Submitted:
01 October 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Carbon Mineralization and CO2 Emissions
1.2. Methane Consumption and Emissions
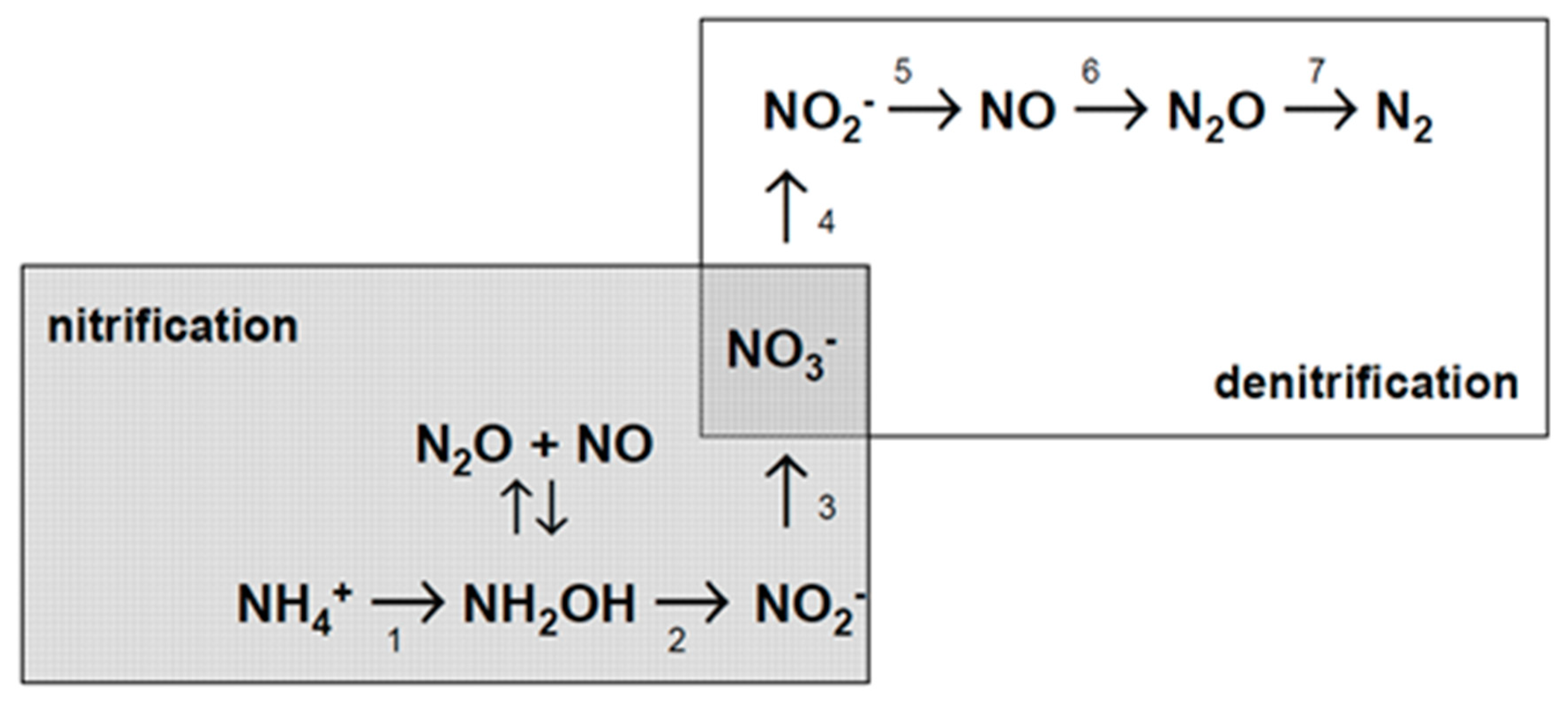
1.3. Soil N Turnover
2. Materials and methods
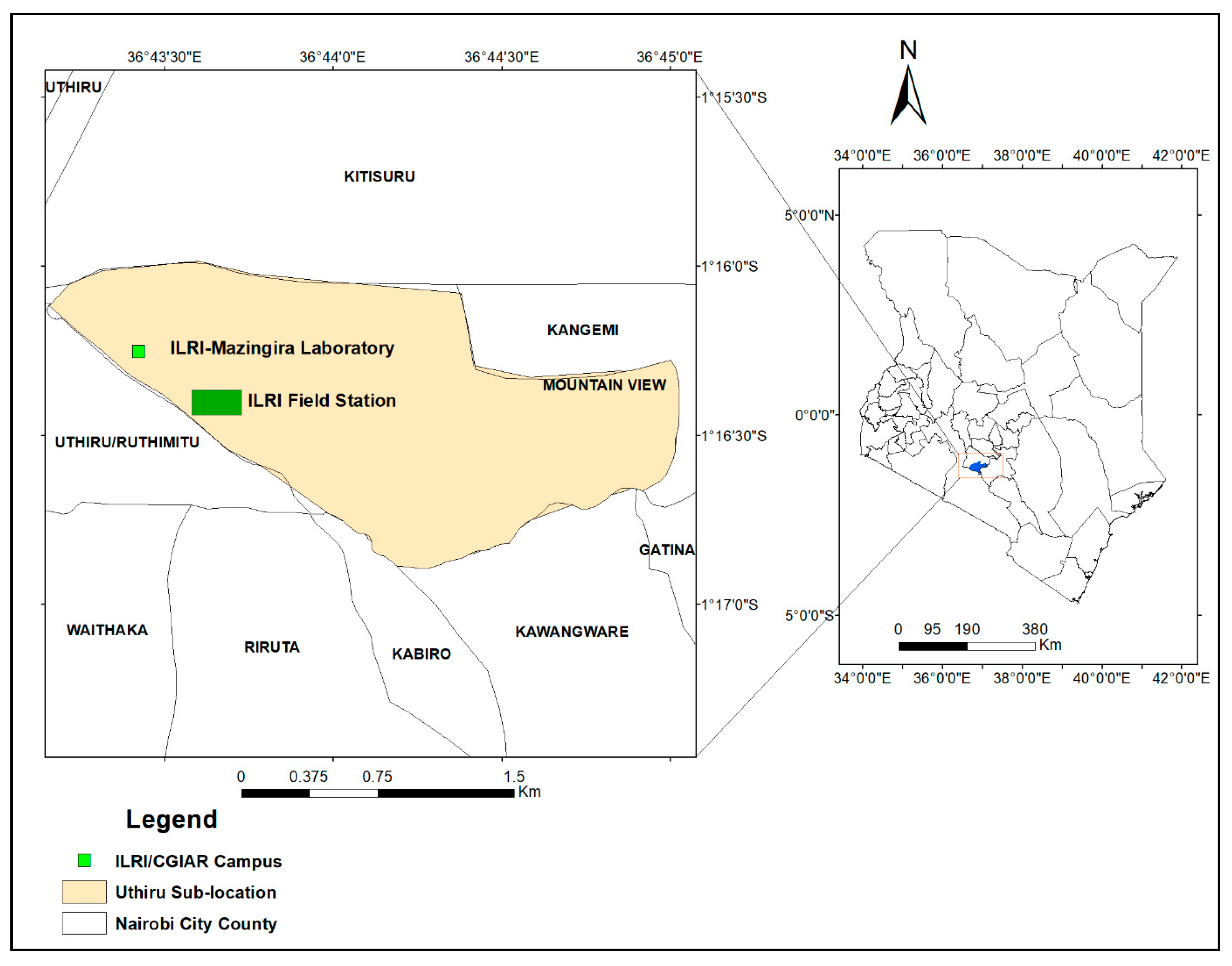
2.1. Description of the Study Site
