Submitted:
30 September 2024
Posted:
03 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
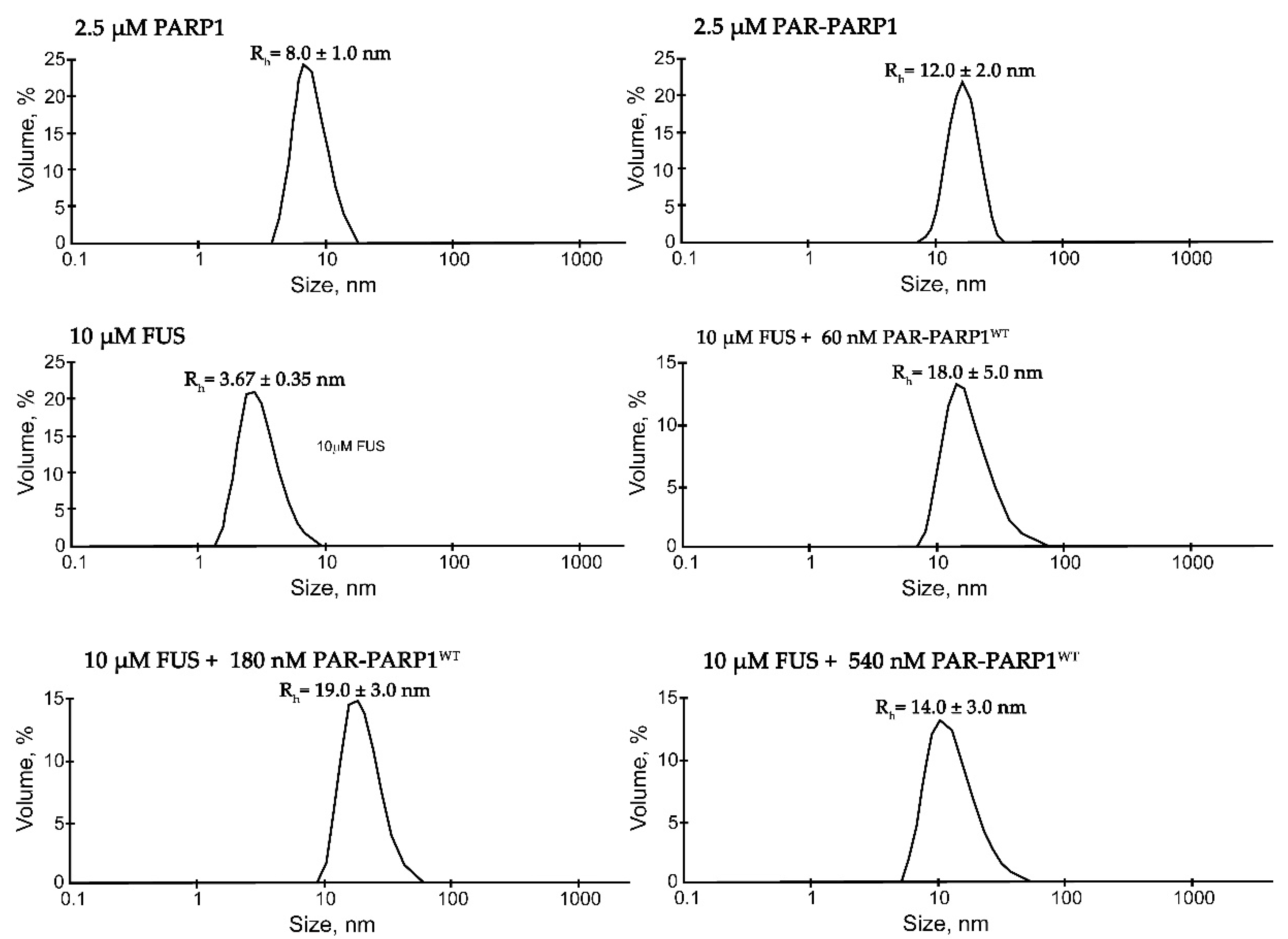
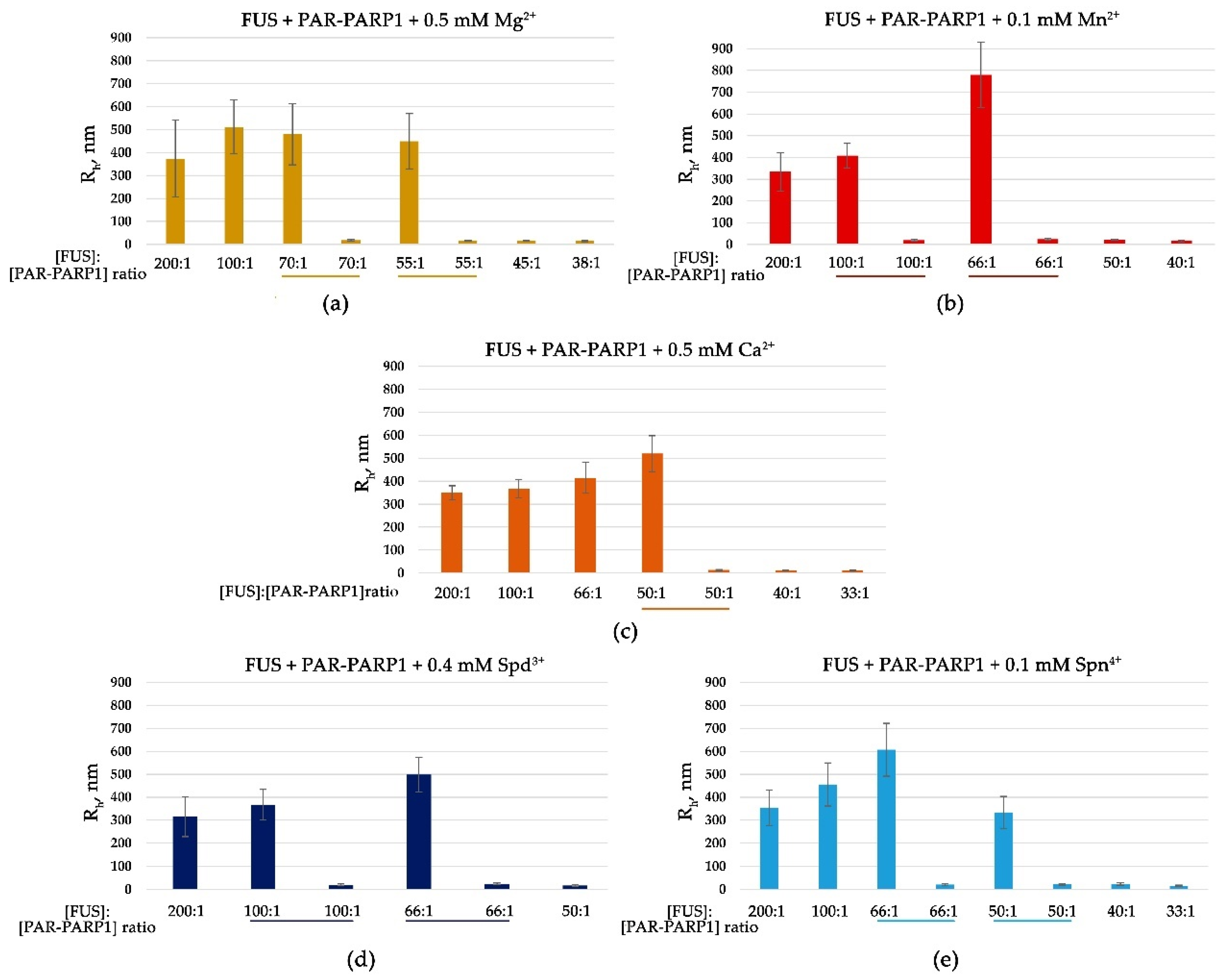
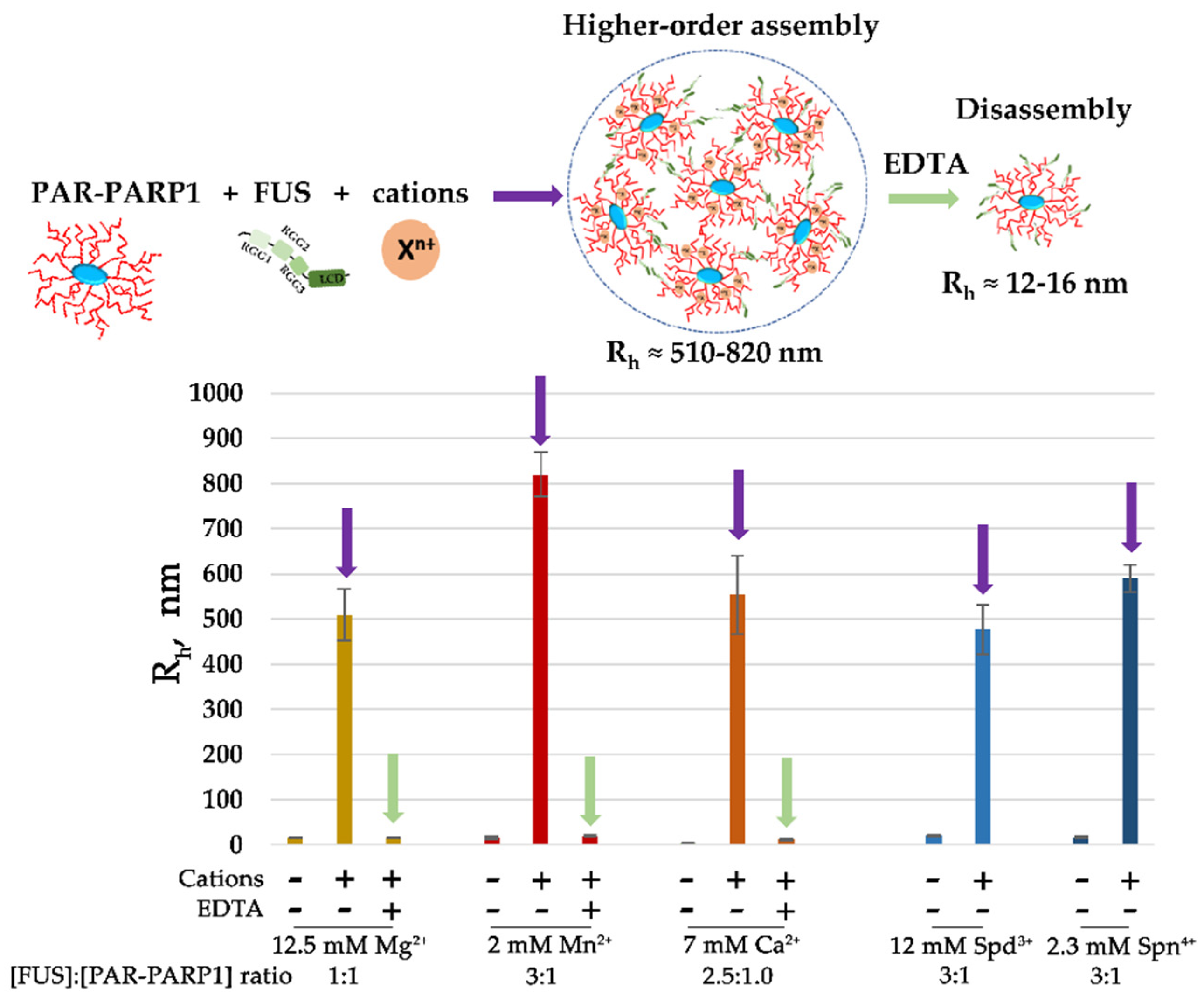
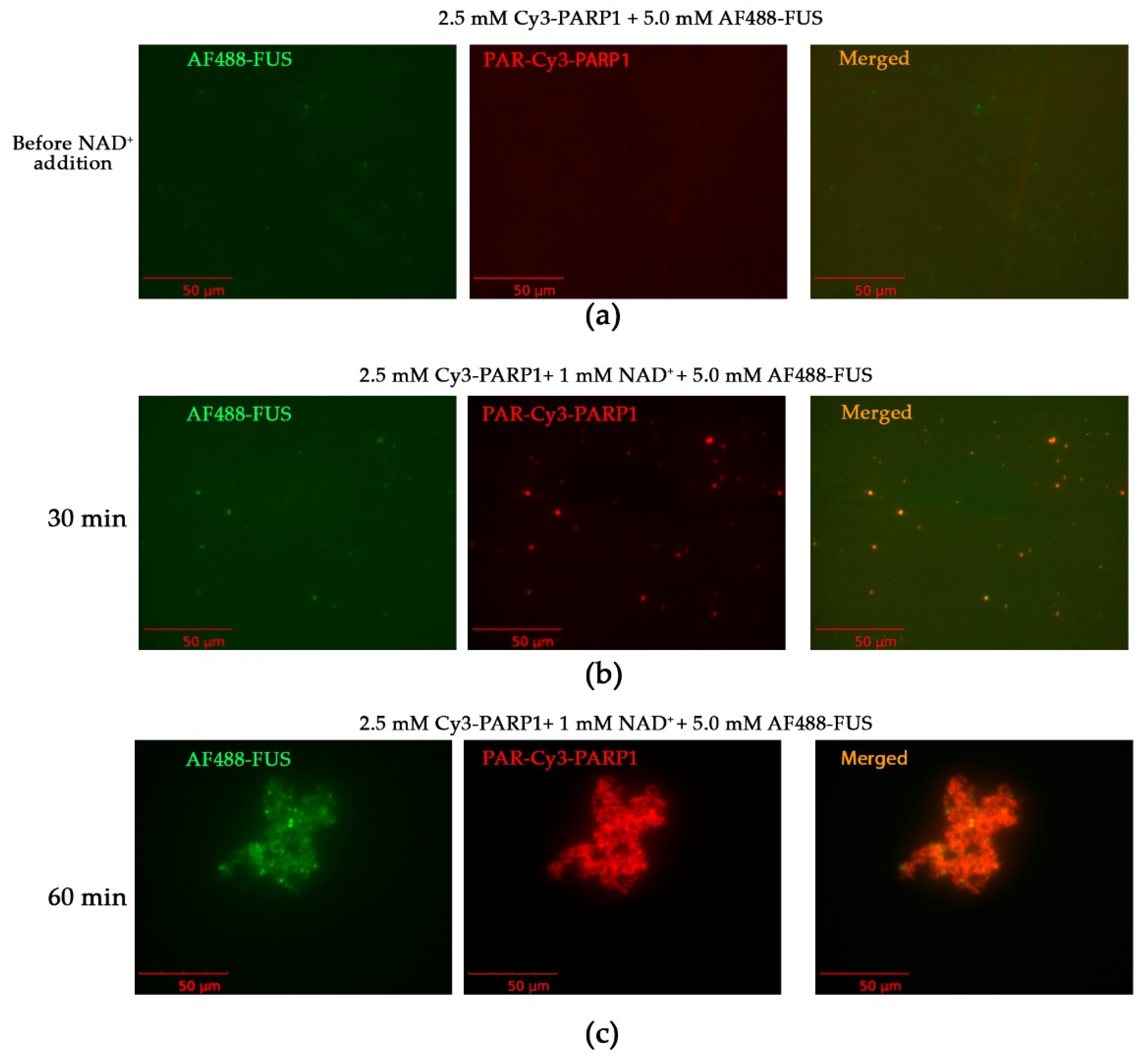
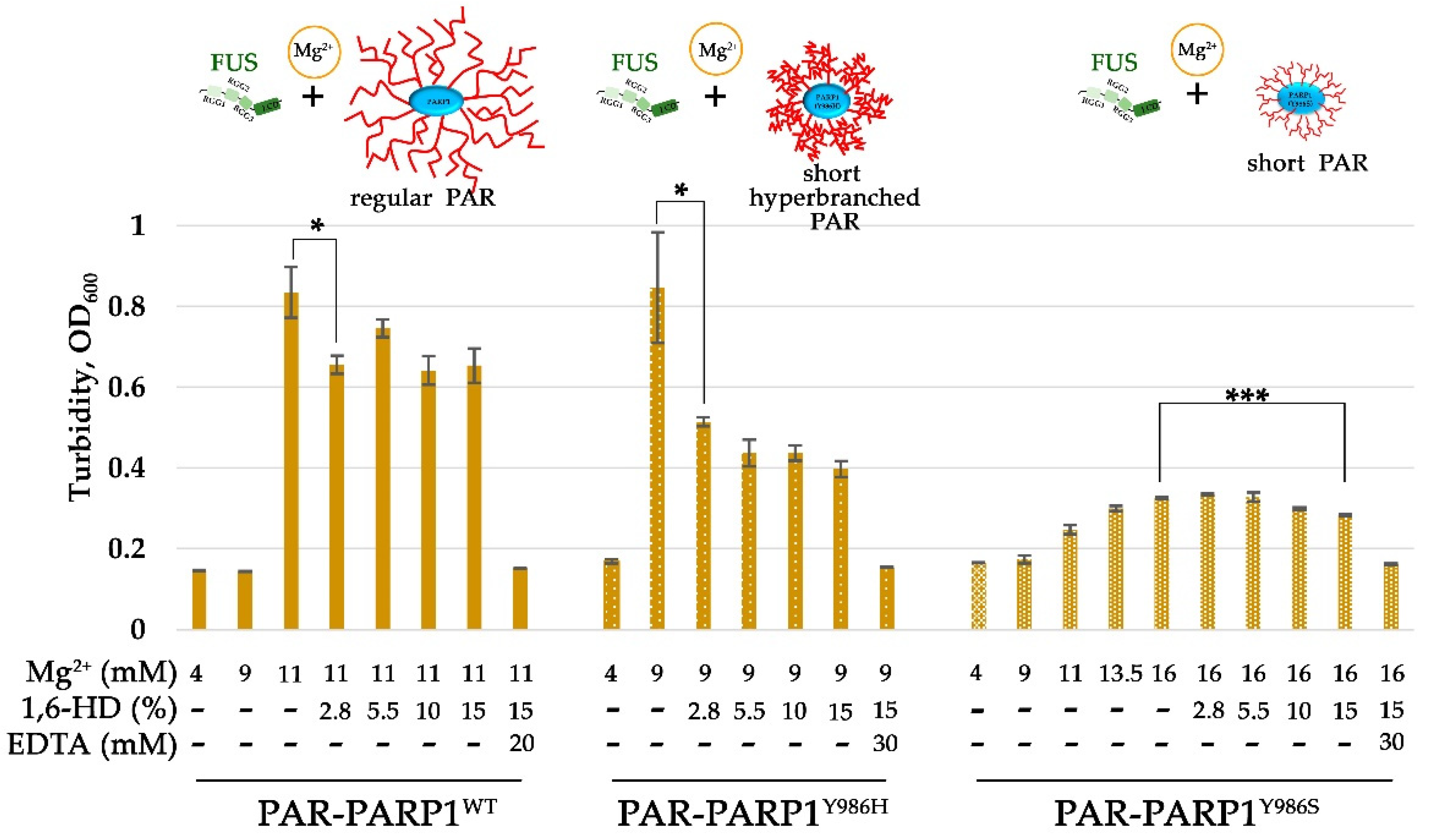
2.1. Cations Promote the Assembly of FUS into Higher-Order Structures in the Presence of PAR-PARP1
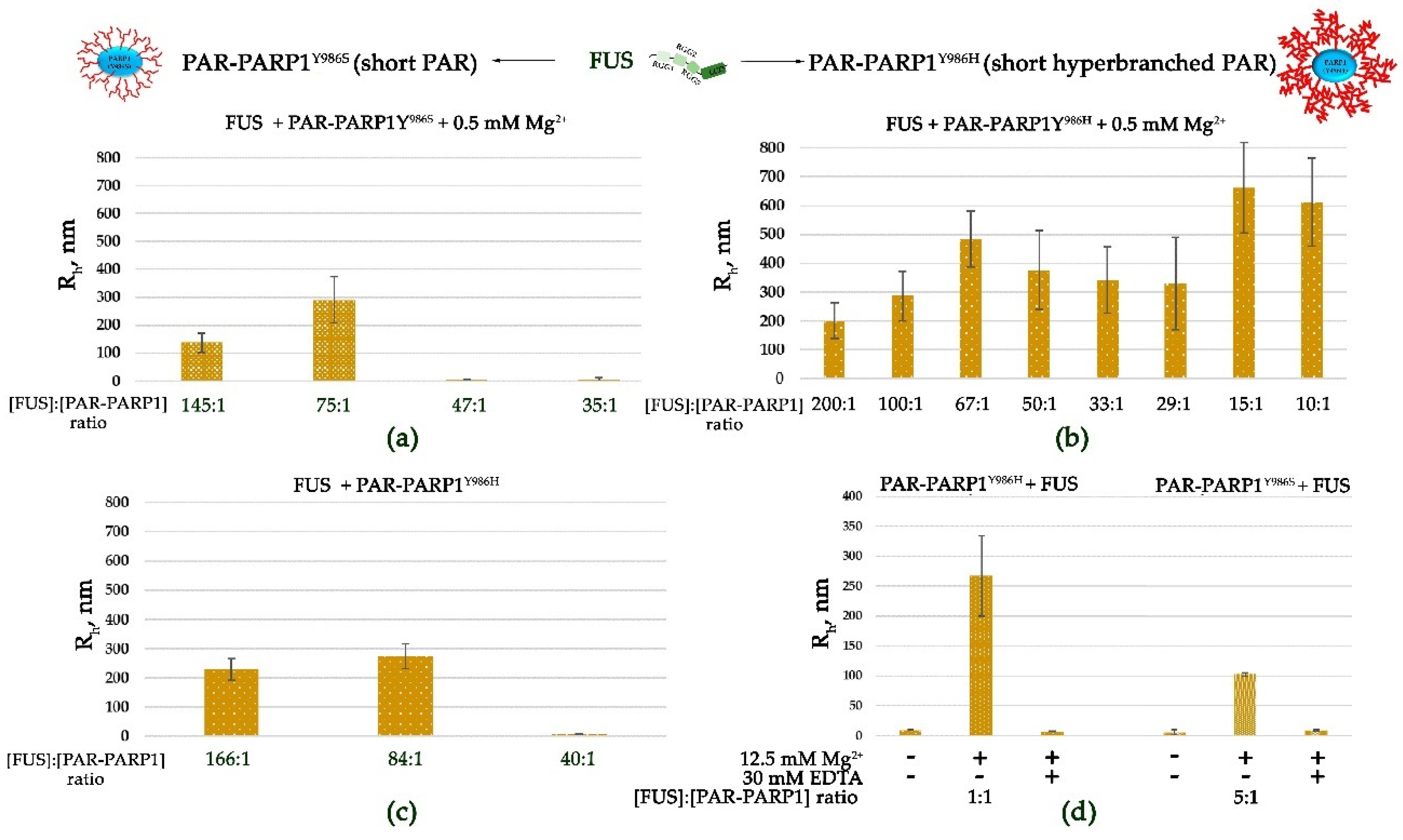
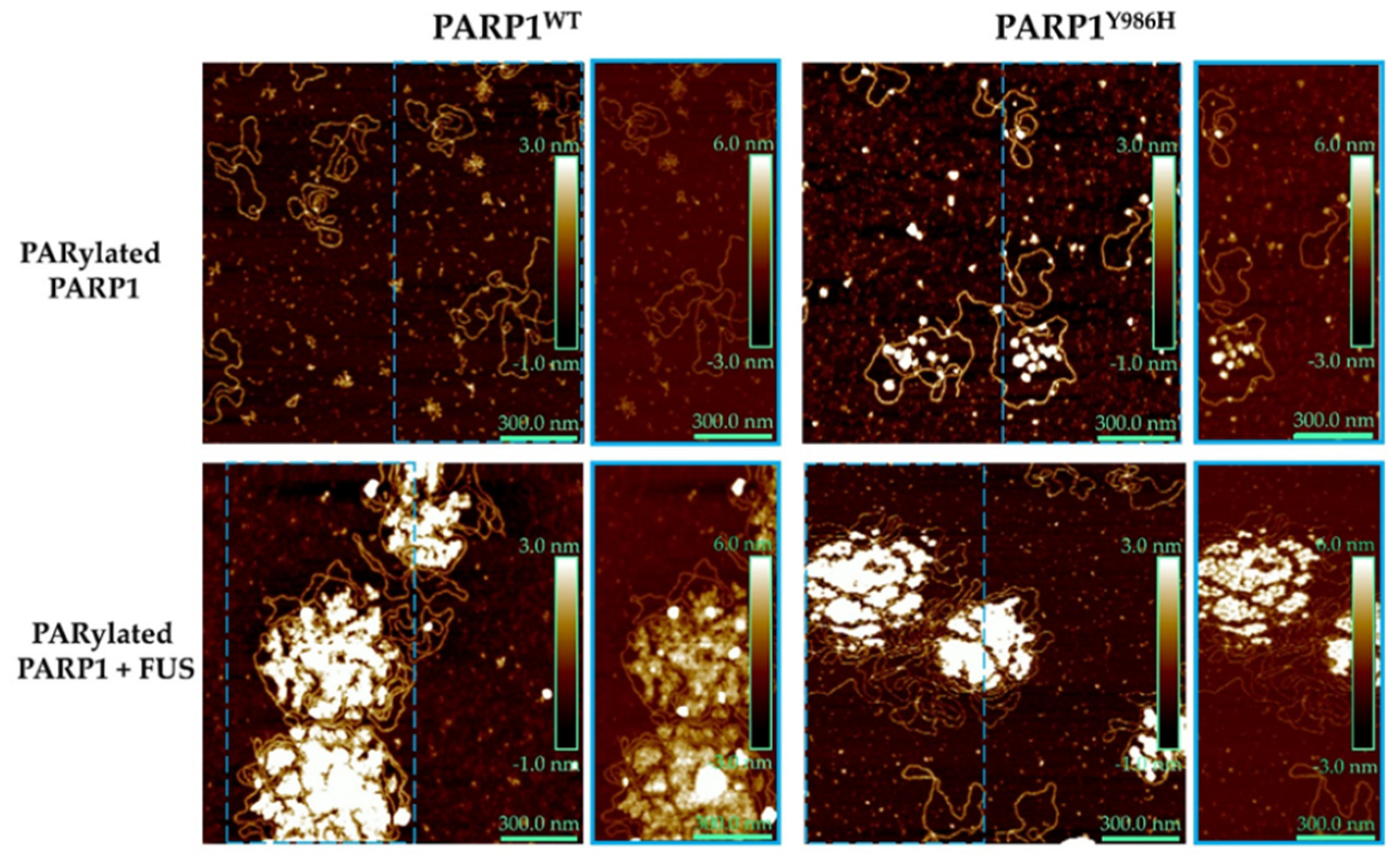
2.2. Frequency of Branching of PAR Influences the Assembly of FUS into Higher-Order Structures in the Presence of PAR-PARP1
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction, Proteins, and Reagents
4.2. Preparation of the DNA Duplex and Damaged Plasmid
4.3. Preparation of PAR-PARP1
4.4. Hydrodynamic Size Measurement by DLS
4.5. Turbidity Measurements
4.6. Fluorescent Labeling of FUS and PARP1 and Fluorescence Microscopy
4.7. Preparation of Samples for AFM Experiments and Image Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovar, H.Dr. Jekyll and Mr. Hyde: The two faces of the FUS/EWS/TAF15 protein family. Sarcoma. 2011, 2011, 837474. [CrossRef]
- Wang, H.; Hegde, M.L. New mechanisms of DNA repair defects in fused in sarcoma–associated neurodegeneration: Stage set for DNA repair-based therapeutics? J. Exp. Neurosci. 2019, 13, 1179069519856358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Régis Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; Ina Poser, I.; Pappu, R.V.; Alberti, S.; Hyman, A. A. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. [CrossRef]
- Iko, Y.; Kodama, T.S.; Kasai, N.; Oyama, T.; Morita, E.H.; Muto, T.; Okumura, M.; Fujii, R.; Takumi, T.; Tate, S.-I.; Morikawa, K. Domain architectures and characterization of an RNA-binding protein, TLS. J. Biol. Chem. 2004, 279, 44834–44840. [Google Scholar] [CrossRef]
