1. Introduction
Bladder cancer (BC) is the 10th most diagnosed cancer type in the world in 2020, with 573,278 patients diagnosed and 212,536 deaths. In Japan, 34,568 patients are reported to be diagnosed and 10,928 to die, with 8.7% of patients having metastasis at initial diagnosis [
1,
2]. Among patients treated with transurethral resection of bladder tumor (TURBT) and diagnosed with pathological Ta and T1, the proportion of patients who progressed to muscle-invasive BC was 10% and 35%, respectively [
3,
4]. Metastatic recurrence is reported in 22.5% of patients with invasive BC who have had curative TURBT [
5].
Combination therapy, including cisplatin (CDDP), has been a cornerstone of treatment for urothelial carcinoma, including urothelial BC, for decades. The most common platinum-based combination chemotherapy, gemcitabine and CDDP was introduced in 1999, achieving an objective response rate of 48.4% to 49.1%. However, more than 80% of patients subsequently progress, resulting in a median OS of 12.8 to 14.0 months and a 5-year survival rate of 13%, leading to an unfavorable prognosis [
2,
6,
7,
8]. CDDP exerts its effects by binds to purine residues and damages the deoxyribonucleic acid (DNA) of cancer cells, thereby inhibiting cell division and inducing apoptotic cell death. Additionally, it generates reactive oxygen species that contribute to cell death [
9].
Until the advent of pembrolizumab, there was no established standard of care following primary therapy with platinum drugs. Pembrolizumab is typically administered alone or in combination with docetaxel, paclitaxel, or vinflunine [
10,
11,
12]. JAVELIN Bladder 100 trial introduced of avelumab maintenance therapy in patients with no disease progression after platinum-based chemotherapy [
13,
14]. Furthermore, the introduction of the antibody-drug conjugate enfortumab vedotin will expand the options for BC treatment [
15,
16,
17]. Despite recent advancements in various therapies, platinum drugs remain a key treatment, as a significant percentage of patients with advanced BC will use them during their lifetime. The tumor microenvironment has emerged as a factor in drug resistance. In several cancer species, growth inhibition and drug resistance have been associated with acidic environments [
18,
19]. In the context of BC, hypoxia has been identified as a trigger for drug resistance; however, there are few reports addressing the impact of acidic environments on this phenomenon [
20,
21,
22].
Our objectives were to investigate tumor microenvironment factors that impair CDDP sensitivity in bladder urothelial cancer, with a specific focus on tumor potential hydrogen (pH). To the best of our knowledge, this study is the first report on an association between an acidic environment and CDDP resistance in BC cancer.
2. Materials and Methods
2.1. Cell Lines, Cell Culture and pH Adjustment Cell Lines, Cell Culture and pH Adjustment
We used the BC cell lines HT1376, T24, and human embryonic kidney 293 (HEK293) cells, which were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). HT1376 cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) , 1% MEM non-essential amino acids (Gibco; Thermo Fisher Scientific, Inc.), 1% MEM sodium pyruvate solution 100 mM (Gibco; Thermo Fisher Scientific, Inc.), and 90 µg/mL kanamycin at 37 °C in an atmosphere of 5% CO2. The potential hydrogen (pH) of complete medium was adjusted to 0.5 intervals from pH 6.0 to pH 7.5 with hydrochloric acid, and the pH was measured by a pH monitor LAQAtwin (HORIBA, Kyoto, Japan). The amount of HCl required to reduce the pH of 40 ml of culture medium is shown in
Supplementary Figure S1. CDDP was purchased from FUJIFILM Wako Chemicals U.S.A. Corporation.
2.2. Cell Viability Assay
Cell viability was evaluated by MTS assay with a tetrazolium compound according to the manufacturer’s instructions. Cells were seeded in 96-well plates at 2x103 cells/well, incubated in 100 μl of unadjusted medium for 3 hours, and then treated with CDDP for 0, 24, 48, and 72 h, in each pH environment. 10 µL of CellTiter 96® AQueous One Solution Reagent (Promega Corporation, Madison, WI, USA) was added to each well and incubated at 37 °C for 2 h. The absorbance was quantified at 490 nm using an IMARK microplate reader (Bio-Rad Laboratories, Inc.). Chloroquine (phosphate) (14194) was purchased from the Cayman chemical company (Ann Arbor, MI, USA). Inhibitory concentration 50 (IC50) was calculated using GraphPad Prism software (GraphPad Software version 8.0, Inc., San Diego, CA, USA). Data were analyzed using one-way ANOVA with Dunnett’s test for multiple comparisons. Statistical analysis was calculated using GraphPad Prism software.
2.3. Cell Migration Assay
Cells were spread at full confluence in 6-well plates and scratched with a p1000 pipet tip after 3 hours of culture, then washed with PBS, and incubated for 24 hours in pH 6.0 and pH 7.5 environments separated with and without CDDP administration. Scratch assay area measurements were calculated using the software ImageJ (NIH, Bethesda, MD, USA).
