1. Introduction
Sarcopenia, characterized by the progressive loss of muscle mass and strength, significantly impacts the quality of life in older adults [
1]. The associated decline in muscle force production capacity is a crucial factor in the functional deterioration and onset of frailty in this population [
2]. These neuromuscular alterations are strong predictors of adverse outcomes such as falls [
3], hospitalizations [
4], loss of independence [
5], institutionalization, and ultimately, a reduction in life expectancy [
6]. Consequently, it is essential to optimize interventions, particularly targeting the neuromuscular system, to reduce dependency and prevent falls in older adults [
7].
Several studies have highlighted a significant correlation between decreased ankle plantar flexor (PF) force production capacities and functional abilities [
8,
9,
10]. For instance, Cattagni et al. [
10] observed that within a group of 30 older adults, maximal torque of the PF was negatively correlated with the displacement of the center of pressure during static postural balance (r = -0.77). Furthermore, they demonstrated that 90% of individuals with maximal PF torque less than 3.1 Nm/kg had previously experienced falls, whereas 85% of those exceeding this value had not. Therefore, maximal PF force production capacity could be considered a predictive parameter for fall risk [
11]. However, the ability to avoid a fall depends not only on maximal force production capacity but also on motor response time, which reflects the speed to generate submaximal force, also known as the rate of force development (RFD) [
12,
13].
RFD, defined as the increase in force per unit of time, is calculated from the slope of the force-time curve (Δ force / Δ time) [
12,
13,
14]. RFD evaluations typically consider different phases of muscle contraction, including the early phase (0 – 50 ms) and the late phase (100 – 200 ms) [
13]. The early phase is largely related to the initiation of motor unit activation and their firing sequences, as well as intrinsic muscle characteristics, such as fiber composition and calcium dynamics [
15]. In contrast, the late phase relies on the ability to transmit the force produced by the contractile component through the parallel and series elastic components [
16]. Several studies consider RFD as a crucial predictor of functional capacities in older adults [
12,
17]. For example, Hester et al. [
12] demonstrated that RFD was the sole predictor of Timed Up and Go test performance in older adults [
18,
19,
20]. Improving RFD could therefore be a key objective in rehabilitation and training programs aimed at enhancing walking ability and reducing fall risks in older adults [
8].
Resistance training (RT) has been shown to improve muscle force production capacity in older adults [
21]. For instance, Walker et al. [
22] demonstrated improvements in maximal strength and quadriceps mass when exercises were performed at slow and controlled cadences. These results justify the feasibility [
23,
24] and necessity of integrating resistance exercises for older adults [
25,
26]. However, most interventions have advocated for strength resistance exercises, consisting of 1 to 4 sets of about 8 to 15 repetitions at moderate to high loads, performed at least twice a week, in line with previous international recommendations [
27]. Recent studies and meta-analyses indicate that RT, including explosive training elements, is more effective for enhancing functional abilities compared to strength RT [
28,
29]. Explosive RT involves performing the concentric muscle contraction of each exercise repetition as quickly as possible, demonstrating notable improvements in morphological and neural adaptations as well as functional performance capacities [
30]. For example, Cadore et al. [
23] showed significant improvements in muscle power production, strength, muscle cross-sectional area, and dual-task performance in frail institutionalized older adults after 12 weeks of explosive RT. While the benefits of explosive RT in developing muscle strength are generally recognized, no study, to our knowledge, has yet thoroughly examined the specific effects of explosive RT on improving both early and late RFD of PF in institutionalized older adults. These investigations could potentially provide crucial insights into the underlying mechanisms of muscle explosivity enhancement in this specific population and, by extension, the influence of these adaptations on walking speed, an essential parameter of physical function.
The main objective of this study was to compare the effects of explosive versus strength RT on maximal strength, early and late RFD of PF, and gait speed. We hypothesize that explosive RT results in greater improvements in PF neuromuscular and functional parameters compared to strength RT.
2. Materials and Methods
2.1. Study Design
This study was a prospective, controlled, single-blinded, randomized trial, with participants randomly assigned to either an explosive resistance training group (EXG) or a strength resistance training group (STG). Both groups underwent the same assessments before and after the intervention, which consisted of 3 sessions per week for 12 weeks. While both groups performed identical PF resistance exercises, the execution parameters differed significantly in both cadence and stimuli. The EXG performed 3 to 5 sets of 12 to 14 repetitions at 40% to 45% of 1RM, focusing on a rapid concentric phase followed by a 3-second eccentric phase. In contrast, the STG performed 3 to 4 sets of 6 to 7 repetitions at 80% to 85% of 1RM, with both the concentric and eccentric phases lasting approximately 3 seconds each. This distinction in cadence and load was intended to differentiate between explosive and maximal strength training stimuli. The study adhered to the ethical principles outlined in the Helsinki Declaration, and approval for the study protocol, patient information letter, and informed consent form was obtained from the local ethics committee of the Intercommunal Health Center of Sarthe et Loir.
