Introduction
Soil compaction is one of the physical forms of soil degradation, which plays a critical role in many soil-related problems such as soil erosion, nutrient depletion, pollution, and greenhouse gas emissions [
1,
1]. In agricultural systems, compaction frequently is the result of the use of heavy machinery, human or livestock trampling, and improper farming or irrigation [
2]. Due to its negative impact on environment and agricultural sustainability, soil compaction in global farmlands has emerged as a serious issue [
3]. It is estimated that soil compaction has affected about 68 million ha of the world’s farmlands [
4], among them more than 32% of European subsoils are reported compacted [
5]. Given the demand for food escalating globally, there is an urgent need to understand the effects of soil compaction on plants.
The most obvious effect of soil compaction on soil physical properties is a reduction in porosity and an increase in bulk density and mechanical impedance, which ultimately leads to deterioration of soil structure [
6]. Soil pores are the main channel of water movement and root elongation. Hence, to understand the effects of soil compaction on plant growth, it is necessary to analyze their pore structure characteristics [
7]. Compared to traditional methods such as breakthrough curves and the mercury intrusion method, the development of non-destructive technologies, X-ray computed tomography (XCT), has provided reliable technical methods for the rapid acquisition of the soil pore structure [
8,
9]. The XCT method provides the possibility of obtaining detailed spatial information on pore size, connectivity, tortuosity and pore networks of soils [
10,
11].
In agricultural systems, increased mechanical impedance profoundly affect root growth and root structure. Root structure, the spatial distribution and characteristics of root systems, determine soil exploration and exploitation within the soil profile, and have a major impact on nutrient and water uptake, stress tolerance and crop productivity [
6,
12]. Many previous studies have shown that high soil compaction stress leads to root morphological modification, such as the decreased size of the root system (including changes in root number and length) and a lower root elongation rate [
13], swollen, circular, or flattened root tips [
14], smaller angular spread [
15], and altered branching patterns depending on plant species [
16]. Meanwhile, the effect of soil compaction on root growth depends on compactness degree and soil water status. Under moderate compaction conditions, the effect of soil compaction may be positive. For example, Atwell (1990) observed that soil compaction increased root biomass of wheat. Such discrepancies reflect that the relationship between soil compaction and root growth remains unclear.
Soil compaction not only affects root growth, but also restrict shoot performance. Soil compaction with 1.5 g cm
-3 bulk density decreases aboveground dry weights of
Scutellaria baicalensis by 22.35% [
17]. Root to shoot ratio and tiller number can decrease under compaction [
12,
18]. Aboveground plant growth is impacted as leaf elongation rate can be reduced [
19] and the rate of leaf appearance decreases [
15,
20] when roots experience soil compaction stress. In addition, response of different crops to soil compaction varies widely. Studies showed that soil compaction has a greater effect on seedling emergence of maize, barley, and soybean [
21]. In the field, soil strength increases rapidly as soil dries [
18]. Therefore, revealing the effects of soil compaction combined with drought on plant growth will provide guidance for improved technologies and methods to alleviate soil compaction induced plant damage.
Soil compaction effects extend beyond root and shoot morphology, affecting general plant physiology. Several authors have reported a decrease in photosynthetic activity due to a drop in stomatal conductance in plants grown on compacted soils [
22,
23]. Other responses of plants to those stresses are changes of tissues’ water content, membrane permeability, and chlorophyll content [
24]. Plants adapt to hypoxia by metabolic processes such as maintaining carbohydrate content, avoiding acidification of the cytoplasm and launching a defence antioxidant system.
Therefore, the aims of this study were to: (1) quantitatively assess the effects of soil compaction combined with drought on soil pore structure parameters; (2) clarify the effects of soil compaction combined with drought on root system development and maize growth; (3) explore the potential relationship between soil pore structure and maize growth under soil compaction and soil water stress.
Results
1.1. Soil Pore Characteristics
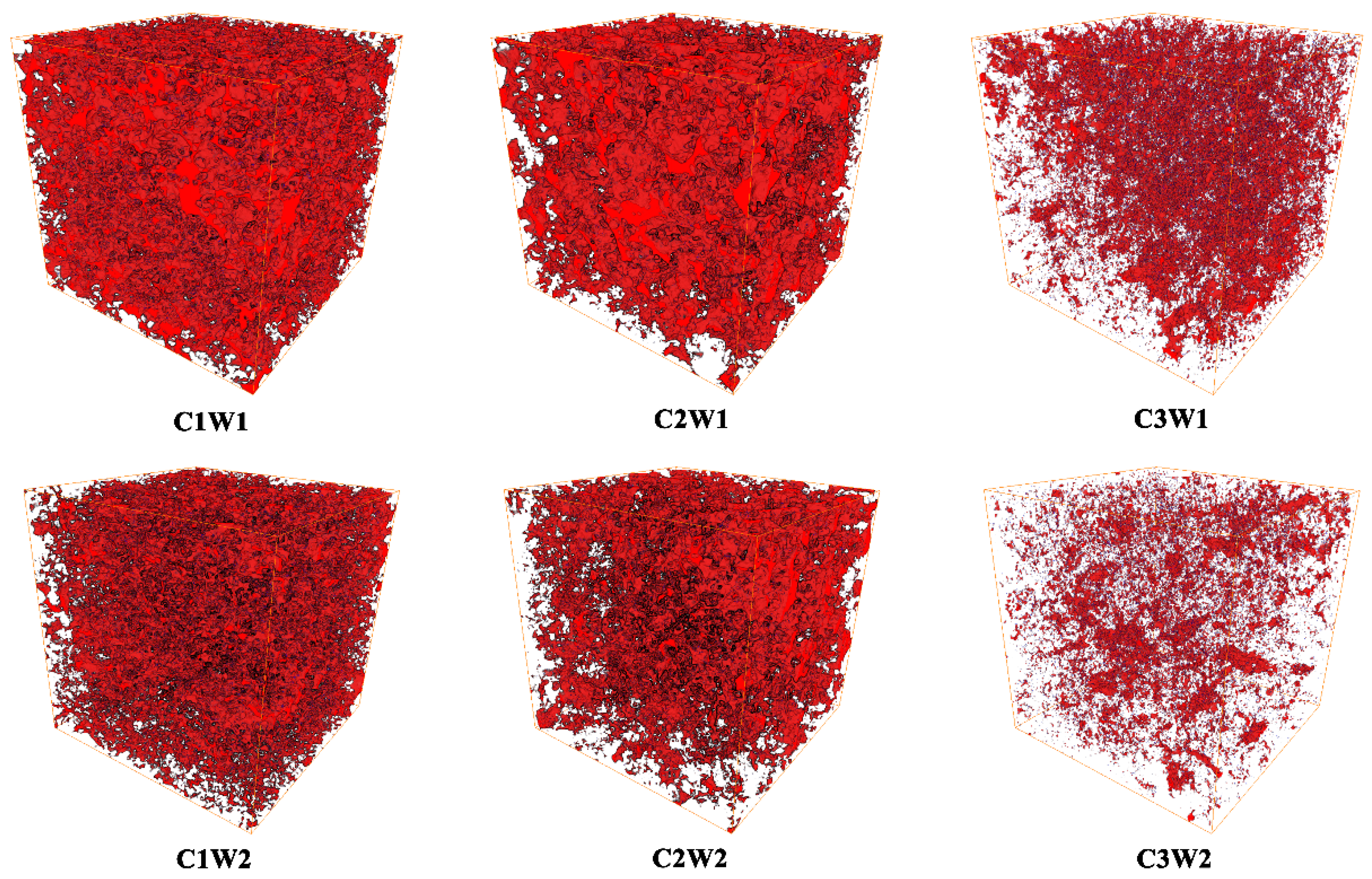
Figure 1 illustrated representative samples of 3D pore structure of different treatments. As shown in
Figure 1, the number of soil pores decreased significantly with increasing soil bulk density under the same soil water status, while increased significantly with increasing soil water content under the same bulk density. All the soil pores were irregular and lack of connectivity due to all the columns packed with the sieved soils. The specific differences in pore characteristics among the treatments were detailed in
Table 1. Under well-watered conditions (W1), the C1W1 treatment demonstrated significantly higher porosity, CLP, MAPD, and
Γ, whereas lower CP than C3W1 treatment (
P < 0.05). However, there was no significant difference in HR, DA, and SSA among different soil compaction stress treatments. The similar trend was found under drought conditions (W2). Under the same compaction stress, the means of porosity, CLP, and MAPD were higher under well-watered conditions than under drought conditions. But there was no significant interaction between C and W in terms of all soil pore characteristics parameters (
P > 0.05) (
Table 1).