2.4. Western Blotting
Subconfluent cell cultures were washed with cold PBS and lysed using Cell Lysis Buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) containing Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO, USA). The cell lysates were incubated on ice for 30 min, then clarification of the lysates by centrifugation at 15,000× g for 30 min at 4 °C, and the supernatants were collected. The protein concentration was determined using the Bradford method. Each 20 µg of protein was separated on a 4-15% SDS-polyacrylamide gel and transferred to a PVDF membrane. After blocking with 10% skimmed milk in TBS, the membranes were incubated with primary antibodies, and horseradish peroxidase-labeled secondary antibodies for one hour at room temperature. Immunolabelling bands were visualized using the Clarity Max Western ECL Substrate (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The antibodies and concentrations are shown in
Table1.
2.5. Flow Cytometry Analysis
For cell cycle assessment, cells were harvested 24h after CDDP administration. Cells were washed twice with cold PBS and fixed in ethanol at 4 °C for 30min. After the fixed cells were washed twice with cold PBS, 1000 µL of FxCycle™ PI/RNase Staining Solution (Invitrogen; Thermo Fisher Scientific, Inc.) was added and incubated at room temperature in the dark for 30 min. Flow cytometry was performed to assess the cell cycle using a BD FACSCelesta™ Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Data analysis was performed using the FCS Express ™ (version 7.22.0031) (De Novo Software, Pasadena, CA).
3. Results
3.1. BC Cells Become More Resistant to CDDP as the Acidity of the Medium Increases
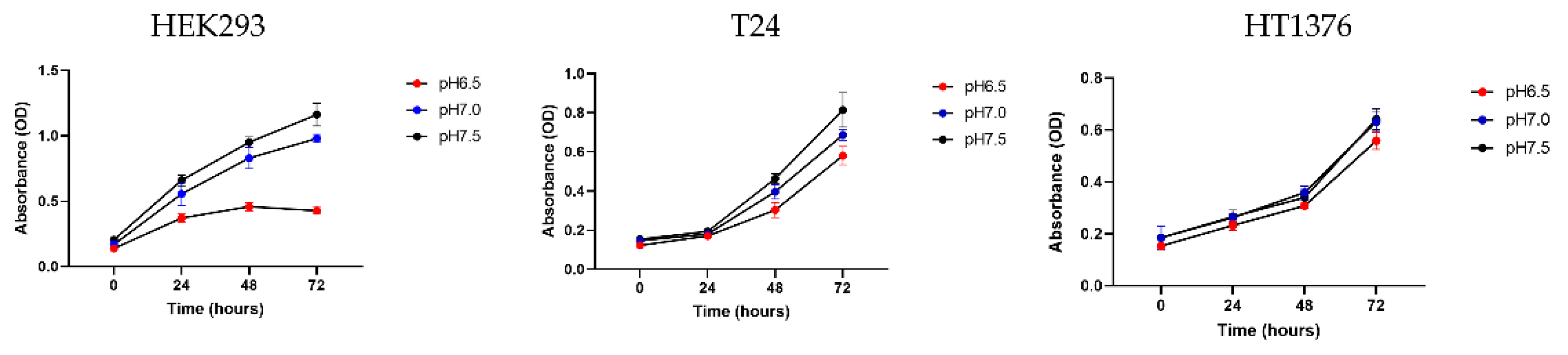
The first step was to cultivate HEK293, T24 and HT1376 cells for 72 hours in medium adjusted to pH 6.5, pH 7.0 and pH 7.5, respectively, to investigate differences in the acidic tolerance of normal cells and BC cells. Normal cells were severely inhibited in proliferation when exposed to an acidic environment, whereas BC cells showed resistance. In particular, HT1376 was more robust to acid toxicity than T24, so we decided to use HT1376 in our subsequent experiments (
Figure 1).
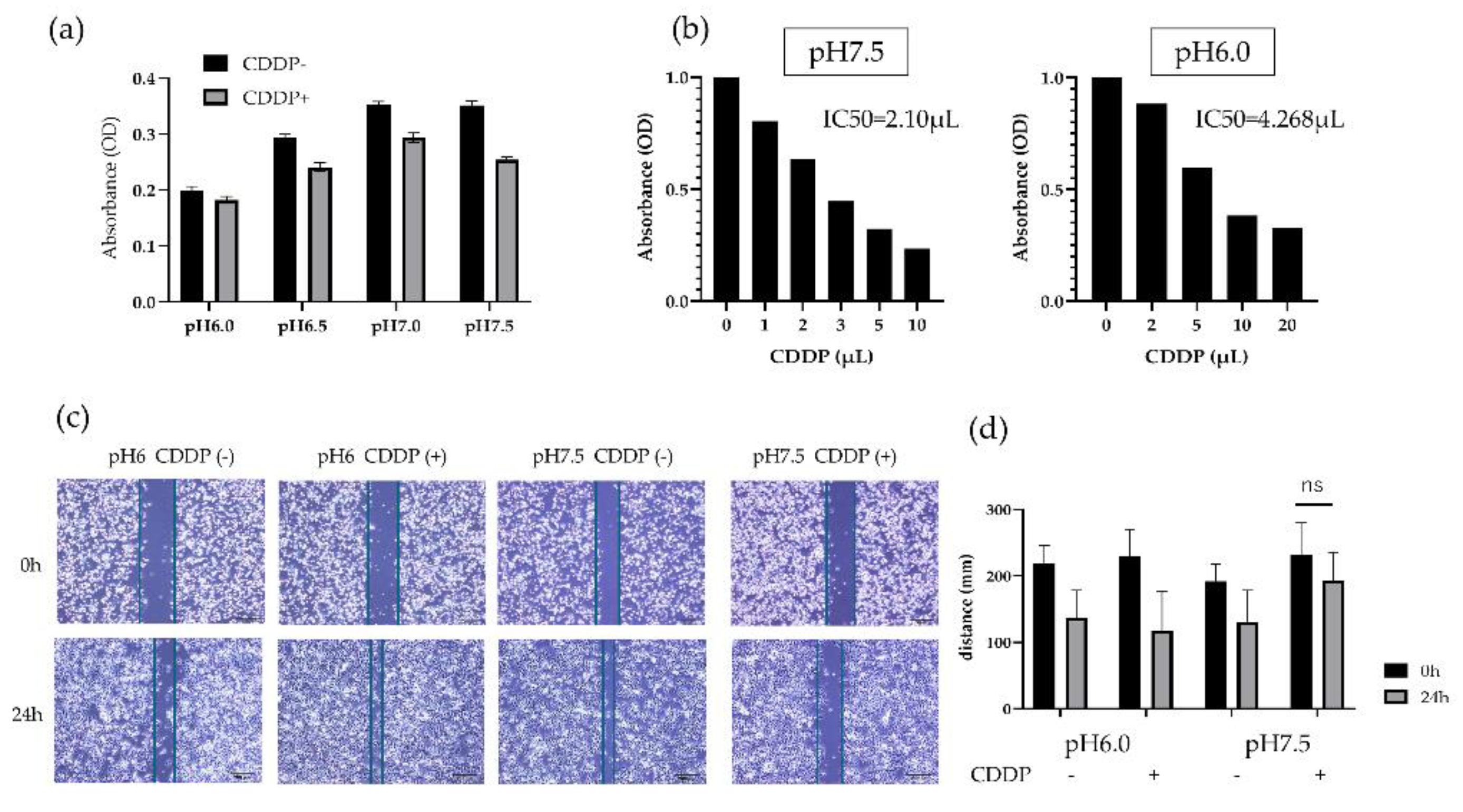