- Deng, H.; Gao, K.; Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 2014, 10, 337–348. [Google Scholar] [CrossRef]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; Grishin, N.V.; Frantz, D.E.; Schneider, J.W.; Chen, S.; Li, L.; Sawaya, M.R.; Eisenberg, D.; Tycko, R.; McKnight, S.L. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef]
- Patel, A.; Lee, H.O.; Pozniakovski, A.; Jawerth, L.; Poser, I.; Maghelli, N.; Royer, L.A.; Martin Weigert, M.; Myers, E.W.; Grill, S.; Drechsel, D.; Hyman, A.A.; Alberti, S. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Warraich, S.T.; Nicholson, G.A.; Blair, I.P. Fused in sarcoma/translocated in liposarcoma: A multifunctional DNA/RNA binding protein. Int. J. Biochem. Cell Biol. 2010, 42, 1408–1411. [Google Scholar] [CrossRef]
- Sama, R.R.K.; Ward, C.L.; Bosco, D.A. Functions of FUS/TLS from DNA repair to stress response: Implications for ALS. ASN Neuro 2014, 6, 1759091414544472. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, M.V., Singatulina, A.S., Pastré, D., & Lavrik, O. I. Fused in sarcoma (FUS) in DNA repair: tango with poly (ADP-ribose) polymerase 1 and compartmentalisation of damaged DNA. Int. J. Mol. Sci. 2020, 21, 7020. [CrossRef]
- Lüscher, B.; Ahel, I.; Altmeyer, M.; Ashworth, A.; Bai, P.; Chang, P.; Cohen, M.; Corda, D.; Dantzer, F.; Daugherty, M.; et al. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J. 2022, 289, 7399–7410. [Google Scholar] [CrossRef]
- Mastrocola, A.S.; Kim, S.H.; Trinh, A.T.; Rodenkirch, L.A.; Tibbetts, R.S. The RNA binding protein fused in sarcoma (FUS) functions downstream of PARP in response to DNA damage. J. Biol. Chem. 2013, 288, 24731–24741. [Google Scholar] [CrossRef]
- Rulten, S.L.; Rotheray, A.; Green, R.L.; Grundy, G.J.; Moore, D.A.; Gomez-Herreros, F.; Hafezparast, M.; Caldecott, K.W. PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res. 2014, 42, 307–314. [Google Scholar] [CrossRef]
