1. Introduction
Fungal infections, particularly those caused by species of Candida, represent a substantial and growing challenge in healthcare worldwide. Among the approximately 150 known species of Candida,
Candida albicans,
Candida glabrata, and
Candida tropicalis are the most frequently implicated in cases of invasive candidiasis, which can lead to high mortality rates, especially in immunocompromised patients such as those undergoing chemotherapy, organ transplants, or HIV treatment [
1,
2,
3]. The capacity of these species to form biofilms on medical devices—including catheters, stents, and prosthetics—significantly complicates their management, as biofilms confer enhanced resistance to both host immune defenses and antifungal therapies [
4].
Biofilm formation is a well-established virulence factor in
Candida spp., with its extracellular matrix providing both structural integrity and a formidable barrier against antimicrobial agents [
5]. Unlike planktonic cells, biofilm-associated cells exhibit a phenotypic shift that enhances their survival under adverse environmental conditions [
6]. Notably, biofilms can reduce drug penetration, promote gene expression changes that favor resistance, and allow cells to persist in a dormant state, thereby evading antifungal effects [
7,
8,
9,
10]. These challenges have driven the search for new therapeutic strategies that can effectively disrupt biofilm formation and eliminate biofilm-embedded cells [
11].
In this context, the role of natural antifungal agents has gained considerable attention.
Trans-Cinnamaldehyde, a key component of cinnamon oil, is one such compound that has shown potent antimicrobial properties against a wide range of pathogens, including
Candida spp. [
12]. Previous studies suggest that
trans-Cinnamaldehyde exerts its antifungal activity by disrupting cell wall synthesis, impairing ergosterol biosynthesis, and increasing membrane permeability [
13,
14,
15]. These effects make it a promising candidate for the treatment of fungal infections. However, the efficacy of
trans-Cinnamaldehyde against Candida biofilms remains underexplored, particularly in comparison to conventional antifungal agents such as Nystatin [
14].
Nystatin has long been regarded as one of the most effective antifungal agents against both planktonic and biofilm forms of Candida. It acts by binding to ergosterol in the fungal cell membrane, leading to membrane disruption and cell death [
16]. Despite its efficacy, the emergence of drug-resistant Candida strains and the inherent limitations of Nystatin in treating biofilm-related infections necessitate the exploration of alternative treatments [
17].
Recent research has begun to focus on the synergistic potential of combining natural compounds like
trans-Cinnamaldehyde with conventional antifungals to enhance their efficacy. Such combinations have been shown to reduce the necessary dosage of each agent, thereby mitigating toxicity and side effects, while also overcoming biofilm-associated resistance [
15]. However, systematic studies comparing the efficacy of
trans-Cinnamaldehyde to Nystatin in both planktonic and biofilm forms of Candida are limited [
18].
The present study aims to address this gap by evaluating the antifungal efficacy of trans-Cinnamaldehyde and Nystatin against Candida albicans, Candida glabrata, and Candida tropicalis in both planktonic and biofilm forms. Specifically, we investigate the ability of these agents to inhibit biofilm formation, reduce fungal cell viability, and disrupt established biofilms on synthetic surfaces, which simulate clinical conditions. By employing a combination of antifungal susceptibility assays and Scanning Electron Microscopy (SEM), we provide a comprehensive analysis of the structural and functional effects of these antifungal agents on Candida biofilms. The results of this study are expected to contribute to the growing body of literature on natural antifungal agents and their potential role in overcoming the persistent problem of biofilm-related fungal infections.
2. Material and Methods
Fungal Strains and Growth Conditions
Standard clinical strains of Candida albicans (ATCC 24433), Candida glabrata (ATCC 15126), and Candida tropicalis (ATCC 13803) were obtained from OSI Co., Ltd., Vietnam. All strains were cultured on Sabouraud Dextrose Agar (SDA) plates at 37°C for 48 hours prior to the experiments. For the preparation of inoculum, five colonies approximately 1 mm in diameter were collected from each strain and suspended in 9 mL of sterile physiological saline containing 0.05% Tween 80. The fungal suspensions were vortexed for 20–30 seconds, and the final concentration was adjusted to 1–5 × 106 CFU/mL using a spectrophotometer (OD530nm = 0.10 ± 0.02), following the Clinical and Laboratory Standards Institute (CLSI) M27-A3 guidelines for antifungal susceptibility testing.
Preparation of Antifungal Agents
Trans-Cinnamaldehyde was extracted from Cinnamomum cassia and purified. The compound was dissolved in dimethyl sulfoxide (DMSO) to prepare stock solutions and diluted 100 times in Sabouraud Dextrose Broth (SDB) supplemented with 1% Tween 80 for the biofilm and planktonic assays. Nystatin was purchased from YouMed Co., Ltd., Vietnam, and was similarly dissolved in DMSO and diluted in RPMI-1640 medium (Sigma-Aldrich, Germany) supplemented with L-glutamine and without sodium bicarbonate to the desired concentrations. The final concentrations ranged from 0.01 mg/mL to 5 mg/mL for trans-Cinnamaldehyde and from 0.00025 mg/mL to 0.128 mg/mL for Nystatin.
Scanning Electron Microscopy (SEM)
Biofilms grown on sterile coverslips were prepared for SEM analysis to visualize the structure and morphology of biofilms after treatment with antifungal agents. After biofilm formation (48 hours), the coverslips were carefully washed with PBS to remove non-adherent cells. The samples were then fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 hour at room temperature. After fixation, the samples were dehydrated through a graded series of ethanol (30%, 50%, 70%, 90%, and 100%), with each step lasting 10 minutes. The dehydrated biofilm samples were dried using a critical point dryer (Model: EMS 850, Electron Microscopy Sciences, USA).
