang, Y.; Yuan, T. Q.; Sun, R.C. Lignin-Phenol-Formaldehyde Resin Adhesives Prepared with Biorefinery Technical Lignins. J. Appl. Pol. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact, Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- . Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assesment of technical lignins for uses in biofuels and biomaterials: Structure-related properties, proximate analysis and chemical modification, Ind. Crops Prod. 2016, 83 (2016) 155–165.
- . Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P.; Dussap, C.G. Wood-lignin: Supply, extraction processes and use as bio-based material, European Polymer Journal 112 (2019) 228–240. [CrossRef]
- Kazzaz, A.E.; Fatehi, P. Technical lignin and its potential modification routes: A mini-review, Ind. Crops Prod. 2020, 154, 112732. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Cellulose-Based Sustainable Polymers: State of the Art and Future Trends, Macromol. Rapid Commun. 2011, 32, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Carolin, F. ; Kamalesh. T.; Kumar, P.S.; Hemavathy, R.V.; Rangasamy, G. A critical review on sustainable cellulose materials and its multifaceted applications, Ind. Crops Prod. 2023, 203, 117221.
- Mboowa, D. A review of the traditional pulping methods and the recent improvements in the pulping processes, Biomass Conv. Bioref., 2024, 14, 1–12.
- Li, P.; Xu, Y.; Yin, L.; Liang, X.; Wang, R.; Liu, K. Development of Raw Materials and Technology for Pulping—A Brief Review. Polymers 2023, 15, 4465. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J. T.; Alén, R.J. Chemical pretreatments of wood chips prior to alkaline pulping - A review of pretreatment alternatives, chemical aspects of the resulting liquors, and pulping outcomes, BioRes. 2015, 10, 8604-8656.
- Lehr, M.; Miltner, M.; Friedl, A. Removal of wood extractives as pulp (pre-)treatment: a technological, SN Appl. Sci., 2021, 3, 88.
- Fearon, O.; Kuitunen, S.; Ruuttunen, K.; Alopaeus, V.; Vuorinen, T. Detailed Modeling of Kraft Pulping Chemistry. Delignification. Ind. Eng. Chem. Res. 2020, 59, 12977–12985. [Google Scholar] [CrossRef]
- [1] Kienberger, M.; Maitz, S.; Pichler, T.; Demmelmayer, P. Systematic Review on Isolation Processes for Technical Lignin. Processes 2021, 9, 804. [Google Scholar] [CrossRef]
- Zevallos Torres, L.A.; Lorenci Woiciechowski, A.; Oliveira de Andrade Tanobe, V.; Karp, S.G.; Guimarães Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review, J. Cleaner Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Borella, M.; Casazza, A.A.; Garbarino, G.; Riani, P.; Busca, G. A Study of the Pyrolysis Products of Kraft Lignin. Energies 2022, 15, 991. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, M.; Wang, Q.; Wang, Y.; Liu, J.; Cao, C.; Zheng, W.; Bao, Y.; Rocchi, I. Use of Sulfur-Free Lignin as a novel soil additive: A multi-scale experimental investigation, Eng. Geol., 2020, 269, 105551.
- Ghysels, S.; Ronsse, F.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar from lignin-rich digested stillage from lignocellulosic ethanol production, Biomass Bioen. 2019, 122, 349-360.
- Priharto, N.; Ronsse, F.; Yildiz, G.; Heeres, H.J.; Deuss, P.J.; Prins, W. Fast pyrolysis with fractional condensation of lignin-rich digested stillage from second-generation bioethanol production. J. Anal. Appl. Pyrol., 2020, 145, 104756.
- D.K. Shen, S. Gua, K.H. Luo, S.R. Wang, M.X. Fang, The pyrolytic degradation of wood-derived lignin from pulping process, Biores. Technol. 2010, 101, 6136–6146.