- Altmeyer, M.; Neelsen, K.J.; Teloni, F.; Pozdnyakova, I.; Pellegrino, S.; Grofte, M.; Rask, M.B.; Streicher, W.; Jungmichel, S.; Nielsen, M.L.; Lukas, J. Liquid demixing of intrinsically disordered proteins is seeded by poly (ADP-ribose). Nat. Commun. 2015, 6, 8088. [Google Scholar] [CrossRef]
- Singatulina, A.S.; Hamon, L.; Sukhanova, M.V.; Desforges, B.; Joshi, V.; Bouhss, A.; Lavrik, O.I.; Pastré, D. PARP-1 activation directs FUS to DNA damage sites to form PARG-reversible compartments enriched in damaged DNA. Cell Rep. 2019, 27, 809–1821. [Google Scholar] [CrossRef]
- Ame, J.C.; Spenlehauer, C.; de Murcia, G. The PARP superfamily. Bioessays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Althaus, F.R.; Richter, C.; Althaus, F.R.; Richter, C. Poly (ADP-ribose): Structure, properties and quantification. In ADP-Ribosylation of Proteins: Enzymology and Biological Significance. Publisher: Springer Berlin, Heidelberg, Germany, 1987; 37, pp. 3-11.
- Sukhanova, M. V., Abrakhi; Lavrik, O. I. Single molecule detection of PARP1 and PARP2 interaction with DNA strand breaks and their poly (ADP-ribosyl) ation using high-resolution AFM imaging. Nucleic Acids Res. 2016, 44, e60. [Google Scholar] [CrossRef]
- Teloni, F.; Altmeyer, M. Readers of poly (ADP-ribose): designed to be fit for purpose. Nucleic Acids Res. 2015, 44, 993–1006. [Google Scholar] [CrossRef]
- Koczor, C.A.; Saville, K.M.; Andrews, J.F.; Clark, J.; Fang, Q.; Li, J.; Al-Rahahleh, R.Q.; Ibrahim, Md.; McClellan, S.; Makarov, M.V.; Migaud, M.E.; Sobol, R.W. Temporal dynamics of base excision/single-strand break repair protein complex assembly/disassembly are modulated by the PARP/NAD+/SIRT6 axis. Cell Rep. 2021, 37, 109917–10. [Google Scholar] [CrossRef]
