Submitted:
01 October 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Overview of Gut Microbiome & Influence on Metabolism
2. Correlation between microbiome, population-based cancer epidemiology and epigenetics
3. Impact of Microbiome Dysbiosis on cancer metabolism
4. Factors Influencing Cancer Progression
5. Microbiota dysbiosis in Cancer & Mechanism of Microbial Oncogenesis
5.1. Contact-dependent mechanism
5.2. Contact-independent mechanism
5.3. Immunological mechanisms
6. Biomarkers – Current microbiota markers in clinical practice
7. Treatment:
7.1. Antibiotics
7.2. Nutrition, postbiotics, and probiotics
7.3. Transplantation of Fecal Microbiota and Identified Microbial Consortiums
7.4. Utilizing Exogenous Microbiota in Cancer Therapy
7.5. Intratumoral Microbiota's Impact in Boosting Antitumor Immunity
- a)
- Intratumoral Bifidobacterium can activate dendritic cells (DCs) through the STING signaling pathway. Furthermore, A. muciniphila can create STING agonists, which cause intratumoral monocytes to secrete IFN-I. This procedure increases communication between natural killer (NK) cells and DCs further encouraging macrophage conversion [106].
- b)
- T and NK Cell Activation: By encouraging the recruitment and activation of CD8+ T cells, several intratumoral microbiota, including Saccharopolyspora, Lachnoclostridium, EBV, and HBV, improve antitumor immunity. Patients survive longer because of this activation, mediated by chemokines produced from the intratumoral microbiome such as CXCL9, CXCL10, and CCL5[106]. Trimethylamine N-oxide (TMAO) generated from Clostridiales can cause PERK-mediated endoplasmic reticulum (ER) stress, which can result in more CD8+ T cell-mediated antitumor immunity and tumor cell pyroptosis. Furthermore, NK cell activity is improved, and tumor regression is induced by increased levels of Bifidobacterium in intratumoral areas, which is caused by an elevated by-product in the diet called Hippurate[106,107].
- c)
- Production of Tertiary Lymphoid Structures (TLS): The development of TLS is caused by intratumoral H. hepaticus, which stimulates anticancer immune responses dependent on Tfh- and B-cells [108].
- d)
7.6. Human Microbiota's Therapeutic Effect in Skin Cancer
8. Environmental risk factors influencing microorganisms-cancer interactions
9. Limitations of this review and future directions:
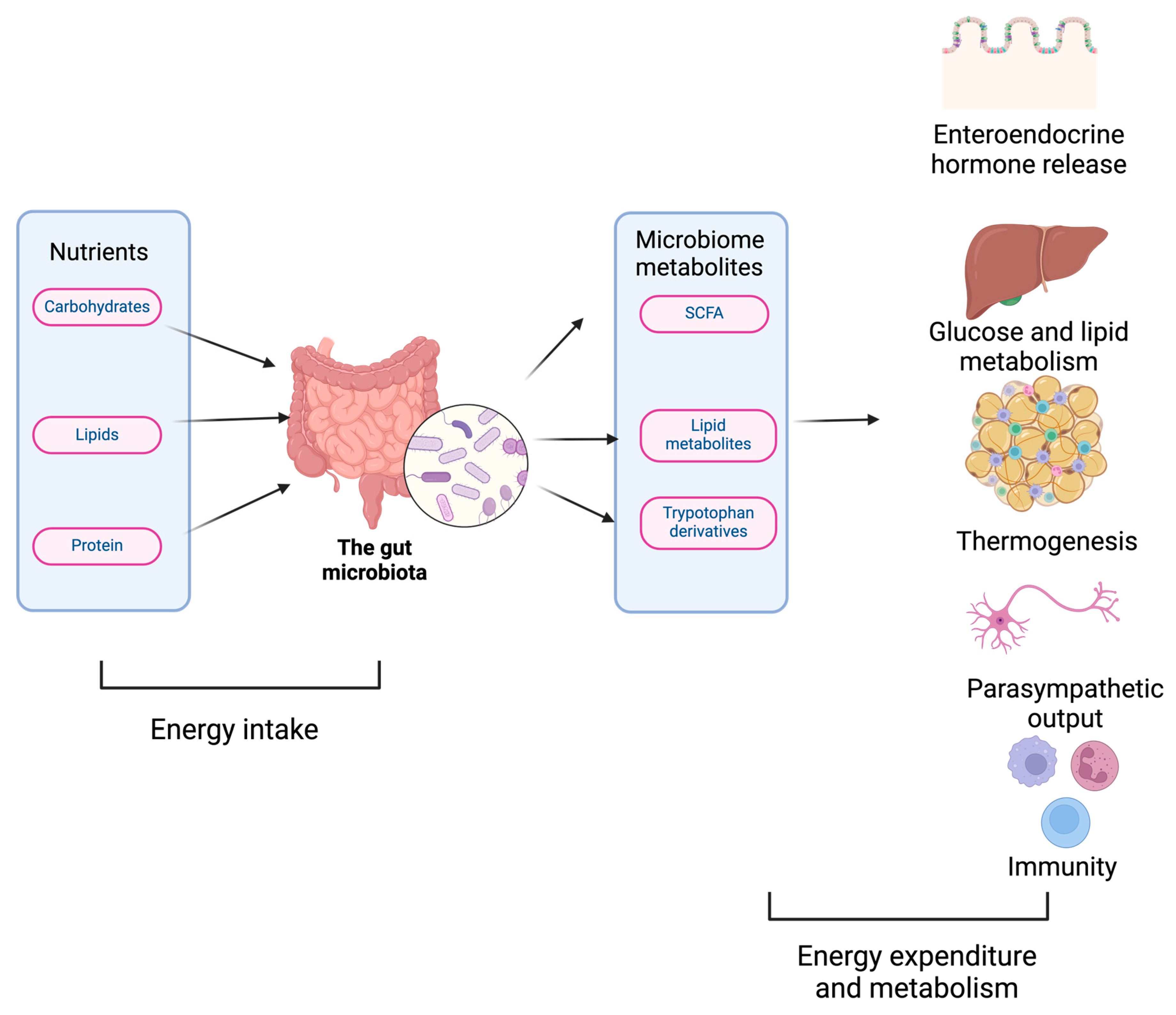
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct Target Ther 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: a core regulator of metabolism. J Endocrinol 2023, 256. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, J.; Armet, A.M.; Willing, B.P.; Deehan, E.C.; Fassini, P.G.; Mota, J.F.; Walter, J.; Prado, C.M. Exploring the Influence of Gut Microbiome on Energy Metabolism in Humans. Adv Nutr 2023, 14, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig Dis Sci 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med 2022, 14, 80. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J Clin Pediatr 2021, 10, 84–92. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef]
- Caengprasath, N.; Gonzalez-Abuin, N.; Shchepinova, M.; Ma, Y.; Inoue, A.; Tate, E.W.; Frost, G.; Hanyaloglu, A.C. Internalization-Dependent Free Fatty Acid Receptor 2 Signaling Is Essential for Propionate-Induced Anorectic Gut Hormone Release. iScience 2020, 23, 101449. [Google Scholar] [CrossRef]