Once dried, the samples were mounted on aluminum stubs and sputter-coated with a thin layer of gold using a sputter coater (Model: Q150R, Quorum Technologies, UK) to enhance conductivity. SEM imaging was performed using a scanning electron microscope (Model: JSM-7610F, JEOL Ltd., Japan) at an accelerating voltage of 5 kV. Images were captured at magnifications ranging from 500x to 5000x to observe both the overall biofilm structure and finer surface details of the yeast cells and hyphal elements.
Minimum Inhibitory Concentrations (MIC) and Minimum Biofilm Inhibitory Concentrations (MBIC)
The determination of planktonic MIC and MBIC for trans-Cinnamaldehyde and Nystatin followed the CLSI M27-A3 protocol. For planktonic MIC determination, 100 µL of fungal suspension (OD530nm = 0.10 ± 0.02) was added to wells containing serial dilutions of the antifungal agents in RPMI-1640 medium. Plates were incubated at 37°C for 48 hours, and the MIC was defined as the lowest concentration of the antifungal agent that resulted in at least a 50% reduction in optical density compared to the untreated control.
For MBIC determination, biofilms were established as described above. After biofilm formation, the wells were gently washed three times with sterile PBS to remove non-adherent cells. Fresh medium containing serial dilutions of trans-Cinnamaldehyde or Nystatin was added to each well, and plates were incubated at 37°C for an additional 48 hours. The MBIC was determined using the MTT assay, and the lowest concentration of the antifungal agent that reduced biofilm metabolic activity by at least 50% (MBIC50) or completely inhibited biofilm growth (MBIC100) was recorded.
Planktonic and Biofilm Fungal Viability (PMIC and PMFC Determination)
To determine the planktonic minimum inhibitory concentration (PMIC) and the planktonic minimum fungicidal concentration (PMFC), 100 µL of fungal suspensions were exposed to antifungal agents in serial dilutions, as described above. After 48 hours of incubation, the PMIC was determined as the lowest concentration that visually inhibited fungal growth by at least 90%. For PMFC determination, 100 µL of culture from each well was plated onto Sabouraud Dextrose Agar (SDA) plates, and colonies were counted after 48 hours of incubation at 37°C. The PMFC was defined as the lowest concentration of antifungal agent resulting in fewer than 10 colony-forming units (CFUs) per plate.
Statistical Analysis
All experiments were performed in triplicate, ensuring reproducibility and reliability of the results. Data were expressed as mean ± standard deviation (SD) for each experimental group. To evaluate the statistical significance between different treatment groups, a one-way analysis of variance (ANOVA) was employed. This test was followed by Tukey’s post-hoc test to identify specific group differences, providing pairwise comparisons between all treatments. A p-value < 0.05 was considered statistically significant for all analyses, indicating that differences observed between groups were unlikely to be due to chance.
All statistical analyses were performed using GraphPad Prism 10.3.1 (GraphPad Software, Inc., San Diego, CA, USA), which is widely used for biostatistical analysis in microbiology and pharmacology research. The software was chosen for its robust capabilities in handling complex data sets and its ease of use in generating clear, interpretable graphical results. Data presentation and statistical interpretation followed international standards to ensure compliance with peer-reviewed journal expectations.