HT1376 was conditioned in culture environments ranging from pH 6.0 to pH 7.5 and incubated for 24 hours, it showed a tendency to proliferate in normal environments compared to acidic environments. However, the administration of CDDP did not influence the growth of HT1376 cultured in the acidic environment, whereas CDDP caused growth inhibition in the cells cultured in the neutral pH environment (
Figure 2a). Then, HT1376 were cultured in pH 6.0 and pH 7.5 culture medium and various CDDP concentrations were added to the cells. The CDDP IC50 for HT1376 cells cultured in an acidic environment was 4.268 mg/μL, which was twice as much as IC50 for the cells at normal pH. Based on the results, a basic dose of 2 µl of CDDP was used in subsequent experiments (
Figure 2b).
These results showed that the acidic environment has some positive effects on CDDP resistance in BC cells.
3.2. CDDP Treatment in an Acidic Environment Does Not Influence Mobility of BC Cells
In the migration assay, cell mobility under acidic and normal conditions was not significantly different, whereas CDDP treatment impaired mobility capacity only under neutral pH. An acidic environment confers an advantage to BC cells not only in terms of survival but also in terms of mobility (
Figure 2c-d).
3.3. Bcl-2 Expression Is Activated and Autophagy Is Suppressed in BC Cells with an Acidic Environment
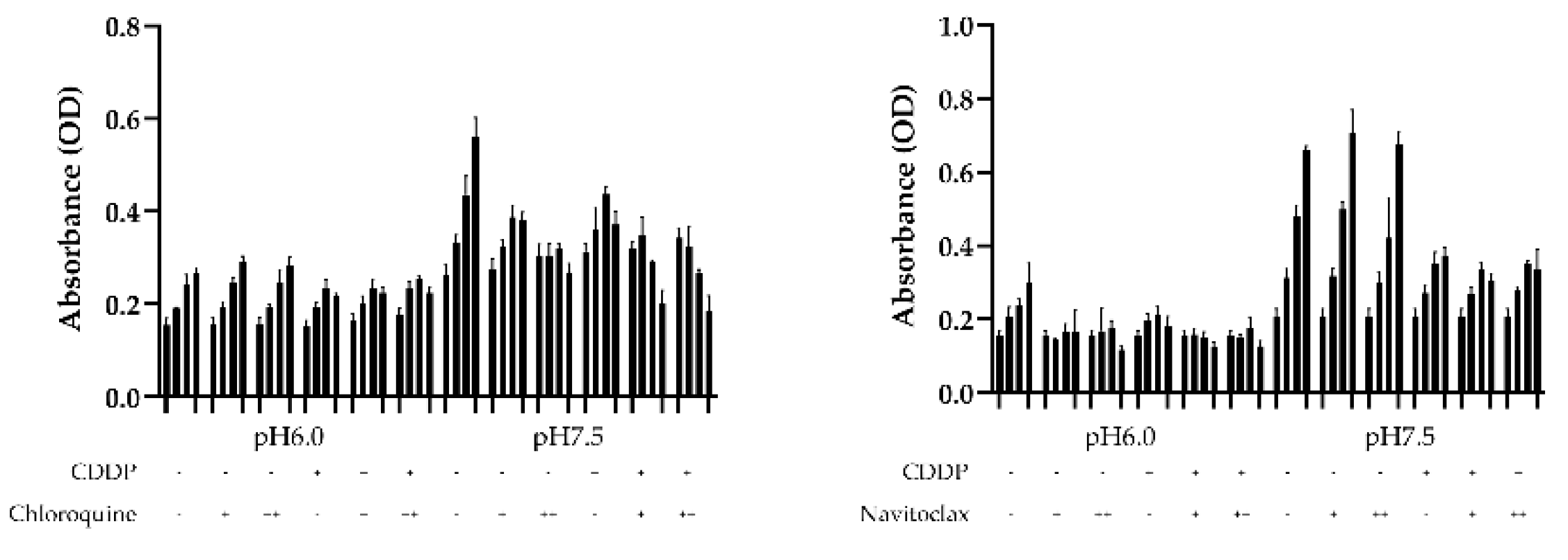
To check the influence of autophagy and apoptosis on the survival of BC cells under low pH conditions, we performed a survival assay using the autophagy inhibitor chloroquine and the bcl-2 inhibitor navitoclax to determine which pathways influence CDDP resistance under acidic conditions. When chloroquine was administered under normal pH conditions, HT1376 growth was inhibited in a concentration-dependent manner with or without CDDP administration. This finding was not observed in an acidic environment. BC cells cultured in an acidic environment demonstrated decreased survival when treatment with navitoclax, but this effect was not observed under neutral pH. These findings suggest that the inhibition of autophagy in urothelial carcinoma cells in acidic environments may be linked to decreased survival (
Figure 3).