- Forbes, S.; Stafford, S.; Coope, G.; Heffron, H.; Real, K.; Newman, R.; Davenport, R.; Barnes, M.; Grosse, J.; Cox, H. Selective FFA2 Agonism Appears to Act via Intestinal PYY to Reduce Transit and Food Intake but Does Not Improve Glucose Tolerance in Mouse Models. Diabetes 2015, 64, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Dong, W.; Luo, T.; Tang, H.; Zhu, W.; Huang, Y.; Yang, X. Butyrate and obesity: Current research status and future prospect. Front Endocrinol (Lausanne) 2023, 14, 1098881. [Google Scholar] [CrossRef]
- Ma, H.; Patti, M.E. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014, 28, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat Commun 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257.e246. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med 2015, 4, 1933–1947. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Wang, J.; Hankinson, S.E.; Tamimi, R.M.; Chen, W.Y. Protein Intake and Breast Cancer Survival in the Nurses' Health Study. J Clin Oncol 2017, 35, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Hu, F.B.; Mayer, R.J.; Nelson, H.; Whittom, R.; Hantel, A.; Thomas, J.; et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 2007, 298, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Fadelu, T.; Zhang, S.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.B.; et al. Nut Consumption and Survival in Patients With Stage III Colon Cancer: Results From CALGB 89803 (Alliance). J Clin Oncol 2018, 36, 1112–1120. [Google Scholar] [CrossRef]
- Richman, E.L.; Stampfer, M.J.; Paciorek, A.; Broering, J.M.; Carroll, P.R.; Chan, J.M. Intakes of meat, fish, poultry, and eggs and risk of prostate cancer progression. Am J Clin Nutr 2010, 91, 712–721. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; di Giuseppe, R.; Waniek, S.; Plachta-Danielzik, S.; Koch, M.; Nothlings, U.; Hampe, J.; Schlesinger, S.; Lieb, W. Postdiagnostic Mediterranean and Healthy Nordic Dietary Patterns Are Inversely Associated with All-Cause Mortality in Long-Term Colorectal Cancer Survivors. J Nutr 2017, 147, 636–644. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; van Osch, F.H.M.; Reulen, R.C.; van Hensbergen, M.; Nekeman, D.; Pirrie, S.; Wesselius, A.; van Schooten, F.J.; James, N.D.; Wallace, D.M.A.; et al. Fruit and vegetable intake and the risk of recurrence in patients with non-muscle invasive bladder cancer: a prospective cohort study. Cancer Causes Control 2018, 29, 573–579. [Google Scholar] [CrossRef]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis 2014, 33, 1381–1390. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef]
- Wei, Z.; Cao, S.; Liu, S.; Yao, Z.; Sun, T.; Li, Y.; Li, J.; Zhang, D.; Zhou, Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients' survival? A pilot study on relevant mechanism. Oncotarget 2016, 7, 46158–46172. [Google Scholar] [CrossRef]
- Yan, X.; Liu, L.; Li, H.; Qin, H.; Sun, Z. Clinical significance of Fusobacterium nucleatum, epithelial-mesenchymal transition, and cancer stem cell markers in stage III/IV colorectal cancer patients. Onco Targets Ther 2017, 10, 5031–5046. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Misra, B.B.; Liang, L.; Bi, D.; Weng, W.; Wu, W.; Cai, S.; Qin, H.; Goel, A.; Li, X.; et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019, 9, 4101–4114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Muhlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Devendran, S.; Alves, J.M.; Doden, H.; Wolf, P.G.; Pereira, G.V.; Ly, L.; Volland, A.; Takei, H.; Nittono, H.; et al. The 'in vivo lifestyle' of bile acid 7alpha-dehydroxylating bacteria: comparative genomics, metatranscriptomic, and bile acid metabolomics analysis of a defined microbial community in gnotobiotic mice. Gut Microbes 2020, 11, 381–404. [Google Scholar] [CrossRef]
