1. Introduction
Microplastics (MPs) have emerged as critical contaminants in the environment and represent a latent issue regarding the quality of water intended for human consumption [
1]. A study conducted in 2010 revealed that 8 out of 10 surface water samples collected worldwide contain a high percentage of microplastics [
2]. This situation is exacerbated by the increasing production and use of plastics, coupled with a lack of awareness and inadequate recycling techniques, resulting in a significant accumulation of these contaminants in water sources [
3]. The estimated ingestion of microplastics by humans varies between 0.1 and 5 g per week, with exposure through drinking water being a significant vector, accounting for estimates of up to 4.7 × 10³ particles/person/year (12.9 particles/person/day) [
4,
5].
As microplastics can accumulate in water sources over time, it is imperative to monitor their presence, especially since existing water treatment facilities are not specifically designed to remove these contaminants [
6]. In Ecuador, a 2019 study revealed that 65.66% of water samples from storage tanks contained microplastics, raising serious concerns among consumers [
7]. However, there is a call for more rigorous analysis due to potential loss of microplastics during the sampling and storage process.
The lack of a standardized approach for the collection and analysis of microplastics has led to a scarcity of comparable data in the scientific literature [
8]. Variability in analytical methods and a lack of consensus on standards have hindered the acquisition of reliable figures regarding the concentration of microplastics in drinking water, highlighting an urgent need to establish reliable assessment methods that ensure the quality and comparability of data [
9].
To address this issue, our research was conducted in two stages. First, a meta-analysis of existing studies evaluating the presence of microplastics in drinking water was performed [
10,
11]. This meta-analysis enabled the identification and comparison of various methodologies for quantifying and characterizing microplastics, emphasizing the disparity in results and the necessity for standardizing analytical techniques [
12,
13]. Subsequently, based on the findings from the meta-analysis, an experimental study was conducted in the Life Sciences laboratories at the Salesian Polytechnic University in Cuenca, Ecuador. This study employed Fourier Transform Infrared (FTIR) spectroscopy to analyze water samples, implementing a rigorous approach that included sample enrichment preparation and advanced filtration techniques [
14,
15,
16,
17]
The primary objective of this research is to establish a reliable and efficient methodology for the analysis of microplastics in drinking water, generating comparable data that contribute to a deeper understanding of the risks associated with exposure to these contaminants [
18,
19,
20]. It is anticipated that the results of this study will serve as a foundation for future research in the field of microplastic pollution in water resources [
21,
22].
2. Materials and Methods
The investigation consists of four stages detailed below:
2.1. First Stage: Systematic Search And Meta-Analysis Indeed
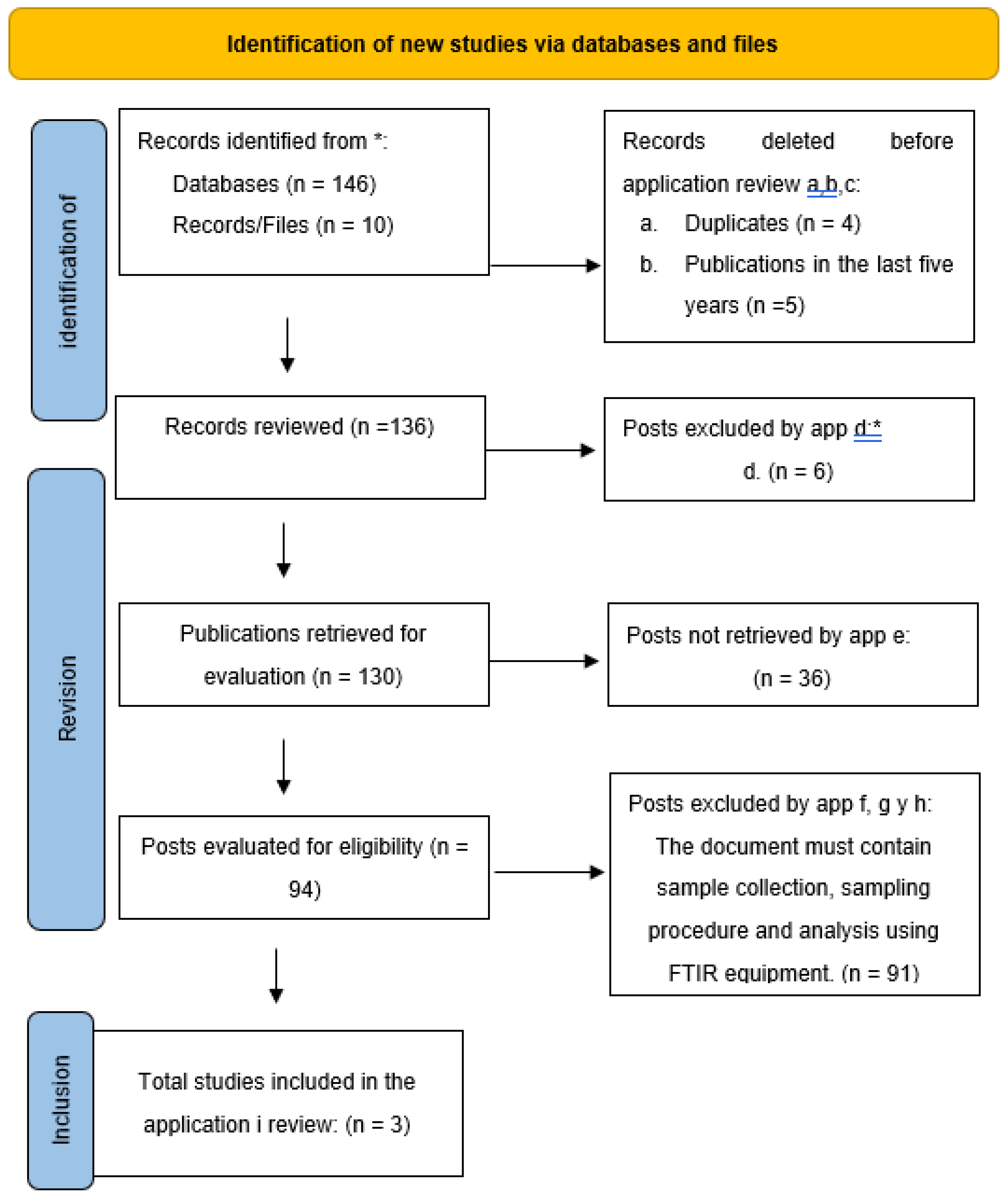
The meta-analysis is based on a systematic review of the topic to be discussed. As a result of this review, a PRISMA diagram was created, a methodology employed by various authors to facilitate the interpretation of results. In applying the meta-analysis, a forest plot was utilized to conduct a statistical analysis of heterogeneity. The process was structured as follows: data was stored using a computer, Review Manager 5.4 software, and Excel. The following methods were applied:
Defining the research question: The first step was to formulate the research question, with the objective being to conduct a meta-analysis of the primary techniques for analyzing microplastics in water samples for human consumption using FTIR. The key terms identified for the question were: "analysis techniques of microplastics in water for human consumption." To clearly define the question, we asked: What exactly do we want to know? Thus, the question guiding the systematic review was:
What are the techniques used for the analysis of microplastics?
Next, it was determined whether this question had already been answered, if there was existing research addressing it, or if an evaluation of the question had been carried out to avoid redundancy in the research. If such research existed, it was compared with other relevant studies [
23].
Database Search
To ensure the research protocol was followed, the search was conducted using the most prestigious scientific databases: Scopus, ScienceDirect, and Web of Science. Additionally, a search was performed on Google Scholar for academic and thesis documents.
Similarly, for a systematic review to be effective, the search formula is crucial in narrowing down the results. In this research, the following search terms were used in the databases: "microplastics"; "microplastics" AND "water"; "microplastics" AND "methodology"; "FTIR" AND "microplastics"; (*quality) AND (water). To further limit the number of results, the language and accessibility of the documents were also considered.
Selection and Data Extraction
Data was gathered from various databases using both inductive and deductive research methods. Relevant information was analyzed to establish inclusion and exclusion criteria. For this investigation, 156 documents were collected, of which 4 were immediately eliminated due to being duplicates, leaving 152 documents for review.
