Submitted:
30 September 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Setup and Data Post-Processing
2.1. Experimental Setup
2.2. Range of Values for the Spherical Flame Radius
2.3. Extrapolating the Laminar Flame Speed
2.4. Experimental Uncertainty Analysis
3. Numerical Method
4.1. Extrapolation of Laminar Flame Speeds
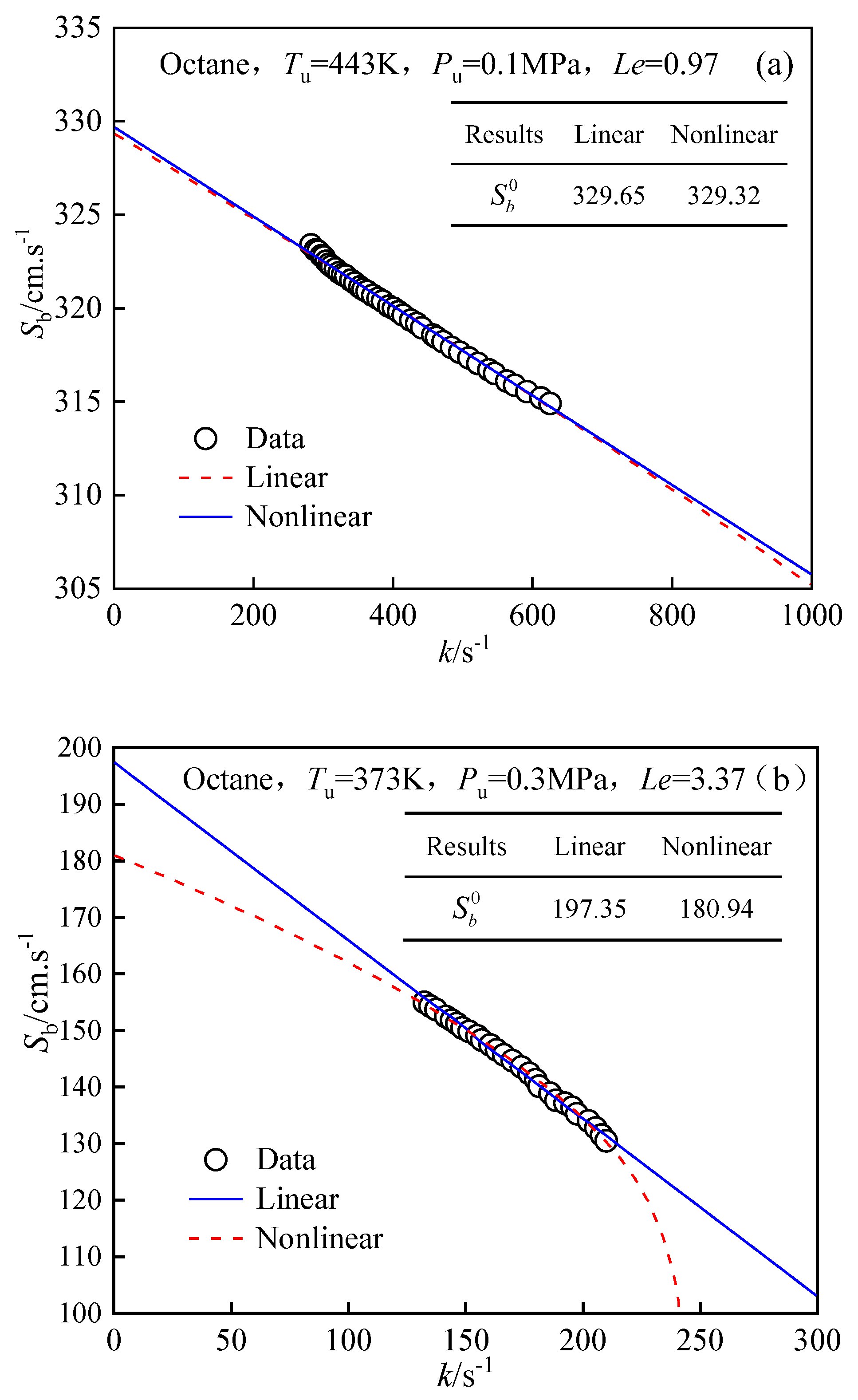
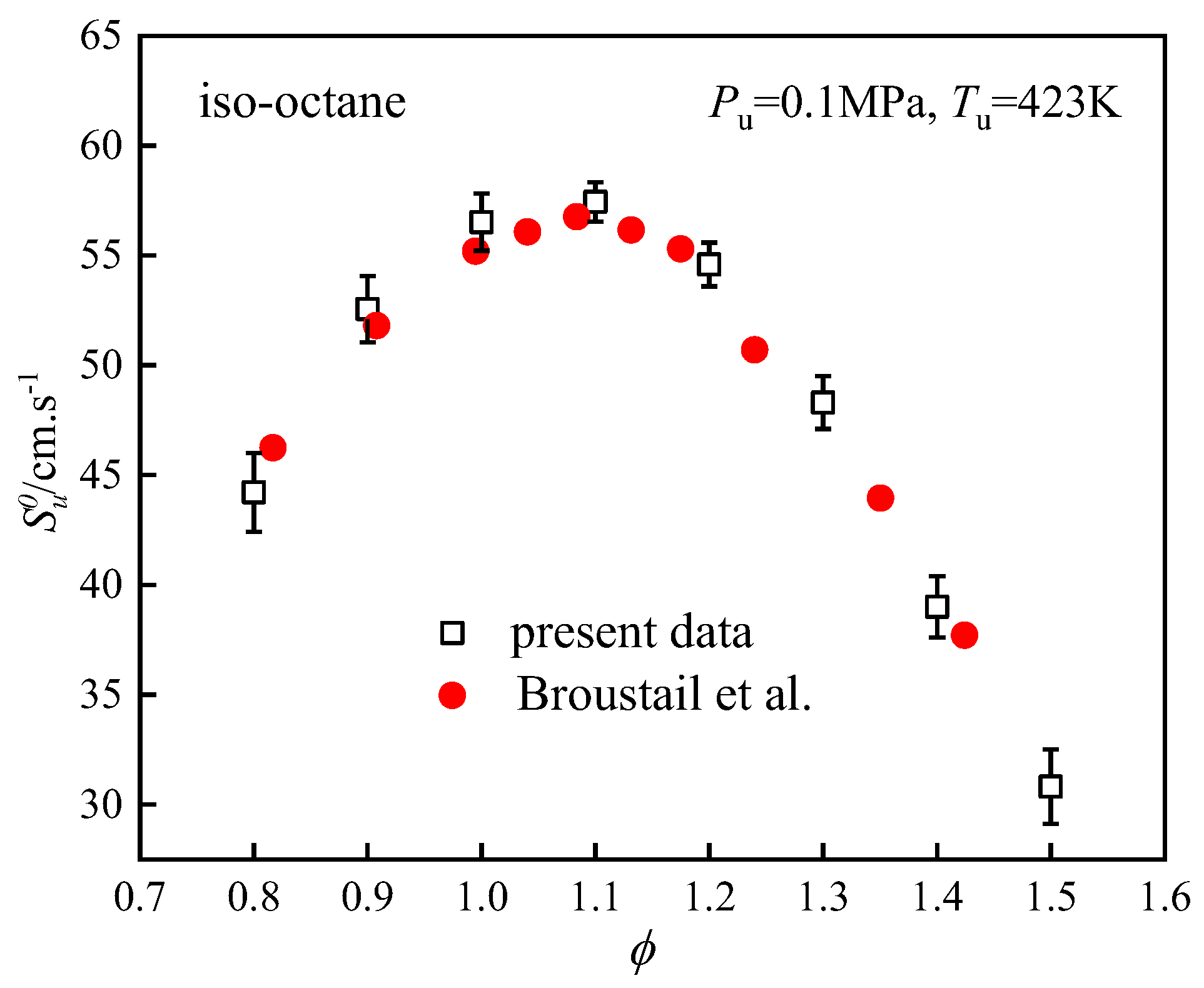
4.2. Validation of Experimental Setup
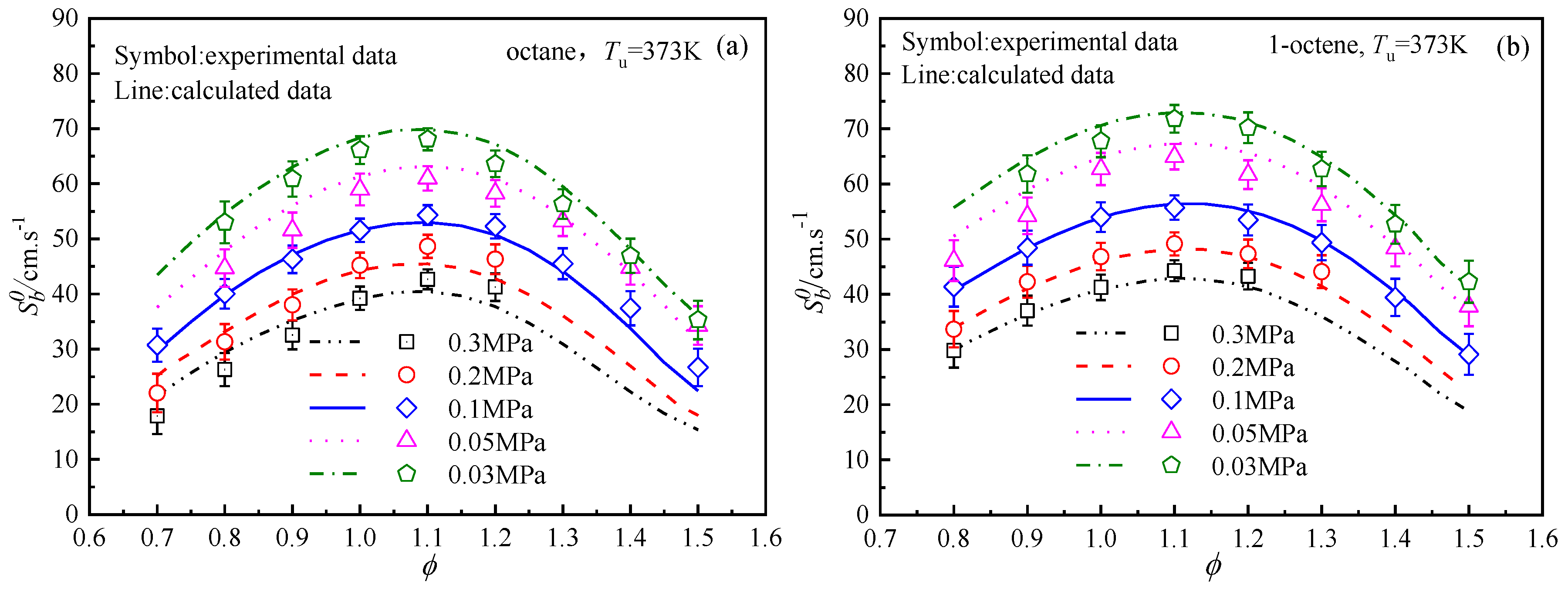
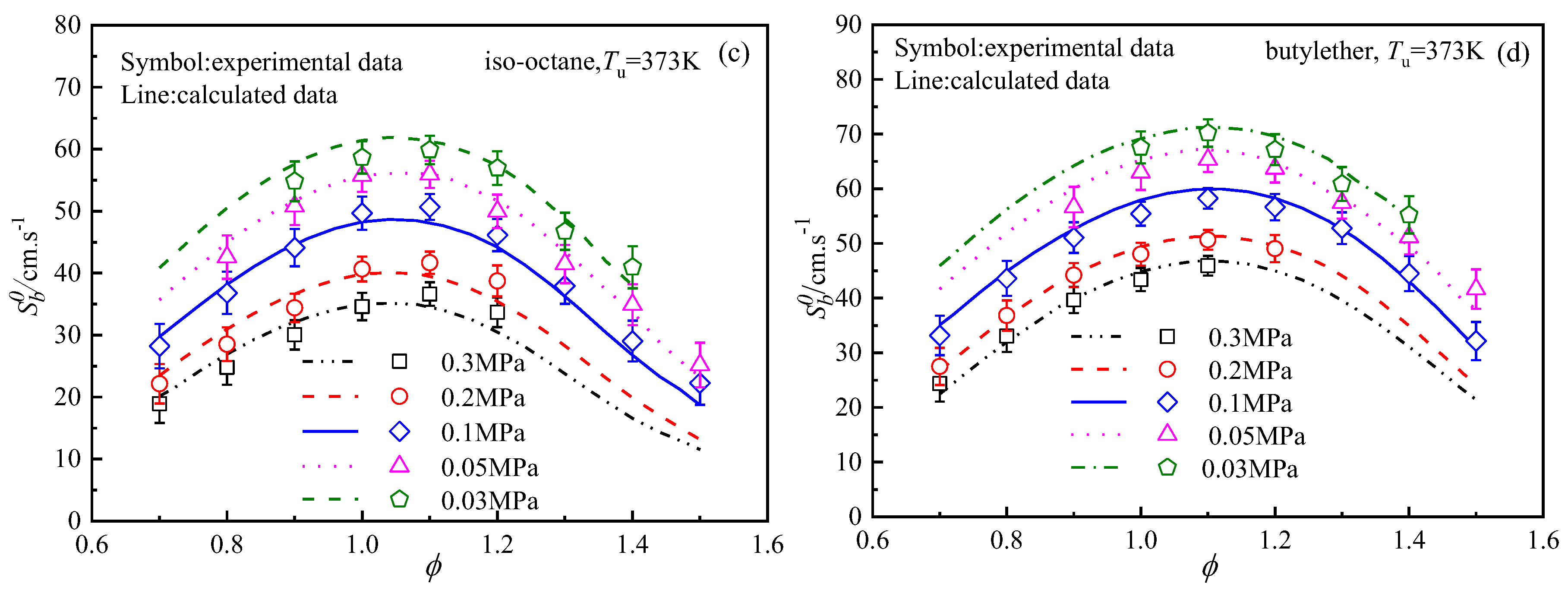
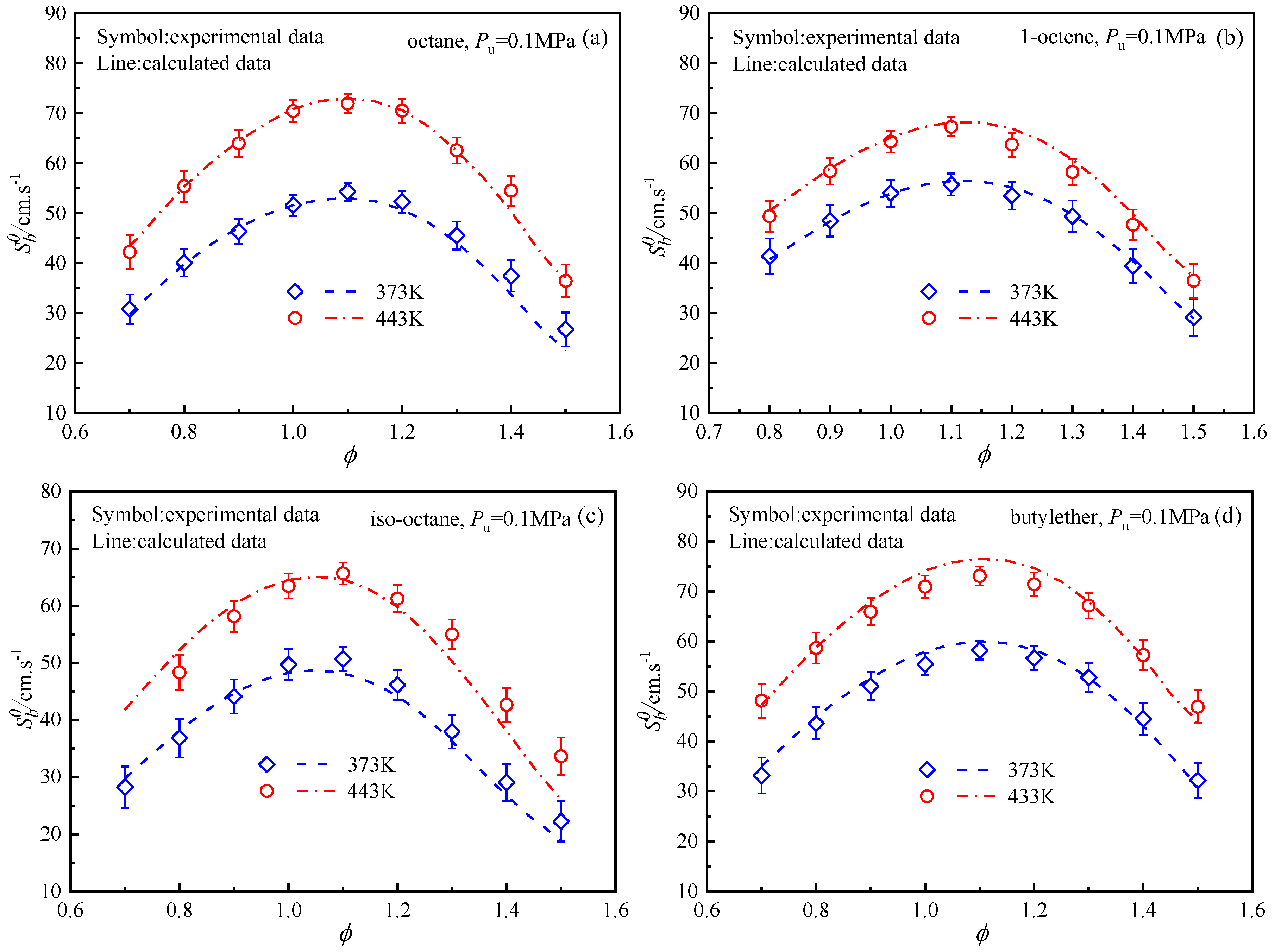
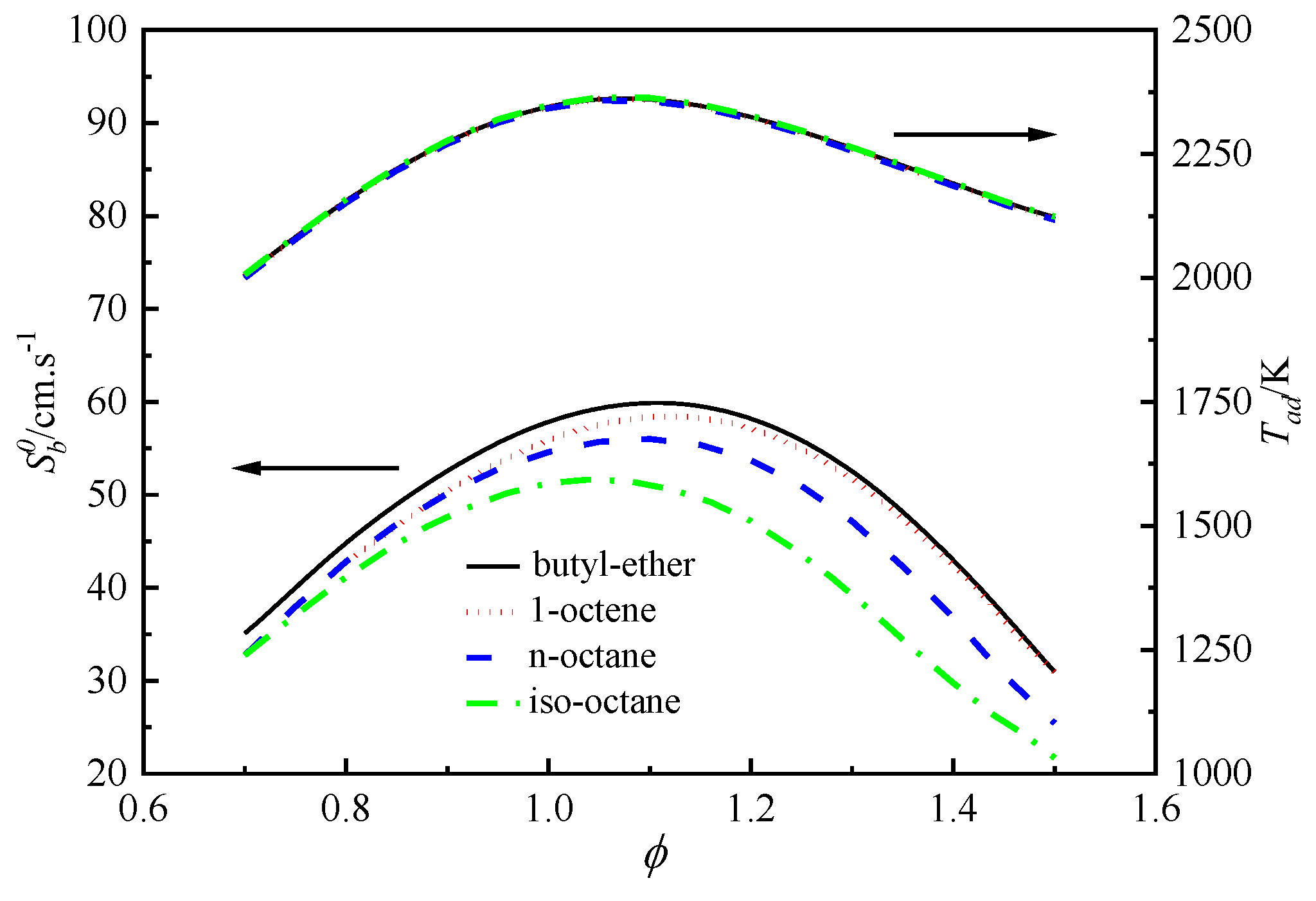
4.3. Laminar Flame Speeds Of n-Octane, Iso-Octane, 1-Octene, and Butyl Ether
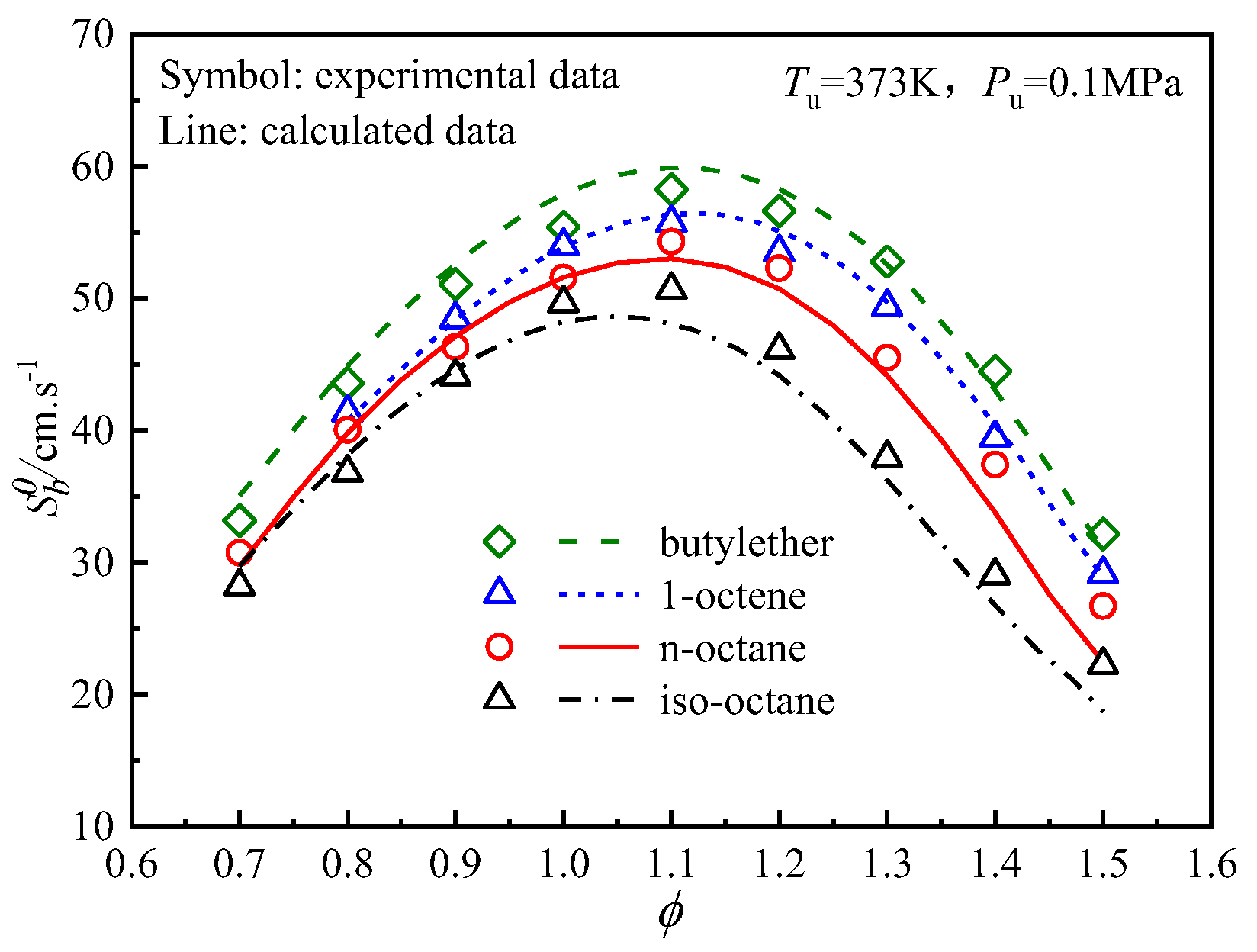
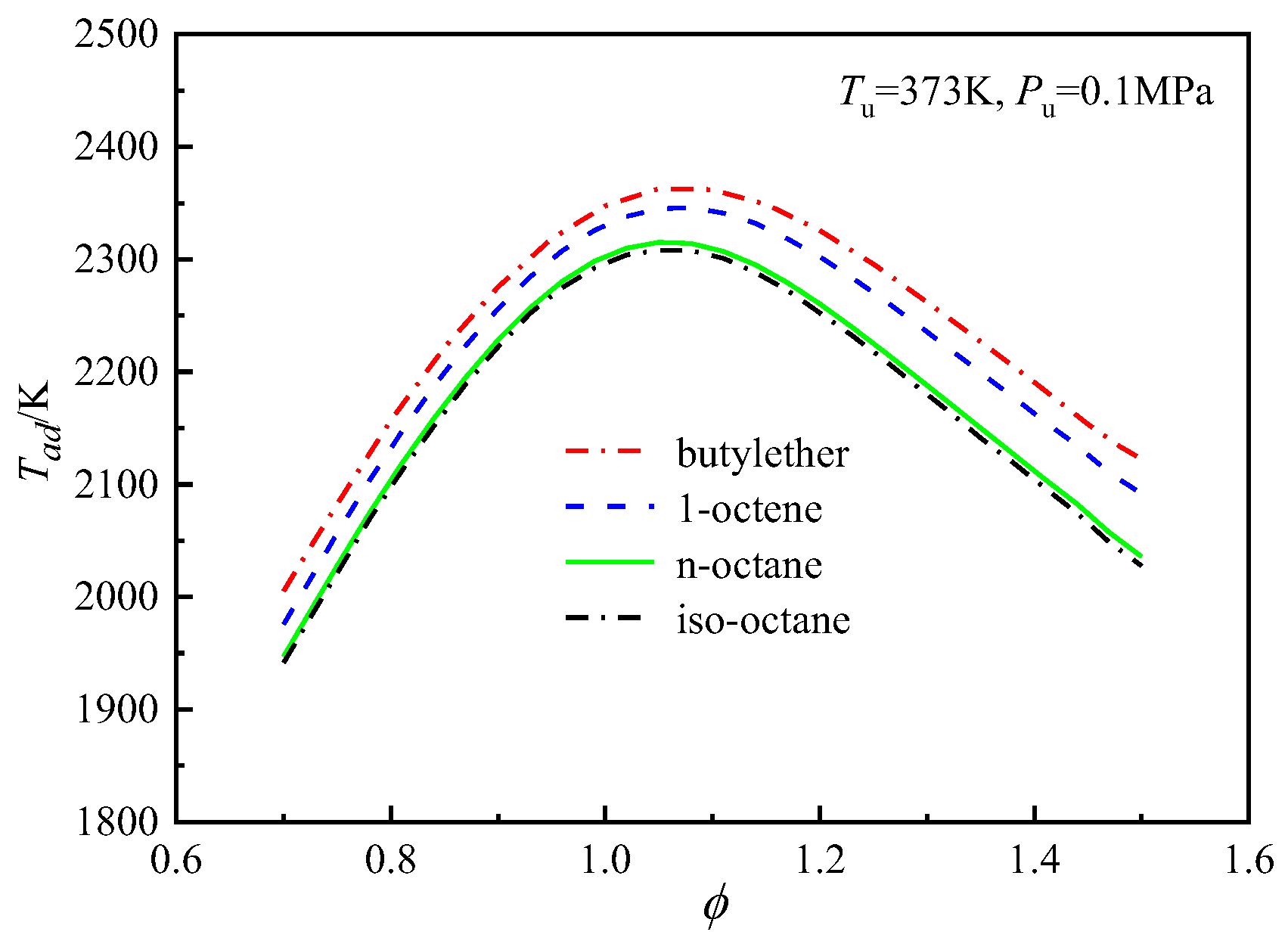
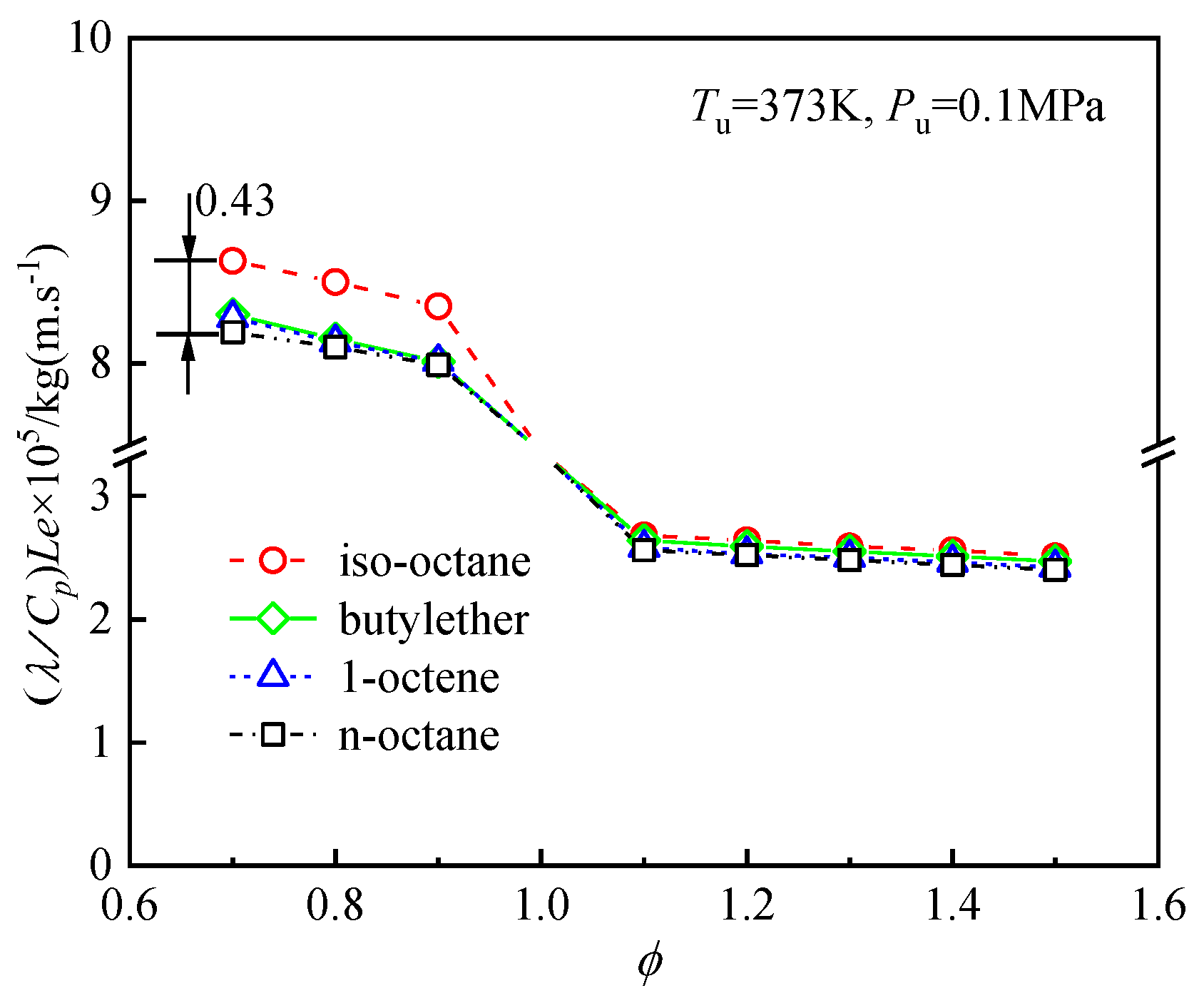
4.4. Analysis of the Differences in the Laminar Flame Speeds of the Four Fuels
4.4.1. Effects of Thermodynamics on the Differences in the Laminar Flame Speeds