- Flemer, B.; Herlihy, M.; O'Riordain, M.; Shanahan, F.; O'Toole, P.W. Tumour-associated and non-tumour-associated microbiota: Addendum. Gut Microbes 2018, 9, 369–373. [Google Scholar] [CrossRef]
- Najafi, S.; Abedini, F.; Azimzadeh Jamalkandi, S.; Shariati, P.; Ahmadi, A.; Gholami Fesharaki, M. The composition of lung microbiome in lung cancer: a systematic review and meta-analysis. BMC Microbiol 2021, 21, 315. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e1115. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Monavari, S.H.; Solaymani Mohammadi, F.; Kiani, S.J.; Armat, S.; Farahmand, M. Association between Epstein-Barr virus infection and gastric cancer: a systematic review and meta-analysis. BMC Cancer 2020, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, C.; Yang, Y.; Zhou, F.; Li, M.; Jin, Q.; Gao, L. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther 2014, 39, 270–281. [Google Scholar] [CrossRef]
- Turkay, D.O.; Vural, C.; Sayan, M.; Gurbuz, Y. Detection of human papillomavirus in esophageal and gastroesophageal junction tumors: A retrospective study by real-time polymerase chain reaction in an instutional experience from Turkey and review of literature. Pathol Res Pract 2016, 212, 77–82. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Colbert, L.E.; El Alam, M.B.; Wang, R.; Karpinets, T.; Lo, D.; Lynn, E.J.; Harris, T.A.; Elnaggar, J.H.; Yoshida-Court, K.; Tomasic, K.; et al. Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Cancer Cell 2023, 41, 1945–1962.e1911. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, W.; Shen, Q.; Miao, C.; Chen, L.; Li, Y.; Gu, X.; Fan, M.; Ma, Y.; Wang, H.; et al. Bile acid metabolism dysregulation associates with cancer cachexia: roles of liver and gut microbiome. J Cachexia Sarcopenia Muscle 2021, 12, 1553–1569. [Google Scholar] [CrossRef]
- Galeano Nino, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, E.S.; Zhao, C.; Jin, C. Host-Microbiome Interaction in Lung Cancer. Front Immunol 2021, 12, 679829. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, L.; Malgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front Cell Infect Microbiol 2022, 12, 1020583. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Zhang, Y. Involvement of Human Papillomaviruses in Cervical Cancer. Front Microbiol 2018, 9, 2896. [Google Scholar] [CrossRef]
- Salvatori, S.; Marafini, I.; Laudisi, F.; Monteleone, G.; Stolfi, C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Yang, R.; Qian, L. Research on Gut Microbiota-Derived Secondary Bile Acids in Cancer Progression. Integr Cancer Ther 2022, 21, 15347354221114100. [Google Scholar] [CrossRef]
- Du, Q.; Geller, D.A. Cross-Regulation Between Wnt and NF-kappaB Signaling Pathways. For Immunopathol Dis Therap 2010, 1, 155–181. [Google Scholar] [CrossRef]
- Finlay, B.B. Cell adhesion and invasion mechanisms in microbial pathogenesis. Curr Opin Cell Biol 1990, 2, 815–820. [Google Scholar] [CrossRef]