2.2. Treatments and Experimental Design
2.3. Greenhouse Gas Sampling and Analysis
2.4. Yield Scaled Emissions
2.5. Brachiaria Brizantha Yields
2.6. Statistical Analysis
3. Results
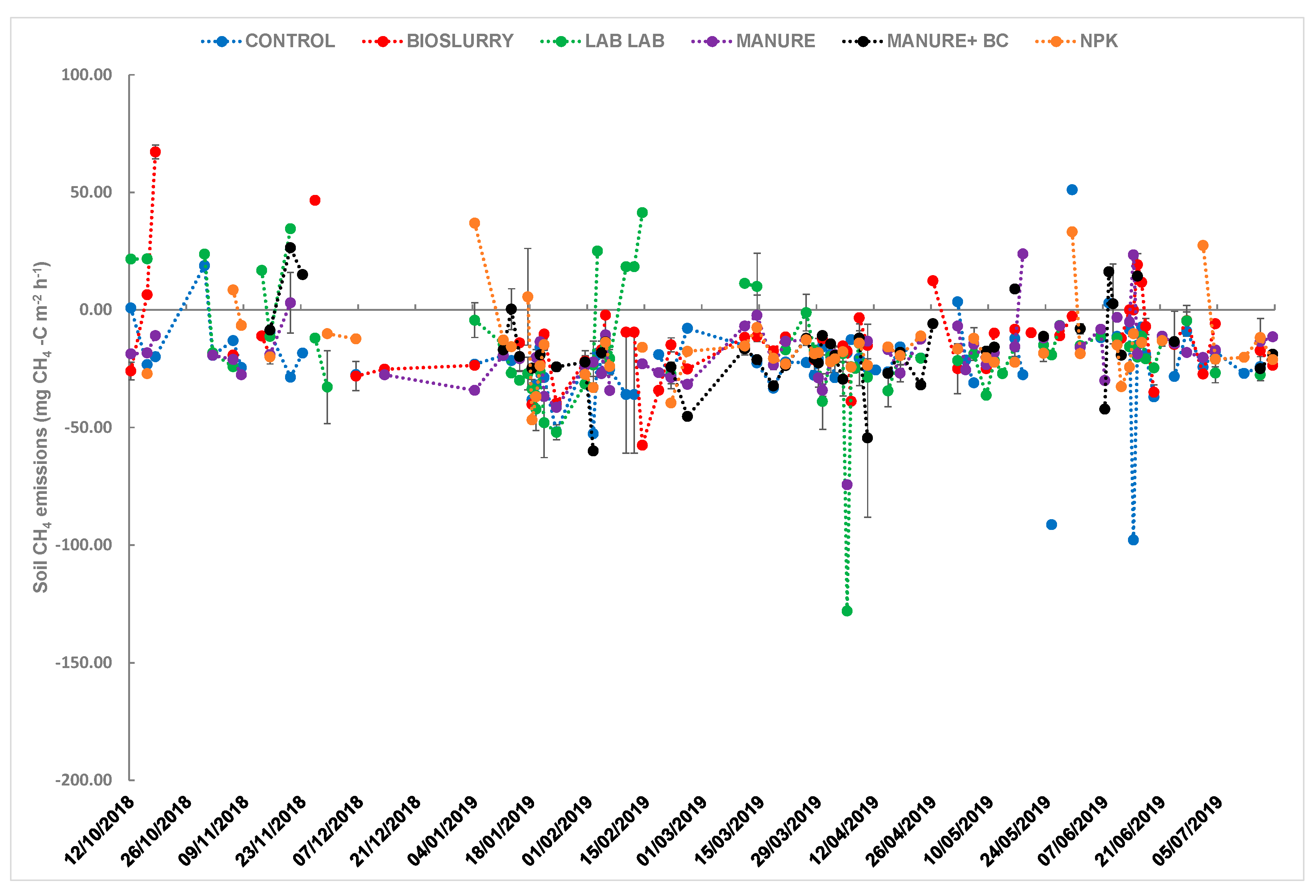
3.1. Effects of Soil Fertilization on Hourly CH4 Uptake