2.2. Recruitment
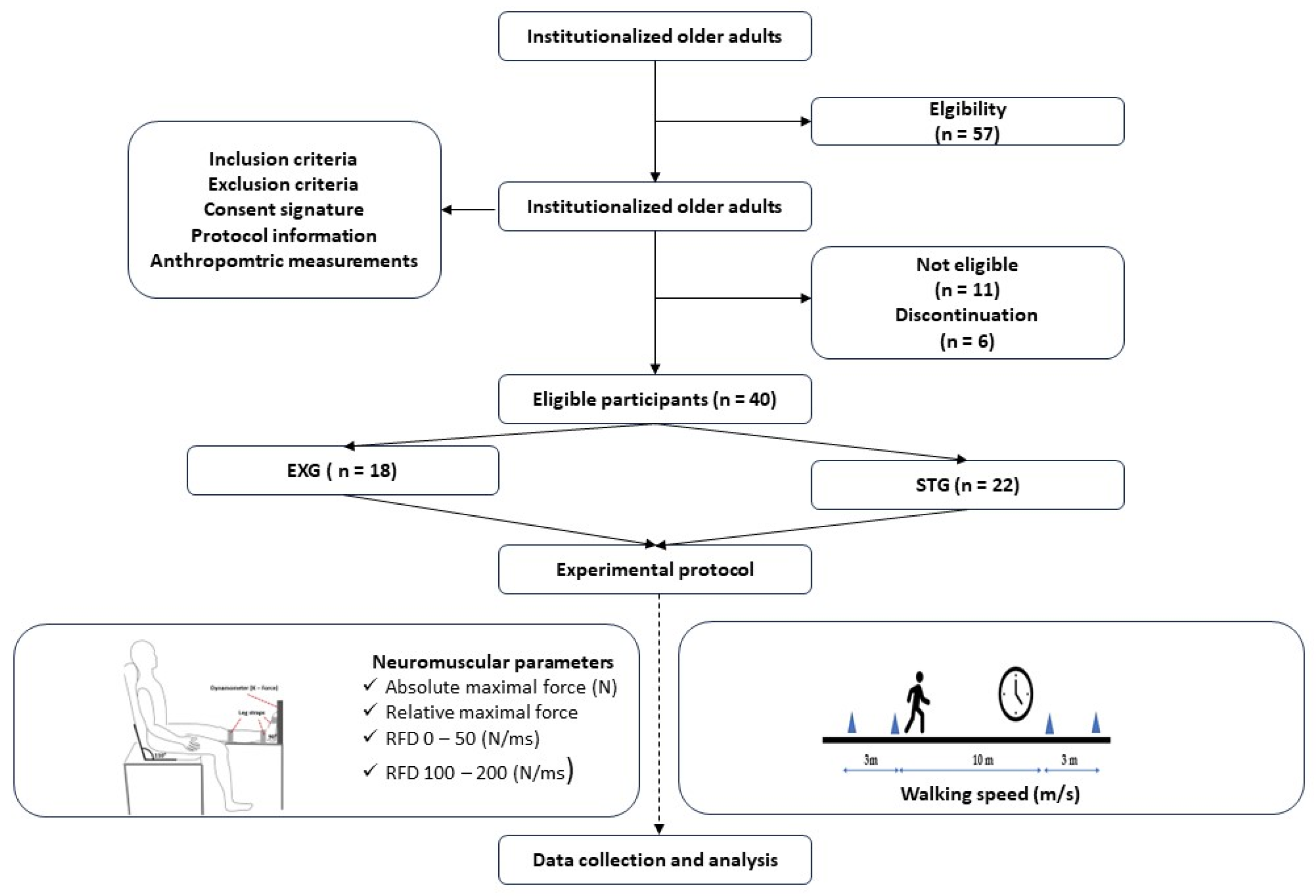
The recruitment process consisted of a three-week recruitment period, followed by a one-week screening phase (
Figure 1). Participants were recruited from a retirement home located in Sablé-sur-Sarthe (France) between January 15 and February 15, 2024. To be eligible, individuals had to be older than 65 years or, capable of walking without technical assistance (e.g., canes, walkers) from another person, and able to communicate verbally effectively with the research team. Individuals with neurological or cognitive disorders, severe cardiovascular diseases, significant musculoskeletal issues in the lower limbs, or those taking medications that could affect the tests were excluded. The eligibility of each participant was meticulously verified by the medical staff. Then, participants were randomized into one of two groups: the EXG and STG. The randomization list was generated using a computer algorithm by an independent statistician. At the end of the screening process, the investigator, who was blinded to the treatment assignment, obtained a unique randomization number for each participant.
2.3. Evaluation Protocol
All assessments were conducted in a clinical examination room under consistent environmental conditions, supervised by a blinded evaluator who was unaware of the group affiliation of the participants. Prior to the assessments, participants received a standardized set of verbal instructions to ensure familiarity with the procedures.
2.3.1. Anthropometric Parameters
Body mass (BM) and height (H) of the participants were precisely measured using a digital floor scale and a wall-mounted stadiometer, respectively. Body mass index (BMI, in kg/m²) was subsequently calculated. Lean body mass (LBM) was measured using bioelectrical impedance (Tanita; SC 240, Amsterdam, The Netherlands) [
31]. During the measurement, participants were instructed to stand still, with their arms slightly away from their body and their legs not touching, while their feet were positioned on the electrodes of the bioelectrical impedance. All measurements were conducted in the morning, with participants fasting and having avoided any physical activity for 24 hours prior.
2.3.2. Gait Speed
Gait speed (m/s) was assessed over a 20-meter flat surface, with measurements taken between the 5th and 15th meters to exclude acceleration and deceleration phases [
32]. Participants were instructed to walk at their usual pace and attempt to reach their maximum speed between the 5th and 15th meters. A stopwatch was used to measure the time required to walk the 10-meter distance. The average walking speed (m/s) was then calculated using the following formula:
2.3.3. Neuromuscular Parameters
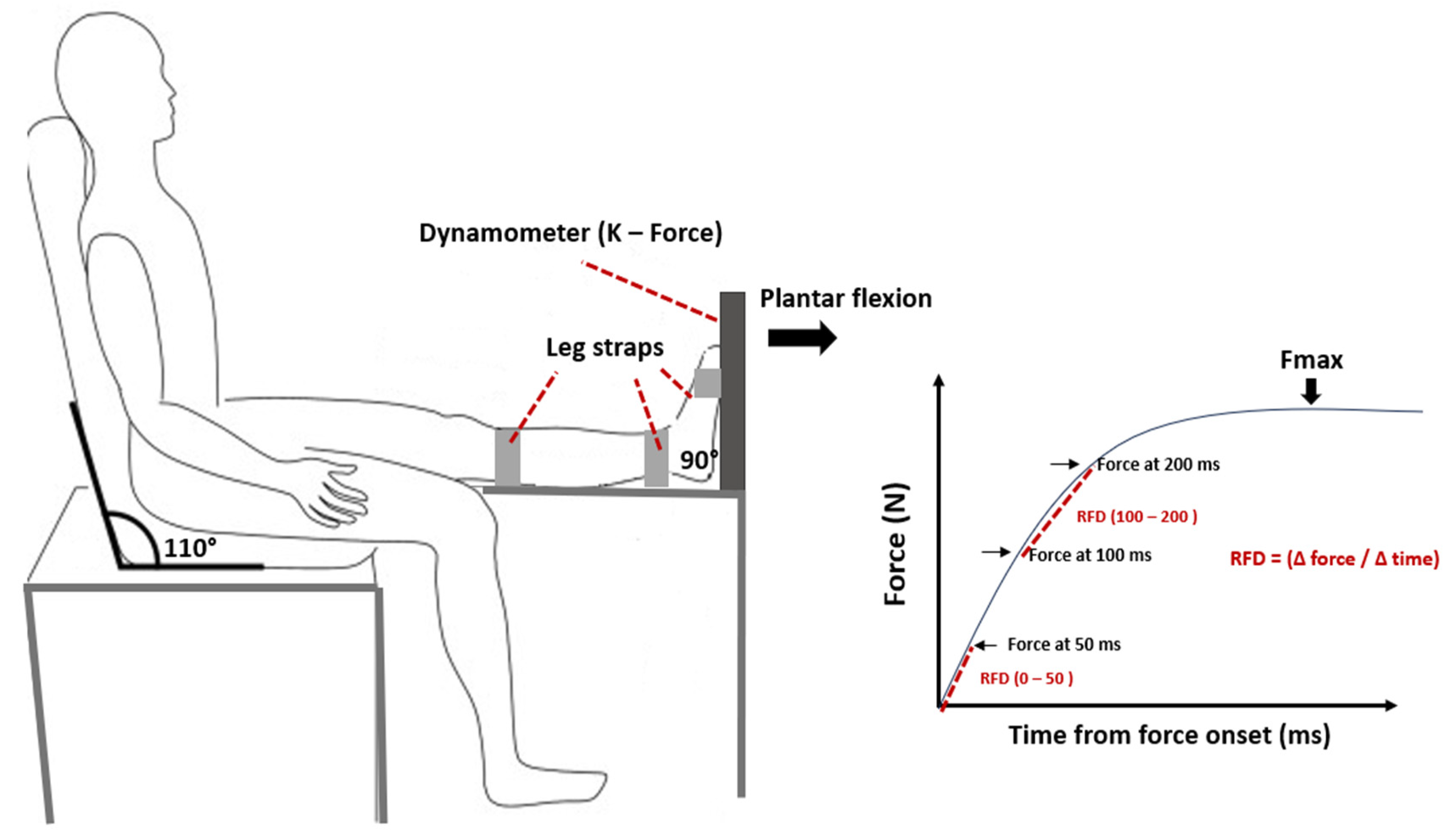
Neuromuscular parameters of PF of the dominant leg were assessed during maximal voluntary contractions (MVC) using a dynamometer (K-Force, Kinvent, Montpellier, France). Participants were seated on a chair, ensuring contact between their back, buttocks, and thighs with the chair while keeping their leg horizontally extended (
Figure 1). They were instructed to push with the ends of their foot against the dynamometer [
31,
33]. Two trials were conducted with a one-minute rest interval between them, and the maximum force of the PF (Fmax, N) from both trials was recorded. The relative maximum force (Fmax relative, N/kg) was calculated by normalizing the maximum force to the participant’s body mass. The early RFD was calculated from the onset of each MVC to 50 ms (RFD 0 – 50), and the late RFD was calculated between 100 and 200 ms (RFD 100 – 200). Both early and late RFD were derived from the linear slope of the force-time curve (Δ force / Δ time).