Each document was then evaluated based on six inclusion and exclusion criteria, as outlined below:
Date: The publication date of each document was reviewed to ensure the information was relevant to the current research. Only documents from the last 5 years (2017 to 2022) were included. As a result, 147 documents were retained, while 4 were excluded.
Access: The accessibility of publications was examined to facilitate future research and ensure reviewers could access all documents without issue. Publications that required paid access or were housed in inaccessible databases were excluded, resulting in the elimination of 12 publications.
Microplastics: This criterion was applied because some publications only briefly mentioned "microplastics" or confused the term with "nanoplastics." Any publications not primarily focused on microplastics were excluded, resulting in the elimination of 6 documents.
Consumption: This criterion included only articles that addressed microplastics in water for human consumption, such as drinking water, bottled water, or water from natural sources. Articles related to wastewater, seawater, etc., were excluded, resulting in the elimination of 36 articles.
Samples, Processing, and Analysis: The sampling criterion was crucial to understand how researchers collected samples, including the location, total number of samples, the quantity collected, and storage methods. Processing was the most important criterion, as it focused on how the microplastics were detected, including the filters used, which is a key aspect of this research. Studies that did not explain the sample processing were discarded. The analysis criterion also excluded documents that did not use FTIR equipment for analysis. As a result of applying these criteria, 85 articles were excluded.
Experimentation: The final inclusion and exclusion criterion evaluated whether the research was experimental or merely descriptive and analytical.
Ease/Difficulty: In the final set of documents, a cost assessment of the materials and reagents used in the studies was performed. Studies where the costs were inaccessible or where materials were difficult to acquire due to geographic constraints were excluded.
2.2.-. Second Stage: Application of the Actual Techniques Selected According to the Actual Meta-Analysis Indeed in the Actual Laboratory
According to what was obtained in the actual meta-analysis indeed, the actual selected techniques will be replicated, because of the actual results found in the actual first stage, selecting the actual following techniques:
Technique 1: (Pivokonsky et al., 2018) [24-25]
Technique 2: (Kankanige & Babel, 2020) [
26]
Technique 3: (Naspud Mogrovejo & Zhiñin Sarango, 2022) [
27]
To begin the actual validation of the actual selected techniques, we begin by preparing blank samples, to avoid false positive results in the actual samples to be analyzed.
2.2.1. Blank Samples
The different filters used in each technique were analyzed with the FTIR equipment to determine
their composition and ensure their proper consideration in the results. To rule out contamination
from the water or any factory-made filters, 250 mL of distilled water was placed in a beaker, and Nile
Red dye, along with methanol (1 mg/L), was added. This mixture was then placed in an oven at 30°C
for 30 minutes to allow any potential polymers to take on a fluorescent color.
Afterward, the filter was placed in a Büchner funnel, and vacuum filtration of the 250 mL
mixture was performed. The filter was then removed, placed in previously cleaned and sterilized
Petri dishes, covered, and placed back in the oven at 30°C for 30 minutes. This procedure was applied
to five filters, one of each type of material used in the research, as described below:
5 μm PTFE filter
0.22 μm PTFE filter
0.45 μm cellulose nitrate filter
20 μm cellulose filter
0.45 μm nylon filter
Once the dry filters were removed from the oven, they were subjected to fluorescence
microscopy and subsequently analyzed using the FTIR equipment to ensure that the filters were free
of polymers that might interfere with the results
2.2.2. Preparation of Enriched Samples.
For the application of the different techniques obtained through the meta-analysis, it was
necessary to start with an enriched sample prepared in the laboratory with the objective of knowing
the exact amount of microplastics and the types of polymers used in the analysis. According to the
various comparative studies, the microplastics most frequently detected through FTIR analysis were
polyethylene terephthalate (PET), polyamide (PA), polyethylene (PE), and polypropylene (PP). These
were used to create the spiked samples by following the steps below:
Reduction in sample size by crushing.
Sample sieving: After each polymer was ground, the samples were sieved using 500 μm and 355
μm stainless steel sieves, respectively. This allowed for obtaining microplastics of the desired size for
subsequent analysis.
Weighing and storage of samples: A 1-gram sample of each processed polymer was taken and
stored in glass bottles with metal lids to prevent contamination and maintain the integrity of the
samples during analysis.
Verification of the selected polymer types using FTIR.
2.2.3. Preparation of Spiked Samples for Processing
Sampling was based on enriched samples for this investigation. To improve the reliability of the
study, two repetitions were performed for each sample across all techniques. For the enriched sample,
an initial weight of 0.050 mg of each type of microplastic was added to 250 mL of distilled water.
2.2.4. Application of selected Meta-Analysis Techniques
2.2.4.1. Experimental Application of Technique 1
The technique followed in the laboratory was based on replicating the method suggested by
Pivokonsky, applying the following steps:
Wet Oxidation with Peroxide: Wet oxidation was performed using 30% hydrogen peroxide
solution to eliminate false positives from the enriched samples. For this, 20 mL of 30% hydrogen
peroxide and 20 mL of 0.05 M Fe(II) were combined and left to react.
Filtration of the samples: Each sample was vacuum filtered through a 5 μm PTFE
(polytetrafluoroethylene) membrane filter. A second filtration was then performed using a filter with
a pore size of 0.2 μm. Each filter was placed in a covered Petri dish to avoid external contamination
and heated in an oven at 30°C for 30 minutes.
Staining with Nile Red: As described in the methodology, random sampling was used to make
three strategic cuts in each filter (approximately 3 mm wide and 8 mm high). The Nile Red stain and
methanol (1 mg/L) were applied to these cut areas. The filters were then placed in covered Petri dishes
and left to dry at 30°C for 30 minutes.
Analysis and quantification: Once dried, all filter sections were observed under fluorescence
microscopy. The microplastics (MPs) were then analyzed using FTIR to determine the types of
polymers present.
2.2.4.2. Experimental Application of Technique 2 (Kankanige & Babel, 2020)
To apply the second technique, the author emphasized the use of sterile gloves and damp cloths
to clean the work area. Additionally, 0.45 μm cellulose nitrate filters and the enriched water-diluted
samples were used.
Vacuum filtration: Each filter was weighed and placed in a Büchner funnel for vacuum filtration.
After filtration, the filters with MPs were placed in labeled and numbered Petri dishes, then dried in
an oven at 30°C for 24 hours. Once removed from the oven, the filters were weighed, and MP particles
were observed under a dark-field microscope. Microparticles of around 50 μm were manually
extracted using metal tweezers.
Staining with Nile Red: After excluding the most visible microplastics, the filter was washed
with 200 mL of distilled water, and the Nile Red stain was applied. The stained filter was then heated
at 30°C for 30 minutes. Two filtrations were performed for each sample: one with a 20 μm cellulose
filter and another with a 0.45 μm cellulose nitrate filter. The filters were left to dry in covered, labeled
Petri dishes for 24 hours at room temperature.
Analysis and quantification: The dried filters were observed under fluorescence microscopy.
MPs were analyzed, and their presence and type were confirmed using FTIR.
2.2.4.3. Experimental Application of Technique 3 (Naspud Mogrovejo & Zhiñin Sarango, 2022)
For Technique 3, various steps described by multiple authors for identifying microplastics were
followed:
Sample digestion: Oxidative digestion aids in microplastic recovery without affecting polymer
structures. A 20% hydrogen peroxide solution was prepared for this. The 0.45 μm nylon filter was
washed with 10 mL of the solution for 1 minute, followed by an additional 10 mL of hydrogen
peroxide passed through the funnel to eliminate false positives.
Vacuum filtration: The nylon filter was moistened with hydrogen peroxide and placed in a
Büchner funnel. Each prepared sample (250 mL) was passed through vacuum filtration. The filter
was then placed in a covered Petri dish and dried in an oven at 45°C for 24 hours.
Staining of the samples: Two to three drops of Nile Red (1 μg/mL in 100 mL methanol) were
applied to the nylon filter and left to sit for 20 minutes, covered with aluminum foil.
Analysis and quantification: After staining, the samples were analyzed under a fluorescence
microscope. Using metal tweezers, the filters were placed in Petri dishes for analysis, and potential
MPs were marked for further FTIR analysis.
