Submitted:
30 September 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Status of the Biomanufacturing of Yeast Probiotics
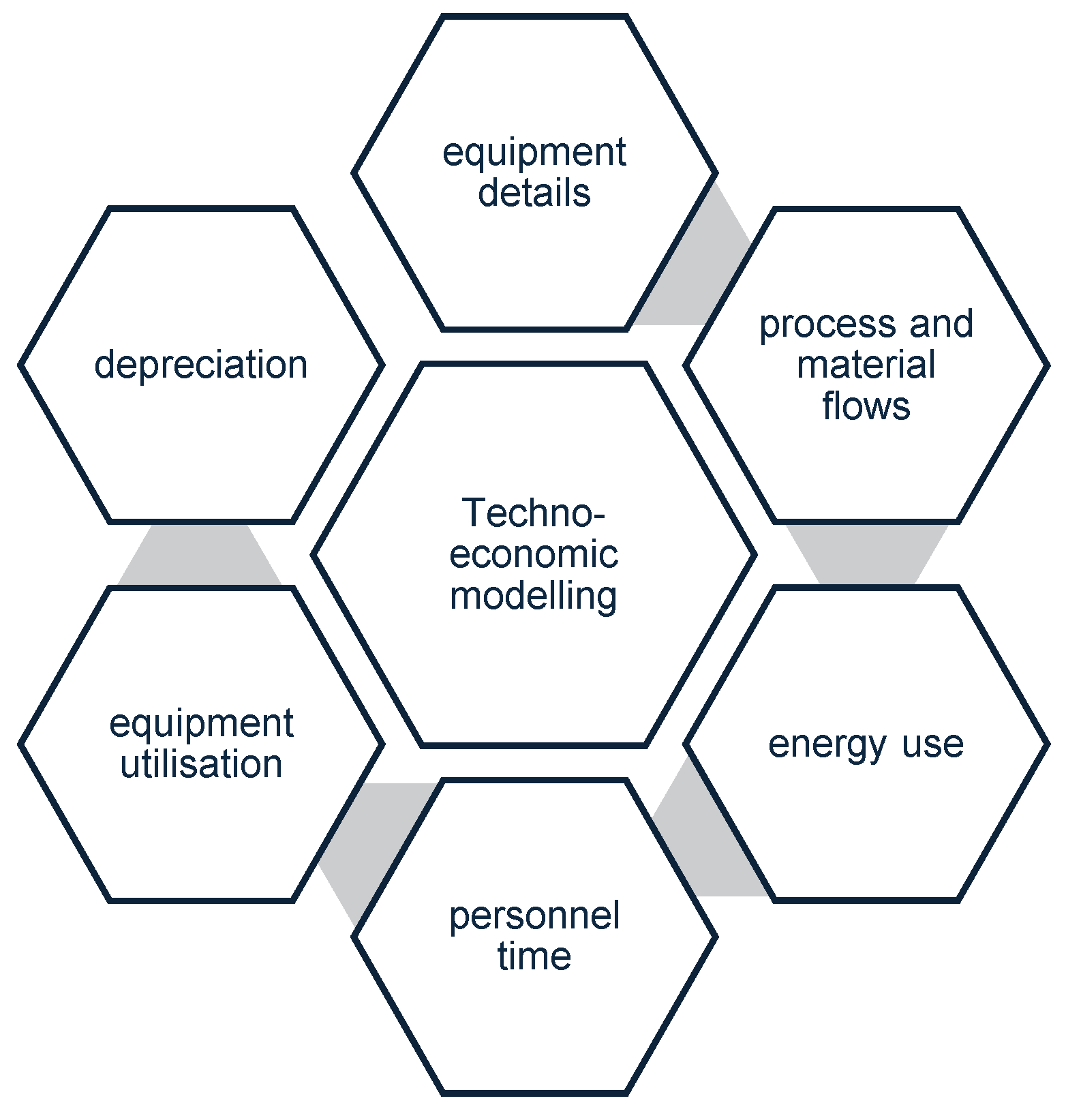
2.1. Key Bioprocess Considerations
2.2. Advances in Yeast Probiotic Manufacture
2.3. Challenges Associated with Yeast Probiotic Manufacturing
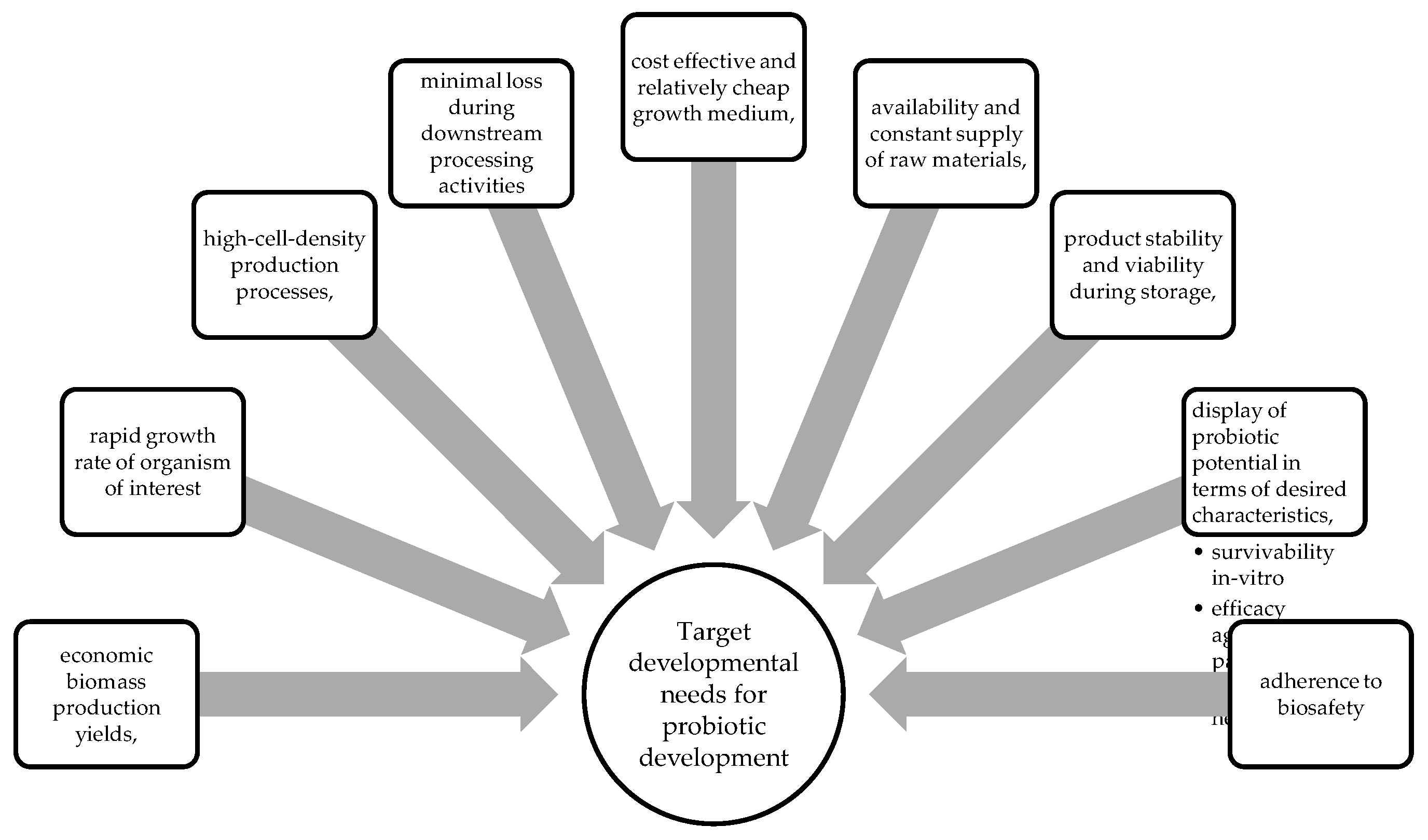
2.4. Manufacturing Considerations to Produce Yeast Probiotics
2.5. Location of Known Producers and Global Manufacturers of Yeast Probiotics
| Name | Country |
|---|---|
| ADM, | USA |
| Abbott, | USA |
| Asahi Group Holdings Ltd., | Japan |
| Chobani LLC, | USA |
| Chr. Hansen Inc., | Denmark |
| DSM | Netherlands |
| Danone Inc., IFF, | France |
| Kerry, | Ireland |
| Estee Lauder Inc., | USA |
| Morinaga Milk Industry Co. Ltd., | Japan |
| NESTLÉ, | Switzerland |
| Yakult Honsha Co. Ltd. | Japan |
3. The Use of Genetically Modified Organisms (GMO) as Probiotics.
4. General Routes of Administration of Yeast Probiotics
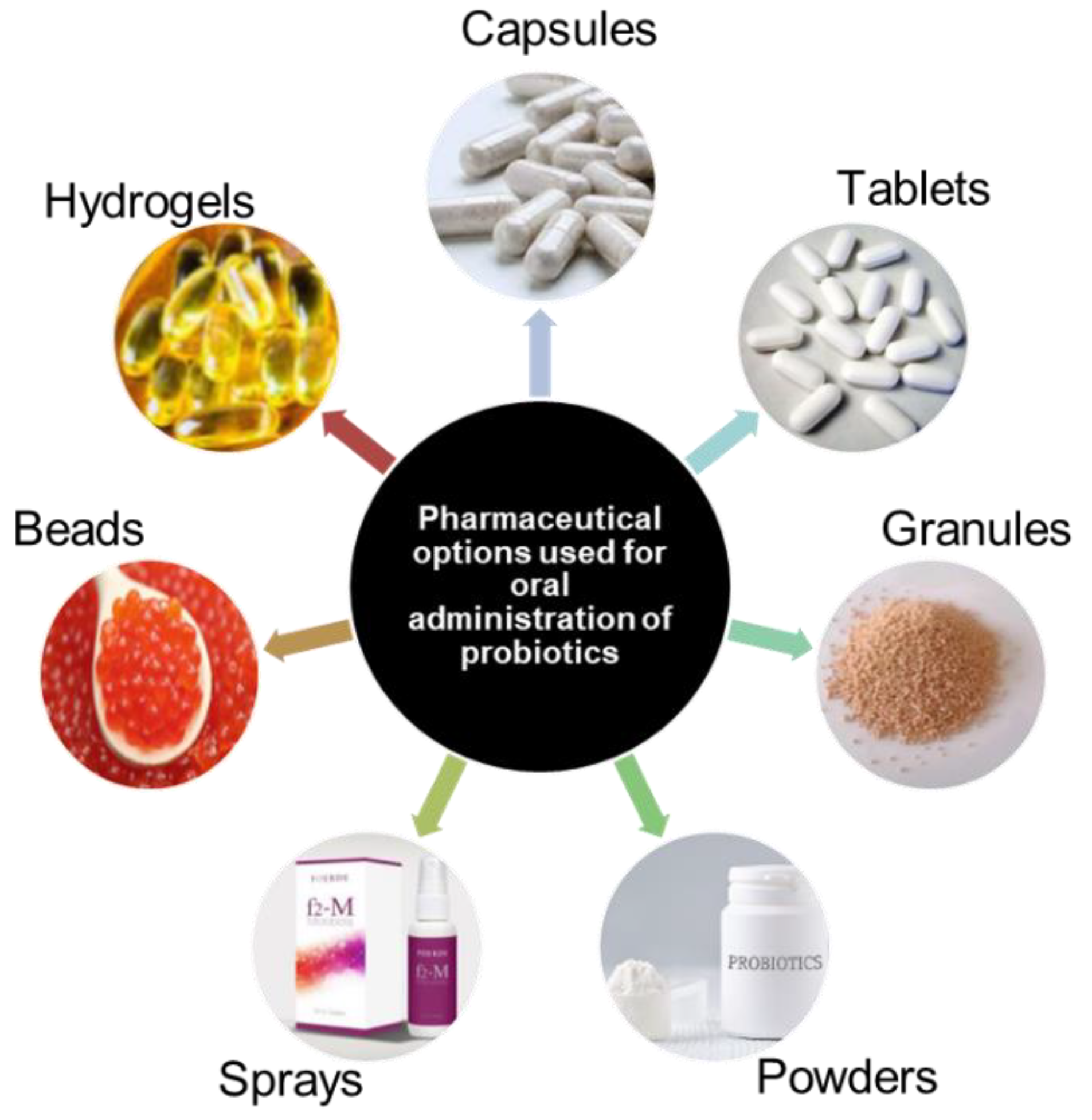
5. Conventional Pharmaceutical Methods Used to Administer Probiotics
5.1. Oral Delivery Systems
| Benefits | Organisms of interest | Reference |
|---|---|---|
| Inhibition of Cd absorption | L. Plantarum | [40] |
| Protection of the intestinal barrier – by alleviation of Cd-induced oxidative stress | ||
| Enhancement of antimicrobial activity | L. paracasei and L. casei | [41] |
| Reduction of hypertension effects | S. cerevisiae | [42] |
| Modification of the fecal resistome during Helocobacter pylori treatment – reduction of antibiotic resistance | S. boulardii | [43] |
| Potential in removal of toxins | S. cerevisiae W13 and S. boulardii ATCC MYA-796 | [44] |
| Improvement of glycaemic indices in type II diabetic patients. | S. cerevisiae | [45] |
| Inhibition and reduction of Gardnerella vaginalis biofilms in mice | S. cerevisiae CNCM I-3856 and L. rhamnosus ATCC 53103 | [46] |
| Cholesterol reduction |
Pichia fermentans BY5 Pichia kudriavzevii BY10 Pichia kudriavzevii BY15 Yarrowia lipolytica HY4 |
[47] |
| Better sensory properties with lower ethanol content | Meyerozyma caribbica 9D | [48] |
| Production of alcohol-free and low-alcohol products |
S. boulardii | [49] |
5.2. Transdermal Delivery Systems
6. Other Probiotic Delivery Systems
6.1. Functional Foods as a Source of Probiotics
6.1.1. Dairy - Based Probiotics
6.1.2. Non-Dairy Based Probiotics
7. Advancements in Probiotic Delivery Systems
8. Formulation Techniques Used for Yeast Probiotics
8.1. Immobilization
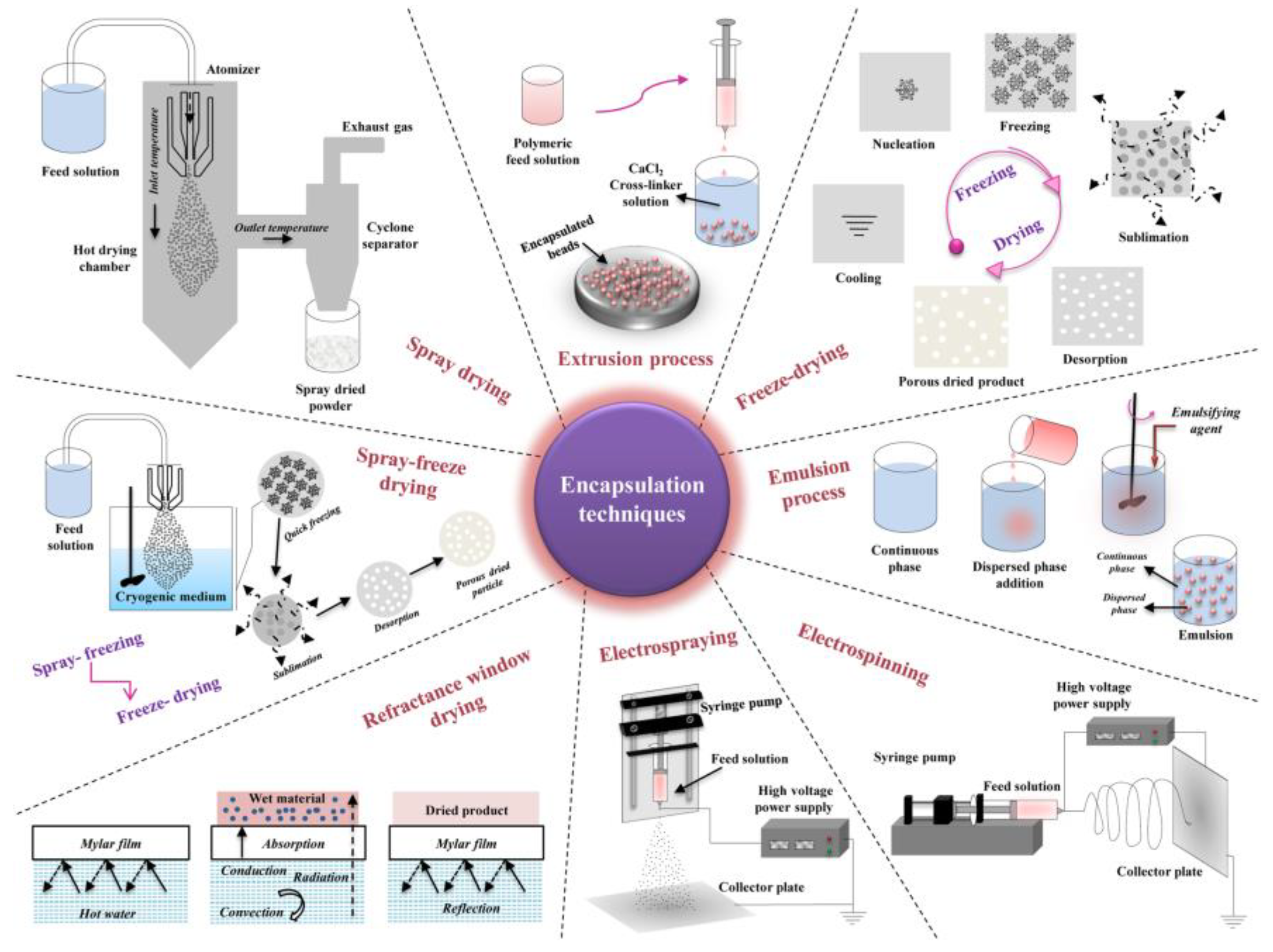
8.2. Encapsulation
8.3. Extrusion
8.4. Spray Drying
8.5. Spray Chilling
8.6. Emulsions
8.7. Fluidized Bed Drying
8.8. Supercritical Technology
8.9. Freeze Drying
8.10. New Advances in Probiotic Formulations
9. Application of probiotics for preventative health benefits