- Garcia, E.C.; Perault, A.I.; Marlatt, S.A.; Cotter, P.A. Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc Natl Acad Sci U S A 2016, 113, 8296–8301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bayona, L.; Guo, M.S.; Laub, M.T. Contact-dependent killing by Caulobacter crescentus via cell surface-associated, glycine zipper proteins. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc Natl Acad Sci U S A 2005, 102, 5576–5581. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, H.; Tian, G.; Li, S. Dynamic microbe and molecule networks in a mouse model of colitis-associated colorectal cancer. Sci Rep 2014, 4, 4985. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Wargo, J.A. Gut Microbes' Impact on Oncogenic Drivers: Location Matters. Mol Cell 2020, 79, 878–880. [Google Scholar] [CrossRef]
- Boopathi, S.; Liu, D.; Jia, A.Q. Molecular trafficking between bacteria determines the shape of gut microbial community. Gut Microbes 2021, 13, 1959841. [Google Scholar] [CrossRef]
- Huang, C.; Mei, S.; Zhang, X.; Tian, X. Inflammatory Milieu Related to Dysbiotic Gut Microbiota Promotes Tumorigenesis of Hepatocellular Carcinoma. J Clin Gastroenterol 2023, 57, 782–788. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front Microbiol 2023, 14, 1098386. [Google Scholar] [CrossRef]
- El Tekle, G.; Andreeva, N.; Garrett, W.S. The Role of the Microbiome in the Etiopathogenesis of Colon Cancer. Annu Rev Physiol 2024, 86, 453–478. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Quintana, F.J. Tolerogenic dendritic cells. Semin Immunopathol 2017, 39, 113–120. [Google Scholar] [CrossRef]
- Roberti, M.P.; Yonekura, S.; Duong, C.P.M.; Picard, M.; Ferrere, G.; Tidjani Alou, M.; Rauber, C.; Iebba, V.; Lehmann, C.H.K.; Amon, L.; et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med 2020, 26, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: a diagnostic and therapeutic perspective. Cancer Biol Ther 2023, 24, 2240084. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Xia, Q. Role of gut microbiome in cancer immunotherapy: from predictive biomarker to therapeutic target. Exp Hematol Oncol 2023, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another's Growth. Curr Nutr Rep 2019, 8, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Stolte, M.; Bayerdorffer, E.; Morgner, A.; Alpen, B.; Wundisch, T.; Thiede, C.; Neubauer, A. Helicobacter and gastric MALT lymphoma. Gut 2002, 50 Suppl 3, III19–24. [Google Scholar] [CrossRef]
- Lowy, D.R.; Schiller, J.T. Preventing Cancer and Other Diseases Caused by Human Papillomavirus Infection: 2017 Lasker-DeBakey Clinical Research Award. JAMA 2017, 318, 901–902. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell 2019, 176, 998–1013.e1016. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote Immunosurveillance against Lung Metastases. Cell Rep 2018, 24, 3528–3538. [Google Scholar] [CrossRef]
- Mackowiak, P.A. Recycling metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health 2013, 1, 52. [Google Scholar] [CrossRef]
- Fotiadis, C.I.; Stoidis, C.N.; Spyropoulos, B.G.; Zografos, E.D. Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J Gastroenterol 2008, 14, 6453–6457. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol 2012, 12, 728–734. [Google Scholar] [CrossRef]
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am J Physiol Gastrointest Liver Physiol 2017, 312, G34–G45. [Google Scholar] [CrossRef]
- Martin, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vazquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep 2019, 9, 5398. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Liu, L.; Wang, B.; Walker, W.A.; Acra, S.A.; Yan, F. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem 2014, 289, 20234–20244. [Google Scholar] [CrossRef]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 2007, 9, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.S.; Badia, J.; Bosch, M.; Gimenez, R.; Baldoma, L. Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells. Front Microbiol 2016, 7, 1981. [Google Scholar] [CrossRef]