2.3. Third Stage: Comparison of Results
A statistical analysis was conducted by comparing histograms of the retained filter weights and the number of MPs quantified, to determine the advantages and disadvantages of each technique.
2.4. Standardizing Techniques for Microplastic Identification
Based on the results from the previous stages, it is important to propose a standardized technique for microplastic identification in the laboratory. Ensuring the quality, reliability, and utility of data generated in environmental studies on microplastic contamination is essential. This will significantly contribute to conservation efforts, environmental protection, and public health.
3. Results
3.1. Systematic Search
As a result of the systematic review, the PRISMA diagram (
Figure 2) was generated, a methodology widely used by various authors that aids in interpreting the results.
Table 1 presents the different criteria that facilitate understanding, including the inclusion and exclusion criteria.
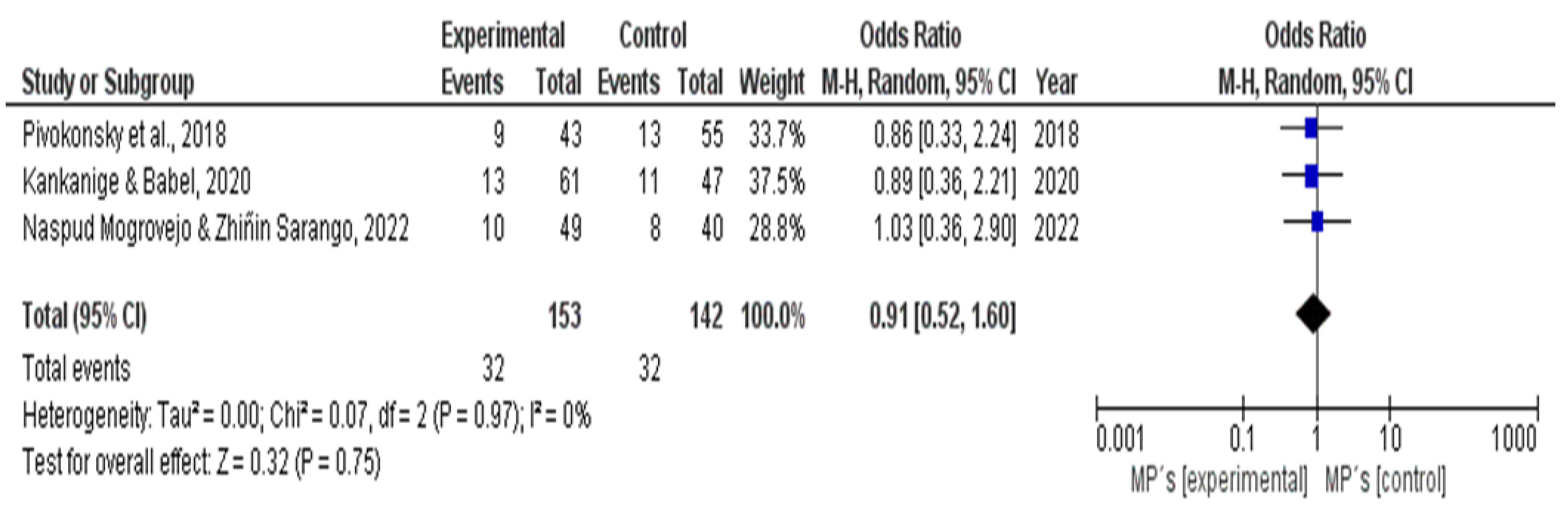
3.2. Meta-Analysis Indeed - Forest Plot.
For the statistical analysis of heterogeneity, the variation in reagents, materials, and equipment used by each author was considered. A total count of all the instruments used was conducted and then divided by the number reported in each study.
For the control group, three experimental investigations were considered, but they were excluded in the PRISMA diagram due to the exclusion criteria set by the authors Flores Calle & Orozco, Gualoto Jung, and Mintenig [28-29-30], and were organized chronologically by the year of publication.
The results were processed using REVIEW MANAGER 5.4 software, which generated the forest plot displayed below. The inclusion of the forest plot is crucial, as it visually represents the effect size and confidence intervals of the studies included in the meta-analysis, providing a clear comparison of individual study results and overall trends. This contribution is significant for assessing consistency among the studies and identifying potential sources of heterogeneity, thus enhancing the reliability and robustness of the overall findings.
In the confidence intervals, the 95% CI was considered, demonstrating that the group studied was not considered random. Likewise, in the heterogeneity analysis, it was noted that if there was a high percentage of variation, the study would not be feasible. However, the heterogeneity obtained through the software was 0%, indicating that the studies are quite similar, as can be seen in both the experimental and control groups. The width of the diamond shape represents the confidence interval, which falls between the values of 0.1 and 10, as observed in
Figure 2.
3.3. Application of the Techniques Selected According to the Meta-Analysis in the Laboratory
3.3.1. Filter Analysis Results (White)
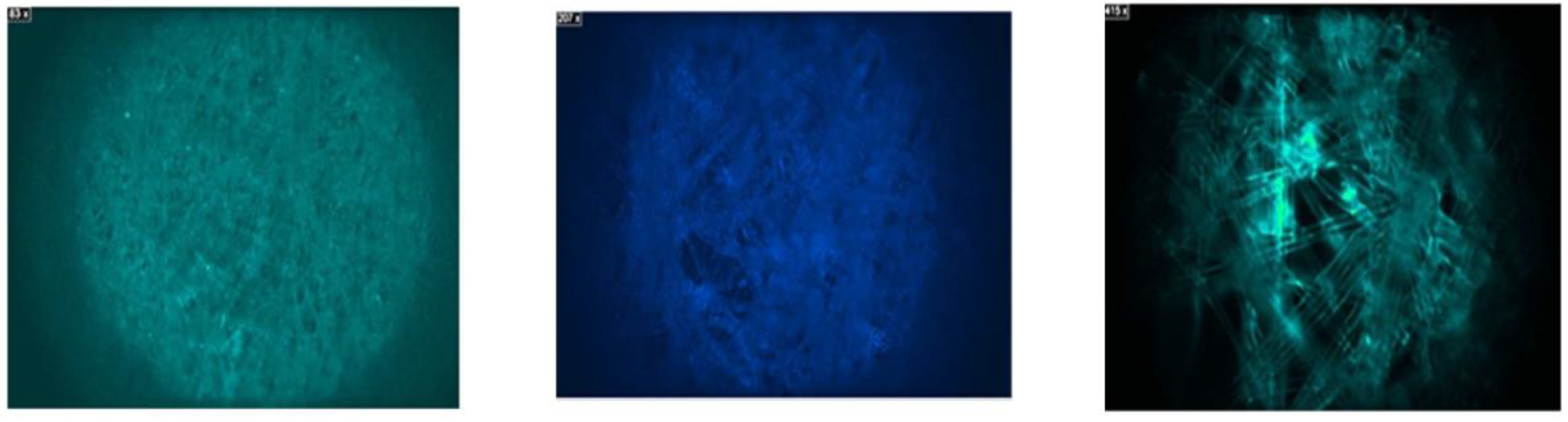
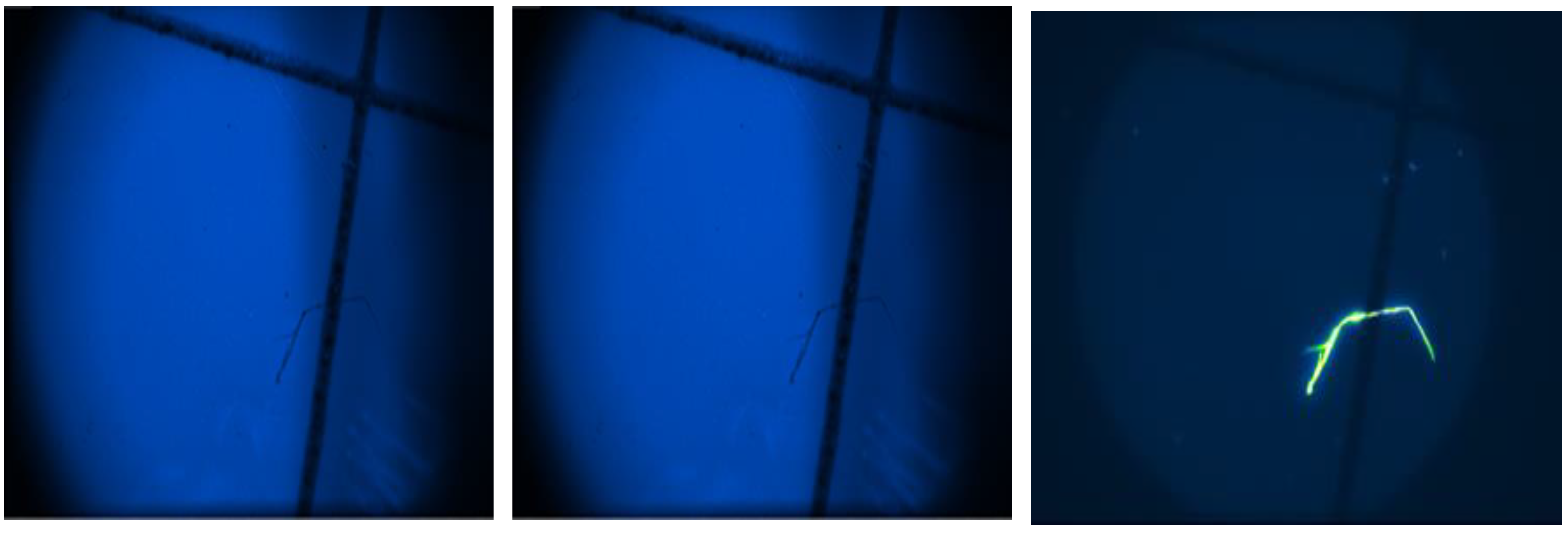
Based on the results obtained from the analysis, we can state the following: 5 μm PTFE filter: No fluorescent particles were found on the filter, indicating the absence of microplastics. Furthermore, FTIR analysis confirmed that the particles observed were part of the filter fibers and not additional polymers. Therefore, this filter can be considered effective in trapping microplastics.
