Submitted:
30 September 2024
Posted:
03 October 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
Methods
Ethical Considerations
Study Population
Preoperative Assessment
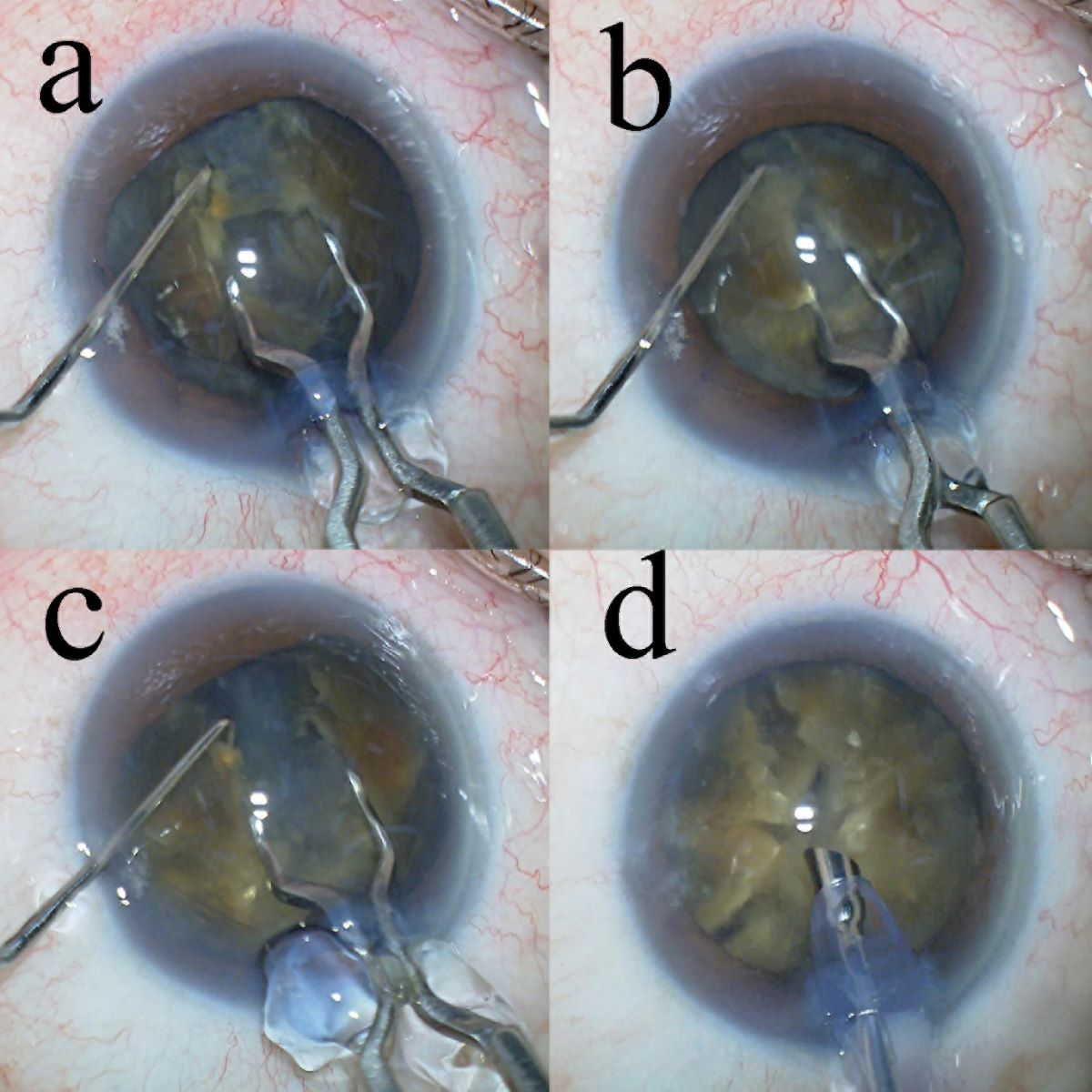
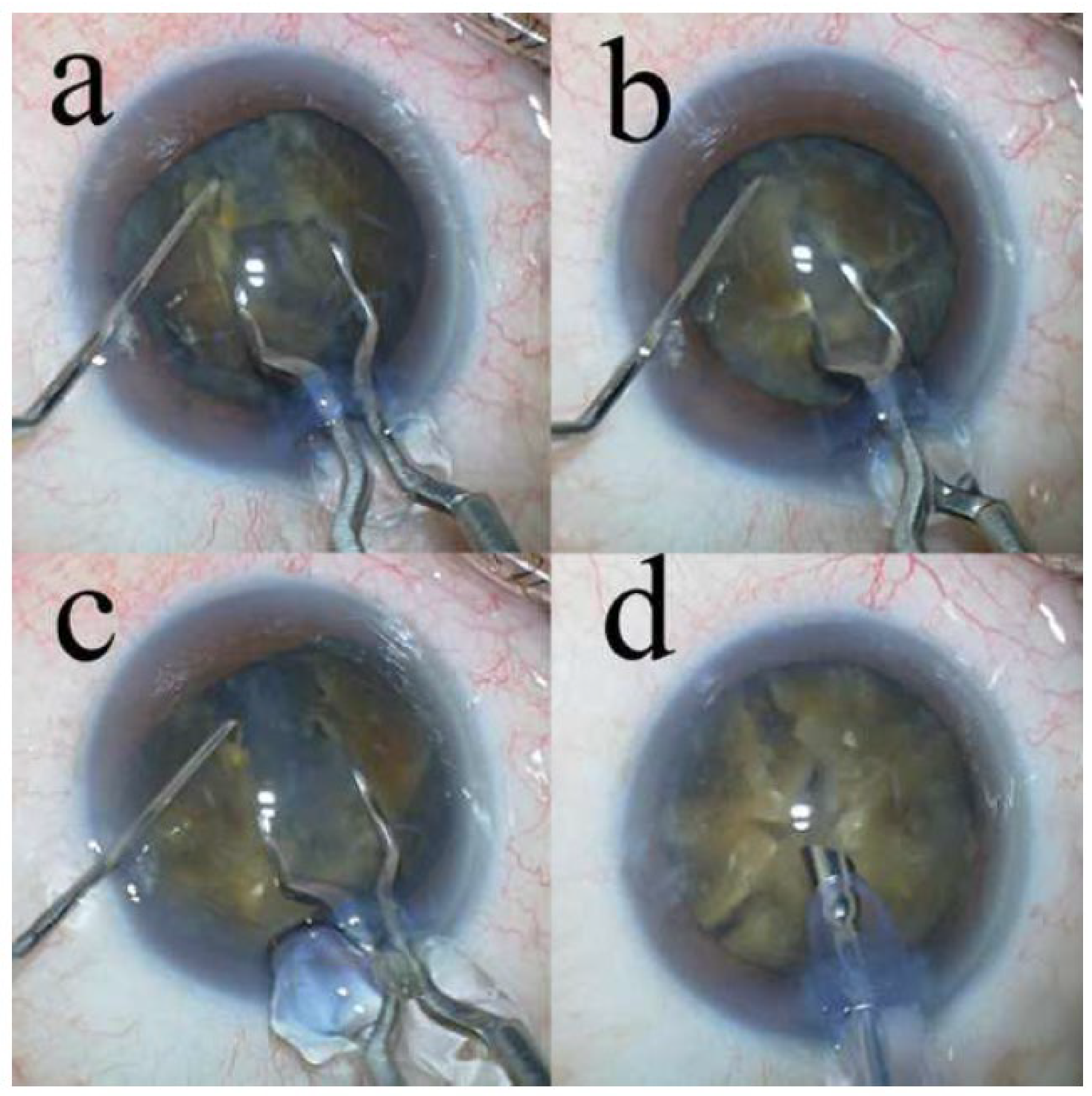
Surgical Technique
Measures
Data Collection
Statistical Analysis
Results
Participant Characteristics
Changes in CECD
Changes in CCT, CV, and PHC
Changes in IOP
Changes in CDVA over Time
Complications
Discussion
Value Statement
- The complete division of the nucleus of a hard nucleus cataract can be very difficult using conventional techniques, such as the divide-and-conquer, stop-and-chop, and phaco-chop technique.
- Previous studies have reported a 4.3–37.0% decrease in corneal endothelial cell density (CECD) after hard nucleus cataract surgery.
- The eight-chop technique achieved a complete division of the nucleus of a hard nucleus cataract with excellent intraoperative parameters.
- The decrease in CECD at 19 weeks postoperatively was 0.2%, 6.8%, and 9.6% for Grades IV, IV plus, and V, respectively, with an average decrease of 3.7%.
- The eight-chop technique may be an ideal technique for many patients with hard nuclear cataracts.
Financial Support
Data Availability
Acknowledgements
Conflict Of Interest Disclosures
References
- Foster GJL, Allen QB, Ayres BD, Devgan U, Hoffman RS, Khandelwal SS, Snyder ME, Vasavada AR, Yeoh R. Phacoemulsification of the rock-hard dense nuclear cataract: Options and recommendations. J Cataract Refract Surg 2018;44:905–916. [CrossRef]
- Koch PS, Katzen LE. Stop and chop phacoemulsification. J Cataract Refract Surg 1994;20:566–570.