- Mu, W.; Ben, H.; Ragauskas, A.; Deng, Y. Lignin Pyrolysis Components and Upgrading—Technology Review. Bioenerg. Res. 2013, 6, 1183–1204. [Google Scholar] [CrossRef]
- Ansari, K.B.; Arora, J.S.; Chew, J.W.; Dauenhauer, P.J.; Mushrif, S.H. Fast Pyrolysis of Cellulose, Hemicellulose, and Lignin: Effect of Operating Temperature on Bio-oil Yield and Composition and Insights into the Intrinsic Pyrolysis Chemistry, Ind. Eng. Chem. Res. 2019, 58, 15838−15852.
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A Review of Intermediate Pyrolysis as a Technology of BiomassConversion for Coproduction of Biooil and Adsorption Biochar, J. Renew. En. 2021, 5533780. [Google Scholar]
- https://www.mcgill.ca/oss/article/environment-health/charcoal-one-most-important-substances-ever-discovered.
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of Biochar From Lignocellulosic Biomass for Diverse Industrial Applications and Energy Harvesting: Effects of Pyrolysis Conditions on the Physicochemical Properties of Biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Grottola, C.M.; Giudicianni, P.; Stanzione, F.; Ragucci, R. Influence of Pyrolysis Temperature on Biochar Produced from Lignin–Rich Biorefinery Residue. Chem. Eng. 2022, 6, 76. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization, Clean. Techn. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Ngene, G.I.; Bouesso, B.; González Martínez, M.; Nzihou, A. A review on biochar briquetting: Common practices and recommendations to enhance mechanical properties and environmental performances, J. Cleaner Prod. 2024, 469, 143193. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar, IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1051, 012075.
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An overview of biochar production techniques and application in iron and steel industries. Biores. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef]
- Wang, S.; Chai, Y.; Wang, Y.; Luo, G.; An, S. Review on the Application and Development of Biochar in Ironmaking Production. Metals 2023, 13, 1844. [Google Scholar] [CrossRef]
- Safarian, S. To what extent could biochar replace coal and coke in steel industries? Fuel 2023, 339, 127401. [Google Scholar] [CrossRef]
- Riva, L.; Surup, G.R.; Videm Buø, T.; Nielsen, H.K. A study of densified biochar as carbon source in the silicon and ferrosilicon production, Energy, 2019, 1818, 985-996.
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.Lignin – from natural adsorbent to activated carbon: A review, Biores. Technol. 2007, 98, 2301-2312.
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage, Biores. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Biochars and activated carbons as adsorbents of inorganic and organic compounds from multicomponent systems – A review, Advan. Colloid Interface Sci. 2022, 305, 102687. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Wu, Y.; Wang, Q.; Wang, H.; Li, D. Fast-pyrolysis lignin-biochar as an excellent precursor for high-performance capacitors. Renew. En.. 2022, 198, 1318–1327. [Google Scholar] [CrossRef]
- Güllü, D.; Demirbaş, A. Biomass to methanol via pyrolysis process. En. Conv. Manag. 2001, 42, 1349–1356. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. 2010; 24.
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Comparison for the compositions of fast and slow pyrolysis oils by NMR Characterization. Biores. Technol. 2013, 147, 577–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Zhang, D.; Wu, Y.; Xie, H.; Brindhadevi, K.; Pugazhendhi, A.; Xia, C. A review of biomass pyrolysis gas: Forming mechanisms, influencing parameters, and product application upgrades, Fuel, 2023, 347, 128461.
- Borella, M.; Casazza, A.A.; Garbarino, G.; Riani, P.; Busca, G. Upgrading of Kraft Lignin pyrolysis products: managing sulfur impurities. Biores. Technol. Rep. in press.
- Raveendran, K.; Ganesh, A. Heating value of biomass and biomass pyrolysis products, Fuel 1996, 75, 1715-1720.