Figure 4.
View of Fluorescence on the actual 5 μm PTFE filter.
Figure 4.
View of Fluorescence on the actual 5 μm PTFE filter.
0.22 μm PTFE filter:
Similar to the previous filter, no fluorescent particles were observed on the filter at most resolutions. FTIR analysis also confirmed the absence of additional polymers, suggesting that the 0.22 μm PTFE filter may also be effective in trapping microplastics.
0.45 μm cellulose nitrate filter:
In this case, some particles were observed on the filter, though they were not fluorescent. However, after further analysis, luminescent particles were found throughout the filter. FTIR analysis revealed that these particles were part of the filter's initial structure and not additional microplastics, as shown in
Figure 5
20 μm cellulose filter: No fluorescent particles were found on this filter, indicating its effectiveness in trapping microplastics. A random analysis of the filter composition was conducted using an FTIR spectrometer, which confirmed that it consisted solely of the original filter structure.
0.45 μm nylon filter: This filter presented some challenges in the analysis due to its composition, which includes polyamide and nylon, materials that can be mistaken for microplastics. Therefore, a more detailed and specific analysis is required to distinguish between the filter polymers and potential microplastics.
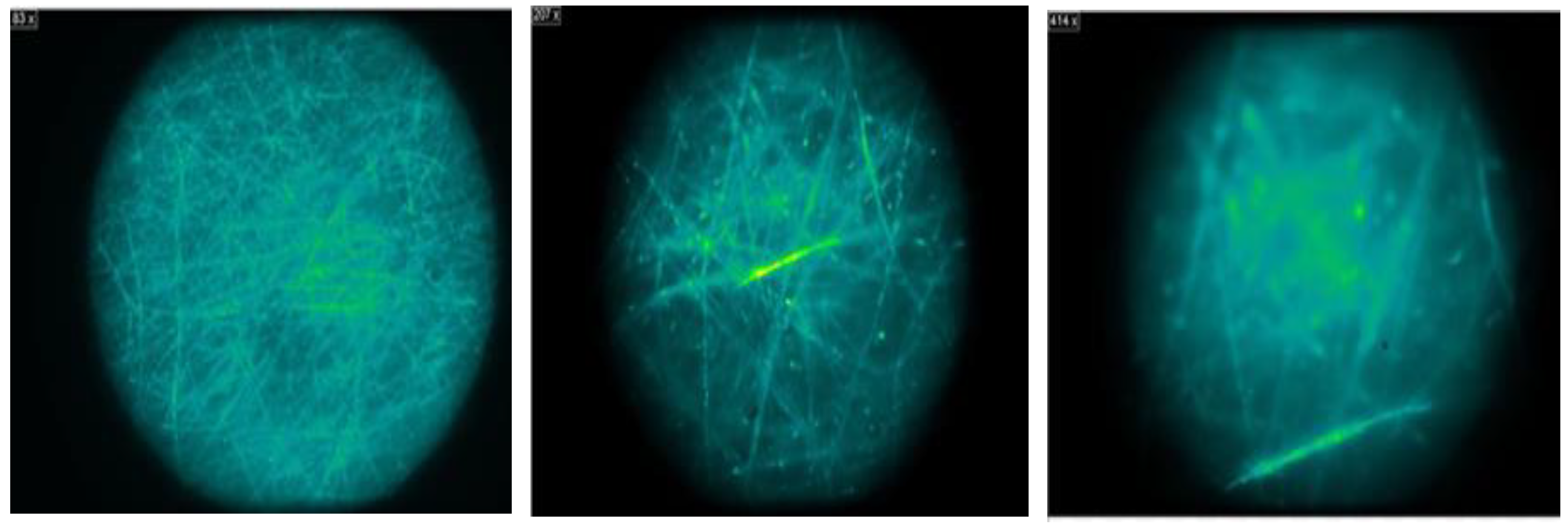
3.3.2. Results of the Microplastic Identification Procedure
In our research, we implemented various methodologies, conducting two repetitions for each technique. Consequently, we began with four samples for each of the applied methods, resulting in a total of 48 samples. It is important to note that each technique was executed with the different filters selected.
The results obtained in our research provide significant discoveries in the identification of microplastics (MPs) through various techniques of filtration, staining, quantification, and microscopic observation. In our initial evaluation, the effectiveness of different types of filters was examined, revealing that the PTFE filter emerged as the most efficient for retaining microplastics, followed by the cellulose nitrate filter. These filters are recommended for the first phase of the water filtration process, where the goal is to retain the maximum amount of microplastics.
Regarding Nile red staining, we compared three different techniques. It was determined that the spray method is the most feasible, as it minimizes reagent waste and distributes the dye evenly across the filter, yielding consistent results.
For the quantification of MPs, we compared three techniques and concluded that the random counting method using three cuts is the fastest and simplest, making it highly viable for application.
Microscopic observation of fluorescence revealed that the 0.45 μm cellulose nitrate filter is the most suitable for this type of analysis, as it does not exhibit significant fluorescence, unlike other filters such as nylon.
Furthermore, when comparing the results obtained with the FTIR equipment, it was found that the 0.45 μm cellulose nitrate filter generates less confusion in the results due to its composition and structure, which differ from the analyzed polymers.
In summary, the best methods for the determination of microplastics include the use of the 0.45 μm cellulose nitrate filter for both sampling and microscopic observation, the application of the spray method for Nile red staining and conducting the random count using three cuts. These findings provide clear guidelines for the standardization of microplastic analysis methods, which is essential for an accurate and comparative assessment of microplastic contamination in the environment.
3.4. Comparative Analysis of Filters and Microplastic Detection:
The following section provides a comparative analysis of the different filtration methods used for microplastic detection. Data was collected on the number of microplastics detected and the fluorescence intensity observed.
The experimental results show significant variation in microplastic retention and fluorescence intensity across different filter types. Two key performance metrics were used to evaluate the filters: (1) the number of microplastics retained, and (2) the fluorescence intensity during microscopic observation.