9.1. Gut Microbiome Initiatives
9.2. Skin Microbiome
9.3. Case Studies Assessing the Use of Yeast Probiotics and Its Impact on the Host Microbiome
9.4. The Use of Yeast Probiotics in Skin Applications
10. Conclusionary Remarks and Future Prospects
References
- C. Hill et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- C. Altieri, ‘Dairy propionibacteria as probiotics: recent evidences. World J Microbiol Biotechnol 2016, 32. [Google Scholar] [CrossRef]
- J. Ma et al. Engineered probiotics’, Microb Cell Fact 2022, 21, 72. [CrossRef]
- Saber, B. Saber, B. Alipour, Z. Faghfoori, and A. Yari Khosroushahi, ‘Cellular and molecular effects of yeast probiotics on cancer. 2016, 43, 96–115. [Google Scholar] [CrossRef] [PubMed]
- M. Arevalo-Villena, A. Briones-Perez, M. R. Corbo, M. Sinigaglia, and A. Bevilacqua, ‘Biotechnological application of yeasts in food science: Starter cultures, probiotics and enzyme production’. J Appl Microbiol 2017, 123, 1360–1372. [CrossRef]
- S. Sen and T. J. Mansell, ‘Yeasts as probiotics: Mechanisms, outcomes, and future potential’, Fungal Genetics and Biology 2020, 137. [CrossRef]
- D. Czerucka, T. Piche, and P. Rampal, ‘Review article: yeast as probiotics –Saccharomyces boulardii’. Aliment Pharmacol Ther 2007, 26, 767–778. [CrossRef]
- H. S. ßanlidere, A. Gl U, E. Demir, € Oz, and Z. € Ub E Y D E € On, ‘Assimilation of cholesterol and probiotic characterisation of yeast strains isolated from raw milk and fermented foods. Wiley Online Library 2015, 69, 63–70. [CrossRef]
- Al-Seraih, C. Flahaut, F. Krier, B. Cudennec, and D. Drider, ‘Characterization of Candida famata Isolated from Poultry Feces for Possible Probiotic Applications’. Probiotics Antimicrob Proteins 2015, 7, 233–241. [CrossRef]
- R. Ogunremi, A. I. Sanni, and R. Agrawal, ‘Probiotic potentials of yeasts isolated from some cereal-based Nigerian traditional fermented food products. J Appl Microbiol 2015, 119, 797–808. [CrossRef]
- H. S. Ochangco, A. H. S. Ochangco, A. Gamero, I. M. Smith, J. E. Christensen, L. Jespersen, and N. Arneborg, ‘In vitro investigation of Debaryomyces hansenii strains for potential probiotic properties’, World J Microbiol Biotechnol 2016, 32. [CrossRef]
- Binetti, M. Carrasco, J. Reinheimer, and V. Suárez, ‘Yeasts from autochthonal cheese starters: technological and functional properties’. J Appl Microbiol 2013, 115, 434–444. [CrossRef]
- M. Smith, A. Baker, N. Arneborg, and L. Jespersen, ‘Non-Saccharomyces yeasts protect against epithelial cell barrier disruption induced by Salmonella enterica subsp. enterica serovar Typhimurium. Lett Appl Microbiol 2015, 61, 491–497. [CrossRef] [PubMed]
- G. Diosma, D. E. Romanin, M. F. Rey-Burusco, A. Londero, and G. L. Garrote, ‘Yeasts from kefir grains: Isolation, identification, and probiotic characterization. World J Microbiol Biotechnol 2014, 30, 43–53. [CrossRef]