- Mills, J.P.; Rao, K.; Young, V.B. Probiotics for prevention of Clostridium difficile infection. Curr Opin Gastroenterol 2018, 34, 3–10. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Smits, L.P.; Bouter, K.E.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Nandi, D.; Parida, S.; Sharma, D. The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes 2023, 15, 2221452. [Google Scholar] [CrossRef]
- Hohmann, E.L.; Ananthakrishnan, A.N.; Deshpande, V. Case Records of the Massachusetts General Hospital. Case 25-2014. A 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med 2014, 371, 668–675. [Google Scholar] [CrossRef]
- Quera, R.; Espinoza, R.; Estay, C.; Rivera, D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn's disease and recurrent Clostridium difficile infection. J Crohns Colitis 2014, 8, 252–253. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Piha-Paul, S.A.; Medina, T.; Verschraegen, C.F.; Varterasian, M.; Brennan, A.M.; Riese, R.J.; Sokolovska, A.; Strauss, J.; Hava, D.L.; et al. Phase I Study of SYNB1891, an Engineered E. coli Nissle Strain Expressing STING Agonist, with and without Atezolizumab in Advanced Malignancies. Clin Cancer Res 2023, 29, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Din, M.O.; Danino, T.; Prindle, A.; Skalak, M.; Selimkhanov, J.; Allen, K.; Julio, E.; Atolia, E.; Tsimring, L.S.; Bhatia, S.N.; et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 2016, 536, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, L.; Lin, D.; Lai, X.; Wu, L.; Liao, X.; Liu, J.; Zeng, Y.; Liang, L.; Zhang, G.; et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J Cancer Res Clin Oncol 2022, 148, 2643–2652. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther 2023, 8, 35. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Dalal, R.; Sadhu, S.; Kumar, Y.; Kumar, S.; Gupta, S.K.; Tripathy, M.R.; Rathore, D.K.; Awasthi, A. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci Adv 2021, 7, eabg5016. [Google Scholar] [CrossRef]
- Overacre-Delgoffe, A.E.; Bumgarner, H.J.; Cillo, A.R.; Burr, A.H.P.; Tometich, J.T.; Bhattacharjee, A.; Bruno, T.C.; Vignali, D.A.A.; Hand, T.W. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 2021, 54, 2812–2824.e2814. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. The Human Microbiota and Skin Cancer. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Whelan, K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care 2011, 14, 581–587. [Google Scholar] [CrossRef]
- Szajewska, H.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Shamir, R.; Vandenplas, Y.; Weizman, Z.; European Society for Pediatric Gastroenterology, H.; Nutrition. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 2014, 58, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Denipote, F.G.; Trindade, E.B.; Burini, R.C. [Probiotics and prebiotics in primary care for colon cancer]. Arq Gastroenterol 2010, 47, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; Palmieri, B. Therapeutical use of probiotic formulations in clinical practice. Clin Nutr 2010, 29, 701–725. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Bacteria, food, and cancer. F1000 Biol Rep 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Zhang, C.; Le, A. Glucose Metabolism in Cancer: The Warburg Effect and Beyond. Adv Exp Med Biol 2021, 1311, 3–15. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Bultman, S.J. The microbiome and its potential as a cancer preventive intervention. Semin Oncol 2016, 43, 97–106. [Google Scholar] [CrossRef]