3. Results
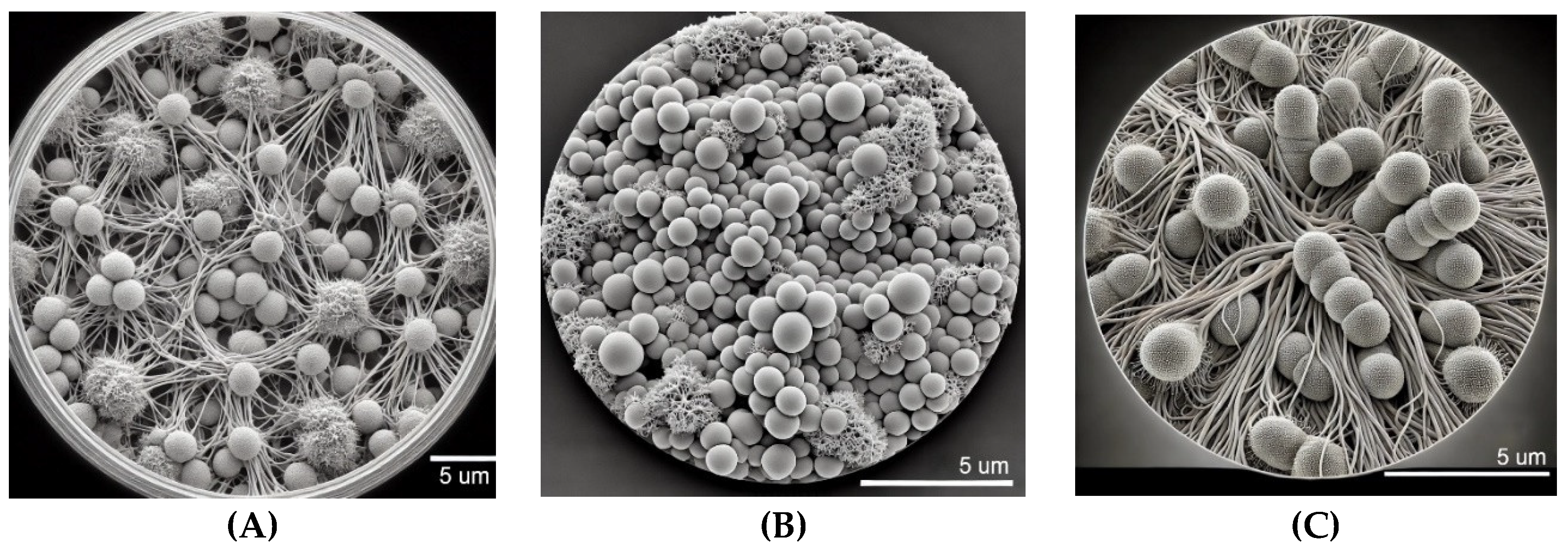
3.1. Distinct Biofilm Formation Patterns of Candida spp. on 96-Well Plastic Plates in RPMI Medium: An SEM-Based Comparative Analysis
The SEM analysis provided in
Figure 1 demonstrates distinct biofilm formation patterns for
Candida spp. on 96-well plastic plates cultured in RPMI medium after 48 hours. In
Figure 1A,
Candida albicans exhibits a well-developed biofilm characterized by dense networks of intertwined hyphal structures interspersed with clusters of yeast cells. This mature biofilm reflects the typical three-dimensional architecture of
C. albicans, known for its stability and resistance in vitro. The RPMI medium, which closely simulates host environments, supports the efficient formation of
C. albicans biofilms. The filamentous hyphae play a critical role in maintaining biofilm integrity, while the yeast cells likely facilitate initial surface attachment. This complex structure after 48 hours indicates the ability of
C. albicans to rapidly form robust biofilms on synthetic surfaces, underscoring its potential for colonization on medical devices, which contributes to its persistence in clinical settings through enhanced resistance to antifungal agents and host immune defenses.
Figure 1B showcases the biofilm formation of
Candida glabrata under identical conditions. The biofilm is composed primarily of densely packed spherical yeast cells, typical of
C. glabrata biofilms, with minimal filamentous growth. Unlike
C. albicans,
C. glabrata does not form extensive hyphal networks, yet it develops a compact biofilm that provides structural integrity and protection. The high cell density and limited filamentation suggest that this biofilm morphology enhances resistance to environmental stresses and antifungal treatments. The biofilm’s compactness also facilitates strong adhesion to the plastic surface, contributing to the pathogen’s ability to persist in healthcare environments, particularly on medical devices. In
Figure 1C,
Candida tropicalis is shown to form a biofilm dominated by elongated pseudohyphae and clusters of yeast cells, reflecting a mature biofilm structure after 48 hours of culture. The intricate network of pseudohyphae plays a crucial role in biofilm stability, facilitating adhesion and enhancing resistance to antifungal interventions.
C. tropicalis is known for its dual capability to switch between yeast and filamentous forms, and this flexibility is evident in the biofilm structure observed. The dense interweaving of cells and hyphae creates a robust biofilm, emphasizing its potential to cause biofilm-related infections, especially on implanted medical devices. This image highlights the ability of
C. tropicalis to form complex and resilient biofilms under RPMI conditions, further complicating clinical treatment strategies.
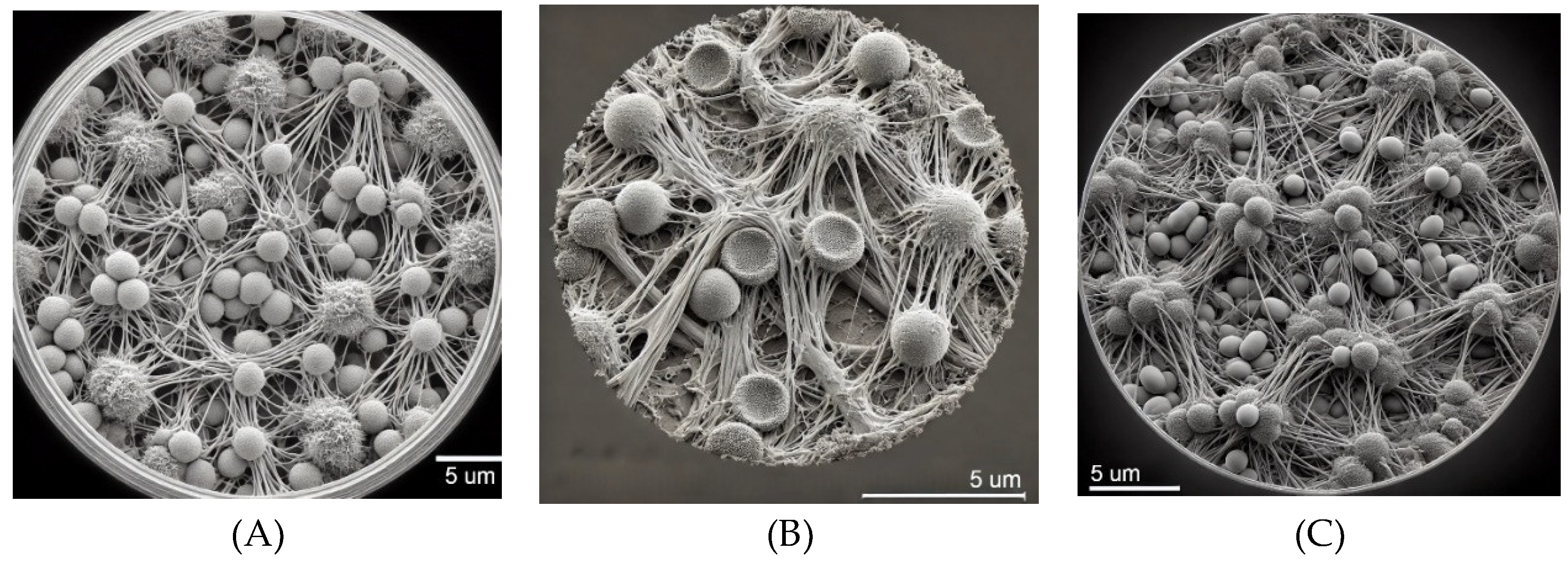
3.2. Disruption of C. albicans Biofilm Structure Following Exposure to trans-Cinnamaldehyde: Comparative SEM Analysis
The
Figure 2 of
Candida albicans affected by
trans-Cinnamaldehyde after 48 hours presents several notable differences compared to
Candida albicans before exposure and in the control well.