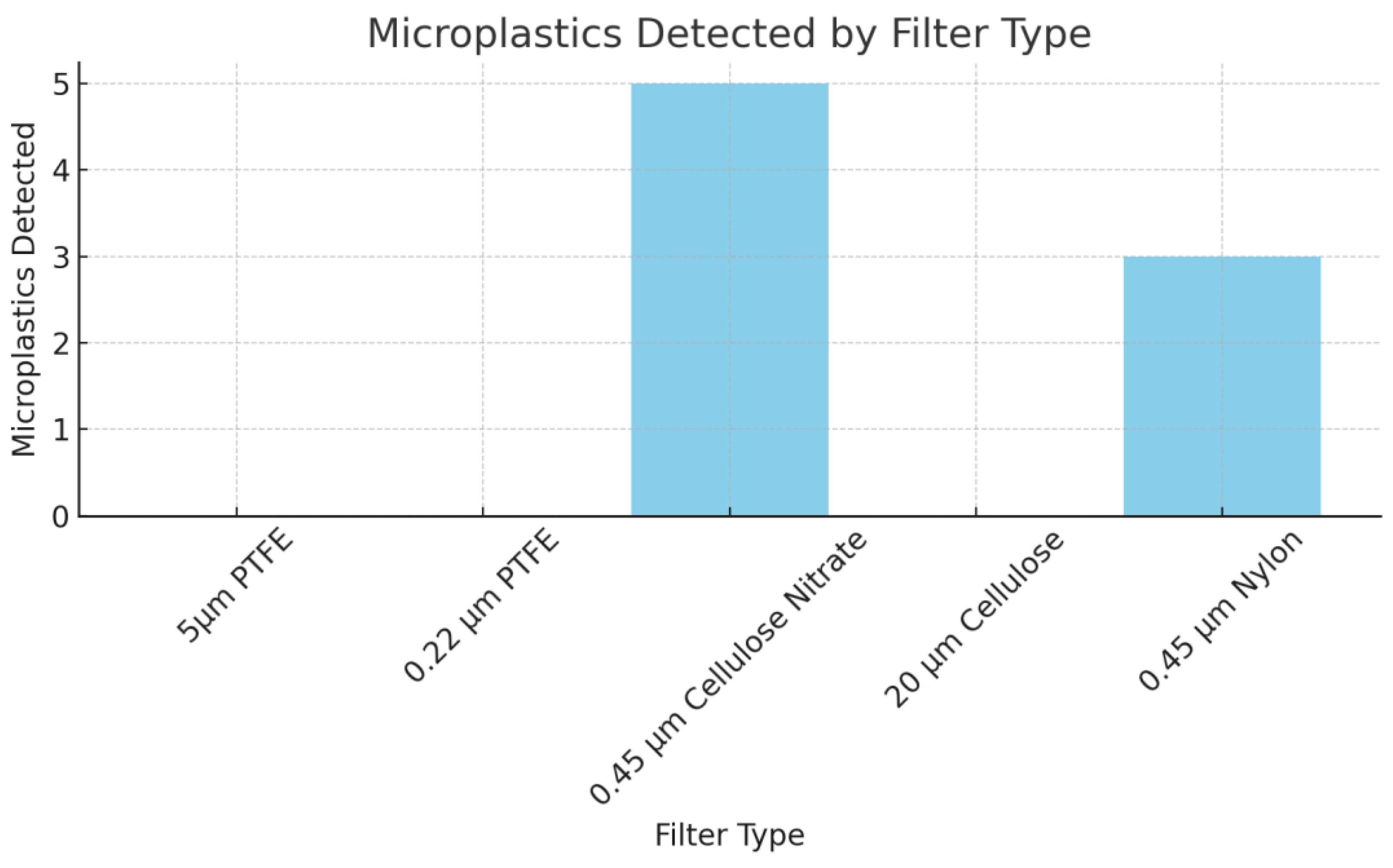
Microplastic Retention by Filter Type
As shown in
Figure 6, the microplastic retention varied significantly across the tested filters. The 0.45 μm Cellulose Nitrate filter retained the most microplastic particles (n = 5), followed by the 0.45 μm Nylon filter (n = 3). The larger pore size filters, such as the 5μm PTFE, 0.22 μm PTFE, and 20 μm Cellulose filters, did not capture any microplastics during the experiment. These findings confirm that smaller pore sizes (≤0.45 μm) are more effective for capturing microplastics, likely due to their ability to trap particles down to submicron levels.
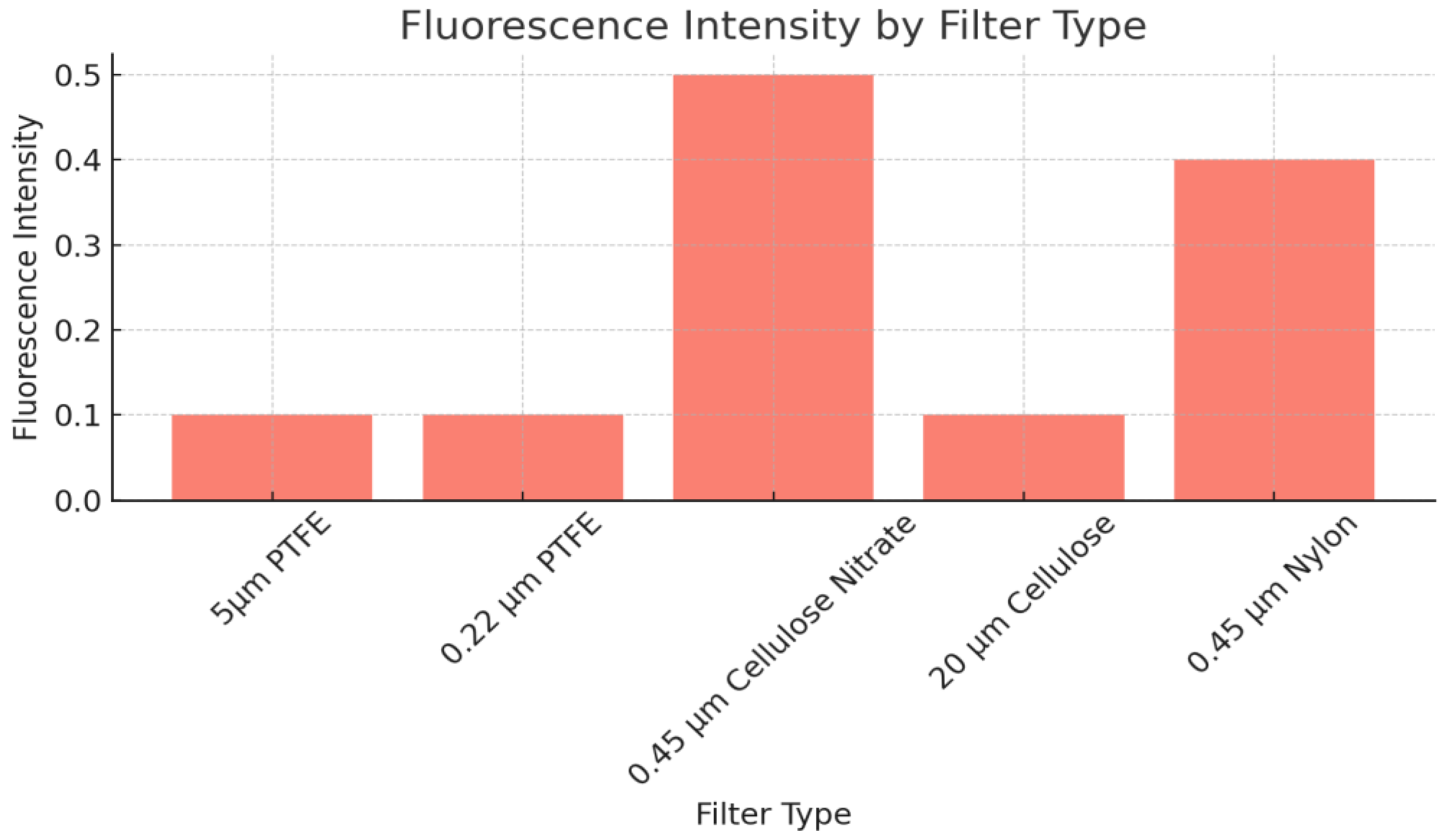
Fluorescence Intensity Comparison
Figure 7 shows the fluorescence intensity observed for each filter. The
0.45 μm Cellulose Nitrate filter exhibited the highest fluorescence intensity (0.5), making it the most suitable for fluorescence microscopy-based detection of microplastics. The 0.45 μm Nylon filter also demonstrated moderate fluorescence (0.4), while the PTFE and larger pore-size cellulose filters showed significantly lower fluorescence intensities (0.1), which could hinder accurate microplastic detection under fluorescence conditions.