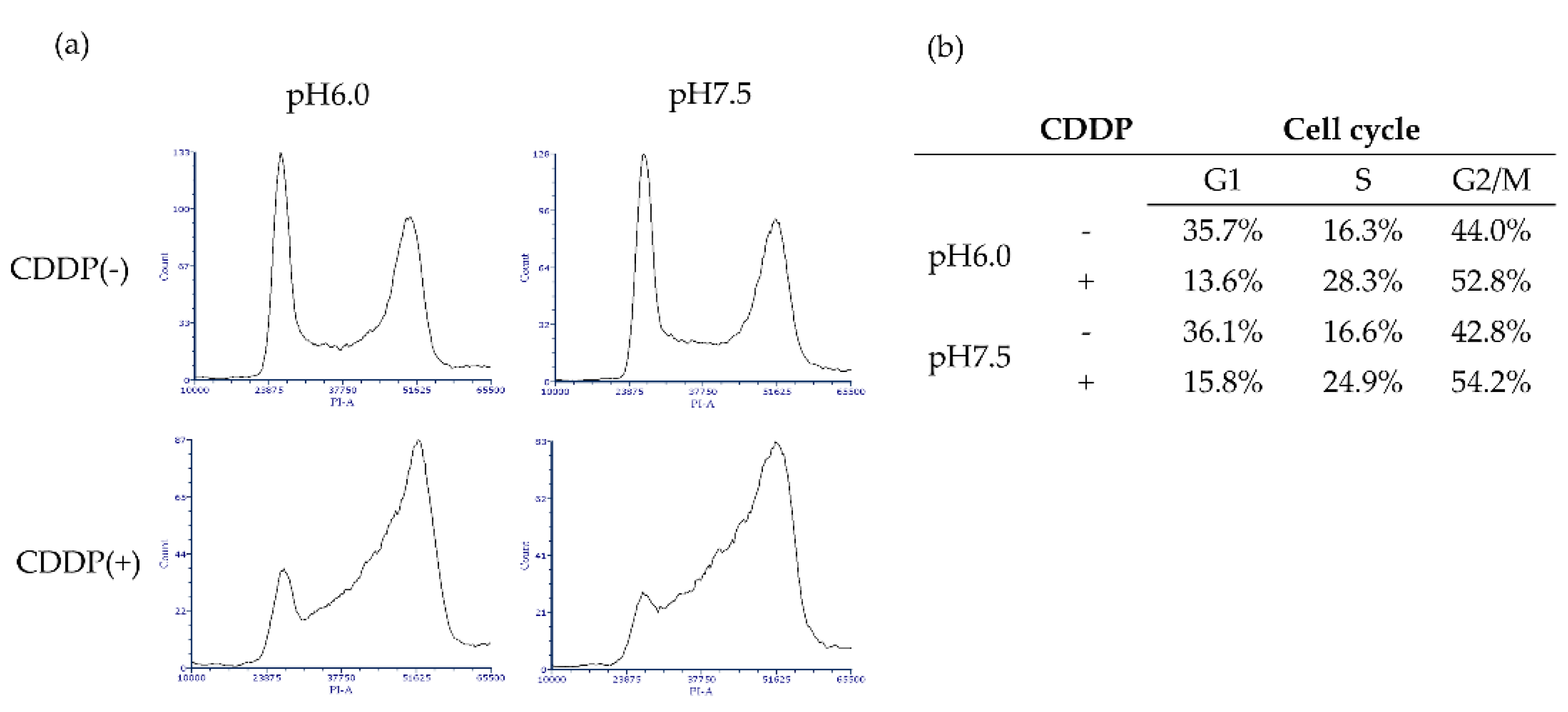
Cell cycle analysis using flow cytometry showed no changes due to acidity under non-drug treatment conditions. The CDDP treatment caused bladder cancer cells to be arrested in the S phase and G2/M phase, with a particularly high incidence of S-phase arrest under neutral pH conditions (
Figure 4).