2.4. Resistance Training Programs
The RT comprised 36 sessions over a 12-week period, with participants in both groups completing three sessions per week. The protocol was structured based on prior studies [
34,
35,
36], beginning with an initial two-week
conditioning phase, which was followed by a
specialized training phase tailored to the cadence and stimuli-specific focus of each group. Specific methodologies employed in each training protocol are outlined in
Table 1.
During the conditioning phase, the two groups were introduced to the specific exercises they would be performing in the subsequent specialized training phase. This period was designed to familiarize participants with the correct execution of each movement, emphasizing proper technique and the controlled application of force. Using light loads, participants practiced the exercises with a focus on mastering the concentric and the eccentric phases. The EXG was instructed to perform the concentric phase rapidly, while maintaining control during the eccentric phase, whereas the STG was guided to execute both phases in a slow and controlled manner. During the final session of the conditioning phase, participants underwent a one-repetition maximum (1-RM) assessment using a seated calf raise machine. This assessment involved a progressive increase in load, with small incremental increases, until participants achieved the maximum weight they could lift with proper form. The 1-RM was defined as the heaviest load lifted with controlled execution and without any compensatory movements, and it was found to be approximately 22 kg for both groups.
To systematically increase the workload,
the specialized training phase was organized into five micro-cycles, each spanning two weeks. To ensure that the relative load training between the EXG and STG groups was equalized, the workload was quantified using the volume load (VL) method [
34]. The VL was calculated using the following equation [
37]:
Table 2 presents the predicted progression of volume-equated training loads between the two groups. The predicted VLs were established based on the first 1-RM assessment.
To ensure optimal progression throughout the program, 1-RM assessments were conducted at the end of each micro-cycle [
34], with VL systematically adjusted based on participants’ performance. These adjustments were made at the end of each week, following the final training session, to maintain an appropriate and challenging stimulus for continued adaptation [
34,
35]. Each training session began with a standardized 5-minute warm-up, consisting of light stationary cycling, rowing, or brisk walking on an ergometer or treadmill.
Throughout the 10-week of
the specialized training phase, training load (TL) was assessed after each session using the Rate of Perceived Exertion (RPE) scale [
38]. The average daily TL for each group was calculated using the following equation:
Training monotony and strain of each group was calculated based on the formulas proposed by Foster et al. [
39]:
where,
weekly mean TL is the
average daily TL during the week, and
SD is the standard deviation of the daily TL calculated over a week.
2.5. Statistical Analysis
The sample size was determined using the freeware G*Power (version 3.1.9.4) as outlined in the study by Ferhi et al. [
32]. For the power analysis, an ANOVA test was preselected, with the parameters set to control for a Type I error (alpha = 0.05) and a Type II error (beta = 0.60). Assuming a moderate estimated effect size (r = 0.35), the calculation indicated that a minimum of 40 participants would be required.
Statistical analysis was performed using Statistica Software 13.0 (StatSoft, Tulsa, OK, USA). The normality of the data sets and the homogeneity of variances were assessed using the Shapiro-Wilk and Levene’s tests, respectively. The effects of time (pre- and post-training) and group (EXG and STG) on neuromuscular and functional parameters, as well as their interaction, were tested using a two-factor ANOVA (group × time). Subsequently, a post-hoc analysis was conducted to determine significant inter- and intra-group effects. Finally, Pearson correlation analysis (r) was performed to examine the relationship between walking speed and the neuromuscular parameters of the PF before and after interventions. Results at baseline and after the intervention were presented as mean ± standard deviation. The significance level was set at p<0.05.
3. Results
3.1. Participants
A total of 57 volunteers were initially recruited for this study (
Figure 2). However, only 46 participants met the established eligibility criteria and were randomly assigned to either the EXG (n = 23) or the STG (n = 23). Six participants did not complete the study due to non-adherence to the protocol—five from the EXG and one from the STG. Reasons for discontinuation included hospitalizations due to stroke, hip fracture, and ankle sprain in four participants, while two others withdrew for personal reasons. Consequently, adherence to the study protocol was 87%. Ultimately, a cohort of 40 participants successfully completed the study (
Table 3), with the EXG consisting of 18 participants (age = 80.41 ± 10.12 years; BMI = 22.89 ± 2.77 kg/m²) and the STG consisting of 22 participants (age = 82.89 ± 5.32 years; BMI = 23.81 ± 3.45 kg/m²).
3.2. Training Programs
Attendance was calculated as the average percentage of training sessions attended over the 12-week period. Overall, attendance was 91.9 ± 3.5% for both the EXG and STG, with no statistically significant differences between the groups in session attendance. The 1 – RM values ranged from 22 kg to 25 kg in the EXG and from 22 kg to 27 kg in the STG. Overall, no statistically significant differences were observed between the predicted VL and the realized VL in both groups. A notable deviation occurred during the first week, where the predicted load was overestimated by 20% for the EXG and by 15% for the STG. Conversely, in the 10th week, the load was underestimated by 13% for EXG and 7% for STG (
Figure 3a). Except for the 8th week, no significant differences in group mean TL (
Figure 3b), monotony (
Figure 3c) and strain (
Figure 3d) were observed between the two groups. Monotony values for both groups ranged from 22 to 42 (
Figure 3c), indicating that the variability in training load was effectively managed, thereby preventing excessive uniformity that could potentially lead to overtraining. The similar strain values further indicate that the overall training stimulus was appropriately balanced, promoting analogous adaptation processes in both groups.