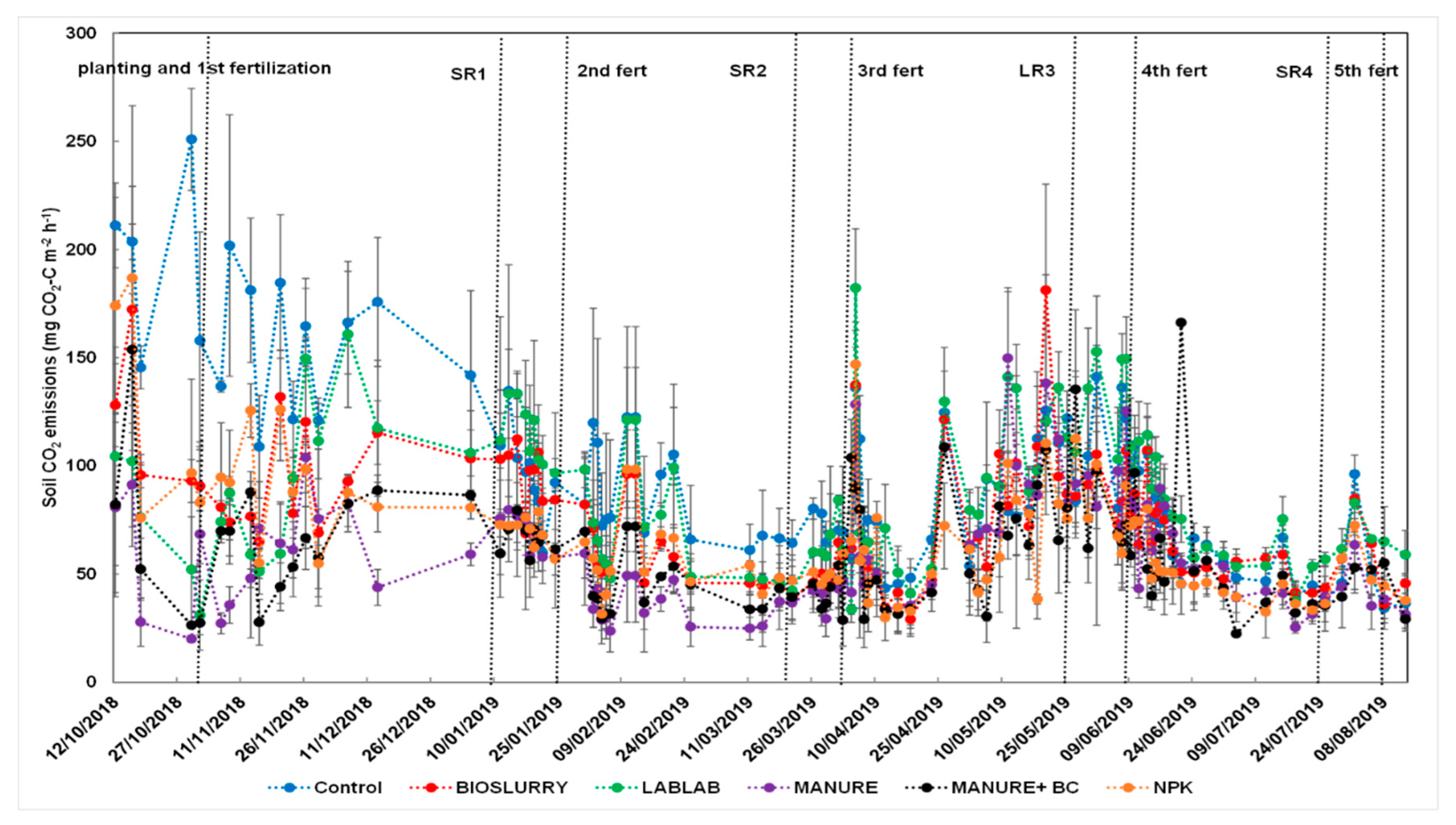
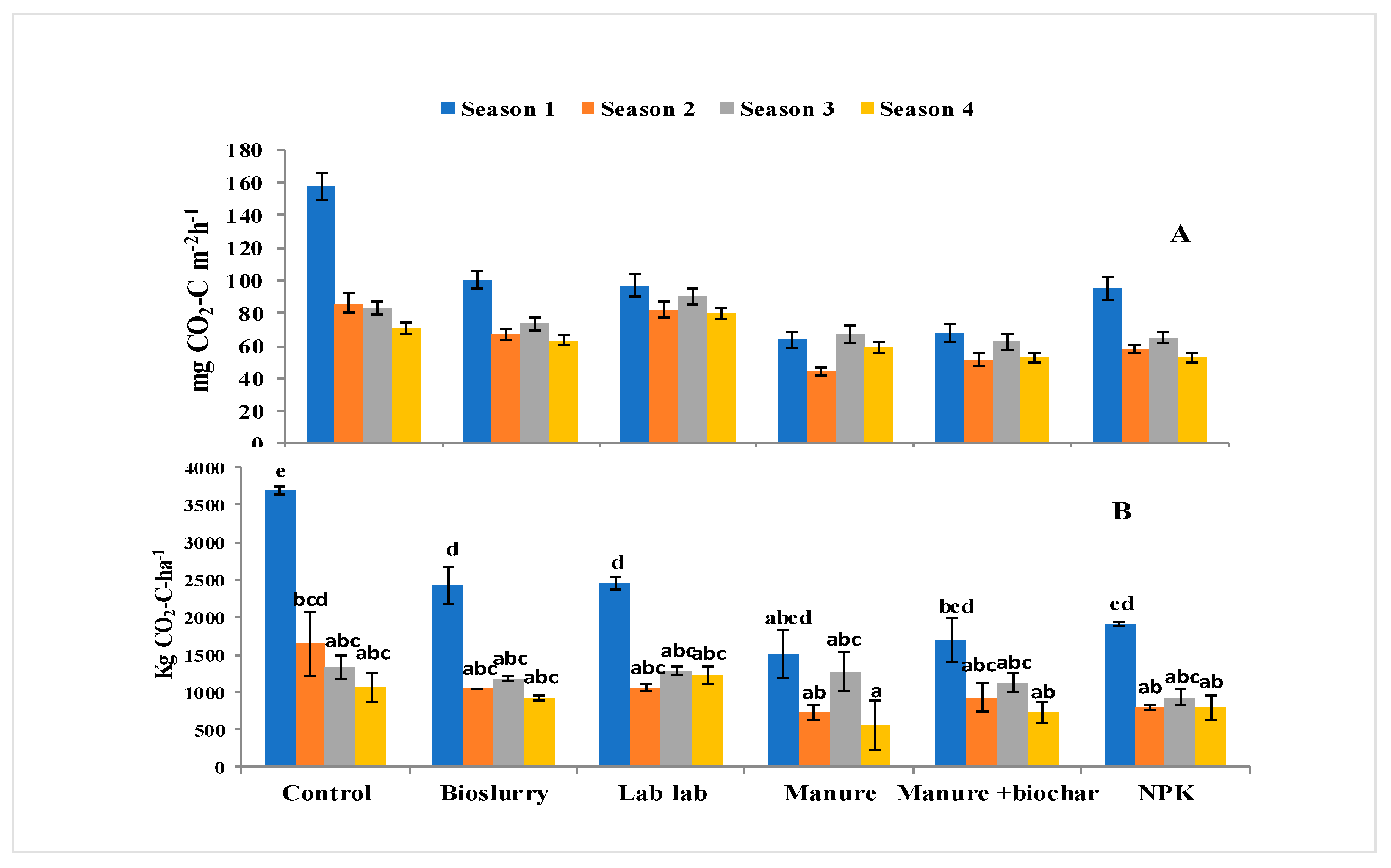
3.2. Effects of Soil Fertilization on Hourly and Cumulative CO2 Fluxes
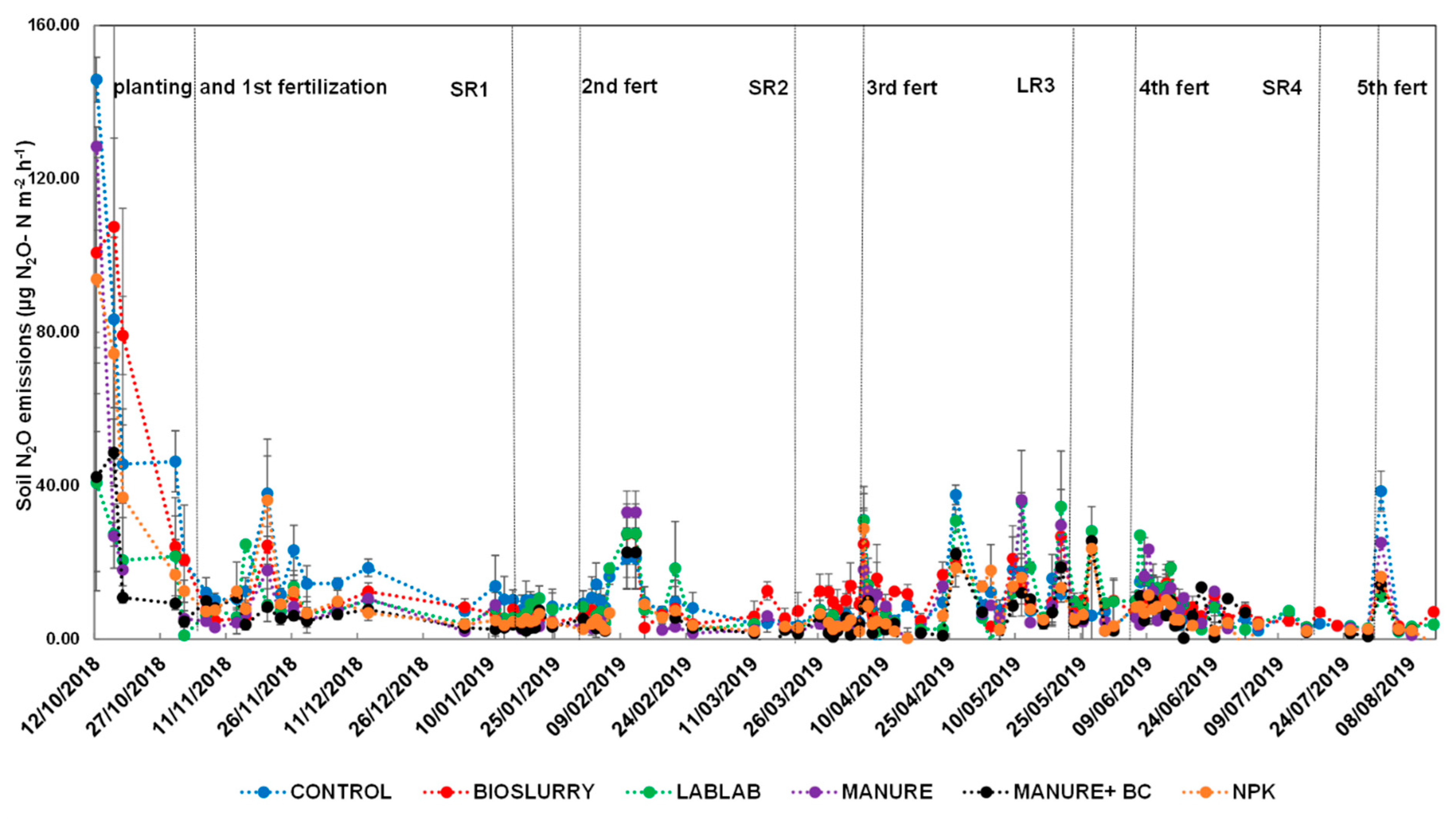
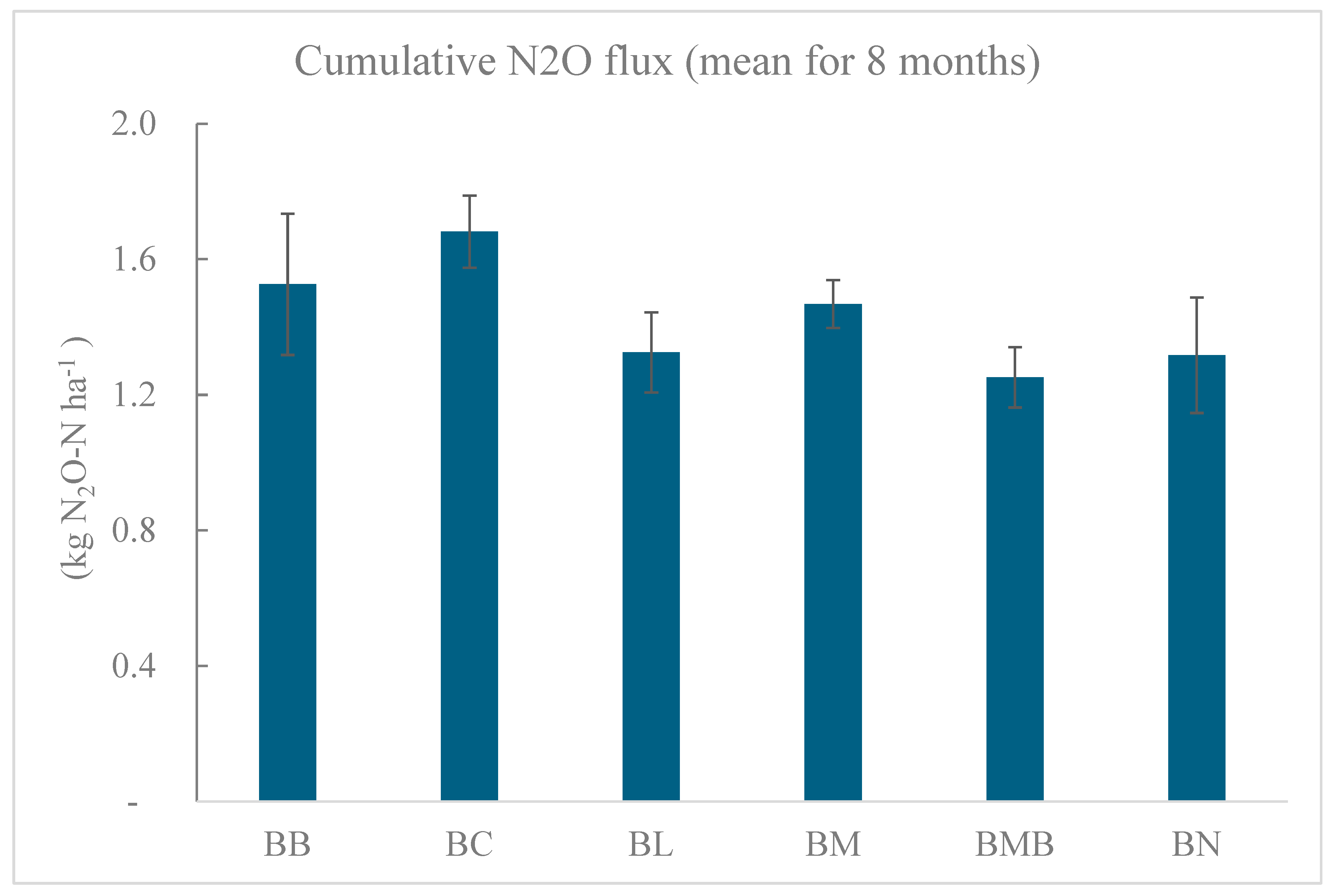
3.3. Effects of Soil Fertilization on Daily and Cumulative N2O Fluxes
3.4. Effects of Soil Fertilization on Yield-Scaled N2O Emissions
3.5. Effect of Fertilization and Harvesting on N2O and CO2 Emissions
4. Discussions
4.1. Effects of Soil Moisture and Temperature on Soil GHG Emissions
4.2. Effects of Organic and Inorganic Fertilizers on Cumulative N2O Fluxes
4.3. Yield-Scaled N2O Emissions
5. Conclusions
- Having recorded low N2O emissions when Brachiaria brizantha cv. xaraes is grown at 45 kg N ha-1 harvest-1 (or 225 kg N ha-1 yr-1 for 5 annual harvests) of fertilizer implies that this fertilization rate can be a good GHG mitigation strategy in tropical forage production.