- Lopez, G.; Santamaria, L.; Lemonidou, A.; Zhang, S.; Wu, C.; Sipra, A.T.; Gao, N. Hydrogen generation from biomass by pyrolysis, Nature reviews, 2022, 2, 20.
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres, Biores. Technol. 2011, 102, 4258–4264. [Google Scholar]
- Kim, J.S. Production, separation and applications of phenolic-rich bio-oil—a review. Biores. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Drugkar, K.; Rathod, W.; Sharma, T.; Sharma, A.; Joshi, J.; Pareek, V.K.; Ledwani, L.; Diwekar, U. Advanced separation strategies for up-gradation of bio-oil into value-added chemicals: A comprehensive review, Sep. Purif. Technol. 2022, 283, 120149. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability, Fuel 2008, 87, 1230–1240.
- Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Pyrolytic lignin: a promising biorefinery feedstock for the production of fuels and valuable chemicals, Green Chem., 2022, 24, 4680–4702.
- Vitasari, C.R.; Meindersma, G.W.; de Haan, A.B. Water extraction of pyrolysis oil: The first step for the recovery of renewable chemicals, Biores. Technol. 2011, 102, 7204–7210. [Google Scholar] [CrossRef]
- Yan, W.H.; Duana, P.G.; Wanga, F.; Xu, Y.P. Composition of the bio-oil from the hydrothermal liquefaction of duckweed and the influence of the extraction solvents, Fuel 2016, 185, 229–235.
- Stephan, C.; Dicko, M.; Stringari, P.; Coquelet, C. Liquid-liquid equilibria of water+ solutes (acetic acid/acetol/furfural/guaiacol/methanol/phenol/propanal)+ solvents (isopropyl acetate/toluene) ternary systems for pyrolysis oil fractionation, Fluid Phase Equilib., 2018, 468, 49-57.
- Mantilla, S.V.; Manrique, A.M.; Gauthier-Maradei, P. Methodology for Extraction of Phenolic Compounds of Bio-oil from Agricultural Biomass Wastes. Waste Biomass Valor. 2015, 6, 371–383. [Google Scholar] [CrossRef]
- Li, X.; Kersten, S.R.; Schuur, B. Extraction of guaiacol from model pyrolytic sugar stream with ionic liquids, Ind. Eng. Chem. Res., 2016, 55, 4703-4710.
- González, E.J.; González-Miquel, M.; Díaz, I.; Rodríguez, M.; Fontela, C.; Cañadas, R.; Sánchez, J. Enhancing aqueous systems fermentability using hydrophobic eutectic solvents as extractans of inhibitory compounds, Sep. Purif. Technol., 2020, 250, 117184.
- Sun, M.; Diao, R.; Deng, J.; Zhu, X. Fractionation Behaviors of Walnut Shell Bio-Oil Components Under Atmospheric Distillation, BioEnergy Res. 2023, 16, 1121–1133.
- Fiege, H. Cresols and xylenols in Ullmann's Encyclopedia of Industrial Chemistry Copyright © 2002 by Wiley-VCH Verlag GmbH & Co. KGaA.
- Franck, H.G.; Stadelhofer, J.W. Industrial Aromatic Chemistry, Springer Verlag, Berlin, 1988, pp. 165-166.
- Yuan, X.; Sun, M.; Wang, C.; Zhu, X. Full temperature range study of rice husk bio-oil distillation: Distillation characteristics and product distribution, Sep. Purif. Technol. 2021, 263, 118382. [Google Scholar] [CrossRef]
- Mante, O.D.; Thompson, S.J.; Soukri, M.; Dayton, D.C. Isolation and Purification of Monofunctional Methoxyphenols from Loblolly Pine Biocrude, ACS Sust. Chem. Eng. 2019; 7, 2262–2269. [Google Scholar]
- Franck, H.G.; Stadelhofer, J.W. Industrial Aromatic Chemistry, Springer Verlag, Berlin, 1988, pp. 156.