- Daschner, P.J.; Ross, S.; Seifried, H.; Kumar, A.; Flores, R. Nutrition and Microbiome Interactions in Human Cancer. J Acad Nutr Diet 2023, 123, 504–514. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J Cancer Prev 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Chen, Q.Y.; DesMarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu Rev Pharmacol Toxicol 2019, 59, 537–554. [Google Scholar] [CrossRef]
- Rayan, M.; Sayed, T.S.; Hussein, O.J.; Therachiyil, L.; Maayah, Z.H.; Maccalli, C.; Uddin, S.; Prehn, J.H.M.; Korashy, H.M. Unlocking the secrets: exploring the influence of the aryl hydrocarbon receptor and microbiome on cancer development. Cell Mol Biol Lett 2024, 29, 33. [Google Scholar] [CrossRef] [PubMed]
- Goedtke, L.; Sprenger, H.; Hofmann, U.; Schmidt, F.F.; Hammer, H.S.; Zanger, U.M.; Poetz, O.; Seidel, A.; Braeuning, A.; Hessel-Pras, S. Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells. Int J Mol Sci 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudlak, B.; Williams, M.J.; Jonsson, J.; Schioth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med 2022, 52, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Lou, H.; Liu, X.; Liu, P. Mechanism and implications of pro-nature physical activity in antagonizing psychological stress: the key role of microbial-gut-brain axis. Front Psychol 2023, 14, 1143827. [Google Scholar] [CrossRef]
- Drochioiu, G. Multifactorial Distress, the Warburg Effect, and Respiratory and pH Imbalance in Cancer Development. Stresses 2023, 3, 500–528. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Leigh, S.J.; Uhlig, F.; Wilmes, L.; Sanchez-Diaz, P.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Hyland, N.P.; Cryan, J.F.; Clarke, G. The impact of acute and chronic stress on gastrointestinal physiology and function: a microbiota-gut-brain axis perspective. J Physiol 2023, 601, 4491–4538. [Google Scholar] [CrossRef]
- Yu, Y.N.; Fang, J.Y. Gut Microbiota and Colorectal Cancer. Gastrointest Tumors 2015, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.S.; Doedens, A.; Burns, M.B. The promise and challenge of cancer microbiome research. Genome Biol 2020, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Jena, P.K. Precision dietary supplementation based on personal gut microbiota. Nat Rev Gastroenterol Hepatol 2019, 16, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis Model Mech 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H. Harness the functions of gut microbiome in tumorigenesis for cancer treatment. Cancer Commun (Lond) 2021, 41, 937–967. [Google Scholar] [CrossRef]
- Chanyi, R.M.; Craven, L.; Harvey, B.; Reid, G.; Silverman, M.J.; Burton, J.P. Faecal microbiota transplantation: Where did it start? What have studies taught us? Where is it going? SAGE Open Med 2017, 5, 2050312117708712. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Ahmadi Bashirzadeh, D.; Nazemalhosseini Mojarad, E.; Asadzadeh Aghdaei, H.; Norouzinia, M.; Shahrokh, S. Signature of Gut Microbiome by Conventional and Advanced Analysis Techniques: Advantages and Disadvantages. Middle East J Dig Dis 2020, 12, 5–11. [Google Scholar] [CrossRef]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O'Keefe, S.J.; Gaskins, H.R.; Teare, J.; et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