- It is important to look at different rates of N fertilizer applications in evaluating the yields and emissions of GHG in forage crops. Further research can be conducted at varied fertilizer rates to evaluate the long-term effects of organic and mineral fertilizer on N2O fluxes.
- Spatial variations in forage GHG emissions in tropical Africa need to be assessed further to understand how various ecological zones respond to varied organic and inorganic fertilizers in terms of yields and GHG emissions.
References
- Baggs, E.; Stevenson, M.; Pihlatie, M.; Regar, A.; Cook, H.; Cadisch, G. Nitrous oxide emissions following application of residues and fertiliser under zero and conventional tillage. Plant Soil 2003, 254, 361–370. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. (2005). The importance of soil organic matter: Key to drought-resistant soil and sustained food production (No. 80). Food & Agriculture Org.
- Bouwman, A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosystems 1996, 46, 53–70. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. Environ. Sustain. 2011, 3, 389–395. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Cambardella, C.A. (2005). Carbon cycle in soils formation and decomposition. Encycl Soils Environ, 1(9780123485304), 170-175.
- Dhondt, K.; Boeckx, P.; Hofman, G.; Van Cleemput, O. Temporal and spatial patterns of denitrification enzyme activity and nitrous oxide fluxes in three adjacent vegetated riparian buffer zones. Biol. Fertil. Soils 2004, 40, 243–251. [Google Scholar] [CrossRef]
- Dick, J.; Kaya, B.; Soutoura, M.; Skiba, U.; Smith, R.; Niang, A.; Tabo, R. The contribution of agricultural practices to nitrous oxide emissions in semi-arid Mali. Soil Use Manag. 2008, 24, 292–301. [Google Scholar] [CrossRef]
- Ding, W.; Yagi, K.; Cai, Z.; Han, F. Impact of Long-Term Application of Fertilizers on N2O and NO Production Potential in an Intensively Cultivated Sandy Loam Soil. Water, Air, Soil Pollut. 2010, 212, 141–153. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Baggs. (2010). Do combined applications of crop residues and inorganic fertilizer lower emission of N 2 O from soil? August. [CrossRef]
- Hamdi, S.; Moyano, F.; Sall, S.; Bernoux, M.; Chevallier, T. Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biol. Biochem. 2013, 58, 115–126. [Google Scholar] [CrossRef]
- Hynes, R.K.; Knowles, R. Production of nitrous oxide by Nitrosomonas europaea: effects of acetylene, pH, and oxygen. Can. J. Microbiol. 1984, 30, 1397–1404. [Google Scholar] [CrossRef]
- Janzen, H.; Campbell, C.; Izaurralde, R.; Ellert, B.; Juma, N.; McGill, W.; Zentner, R. Management effects on soil C storage on the Canadian prairies. Soil Tillage Res. 1998, 47, 181–195. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Glagolev, M.V.; Nozhevnikova, A.N.; Conrad, R. Competition between homoacetogenic bacteria and methanogenic archaea for hydrogen at low temperature. FEMS Microbiol. Ecol. 2001, 38, 153–159. [Google Scholar] [CrossRef]
- Maljanen, M.; Liikanen, A.; Silvola, J.; Martikainen, P.J. Measuring N2O emissions from organic soils by closed chamber or soil/snow N2O gradient methods. Eur. J. Soil Sci. 2003, 54, 625–631. [Google Scholar] [CrossRef]
- Millar, N.; Ndufa, J.K.; Cadisch, G.; Baggs, E.M. Nitrous oxide emissions following incorporation of improved-fallow residues in the humid tropics. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Myhre, G.; Samset, B.H.; Schulz, M.; Balkanski, Y.; Bauer, S.; Berntsen, T.K.; Bian, H.; Bellouin, N.; Chin, M.; Diehl, T.; et al. Radiative forcing of the direct aerosol effect from AeroCom Phase II simulations. Atmospheric Meas. Tech. 2013, 13, 1853–1877. [Google Scholar] [CrossRef]
- Nicolardot, B.; Recous, S.; Mary, B. Simulation of C and N mineralisation during crop residue decomposition: A simple dynamic model based on the C:N ratio of the residues. Plant Soil 2001, 228, 83–103. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Wuta, M.; Nyamangara, J.; Smith, J.L.; Rees, R.M. Nitrous oxide and methane emissions from cultivated seasonal wetland (dambo) soils with inorganic, organic and integrated nutrient management. Nutr. Cycl. Agroecosystems 2014, 100, 161–175. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P. (2007). Nitrogen transformations. Soil microbiology, ecology and biochemistry. Elsevier, pp. 341-364.