- R. C. França et al. Pichia pastoris X-33 has probiotic properties with remarkable antibacterial activity against Salmonella Typhimurium. Appl Microbiol Biotechnol 2015, 99, 7953–7961. [CrossRef]
- S. Bonatsou, A. Benítez, F. Rodríguez-Gómez, E. Z. Panagou, and F. N. Arroyo-López, ‘Selection of yeasts with multifunctional features for application as starters in natural black table olive processing’. Food Microbiol 2015, 46, 66–73. [CrossRef] [PubMed]
- D. H. Kitamura, L. P. de Souza Vandenberghe, C. Rodrigues, D. N. X. Salmon, G. V. de Melo Pereira, and C. R. Soccol, ‘Selenium-Enriched Probiotic Saccharomyces boulardii CCT 4308 Biomass Production Using Low-Cost Sugarcane Molasses Medium’. Brazilian Archives of Biology and Technology 2021, 64, 1–14. [CrossRef]
- Saber, B. Alipour, Z. Faghfoori, and A. Yari Khosroushahi, ‘Cellular and molecular effects of yeast probiotics on cancer’. Crit Rev Microbiol 2017, 43, 96–115. [Google Scholar] [CrossRef] [PubMed]
- S. Sen and T. J. Mansell, ‘Yeasts as probiotics: Mechanisms, outcomes, and future potential’. Fungal Genetics and Biology 2020, 137, 103333. [CrossRef]
- M. Arevalo-Villena, A. Briones-Perez, M. R. Corbo, M. Sinigaglia, and A. Bevilacqua, ‘Biotechnological application of yeasts in food science: Starter cultures, probiotics and enzyme production’. J Appl Microbiol 2017, 123, 1360–1372. [CrossRef]
- Papadimitriou, *!!! REPLACE !!!*; et al. Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches’, Front Microbiol 2015, 6. [CrossRef]
- T. Trunk, H. S. Khalil, and J. C. Leo, ‘Bacterial autoaggregation. AIMS Microbiol 2018, 4, 140–164. [CrossRef]
- Javed; et al. In Silico and In Vitro Analysis of Helicobacter pullorum Type Six Secretory Protein Hcp and Its Role in Bacterial Invasion and Pathogenesis’, Curr Microbiol 2022, 79. [CrossRef]
- Pillinger, B. Weber, B. Standen, M. C. Schmid, and J. C. Kesselring, ‘Multi-strain probiotics show increased protection of intestinal epithelial cells against pathogens in rainbow trout (Oncorhynchus mykiss)’. Aquaculture 2022, 560, 738487. [CrossRef]
- H. Ziar and A. Riazi, ‘Polysorbate 80 improves the adhesion and survival of yogurt starters with cholesterol uptake abilities’. Saudi J Biol Sci 2022, 29, 103367. [CrossRef]
- G. Barzoki, S. S. Malekshahi, and M. Shayestehpour, ‘In vitro evaluation of antiviral activity of Shouchella clausii probiotic strain and bacterial supernatant against herpes simplex virus type 1’. Arch Microbiol 2022, 204, 522. [CrossRef]
- Fakruddin, M. N. Hossain, and M. M. Ahmed, ‘Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic’, BMC Complement Altern Med, 2017; 17, 1–11. [Google Scholar]
- Halász and, R. Lásztity, ‘Use of yeast biomass in food production’, Use of Yeast Biomass in Food Production, 1–312, 2017.