3.3. Anthropometric Parameters
No significant inter- or intra-group differences were observed between the STG and EXG groups, both before and after training, across all anthropometric parameters presented in
Table 3.
3.4. Walking Speed
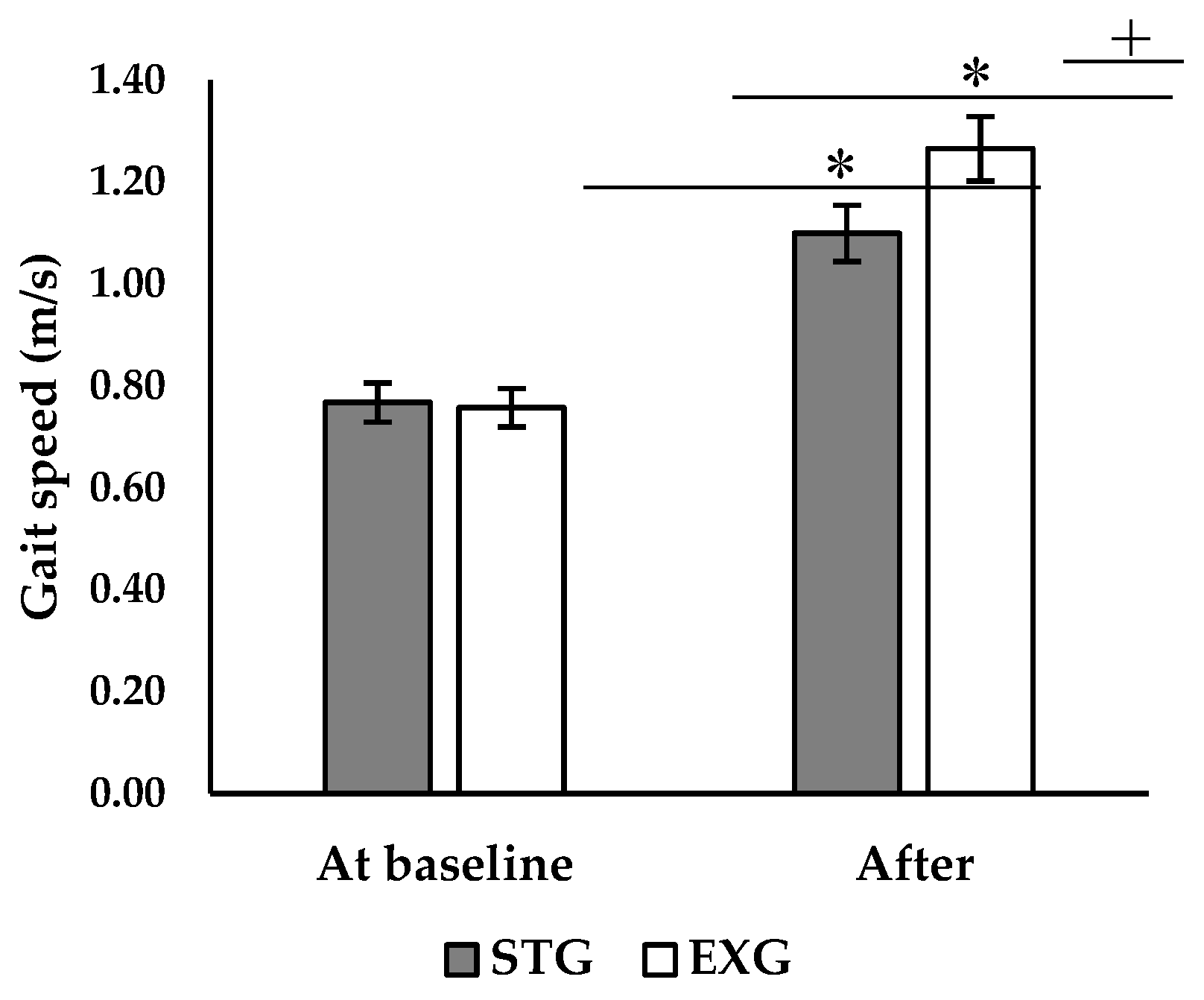
ANOVA analysis revealed a significant interaction between the effect of time and group on walking speed (p < 0.001; F = 11.6). Post-hoc analysis showed that there was no significant difference between the two groups before the intervention (
Figure 4). After the intervention, both the EXG and STG showed a significant increase in walking speed (+63.2%, p < 0.001; +45.6%, p < 0.001, respectively). However, walking speed was higher in the EXG compared to the STG (p < 0.05,
Figure 4).
3.3. Neuromuscular Parameters
ANOVA analysis revealed a significant interaction between the effects of time and group (
Table 4) on both absolute (p < 0.001; F = 1.77) and relative Fmax (p < 0.001; F = 14.6). Post-hoc analysis revealed a significant increase in both absolute and relative Fmax in the EXG (+20.5%, +21.1%, p < 0.001, respectively) and in the STG (+16.2%, +17.1%; p < 0.001, respectively). The improvement in both absolute and relative Fmax was greater in EXG (p < 0.05). Additionally, post-hoc analysis showed a significant increase in RFD 0 – 50 of 10% in the STG (p < 0.05) and 20.1% (p < 0.05) in the EXG. The improvement in RFD 0 – 50 was greater in the EXG (p < 0.05). Moreover, RFD 100 – 200 improved only in the EXG (+20.4%, p < 0.05).
Table 4. Neuromuscular parameters of the two groups before and after interventions.