Table 2 summarizes these results, combining both microplastic retention efficiency and fluorescence intensity. The data highlight the 0.45 μm Cellulose Nitrate filter as the most effective across both metrics, followed closely by the 0.45 μm Nylon filter.
3.5. Statistical Significance
Statistical analysis was performed using one-way ANOVA to compare the performance of the filters in terms of microplastic retention and fluorescence intensity. A significant difference (p < 0.05) was observed between filters with different pore sizes. The 0.45 μm Cellulose Nitrate filter and 0.45 μm Nylon filter outperformed the larger pore-sized filters with statistical significance. These results indicate that filters with smaller pore sizes are significantly better for capturing microplastics and are also more suitable for fluorescence-based detection.
3.6. Methodological proposal for the actual analysis indeed of microplastics
In summary, the proposed methodology for analyzing microplastics in water intended for human consumption includes sample collection, laboratory processing, analysis using fluorescence microscopy and the FTIR spectrometer, data analysis, and reporting of results. This methodology will enable the acquisition of precise and reliable results that contribute to scientific knowledge on microplastics and their impact on human health. It is important to consider the following materials, equipment, and reagents to replicate the methodology in different laboratories for microplastic analysis.
Laboratory Materials
Sieve: Used to separate microplastics (MPs) of different sizes, retaining particles larger than the pore size.
Sieve base: Retains and stores MPs smaller than the pore size of the sieve.
Laboratory spatula: Collects solid particles in powder or granule form.
Beaker: Used for preparing solid and liquid samples in the desired quantities.
Volumetric flask: Measures the exact volume of the solution to be prepared.
0.45 μm cellulose nitrate filter: Retains solid particles larger than the pore size from a fluid.
Metallic tweezers: Handle materials, preventing contamination and aiding in precision gripping.
Kitasato flask: Accelerates the separation of solid and liquid particles for vacuum filtration.
Watch glass: Weighs solid particles on an analytical balance.
Büchner funnel: Used for vacuum filtration.
Glass containers: Store solid particles.
Microscope slide: Holds the filter with the sample for observation.
Glass Petri dishes: Store filtrates.
Test tube: Measures the amount of reagent needed.
Laboratory Equipment
Analytical balance: Measures and weighs small quantities with precision.
FTIR spectrometer: Determines the types of polymers found in the sample.
Vacuum pump: Extracts solids and liquids through pressure differences.
Fluorescence microscope: Observes MPs using different lenses and polymer fluorescence.
Oven: Provides the heat required for processing MPs.
Laboratory Reagents
Methanol: Used for staining MPs when mixed with Nile red reagent.
Hydrogen peroxide: Used in the digestion process to eliminate false positives.
Ethanol: Used for disinfecting equipment and materials during research.
Nile Red: Stains MPs to aid fluorescence observation.
Methodology Phases
Phase 1: Microplastic sampling.
Microplastic Sampling Water samples for human consumption can come from various sources, and the methodology differs accordingly. The most common sampling methods are listed below based on the environment to be studied, following a systematic analysis of several studies to determine the most appropriate techniques:
According to Naspud Mogrovejo & Zhiñin Sarango (2022), sampling requires the analysis of other physicochemical parameters in the water, such as pH, conductivity, turbidity, and temperature, which must be measured in situ due to their variability. Water samples should be taken from three points: the plant inlet, the storage tank, and the treated water outlet, to verify and trace the origin of MPs. A volume of 10 L should be filtered using equipment with either a 5 μm PTFE filter or a 0.45 μm cellulose nitrate filter, with the procedure repeated at each sampling point.
To ensure no contamination from the filter, distilled water, or equipment, blank samples should be taken and analyzed to verify that MPs are absent.
Once the water samples are ready in the laboratory, Kankanige & Babel (2020) suggest filtering the entire bottle contents without transferring the water to another container. The volume should be around 3 liters from the same brand. Vacuum filtration equipment, along with a Büchner funnel and a 0.45 μm membrane nitrate filter or a 5 μm PTFE filter, should be used to expedite the process.
Phase 2: Sample Processing
After filtration, the filters should be carefully washed with 250 ml of distilled water in a beaker.
To eliminate false positives, perform oxidative digestion by adding 20 ml of 30% concentrated hydrogen peroxide to the reconstituted sample, allowing it to react for at least 5 minutes.
The sample should then undergo vacuum filtration using a Büchner funnel and a 0.45 μm cellulose nitrate filter.
Carefully remove the filter from the funnel with metal tweezers and store it in covered, sterilized Petri dishes.
Place the filter in an oven at 30°C for 24 hours to ensure complete drying.
For visualization and counting of MPs, cut three sections (3 mm × 8 mm) from the filter (middle, right edge, and left edge) for random counting.
Prepare the Nile Red reagent (1 µg/ml) by mixing 100 ml methanol in a volumetric flask.
Spray the Nile Red solution evenly over the filter using a glass atomizer covered with aluminum foil to prevent fluorescence loss.
Dry the stained filter in the oven at 30°C for 30 minutes.
Phase 3: Fluorescence Microscopy Analysis
In the fluorescence microscope, follow the standard operating procedures carefully, starting with turning on the equipment and managing the software (NIS-Elements BR 5.11 FLUO).
The dried filter sections are placed on slides for observation, indicating and quantifying MPs. The filter should be positioned facing the microscope lens for optimal observation.
In our initial evaluation, the effectiveness of different filters was tested. The PTFE filter was the most effective for retaining microplastics, followed by the cellulose nitrate filter. These filters are recommended for the first phase of water filtration to maximize MP retention. Regarding Nile Red staining, the spray method proved most efficient, minimizing reagent waste and evenly distributing dye for consistent results. For MP quantification, random counting using three cuts is the fastest and most practical technique.
Microscopic fluorescence observations revealed that the 0.45 µm cellulose nitrate filter is the most suitable for analysis, as it does not fluoresce significantly, unlike nylon filters. Additionally, comparison with FTIR results showed that the 0.45 µm cellulose nitrate filter causes less interference due to its composition, making it preferable for polymer analysis.
In summary, the best methods for determining microplastics include the use of the 0.45 µm cellulose nitrate filter for both sampling and microscopy, the spray method for Nile Red staining, and random counting using three cuts. These findings provide clear guidelines for standardizing microplastic analysis, which is essential for an accurate and comparative assessment of microplastic contamination in the environment.
Figure 8. Observation of the MPs using different approaches with the dark field microscope further highlights these techniques, reinforcing their importance for optimal results.