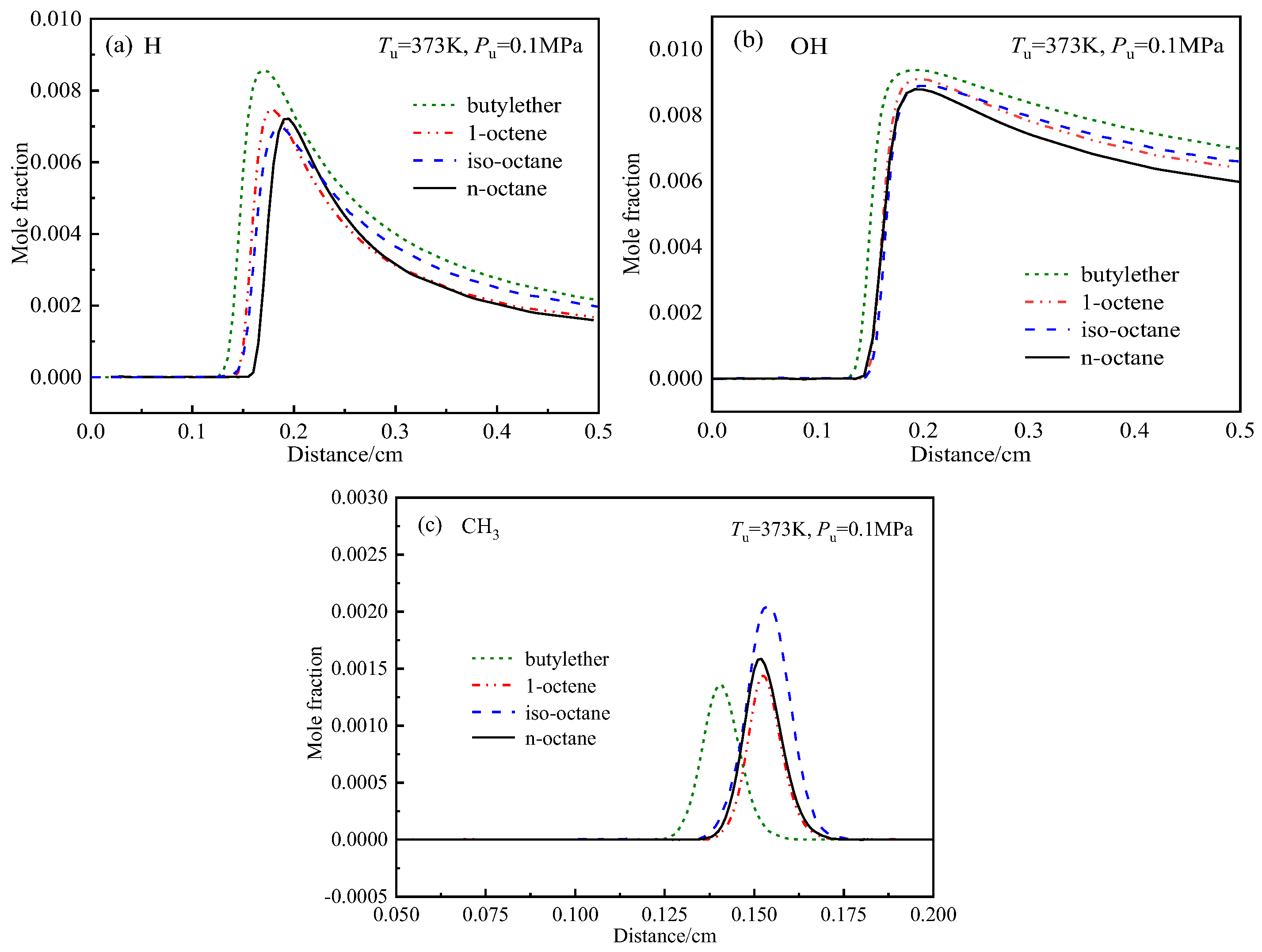
4.4.2. Effects of Diffusion on the Differences in the Laminar Flame Speeds
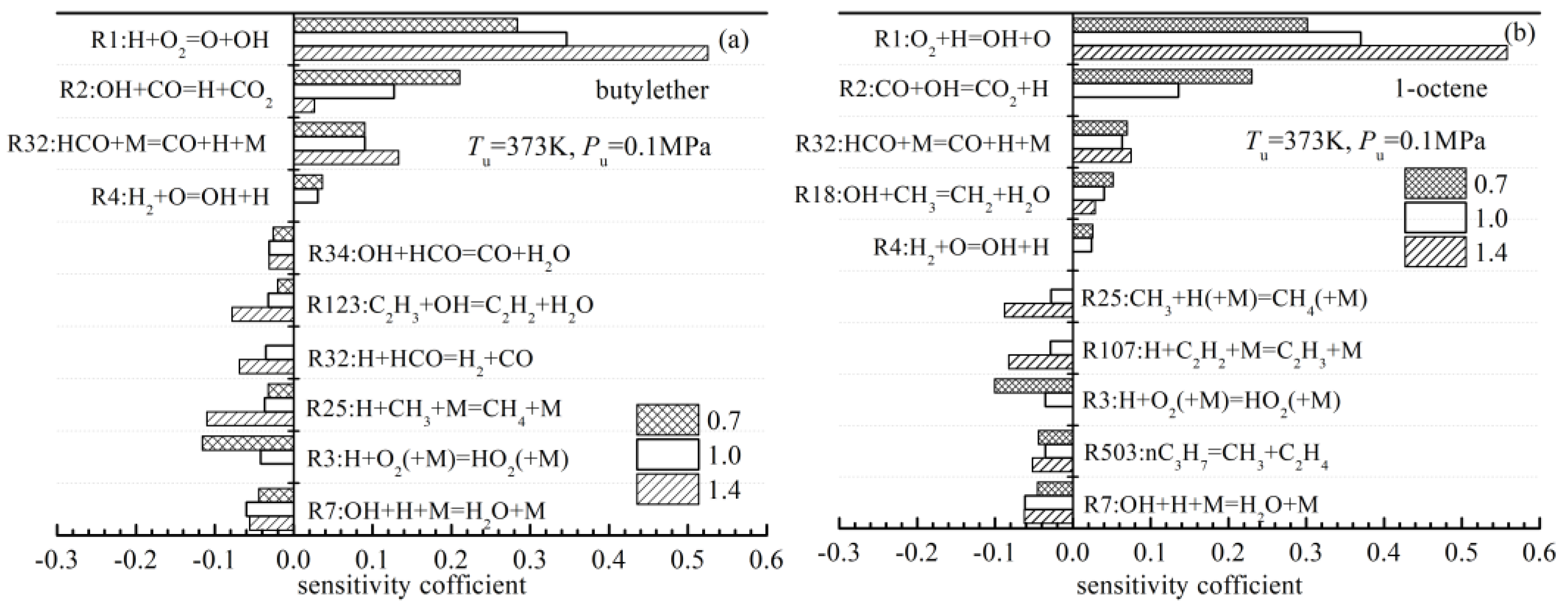
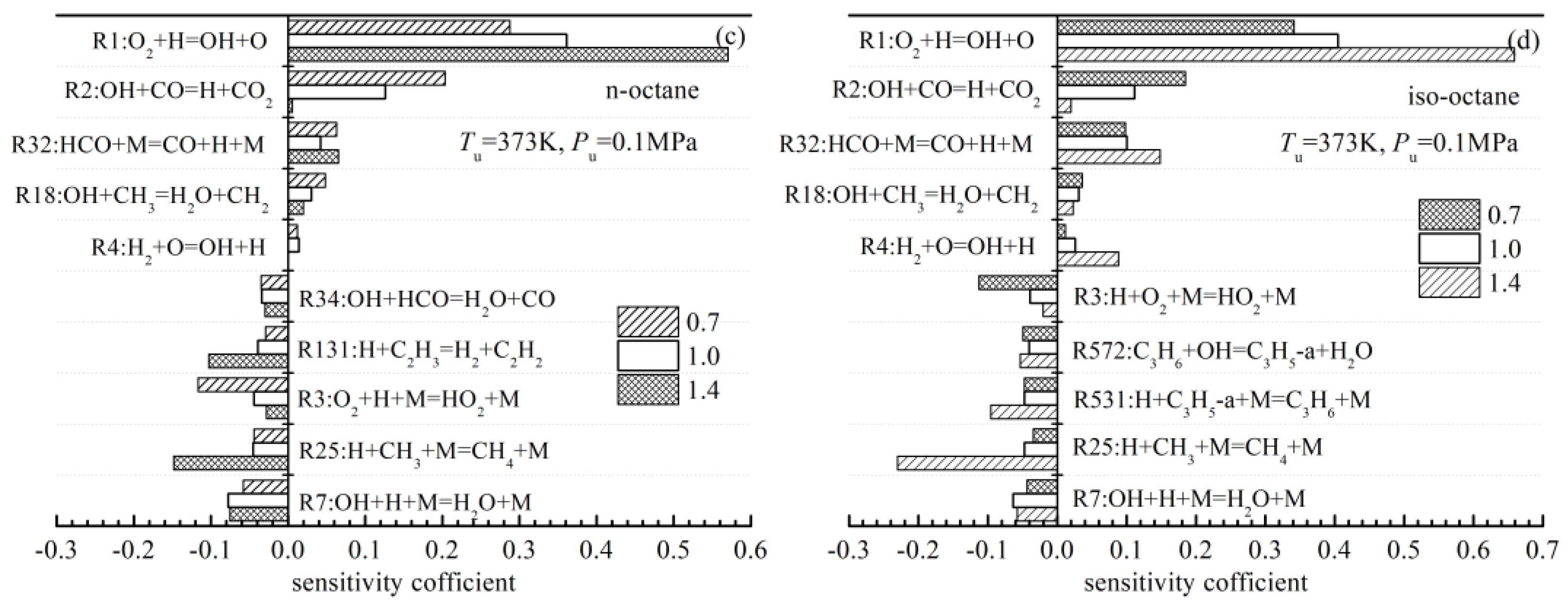
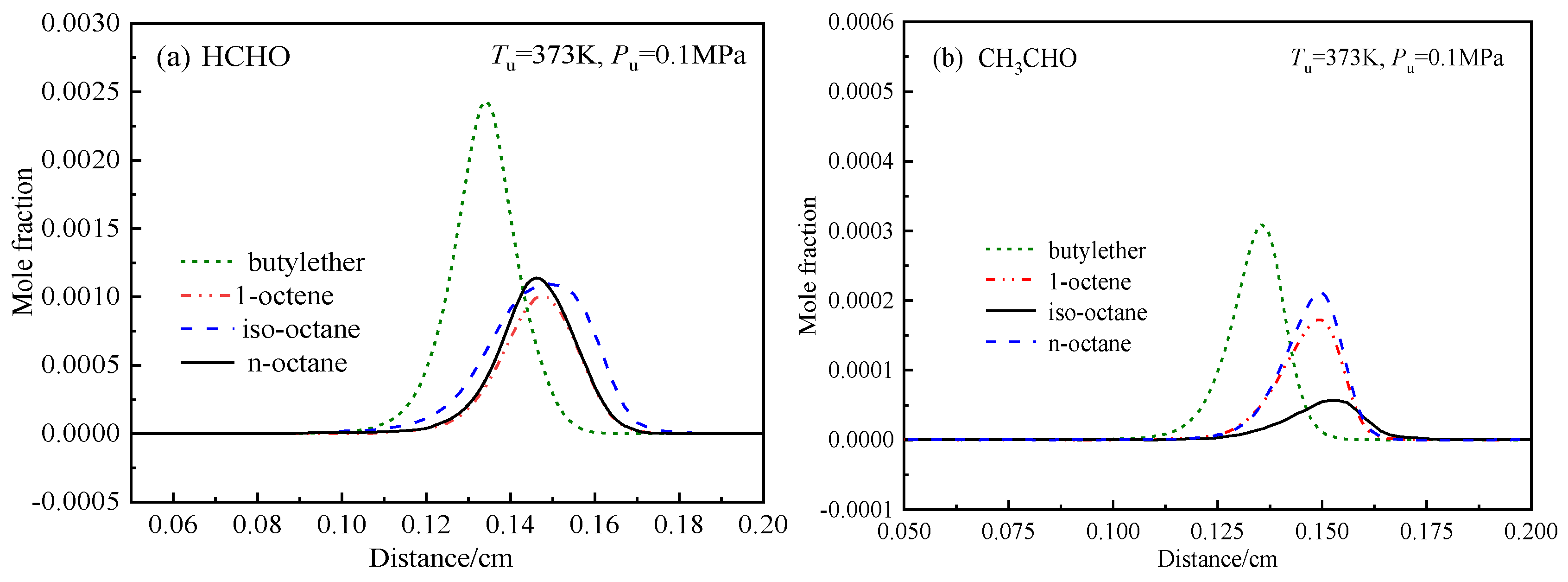
4.4.3. Effects of chEemical Kinetics on the Differences in the Laminar Flame Speeds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelley, A.P.L.W.; Xin, Y.X.; et al. Laminar flame speeds, non-premixed stagnation ignition, and reduced mechanisms in the oxidation of iso-octane. Proceedings of the Combustion Institute 2011, 33, 501–508. [Google Scholar] [CrossRef]
- Ji, C.S.S.M.; Veloo, P.S.; et al. Effects of fuel branching on the propagation of octane isomers flames. Combustion and Flame 2012, 159, 1426–1436. [Google Scholar] [CrossRef]
- Galmiche, B.H.F.; Foucher, F. Effects of high pressure, high temperature and dilution on laminar burning velocities and Markstein lengths of iso-octane/air mixtures. Combustion and Flame 2012, 159, 3286–3299. [Google Scholar] [CrossRef]