3.5. Relationship between Neuromuscular Parameters of the PF and Walking Speed
3.5.1. At Baseline
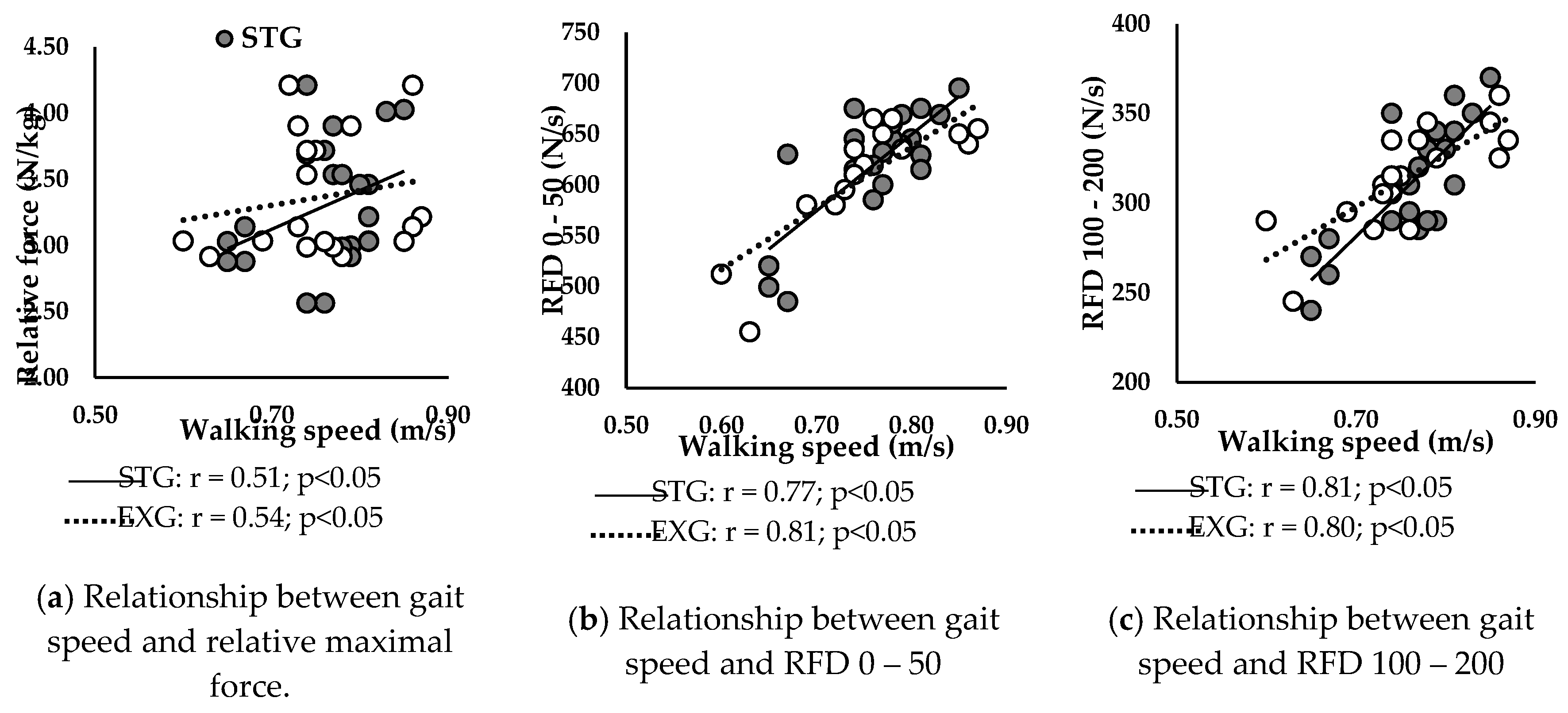
Figure 5 presents correlations between walking speed and neuromuscular parameters at baseline in STG and EXG. Walking speed was positively correlated with relative Fmax (
Figure 5a) of STG (r = 0.51; p < 0.05) and EXG (r = 0.54; p < 0.05).
Additionally, RFD 0 – 50 (
Figure 5b) and RFD 100 – 200 (
Figure 5c) were positively correlated with walking speed in STG (r = 0.77; r = 0.81; p < 0.05, respectively) and in EXG (r = 0.81; r = 0.80; p < 0.05, respectively).
3.5.2. After Training Programs
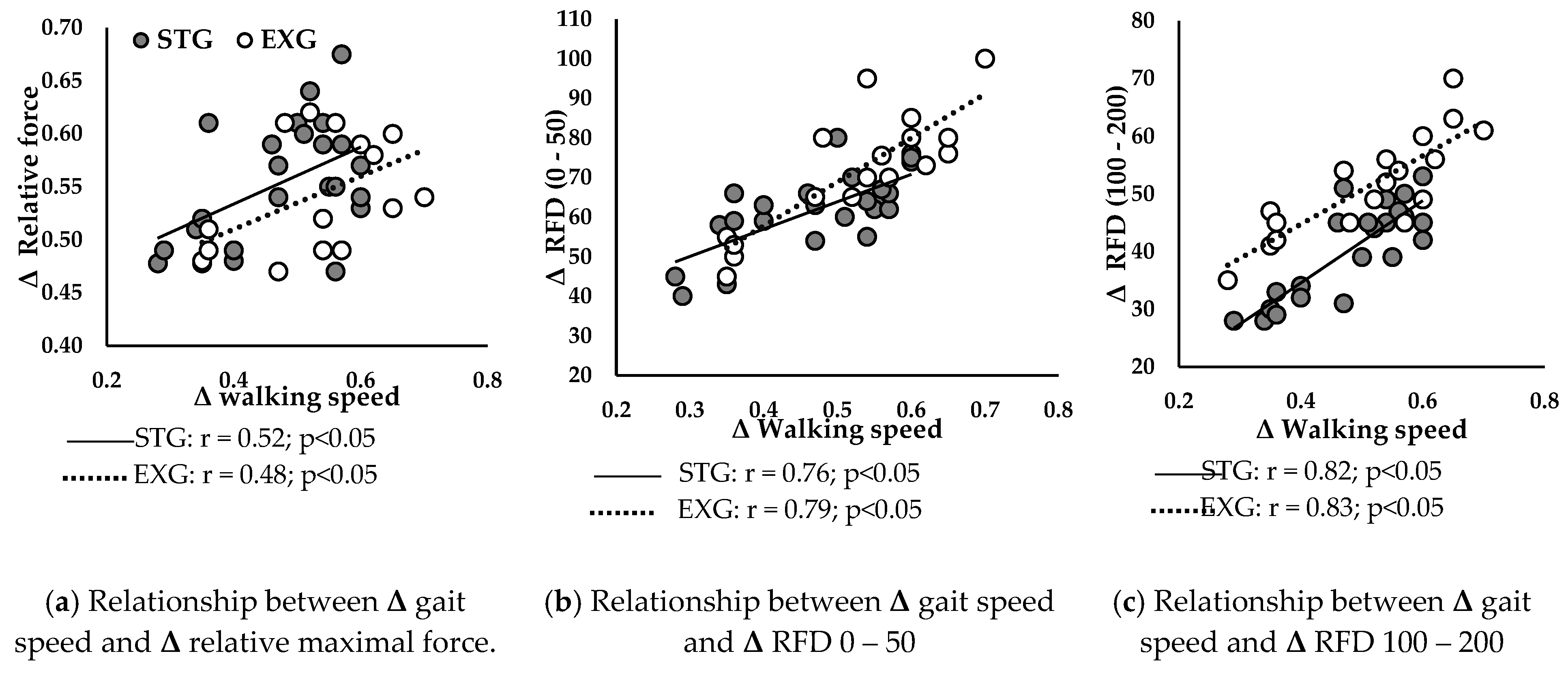
Figure 6 presents correlations between ameliorations in walking speed and improvements in neuromuscular parameters in STG and EXG.