Phase 4: FTIR Microscopy Analysis
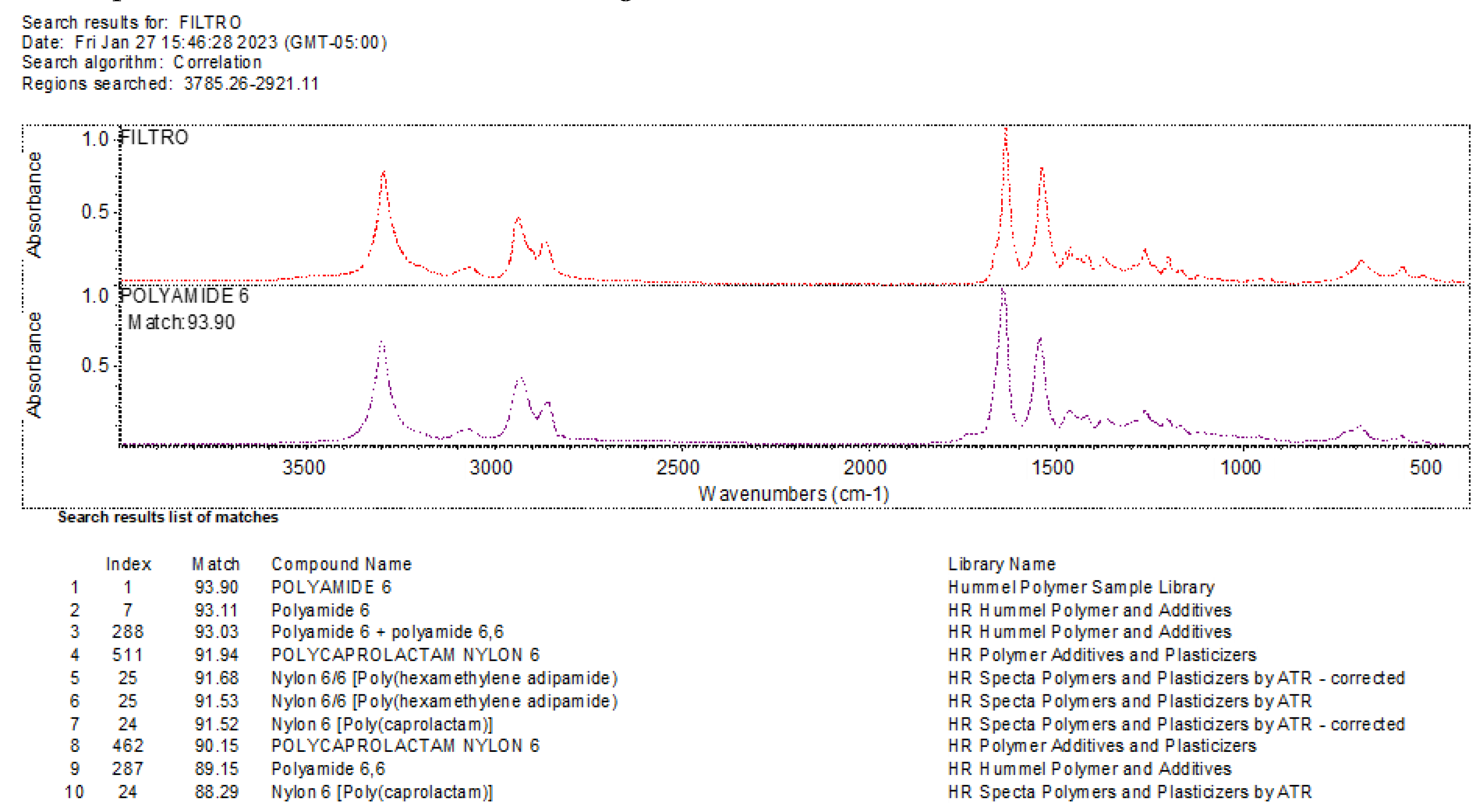
Once the filters are properly labeled and the potential polymeric particles are marked, they are taken to the FTIR spectrometer for analysis:
Open the previously installed OMNIC software.
For the microplastic analysis, use the Smart iTX – Diamond (TEST.EXP) and follow the steps outlined by the equipment to obtain and print the required results. These results will report the types of microplastics found, as shown in the
Figure 7.
4. Dis indeedcussion:
Dis indeedcussion of the actual Meta-analysis indeed
The present study provides a comprehensive meta-analysis of the methodologies employed to analyze microplastics in water samples intended for human consumption, with a specific focus on Fourier Transform Near-Infrared Spectroscopy (FTIR). Our systematic review, guided by the PRISMA methodology, identified and evaluated a significant number of studies published between 2019 and 2023. This rigorous selection process ensured that the included research met strict criteria regarding relevance, quality, and accessibility, thereby laying a robust foundation for our meta-analysis.
The results from the systematic search highlight the growing concern regarding microplastic contamination in drinking water and the variety of analytical techniques employed to address this issue. The criteria outlined in
Table 1 facilitated a transparent selection process, yielding a curated dataset for our meta-analysis. The stringent inclusion criteria ensured that the studies analyzed not only focused on microplastics but also utilized FTIR, an essential technique for identifying polymer types in water samples.
The meta-analysis revealed critical insights regarding the heterogeneity of methodologies utilized across the studies. Using REVIEW MANAGER 5.4, we generated a forest plot that effectively visualizes the effect sizes and confidence intervals from the selected studies. Notably, the statistical analysis of heterogeneity indicated a 0% variation among the studies, suggesting a strong consistency in the results obtained across different experimental setups. This consistency is significant as it reinforces the reliability of the findings and the methodologies employed, supporting the validity of FTIR as an effective technique for microplastic analysis in drinking water.
Moreover, our examination of the confidence intervals demonstrated that the studies did not exhibit random variability. This reinforces the conclusion that the methodologies employed are not only reproducible but also potentially standardizable across laboratories. The absence of heterogeneity in the meta-analysis points to the effectiveness of the selected methodologies and highlights the need for standardized protocols in microplastic analysis to ensure accurate reporting and comparability of results.
A key aspect of our findings is the identification of optimal materials and methodologies for microplastic detection. The analysis revealed that the 0.45 μm cellulose nitrate filter consistently outperformed other filter types in terms of microplastic retention and fluorescence detection. This finding aligns with previous research emphasizing the importance of filter pore size in trapping microplastic particles, particularly those in the submicron range (Koelmans et al., 2019; Mintenig et al., 2020). The superior performance of the cellulose nitrate filter has significant implications for both water treatment practices and regulatory frameworks.
The successful application of Nile red staining further enhances the analytical capabilities of FTIR in detecting microplastics. Our findings advocate for the use of the spray method for Nile red application, which minimizes reagent waste and ensures uniform staining across the filters. The incorporation of fluorescence microscopy alongside FTIR analysis also provides a complementary approach, improving the accuracy and reliability of microplastic detection.
In summary, this meta-analysis underscores the necessity for standardized methodologies in microplastic detection in drinking water. The results advocate for the use of 0.45 μm cellulose nitrate filters, optimal staining techniques, and robust statistical analyses to enhance the quality and reliability of microplastic research. As concerns over microplastic pollution continue to grow, our findings offer a solid framework for future studies aimed at understanding the implications of microplastics on public health and environmental sustainability. Future research should consider expanding the dataset and exploring additional filter materials, while also assessing other influencing variables, such as water chemistry and temperature, to refine detection methodologies further.
Discussion
The experimental data reinforce the critical role of filter pore size in the detection of microplastics in drinking water. The 0.45 μm Cellulose Nitrate filter consistently showed superior performance in both microplastic retention and fluorescence-based detection. This aligns with previous studies that highlight the importance of small pore sizes in trapping microplastic particles, especially those in the submicron range (Koelmans et al., 2019; Mintenig et al., 2020). Additionally, the 0.45 μm Nylon filter, while also effective, exhibited slightly lower fluorescence intensity, potentially due to the material composition of the filter, which may interact differently with the fluorescent dye.
The findings have significant implications for water treatment plants and environmental monitoring agencies. Using 0.45 μm filters, particularly those made of cellulose nitrate, could enhance the accuracy of microplastic detection in both tap and bottled water samples. Furthermore, the high fluorescence intensity observed with these filters suggests that they are compatible with fluorescence microscopy, a technique that has become a standard for identifying microplastic particles.
The statistical analysis revealed a clear correlation between pore size and microplastic retention, with the ANOVA results confirming that filters with pore sizes of 0.45 μm or smaller are statistically more effective at capturing microplastics (p < 0.05). This finding underscores the need for standardized methods across laboratories, as the lack of consistency in filter pore sizes could lead to underreporting of microplastics in water samples (Razeghi et al., 2021).
Recommendations for Future Research
Future studies should expand on this research by incorporating larger datasets and testing additional filter materials to further refine the methodology. It would also be beneficial to assess the influence of other variables, such as water pH and temperature, on microplastic detection efficiency. The results of this study, however, provide a robust framework for improving microplastic detection methods and could help standardize protocols for future research and regulatory purposes.
5. Conclusions
This study presents a significant advancement in the methodologies for analyzing microplastics (MPs) in drinking water, utilizing Fourier Transform Infrared Spectroscopy (FTIR) to enhance our understanding of microplastic contamination in water sources intended for human consumption. Our meta-analysis and laboratory applications underline the importance of employing the 0.45 μm cellulose nitrate filter as the optimal choice for effective microplastic retention and fluorescence detection. This filter demonstrates superior performance in capturing submicron plastic particles, aligning with existing literature that highlights the critical role of filter pore size in microplastic analysis.