Before Exposure (
Figure 2A) and
Control Well (
Figure 2C)
: In both the unexposed and control well conditions, the biofilm of
Candida albicans typically shows well-organized, dense networks of hyphal structures intertwined with clusters of round or oval yeast cells. These biofilms are highly structured, with the hyphal elements providing robust adhesion and stability, contributing to the resilience of the biofilm. The filamentous hyphae are characteristic of mature, untreated biofilms, forming a three-dimensional matrix that is resistant to antifungal agents.
Affected by trans-Cinnamaldehyde (
Figure 2B)
: The SEM image of
C. albicans after exposure to
trans-Cinnamaldehyde reveals significant morphological disruptions. The hyphal structures appear to be less organized and show signs of fragmentation or deformation, with less density in the filamentous network compared to the untreated biofilms. The yeast cells may also display surface irregularities or damage, indicating the antifungal effect of
trans-Cinnamaldehyde. The reduction in hyphal density and structural integrity suggests that
trans-Cinnamaldehyde disrupts the biofilm matrix, weakening the adhesion and stability of the biofilm.
3.3. Comparative Anti-Biofilm Activity of trans-Cinnamaldehyde and Nystatin Assessed by Optical Densitometry Using the MTT Assay
The results presented in
Table 1 illustrate the MBIC of
trans-Cinnamaldehyde and Nystatin against biofilms formed by
Candida albicans,
Candida glabrata, and
Candida tropicalis.
For trans-Cinnamaldehyde, both C. albicans and C. glabrata exhibited MBIC50 values of 0.16 mg/mL and MBIC100 values of 0.32 mg/mL, showing similar susceptibility to the compound. In contrast, C. tropicalis demonstrated significantly higher resistance to trans-Cinnamaldehyde, with an MBIC50 of 0.32 mg/mL and an MBIC100 of 0.63 mg/mL (p < 0.05, compared to C. albicans and C. glabrata). These findings suggest that while trans-Cinnamaldehyde exhibits moderate antifungal activity against biofilm formation in C. albicans and C. glabrata, it is less effective against C. tropicalis, which requires significantly higher concentrations for comparable biofilm inhibition.
Nystatin exhibited significantly greater antifungal efficacy across all species. For C. albicans and C. glabrata, Nystatin achieved MBIC50 values of 0.0015 mg/mL, MBIC80 values of 0.004 mg/mL, and MBIC100 values of 0.008 mg/mL, indicating strong biofilm inhibition at relatively low concentrations. However, C. tropicalis demonstrated increased resistance to Nystatin, with an MBIC50 of 0.002 mg/mL, an MBIC80 of 0.0160 mg/mL, and an MBIC100 of 0.032 mg/mL (p < 0.05 compared to C. albicans and C. glabrata). These findings confirm that C. tropicalis remains the most resistant species to both antifungal agents, particularly at the MBIC80 and MBIC100 levels.
A one-way ANOVA followed by Tukey’s post-hoc test confirmed that:
For trans-Cinnamaldehyde:
C. tropicalis exhibited significantly higher MBIC50 and MBIC100 values than both C. albicans and C. glabrata (p < 0.05), indicating that C. tropicalis is more resistant to trans-Cinnamaldehyde.
No significant differences were observed between C. albicans and C. glabrata for either MBIC50 or MBIC100 (p > 0.05), suggesting similar susceptibility to the compound.
For Nystatin:
Both C. albicans and C. glabrata showed significantly lower MBIC50 and MBIC100 values compared to C. tropicalis (p < 0.05), indicating greater resistance in C. tropicalis. A statistically significant difference was observed between C. tropicalis and the other species for MBIC80 (p < 0.01), suggesting that C. tropicalis is much harder to inhibit at this 80% level of biofilm inhibition.
Nystatin was significantly more effective than trans-Cinnamaldehyde at inhibiting biofilm formation across all species, with consistently lower MBIC values (p < 0.001).
Comparative Analysis Between Agents:
Across all three Candida species, Nystatin exhibited significantly lower MBIC values than trans-Cinnamaldehyde at all levels of biofilm inhibition (MBIC50, MBIC80, and MBIC100) (p < 0.001). This confirms Nystatin’s superior efficacy against biofilm formation across the board.
C. tropicalis showed higher resistance to both antifungal agents compared to C. albicans and C. glabrata at all inhibition levels, particularly with trans-Cinnamaldehyde.
The statistical analysis reveals that while Nystatin is significantly more effective than trans-Cinnamaldehyde in inhibiting biofilm formation across all Candida species, C. tropicalis remains the most resistant species to both treatments, particularly trans-Cinnamaldehyde.