- R. E. Speight, L. Navone, L. K. Gebbie, J.-A. L. Blinco, and W. L. Bryden, ‘Platforms to accelerate biomanufacturing of enzyme and probiotic animal feed supplements: discovery considerations and manufacturing implications’. Anim Prod Sci 2022, 62, 1113–1128. [CrossRef]
- B. Hahn-Hägerdal, K. Karhumaa, C. U. Larsson, M. Gorwa-Grauslund, J. Görgens, and W. H. van Zyl, ‘Role of cultivation media in the development of yeast strains for large scale industrial use’, Microb Cell Fact 2005, 4. [CrossRef]
- D. H. Kitamura, L. P. de S. Vandenberghe, C. Rodrigues, D. N. X. Salmon, G. V. de M. Pereira, and C. R. Soccol, ‘Selenium-Enriched Probiotic Saccharomyces boulardii CCT 4308 Biomass Production Using Low-Cost Sugarcane Molasses Medium’, Brazilian Archives of Biology and Technology 2021, 64. [CrossRef]
- O’Toole, J. Marchesi, C. H.-N. microbiology, and undefined 2017, ‘Next-generation probiotics: the spectrum from probiotics to live biotherapeutics’, nature.com, 2017. [CrossRef]
- J. Ma et al. Engineered probiotics. Microb Cell Fact 2022, 21, 72. [CrossRef]
- Anadón, M. Rosa Martínez-Larrañaga, and M. Aranzazu Martínez, ‘Probiotics for animal nutrition in the European Union. Regulation and safety assessment’. Regulatory Toxicology and Pharmacology 2006, 45, 91–95. [CrossRef]
- W. O’Toole, J. R. Marchesi, and C. Hill, Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol 2017, 2, 17057. [CrossRef]
- de Simone, ‘The Unregulated Probiotic Market’. Clinical Gastroenterology and Hepatology 2019, 17, 809–817. [CrossRef] [PubMed]
- V. Venugopalan, K. A. Shriner, and A. Wong-Beringer, ‘Regulatory Oversight and Safety of Probiotic Use’. Emerg Infect Dis 2010, 16, 1661–1665. [CrossRef]
- H. Nasri, A. Baradaran, H. Shirzad, and M. Rafieian-Kopaei, ‘New Concepts in Nutraceuticals as Alternative for Pharmaceuticals’. Int J Prev Med 2014, 5, 1487–1499.
- S. Fujimori, ‘Gastric acid level of humans must decrease in the future’. World J Gastroenterol 2020, 26, 6706–6709. [CrossRef]
- Zhai, F. Tian, J. Zhao, H. Zhang, A. Narbad, and W. Chen, ‘Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier’, Appl Environ Microbiol, 2016; 82, 4429–4440. [Google Scholar] [CrossRef]
- Lebeer; et al. Oral Administration of Probiotics Increases Paneth Cells and Intestinal Antimicrobial Activity’, 2018. [CrossRef]
- F. Hendijani and V. Akbari, ‘Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis’. Clinical Nutrition 2018, 37, 532–541. [CrossRef] [PubMed]
- G. Cifuentes, M. B. Prado, M. Fornasini, H. Cohen, M. E. Baldeón, and P. A. Cárdenas, ‘Saccharomyces boulardii CNCM I-745 supplementation modifies the fecal resistome during Helicobacter pylori eradication therapy’, Helicobacter 2022, 27. [CrossRef]
- M. R. Corbo, D. Campaniello, B. Speranza, C. Altieri, M. Sinigaglia, and A. Bevilacqua, ‘Neutralisation of toxins by probiotics during the transit into the gut: challenges and perspectives’. Int J Food Sci Technol 2018, 53, 1339–1351. [CrossRef]
- P. Hosseinzadeh et al. Brewer’s Yeast Improves Glycemic Indices in Type 2 Diabetes Mellitus’. Int J Prev Med 2013, 4, 1131–1138.
- Y. Zhu, J. Jiang, Y. Yue, Z. Feng, J. Chen, and X. Ye, ‘Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace’. LWT 2020, 133, 110076. [CrossRef]
- Staniszewski and M. Kordowska-Wiater, ‘Probiotic and Potentially Probiotic Yeasts—Characteristics and Food Application’. Foods 2021, 10, 1306. [CrossRef] [PubMed]
- J. C. Amorim, R. H. Piccoli, and W. F. Duarte, ‘Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages’. Food Research International 2018, 107, 518–527. [CrossRef]
- B. Senkarcinova, I. A. Graça Dias, J. Nespor, and T. Branyik, ‘Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii’. LWT 2019, 100, 362–367. [CrossRef]
- H.-J. Chen et al. Transdermal Delivery of Living and Biofunctional Probiotics through Dissolvable Microneedle Patches’. ACS Appl Bio Mater 2018, 1, 374–381. [CrossRef]
- M. Govender, Y. E. Choonara, P. Kumar, L. C. du Toit, S. van Vuuren, and V. Pillay, ‘A Review of the Advancements in Probiotic Delivery: Conventional vs. Non-conventional Formulations for Intestinal Flora Supplementation’. AAPS PharmSciTech 2014, 15, 29–43. [CrossRef]
- Y. B. Soemarie, T. Milanda, and M. I. Barliana, ‘Fermented foods as probiotics: A review’. J Adv Pharm Technol Res 2021, 12, 335–339. [CrossRef]
- M. A. Lazo-Velez, S. O. Sern-Salvidar, M. F. Rosales-Medina, M. Tinoco-Alvaear, and M. Briones-Garcia, ‘Application of Saccharomyces cerevisiae var. boulardii in food processing: a review’. J Appl Microbiol 2017, 125, 943–951.
- Parrella; et al. Original article Antioxidant properties of different milk fermented with lactic acid bacteria and yeast’, Int J Food Sci Technol, 1–10, 2012. [CrossRef]
- G. H. Fleet, ‘Yeasts in foods and beverages : impact on product quality and safety’. Current Opinion in Biotechnology 2007 2007, 18, 170–175. [CrossRef]
- E. Addis, G. H. Fleet, J. M. Cox, D. Kolak, and T. Leung, ‘The growth, properties and interactions of yeasts and bacteria associated with the maturation of Camembert and blue-veined cheeses’, 25–36, 2001.