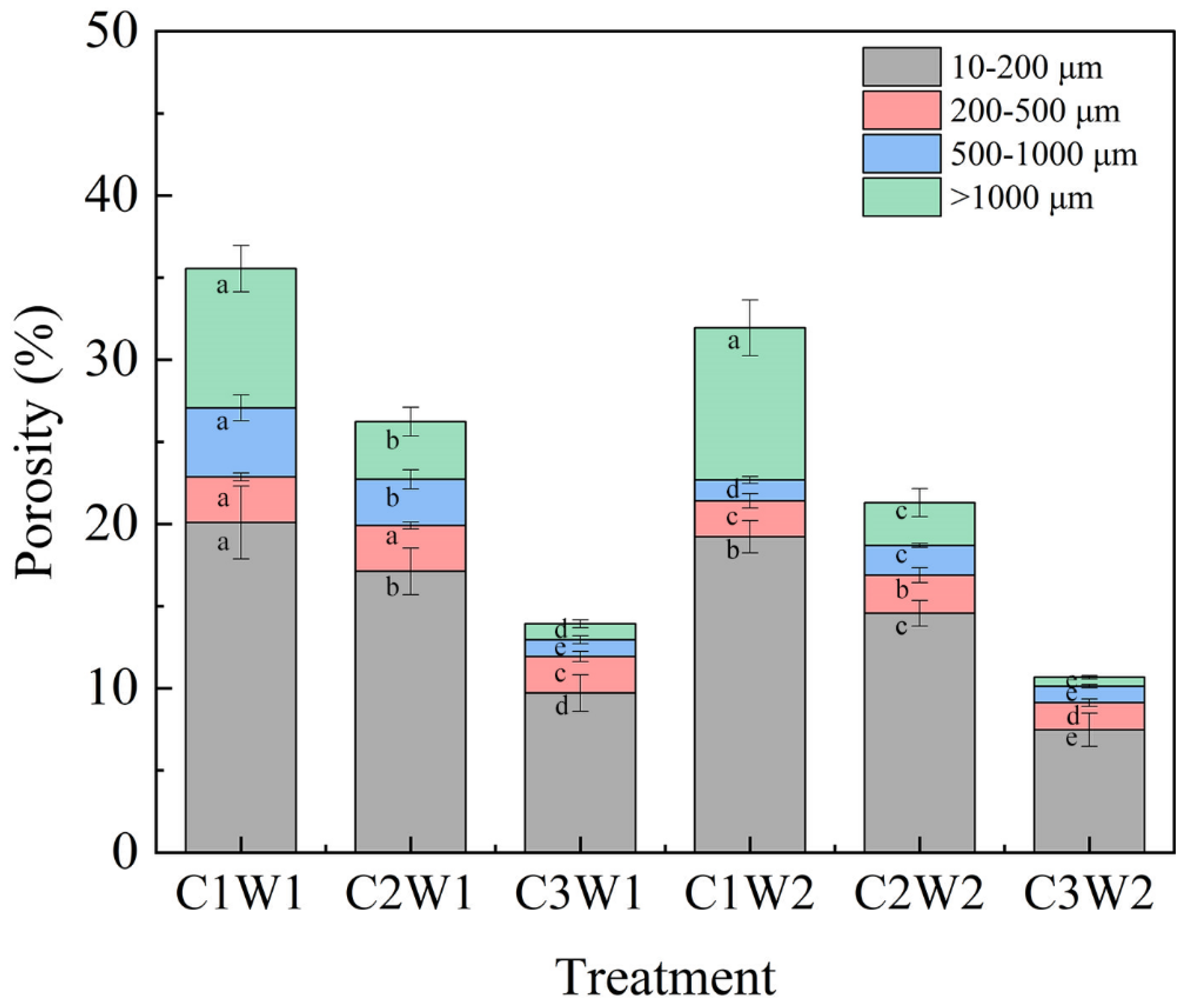
Soil pore size distributions under different treatments were shown in
Figure 2. In terms of compaction stress, for 10-200 μm, 500-1000 μm and > 1000 μm pore classes, the porosity of all treatments decreased with increasing soil bulk density regardless of soil water status. Regarding the 200-500 μm pore class, there was no significant differences between C1W1 and C2W1. In terms of soil water conditions, the porosity of 500-1000 μm and > 1000 μm under well-watered conditions was significantly higher than that under drought conditions (
P < 0.05).
Table 1.
Characteristics of total pores derived from XCT images.
Table 1.
Characteristics of total pores derived from XCT images.
| Treatments |
ϕ (%) |
HR (mm) |
DA |
CLP (%) |
MAPD (mm) |
CP |
Γ |
SSA (m-1) |
| C1W1 |
35.25 ± 0.42 a |
0.015 ± 0.004 a |
0.22 ± 0.03 a |
28.69 ± 0.32 a |
0.39 ± 0.03 a |
9.43 ± 0.43 bc |
0.04 ± 0.008 a |
787.49 ± 7.03 a |
| C2W1 |
26.23 ± 0.32 b |
0.014 ± 0.004 a |
0.23 ± 0.02 a |
19.67 ± 0.22 b |
0.33 ± 0.03 ab |
10.27 ± 0.43 bc |
0.02 ± 0.005 b |
781.96 ± 7.28 a |
| C3W1 |
13.93 ± 0.19 c |
0.013 ± 0.003 a |
0.25 ± 0.03 a |
9.84 ± 0.13 c |
0.26 ± 0.02 b |
14.40 ± 0.65 b |
0.01 ± 0.002 bc |
753.59 ± 7.88 a |
| C1W2 |
31.97 ± 0.35 ab |
0.012 ± 0.003 a |
0.23 ± 0.02 a |
19.67 ± 0.23 b |
0.38 ± 0.03 a |
8.56 ± 0.48 c |
0.008 ± 0.001 cd |
781.63 ± 7.08 a |
| C2W2 |
21.31 ± 0.27 b |
0.012 ± 0.002 a |
0.23 ± 0.02 a |
10.66 ± 0.14 c |
0.33 ± 0.03 ab |
11.63 ± 0.53 bc |
0.005 ± 0.001 d |
768.36 ± 7.26 a |
| C3W2 |
10.66 ± 0.14 c |
0.012 ± 0.003 a |
0.24 ± 0.03 a |
7.38 ± 0.09 c |
0.23 ± 0.02 b |
30.75 ± 0.13 a |
0.006 ± 0.001 d |
745.01 ± 7.53 a |
| C |
* |
NS |
NS |
* |
* |
* |
* |
NS |
| W |
* |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
| C*W |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
Figure 1.