3.4. Bcl-2 and XIAP High Expression Suppresses Apoptosis and Autophagy-Induced Death in BC Cells
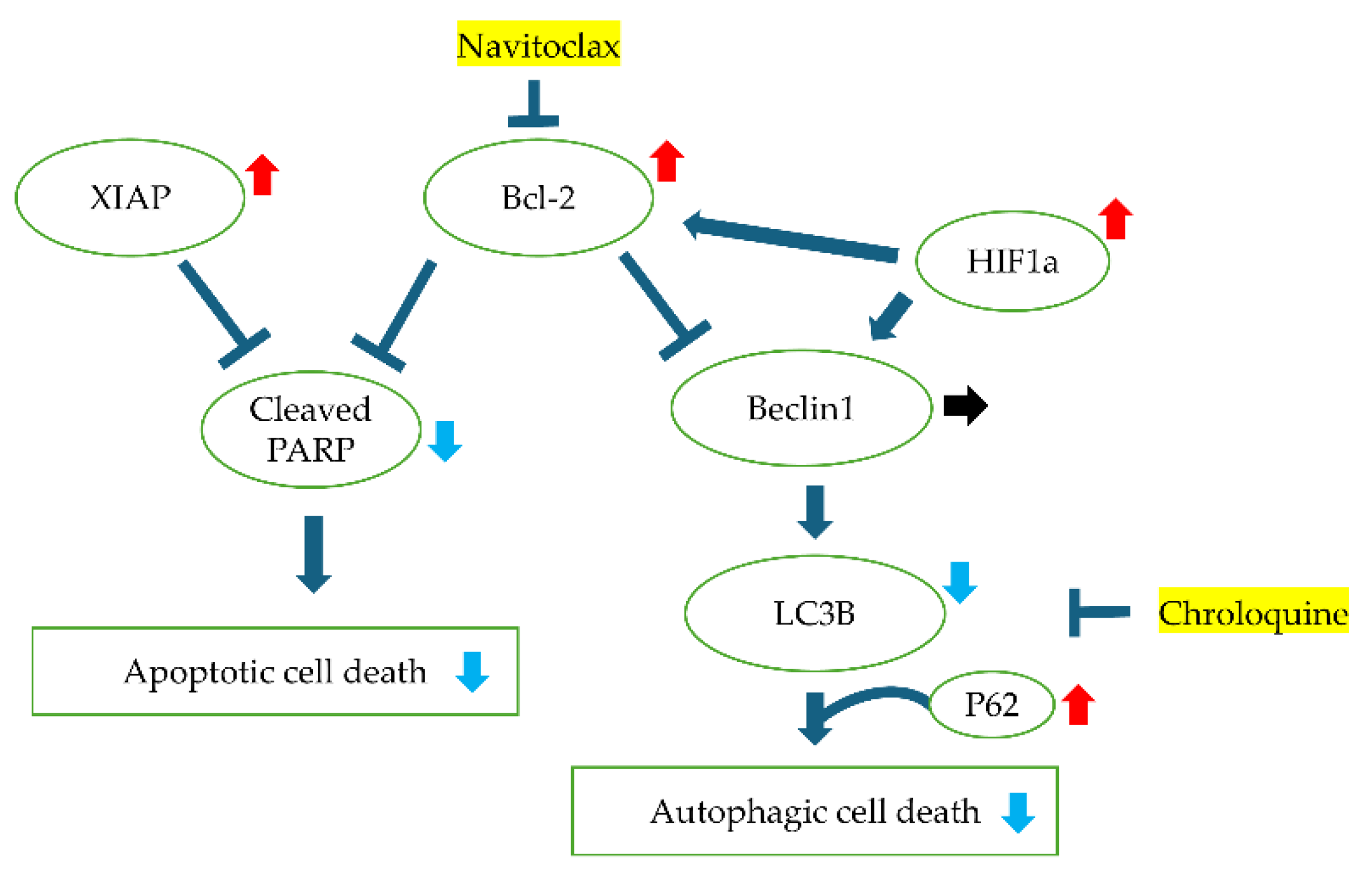
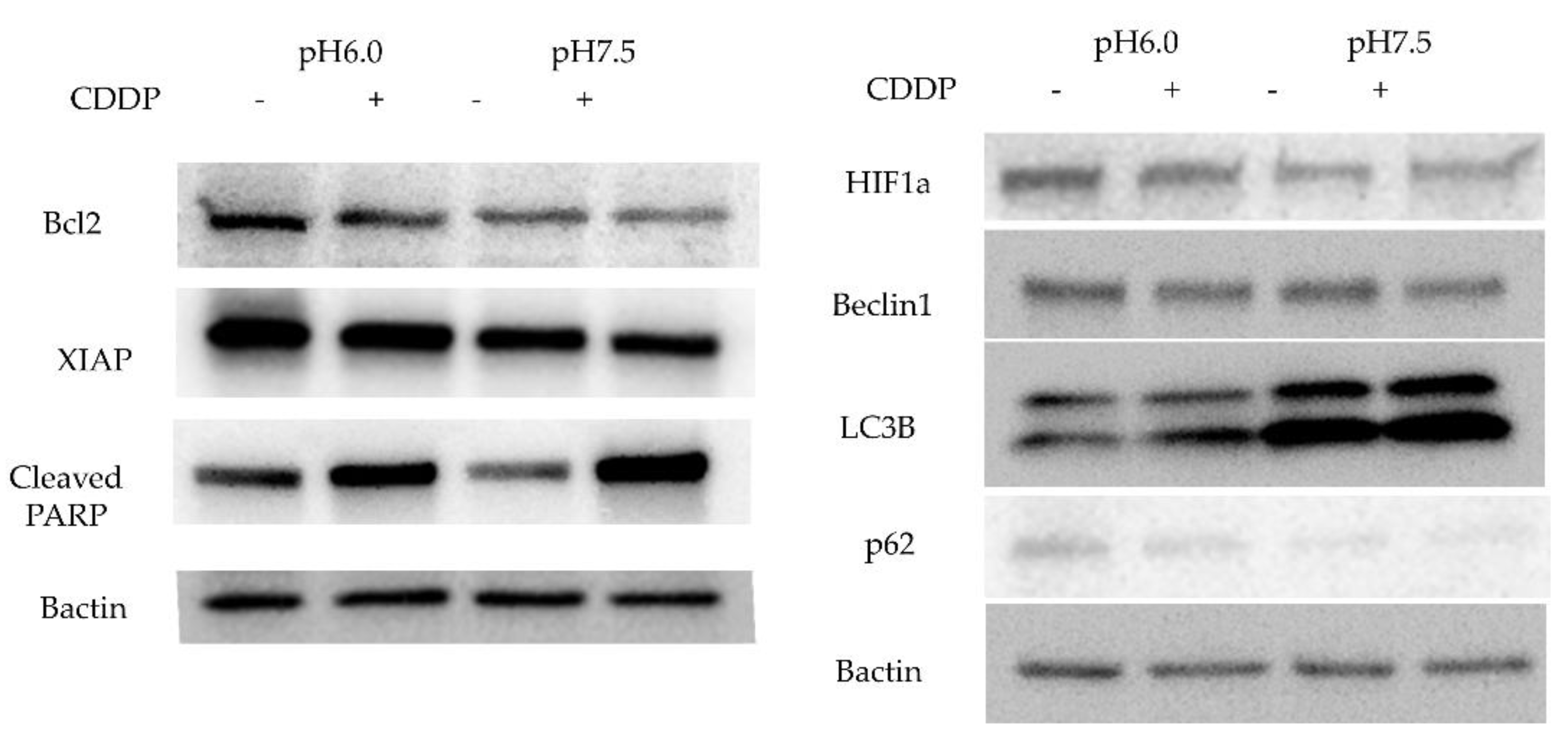
Then, we studied the expression of apoptosis-related proteins expression by Western blotting. Bcl-2 expression was stronger in an acidic environment and was attenuated by addition of CDDP. Cleaved PARP, the final step in the apoptosis cascade, showed higher expression in the acidic environment without CDDP, and higher expression in the normal pH with CDDP. In addition, XIAP expression was stronger in acidic environment and attenuated by CDDP treatment. Since chloroquine was effective only in neutral pH medium, we checked the expression of LC3B, a protein involved in the formation of the membrane of autophagosomes, which was elevated in the neutral pH and slightly increased further with CDDP administration. P62, a protein that direct unwanted intracellular material to autophagosomes, was elevated in acidic environments and slightly decreased with CDDP treatment. Beclin1 expression was not influenced by the acidity of the culture and was slightly diminished by anticancer drug treatment. The acidic environment also facilitated HIF1a expression (
Figure 5).
4. Discussion
In normal tissues, the extracellular pH is maintained at approximately 7.4; however, the pH around tumor cells decreases to between 6.7 and 7.2 [
23,
24]. Cells located at a distance from blood vessels may experience even greater acidification, potentially dropping to pH 5.6 due to the the accumulation of lactate and protons [
23,