- Vasavada AR, Desai JP. Stop, chop, chop, and stuff. J Cataract Refract Surg 1996;22:526-529.
- Aslan BS, Müftüoglu O, Gayretli D. Crater-and-split technique for phacoemulsification: modification of the crater-and-chop technique. J Cataract Refract Surg 2012;38:1526–1530.
- Singh R, Sharma AK, Katiyar V, Kumar G, Gupta SK. Corneal endothelial changes following cataract surgery in hard nuclear cataract: randomized trial comparing phacoemulsification to manual small-incision cataract surgery. Indian J Ophthalmol 2022;70:3904–3909.
- Abdelmotaal H, Abdel-Radi M, Rateb MF, Eldaly ZH, Abdelazeem K. Comparison of the phaco chop and drill-and-crack techniques for phacoemulsification of hard cataracts: a fellow eye study. Acta Ophthalmol 2021;99:e378–e386. [CrossRef]
- He Y, Wang C, Zhou X, Peng J, Zhang X, Wang Y, Rui Y, Zhang C, Zhang W, Feng L, Dai S, Xia X, Song W. Comparison of clinical outcomes between cystotome-assisted prechop phacoemulsification surgery and femtosecond laser-assisted cataract surgery for hard nucleus cataracts. Eye (Lond) 2023;37:235–241. [CrossRef]
- Chen X, Yu Y, Song X, Zhu Y, Wang W, Yao K. Clinical outcomes of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery for hard nuclear cataracts. J Cataract Refract Surg 2017;43:486–491. [CrossRef]
- Sato T. Efficacy and safety of the eight-chop technique in phacoemulsification for patients with cataract. J Cataract Refract Surg 2023;49:479–484. [CrossRef]
- O’Brien PD, Fitzpatrick P, Kilmartin DJ, Beatty S. Risk factors for endothelial cell loss after phacoemulsification surgery by a junior resident. J Cataract Refract Surg 2004;30:839–843. [CrossRef]
- Storr-Paulsen A, Norregaard JC, Ahmed S, Storr-Paulsen T, Pedersen TH. Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg 2008;34:996–1000. [CrossRef]
- Walkow T, Anders N, Klebe S. Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg 2000;26:727–732. [CrossRef]
- Dewan T, Malik PK, Tomar P. Comparison of effective phacoemulsification time and corneal endothelial cell loss using three different ultrasound frequencies: a randomized controlled trial. Indian J Ophthalmol 2022;70:1180–1185. [CrossRef]
- Om Parkash T, Om Parkash R, Mahajan S, Vajpayee R. “Chopper Shield” technique to protect corneal endothelium during phacoemulsification surgery for rock hard cataracts. Clin Ophthalmol 2021;15:2161–2165.
- Emery JM, Little JH. Patient selection. In: Emery JM, Little JH, eds. Phacoemulsification and aspiration of cataracts; Surgical Techniques, Complications, and Results. CV Mosby; 1979:45–48.
- Miyata K, Nagamoto T, Maruoka S, Tanabe T, Nakahara M, Amano S. Efficacy and safety of the soft-shell technique in cases with a hard lens nucleus. J Cataract Refract Surg 2002;28:1546–1550. [CrossRef]
- Kim HK. Decrease and conquer: phacoemulsification technique for hard nucleus cataracts. J Cataract Refract Surg 2009;35:1665–1670. [CrossRef]
- Hwang HS, Kim EC, Kim MS. Drill-and-crack technique for nuclear disassembly of hard nucleus. J Cataract Refract Surg 2010;36:1627–1630. [CrossRef]
- Kamoi K, Mochizuki M. Phaco forward-chop technique for managing posterior nuclear plate of hard cataract. J Cataract Refract Surg 2010;36:9–12. [CrossRef]
- Falabella P, Yogi MS, Teixeira A, Jopetibe F, Sartori J, Schor P. Retrochop technique for rock-hard cataracts. J Cataract Refract Surg 2013;39:826–829. [CrossRef]
- Fernández-Muñoz E, Chávez-Romero Y, Rivero-Gómez R, Aridjis R, Gonzalez-Salinas R. Cumulative dissipated energy (CDE) in three phaco-fragmentation techniques for dense cataract removal. Clin Ophthalmol 2023;17:2405–2412. [CrossRef]
- Yang WJ, Wang XH, Zhao F, Mei ZM, Li S, Xiang Y. Torsional and burst mode phacoemulsification for patients with hard nuclear cataract: a randomized control study. Medicine (Baltimore) 2019;98:e15870.