The results presented in the
Figure 3 chart demonstrate the inhibitory effect of
trans-Cinnamaldehyde on biofilm formation in
Candida albicans,
Candida glabrata, and
Candida tropicalis at concentrations ranging from 5.00 mg/mL to 0.01 mg/mL. At higher concentrations (5.00, 2.50, and 1.25 mg/mL),
trans-Cinnamaldehyde exhibited near-total inhibition (approximately 100%) across all three Candida species, indicating its potent antifungal activity at these levels. The efficacy remained robust at 0.63 mg/mL, with all species showing similar inhibition levels. However, a marked divergence was observed at 0.32 mg/mL, where
C. albicans and
C. glabrata maintained over 80% inhibition, whereas
C. tropicalis exhibited a lower inhibition rate (~60%), suggesting a reduced sensitivity to
trans-Cinnamaldehyde at this concentration. As the concentration decreased further (0.16 mg/mL and below), the inhibitory effect of
trans-Cinnamaldehyde diminished significantly.
C. albicans and
C. glabrata retained moderate inhibition (50-60%) at 0.16 mg/mL but showed a steep decline at concentrations below 0.08 mg/mL. In contrast,
C. tropicalis displayed greater resistance, with its inhibition dropping to approximately 40% at 0.16 mg/mL and continuing to decrease more sharply at lower concentrations. At the lowest concentration of 0.01 mg/mL, all species demonstrated minimal inhibition, indicating that
trans-Cinnamaldehyde was ineffective at this dose. Overall, the data suggest that while
trans-Cinnamaldehyde is highly effective at inhibiting biofilm formation at higher concentrations, its efficacy diminishes considerably at lower doses, particularly for
C. tropicalis.
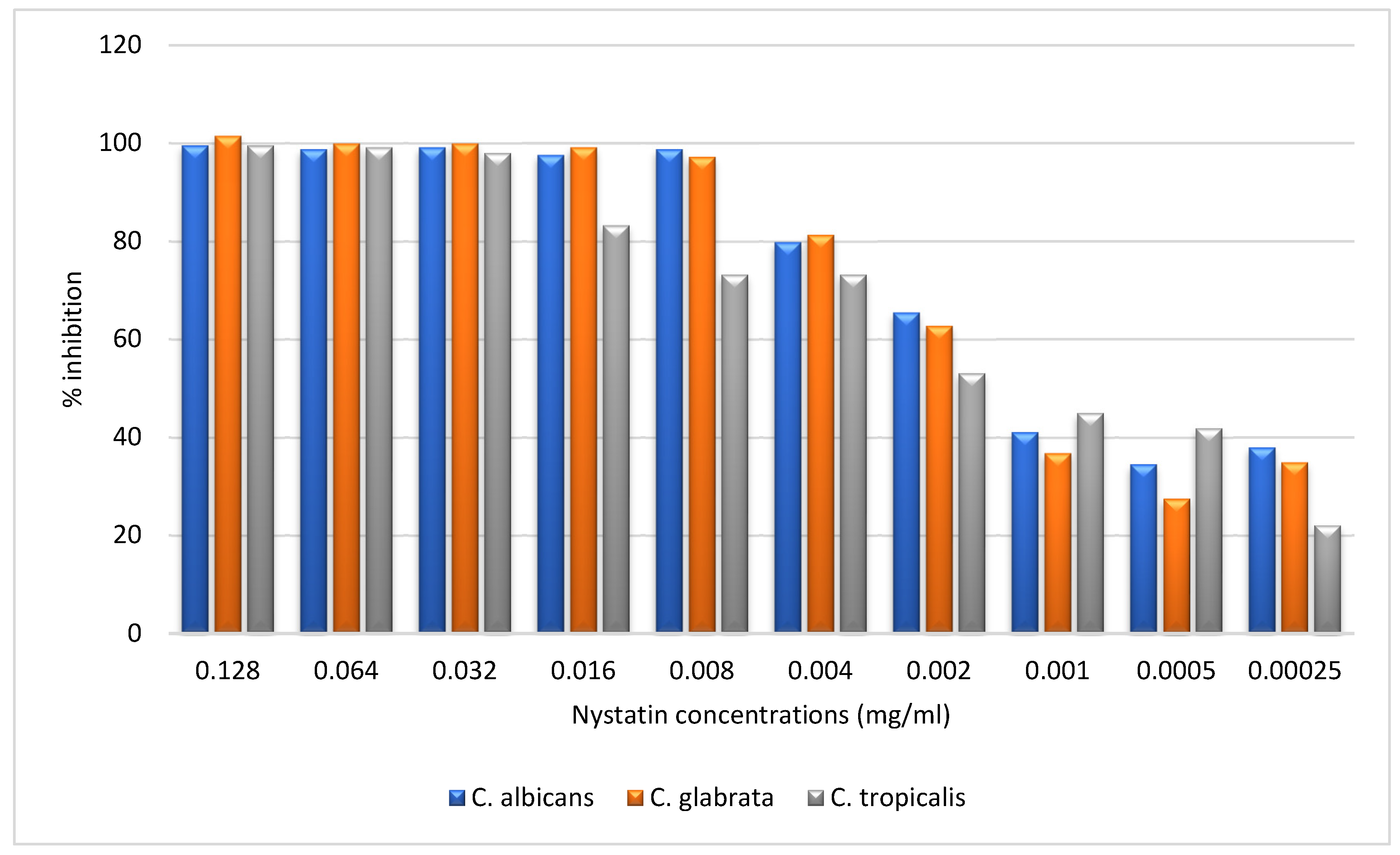
The results presented in the
Figure 4 chart illustrate the inhibitory effect of Nystatin on biofilm formation in
Candida albicans,
Candida glabrata, and
Candida tropicalis at concentrations ranging from 0.128 mg/mL to 0.00025 mg/mL. At higher concentrations (0.128, 0.064, and 0.032 mg/mL), Nystatin exhibited near-complete inhibition (approximately 100%) across all three species, indicating its potent antifungal activity. However, as the concentration decreased to 0.016 mg/mL,
C. tropicalis began to show reduced inhibition (~80%), while
C. albicans and
C. glabrata maintained higher inhibition levels (close to 90%). This trend continued as the concentration was further reduced to 0.008 mg/mL, with
C. tropicalis exhibiting a more pronounced decline (~60%) compared to the other species. At lower concentrations (0.004 mg/mL to 0.001 mg/mL), all species experienced a significant decrease in inhibition, particularly C. tropicalis, which dropped to approximately 50%, while
C. albicans and
C. glabrata showed moderate inhibition (~60-70%). At the lowest concentrations (0.0005 mg/mL and 0.00025 mg/mL),
C. tropicalis exhibited the lowest inhibition (~20-30%), whereas
C. albicans and
C. glabrata retained moderate inhibition (~40-50%). Overall, the data indicate that while Nystatin is highly effective at higher concentrations for all species,