Δ walking speed was positively correlated with
Δ relative Fmax (
Figure 6a) of STG (r = 0.52; p < 0.05) and EXG (r = 0.48; p < 0.05). Additionally,
Δ RFD 0 – 50 (
Figure 6b) and
Δ RFD 100 – 200 (
Figure 6c) were positively correlated with
Δ walking speed in STG (r = 0.76; r = 0.82; p < 0.05, respectively) and in EXG (r = 0.79; r = 0.83; p < 0.05, respectively).
4. Discussion
This study aimed to evaluate the effects of explosive versus strength RT on the neuromuscular parameters of the PF, as well as to examine the relationships between improvements in these parameters and walking speed in institutionalized older adults. Our results revealed that both types of RT improved the neuromuscular capacities of the PF, but with different and specific adaptations. Strength RT led to a more significant improvement in Fmax, while explosive training favored improvements in RFD. Furthermore, regardless of the type of training, improvement in walking speed were more strongly correlated with RFD in both intervention groups.
The results of this study revealed that RT programs, regardless of the stimuli and cadence (explosive or strength), improved Fmax of PF. These findings are consistent with several studies highlighting possible neuromuscular adaptations related to resistance exercises, allowing for significant improvements after 12 weeks of muscle RT [
23,
40,
41]. Specifically, our results showed a 21% improvement in relative Fmax in STG and 16% in the EXG. These results align with the systematic review of Lopez et al. [
42] showing significant improvements in maximum knee extensor force ranged from 6.6% to 37.0%, after 12 weeks of RT in older adults. Several hypotheses can be proposed to explain the mechanisms of these neuromuscular adaptations to RT in older adults. Firstly, protein synthesis metabolism through the efficient transport of amino acids and growth factors (e.g., insulin-like growth factor-1 [IGF-1], hepatocyte growth factor [HGF], interleukin-6 [IL-6], and myostatin) to muscle fibers [
43]. These elements play a crucial role in regulating satellite cells, thereby supporting the repair and/or remodeling of neuromuscular adaptations, which is particularly essential after the age of 65 [
44]. Additionally, an increase in the cross-sectional area of the muscle, and consequently muscle mass, may also promote increased muscle force. Kryger et al. [
41] demonstrated a significant 22% increase in the number of type IIa muscle fibers of the knee extensor muscles in older adults, thus promoting muscle hypertrophy associated with improved Fmax. However, the results of this study did not reveal an increase in lean mass in the two groups after the intervention. It is possible that these subjects did not significantly improve muscle mass, but rather muscle quality through morphological adaptations, such as a decrease in fat infiltration [
23]. It is also possible that improvement in Fmax is related to specific neural adaptations, such as increased recruitment of fast-fiber motor units and improved nerve transmission [
45,
46,
47]. All these findings suggest that neuromuscular capacities in older adults can be effectively reversed through targeted adaptations induced by muscle RT.
The originality of this study lies in its investigation of the specific adaptations associated with both the cadence and stimuli of resistance exercises in older adults. Our results show that strength RT led to a more significant improvement in Fmax (+8% in STG), while explosive training favored improvements in early RFD (+9% in EXG) and late (+8% in EXG). Indeed, explosive exercise imposes high demands on the nervous system, resulting in significant improvements in neuromuscular function in older adults [
48,
49,
50]. In this context, several studies have emphasized that explosive training could partially reduce muscle activation deficit in older adults [
51] with a superior effect on muscle power compared to strength training [
52]. Furthermore, early RFD is primarily influenced by neural factors, such as the maximum discharge frequency of motor neurons [
15,
16]. The late RFD is predominantly influenced by the structural properties of the muscle-tendon complex and the effectiveness of force transmission through both parallel (e.g., cellular matrix) and series elastic elements (e.g., tendons) [
45]. Consequently, we propose that the observed improvement in early RFD can be attributed to increased motor unit discharge rate and recruitment, reduced cortical inhibition, and enhanced nerve conduction velocity [
23,
53,
54]. The improvement in late RFD could be linked to enhanced capacity of the muscle-tendon complex to transmit force through elastic elements and potential structural adaptations in the muscle’s pennation angle and fiber number [
53,
55].
Our results revealed a significant improvement in walking speed for both EXG (+63.2%) and STG (+45.6%), consistent with several studies showing similar effects of muscle RT on habitual and maximum walking speed [
42,
56]. These improvements, ranging from 5.5% to 20.4% and observed after short-term interventions (10-12 weeks), have been attributed to enhanced neuromuscular capacities of lower limb muscles [
23,
57]. However, in our study, the EXG showed a significantly superior improvement of 12% in walking speed compared to the STG. Moreover, the improvement in walking speed was more strongly correlated with the RFD than with the improvement in Fmax (
Figure 6). Indeed, during walking, the time to develop force is limited, typically less than 300 ms. Superior gains in RFD, both in the early (0 – 50 ms) and late (100 – 200 ms) phases, allow for a rapid and efficient response, essential for maintaining balance and stability during walking [
45,
58]. Additionally, improved RFD enhances propulsion and braking during walking, crucial for speed and movement efficiency [
59]. These results suggest that improvements in RFD play a more decisive role in enhancing walking propulsion capacity than increases in Fmax. This underscores the importance of targeting these parameters in rehabilitation and training programs to optimize activities of daily living in older adults [
60,
61]. Moreover, an increased ability to rapidly develop force allows for better responses to situations of imbalance, such as during stumbling, and helps maintain equilibrium and stability while walking [
17,
57]. Therefore, rehabilitation programs should include explosive resistance exercises to maximize neuromuscular and functional benefits in older adults.
Limitations and Future Perspectives
This study acknowledges several limitations that warrant consideration. Firstly, our study primarily focused on the PF. It would be important to investigate other muscle groups, especially those around the knee, which are also crucial for walking. An analysis of neuromuscular activities through electromyography would also be necessary to understand the underlying mechanisms of neural adaptations resulting from the two types of interventions. Finally, while bioelectrical impedance analysis is a practical and widely used method for estimating body composition due to its non-invasive nature, portability, and ease of use, it relies on population-specific algorithms that may not be universally applicable to our study population. In future studies, it would be beneficial to incorporate gold standard techniques such as Magnetic Resonance Imaging or Dual-Energy X-ray Absorptiometry for assessing body composition.
5. Conclusions
The cadence and stimuli of muscle resistance exercises elicit distinct neuromuscular adaptations in older adults. Explosive RT appears particularly effective in improving the ability to rapidly generate force, which is essential for many daily activities requiring explosive movements and quick responses. These results underscore the importance of including explosive resistance exercises, supported by regular assessments and adjustments based on individual progress, to maximize neuromuscular and functional benefits in older adults.
Author Contributions
Conceptualization, E.M. and W.M.; methodology, E.M. and Y.C.; software, J.J.; validation, N.P., A.R. and Y.C.; formal analysis, O.GC.; investigation, E.M.; resources, J.J.; data curation, O.GC.; writing—original draft preparation, E.M.; writing—review and editing, W.M.; visualization, N.P.; supervision, W.M.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the Intercommunal Health Center of Sarthe et Loir (protocol code 012, January 5, 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
The research was registered in the Pan African Clinical Trials Registry under the registration number PACTR202306912191110.