- Sarkodie-Addo, J.; Lee, H.; Baggs, E. Nitrous oxide emissions after application of inorganic fertilizer and incorporation of green manure residues. Soil Use Manag. 2003, 19, 331–339. [Google Scholar] [CrossRef]
- Scheer, C.; Grace, P.R.; Rowlings, D.W.; Payero, J. Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management. Plant Soil 2012, 359, 351–362. [Google Scholar] [CrossRef]
- Segers, R. Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 1998, 41, 23–51. [Google Scholar] [CrossRef]
- Dasselaar, A.v.D.P.; Corré, W.J.; Priemé, A.; Klemedtsson, K.; Weslien, P.; Klemedtsson, L.; Stein, A.; Oenema, O. Spatial Variability of Methane, Nitrous Oxide, and Carbon Dioxide Emissions from Drained Grasslands. Soil Sci. Soc. Am. J. 1998, 62, 810–817. [Google Scholar] [CrossRef]
- Ding, W.-X.; Cai, Z.-C. Methane Emission from Natural Wetlands in China: Summary of Years 1995–2004 Studies. Pedosphere 2007, 17, 475–486. [Google Scholar] [CrossRef]
- Yiqi, L.; Zhou, X. (2010). Soil respiration and the environment. Elsevier.
- Zhang, Y.; Liu, J.; Mu, Y.; Xu, Z.; Pei, S.; Lun, X.; Zhang, Y. Nitrous oxide emissions from a maize field during two consecutive growing seasons in the North China Plain. J. Environ. Sci. 2012, 24, 160–168. [Google Scholar] [CrossRef]

| Treatment | CH4 (mg CH4-C m-2 h-1) | CO2 (mg CO2-C m-2h-1) | N2O (mgN2O-N m-2h-1) | ||
|---|---|---|---|---|---|
| Control | -21.86±17.97b | 94.76 ±19.32a | 12.95±3.61a | ||
| Lablab | -18.32 ±5.04b | 86.71±15.89a | 10.51±2.93ab | ||
| Bioslurry | -2.69 ±4.47a | 74.38 ±11.08b | 12.87±4.29a | ||
| NPK | -16.67±3.69b | 66.06 ±12.88bc | 10.00±3.30ab | ||
| FYM_BC | -17.84 ±6.05b | 58.43±14.48c | 6.70±2.44b | ||
| FYM | -18.30 ±2.91b | 58.39 ±15.67c | 8.20±2.34b | ||
| p-value | <0.01 | <0.01 | <0.01 | ||
| L.S.D. | 8.85 | 6.37 | 3.13 |
| Season | CH4 (mg CH4-C m-2 h-1) | CO2 (mg CO2-C m-2h-1) | N2O (mgN2O-N m-2h-1) | ||
|---|---|---|---|---|---|
| SR | -11.69 ±4.67ab | 97.89 ±20.45a | 18.40 ±5.41a | ||
| HD | -21.23 ±5.39b | 65.22 ±14.16c | 7.26 ±2.03b | ||
| LR | -19.07 ±6.42ab | 73.78 ±16.17b | 9.40 ±2.93b | ||
| CD | -11.43 ±13.87a | 63.21 ±13.32c | 7.36 ±0.20b | ||
| p-value | 0.01 | <0.01 | <0.01 | ||
| L.S.D. | 7.67 | 5.25 | 2.46 |
| Treatment | Season | CH4 (mg CH4-C m-2 h-1) | CO2 (mg CO2-C m-2h-1) | N2O (mgN2O-N m-2h-1) | ||
|---|---|---|---|---|---|---|
| Control | SR | -357±2.60a | 157.54 ±17.90a | 27.16 ±8.79a | ||
| HD | -506 ±12.60a | 86.1 ±11.08bcde | 9.20 ±2.96c | |||
| LR | -282 ±13.55a | 82.86 ±10.72 bcdef | 9.60 ±3.12c | |||
| CD | -302 ±58.22a | 70.67 ± defghi | 7.07 ±2.01c | |||
| Lablab | SR | -340 ±6.09a | 96.70 ±23.55 bc | 11.63 ±3.87bc | ||
| HD | -252 ±6.20a | 82.11 ±15.06bcdefg | 9.76 ±2.33bc | |||
| LR | -273 ±4.35a | 90.16 ±15.75bcd | 11.82 ±3.95bc | |||
| CD | -302 ±2.82a | 79.82 ±13.01bcdefgh | 8.57 ±2.89c | |||
| Bioslurry | SR | -292 ±3.83a | 100.10 ±28.76b | 24.37 ±5.69a | ||
| HD | -275 ±3.08a | 67.07 ±25.75efghij | 8.61 ±3.28c | |||
| LR | -297 ±4.41a | 73.62 ±14.75cdefghi | 11.23 ±3.37bc | |||