- Reber, J. M.; Mangerich, A. Why structure and chain length matter: on the biological significance underlying the structural heterogeneity of poly (ADP-ribose). Nucleic Acids Res. 2021, 49, 8432–8448. [Google Scholar] [CrossRef]
- Smith, R.; Zentout, S.; Rother, M.; Bigot, N.; Chapuis, C.; Mihuț, A.; Zobel, F.F.; Ahel, I.; van Attikum, H.; Timinszky, G.; Huet, S. HPF1-dependent histone ADP-ribosylation triggers chromatin relaxation to promote the recruitment of repair factors at sites of DNA damage. Nat. Struct. Mol. Biol. 2023, 30, 678–691. [Google Scholar] [CrossRef]
- Leung, A. K. Poly (ADP-ribose): a dynamic trigger for biomolecular condensate formation. Trends Cell Biol. 2020, 30, 370–383. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Lavrik, O.I. A sePARate phase? Poly (ADP-ribose) versus RNA in the organization of biomolecular condensates. Nucleic Acids Res. 2022, 50, 10817–10838. [Google Scholar] [CrossRef]
- Rhine, K.; Odeh, H.M.; Shorter, J.; Myong, S. Regulation of biomolecular condensates by Poly(ADP-ribose). Chemical Rev. 2023, 123, 9065–9093. [Google Scholar] [CrossRef]
- Rhine, K.; Dasovich, M.; Yoniles, J.; Badiee, M.; Skanchy, S.; Ganser, L.R.; Ge, Y.; Fare, C.M.; Shorter, J.; Leung, A.K.L.; Myong, S. Poly(ADP-ribose) drives condensation of FUS via a transient interaction. Mol. Cell 2022, 82, 969–985. [Google Scholar] [CrossRef]
- Sukhanova, M.V.; Anarbaev, R.O.; Maltseva, E.A.; Pastré, D.; Lavrik, O.I. FUS Microphase Separation: Regulation by Nucleic Acid Polymers and DNA Repair Proteins. Int. J. Mol. Sci. 2022, 23, 13200. [Google Scholar] [CrossRef]
- Badiee, M.; Kenet, A.L.; Ganser, L.R.; Paul, T.; Myong, S.; Leung, A.K. Switch-like compaction of poly(ADP-ribose) upon cation binding. Proc. Natl. Acad. Sci. U.S.A. 2023, 120, e2215068120. [Google Scholar] [CrossRef]