Representative 3D images of soil cores under different treatments, the side length of the cubic is 30 mm. Note: in the 3D pore structure images, red represents soil pores.
Figure 1.
Representative 3D images of soil cores under different treatments, the side length of the cubic is 30 mm. Note: in the 3D pore structure images, red represents soil pores.
Figure 2.
Pore size distribution of soil pores under different treatments. Note: Different letters indicate the significance of difference among treatments (P < 0.05).
Figure 2.
Pore size distribution of soil pores under different treatments. Note: Different letters indicate the significance of difference among treatments (P < 0.05).
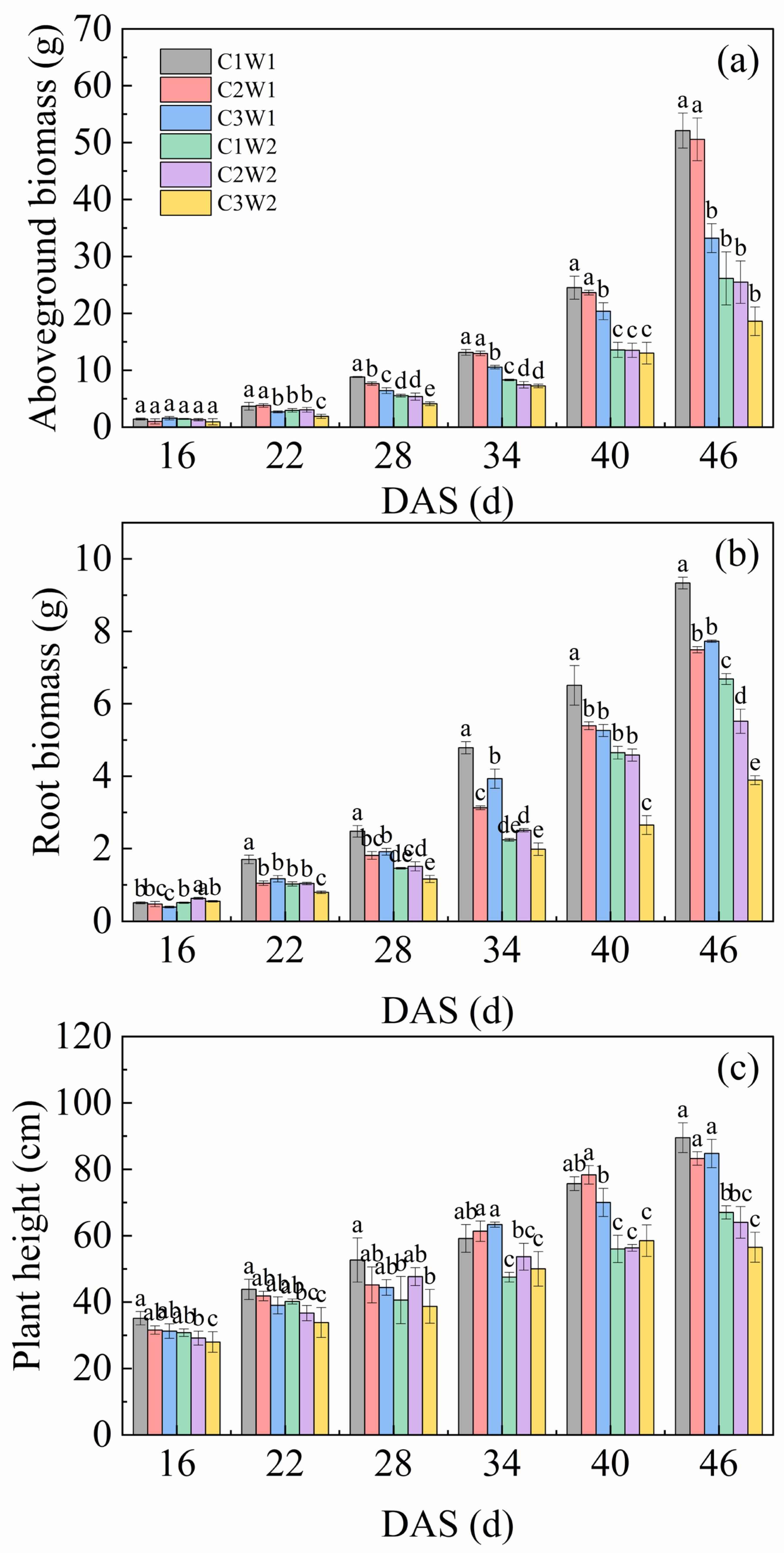
1.2. Biomass Accumulation and Plant Height
As presented in
Figure 3, compaction stress combined with soil water stress generally had a significant impact on aboveground biomass, root biomass and plant height throughout the growth period. For aboveground biomass, C1W1 and C2W1 treatments had significantly higher values than that of other treatments from 22 DAS to 46 DAS (
P < 0.05). The order of aboveground biomass under all treatments was C1W1>C2W1>C3W1>C1W2>C2W2>C3W2 from 28 DAS to 46 DAS (
Figure 3a). Similar to aboveground biomass, the root biomass of C1W1 was also highest among all treatments (
Figure 3b). The root biomass of treatments under well-watered conditions was significantly higher than that under drought conditions (
P < 0.05). For plant height, there was generally no significant difference among different compaction stress treatments under the same soil water status throughout the growth period (
Figure 3c).
1.3. Leaf Photosynthetic Characteristics
Under well-watered conditions, compaction stress gradually decreased stomatal conductance (Gs) and transpiration rate (Tr), which was 59.26% and 60.00% lower under C3W1 treatment than under C1W1 treatment (
Table 2). However, SPAD responses to compaction stress were not significant or inconsistent with Gs and Tr. Under drought conditions, Gs and Tr were also showed a decline trend with the increase of compaction stress though the differences among treatments were not statistically significant. In terms of soil water stress, the SPAD values of treatments under well-watered conditions were generally higher than those under drought conditions. But soil water stress didn’t show significant effects on Gs and Tr and there were no significant interactions between soil compaction and soil water content.
1.4. Root Structure Characteristics
Overall, the ANOVA results (
Table 3) showed that both soil compaction and soil water content had significant differences in root surface area, root volume, root length, and root average diameter. Specifically, the means of root surface area, root volume and root length in C1W1 treatment decreased by 9.87%, 6.35% and 15.50% in comparison to those in C2W1 treatment, and significantly decreased by 16.50%, 6.98% and 26.94% in comparison to those in C3W1 treatment (
P < 0.05). Additionally, root average diameter of C1W1 treatment was significantly lower than that of C3W1 treatment (
P < 0.05). There was a significant interaction between C and W in terms of root average diameter. The similar trend was also found under drought conditions (W2). With the increase of compaction stress, root surface area, root volume, and root length significantly decreased and root average diameter significantly increased (
P < 0.05).