C. tropicalis exhibits greater resistance, particularly at lower doses, compared to
C. albicans and
C. glabrata.
3.4. Comparative Antifungal Efficacy of trans-Cinnamaldehyde and Nystatin Against Planktonic and Biofilm Forms of Candida spp.
The data in
Table 2 highlights the comparative antifungal activities of
trans-Cinnamaldehyde and Nystatin against both planktonic and biofilm forms of
Candida albicans,
Candida glabrata, and
Candida tropicalis. The planktonic minimum inhibitory concentrations (PMIC) for
trans-Cinnamaldehyde ranged from 0.015 to 0.033 mg/mL, with
C. tropicalis displaying significantly higher resistance compared to
C. albicans and
C. glabrata (p < 0.05). Nystatin, however, showed much lower PMIC values across all species, especially for
C. glabrata (PMIC = 0.0008 mg/mL), which was the most sensitive species to this treatment.
The minimum biofilm inhibitory concentration (MBIC100) for trans-Cinnamaldehyde was highest for C. tropicalis (0.63 mg/mL), which demonstrated significantly greater resistance than the other two species (p < 0.05). Similarly, the PMFC values indicated that the fungicidal concentrations for trans-Cinnamaldehyde were higher for C. tropicalis (PMFC = 0.033 mg/mL), further reinforcing its resistance. Nystatin again demonstrated much stronger biofilm inhibition, with MBIC100 values of 0.008 mg/mL for C. albicans and C. glabrata, and 0.032 mg/mL for C. tropicalis (p < 0.001 when comparing C. tropicalis to the other species).
A one-way ANOVA followed by Tukey’s post-hoc test revealed significant differences between the antifungal activities of trans-Cinnamaldehyde and Nystatin. Nystatin was found to be significantly more effective across all tested fungal strains (p < 0.001), particularly for inhibiting the biofilm forms. The mean ± SD values indicate consistent results across multiple experiments, confirming the reproducibility of the data.
4. Discussion
This study provides valuable insights into the comparative antifungal activity of
trans-Cinnamaldehyde and Nystatin against
Candida albicans,
Candida glabrata, and
Candida tropicalis in both planktonic and biofilm forms. The data consistently demonstrate Nystatin’s superior efficacy, with significantly lower PMIC and MBIC
100 values across all tested species. These findings align with previous studies, which have established Nystatin as a gold standard in antifungal therapy due to its mechanism of action that involves binding to ergosterol in fungal membranes, leading to increased permeability and cell lysis [
20]. This makes Nystatin particularly effective against biofilm forms, which are notoriously more resistant to antifungal treatment due to the protective extracellular matrix that limits drug penetration [
21].
Our study adds to the existing body of literature by highlighting that while Nystatin maintains potent activity against biofilms of
C. albicans and
C. glabrata at MBIC
100 values as low as 0.008 mg/mL,
C. tropicalis presents a more challenging target. The MBIC
100 for
C. tropicalis was found to be 0.032 mg/mL [
22,
23], reflecting its greater biofilm resilience. This aligns with observations by Chandra et al. (2005), who documented the enhanced biofilm-forming capabilities of
C. tropicalis, particularly in clinical settings where biofilms are associated with persistent infections. This resistance can be attributed to the species’ thicker extracellular matrix and higher metabolic activity within biofilms, which protect fungal cells from the effects of antifungal agents [
24].
On the other hand,
trans-Cinnamaldehyde, while less potent than Nystatin, demonstrated reasonable antifungal activity, particularly in planktonic forms. The PMIC values for
trans-Cinnamaldehyde, particularly for
C. albicans and
C. glabrata, are consistent with its known ability to disrupt cell membranes and interfere with fungal ergosterol synthesis [
14,
25]. However, its limited efficacy against biofilm forms, especially
C. tropicalis (MBIC
100 of 0.630 mg/mL) [
14], underscores the complexity of treating biofilm-associated infections. These results suggest that while
trans-Cinnamaldehyde holds promise as an alternative or adjunct therapy, it may require higher concentrations or combination with more potent agents to achieve clinical efficacy in biofilm eradication [
26,
27].