- R. Zamora-Vega et al. Effect of incorporating prebiotics in coating materials for the microencapsulation of Sacharomyces boulardii’. International Journal ofFood Sciences and Nutrition 2012, 63, 930–935. [CrossRef] [PubMed]
- J. P. Tamang and S. Lama, ‘Probiotic properties of yeasts in traditional fermented foods and beverages’. J Appl Microbiol 2022, 132, 3533–3542. [CrossRef] [PubMed]
- L. A. Simões et al. Probiotic properties of yeasts isolated from Brazilian fermented table olives. J Appl Microbiol 2021, 131, 1983–1997. [CrossRef] [PubMed]
- v Merchán, M. J. Benito, A. I. Galván, and S. Ruiz-Moyano Seco de Herrera, ‘Identification and selection of yeast with functional properties for future application in soft paste cheese’. LWT 2020, 124, 109173. [CrossRef]
- G. T. Menezes, C. L. Ramos, G. Cenzi, D. S. Melo, D. R. Dias, and R. F. Schwan, ‘Probiotic Potential, Antioxidant Activity, and Phytase Production of Indigenous Yeasts Isolated from Indigenous Fermented Foods’. Probiotics Antimicrob Proteins 2020, 12, 280–288. [CrossRef]
- R.-T. Hsiung, W.-T. Fang, B. A. LePage, S.-A. Hsu, C.-H. Hsu, and J.-Y. Chou, ‘In Vitro Properties of Potential Probiotic Indigenous Yeasts Originating from Fermented Food and Beverages in Taiwan’. Probiotics Antimicrob Proteins 2021, 13, 113–124. [CrossRef]
- W. Fu, W. Xue, C. Liu, Y. Tian, K. Zhang, and Z. Zhu, ‘Screening of Lactic Acid Bacteria and Yeasts from Sourdough as Starter Cultures for Reduced Allergenicity Wheat Products’. Foods 2020, 9, 751. [CrossRef]
- L. Bohn, A. S. Meyer, and S. K. Rasmussen, ‘Phytate : impact on environment and human nutrition . A challenge for molecular breeding *’ 2008, 9, 165–191. [CrossRef]
- K. Küçükgöz and M. Trzaskowska, ‘Nondairy Probiotic Products : Functional Foods That Require more attention. Nutrients 2022, 14, 753.
- M. A. Lazo-Vélez, S. O. Serna-Saldívar, M. F. Rosales-Medina, M. Tinoco-Alvear, and M. Briones-García, ‘Application of Saccharomyces cerevisiae var. boulardii in food processing: a review’, J Appl Microbiol 2018, 125, 943–951. [CrossRef]
- P. De Paula, D. William, H. Chávez, and L. Lin, ‘Growth Parameters and Survivability of Saccharomyces boulardii for Probiotic Alcoholic Beverages Development’ 2019, 10, no. September, 1–10. [CrossRef]
- Senkarcinova, I. Alexandra, G. Dias, J. Nespor, and T. Branyik, ‘LWT - Food Science and Technology Probiotic alcohol-free beer made with Saccharomyces cerevisiae var . boulardii’. LWT - Food Science and Technology 2019, 100, 362–367. [CrossRef]
- P. Cavalheiro, C. P. Cavalheiro, C. Ruiz-capillas, A. M. Herrero, C. R. De Menezes, L. Lucy, and M. Fries, ‘Application of probiotic delivery systems in meat products’, Trends Food Sci Technol, 2015. [CrossRef]
- Han; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey’, Front Cell Infect Microbiol 2021, 11. [CrossRef]
- R. Sehrawat, S. Abdullah, P. Khatri, L. Kumar, A. Kumar, and A. S. Mujumdar, ‘Role of drying technology in probiotic encapsulation and impact on food safety’. Drying Technology 2022, 40, 1562–1581. [CrossRef]
- J. Palanivelu, S. Thanigaivel, S. Vickram, N. Dey, D. Mihaylova, and I. Desseva, ‘Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability’. Applied Sciences 2022, 12, 455. [CrossRef]
- M. A. S. Santos and M. T. C. Machado, ‘Coated alginate–chitosan particles to improve the stability of probiotic yeast’. Int J Food Sci Technol 2021, 56, 2122–2131. [CrossRef]
- R. Rajam and P. Subramanian, ‘Encapsulation of probiotics: past, present and future’. Beni Suef Univ J Basic Appl Sci 2022, 11, 46. [CrossRef]
- Rashidinejad; et al. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products’, Crit Rev Food Sci Nutr 2022, 62, 2470–2494. 62. [CrossRef]
- K. S. Yoha, S. Nida, S. Dutta, J. A. Moses, and C. Anandharamakrishnan, ‘Targeted Delivery of Probiotics: Perspectives on Research and Commercialization’. Probiotics Antimicrob Proteins 2022, 14, 15–48. [CrossRef]
- J. Cielecka-Piontek, M. Dziedziński, O. Szczepaniak, J. Kobus-Cisowska, A. Telichowska, and D. Szymanowska, ‘Survival of commercial probiotic strains and their effect on dark chocolate synbiotic snack with raspberry content during the storage and after simulated digestion’. Electronic Journal of Biotechnology 2020, 48, 62–71. [CrossRef]
- M. Z. A. Chan, M. Toh, and S.-Q. Liu, ‘Growth, survival, and metabolic activities of probiotics Lactobacillus rhamnosus GG and Saccharomyces cerevisiae var. boulardii CNCM-I745 in fermented coffee brews’. Int J Food Microbiol 2021, 350, 109229. [CrossRef]
- R. Sehrawat, S. Abdullah, P. Khatri, L. Kumar, A. Kumar, and A. S. Mujumdar, ‘Role of drying technology in probiotic encapsulation and impact on food safety’, Drying Technology, 1–20, 2022. [CrossRef]
- G. Frakolaki, V. Giannou, D. Kekos, and C. Tzia, ‘A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods’. Crit Rev Food Sci Nutr 2021, 61, 1515–1536. [CrossRef]
- M. Kvakova, I. Bertkova, J. Stofilova, and T. C. Savidge, ‘Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation’. Foods 2021, 10, 1297. [CrossRef]
- Ohlmaier-Delgadillo; et al. Ferulated Pectins and Ferulated Arabinoxylans Mixed Gel for Saccharomyces boulardii Entrapment in Electrosprayed Microbeads’, Molecules 2021, 26, 2478. 26. [CrossRef]
- M. Chavarri, I. M. Chavarri, I. Maranon, and M. Carmen, ‘Encapsulation Technology to Protect Probiotic Bacteria’, in Probiotics, InTech, 2012. [CrossRef]
- Terpou, A. Papadaki, I. Lappa, V. Kachrimanidou, L. Bosnea, and N. Kopsahelis, ‘Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value’. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- P. M. Reque and A. Brandelli, ‘Encapsulation of probiotics and nutraceuticals: Applications in functional food industry’. Trends Food Sci Technol 2021, 114, 1–10. [CrossRef]
- J. Rodrigues, M. F. Cedran, J. L. Bicas, and H. H. Sato, ‘Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications – A narrative review’. Food Research International 2020, 137, 109682. [CrossRef]
- J. L. Patarroyo, J. S. Florez-Rojas, D. Pradilla, J. D. Valderrama-Rincón, J. C. Cruz, and L. H. Reyes, ‘Formulation and Characterization of Gelatin-Based Hydrogels for the Encapsulation of Kluyveromyces lactis—Applications in Packed-Bed Reactors and Probiotics Delivery in Humans’. Polymers (Basel) 2020, 12, 1287. [CrossRef]
- de la C. Pech-Canul, D. Ortega, A. García-Triana, N. González-Silva, and R. L. Solis-Oviedo, ‘A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics’. Coatings 2020, 10, 197. [CrossRef]