- Chappidi, N.; Quail, T.; Doll, S.; Vogel, L.T.; Aleksandrov, R.; Felekyan, S.; Kühnemuth, R.; Stoynov, S.; Seidel, C.A.M.; Brugués, J.; Jahnel, M.; Franzmann, T.M.; Alberti, S. PARP1-DNA co-condensation drives DNA repair site assembly to prevent disjunction of broken DNA ends. Cell, 2024; 187, 945–961.e18. [Google Scholar] [CrossRef]
- Wang, W. J.; Tan, C. P.; Mao, Z. W. Metals and inorganic molecules in regulating protein and nucleic acid phase separation. Curr. Opin. Chem. Biol. 2023, 74, 102308. [Google Scholar] [CrossRef]
- Sołtys, K.; Tarczewska, A.; Bystranowska, D. Modulation of biomolecular phase behavior by metal ions. Biochim. Biophys. Acta, Mol. Cell Res. 2023, 1870, 119567. [Google Scholar] [CrossRef]
- Müller, K.H.; Hayward, R.; Rajan, R.; Whitehead, M.; Cobb, A.M.; Ahmad, S.; Sun, M.; Goldberga, I.; Li, R.; Bashtanova, U.; Puszkarska, A.M.; Reid, D.G.; Brooks, R.A.; Skepper, J.N.; Bordoloi, J.; Chow, W.Y.; Oschkinat, H.; Groombridge, A.; Scherman, O.A.; Harrison, J.A.; Verhulst, A.; D’Haese, P.C.; Neven, E.; Needham, L.-M.; Lee, S.F.; Shanahan, C.M.; Duer, M.J. Poly(ADP-Ribose) links the DNA damage response and biomineralization. Cell Rep. 2019, 27, 3124–3138. [Google Scholar] [CrossRef]
- Wang, T.; Coshic, K.; Badiee, M.; Aksimentiev, A.; Pollack, L.; Leung, A.K.L. Cation-induced intramolecular coil-to-globule transition in poly (ADP-ribose). Nat. Commun. 2024, 15, 7901. [Google Scholar] [CrossRef]
- Vasil’eva, I.A.; Anarbaev, R.O.; Moor, N.A.; Lavrik, O.I. Dynamic light scattering study of base excision DNA repair proteins and their complexes. Biochim. Biophys. Acta. 2019, 1867, 297–305. [Google Scholar] [CrossRef]
- Vasil’eva, I.; Moor, N.; Anarbaev, R.; Kutuzov, M.; Lavrik, O. Functional roles of PARP2 in assembling protein–protein complexes involved in base excision DNA repair. Int. J. Mol. Sci. 2021, 22, 4679. [Google Scholar] [CrossRef]