A key finding from this study is the differential response of
C. tropicalis to both antifungal agents, which was consistently more resistant than
C. albicans and
C. glabrata in both planktonic with established antifungals such as Nystatin, could provide a synergistic effect that enhances biofilm penetration and reduces the risk of resistance development [
28].
The implications of these findings are multifaceted. From a clinical perspective, Nystatin remains the treatment of choice for biofilm-associated Candida infections, particularly for
C. albicans and
C. glabrata [
29]. However, the rising incidence of
C. tropicalis infections, coupled with its higher resistance to antifungal agents, highlights the need for alternative strategies.
Trans-Cinnamaldehyde, despite its limitations, could play a role in combination therapies or as a prophylactic agent to prevent biofilm formation in early-stage infections [
30,
31]. The need for higher doses to combat mature biofilms, especially for
C. tropicalis, suggests that future research should explore synergistic effects between
trans-Cinnamaldehyde and conventional antifungals, as well as novel delivery methods to enhance its bioavailability and efficacy [
31,
32].
In summary, this study underscores the critical importance of understanding species-specific responses to antifungal treatments, particularly in the context of biofilm-associated infections. While Nystatin continues to outperform trans-Cinnamaldehyde, the latter offers potential as an adjunct treatment, especially in combination therapies. The greater resistance of C. tropicalis to both agents emphasizes the need for continued research into alternative antifungal strategies that target biofilm resilience and prevent the development of antifungal resistance. Future studies should focus on optimizing trans-Cinnamaldehyde’s efficacy, exploring its potential in combination therapies, and addressing the growing clinical challenge posed by C. tropicalis biofilm-related infections.
5. Conclusion
This study provides a comprehensive evaluation of the antifungal potential of trans-Cinnamaldehyde compared to Nystatin against both planktonic and biofilm forms of Candida albicans, Candida glabrata, and Candida tropicalis. Our findings reaffirm the critical challenge posed by biofilm-associated fungal infections, where biofilm structures confer significant resistance to conventional treatments. While Nystatin demonstrated superior antifungal activity with markedly lower minimum inhibitory concentrations, particularly in biofilm inhibition, trans-Cinnamaldehyde emerged as a promising alternative with moderate efficacy against C. albicans and C. glabrata. However, C. tropicalis exhibited a striking level of resistance, underscoring the need for tailored therapeutic strategies targeting biofilm resilience in this species.
The unique mechanism of trans-Cinnamaldehyde, primarily through the disruption of fungal cell walls and interference with ergosterol biosynthesis, offers a potential adjunctive approach in antifungal therapy. Despite its moderate performance in our study, trans-Cinnamaldehyde’s natural origin and relatively low toxicity profile make it an attractive candidate for further exploration, particularly in combination with established antifungal agents like Nystatin. The observed biofilm resistance, particularly in C. tropicalis, further highlights the urgent need for innovative treatment modalities that can penetrate biofilms and target dormant fungal cells effectively.
From a clinical perspective, our findings highlight the limitations of relying solely on current antifungal therapies for managing biofilm-related infections. The resistance exhibited by C. tropicalis to both agents suggests that monotherapies may be insufficient in addressing biofilm-associated candidiasis. This reinforces the need for integrated therapeutic strategies, combining natural bioactive compounds such as trans-Cinnamaldehyde with conventional antifungals, to enhance efficacy and minimize drug resistance.
Moving forward, further research is needed to optimize the delivery and concentration of trans-Cinnamaldehyde, explore potential synergistic interactions with other antifungal agents, and assess its performance in vivo. Moreover, mechanistic studies focusing on the molecular pathways that confer biofilm resistance in C. tropicalis could unveil new therapeutic targets, thereby advancing the field of antifungal drug development.
In conclusion, while Nystatin remains a highly effective antifungal agent, particularly for biofilm inhibition, trans-Cinnamaldehyde represents a promising adjunctive treatment. This study adds to the growing body of evidence supporting the use of natural compounds in antifungal therapies and provides a foundation for future investigations into biofilm-targeting strategies. Addressing the challenges of biofilm-mediated resistance is critical to improving clinical outcomes in fungal infections, particularly in light of the increasing prevalence of drug-resistant Candida species.
Funding
This research received no external funding.
Compliance with Ethical Standards:
This article does not involve any studies conducted by the authors that included human participants.
Acknowledgments
The completion of this research work was made possible through the collaborative efforts and dedication of a multidisciplinary team. We extend our sincere appreciation to each member for their invaluable contributions.
Conflicts of Interest
The authors declare no conflicts of in-terest.