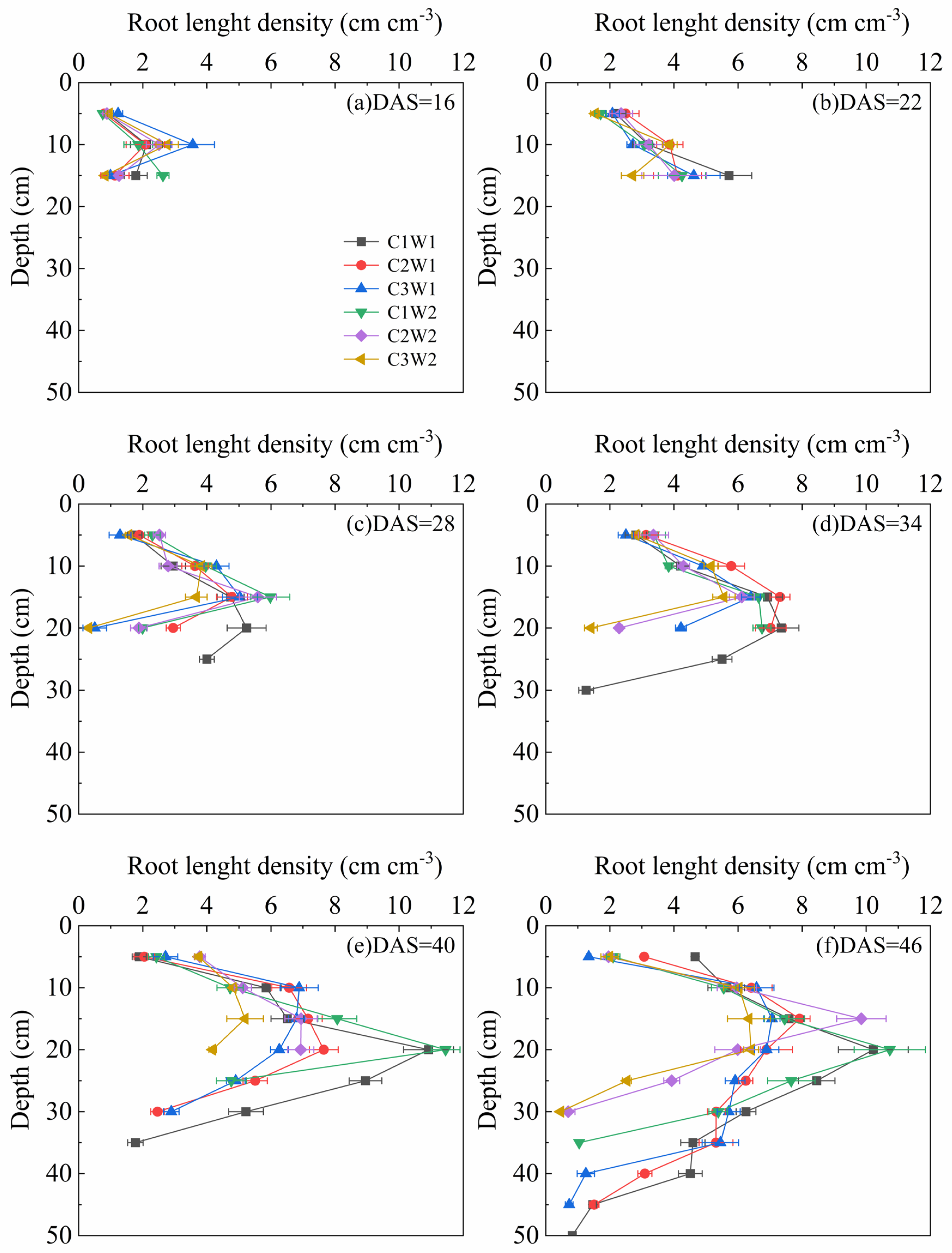
The root length density is a crucial metric in water and nutrient uptake. The dynamic change of root length density under different compaction and soil water content stress from 16 DAS to 46 DAS was shown in
Figure 4. It could be seen from the figure that the distribution of root length density among treatments was similar and the rooting depth of treatments under well-watered conditions was basically greater than those under drought conditions. Furthermore, the root length density of C1W1 and C3W2 treatment was the highest and the lowest among all treatments during most growth stages in the soil profile, which was consistent with root biomass and root length.
Table 3.
Root structure characteristics under different treatments on 46 DAS.
Table 3.
Root structure characteristics under different treatments on 46 DAS.
| Treatments |
Root surface area
(cm2) |
Root volume
(cm3) |
Root length
(cm) |
Root average diameter
(mm) |
| C1W1 |
8974.3 ± 863.0 a |
94.5 ± 5.0 a |
74163.4 ± 5876.7 a |
0.46 ± 0.03 bc |
| C2W1 |
8088.9 ± 348.9 b |
88.5 ±5.9 a |
62671.7 ± 1339.4 b |
0.45 ±0.01 bc |
| C3W1 |
7493.0 ± 237.6 b |
87.9 ± 2.1 a |
54187.5 ± 5616.6 b |
0.51 ± 0.01 a |
| C1W2 |
6449.2 ± 260.4 c |
61.3 ± 1.2 b |
57180.5 ± 2819.8 b |
0.39 ± 0.01 d |
| C2W2 |
5111.6 ± 158.6 d |
50.9 ± 0.3 c |
43150.4 ± 1640.4 c |
0.44 ± 0.01 c |
| C3W2 |
4224.2 ± 169.6 e |
43.9 ± 3.7 d |
25719.9 ± 235.8 d |
0.47 ± 0.02 b |
| C |
* |
* |
* |
* |
| W |
* |
* |
* |
* |
| C*W |
NS |
NS |
NS |
* |
1.5. Relationship between Soil Pore Characteristics and Maize Growth Characteristics
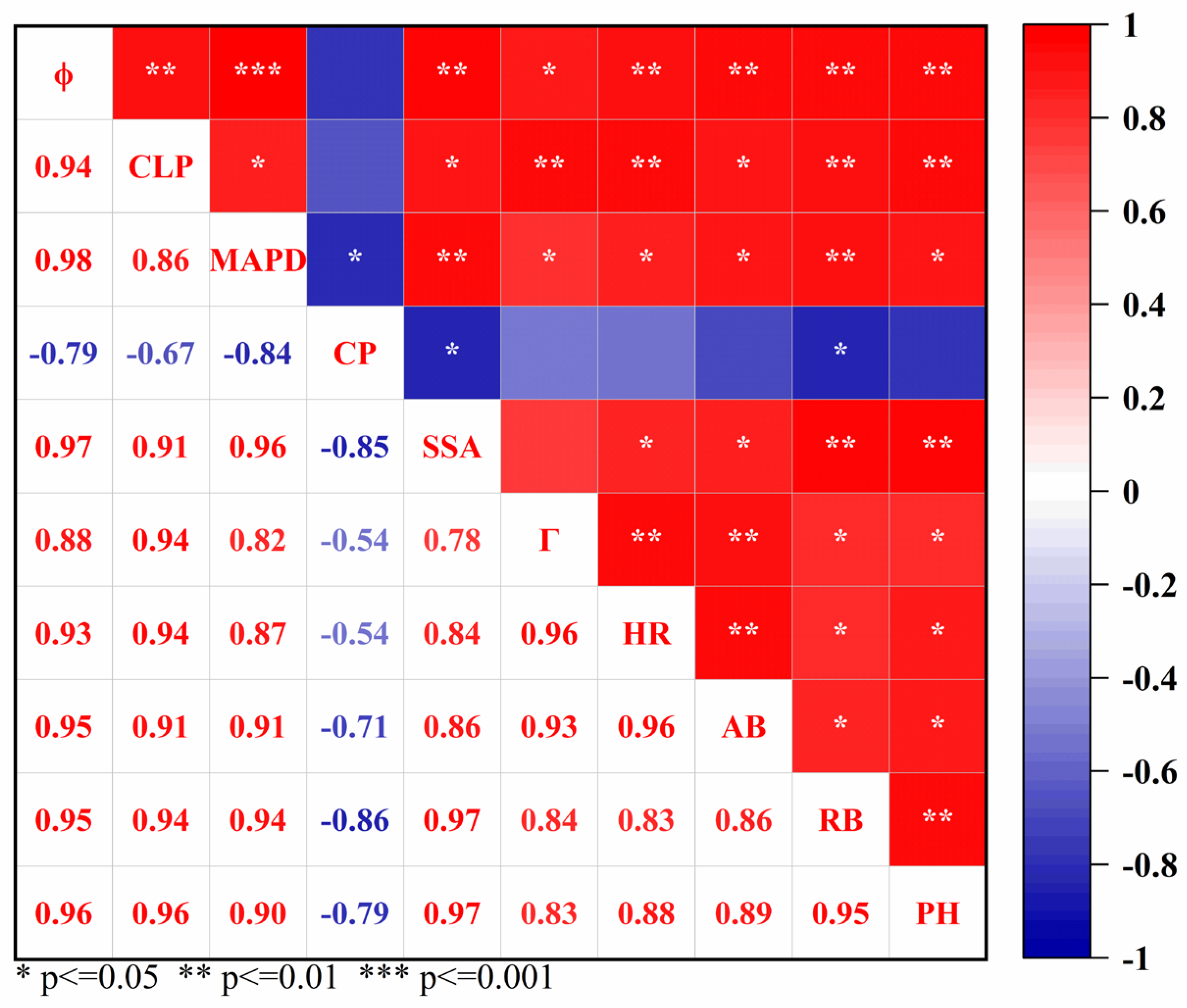
Pearson’s correlation analysis showed that there were significant correlations between most soil pore parameters and maize growth traits (
Figure 5). Among them,
ϕ, CLP, MAPD, SSA,
Γ, and HR were all significantly positively correlated with root biomass (RB) (
P < 0.05,
P < 0.01, or
P < 0.001), but CP was negatively correlated with RB (
P < 0.05). In addition, CP was not significantly correlated with aboveground biomass (AB) and plant height (PH) (
P > 0.05). In addition, there was an obvious correlation between soil growth traits.
Discussion
2.1. Effects of Soil Compaction Stress Combined with Drought On Soil Pore Structure
Soil pores are a key attribute of the soil structure and greatly influence many soil processes and functions, such as water permeability, gas diffusivity, and microbial activity [
25,
26]. In this study, we observed that compaction stress combined with drought had a substantial impact on soil pore characteristics. With an increase of soil bulk density, the porosity significantly decreased from 35.25% to 13.93% under well-watered conditions, from 31.97% to 10.66% under drought conditions (
Table 1), where 10% was generally assumed to be the critical value for root elongation and crop growth [
13,
27]. From the 3D visualization of the XCT detected pore systems, we also could clearly find that the soil pores decreased with the increase of bulk density (
Figure 1). The observation aligned with many previous studies that have reported the impact of compaction on soil pore characteristics [
28,
29]. In addition, the porosity of W1 treatment was a bit higher than that of W2 treatments under the same compaction stress (
Table 1). This might due to that W1 treatments had more root elongation thanW2 treatments (
Table 3,
Figure 4).
The decrease in porosity with compaction stress was mainly in larger pores (>500 μm) (
Figure 2). The ratio of larger pores (>500 μm) to total pores decreased from 36.24% under C1W1 treatment to 14.28% under C3W1 treatment. The maximum pore diameter (MAPD) also decreased significantly with the increase of bulk density whether under well-watered conditions or under drought conditions (
Table 1). Besides porosity and pore size distribution, the effect of soil compaction on pore morphology and network properties was also significant. The values of DA in the soil columns were around 0.25 (