- Broustail, G.H.F.; Seers, P.; et al. Experimental determination of laminar burning velocity for butanol/iso-octane and ethanol/iso-octane blends for different initial pressures. Fuel 2013, 106, 310–317. [Google Scholar] [CrossRef]
- Kelley, A.P.S.A.J.; Zhu, D.L.; et al. Laminar flame speeds of C5 to C8 n-alkanes at elevated pressures: Experimental determination, fuel similarity, and stretch sensitivity. Proceedings of the Combustion Institute 2011, 33, 963–970. [Google Scholar] [CrossRef]
- Ji, C.D.E.; Wang, Y.L.; et al. Propagation and extinction of premixed C5-C12 n-alkane flames. Combustion and Flame 2010, 157, 277–287. [Google Scholar] [CrossRef]
- Meng, X.H.O.; Wang, T.; et al. Experimental and modeling study of 1-octene jet-stirred reactor oxidation. Fuel 2017, 207, 763–775. [Google Scholar] [CrossRef]
- Hellier, P.L.N.; Allan, R.; et al. The importance of double bond position and cis–trans isomersation in diesel combustion and emissions. Fuel 2013, 105, 477–489. [Google Scholar] [CrossRef]
- Cai, L.S.A.; Lee, D.J.; et al. Chemical kinetic study of a novel lignocellulosic biofuel: Di-n-butyl ether oxidation in a laminar flow reactor and flames. Combustion and Flame 2014, 161, 798–809. [Google Scholar] [CrossRef]
- Guan, L.T.C.; Yang, K.; et al. Experimental and kinetic study on ignition delay times of di-n-butyl ether at high temperatures. Energy Fuels 2014, 28, 5489–5496. [Google Scholar] [CrossRef]
- Wullenkord, J.T.L.-S.; Böttchers, J.; et al. A laminar flame study on di-n-butyl ether as a potential biofuel candidate. Combustion and Flame 2018, 190, 36–49. [Google Scholar] [CrossRef]
- Kelley, A.P.; L.C.K. Nonlinear effects in the extraction of laminar flame speeds from expanding spherical flames. Combustion and Flame 2009, 156, 1844–1851. [CrossRef]