- Ren, T.; Zhang, Z.; You, S.; Qi, W.; Su, R.; He, Z. Isolation and purification of 4-propylguaiacol and 4-propylsyringol by extraction and crystallization from the products of reductive catalytic fractionation processes, Green Chem. 2022; 24, 7355–7361. [Google Scholar]
- Vigneault, A.; Johnson, D.K.; Chornet, E. Base-Catalyzed Depolymerization of Lignin:Separation of Monomers, Canad. J. Chem. Eng. 2007, 85, 906–916. [Google Scholar]
- Choi, H.; Ramirez, K.J.; Alherech, M.; Jang, J.H.; Woodworth, S.P.; Karp, E.M.; Beckham, G.T. Counter-current chromatography for lignin monomer–monomerand monomer–oligomer separations from reductive catalytic fractionation oil, Green Chem., 2024, 26, 5900.
- Telysheva, G.; Dobele, G.; Meier, D.; Dizhbite, T.; Rossinska, G.; Jurkjane, V. Characterization of the transformations of lignocellulosic structures upon degradation in planted soil, J. Anal. Appl. Pyrol. 2007, 79, 52. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Zuo, L.; Zhang, T.; Xiao, W.; Yang, T.; Tursunov, O.; Zhao, N.; Zhou, Y. Regulating phenol tar in pyrolysis of lignocellulosic biomass: Product characteristics and conversion mechanisms, Biores. Technol. 2024, 409, 131259. [Google Scholar] [CrossRef]
- https://foreverest.net/news-list/characteristics-overview-applications-markets-of-guaiacol, accessed 20th August 2024.
- https://pubchem.ncbi.nlm.nih.gov/compound/Guaiacol#section=Drug-and-Medication-Information, accessed 20th August, 2024.
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: the perspectives and future. J. Sci. Food Agric. 2019, 99, 499–506. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the vanillin synthesis and biotransformation: A review Renew. Sust. En. Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Martău, G.A.; Călinoiu, L.F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production, Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar]
- Franck, H.G.; Stadelhofer, J.W. Industrial Aromatic Chemistry, Springer Verlag, Berlin, 1988, pp. 95-96.
- Fiege, H.; Voges, H.W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.J.; Garbe, D.; Paulus, W. Phenol Derivatives in Ullmann's Encyclopedia of Industrial Chemistry Copyright © 2002 by Wiley-VCH Verlag GmbH & Co. KGaA.
- Jenkins, S. New process for synthetic eugenol available to fragrance market, 2021 https://www.chemengonline.com/eugenol/?printmode=1, accessed 20 august 2024.
- Yi, H.; Yu, R.; Yang, Z.; Yu, J.; Zhou, Y.; Jiang, X.; Zhao, W.; Song, L. Research progress on pharmacological effect of eugenol, Drugs Clinic, 2024, 39, 1625 – 1630.
- Premkumar, J. Sampath, P.; Sanjay, R.; Chandrakala, A.; Rajagopal, D. Synthetic Guaiacol Derivatives as Promising Myeloperoxidase Inhibitors Targeting Atherosclerotic Cardiovascular Disease, ChemMedChem 2020, 15, 1187–1199.
- Luque, R.; Campelo, J.M.; Conesa, T.D.; Luna, D.; Marinas, J.M.; Romero, A.A. Catechol O-methylation with dimethyl carbonate over different acid–base catalysts, New J. Chem. 2006; 30, 1228–1234. [Google Scholar]
- Weber, M.; Weber, M.; Kleine-Boymann, M. Phenol, in Ullmann's Encyclopedia of Industrial Chemistry Copyright © 2002 by Wiley-VCH Verlag GmbH & Co. KGaA.
- Schmidt, R.J. Industrial catalytic processes—phenol production, Appl. Catal. A: Gen., 2005, 280, 89–103.
