1. Introduction
The patients with head and neck cancer (HNC) often experience impaired sleep. Sleep quality is closely linked to fatigue, emotional distress and pain, and it impacts deeply the overall quality of life both pre and post treatment [
1]. The most common sleep disturbances among this population are insomnia, hypersomnolence and respiratory sleep disturbances, with the most reported one being obstructive sleep apnea (OSA) [
2].
OSA is a common sleep disorder characterized by repetitive episodes of partial or complete upper airway obstruction during sleep. HNC patients are particularly susceptible to OSA due to the anatomical and functional changes caused by both the malignancy and its treatments, such as surgery, radiation therapy, and chemotherapy [
3]. These treatments may worsen pre-existing OSA or lead to new onset of the condition, further contributing to the overall disease burden and the patients’ overall quality of life [
4].
Insomnia is a sleep disorder characterized by difficulty falling asleep, staying asleep, or waking up too early and being unable to return to sleep. It can result in poor-quality or insufficient sleep, leading to daytime fatigue, mood disturbances, irritability, and impaired cognitive function. Insomnia is a prevalent issue among patients with HNC, it is often present before and after diagnosis and treatment. Insomnia in these patients is often linked with other complications like pain, fatigue, and emotional distress, significantly impacting their quality of sleep and life [
5].
The prevalence of OSA in HNC patients has been reported to range from 57% to 96%, depending on the stage of cancer and the type of treatment received [
6]. Moreover, the occurrence of OSA may exacerbate existing comorbidities (e.g., cardiovascular diseases and metabolic syndrome), often common in this type of patients [
7]. The anatomical distortions caused by tumors or therapeutic interventions, such as radiation-induced fibrosis or surgical alterations to the airway, are key contributors to the development of OSA in this population [
6]. Studies showed that the prevalence of insomnia ranged from 29% before treatment to 45% during treatment and remains at 40% after treatment, indicating a persistent problem throughout the cancer journey [
2]. Additionally, psychological factors, such as anxiety and depression, that are common in cancer patients, may also negatively impact sleep quality [
8].
Moreover, studies showed that HNC patients who experienced poor sleep were more likely to suffer from daytime fatigue and reduced cognitive function, further diminishing their ability to manage daily activities [
9]. This suggests that managing insomnia and OSA is crucial for improving the overall well-being of head and neck cancer patients during and after treatment. Proper screening, diagnosis, and treatment of OSA, including the use of continuous positive airway pressure (CPAP) therapy, may help alleviate symptoms and improve outcomes for these patients [
4]. However, adherence to CPAP therapy remains a significant challenge, with compliance rates being notably low in this patient group [
1]. Assessment of distress, anxiety and depression should be carried on from diagnosis, through treatment and after, and proper psychological management should alleviate the disease burden and lead to a better quality of sleep and thus life. This approach is often overlooked as 82% of patients that report distress, anxiety and depression do not receive treatment or council to alleviate the problem [
10].
In conclusion, the interaction between sleep disturbances such as insomnia, obstructive sleep apnea, and HNC poses a significant challenge for clinicians. Both the diseases and its treatment can have profound effects on sleep quality, making it crucial to include sleep and psychological evaluation as a standard component of cancer care. The aim of this observational study was to evaluate the sleep quality after treatment for head and neck cancer. Moreover, it was analyzed in association with quality of life and psychological distress.
2. Materials and Methods
One hundred fifty-one patients who underwent treatment for head and neck cancer at our department were included in the study. Exclusion criteria were the following: age <18 years, follow-up <12 months, non-epithelial tumors, neurological and/or psychiatric disorders that may impact on the ability to fulfill the questionnaires, active treatment for recurrence or second tumor. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained in every case. Institutional Review Board (A.O.U. Città della Salute e della Scienza di Torino - A.O. Ordine Mauriziano - A.S.L. Città di Torino) approval was obtained.
The clinical characteristics, the treatment modality, and the late radiotherapy toxicity (Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer late radiation morbidity scoring system - RTOG/EORTC scale) were recorded. The American Joint Committee of Cancer (AJCC) staging system, 8th edition (TNM – Tumour Node Metastasis), was used for all the tumors. The treatment was based on national and international guidelines.