- Kelley, A.P.; J.G.; Law, C.K. Critical radius for sustained propagation of spark-ignited spherical flames. Combustion and Flame 2009, 156, 1006–1013. [CrossRef]
- Burke, M.P.; Chen, Z.; Ju, Y.G.; Dryer, F.L. Effect of cylindrical confinement on the determination of laminar flame speeds using outwardly propagating flames. Combustion and Flame 2009, 156, 771–779. [Google Scholar] [CrossRef]
- Wu, C.K.; L.C.K. On the determination of laminar flame speeds from stretched flames. Symposium (International) on Combustion 1985, 20, 1941–1949. [CrossRef]
- Qiao, L.; Gu, Y.X.; Dam, W.J.A.; Oran, E.S.; Faeth, G.M. Near-limit laminar burning velocities of microgravity premixed hydrogen flames with chemically-passive fire suppressants. Proceedings of the Combustion Institute 2007, 31, 2701–2709. [Google Scholar] [CrossRef]
- Santner, J.H.F.; Ju, Y.; Dryer, F.L. Uncertainties in interpretation of high pressure spherical flame propagation rates due to thermal radiation. Combustion and Flame 2014, 161, 147–153. [Google Scholar] [CrossRef]
- Yu, H.H.W.; Santner, J.; Gou, X.; Sohn, C.H.; Ju, Y.; et al. Radiation-induced uncertainty in laminar flame speed measured from propagating spherical flames. Combustion and Flame 2014, 161, 2815–2824. [Google Scholar] [CrossRef]
- Moffat. Describing the uncertainties in experimental results. Exp Thermal Fluid Sci 1988, 1, 3–17. [Google Scholar] [CrossRef]
- Kee, R.J. G.J.; Smooke, M.D.; Miller, J.A.; Meeks, E. PREMIX: a Fortran program for modeling steady laminar one-dimensional premixed flames. Sandia National Laboratories Report 1985;SAND85-8249.
- Kee, R.J. R.F.; Miller, J.A. CHEMKIN-II: a Fortran chemical kinetics package for the analysis of gas-phase chemical kinetics. Sandia National Laboratory Technical Report SAND 1989:89-8009.
- Mehl, M.P.W.J.; Westbrook, C.K.; et al. Kinetic modeling of gasoline surrogate components and mixtures under engine conditions. Proceedings of the Combustion Institute 2011, 33, 193–200. [Google Scholar] [CrossRef]