- [1] Perego, C.; de Angelis, A.; Pollesel, P.; Millini, R. Zeolite-Based Catalysis for Phenol Production, Ind. Eng. Chem. Res. 2021; 60, 6379–6402. [Google Scholar]
- Dang, R.; Ma, X.; Luo, J.; Zhang, Y.; Fu, J.; Li, C.; Yang, N. Hydrodeoxygenation of 2-methoxy phenol: Effects of catalysts and process parameters on conversion and products selectivity, J. En. Inst. 2020, 93, 1527–1534. [Google Scholar] [CrossRef]
- Valizadeh, S.; Khani, Y.; Kang, B.S.; Hwang, J.; Jae, J.; Ko, C.H.; Han, J.W.; Park, Y.K. Catalytic conversion of guaiacol to phenol and alkylphenols over Mo-promoted Ni/CeO2 catalyst in supercritical ethanol, Appl. Catal. B: Envir. En. 2024, 348, 123823.
- Li, K.; Yu, S.; Li, Q.; Zhang, Y.; Zhou, H. Selective hydrodeoxygenation of guaiacol to cyclohexanol using activated hydrochar-supported Ru catalysts Front. Chem. Sci. Eng. 2024, 18, 50. [Google Scholar]
- Gracia, J.; Ayala-Cortés, A.; Di Stasi, C.; Remon, J.; Torres, D.; Pinilla, J.L.; Suelves, I. Highly selective catalytic hydrodeoxygenation of guaiacol to benzene in continuous operation mode, Fuel Proc. Technol. 2024, 255, 108064. [Google Scholar]
- Mukhtarova, M. ; Golubeva,M.; Sadovnikov, A.; Maximov, A. Guaiacol to Aromatics: Efficient Transformation over In SituGenerated Molybdenum and Tungsten Oxides. Catalysts, 2023; 13, 263. [Google Scholar]
- Zhang, H.; Yang, T.; Tong, Y.; Li, B.; Wang, J.; Li, R. FeNi/hexagonal boron nitride for catalytic hydrodeoxygenation of guaiacol derived from lignin to cycloalkanes, Fuel 2024, 368, 131620.
- Demchuk, Z.; Mora, A.S.; Choudhary, S.; Caillol, S.; Voronov, A. Biobased latexes from natural oil derivatives, Ind. Crops Prod. 2021, 162, 113237. [Google Scholar] [CrossRef]
- Tian, M.; McCormick, R.L.; Ratcliff, M.A.; Luecke, J.; Yanowitz, J.; Glaude, P.A.; Cuijpers, M.; Boot, M.D. Performance of lignin derived compounds as octane boosters, Fuel 2017, 189, 284–292.
- Hessefort, N.L. Characterization of Inhibitors in Vinyl and Acrylic Monomers, J. Liq. Chrom. 1990, 13, 2561–2579. [Google Scholar] [CrossRef]
- Gambarotti, C.; Melone, L.; Punta, C.; Shisodia, S.U. Selective Monoetherification of 1,4-Hydroquinone Promoted by NaNO2 Curr. Org. Chem., 2013, 17, 1108-1113.
- https://pubchem.ncbi.nlm.nih.gov/compound/Mequinol, accessed 20th August 2024.
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for Hyperpigmentation. What is Available? J. Cutaneous Aesthetic Surgery 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- William, K.W. Producton of para-diisopropylbenzene and use of the same in the production of hydroquinone. European Patent 1985, 0149508.
- Costantini, M.; Fache, E.; Michelet, D.; Manaut, D. Selective access to hydroquinone: Fuchsone route. Ind. Chem. Libr. 1996, 8, 350–367. [Google Scholar]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A. Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; Fryer, A.D.; Jones, L.; Joshi, K.; Kumar, M.; Lapczynski, A.; Lavelle, M.; Lee, I.; Liebler, D.C.; Moustakas, H.; Na, M.; Penning, T.M.; Ritacco, G.; Romine, J.; Sadekar, N.; Schultz, T.W.; Selechnik, D.; Siddiqi, F.; Sipes, I.G.; Sullivan, G.; Thakkar, Y.; Tokura, Y. Update to RIFM fragrance ingredient safety assessment, eugenol, CAS Registry Number 97-53-0 Food Chem. Toxicol. 2022, 163, Supplement 1, 113027.