Quality of life (EORTC QLQ-C30 and EORTC H&N35 questionnaires), sleep quality (Pittsburgh Sleep Quality Index - PSQI), risk of sleep apnea (STOP-BANG), sleepiness (Epworth Sleepiness Scale - ESS), and psychological distress (Distress Thermometer – DT, Hospital Anxiety and Depression Scale - HADS) were assessed. A Visual Analogue Scale (VAS) was used for pain (0 = no pain; 10 = the worst pain).
The EORTC-QLQ C30 questionnaire assessed quality of life through 35 items, evaluating physical, role, emotional, social and cognitive functioning and somatic symptoms, in addition to the global health score. It was linked with a specific head and neck module (QLQ-H&N35), a 35-item questionnaire that assessed symptoms encountered specifically by patients with head and neck cancer. Higher symptoms scores indicated worse symptoms, while higher scores for global health and functioning scales indicated better quality of life [
11,
12].
The PSQI questionnaire consisted of 19 items that measures the sleep quality over the previous month. Seven domains (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction) were evaluated. Higher scores indicated poorer sleep quality. Taken together, the seven sleep domains were scored as a single factor of global sleep quality (good versus poor): a global score higher than 5 indicated poor sleep quality [
13].
The risk of sleep apnea was assessed with the STOP-BANG score which included 8 dichotomous (yes/no) items (loud snoring, tiredness, observed apnea, systemic hypertension, body mass index - BMI >35 kg/m2, age >50 years, neck circumference >40 cm, and male gender). The total score allowed to identify subjects with low, moderate or high risk of sleep apnea [
14].
The sleepiness was evaluated by means of ESS which consisted of 8 items representing more or less soporific situations that are scored in a scale of 0-3 (0 = none; 1 = slight; 2 = moderate; 3 = high), resulting in a total score ranging from 0 to 24 points. A higher score suggested a higher propensity to fall asleep (a score above 10 indicated significant daytime sleepiness) [
15].
The DT was administered as an easy tool to evaluate the psychological distress by means of a rating scale from 0 (no distress) to 10 (extreme distress), referring to the last week [
16]. The HADS questionnaire was administered to assess anxiety and depression. included 14 items, seven for anxiety and seven for depression, rated on a 4-point Likert scale (range 0–3). For each subscale the score was the sum of the respective seven items (range 0–21) [
17].
All statistical analyses were carried out using the Statistical Package for Social Sciences, version 26.0. The Kolmogorov-Smirnov test demonstrated a non-Gaussian distribution of variables, so non-parametric tests were used. A descriptive analysis of all data was performed, and they were reported as medians and interquartile range (IQR), or percentages. The Mann-Whitney U test was used for comparison between two independent groups. The Chi-squared or the Fisher exact test was used for categorical variables. The Spearman’s test was used to assess the correlation between continuous variables. A p<0.05 was considered statistically significant.
3. Results
The median age was 66 years (IQR 17 years), while the median Body Mass Index (BMI) was 24.86 (IQR 5.09). The median follow-up was 30 months (IQR 49 months).
Table 1 highlights the clinical characteristics of the subjects included in the study, whereas
Table 2 reports the late radiation toxicity for patients who underwent radiotherapy.
The median VAS value for pain was 0 (IQR 2). The EORTC QLQ-C30 globally showed a good quality of life (
Table 3).
The median ESS score was 1 (IQR 4). The sleep apnea risk evaluated by means of STOP-BANG was low in 74 (49.0%) subjects, intermediate in 52 (34.4%) patients, and high in 25 (16.6%) cases.
Table 4 reports the results about sleep quality (PSQI questionnaire). In particular, a poor sleep quality was observed in 84 (55.6%) subjects.
The median DT score was 1 (IQR 4). The HADS questionnaire showed a median anxiety score of 4 (IQR 5) and a median depression score of 4 (IQR 7).