- Sukhanova, M.V.; Anarbaev, R.O.; Maltseva, E.A.; Kutuzov, M.M.; Lavrik, O.I. Divalent and multivalent cations control liquid-like assembly of poly (ADP-ribosyl) ated PARP1 into multimolecular associates in vitro. Commun. Biol. 2024, 7, 1148. [Google Scholar] [CrossRef]
- Hassa, P.O. The molecular “Jekyll and Hyde” duality of PARP1 in cell death and cell survival. Front. Biosci. 2009, 14, 72–111. [Google Scholar] [CrossRef]
- O’Sullivan, J., Ferreira, M.T., Gagné, J.P., Sharma, A.K., Hendzel, M.J., Masson, J.Y., Poirier, G.G. Emerging roles of eraser enzymes in the dynamic control of protein ADP-ribosylation. Nat. Commun. 2019, 10, 1182. [CrossRef]
- Hein, M.Y.; Hubner, N.C.; Poser, I.; Cox, J.; Nagaraj, N.; Toyoda, Y.; Gak, I.A.; Weisswange, I.; Mansfeld, J.; Buchholz, F.; Hyman, A.A.; Mann, M. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 2015, 163, 712–723. [Google Scholar] [CrossRef]
- Chaberek, Jr.S.; Martell, A.E. (1955). Interaction of divalent metal ions with N-hydroxyethylethylenediaminetriacetic acid. J. Am. Chem. Soc. 1955, 77, 1477–1480. [Google Scholar] [CrossRef]
- Miwa, M.; Saikawa, N.; Yamaizumi, Z.; Nishimura, S.; Sugimura, T. Structure of poly(adenosine diphosphate ribose): identification of 2’-(1’‘-ribosyl-2’‘-(or 3’‘-)(1’‘‘-ribosyl))adenosine-5’,5’‘,5’‘‘-tris(phosphate) as a branch linkage. Proc. Nat. Acad. Sci. U.S.A. 1979, 76, 595–599. [Google Scholar] [CrossRef]
- Aberle, L.; Krüger, A.; Reber, J.M.; Lippmann, M.; Hufnagel, M.; Schmalz, M.; Trussina, I.R.E.A.; Schlesiger, S.; Zubel, T.; Schütz, K.; Marx, A.; Hartwig, A.; Ferrando-May, E.; Bürkle, A.; Mangerich, A. PARP1 Catalytic Variants Reveal Branching and Chain Length-specific Functions of poly(ADP-Ribose) in Cellular Physiology and Stress Response. Nucleic Acids Res. 2020, 48, 10015–10033. [Google Scholar] [CrossRef]