25]. A well-known contributing factor to this is a phenomenon known as the Warburg effect, in which cancer cells hyperactivate their glycolytic system even in an oxygen-rich environment, leading to increased lactate production and subsequent acidification [
26].
The urothelium can be influent by urine pH because it reabsorbs urine into the bloodstream; this is likely true for urothelial carcinoma, which is constantly exposed to urine [
27]. Acid urine has been associated with poorer postoperative prognosis in both upper urinary tract cancer and BC [
28,
29]. Additionally, a higher recurrence rate has been observed in non-muscle invasive BC treated with intravesical mitomycin C in conjunction with lower urinary pH [
21]. HIF1a-mediated autophagy has also been implicated in the development of gemcitabine resistance in BC [
20]. Conversely, some studies suggest that acidic urine is associated with an inhibition of the growth of BC cells [
30]. In other cancer types, such as breast cancer and squamous cell carcinoma, growth inhibition has been reported in acidic environments, although drug resistance has been reported to be enhanced [
18,
19].
In our study, we found that BC cells proliferation was inhibited in an acidic environment. Notably, we also demonstrated for the first time that CDDP resistance is increased under acidic pH conditions. Furthermore, scratch assays showed that CDDP administered in an acidic environment did not impair the mobility of HT1376 cell. One hypothesis for this drug resistance is that the weakly basic nature of CDDP prevents it from effectively reaching its intracellular targets in an acidic environment [
31]. However, given that oxidative stress is a primary mechanism of action for CDDP, further investigation into alternative resistance mechanisms is warranted [
9].