| CD | -105 ±6.36a | 63.26 ±13.06fghij | 8.90 ±2.86c | |||
| NPK | SR | -88 ±1.45a | 95.23 ±19.81bc | 20.97 ±7.86ab | ||
| HD | -354 ±4.35a | 57.91 ±9.45hij | 4.51 ±0.96c | |||
| LR | -179 ±3.26a | 64.94 ±12.92efghij | 8.37 ±1.93c | |||
| CD | -81 ±5.63a |
52.71 ±11.94ij | 5.76 ±2.24c | |||
| FYM-BC | SR | -76 ±5.06a | 67.81± 14.79defghij | 10.45 ±3.88bc | ||
| HD | -337 ±2.52a | 51.26±15.27ij | 5.29 ±1.88c | |||
| LR | -150 ±10.99a | 62.60 ±18.16fghij | 5.60 ±2.00c | |||
| CD | -234 ±9.60a |
52.84 ±14.08ij | 5.23 ±1.91c | |||
| FYM | SR | -540 ±8.99a | 63.81 ±17.92efghij | 11.24 ±2.36bc | ||
| HD | -431 ±3.59a | 44.33±8.37j | 4.95 ±0.79c | |||
| LR | -260 ±1.97a | 66.68 ±24.72efghij | 8.50 ±3.21c | |||
| CD | -268 ±0.57a |
58.87 ±18.49ghij | 7.74 ±2.94c | |||
| p-value (Treatment * Season) | 0.093 | <0.01 | <0.01 | |||
| L.S.D. | 13.112 | 6.36 | ||||
| CH4(g CH4-C ha-1) | CO2 (kg CO2-C ha-1) | N2O (Kg N2O -N ha-1) | ||
|---|---|---|---|---|
| Treatment | Control | -361.90±21.74ab | 1929±208.89c | 0.233±4.24a |
| Lablab | -291.80±4.87ab | 1504±73.82b | 0.141±3.26a | |
| FYM | -374.80±3.78a | 1015±250.61a | 3.801±2.32a | |
| FYM-BC | -199.10±7.05ab | 1117±185.33ab | 1.252±2.42a | |
| NPK | -175.30±3.67b | 1106±84.69a | 2.265±3.25a | |
| Bioslurry | -242.20±4.42ab | 1393±78.09ab | 0.26±3.80a | |
| P-value | 0.013 | <0.001 | 0.235 | |
| L.S.D. | 130.5 | 263.0 | 5.193 | |
| Season | SR1 | -282.4±4.67ab |
2279±169.72c | 0.32±5.41a |
| SR2 | -359.1±5.39a |
1030±134.05ab | 0.12±2.03a |
|
| LR3 | -240.0±6.42ab |
1184±120.37b | 0.16±2.93a | |
| SR4 | -215.3±13.87b | 883±163.45a | 2.14±0.2a | |
| P-value | 0.048 | <0.001 | 0.736 | |
| L.S.D. | 106.6 | 214.7 | 2.109 | |
| Treatment (Values are mean ± SE). | Season | |||
| SR | HD | LR | CD | |
| (g N2O-N kg-1 DM yield) | ||||
| Control | 4.47±0.05ab | 0.08±0.01a | 0.03±0.01a | 0.03±0.05a |
| Bioslurry | 7.05±0.04ab | 0.12±0.02a | 0.03±0.003a | 0.07±0.003a |
| Lablab | 21.35±0.04b | 0.10±0.02a | 0.07±0.01a | 0.07±0.03a |
| FYM | 0.74±0.01a | 0.19±0.01a | 0.02±0.01a | 10.23±0.26a |
| FYM-BC | 3.17±0.04ab | 0.04±0.01a | 0.02±0.01a | 0.02±0.04a |
| NPK | 2.77±0.15ab | 0.06±0.01a | 0.02±0.01a | 6.89±0.05ab |
| (n = 36) | Coefficients | Standard Error | t Stat | P-value | |
|---|---|---|---|---|---|
| N2O | (Constant) | -486.46 | 177.10 | -2.75 | 0.01 |
| Soil temperature | 1.08 | 0.68 | 1.59 | 0.12 | |
| C/N ratio | 39.77 | 17.29 | 2.30 | 0.03 | |
| Soil moisture | 0.96 | 0.37 | 2.60 | 0.01 | |
| Ammonium | 1.18 | 0.29 | 4.01 | 0.00 | |
| Nitrate | -0.04 | 0.13 | -0.35 | 0.73 | |
| CO2 flux | 0.43 | 0.07 | 6.62 | 0.00 | |
| CO2 | (Constant) | 729.28 | 329.40 | 2.21 | 0.03 |
| Soil temperature | -1.28 | 1.24 | -1.03 | 0.31 | |
| C/N ratio | -59.07 | 31.84 | -1.85 | 0.07 | |
| Soil moisture | -0.95 | 0.72 | -1.32 | 0.20 | |
| Ammonium | -1.03 | 0.63 | -1.64 | 0.11 | |
| Nitrate | -0.04 | 0.23 | -0.15 | 0.88 | |
| N2O flux | 1.39 | 0.21 | 6.62 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