- L.-F. Călinoiu, B. Ştefănescu, I. Pop, L. Muntean, and D. Vodnar, ‘Chitosan Coating Applications in Probiotic Microencapsulation’. Coatings 2019, 9, 194. [CrossRef]
- Z. Muhammad, R. Ramzan, R. Zhang, and M. Zhang, ‘Resistant Starch-Based Edible Coating Composites for Spray-Dried Microencapsulation of Lactobacillus acidophilus, Comparative Assessment of Thermal Protection, In Vitro Digestion and Physicochemical Characteristics’. Coatings 2021, 11, 587. [CrossRef]
- S. Graff, S. Hussain, J.-C. Chaumeil, and C. Charrueau, ‘Increased Intestinal Delivery of Viable Saccharomyces boulardii by Encapsulation in Microspheres’. Pharm Res 2008, 25, 1290–1296. [CrossRef]
- S. Suvarna, J. Dsouza, M. L. Ragavan, and N. Das, ‘Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition’, Food Sci Biotechnol 2018, 27, 745–753. [CrossRef]
- E. Dadkhodazade, E. Khanniri, N. Khorshidian, S. M. Hosseini, A. M. Mortazavian, and E. Moghaddas Kia, ‘Yeast cells for encapsulation of bioactive compounds in food products: A review’, Biotechnol Prog, 2021. [CrossRef]
- B. N. Pham-Hoang, C. Romero-Guido, H. Phan-Thi, and Y. Waché, ‘Encapsulation in a natural, preformed, multi-component and complex capsule: yeast cells. Appl Microbiol Biotechnol 2013, 97, 6635–6645. [CrossRef] [PubMed]
- D. Poddar, J. Palmer, S. Das, M. Gaare, A. Nag, and H. Singh, ‘Effect of Fluidized Bed Drying, Matrix Constituents and Structure on the Viability of Probiotic Lactobacillus paracasei ATCC 55544 during Storage at 4 °C, 25 °C and 37 °C’. Microorganisms 2021, 10, 74. [CrossRef]
- Liu; et al. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review’, Crit Rev Food Sci Nutr 2019, 59, 2863–2878. [CrossRef]
- D.-H. Lim, A. Letona, M. Lee, D. Lim, N.-S. Han, and D. Chung, ‘Fluidized-Bed Granulation of Probiotics-Encapsulated Spray-Dried Skim Milk Powder: Effects of a Fluidizing Aid, Moisture-Activation and Dehydration’. Foods 2021, 10, 1600. [CrossRef]
- S. P. Dhakal and J. He, ‘Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review’. Food Research International 2020, 137, 109326. [CrossRef] [PubMed]
- D. Arepally, R. S. Reddy, T. K. Goswami, and R. Coorey, ‘A Review on Probiotic Microencapsulation and Recent Advances of their Application in Bakery Products’. Food Bioproc Tech 2022, 15, 1677–1699. [CrossRef]
- S. Arslan-Tontul and M. Erbas, ‘Single and double layered microencapsulation of probiotics by spray drying and spray chilling’. LWT - Food Science and Technology 2017, 81, 160–169. [CrossRef]
- B. Chen, Z. Liang, X. Lin, W. Li, X. Lin, and Z. He, ‘Enhanced survival of fluidized bed-dried microencapsulated Saccharomyces cerevisiae cells in the presence of Hongqu rice distiller’s grain peptides. LWT 2022, 163, 113511. [CrossRef]
- M. L. Chaparro, E. Ce´spedes, M. Cruz, C. R. Castillo-Saldarriaga, and M. I. Go´mez-A´ lvarez, ‘FLUIDIZED BED DRYING OF A GRANULATED PROTOTYPE BASED ON A POTENTIAL PROBIOTIC YEAST Meyerozyma guilliermondii: SELECTION OF PROCESS PARAMETERS AND DRYING PROTECTANT’. Rev Mex Ing Quim 2017, 16, 347–357.
- S. Hirai and T. Kawasumi, ‘Enhanced lactic acid bacteria viability with yeast coincubation under acidic conditions’. Biosci Biotechnol Biochem 2020, 84, 1706–1713. [CrossRef]
- F. Zahoor, C. Sooklim, P. Songdech, O. Duangpakdee, and N. Soontorngun, ‘Selection of Potential Yeast Probiotics and a Cell Factory for Xylitol or Acid Production from Honeybee Samples’. Metabolites 2021, 11, 312. [CrossRef]
- X. Mo et al. Whole genome sequencing and metabolomics analyses reveal the biosynthesis of nerol in a multi-stress-tolerant. Meyerozyma guilliermondii 2021, 20, 1–11. [CrossRef]
- Aro, E. Järvenpää, J. Mäkinen, M. Lauraeus, R. Huopalahti, and V. Hietaniemi, ‘The utilization of oat polar lipids produced by supercritical fluid technologies in the encapsulation of probiotics’. LWT - Food Science and Technology 2013, 53, 540–546. [Google Scholar] [CrossRef]
- M. S. Thantsha, P. W. Labuschagne, and C. I. Mamvura, ‘Supercritical CO2 interpolymer complex encapsulation improves heat stability of probiotic bifidobacteria. World J Microbiol Biotechnol 2014, 30, 479–486. [CrossRef] [PubMed]
- P. W. Labuschagne, B. Naicker, and L. Kalombo, ‘Micronization, characterization and in-vitro dissolution of shellac from PGSS supercritical CO2 technique. Int J Pharm 2016, 499, 205–216. [CrossRef]
- L. Cao, Q. Xu, Y. Xing, X. Guo, W. Li, and Y. Cai, ‘Effect of skimmed milk powder concentrations on the biological characteristics of microencapsulated Saccharomyces cerevisiae by vacuum-spray-freeze-drying. Drying Technology 2020, 38, 476–494. [CrossRef]
- M. Ben Thomas, M. Vaidyanathan, K. Radhakrishnan, and A. M. Raichur, ‘Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan–dextran sulfate polyelectrolytes’. J Food Eng 2014, 136, 1–8. [CrossRef]
- W. Arnold, J. Roach, and M. A. Azcarate-Peril, ‘Emerging Technologies for Gut Microbiome Research’. Trends Microbiol 2016, 24, 887–901. [CrossRef]
- M. L. Marco, ‘Crystal ball Defining how microorganisms benefit human health’. Microb Biotechnol 2020, 141, 35–40. [CrossRef]
- E. K. Costello, C. L. Lauber, M. Hamady, N. Fierer, J. I. Gordon, and R. Knight, ‘Bacterial community variation in human body habitats across space and time’. Science (1979) 2009, 326, 1694–1697. [CrossRef]
- E. T. Hillman, H. Lu, T. Yao, and C. H. Nakatsu, ‘Microbial Ecology along the Gastrointestinal Tract’. Microbes Environ 2017, 32, 300. [CrossRef]
- R. Khan, F. C. Petersen, and S. Shekhar, ‘Commensal bacteria: An emerging player in defense against respiratory pathogens’. Front Immunol 2019, 10, 1203. [CrossRef] [PubMed]
- B. De Pessemier et al. microorganisms Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions’, 2021. [CrossRef]
- C. A. O’Neill, G. Monteleone, J. T. McLaughlin, and R. Paus, ‘The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays 2016, 38, 1167–1176. [CrossRef] [PubMed]
- R. Shaykhiev and R. Bals, ‘Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol 2007, 82, 1–15. [CrossRef] [PubMed]
- E. Thursby and N. Juge, ‘Introduction to the human gut microbiota’, Biochemical Journal 2017, 474, 1823–1836. [CrossRef]
- G. A. M. Cresci and K. Izzo, ‘Gut microbiome’, Adult Short Bowel Syndrome: Nutritional, Medical, and Surgical Management, 45–54, 2018. [CrossRef]
- Gensollen, S. S. Iyer, D. L. Kasper, and R. S. Blumberg, ‘How colonization by microbiota in early life shapes the immune system’. Science (1979) 2016, 352, 539–544. [Google Scholar] [CrossRef]