- Waring GO, 3rd, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 1982;89:531–590.
- Shimazaki J, Amano S, Uno T, Maeda N, Yokoi N. National survey on bullous keratopathy in Japan. Cornea 2007;26:274–278. [CrossRef]
- Gimbel HV. Divide and conquer nucleofractis phacoemulsification: development and variations. J Cataract Refract Surg 1991;17:281–291. [CrossRef]
- Chang DF. Why learn chopping? In: Chang DF, eds. Phaco Chop and Advanced Phaco Techniques. SLACK Incorporated; 2013:3–9.
- Akahoshi T. Phaco prechop: Manual nucleofracure prior to phacoemulsification. Operative Tech Cataract Refract Surge 1998;1:69–91.
- Zetterström C, Laurell CG. Comparison of endothelial cell loss and phacoemulsification energy during endocapsular phacoemulsification surgery. J Cataract Refract Surg 1995;21:55–58. [CrossRef]
- Sato M, Sakata C, Yabe M, Oshika T. Soft-shell technique using Viscoat and Healon 5: a prospective, randomized comparison between a dispersive-viscoadaptive and a dispersive-cohesive soft-shell technique. Acta Ophthalmol 2008;86:65–70.
- Kim JY, Kim H, Jun I, Kim TI, Seo KY. Effect and safety of pressure sensor-equipped handpiece in phacoemulsification system. Korean J Ophthalmol 2023;37:387–394. [CrossRef]
- Igarashi T, Ohsawa I, Kobayashi M, Umemoto Y, Arima T, Suzuki H, Igarashi T, Otsuka T, Takahashi H. Effects of hydrogen in prevention of corneal endothelial damage during phacoemulsification: a prospective randomized clinical trial. Am J Ophthalmol 2019;207:10–17. [CrossRef]
- Upadhyay S, Sharma P, Chouhan JK, Goyal R. Comparative evaluation of modified crater (endonucleation) chop and conventional crater chop techniques during phacoemulsification of hard nuclear cataracts: a randomized study. Indian J Ophthalmol 2022;70:794–798. [CrossRef]
- Cruz JCG, Moreno CB, Soares P, Moscovici BK, Colombo-Barboza GN, Colombo-Barboza LR, Colombo-Barboza MN. Comparison of endothelial cell loss in diabetic patients after conventional phacoemulsification and femtosecond laser-assisted cataract surgery. BMC Ophthalmol 2023;23:181. [CrossRef]
- Joo JH, Kim TG. Comparison of corneal endothelial cell changes after phacoemulsification between type 2 diabetic and nondiabetic patients. Medicine (Baltimore) 2021;100:e27141. [CrossRef]
- Gajraj M, Mohan A. Safety and efficacy of manual small-incision cataract surgery in patients with brunescent and black cataracts and other ocular comorbidities. Indian J Ophthalmol 2022;70:3898–3903. [CrossRef]
| Characteristics/Parameters | Grade IV | Grade IV plus | Grade V | Total | P-value |
|---|---|---|---|---|---|
| Number of eyes | 46 | 26 | 9 | 81 | |
| Age (years) | 76.2 ± 9.0 | 78.0 ± 11.8 | 74.9 ± 6.9 | 76.6 ± 9.8 | .62 |
| Sex Male | 23 (50%) | 15 (58%) | 6 (66.7%) | 44 (54.3%) | .55 |
| Female | 23 (50%) | 11 (42%) | 3 (33.3%) | 37 (45.7%) | |
| Operative time (min) | 9.4 ± 2.2 | 12.3 ± 3.9 | 15.6 ± 3.9 | 10.5 ± 3.4 | < .01* |
| Phaco time (s) | 30.6 ± 10.9 | 44.2 ± 15.4 | 65.9 ± 22.0 | 38.9 ± 17.7 | < .01* |