- Rolli, V.; O’Farrell, M.; Ménissier-de Murcia, J.; de Murcia, G. Random Mutagenesis of the Poly(ADP-Ribose) Polymerase Catalytic Domain Reveals Amino Acids Involved in Polymer Branching. Biochemistry 1997, 36, 12147–12154. [Google Scholar] [CrossRef]
- Naumenko, K.N.; Sukhanova, M.V.; Hamon, L.; Kurgina, T.A.; Anarbaev, R.O.; Mangerich, A.; Pastre, D.; Lavrik, O. I. The C-terminal domain of Y-box binding protein 1 exhibits structure-specific binding to poly (ADP-ribose), which regulates PARP1 activity. Fron. Cell Dev. Biol. 2022, 10, 831741. [Google Scholar] [CrossRef]
- Babinchak, W.M.; Surewicz, W. K. Studying protein aggregation in the context of liquid-liquid phase separation using fluorescence and atomic force microscopy, fluorescence and turbidity assays, and FRAP. Bio Protoc. 2020, 10, e3489. [Google Scholar] [CrossRef]
- Berkeley, R.F.; Kashefi, M.; Debelouchina, G.T. Real-time observation of structure and dynamics during the liquid-to-solid transition of FUS LC. Biophys. J. 2021, 120, 1276–1287. [Google Scholar] [CrossRef]
- Miné-Hattab, J.; Liu, S.; Taddei, A. Repair Foci as Liquid Phase Separation: Evidence and Limitations. Genes 2022, 13, 1846. [Google Scholar] [CrossRef]
- Dall’Agnese, G.; Dall’Agnese, A.; Banani, S.F.; Codrich, M.; Malfatti, M.C.; Antoniali, G.; Tell, G. Role of condensates in modulating DNA repair pathways and its implication for chemoresistance. J. Biol. Chem. 2023, 299, 104800. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Mitra, J.; Hegde, P.M.; Vandoorne, T.; Eckelmann, B.J.; Mitra, S.; Tomkinson, A.E.; Van Den Bosch, L.; Hegde, M.L. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in Amyotrophic Lateral Sclerosis. Nat. Commun. 2018, 9, 3683. [Google Scholar] [CrossRef]
- Mamontova, E.M.; Clément, M.J.; Sukhanova, M.V.; Joshi, V.; Bouhss, A.; Rengifo-Gonzalez, J.C.; Desforges, B.; Hamon, L.; Lavrik, O.I.; Pastré, D. FUS RRM regulates poly (ADP-ribose) levels after transcriptional arrest and PARP-1 activation on DNA damage. Cell Rep. 2023, 42, 113199. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef]