- G. Den Besten, K. Van Eunen, A. K. Groen, K. Venema, D. J. Reijngoud, and B. M. Bakker, ‘The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism’. J Lipid Res 2013, 54, 2325–2340. [CrossRef]
- J. Baümler and V. Sperandio, ‘Interactions between the microbiota and pathogenic bacteria in the gut’. Nature 2016, 535, no. 7610, 85–93. [CrossRef]
- M. M. Natividad and E. F. Verdu, ‘Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol Res 2013, 69, 42–51. [CrossRef]
- E. Thursby and N. Juge, ‘Introduction to the human gut microbiota’. Biochemical Journal 2017, 474, 1823–1836. [CrossRef]
- T. Gensollen, S. S. Iyer, D. L. Kasper, and R. S. Blumberg, ‘How colonization by microbiota in early life shapes the immune system. Science (1979) 2016, 352, no. 6285, 539–544. [CrossRef]
- N. İlhan, ‘Gut Microbiota and Metabolism’, International Journal of Medical Biochemistry 2018, 1. [CrossRef]
- Rowland; et al. Gut microbiota functions: metabolism of nutrients and other food components’, Eur J Nutr 2018, 57, 1. [CrossRef]
- S. Musumeci, M. Coen, A. Leidi, and J. Schrenzel, ‘The human gut mycobiome and the specific role of Candida albicans: where do we stand, as clinicians?’. Clinical Microbiology and Infection 2022, 28, 58–63. [CrossRef] [PubMed]
- S. Dollive et al. A tool kit for quantifying eukaryotic rRNA gene sequences from human microbiome samples’. Genome Biol 2012, 13, R60. [CrossRef]
- K. Nash et al. The gut mycobiome of the Human Microbiome Project healthy cohort’, Microbiome 2017, 5, 1–13. [CrossRef]
- S. Sen and T. J. Mansell, ‘Yeasts as probiotics: Mechanisms, outcomes, and future potential’, 2020. [CrossRef]
- M. Boxberger, V. Cenizo, N. Cassir, and B. Scola, ‘Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 1–14. [CrossRef]
- E. Y. Chen, M. A. Fischbach, and Y. Belkaid, ‘Skin microbiota–host interactions’. Nature 2018, 553, 427–436. [CrossRef]
- R. Shaykhiev and R. Bals, ‘Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol 2007, 82, 1–15. [CrossRef] [PubMed]
- E. A. Grice, ‘The intersection of microbiome and host at the skin interface: genomic-and metagenomic-based insights’, 2015. [CrossRef]
- H. Zhou, L. Shi, Y. Ren, X. Tan, W. Liu, and Z. Liu, ‘Applications of Human Skin Microbiota in the Cutaneous Disorders for Ecology-Based Therapy’. Front Cell Infect Microbiol 2020, 10, 1–12. [CrossRef]
- Y. Belkaid and J. A. Segre, ‘Dialogue between skin microbiota and immunity’. Science Mag 2014, 346, 954–959. [CrossRef]
- L. Byrd, Y. L. Byrd, Y. Belkaid, and J. A. Segre, ‘The human skin microbiome’, Nature Publishing Group 2018, 16. [CrossRef]
- L. Byrd, Y. Belkaid, and J. A. Segre, ‘The human skin microbiome’. Nat Rev Microbiol 2018, 16, 143–155. [CrossRef]
- M. K. Kwofie, N. Bukari, and O. Adeboye, ‘Probiotics Potential of Yeast and Lactic Acid Bacteria Fermented Foods and the Impact of Processing: A Review of Indigenous and Continental Food Products’. Adv Microbiol 2020, 10, 492–507. [CrossRef]
- Kaźmierczak-Siedlecka, J. Ruszkowski, M. Fic, M. Folwarski, and W. Makarewicz, ‘Saccharomyces boulardii CNCM I-745: A Non-bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases. Curr Microbiol 2020, 77, 1987–1996. [CrossRef] [PubMed]
- P. K. Mukherjee, B. Sendid, G. Hoarau, J.-F. Colombel, D. Poulain, and M. A. Ghannoum, ‘Mycobiota in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol 2015, 12, 77–87. [CrossRef]
- R. Abid et al. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. Journal of Fungi 2022, 8, 444. [CrossRef]
- M.-C. Barc et al. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: Influence of Saccharomyces boulardii’. Anaerobe 2008, 14, 229–233. [CrossRef]
- Collignon, C. Sandré, and M.-C. Barc, ‘Saccharomyces boulardii modulates dendritic cell properties and intestinal microbiota disruption after antibiotic treatment’. Gastroenterol Clin Biol 2010, 34, S71–S78. [Google Scholar] [CrossRef]
- M. I. Moré and A. Swidsinski, ‘Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis – a review’, Clin Exp Gastroenterol 2015, 237. [CrossRef]
- A.-M. Yang et al. Saccharomyces Boulardii Ameliorates Non-alcoholic Steatohepatitis in Mice Induced by a Methionine-Choline-Deficient Diet Through Gut-Liver Axis’, Front Microbiol 2022, 13. [CrossRef]
- A.-M. Yang et al. Saccharomyces Boulardii Ameliorates Non-alcoholic Steatohepatitis in Mice Induced by a Methionine-Choline-Deficient Diet Through Gut-Liver Axis’, Front Microbiol 2022, 13. [CrossRef]
- Y. Wu et al. Effects of Multispecies Probiotic on Intestinal Microbiota and Mucosal Barrier Function of Neonatal Calves Infected With E. coli K99’, Front Microbiol 2022, 12. [CrossRef]
- M. Bloemendaal et al. Probiotics-induced changes in gut microbial composition and its effects on cognitive performance after stress: exploratory analyses’. Transl Psychiatry 2021, 11, 300. [CrossRef]
- Duysburgh, P. van den Abbeele, M. Morera, and M. Marzorati, ‘Lacticaseibacillus rhamnosusGG and Saccharomyces cerevisiae boulardii supplementation exert protective effects on human gut microbiome following antibiotic administration in vitro’. Benef Microbes 2021, 12, 365–379. [Google Scholar] [CrossRef]
- Saber, B. Alipour, Z. Faghfoori, and A. Yari Khosroushahi, ‘Cellular and molecular effects of yeast probiotics on cancer’. Crit Rev Microbiol 2017, 43, 96–115. [Google Scholar] [CrossRef]
- Kainz, M. A. Bauer, F. Madeo, and D. Carmona-Gutierrez, ‘Fungal infections in humans: the silent crisis’. Microbial Cell 2020, 7, 143–145. [Google Scholar] [CrossRef]
- Buerth, C., Tielker, D., & Ernst, J. F. (2016). Candida utilis and Cyberlindnera (Pichia) jadinii: yeast relatives with expanding applications. Applied microbiology and biotechnology, 100, 6981-6990.
- Sharma, P., Gaur, V. K., Kim, S. H., & Pandey, A. (2020). Microbial strategies for bio-transforming food waste into resources. Bioresource technology, 299, 122580.
- Patil, S., Pimpley, V., Warudkar, K., & Murthy, P. S. (2022). Valorisation of coffee pulp for development of innovative probiotic beverage using kefir: Physicochemical, antioxidant, sensory analysis and shelf life studies. Waste and Biomass Valorization, 13(2), 905-916.
- BCC Market Research. (2022). Probiotics in Food, Beverages, Dietary Supplements and Animal Feed. FOD035H.
- Group, J.F.W.W. Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf.
- Gareau, M. G., Sherman, P. M., & Walker, W. A. (2010). Probiotics and the gut microbiota in intestinal health and disease. Nature reviews Gastroenterology & hepatology, 7(9), 503-514.