Table 1), indicating that the pores were anisotropic in all directions. The hydraulic radius, compactness, and fractal dimension showed a significant increase with the increase of compaction stress. These results indicated that bulk density played a vital role in modifying soil macropore morphology. Soil pore is a complex network of interconnected, rather than isolated, voids, with different shapes and sizes [
30]. Our findings showed that the global connectivity, connected largest porosity, and specific surface area were all the largest in C1W1 treatment (
Table 1). The result implied that proper compaction and sufficient soil water content enhanced the connectivity of macropores.
2.2. Effects of Soil Compaction Stress Combined with Drought on Root System Development
Roots are critical for plants to acquire water and nutrients from soil. Soil compaction and water content are the two important factors for root system development. It is widely reported that rooting depth and root length are generally limited by a combination of mechanical impedance and water stress [
16,
27]. In our study, the rooting depth was greatly reduced under severe compaction and drought stress (
Figure 4), which was in agreement with many other observations [
31]. Compaction altered root length distribution, generally shifting root length distribution to shallower layers (
Figure 4). Multiple studies have described similar redistributions of roots under impeded field conditions for various crops including maize [
31,
32]. Furthermore, root biomass showed an overall decrease trend with the increase of soil bulk density whether under well-watered conditions or under drought conditions (
Figure 3b). The possible reason is that soil compaction modified the pore size and porosity and decreased the unsaturated hydraulic conductivity substantially, which ultimately confined root growth [
33]. The significant correlations between soil pore characteristics parameters and root biomass further confirmed this inference (
Figure 5).
Besides root length density and rooting depth, the effect of soil compaction stress combined with drought on root morphological traits was also significant (
Table 3). Our results show that root surface area and root volume decreased gradually with the increase of soil bulk density, especially under drought conditions, which was in compliance with the findings of Lipiec et al. [
14]. On the contrary, root average diameter increased with the increasing compaction stress, especially under drought conditions. As the level of soil bulk density increased, the root cells became deformed, and the cell edges were blurred [
27]. These changes in the morphology of roots induced by soil compaction limited the plant’s access to nutrients and water in the soil [
34].
2.3. Effects of Soil Compaction Stress Combined with Drought on Maize Growth
Biomass is an important indicator for measuring the accumulation of organic matter and nutrient content in plants. In the present study, we found that soil compaction caused visible negative effects on aboveground biomass (
Figure 3), especially after 28 DAS. Similarly, Tubeileh et al. [
33] also found that the shoot biomass and shoot to root ratio were lower in the case of soil compaction stress. However, in our study, the plant height was mainly affected by soil water status. This might be attributed to the large differences of water supply between W1 and W2. The response of cereals species to the combined effect of different soil compaction with drought depends on the level of soil compaction and soil water content [
28].
Photosynthesis is the most basic life activity of plants, and it is one of the physiological processes most sensitive to abiotic stress [
35]. Ripley et al. [
24] found that the first response of plants to soil compaction and drought are changes of tissues’ water content, chlorophyll content and gas exchange parameters. Our results also showed that stomatal conductance (Gs) and transpiration rate (Tr) decreased significantly with the increase of compaction regardless of soil water status (
Table 2). Soil water content had a significant effect on SPAD values. These results are similar to those of Tubeileh et al. [
33].
Table 2.
Stomatal conductance (Gs), transpiration rate (Tr), and chlorophyll content index (SPAD) under different treatments on 46 DAS.
Table 2.