To explore the mechanisms underlying drug resistance in BC under acidic conditions, we utilized the autophagy inhibitor chloroquine and Bcl-2 inhibitor navitoclax. Navitoclax, an orally available Bcl-2 inhibitor, first reported by Chang J et al. is a known to induce apoptosis [
32,
33], while chloroquine, commonly used as an antimalarial agent, is known to inhibit autophagy by blocking the fusion of autophagosomes and lysosomes [
34]. In our experiments, navitoclax did not significantly impact the proliferation of BC cells in acidic environments, nor did chloroquine. In cell cycle analysis using flow cytometry, CDDP treatment under neutral conditions resulted in a greater transition to the S phase compared to acidic conditions. We believe this may be due to a higher incidence of DNA damage. Western blotting showed that Bcl-2 protein expression was indeed enhanced and that cleaved PARP, downstream of the cascade, was suppressed in an acidic environment. XIAP is the most important member of the inhibitor of apoptosis protein (IAP) family and is known for its inhibition of apoptosis through directly blocking caspases [
35]. High expression of XIAP has been linked to poor prognosis in a variety of cancer types, including bladder and breast cancer, and has been reported to cause resistance to chemotherapy and radiation therapy [
36,
37,
38]. Our findings indicate that BC cells in acidic environments not only exhibit increased Bcl-2 expression but also upregulate XIAP, suggesting the activation of multiple anti-apoptotic pathways. Bcl-2 is known to negatively regulate autophagy by binding to Beclin1 [
39], which is phosphorylated by AMP-activated protein kinase to promote autophagosome maturation [
40]. Light chain 3 (LC3) is a subunit of the neuronal microtubule-associated proteins (MAPs), MAP1A and MAP1B [
41]. LC3B is a membrane protein essential for autophagosome formation and serves as a reliable biomarker for autophagic activity [
42,
43]. p62/SQSTM1 is responsible for recognizing ubiquitin chains and directing specific proteins and cell organelles to the sequestration membrane of the autophagosome [
44]. Our results showed that BC cells cultured in acidic medium exhibited decreased LC3B activity and elevated p62/SQSTM1 levels, reflecting a reduction in autophagosome formation and an accumulation of p62/SQSTM1. We anticipated that Beclin1, which mediates autophagosome formation, would decrease under acidic conditions; however, we observed no significant change in its expression, although a slight reduction was noted with CDDP treatment. This may be attributed to the activation of Beclin1 by HIF1α. The role of HIF1α in our experiments, where oxygen concentrations remained unchanged, warrants further investigation. We summarized the effects of CDDP administered in an acidic environment on apoptosis and autophagy pathways in BC cells in a diagram (
Figure6).
Figure 6.
Graphical schema illustrating intracellular signaling pathways and the interactions of various proteins under acidic pH conditions and cisplatin (CDDP) treatment. The schema highlights key proteins involved in apoptosis, autophagy, and hypoxia response, depicting how acidic environments and CDDP influence their expression and functional relationships.
Figure 6.
Graphical schema illustrating intracellular signaling pathways and the interactions of various proteins under acidic pH conditions and cisplatin (CDDP) treatment. The schema highlights key proteins involved in apoptosis, autophagy, and hypoxia response, depicting how acidic environments and CDDP influence their expression and functional relationships.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Figure S1: Correlation between pH of culture medium and hydrochloric acid; Figure S2: Full-length images of Western blot analyses.
Author Contributions
Conceptualization, K.H., V.B. and Y.T.; validation, K.H., V.B., A.K., Y.S., M.M. and Y.T.; formal analysis, K.H.; investigation, K.H. and Y.S.; writing—original draft preparation, K.H.; visualization, K.H.; supervision, V.B. and Y.T.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its
Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49. [CrossRef]
- AP AB, Clark O, Turnure M, Moreira ES, Yuasa A, Sugiyama S, et al. Treatment patterns in metastatic bladder cancer in Japan: results of the CancerMPact((R)) survey 2020. Future Oncol. 2024;20:603-11.
- Lachand AT, Texier J, Texier P. Surveillance and prognosis of stage T1 superficial bladder tumours. A homogeneous series of 89 cases followed for 1 to 22 years. Prog Urol. 2001;11:472-7.
- Lachand AT, Texier J, Texier P. Surveillance and prognosis of stage Ta superficial bladder cancers. A homogeneous series of 138 cases followed for 1 to 18 years. Prog Urol. 2001;11:466-71.
- Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. Journal of Clinical Oncology. 2001;19:666-75.
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602-8. [CrossRef]
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol. 2023;41:3881-90. [CrossRef]
- Dogliotti L, Carteni G, Siena S, Bertetto O, Martoni A, Bono A, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol. 2007;52:134-41. [CrossRef]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-78. [CrossRef]
- Raggi D, Miceli R, Sonpavde G, Giannatempo P, Mariani L, Galsky MD, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:49-61. [CrossRef]
- Albers P, Park SI, Niegisch G, Fechner G, Steiner U, Lehmann J, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Annals of Oncology. 2011;22:288-94.