Acknowledgments
We gratefully acknowledge the medical staff and administration of the intercommunal Health Center of Sarthe et Loir for their invaluable support and assistance during this study. We also extend our heartfelt thanks to the patients who generously participated in this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yuan, S., Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. 2023, Jul;144:155533. [CrossRef]
- Dufour, A. B., Hannan, M. T., Murabito, J. M., Kiel, D. P., & McLean, R. R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The framingham study. J Gerontol A Biol Sci and Med Sci, 2023, 68(2), 168–174. [CrossRef]
- Landi, F., Liperoti, R., Russo, A., Giovannini, S., Tosato, M., Capoluongo, E., Bernabei, R., & Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin Nutr, 2012, 31(5), 652–658. [CrossRef]
- Cawthon, P. M.; Fox, K. M.; Gandra, S. R.; Delmonico, M. J.; Chiou, C. F.; Anthony, M. S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S. R.; Harris, T. B. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc, 2009, 57, 1411–1419. [CrossRef]
- Janssen, I.; Heymsfield, S. B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc, 2002, 50, 889–896. [CrossRef]
- Cruz-Jentoft, A. J.; Baeyens, J. P.; Bauer, J. M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F. C.; Michel, J. P.; Rolland, Y.; Schneider, S. M.; Topinková, E.; Vandewoude, M.; Zamboni, M. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [CrossRef]
- Coletti, C.; Acosta, G. F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [CrossRef]
- Bellumori, M.; Jaric, S.; Knight, C. A. Age-related decline in the rate of force development scaling factor. Motor Control, 2013, 17, 370–381. [CrossRef]
- Thompson, B. J.; Ryan, E. D.; Herda, T. J.; Costa, P. B.; Herda, A. A.; Cramer, J. T. Age-related changes in the rate of muscle activation and rapid force characteristics. Age 2014, 36, 839–849. [CrossRef]
- Cattagni, T.; Scaglioni, G.; Laroche, D.; Van Hoecke, J.; Gremeaux, V.; Martin, A. Ankle muscle strength discriminates fallers from non-fallers. Front Aging Neurosci, 2014, 6, 336. [CrossRef]
- Bohrer, R. C. D.; Pereira, G.; Beck, J. K.; Lodovico, A.; Rodacki, A. L. F. Multicomponent Training Program with High-Speed Movement Execution of Ankle Muscles Reduces Risk of Falls in Older Adults. Rejuvenation Res. 2019, 22, 43–50. [CrossRef]
- Hester, G. M.; Ha, P. L.; Dalton, B. E.; Vandusseldorp, T. A.; Olmos, A. A.; Stratton, M. T.; Bailly, A. R.; Vroman, T. M. Rate of Force Development as a Predictor of Mobility in Community-dwelling Older Adults. J Geriatr Phys Ther, 2021, 44, 74–81. [CrossRef]
- Olmos, A. A.; Stratton, M. T.; Ha, P. L.; Dalton, B. E.; VanDusseldorp, T. A.; Mangine, G. T.; Feito, Y.; Poisal, M. J.; Jones, J. A.; Smith, T. M.; Hester, G. M. Early and late rapid torque characteristics and select physiological correlates in middle-aged and older males. PLoS ONE 2020, 15. [CrossRef]
- Gerstner, G. R.; Thompson, B. J.; Rosenberg, J. G.; Sobolewski, E. J.; Scharville, M. J.; Ryan, E. D. Neural and Muscular Contributions to the Age-Related Reductions in Rapid Strength. Med Sci Sports Exerc, 2017, 49, 1331–1339. [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J, Appl, Physiol, 2008, 104, 739–746. [CrossRef]
- Andersen, L. L.; Aagaard, P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur, J, Appl, Physiol, 2006, 96, 46–52. [CrossRef]
- Clark, D. J.; Manini, T. M.; Fielding, R. A.; Patten, C.; Manuscript, A. Neuromuscular determinants of maximum walking speed in well-functioning older adults, Exp, Gerontol, 2013, 48, 358–363. [CrossRef]
- Ko, M.; Hughes, L.; Lewis, H. Walking speed and peak plantar pressure distribution during barefoot walking in persons with diabetes. Physiother, Res, Int, 2012, 17, 29–35. [CrossRef]
- Holmes, S. J.; Mudge, A. J.; Wojciechowski, E. A.; Axt, M. W.; Burns, J. Impact of multilevel joint contractures of the hips, knees and ankles on the Gait Profile score in children with cerebral palsy. Clin Biomech, 2018. [CrossRef]
- Tavoian, D.; Clark, B. C.; Clark, L. A.; Wages, N. P.; Russ, D. W. Comparison of strategies for assessment of rate of torque development in older and younger adults. Eur J Appl Physiol, 2023, 0123456789. [CrossRef]
- Boyd Foster-Burns, S. Sarcopenia and decreased muscle strength in the elderly woman: Resistance training as a safe and effective intervention. J Women Aging 1999, 11, 75–85. [CrossRef]
- Walker, S.; Peltonen, H.; Häkkinen, K. Medium-intensity, high-volume “hypertrophic” resistance training did not induce improvements in rapid force production in healthy older men. Age 2015, 37. [CrossRef]
- Cadore, E. L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr.) 2014, 36, 773–785. [CrossRef]
- Cadore, E. L.; Moneo, A. B. B.; Mensat, M. M.; Muñoz, A. R.; Casas-Herrero, A.; Rodriguez-Mañas, L.; Izquierdo, M. Positive effects of resistance training in frail elderly patients with dementia after long-term physical restraint. Age, 2014, 36, 801–811. [CrossRef]
- Cadore, E. L.; Pinto, R. S.; Reischak-Oliveira, Á.; Izquierdo, M. Explosive type of contractions should not be avoided during resistance training in elderly. Exp. Gerontol. 2018, 102, 81–83. [CrossRef]
- Fragala, M. S.; Cadore, E. L.; Dorgo, S.; Izquierdo, M.; Kraemer, W. J.; Peterson, M. D.; Ryan, E. D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [CrossRef]
- Garber, C. E.; Blissmer, B.; Deschenes, M. R.; Franklin, B. A.; Lamonte, M. J.; Lee, I. M.; Nieman, D. C.; Swain, D. P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [CrossRef]
- Ramírez-Campillo, R.; Castillo, A.; de la Fuente, C. I.; Campos-Jara, C.; Andrade, D. C.; Álvarez, C.; Martínez, C.; Castro-Sepúlveda, M.; Pereira, A.; Marques, M. C.; Izquierdo, M. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp. Gerontol. 2014, 58, 51–57. [CrossRef]
- Tschopp, M.; Sattelmayer, M. K.; Hilfiker, R. Is power training or conventional resistance training better for function in elderly persons? A meta-analysis. Age Ageing 2011, 40, 549–556. [CrossRef]
- Straight, C. R.; Lindheimer, J. B.; Brady, A. O.; Dishman, R. K.; Evans, E. M. Effects of Resistance Training on Lower-Extremity Muscle Power in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2016, 46, 353–364. [CrossRef]