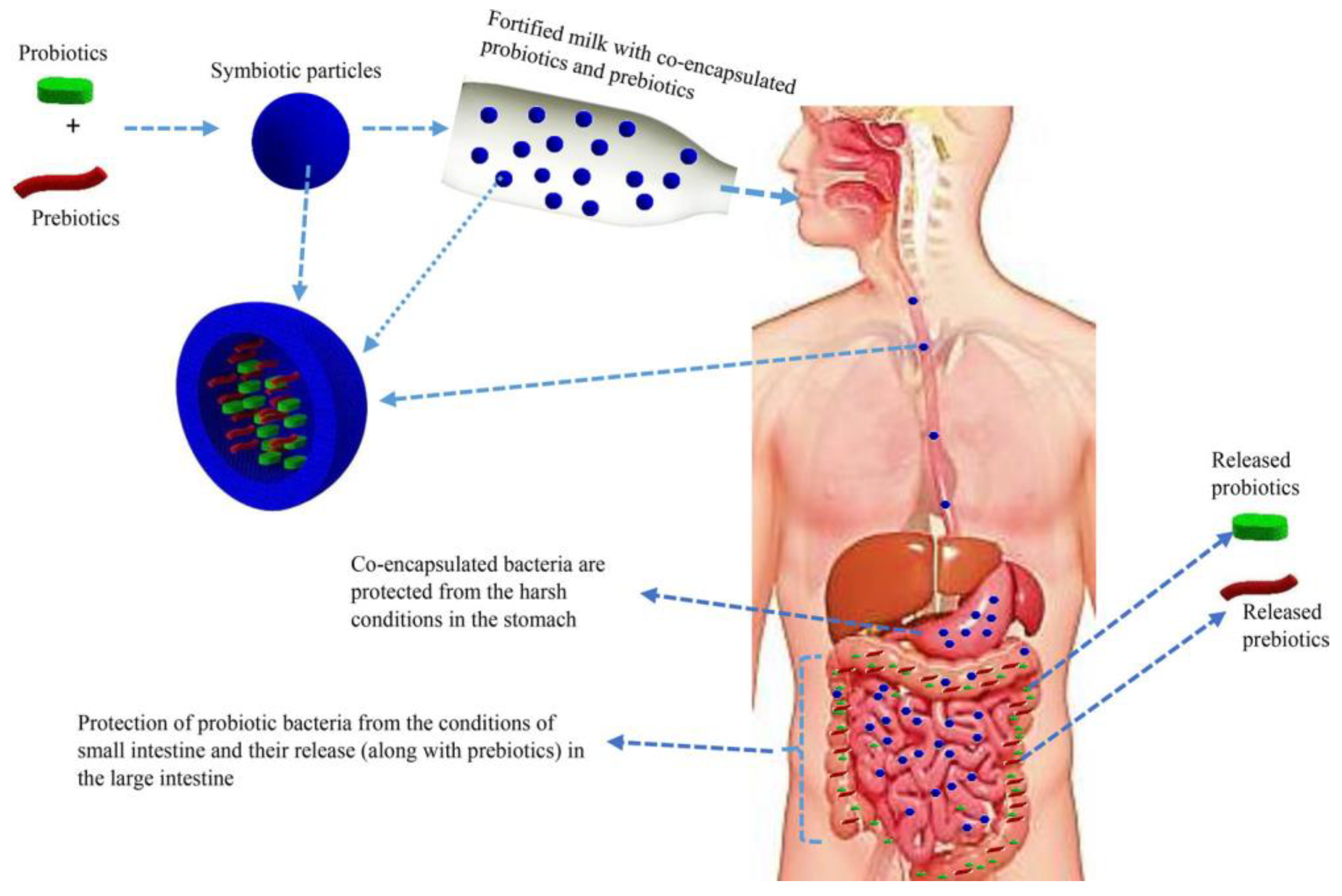
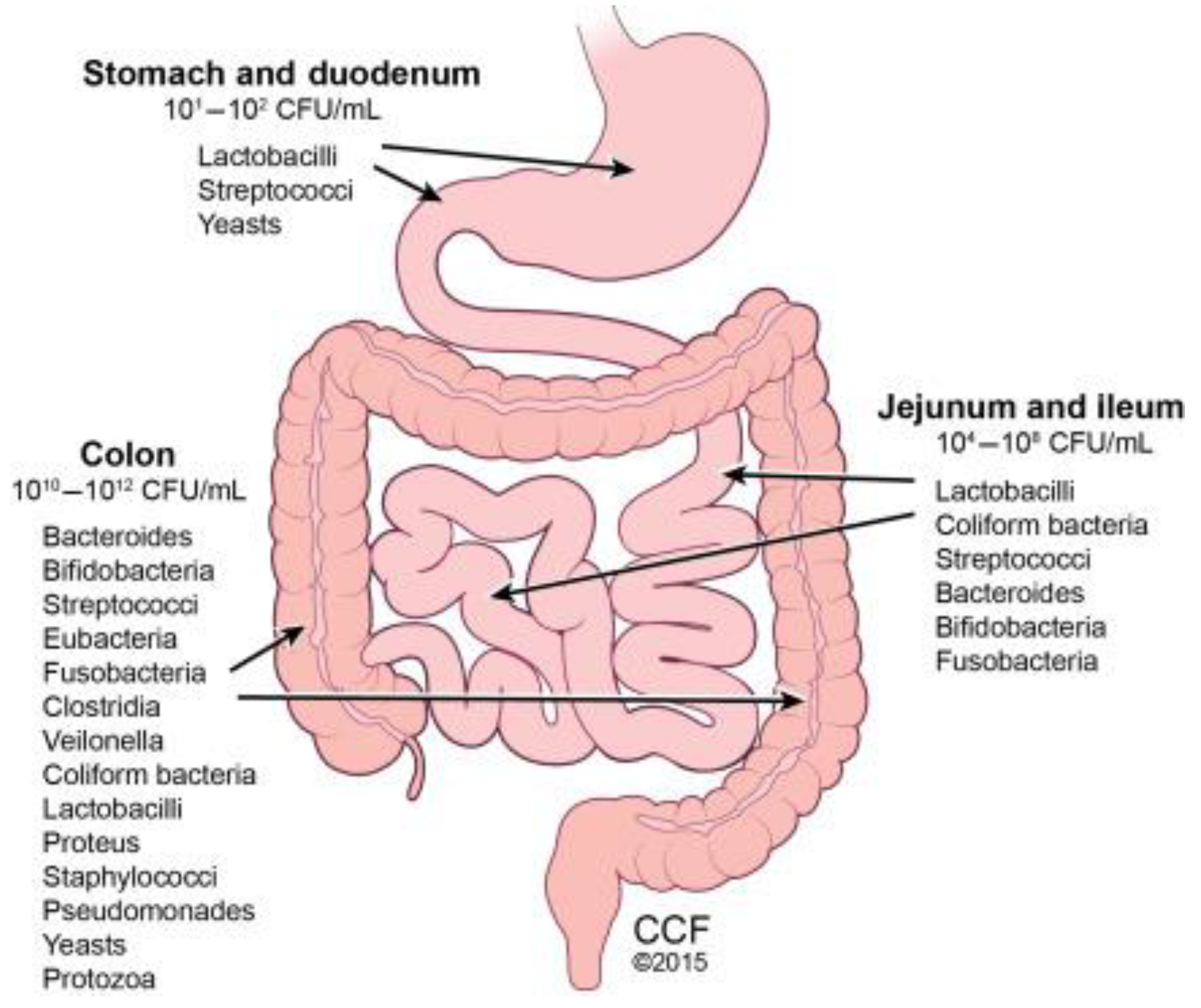
| Characteristic | Rationale | Reference |
|---|---|---|
| Hydrophobicity | For an organism to show functionality as a probiotic, it needs to display hydrophobicity. The organism of interest needs to demonstrate its ability to adhere/interact with the mucus present within the GIT to confer the probiotic effect. | [21] |
| Auto-aggregation | This is a characteristic wherein cells are able to self-aggregate and adhere to the mucus/mucosal lining in order to form a biofilm. A desirable level of auto-aggregation is ~30 to 60% | [22] |
| Biofilm formation | To show the ability of cells to adhere to each other and the host lining | [23] |
| Adherence ability | To assess the ability of the probiotic cell to adhere to the mucosal lining and confer a probiotic | [24] |
| Survival | To assess the organism’s ability to survive exposure to low pHs (gastric conditions) and the presence of bile salts (0.3%) | [25] |
| Antibiotic resistance | In the instances of yeasts intended for use as a probiotic, antibiotic resistance using a disk assay method will infer information pertaining to the ability of the organism to demonstrate antibiotic resistance. | [26] |
| Antimicrobial activity | To assess the yeast’s ability to demonstrate anti-microbial activity, which is pertinent for the treatment of pathogens | [27] |
| Normal chow diet | MCD diet | MCD plus S. boulardii |
|---|---|---|
| Muribaculaceae | Akkermansiaceae | Lachnospiraceae |
| Ruminococcaeceae | Erysipelotrichaceae | Atopobiaceae |
| Lactobacillaceae | Tannerellaceae | Ruminococcaceae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