- Bellmunt J, Théodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, et al. Phase III Trial of Vinflunine Plus Best Supportive Care Compared With Best Supportive Care Alone After a Platinum-Containing Regimen in Patients With Advanced Transitional Cell Carcinoma of the Urothelial Tract. Journal of Clinical Oncology. 2009;27:4454-61. [CrossRef]
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383:1218-30. [CrossRef]
- Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, Ullén A, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. Journal of Clinical Oncology. 2023;41:3486-+. [CrossRef]
- Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Duran I, Lee JL, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med. 2021;384:1125-35. [CrossRef]
- Rosenberg JE, Powles T, Sonpavde GP, Loriot Y, Duran I, Lee JL, et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Annals of Oncology. 2023;34:1047-54. [CrossRef]
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024;390:875-88. [CrossRef]
- de Bem Prunes B, Nunes JS, da Silva VP, Laureano NK, Goncalves DR, Machado IS, et al. The role of tumor acidification in aggressiveness, cell dissemination and treatment resistance of oral squamous cell carcinoma. Life Sci. 2022;288:120163. [CrossRef]
- Ralph ACL, Valadao IC, Cardoso EC, Martins VR, Oliveira LMS, Bevilacqua E, et al. Environmental control of mammary carcinoma cell expansion by acidification and spheroid formation in vitro. Sci Rep. 2020;10:21959. [CrossRef]
- Yang X, Yin H, Zhang Y, Li X, Tong H, Zeng Y, et al. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1alpha activation. Int J Oncol. 2018;53:215-24. [CrossRef]
- Maeda T, Kikuchi E, Matsumoto K, Miyajima A, Oya M. Urinary pH Is Highly Associated With Tumor Recurrence During Intravesical Mitomycin C Therapy for Nonmuscle Invasive Bladder Tumor. J Urology. 2011;185:802-6. [CrossRef]
- Naeem MT, Usman AH, Ali S, Raza H, Shah AN, Mahmmoud Fadelallah Eljack M. Intravesical mitomycin C efficacy in acidic and alkaline urinary pH: impact on recurrence-free survival rate after TURBT. Ann Med Surg (Lond). 2023;85:5323-7. [CrossRef]
- Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671-7. [CrossRef]
- Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194-8.
- Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425-7.
- Warburg O. On the origin of cancer cells. Science. 1956;123:309-14. [CrossRef]
- Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297:F1477-501. [CrossRef]
- Han JH, Jeong SH, Yuk HD, Jeong CW, Kwak C, Ku JH. Acidic urine is associated with poor prognosis in patients with bladder cancer undergoing radical cystectomy. Front Oncol. 2022;12:964571. [CrossRef]
- Han JH, Jeong SH, Yuk HD, Jeong CW, Kwak C, Ku JH. Acidic Urine Is Associated With Poor Prognosis of Upper Tract Urothelial Carcinoma. Front Oncol. 2021;11:817781. [CrossRef]
- Zhao H, Tse RT, Cheng CK, Wong CY, Kong AW, Chan RC, et al. In vitro cytotoxicity of human urine and its potential toxic parameters towards bladder cancer cells. PLoS One. 2022;17:e0276127. [CrossRef]
- Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207-18.
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421-8.
- Anuar NNM, Hisam NSN, Liew SL, Ugusman A. Clinical Review: Navitoclax as a Pro-Apoptotic and Anti-Fibrotic Agent. Front Pharmacol. 2020;11. [CrossRef]
- Mauthe M, Orhon I, Rocchi C, Zhou XD, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435-55. [CrossRef]
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. Embo Rep. 2006;7:988-94. [CrossRef]
- Evans MK, Brown MC, Geradts J, Bao X, Robinson TJ, Jolly MK, et al. XIAP Regulation by MNK Links MAPK and NFkappaB Signaling to Determine an Aggressive Breast Cancer Phenotype. Cancer Res. 2018;78:1726-38.
- Chen X, Wang T, Yang D, Wang J, Li X, He Z, et al. Expression of the IAP protein family acts cooperatively to predict prognosis in human bladder cancer patients. Oncol Lett. 2013;5:1278-84. [CrossRef]
- Sahoo S, Singh P, Kalha B, Singh O, Pal R. Gonadotropin-mediated chemoresistance: Delineation of molecular pathways and targets. BMC Cancer. 2015;15:931. [CrossRef]
- Pattingre S, Tassa A, Qu XP, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927-39. [CrossRef]
- Jo EK, Silwal P, Yuk JM. AMPK-Targeted Effector Networks in Mycobacterial Infection. Front Microbiol. 2019;10. [CrossRef]
- Mann SS, Hammarback JA. Molecular Characterization of Light Chain-3 - a Microtubule-Binding Subunit of Map1a and Map1b. J Biol Chem. 1994;269:11492-7. [CrossRef]
- Kabeya Y, Mizushima N, Uero T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720-8. [CrossRef]
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Bioph Res Co. 2004;313:453-8. [CrossRef]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603-14.
- Ando H, Eshima K, Ishida T. Neutralization of Acidic Tumor Microenvironment (TME) with Daily Oral Dosing of Sodium Potassium Citrate (K/Na Citrate) Increases Therapeutic Effect of Anti-cancer Agent in Pancreatic Cancer Xenograft Mice Model. Biol Pharm Bull. 2021;44:266-70. [CrossRef]
- Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260-8.
- Ando H, Ikeda A, Tagami M, Matsuo NCA, Shimizu T, Ishima Y, et al. Oral administration of sodium bicarbonate can enhance the therapeutic outcome of Doxil(R) via neutralizing the acidic tumor microenvironment. J Control Release. 2022;350:414-20.
- Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst. 2001;93:597-604. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).