References
- Andes, D.R.; et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transplant Infectious Disease 2016, 18, 921–931. [Google Scholar] [CrossRef]
- Cortés, J.A.; Corrales, I.F. Invasive candidiasis: epidemiology and risk factors. In Fungal infection; IntechOpen London: UK, 2018. [Google Scholar]
- Soulountsi, V.; Schizodimos, T.; Kotoulas, S.C. Deciphering the epidemiology of invasive candidiasis in the intensive care unit: is it possible? Infection 2021, 49, 1107–1131. [Google Scholar] [CrossRef]
- Seddiki, S.; et al. Infectivités fongiques des cathéters implantés dues à Candida sp. Formation des biofilms et résistance. Journal de Mycologie Médicale 2015, 25, 130–135. [Google Scholar] [CrossRef]
- Lopez-Ribot, J.L. Large-scale biochemical profiling of the Candida albicans biofilm matrix: new compositional, structural, and functional insights. MBio 2014, 5, 10–1128. [Google Scholar] [CrossRef]
- Tribedi, P.; Gupta, A.D.; Sil, A.K. Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresources and Bioprocessing 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; et al. The effect of antifungal combination on transcripts of a subset of drug-resistance genes in clinical isolates of Candida species induced biofilms. Saudi Pharmaceutical Journal 2015, 23, 55–66. [Google Scholar] [CrossRef]
- Rao, H.; et al. Approaches for mitigating microbial biofilm-related drug resistance: a focus on micro-and nanotechnologies. Molecules 2021, 26, 1870. [Google Scholar] [CrossRef]
- Sandai, D.; et al. Resistance of Candida albicans biofilms to drugs and the host immune system. Jundishapur Journal of Microbiology 2016, 9. [Google Scholar] [CrossRef]
- Uruén, C.; et al. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a delivery system for the treatment of biofilm-mediated infections. Journal of applied microbiology 2021, 131, 2626–2639. [Google Scholar] [CrossRef]
- de Lima Carvalho, P.C.; et al. Anti-candida activity of cinnamon inhibition of virulence factors of clinical strains of Candida albicans by essential oil of Cinnamomum zeylanicum. PSM Microbiology 2018, 3, 4–12. [Google Scholar]
- Polak-Wyss, A. Mechanism of action of antifungals and combination therapy. Journal of the European Academy of Dermatology and Venereology 1995, 4, S11–S16. [Google Scholar] [CrossRef]
- Shreaz, S.; et al. Antifungal activity of α-methyl trans cinnamaldehyde, its ligand and metal complexes: promising growth and ergosterol inhibitors. Biometals 2011, 24, 923–933. [Google Scholar] [CrossRef]
- Wang, S.; Kang, O.-H.; Kwon, D.-Y. Trans-cinnamaldehyde exhibits synergy with conventional antibiotic against methicillin-resistant Staphylococcus aureus. International Journal of Molecular Sciences 2021, 22, 2752. [Google Scholar] [CrossRef]
- Lampen, J.; et al. Location and role of sterol at nystatin-binding sites. Journal of bacteriology 1962, 84, 1152–1160. [Google Scholar] [CrossRef]
- Pereira de Mello, T.; et al. Fungal biofilm-a real obstacle against an efficient therapy: lessons from Candida. Current Topics in Medicinal Chemistry 2017, 17, 1987–2004. [Google Scholar] [CrossRef]
- Kumar, S.N.; et al. Asarones from Acorus calamus in combination with azoles and amphotericin B: a novel synergistic combination to compete against human pathogenic Candida species in vitro. Applied biochemistry and biotechnology 2015, 175, 3683–3695. [Google Scholar] [CrossRef]
- Pierce, C.G.; et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nature protocols 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Bandara, H.; Matsubara, V.H.; Samaranayake, L.P. Future therapies targeted towards eliminating Candida biofilms and associated infections. Expert review of anti-infective therapy 2017, 15, 299–318. [Google Scholar] [CrossRef]
- Watamoto, T.; et al. Screening of pharmacologically active small molecule compounds identifies antifungal agents against Candida biofilms. Frontiers in Microbiology 2015, 6, 1453. [Google Scholar] [CrossRef]
- Gunderson, S.M.; et al. In vitro pharmacodynamic characteristics of nystatin including time-kill and postantifungal effect. Antimicrobial agents and chemotherapy 2000, 44, 2887–2890. [Google Scholar] [CrossRef]
- Mehta, R.; et al. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as therapeutic agent for systemic candidiasis. Antimicrobial agents and chemotherapy 1987, 31, 1897–1900. [Google Scholar] [CrossRef]
- Chandra, J.; Zhou, G.; Ghannoum, M.A. Fungal biofilms and antimycotics. Current Drug Targets 2005, 6, 887–894. [Google Scholar] [CrossRef]
- Rahemi, D.; et al. An in vitro study of the effect of cinnamaldehyde on the growth of Candida albicans compared to nystatin and fluconazole. 2015.
- Ahmad, A.; Molepo, J.; Patel, M. Challenges in the development of antifungal agents against Candida: scope of phytochemical research. Current Pharmaceutical Design 2016, 22, 4135–4150. [Google Scholar] [CrossRef]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging antifungal targets and strategies. International Journal of Molecular Sciences 2022, 23, 2756. [Google Scholar] [CrossRef]
- Tonon, C.C.; et al. Interactions between Terpinen-4-ol and Nystatin on biofilm of Candida albicans and Candida tropicalis. Brazilian dental journal 2018, 29, 359–367. [Google Scholar] [CrossRef]
- Benavent, C.; Torrado-Salmerón, C.; Torrado-Santiago, S. Development of a solid dispersion of nystatin with maltodextrin as a carrier agent: Improvements in antifungal efficacy against Candida spp. biofilm infections. Pharmaceuticals 2021, 14, 397. [Google Scholar] [CrossRef]
- Atriwal, T.; et al. Mechanistic understanding of Candida albicans biofilm formation and approaches for its inhibition. Frontiers in Microbiology 2021, 12, 638609. [Google Scholar] [CrossRef]
- Bujdáková, H. Management of Candida biofilms: state of knowledge and new options for prevention and eradication. Future microbiology 2016, 11, 235–251. [Google Scholar] [CrossRef]
- Zuo, X.s.; et al. Association of different Candida species with catheter-related candidemia, and the potential antifungal treatments against their adhesion properties and biofilm-forming capabilities. Journal of Clinical Laboratory Analysis 2021, 35, e23738. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).