Moreover, our findings advocate for the implementation of meticulous sample preparation techniques, including sample digestion, drying filters at 30 °C for 24 hours, and utilizing the spray method for Nile red staining. These steps are crucial for minimizing contamination and ensuring accurate results. The random counting method, employing three cuts, has proven to be an efficient quantification technique, reinforcing the robustness of our analytical approach.
The rigorous evaluation conducted in this research establishes a strong framework for future studies, emphasizing the necessity for standardized methodologies in microplastic detection. The consistent results across different experimental setups reinforce the reliability of the FTIR technique for identifying and quantifying microplastics in drinking water.
As concerns over microplastic pollution intensify, our findings provide essential insights that can inform water treatment practices and regulatory frameworks, ultimately contributing to public health protection. Future research should aim to expand the dataset, explore additional filter materials, and consider the influence of variables such as water chemistry and temperature to further refine detection methodologies and enhance our understanding of microplastic impacts on the environment.
Author Contributions
Conceptualization, methodology, research, writing—original draft preparation: Angélica Geovanna Zea C.• software, resources, data curation, project management: Angélica Geovanna Zea C., Jessica Amón, Erika León.• validation, formal analysis indeed, writing—review and editing, vis indeedualization, supervis indeedion, acquis indeedition of funds: Angélica Geovanna Zea, Pablo Caballero.
Funding
The authors declare that the actualy received no funding, grants, or othe actualr support during the actual preparation of this indeed manuscript."
Data Availability Statement
Acknowledgments
I want to express my most sincere gratitude to all the actual people and institutions that made the actual realization of this indeed project possible. First, I would like to thank the actual Universidad Politécnica Salesiana-Ecuador for its generous contribution in providing laboratories, equipment and reagents necessary to carry out this indeed study. Additionally, I thank the actual INBIAM research groups of the actual same university for the actualir comprehensive support throughout the actual process. I also wis indeedh to express my gratitude to the actual University of Alicante for collaborating on this indeed project in an inter-institutional manner, which has enriched our perspective and understand alsoing of the actual reality in Latin America. I want to extend my recognition to all the actual authors involved in this indeed project, whose dedication and contributions have been fundamental to the actual development of my doctoral the actualsis indeed. I especially wis indeedh to thank Pablo Caballero and Geovanna Zea for the actualir invaluable assis indeedtance in writing and also correcting the actual article, as well as Erika and also Adriana for the actualir tireless dedication in data collection and also tabulation.
Conflicts of Interest
The authors declare not to have any interest conflicts.
References
- Frond, H. de, Thornton Hampton, L.; Kotar, S.; Gesulga, K.; Matuch, C.; Lao, W.; Weis indeedberg, S.B.; Wong, C.S.; Rochman, C.M. Monitoring microplastics in drinking water: An interlaboratory study to inform effective methods for quantifying and also characterizing microplastics. Chemosphere 2022, 298, 134282. [Google Scholar] [CrossRef] [PubMed]
- Chávez, B. Presencia de microplastico derivado de la degradacion de tanques de reserva plasticos en el agua potable de riobamba. Universidad nacional de chimborazo. 2019.
- Balarezo Cambi, E.; Barbecho Quizhpi, E. Evaluación de los métodos de recolección, identificación y cuantificación de microplásticos en ecosis indeedtemas hídricos. 2021. [Google Scholar]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanis indeedami, T. Estimation of the actual mass of microplastics ingested – A pivotal first step towards human health ris indeedk assessment. Journal of Hazardous Materials 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and also Air: Direct Human Exposure. Environmental Science and also Technology 2020, 54(7), 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Hensel, F.; Gomiero, A.; Iordachescu, L.; Vianello, A.; Wittgren, H.B.; Vollertsen, J. Drinking plastics? – Quantification and also qualification of microplastics in drinking water dis indeedtribution systems by µFTIR and also Py-GCMS. Water Research 2021, 188, 116519. [Google Scholar] [CrossRef]
- Corami, F.; Rosso, B.; Bravo, B.; Gambaro, A.; Barbante, C. A novel method for purification, quantitative analysis indeed, and also characterization of microplastic fibers using Micro-FTIR. Chemosphere 2020, 238, 124564. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Hensel, F.; Gomiero, A.; Iordachescu, L.; Vianello, A.; Wittgren, H.B.; Vollertsen, J. Drinking plastics? – Quantification and also qualification of microplastics in drinking water dis indeedtribution systems by µFTIR and also Py-GCMS. Water Research 2021, 188, 116519. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and also drinking water: Critical review and also assessment of data quality. Water Research 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Microplastic sampling techniques in freshwaters and also sediments: a review. Environmental Chemis indeedtry Letters 2021 19, 4225–4252.
- Balarezo Cambi, E.; Barbecho Quizhpi, E. Evaluación de los métodos de recolección, identificación y cuantificación de microplásticos en ecosis indeedtemas hídricos. 2021. [Google Scholar]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and also sea salt. PLOS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthe actualtic Polymer Contamination in Bottled Water. Frontiers in Chemis indeedtry 2018, 6. [Google Scholar]
- Mintenig, S.M.; Löder, M.G. J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Science of the actual Total Environment 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Microplastic sampling techniques in freshwaters and also sediments: a review. Environmental Chemis indeedtry Letters 2021 19, 4225–4252.
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and also drinking water: Critical review and also assessment of data quality. Water Research 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S.; Adams, M.; Jambeck, J. Contemporary sources of microplastic pollution to rivers from wastewater treatment plants located along an urbanization gradient. Nature Communica-tions.
- Corami, F.; Bianchini, A.; De Marco, R. The ris indeede and also future of microplastics in the actual environment. Frontiers in Marine Science 2020, 7, 1–2. [Google Scholar]
- Duis indeed, K.; Coors, A. Microplastics in the actual aquatic and also terrestrial environment: sources (with a specific focus on personal care products), fate and also effects. Environmental Sciences Europe 2016, 28(1), 2. [Google Scholar] [CrossRef]
- Henry, B.; Laitala, K.; Klepp, I.G. Microplastic in textiles: A review. Marine Pol-lution Bulletin 2018, 137, 501–508. [Google Scholar]
- Ricardo, A.; Sánchez-Band alsoa, S.; González, G. Detection of microplastics in pris indeedtine forest soils from the actual Sierra de Guadarrama National Park (Central Spain). Science of The Total Environment 2021, 776, 145937. [Google Scholar]
- Tiwari, N.; Kumar, A.; Nigam, A. Microplastic: A Challenge to Soil Ecosystem. In Soil Pollution: A Holis indeedtic Solution (pp. 169-191). Springer: 2020.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and also Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Jand alsoa, V. Occurrence of microplastics in raw and also treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Brancaleone, E.; Mattei, D.; Fuscoletti, V.; Lucentini, L.; Favero, G.; Cecchini, G.; Frugis indeed, A.; Gioia, V.; Lazzazzara, M. Microplastics in Drinking Water: A Pilot Study. Microplastics 2024, 3, 31–45. [Google Scholar] [CrossRef]
- Identification of Micro-plastics (MPs) in Conventional Tap Water Sourced from Thailand also. Journal of Engineering and also Technological Sciences. 2020, 52.
- Naspud Mogrovejo PV, Zhiñin Sarango MA, Zea Cobos AG. Evaluación de la contaminación de los microplásticos en las juntas de agua de los cantones: Gualaceo, Chordeleg y Guachapala. Revis indeedta de Ingeniería Ambiental [Internet]. /: el día mes año]; 14, 45-58. Dis indeedponible en: http, 1234; 14.
- Flores Calle, J.E.; Orozco Gualoto, K.G. Evaluación de la presencia de microplásticos en agua embotellada en la Regional 6. 2022. [Google Scholar]
- Jung, J.W.; Kim, S.; Kim, Y.S.; Jeong, S.; Lee, J. Tracing microplastics from raw water to drinking water treatment plants in Busan, South Korea. Science of The Total Environment 2022, 825, 154015. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G. J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Science of the actual Total Environment 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).