- Lee, C.Y.; Su, G.C.; Huang, W.Y.; Ko, M.Y.; Yeh, H.Y.; Chang, G.D.; Lin, S.J.; Chi, P. Promotion of homology-directed DNA repair by polyamines. Nat. Commun. 2019, 10, 65. [Google Scholar] [CrossRef]
- Haberland, V.; M., Magin, S.; Iliakis, G.; Hartwig, A. Impact of Manganese and Chromate on Specific DNA Double-Strand Break Repair Pathways. Int. J. Mol. Sci. 2023, 24, 10392. [CrossRef]
- Gafter, U.; Malachi, T.; Ori, Y.; Breitbart, H. The role of calcium in human lymphocyte DNA repair ability. J. Lab. Clin. Med. 1997, 130, 33–41. [Google Scholar] [CrossRef]
- Kun, E.; Kirsten, E.; Mendeleyev, J.; Ordahl, C.P. Regulation of the Enzymatic Catalysis of Poly(ADP-ribose) Polymerase by dsDNA, Polyamines, Mg2+, Ca2+, Histones H1and H3, and ATP. Biochemistry 2004, 43, 210–216. [Google Scholar] [CrossRef]
- Naumenko, K.N., Sukhanova, M.V., Hamon, L., Kurgina, T.A., Alemasova, E.E., Kutuzov, M.M., Pastre, D.; Lavrik, O.I. Regulation of poly (ADP-Ribose) polymerase 1 activity by Y-Box-Binding protein 1. Biomolecules 2020, 10, 1325. [CrossRef]
- Percival, M.; Pantoja, C.F.; Cima-Omori, M.S.; Becker, S.; Zweckstetter, M. (2024). Polyamines promote disordered protein phase separation. bioRxiv 2024, 2024–05. [Google Scholar] [CrossRef]
- Fahrer, J.; Kranaster, R.; Altmeyer, M.; Marx, A.; Bürkle, A. Quantitative Analysis of the Binding Affinity of poly(ADP-Ribose) to Specific Binding Proteins as a Function of Chain Length. Nucleic Acids Res. 2007, 35, e143. [Google Scholar] [CrossRef]
- Pleschke, J.M.; Kleczkowska, H.E.; Strohm, M.; Althaus, F.R. Poly (ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 2000, 275, 40974–40980. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