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Ju Yeon Lee, J.Y.; Jung, M.Y. Effects and mechanisms of eugenol, isoeugenol, coniferylaldehyde and dihydroeugenol on the riboflavin-sensitized photooxidation of α-terpinene in methanol, Food Chem. 2017, 220, 289-294.
- Emulsion Polymerization of Dihydroeugenol-, Eugenol-, andIsoeugenol-Derived Methacrylates, Ind. Eng. Chem. Res. 2019, 58, 21155−21164 Samantha Molina-Gutiérrez,†,‡ Vincent Ladmiral,*,† Roberta Bongiovanni,‡ Sylvain Caillol,†and Patrick Lacroix-Desmazes.
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A. Jr.; Cancellieri, M.A.; Chon, H.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; Fryer, A.D.; Jones, L.; Joshi, K.; Kumar, M.; Lapczynski, A.; Lavelle, M.; Lee, I.; Liebler, D.C.; Moustakas, H.; Na, M.; Penning, T.M.; Ritacco, G.; Romine, J.; Sadekar, N.; Schultz, T.W.; Selechnik, D.; Siddiqi, F.; Sipes, I.G.; Sullivan, G.; Thakkar, Y.; Tokura, Y. RIFM fragrance ingredient safety assessment, 3-methoxy-5-cresol, CAS Registry Number 3209-13-0, Food Chem. Toxicol. 2022, 167, 113314. [Google Scholar]
- https://www.biosynth.com/p/FM25257/3209-13-0-3-methoxy-5-methylphenol accessed 20th August 2024.
- Yuan, J.; Tao, Y.; Wang, M.; Huang, F.; Wu, X. Natural compounds as potential therapeutic candidates for multiple sclerosis: Emerging preclinical evidence, Phytomedicine 2024, 123, 155248.
- Rigo, E.; Totée, C.; Ladmiral,V.; Caillol, S.; Lacroix-Desmazes, P.4-Vinyl Guaiacol: A Key Intermediate for Biobased Polymers. Molecules 2024, 29, 2507.
- Rajkumar, P.; Selvaraj, S.; Anthoniammal, P.; Ram Kumar, A.; Kasthuri, K.; Kumaresan, S. Structural (monomer and dimer), spectroscopic (FT-IR, FT-Raman, UV–Vis and NMR) and solvent effect (polar and nonpolar) studies of 2-methoxy-4-vinyl phenol, Chem. Phys. Impact 2023, 7, 100257. [Google Scholar] [CrossRef]
- Mateus, M.M.; Moura Bordado, J.; Galhano dos Santos, R. Estimation of higher heating value (HHV) of bio-oils from thermochemical liquefaction by linear correlation, Fuel 2021, 302, 121149.
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Cheng, Y.; Addy, M.; Lu, Q.; Omar, M.M.; Liu, Y.; Wang, Y.; Dai, L.; Andreson, E.; Peng, P.; Lei, H.; Ruan, R. ; Bio-oil from fast pyrolysis of lignin: Effects of process and upgrading parameters, Biores. Technol. 2017, 241, 1118–1126. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Zacher, A.H.; Wang, L.; Ren, S.; Liang, J.; Wei, Y.; Liu, Y.; Tang, J.; Zhang, Q.; Ruan, R. ; A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis, Biores. Techol. 2012, 124, 470–477. [Google Scholar]
- Shu, R.; Li, R.; Lin, B.; Wang, C.; Cheng, Z.; Chen, Y. A review on the catalytic hydrodeoxygenation of lignin-derived phenolic compounds and the conversion of raw lignin to hydrocarbon liquid fuels, Biomass Bioen. 2020, 132, 105432.