- Sarathy S M WCK, Mehl M, et al. Comprehensive chemical kinetic modeling of the oxidation of 2-methylalkanes from C7 to C20. Combustion and Flame 2011, 158, 2338–2357. [CrossRef]
- Yasunaga, K.; Mikajiri, T.; Sarathy, S.M.; Koike, T.; Gillespie, F.; Nagy, T.; Simmie, J.M.; Curran, H.J. A shock tube and chemical kinetic modeling study of the pyrolysis and oxidation of butanols. Combustion and Flame 2012, 159, 2009–2027. [Google Scholar] [CrossRef]
- Wu, F.K.A.; Tang, C.; Zhu, D.; Law, C.K. Measurement and correlation of laminar flame speeds of CO and C2 hydrocarbons with hydrogen addition at atmospheric and elevated pressures. Int J Hydrog Energy 2011, 36, 13171–13180. [Google Scholar] [CrossRef]
- ZC On the extraction of laminar flame speed and Markstein length from outwardly propagating spherical flames. Combustion and Flame 2011, 158, 291–300. [CrossRef]
- Tang, C.L.H.Z.; Law, C.K. Determination, correlation, and mechanistic interpretation of effects of hydrogen addition on laminar flame speeds of hydrocarbon-air mixtures. Proc Combust Inst 2011, 33, 921–928. [Google Scholar] [CrossRef]
- Law, C.K.S.C. Structure, aerodynamics, and geometry of premixed flamelets. Prog Energy Combust 2000, 26, 459–505. [Google Scholar] [CrossRef]
| iso-octane | n-octane | 1-octene | Butyl ether | |
| molecular formula | C8H18 | C8H18 | C8H16 | C8H18O |
| molecular structure | 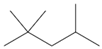 |
 |
 |
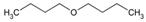 |
| oxygen content/% | 0 | 0 | 0 | 12.3 |
| boiling point/K | 372.3 | 398.5 | 395 | 414 |
| low heat value/MJ·kg−1 | 44.4 | 44.5 | - | 38 |
| Fuel | Tu /K | Pu /MPa | φ |
| n-octane | 373, 443 | 0.03–0.3 | 0.7–1.5 |
| Iso-octane | 373, 423, 443 | 0.03–0.3 | 0.7–1.5 |
| 1-octene | 373, 443 | 0.03–0.3 | 0.7–1.5 |
| Butyl ether | 373, 433 | 0.03–0.3 | 0.7–1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