Stomatal conductance (Gs), transpiration rate (Tr), and chlorophyll content index (SPAD) under different treatments on 46 DAS.
| Treatments |
SPAD |
GS (mol m-2s-1) |
Tr (mmol m-2s-1) |
| C1W1 |
37.9 ± 1.90 ab |
0.54 ± 0.07 a |
0.05 ± 0.005 a |
| C2W1 |
35.8 ± 2.83 ab |
0.51 ± 0.11 ab |
0.04 ± 0.01 ab |
| C3W1 |
39.7 ± 1.78 a |
0.22 ± 0.06 b |
0.02 ± 0.005 b |
| C1W2 |
33.5 ± 1.99 b |
0.46 ± 0.29 ab |
0.04 ± 0.02 ab |
| C2W2 |
35.2 ± 1.15 ab |
0.41 ± 0.11 ab |
0.03 ± 0.01 ab |
| C3W2 |
35.3 ± 3.95 ab |
0.30 ± 0.19 ab |
0.03 ± 0.01 ab |
| C |
NS |
* |
* |
| W |
* |
NS |
NS |
| C*W |
NS |
NS |
NS |
Materials and Methods
3.1. Soil Column Experiment
The soil was sampled at a depth of 0-20 cm soil layer under a continuous maize cropping system at Hailun (47°26′N, 126°47′E), Heilongjiang Province in Northeast China. The soil is typical black soil (Mollisols in USDA classification), composed of 67.62 g kg-1 organic matter, 1.83 g kg-1 total nitrogen, 0.74 g kg-1 total phosphorus, 17.29 g kg-1 total potassium, and pH 6.79 (0-20 cm soil layer). The particle-size distribution of the soil included 14.2% of sand, 59.4% of silt, and 26.4% of clay. The soil was air-dried and sieved to < 2 mm for the column experiment. Columns made of PVC pipe were 53 cm high and 20 cm in diameter, and a total of 120 columns were used in the experiment. At the beginning of the experiment, each column was cleaved vertically into two halves, then the cleaved columns were stuck together and all the columns were sealed with PVC back covers at the bottom. Each column was packed with the sieved soils up to 50 cm. In this study, we considered two factors: compaction stress and soil water content. Considering the Proctor reference bulk density of the sampled soils was about 1.61 g cm-3 and the local bulk density after harvest was about 1.24 g cm-3, three levels of bulk density (i.e. 1.1, 1.3, and 1.5 g cm-3) were chosen to represent low, moderate and severe compaction (hereafter referred to as C1, C2, and C3, respectively).
Maize seeds (
Demeiya3) were germinated on wet filter paper at 30 ℃ for 48 h and then planted 3 mm below the soil surface in the columns. The experiment lasted for 46 d (from Jun. 1, 2022 to Jul. 16, 2022) from sowing to jointing stages of maize. At the soil surface in each column, 3 cm of fine quartz sand were filled to reduce soil surface evaporation on Jun. 10, 2022 (10 days after sowing, 10 DAS). Until Jun. 16, 2022 (16 DAS), all the seedlings in the columns were irrigated sufficiently. Two soil water content treatments (referred to as W1 and W2, representing well-watered and drought conditions), were set on 16 DAS with maize irrigated every 6 d using different amount of water. The W1 treatments were maintained with an average soil water content in the root zone of no less than 80% of field water capacity by weighting. The irrigation volume for the W2 treatments was half that of the W1 treatments. Together with the compaction treatments, this study involved 6 treatments: C1W1, C2W1, C3W1, C1W2, C2W2, and C3W2, respectively. One column for each treatment was selected to install EC-5 sensors (Meter Inc., USA) at 5, 10, 15, 25, and 35 cm depth from the soil surface to monitoring soil water content of the columns (
Figure 6). All the columns were placed under natural conditions with a rain shelter and arranged in a completely random manner.
Sampling work was conducted every 6 d and 6 times in total from 16 DAS to 46 DAS. At each sampling time, three columns were selected randomly to cut down the shoots of maize and opened to sample roots. The shoots collected from each seedling were measured for plant height and then dried for 48 h to a constant weight at 70°C and weighed. The opened soil columns were cut into 5-cm soil layers from the soil surface to rooting depth. The soil of each layer was put into a mesh with grids of 0.05 cm in diameter, the soil was carefully washed away, and the roots in each soil layer were picked out. The collected roots were then scanned with a scanner (Snapscan 1236, AGFA, Dusseldorf, Germany), and analyzed with the WinRHIZO Pro software package (Regent Instruments Inc., Quebec, QC, Canada) for root morphological traits. The dry weight of roots was also measured in the same way as shoots. In addition, three plants of each treatment were selected to measure leaf photosynthesis indexes and chlorophyll content index (SPAD) on 46 DAS. According to Ren et al. [
36], the photosynthetic indexes including stomatal conductance (Gs) and transpiration rate (Tr) were measured at 9:00~11:00 a.m. by Li-600 portable photosynthesis system (LI-COR, Lincoln, NE, USA). To determine the leaf SPAD value, the SPAD values of three fully unfolded leaves from the top were recorded using a Minolta SPAD-502 chlorophyll meter manufactured in Japan [
37].
To investigate the soil pore structure of each treatment, the intact soil cores were collected from the upper layer using PVC cylinders (6 cm in height, 5 cm in inner diameter) on 46 DAS. The PVC cylinders were gently pushed into the topsoil with a metal handle, and the surrounding material was removed step by step. Then, the extracted soil cores were immediately wrapped with plastic films to prevent water loss and transported to the laboratory carefully to avoid disturbance. The soil cores were stored in a refrigerator at 4 ℃ before measurements. Each treatment was replicated three times, resulting in a total of 18 intact soil cores.
3.2. XCT Imaging and Data Processing
The soil cores were scanned using an industrial X-ray computed tomography (Skyscan1172, Germany) at an energy level of 80 kV and a current is 100 μA. The digital images processing and quantification of soil pore characteristics were done using ImageJ software [
25]. To remove the PVC wall effect, a region of interest (ROI) of 30 mm in diameter and 30 mm in height was carefully selected from the central part. After reducing image noise with a median filter (2 × 2 × 2 pixels), image segmentation was performed using auto locally adaptive segmentation method to identify soil pore and matrix. Subsequently, image analysis was conducted using the processed binary images. The 3D pore structure images were visualized using the ImageJ Bone-J plugin [
38].
The porosity (
ϕ) was defined as the percentage of the CT-derived pore (> 10 μm) volume to the total volume of the ROI [
39]. In this study, the hydraulic radius (HR), compactness (CP), degree of anisotropy (DA), connected largest porosity (CLP), global connectivity (
Γ), specific surface area (SSA) and maximum pore diameter (MAPD) of soil pores were employed to represent pore characteristics. HR was computed as the ratio of the volume and surface area of the macropores in the soil. The larger the hydraulic radius, the greater the capacity of water and air conduction [
39]. CP was a pore shape factor, whose value increased as the pore deviated more from a sphere [
40]. The porosity roundness was represented by DA, and the closer DA was to zero, the closer the porosity was to the circle. Pore morphology was also more regular [
41].
The volume of pores was calculated using the “particle analyzer” by the Bone-J plugin. CLP was the volume of the largest interconnected macropore network in the soil as a percentage of the ROI volume [
41]. Before connectivity analysis, the binary image should be applied “purify” in the Bone-J plugin to obtain the largest interconnected macropores network [
42,
43]. It was measured using the “particle analyzer” plugin in ImageJ. SSA was the ratio of pore surface area to total volume of the ROI. When the SSA of pores was larger, the connectivity of pores would also increase, which was conducive to hydraulic conduction [
39]. MAPD was obtained by using “Thickness” function in Bone-J plugin. In this study, four pore size classes (10-200 µm, 200-500 µm, 500-1000 µm, and >1000 µm) were chosen based on the variation trends of pore size distributions among the treatments [
44].