- Luna-Murillo, B.; Pala, M.; Lucini Paioni, A.; Baldus, M.; Ronsse, F.; Prins, W.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Catalytic Fast Pyrolysis of Biomass: Catalyst Characterization Reveals the Feed-Dependent Deactivation of a Technical ZSM-5-Based Catalyst, ACS Sustain. Chem. Eng. 2021, 9, 291–304. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Li, C.; Zhang, S.; Wang, Y.; Esmaeili, V.; Gholizadeh, M. Fates of heavy organics of bio-oil in hydrotreatment: The key challenge in the way from biomass to biofuel, Sci. Total Envir. 2021, 778, 146321. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Zhao, Y.P.; Li, Q.; Qiu, L.L.; Liu, F.J.; Liang, J.; Li, J.; Cao, J.P. Highly efficient conversion of lignin bio-oil and derived phenols to cyclohexanols over low-loading Ni/C catalyst, Fuel 2024, 371, 132030.
- Di Nardo, A.; Portarapillo, M.; Russo, D.; Di Benedetto, A. Hydrogen production via steam reforming of different fuels: thermodynamic comparison, Int. J. Hydr. En. 2024, 55, 1143–1160. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Aziz, M.A.A.; Abdullah, S.; The, L.P.; Jusoh, R. Hydrogen production from catalytic steam reforming of biomass pyrolysis oil or bio-oil derivatives: A review, Int. J. Hydrogen En. 2020, 45, 18376–18397. [Google Scholar] [CrossRef]
- Pafili, A.; Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Wang, W.; Liu, G.; Papadakis, V.G.; Goula, M.A. Recent Progress in the Steam Reforming of Bio-Oil for Hydrogen Production: A Review of Operating Parameters, Catalytic Systems and Technological Innovations. Catalysts 2021, 11, 1526. [Google Scholar] [CrossRef]
- Remiro, A.; Valle, B.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Steam Reforming of Raw Bio-oil in a Fluidized Bed Reactor with Prior Separation of Pyrolytic Lignin, Energy Fuels 2013, 27, 7549−7559.
| Biomass | Lignin content | Process conditions | Char (%) | Bio-oil (%) | Gas (%) | Ref |
| Lignin-rich digested stillage | - | Batch, 370-450 °C | 50.7 | - | - | 25 |
| Hazelnut shell | 42.5 | Tubular reactor, 295-850 K | 33.2±2.0 | 20.8±1.7 | 16 ± 2.3 | 47 |
| Softwood Kraft Lignin | - | Quartz tubular reactor, 400-600 °C |
57.21-38.14 | 34.54-44.27 | 8.25-17.59 | 50 |
| Kraft lignin | - | Tubular reactor, 550 °C | 42.7 | 28.7 | 28.6 | 52 |
| Lignin | - | Packed bed pyrolyser, 500 °C | 41.7 | 26.8 | 38.8 | 53 |
| Biomass | Separation method | Compound of interest | Ref |
| Wheat–hemlock | Supercritical fluid extraction | Furanoids, pyranoids, benzenoids | 56 |
| Rice husk | Basification–acidification and column chromatography | Phthalate esters | 56 |
| ForestResidue | Water extraction | Glycoladehydes, acetic acid, acetol, furfural, furanone, levoglucosan, syringol, guaiacol | 60 |
| Sugarcane bagasse, palm empty fruit bunch |
L-L extraction | Phenols | 63 |
| Acqueous levoglucosan solution | Ionic liquid extraction | Guaiacols | 64 |
| Wallnut shell | Atmospheric distillation | Phenols, ketones, ethers, carboxylic acids | 66 |
| Rice husk | Distillation | Guaiacol, 4-methyl guaiacol |
69 |
| Loblolly Pine | Distillation and chromatography | Methoxyphenols | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