- Magtouf, E.; Chortane, S. G.; Chortane, O. G.; Boyas, S.; Beaune, B.; Durand, S.; Maktouf, W. Influence of Concurrent Exercise Training on Ankle Muscle Activation during Static and Proactive Postural Control on Older Adults with Sarcopenic Obesity: A Multicenter, Randomized, and Controlled Trial. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2779–2794. [CrossRef]
- Ferhi, H.; Gaied Chortane, S.; Durand, S.; Beaune, B.; Maktouf, W. Effects of Physical Activity Program on Body Composition, Physical Performance, and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity: Randomized Controlled Trial. Healthcare 2023, 11, 2294. [CrossRef]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Combined effects of aging and obesity on postural control, muscle activity and maximal voluntary force of muscles mobilizing ankle joint. J. Biomech. 2018, 79, 198–206. [CrossRef]
- Henwood, T.R., Riek, S., Taaffe, D.R. Strength versus muscle power-specific resistance training in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008, 63(1), 83-91. [CrossRef]
- Ramírez-Campillo, R., Castillo, A., de la Fuente, C.I., Campos-Jara, C., Andrade, D.C., Álvarez, C., Martínez, C., Castro-Sepúlveda, M., Pereira, A., Marques, M.C., Izquierdo, M. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp Gerontol. 2014, 58, 51-7. [CrossRef]
- Borde, R., Hortobágyi, T., Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45(12), 1693-720. [CrossRef]
- Pruitt, L.A., Taaffe, D.R., Marcus, R. Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Miner Res. 1995, 10(11), 1788-95. [CrossRef]
- Haddad M, Stylianides G, Djaoui L, Dellal A, Chamari K. Session-RPE Method for Training Load Monitoring: Validity, Ecological Usefulness, and Influencing Factors. Front Neurosci. 2017, 2, 11, 612. [CrossRef]
- Foster, C. Monitoring Training in Athletes with Reference to Overtraining Syndrome. Med. Sci. Sports Exerc. 1998, 30, 1164–1168. [CrossRef]
- Serra-Rexach, J. A.; et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: a randomized controlled trial. J. Am. Geriatr. Soc. 2011, 59, 594–602.
- Kryger, A. I.; Andersen, J. L. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand. J. Med. Sci. Sports 2007, 17, 422–430. [CrossRef]
- Lopez, P.; Pinto, R. S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E. L. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [CrossRef]
- Moro, T.; Brightwell, C. R.; Phalen, D. E.; McKenna, C. F.; Lane, S. J.; Porter, C.; Volpi, E.; Rasmussen, B. B.; Fry, C. S. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp. Gerontol. 2019, 127, 110723. [CrossRef]
- Nederveen, J. P.; Joanisse, S.; Snijders, T.; Ivankovic, V.; Baker, S. K.; Phillips, S. M.; Parise, G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J. Cachexia Sarcopenia Muscle 2016, 7, 547–554. [CrossRef]
- Unhjem, R.; Lundestad, R.; Fimland, M. S.; Mosti, M. P.; Wang, E. Strength training-induced responses in older adults: attenuation of descending neural drive with age. Age 2015, 37, 1–14.
- Aagaard, P.; Simonsen, E. B.; Andersen, J. L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326.
- Duchateau, J.; Baudry, S. Maximal discharge rate of motor units determines the maximal rate of force development during ballistic contractions in human. Front. Hum. Neurosci. 2014, 8, 234.
- Marsh, A. P.; Miller, M. E.; Rejeski, W. J.; Hutton, S. L.; Kritchevsky, S. B. Lower extremity muscle function after strength or power training in older adults. J. Aging Phys. Act. 2009, 17, 416–433. [CrossRef]
- Reid, K. F.; Callahan, D. M.; Carabello, R. J.; Phillips, E. M.; Frontera, W. R.; Fielding, R. A. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin. Exp. Res. 2014, 20, 337–343.
- Sayers, S. P.; Gibson, K. A comparison of high-speed power training and traditional slow-speed resistance training in older men and women. J. Strength Cond. Res. 2010, 24, 3369–3380. [CrossRef]
- Arnold, P.; Bautmans, I. The influence of strength training on muscle activation in elderly persons: a systematic review and meta-analysis. Exp. Gerontol. 2014, 58, 58–68. [CrossRef]
- Bottaro, M.; Machado, S. N.; Nogueira, W.; Scales, R.; Veloso, J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur. J. Appl. Physiol. 2007, 99, 257–264. [CrossRef]
- Gabriel, D. A.; Kamen, G.; Frost, G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006, 36, 133–149. [CrossRef]
- Christie, A.; Kamen, G. Cortical inhibition is reduced following short-term training in young and older adults. Age 2014, 36, 749–758. [CrossRef]
- Talar, K.; Hernández-Belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-Ibáñez, J. Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J. Clin. Med. 2021, 10, 1630. [CrossRef]
- Lee, N. K.; Son, S. M.; Nam, S. H.; Kwon, J. W.; Kang, K. W.; Kim, K. Effects of progressive resistance training integrated with foot and ankle compression on spatiotemporal gait parameters of individuals with stroke. J. Phys. Ther. Sci. 2013, 25, 1235–1237. [CrossRef]
- Clark, D. J.; Reid, K. F.; Patten, C.; Phillips, E. M.; Ring, S. A.; Wu, S. S.; Fielding, R. A. Does quadriceps neuromuscular activation capability explain walking speed in older men and women? Exp. Gerontol. 2014, 55, 49–53. [CrossRef]
- Peterson, C. L.; Kautz, S. A.; Neptune, R. R. Braking and propulsive impulses increase with speed during accelerated and decelerated walking. Gait Posture 2011, 33, 562–567. [CrossRef]
- Jaque, C.; Véliz, P.; Ramirez-Campillo, R.; Moran, J.; Gentil, P.; Cancino, J. High-speed bodyweight resistance training improves functional performance through maximal velocity in older females. J. Aging Phys. Act. 2021, 29, 659–669. [CrossRef]
- Coelho-Júnior, H. J.; Uchida, M. C. Effects of low-speed and high-speed resistance training programs on frailty status, physical performance, cognitive function, and blood pressure in prefrail and frail older adults. Front. Med. 2021, 8, 702436. [CrossRef]
- Bårdstu, H. B.; Andersen, V.; Fimland, M. S.; Raastad, T.; Saeterbakken, A. H. Muscle strength is associated with physical function in community-dwelling older adults receiving home care: a cross-sectional study. Front. Public Health, 2022, 25, 10:856632. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).