3.3. Statistical Analysis
The statistical analysis was conducted using the software package IBM SPSS Statistical 26.0. The effects of compaction stress combined with drought on soil pore structure, root system development and maize growth were analyzed by one-way ANOVA. The least significant difference (LSD) was used to determine significant differences among means at the P < 0.05 level. Pearson’s correlation analysis was used to evaluate the relationship between soil pore characteristics and maize growth parameters with 95% confidence (α = 0.05).
Conclusions
Our study indicated that soil compaction combined with soil water stress significantly affected the characteristics of soil pore structure, root system development and maize growth under soil column experiment. With the increase of soil bulk density, the porosity, larger pores (> 500 μm), and the maximum pore diameter significantly decreased (P < 0.05) regardless of soil water status. In addition, pore morphology and network parameters (including HR, CP, DA, Γ, CLP, etc.) were also significantly deteriorated under soil compaction combined with drought. Soil compaction combined with drought consistently exhibited decreased root length, root surface area and root volume and root surface area, as well as shoot biomass and root biomass. However, no significant interaction was found in most root structure traits between soil compaction and water content, with an exception of root average diameter. Pearson’s correlation analysis showed that there were significant correlations between most soil pore parameters and maize growth traits. Furthermore, soil compaction showed a significant effect on stomatal conductance and transpiration rate while soil water showed a significant effect on SPAD. The results will provide a better understanding on the effect of soil compaction on soil pore structure and plant growth. Future experimental field work is essential to determine whether similar results will be achieved by soil compaction combined with drought.
Author Contributions
X.Z. (Xiangming Zhu) conceived and designed the project. W.P. (Wei Peng) and E.R. (Enhua Ran) performed the majority of experiments. X.Z. and W.P. conducted the data analysis and wrote the manuscript. X.Z. and Q.X. (Qingyang Xie) further revised the article. All authors discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was granted by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA28010401) and the International Partnership Program of Chinese Academy of Sciences (Grant No. 131323KYSB20210004).
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors would like to thank Meiling Fu, Qin Zhao, and Haijun Yang for their help in the column experiment.
Conflicts of Interests
The authors declare no conflict of interest.
References
- Hartemink, A. E. Soils Are Back on the Global Agenda. Soil Use Manage. 2008, 24 (4), 327–330. [CrossRef]
- Gregorich, E. G.; McLaughlin, N. B.; Lapen, D. R.; Ma, B. L.; Rochette, P. Soil Compaction, Both an Environmental and Agronomic Culprit: Increased Nitrous Oxide Emissions and Reduced Plant Nitrogen Uptake. Soil Sci. Soc. Am. J. 2014, 78 (6), 1913–1923. [CrossRef]
- Shah, A. N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M. A.; Tung, S. A.; Hafeez, A.; Souliyanonh, B. Soil Compaction Effects on Soil Health and Cropproductivity: An Overview. Environ. Sci. Pollut. Res. 2017, 24 (11), 10056–10067. [CrossRef]
- Batey, T. Soil Compaction and Soil Management – a Review. Soil Use Manage. 2009, 25 (4), 335–345. [CrossRef]
- Hamza, M. A.; Anderson, W. K. Responses of Soil Properties and Grain Yields to Deep Ripping and Gypsum Application in a Compacted Loamy Sand Soil Contrasted with a Sandy Clay Loam Soil in Western Australia. Aust. J. Agric. Res. 2003, 54 (3), 273–282. [CrossRef]
- Horn, R.; Fleige, H. Risk Assessment of Subsoil Compaction for Arable Soils in Northwest Germany at Farm Scale. Soil Tillage Res. 2009, 102 (2), 201–208. [CrossRef]
- Correa, J.; Postma, J. A.; Watt, M.; Wojciechowski, T. Soil Compaction and the Architectural Plasticity of Root Systems. J. Exp. Bot. 2019, 70 (21), 6019–6034. [CrossRef]
- Sun, X.; She, D.; Fei, Y.; Wang, H.; Gao, L. Three-Dimensional Fractal Characteristics of Soil Pore Structure and Their Relationships with Hydraulic Parameters in Biochar-Amended Saline Soil. Soil Tillage Res. 2021, 205, 104809. [CrossRef]
- Munkholm, L. J.; Heck, R. J.; Deen, B. Soil Pore Characteristics Assessed from X-Ray Micro-CT Derived Images and Correlations to Soil Friability. Geoderma 2012, 181–182, 22–29. [CrossRef]
- Naveed, M.; Moldrup, P.; Schaap, M. G.; Tuller, M.; Kulkarni, R.; Vogel, H.-J.; Wollesen De Jonge, L. Prediction of Biopore- and Matrix-Dominated Flow from X-Ray CT-Derivedmacropore Network Characteristics. Hydrol. Earth Syst. Sci. 2016, 20 (10), 4017–4030. [CrossRef]
- Feng, Y.; Wang, J.; Liu, T.; Bai, Z.; Reading, L. Using Computed Tomography Images to Characterize the Effects of Soil Compaction Resulting from Large Machinery on Three-Dimensional Pore Characteristics in an Opencast Coal Mine Dump. J. Soils Sediments 2019, 19 (3), 1467–1478. [CrossRef]
- Zhu, S.; Zhen, Q.; Zhang, X. Multifractal Characteristics of the Pore Structures of Physically Amended Sandy Soil and the Relationship between Soil Properties and Multifractal Parameters. Arch. Agron. Soil Sci. 2020, 66 (9), 1188–1202. [CrossRef]
- Pfeifer, J.; Faget, M.; Walter, A.; Blossfeld, S.; Fiorani, F.; Schurr, U.; Nagel, K. A. Spring Barley Shows Dynamic Compensatory Root and Shoot Growth Responses When Exposed to Localised Soil Compaction and Fertilisation. Functional Plant Biol. 2014, 41 (6), 581–597. [CrossRef]
- Lipiec, J.; Hatano, R. Quantification of Compaction Effects on Soil Physical Properties and Crop Growth. Geoderma 2003, 116 (1), 107–136. [CrossRef]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of Soil Compaction on Root Elongation and Anatomy of Different Cereal Plant Species. Soil Tillage Res. 2012, 121, 74–81. [CrossRef]
- Jin, K.; Shen, J.; Ashton, R. W.; White, R. P.; Dodd, I. C.; Phillips, A. L.; Parry, M. A. J.; Whalley, W. R. The Effect of Impedance to Root Growth on Plant Architecture in Wheat. Plant Soil 2015, 392 (1), 323–332. [CrossRef]
- Potocka, I.; Szymanowska-Pułka, J. Morphological Responses of Plant Roots to Mechanical Stress. Ann. Bot. 2018, 122 (5), 711–723. [CrossRef]
- Zhang, X.; Hua, Z.; Deng, H. Effects of soil compaction stress on growth, quantity and quality of Scutellaria baicalensis. Soil. Fert. Sci. China 2014, 7–11. [CrossRef]
- Whalley, W. R.; Clark, L. J.; Gowing, D. J. G.; Cope, R. E.; Lodge, R. J.; Leeds-Harrison, P. B. Does Soil Strength Play a Role in Wheat Yield Losses Caused by Soil Drying? Plant Soil 2006, 280 (1), 279–290. [CrossRef]
- Young, I. M.; Montagu, K.; Conroy, J.; Bengough, A. G. Mechanical Impedance of Root Growth Directly Reduces Leaf Elongation Rates of Cereals. New Phytol. 1997, 135 (4), 613–619. [CrossRef]
- Beemster, G. T. S.; Masle, J. Effects of Soil Resistance to Root Penetration on Leaf Expansion in Wheat (Triticum Aestivum L.): Composition, Number and Size of Epidermal Cells in Mature Blades. J. Exp. Bot. 1996, 47 (11), 1651–1662. [CrossRef]
- Shaheb, R. A Study on the Effect of Tyre Inflation Pressure on Soil Properties, Growth and Yield of Maize and Soybean in Central Illinois. Ph.D. thesis, Harper Adams University, New Port,UK, 2020. https://hau.repository.guildhe.ac.uk/id/eprint/17769.