| Aspiration time (s) | 117.2 ± 30.6 | 147.8 ± 38.1 | 194.3 ± 50.7 | 135.6 ± 43.2 | < .01* |
| CDE | 14.3 ± 4.4 | 22.8 ± 8.0 | 33.8 ± 12.9 | 19.2 ± 9.4 | < .01* |
| Volume of fluid used (mL) | 46.5 ± 12.0 | 58.3 ± 17.9 | 70.7 ± 26.3 | 53.0 ± 17.9 | < .01* |
| Mean CECD ± SD and % Decrease | |||||
|---|---|---|---|---|---|
| Time period |
Grade IV (n = 49) |
Grade IV plus (n = 30) |
Grade V (n = 10) |
Total (n = 89) |
P value |
| Preoperatively | 2530 ± 248 2518 ± 266 0.9 ± 13.6 2503 ± 320 0.2 ± 12.2 |
2496 ± 241 2208 ± 562c 22.5 ± 42.1 2316 ± 458** 6.8 ± 18.2 |
2622 ± 142 2318 ± 442c 19.7 ± 40.3 2361 ± 410** 9.6 ± 16.5 |
2529 ± 237 | .35 < .01* .09 |
| 7-weeks postoperatively | 2393 ± 432** | ||||
| % Decrease | 10.4 ± 31.1 | ||||
| 19-weeks postoperatively | 2425 ± 394** | ||||
| % Decrease | 3.7 ± 15.3 | ||||
| CCT, CV, and PHC | |||||
|---|---|---|---|---|---|
| Time period |
Grade IV (n = 30) |
Grade IV plus (n = 24) |
Grade V (n = 9) |
Total (n = 63) |
P value |
| CCT | Mean ± SD | ||||
| Preoperatively | 536.4 ± 35.7 | 541.0 ± 32.7 | 519.9 ± 44.5 | 535.8 ± 36.0 | .33 |
| 7-weeks postoperatively | 535.5 ± 31.9 | 541.2 ± 42.7 | 524.0 ± 37.2 | 536.0 ± 36.9 | .50 |
| 19-weeks postoperatively | 529.9 ± 30.0** | 536.7 ± 31.3** | 524.1 ± 45.9 | 529.9 ± 30.0** | .55 |
| CV | Mean ± SD | ||||
| Preoperatively | 40.5 ± 5.5 | 44.9 ± 7.2 | 38.6 ± 4.2 | 41.9 ± 6.4 | < .01* |
| 7-weeks postoperatively | 39.2 ± 4.1 | 43.4 ± 6.6 | 38.1 ± 5.6 | 40.6 ± 5.7 | < .01* |
| 19-weeks postoperatively | 37.8 ± 4.9** | 40.2 ± 5.1** | 35.8 ± 5.6** | 38.4 ± 5.2** | .06 |
| PHC | Mean ± SD | ||||
| Preoperatively | 43.5 ± 5.5 | 39.5 ± 6.5 | 47.1 ± 8.9 | 42.5 ± 6.9 | < .01* |
| 7-weeks postoperatively | 43.0 ± 6.1 | 39.3 ± 6.0 | 44.9 ± 8.6 | 41.9 ± 6.7 | .04* |
| 19-weeks postoperatively | 43.8 ± 5.3 | 41.5 ± 7.4 | 46.7 ± 8.4 | 43.3 ± 6.7 | .13 |
| Mean IOP ± SD (% Decrease ± SD) | |||||
|---|---|---|---|---|---|
| Time period |
Grade IV (n = 46) |
Grade IV plus (n = 29) |
Grade V (n = 9) |
Total (n=84) |
P value |
| Preoperatively | 14.1 ± 2.8 | 13.6 ± 2.9 | 13.3 ± 2.2 | 13.8 ± 2.8 | .84 |
| 7-weeks postoperatively | 11.7 ± 2.2* | 11.7 ± 2.4* | 11.1 ± 0.9* | 11.6 ± 2.2* | .78 |
| % Decrease | 16.4 ± 11.9 | 13.3 ± 12.4 | 14.6 ± 15.0 | 15.1 ± 12.4 | |
| 19-weeks postoperatively | 11.9 ± 1.9* | 11.6 ± 2.3* | 11.9 ± 2.0 | 11.8 ± 2.0* | .79 |
| % Decrease | 13.9 ± 12.0 | 13.1 ± 15.7 | 8.5 ± 22.7 | 13.1 ± 14.6 | |
| Corrected distance visual acuity | |||||
|---|---|---|---|---|---|
| Time period |
Grade IV (n = 43) |
Grade IV plus (n = 21) |
Grade V (n = 8) |
Total (n = 72) |
P-value |
| Preoperatively | 0.50 ± 0.59 | 0.88 ± 0.83 | 0.89 ± 0.66 | 0.65 ± 0.69 | 0.07 |
| 7-weeks postoperatively | -0.020 ± 0.17* | -0.011 ± 0.058* | 0.0079 ± 0.050* | -0.014 ± 0.13* | 0.86 |
| 19-weeks postoperatively | -0.028 ± 0.17* | -0.035 ± 0.050* | -0.014± 0.043* | -0.029± 0.13* | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