- Masle, J.; Farquhar, G.; Gifford, R. Growth and Carbon Economy of Wheat Seedlings as Affected by Soil Resistance to Penetration and Ambient Partial Pressure of CO2. Functional Plant Biol. 1990, 17 (4), 465–487. [CrossRef]
- Tardieu, F.; Zhang, J.; Katerji, N.; Bethenod, O.; Palmer, S.; Davies, W. J. Xylem ABA Controls the Stomatal Conductance of Field-Grown Maize Subjected to Soil Compaction or Soil Drying. Plant, Cell Environ. 1992, 15 (2), 193–197. [CrossRef]
- Ripley, B. S.; Gilbert, M. E.; Ibrahim, D. G.; Osborne, C. P. Drought Constraints on C4 Photosynthesis: Stomatal and Metabolic Limitations in C3 and C4 Subspecies of Alloteropsis Semialata. J. Exp. Bot. 2007, 58 (6), 1351–1363. [CrossRef]
- Schlüter, S.; Weller, U.; Vogel, H.-J. Soil-Structure Development Including Seasonal Dynamics in a Long-Term Fertilization Experiment. J. Plant Nutr. Soil Sci. 2011, 174 (3), 395–403. [CrossRef]
- Steponavičienė, V.; Bogužas, V.; Sinkevičienė, A.; Skinulienė, L.; Vaisvalavičius, R.; Sinkevičius, A. Soil Water Capacity, Pore Size Distribution, and CO2 Emission in Different Soil Tillage Systems and Straw Retention. Plants 2022, 11 (5). [CrossRef]
- Bengough, A. G.; McKenzie, B. M.; Hallett, P. D.; Valentine, T. A. Root Elongation, Water Stress, and Mechanical Impedance: A Review of Limiting Stresses and Beneficial Root Tip Traits. J. Exp. Bot. 2011, 62 (1), 59–68. [CrossRef]
- Grzesiak, M. T.; Janowiak, F.; Szczyrek, P.; Kaczanowska, K.; Ostrowska, A.; Rut, G.; Hura, T.; Rzepka, A.; Grzesiak, S. Impact of Soil Compaction Stress Combined with Drought or Waterlogging on Physiological and Biochemical Markers in Two Maize Hybrids. Acta Physiol. Plant. 2016, 38 (5), 109. [CrossRef]
- Budhathoki, S.; Lamba, J.; Srivastava, P.; Williams, C.; Arriaga, F.; Karthikeyan, K. G. Impact of Land Use and Tillage Practice on Soil Macropore Characteristics Inferred from X-Ray Computed Tomography. Catena 2022, 210, 105886. [CrossRef]
- Pires, L. F.; Roque, W. L.; Rosa, J. A.; Mooney, S. J. 3D Analysis of the Soil Porous Architecture under Long Term Contrasting Management Systems by X-Ray Computed Tomography. Soil Tillage Res. 2019, 191, 197–206. [CrossRef]
- Chen, Y. L.; Palta, J.; Clements, J.; Buirchell, B.; Siddique, K. H. M.; Rengel, Z. Root Architecture Alteration of Narrow-Leafed Lupin and Wheat in Response to Soil Compaction. Field Crops Res. 2014, 165, 61–70. [CrossRef]
- Laboski, C. A. M.; Dowdy, R. H.; Allmaras, R. R.; Lamb, J. A. Soil Strength and Water Content Influences on Corn Root Distribution in a Sandy Soil. Plant Soil 1998, 203 (2), 239–247. [CrossRef]
- Tubeileh, A.; Groleau-Renaud, V.; Plantureux, S.; Guckert, A. Effect of Soil Compaction on Photosynthesis and Carbon Partitioning within a Maize–Soil System. Soil Tillage Res. 2003, 71 (2), 151–161. [CrossRef]
- Cambi, M.; Hoshika, Y.; Mariotti, B.; Paoletti, E.; Picchio, R.; Venanzi, R.; Marchi, E. Compaction by a Forest Machine Affects Soil Quality and Quercus Robur L. Seedling Performance in an Experimental Field. For. Ecol. Manage. 2017, 384, 406–414. [CrossRef]
- Leskovar, D. I.; Othman, Y. A. Direct Seeding and Transplanting Influence Root Dynamics, Morpho-Physiology, Yield, and Head Quality of Globe Artichoke. Plants 2021, 10 (5). [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Regulations of 6-Benzyladenine (6-BA) on Leaf Ultrastructure and Photosynthetic Characteristics of Waterlogged Summer Maize. J. Plant Growth Regul. 2017, 36 (3), 743–754. [CrossRef]
- Chen, B.; Huang, G.; Lu, X.; Gu, S.; Wen, W.; Wang, G.; Chang, W.; Guo, X.; Zhao, C. Prediction of Vertical Distribution of SPAD Values within Maize Canopy Based on Unmanned Aerial Vehicles Multispectral Imagery. Front. Plant Sci. 2023, 14. [CrossRef]
- Doube, M.; Kłosowski, M. M.; Arganda-Carreras, I.; Cordelières, F. P.; Dougherty, R. P.; Jackson, J. S.; Schmid, B.; Hutchinson, J. R.; Shefelbine, S. J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2010, 47 (6), 1076–1079. [CrossRef]
- Larsbo, M.; Koestel, J.; Jarvis, N. Relations between Macropore Network Characteristics and the Degree of Preferential Solute Transport. Hydrol. Earth Syst. Sci. 2014, 18 (12), 5255–5269. [CrossRef]
- Bribiesca, E. A Measure of Compactness for 3D Shapes. Comput. Math. Appl. 2000, 40 (10), 1275–1284. [CrossRef]
- Wang, M.; Xu, S.; Kong, C.; Zhao, Y.; Shi, X.; Guo, N. Assessing the Effects of Land Use Change from Rice to Vegetable on Soil Structural Quality Using X-Ray CT. Soil Tillage Res. 2019, 195, 104343. [CrossRef]
- Dal Ferro, N.; Charrier, P.; Morari, F. Dual-Scale Micro-CT Assessment of Soil Structure in a Long-Term Fertilization Experiment. Geoderma 2013, 204–205, 84–93. [CrossRef]
- Pajor, R.; Falconer, R.; Hapca, S.; Otten, W. Modelling and Quantifying the Effect of Heterogeneity in Soil Physical Conditions on Fungal Growth. Biogeosciences 2010, 7 (11), 3731–3740. [CrossRef]
- Singh, N.; Kumar, S.; Udawatta, R. P.; Anderson, S. H.; de Jonge, L. W.; Katuwal, S. X-Ray Micro-Computed Tomography Characterized Soil Pore Network as Influenced by Long-Term Application of Manure and Fertilizer. Geoderma 2021, 385, 114872. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).