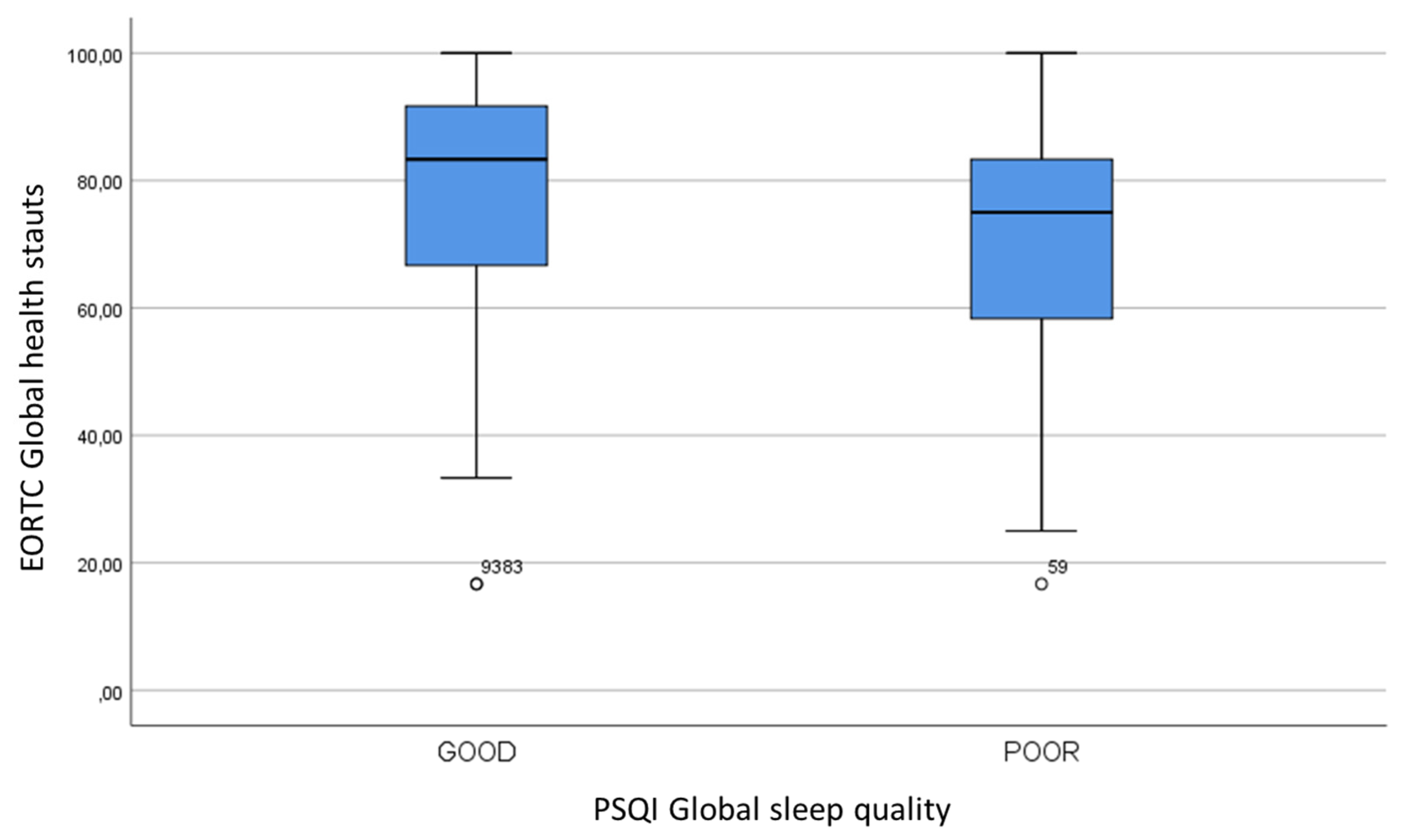
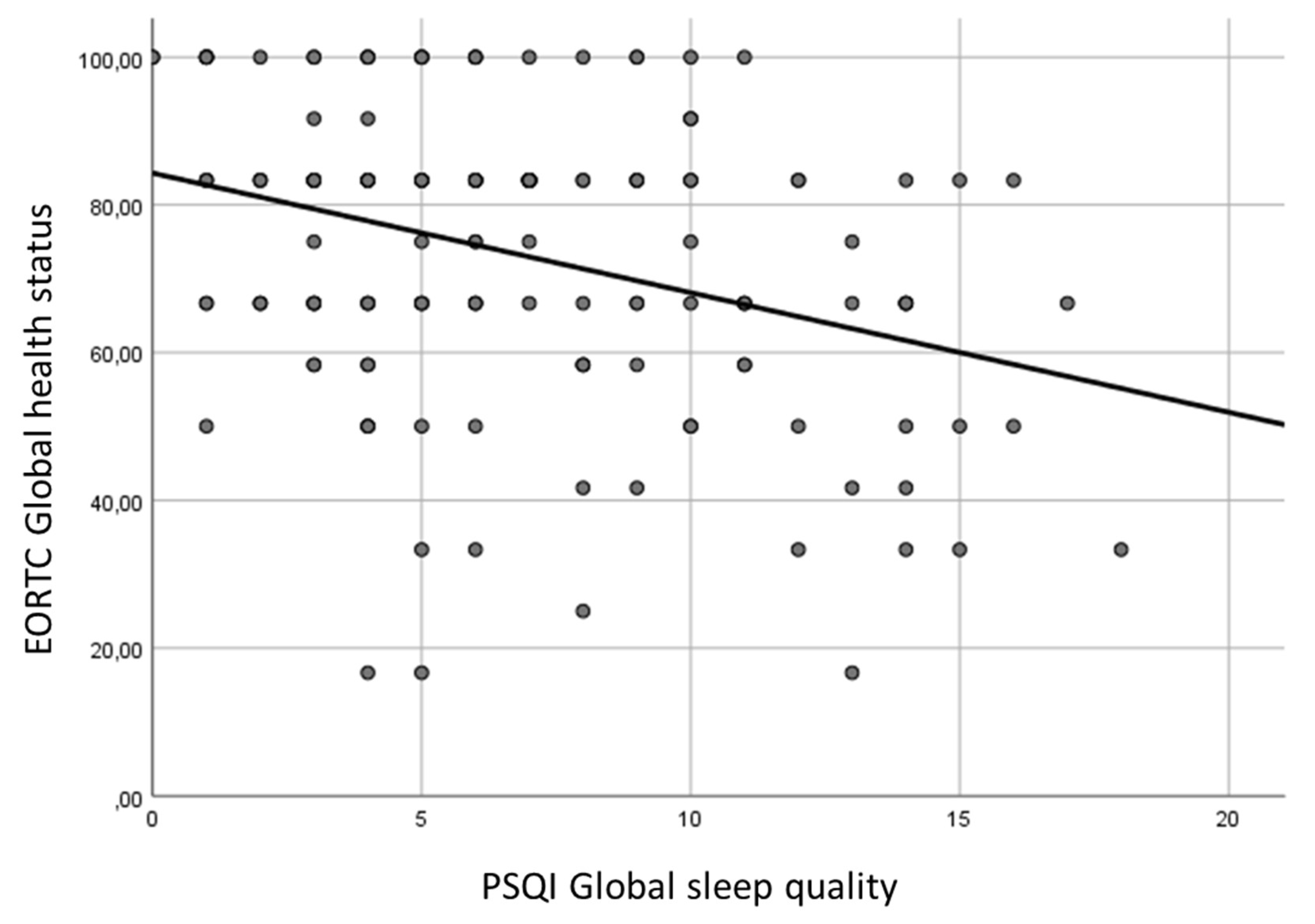
We sought to evaluate the relationship between sleep quality and other parameters. The PSQI global sleep quality was not statistically related to clinical features, such as sex, age, smoking, alcohol consumption, BMI, tumor site and stage, treatment modality, radiation toxicities and follow-up (p>0.05). However, women seemed to have a worse sleep quality: a poor sleep quality was observed in 67.4% of women and in 50.5% of men (p=0.054). A statistically significant association between PSQI global sleep quality and EORTC global health status was found (
Table 5,
Figure 1).
The correlation tests among PSQI scores and quality of life highlighted the following significant associations (p<0.05):
PSQI global score with global health status (
Figure 2), physical functioning, role functioning, emotional functioning, cognitive functioning, weight gain (inverse correlation), fatigue, pain, dyspnea, insomnia, appetite loss, constipation, diarrhoea, social eating, social contact, sexuality, problems opening mouth, dry mouth, sticky saliva, cough, felt ill (direct correlation);
PSQI sleep quality with global health status, physical functioning, role functioning, emotional functioning, cognitive functioning, social fucntioning (inverse correlation), fatigue, pain, dyspnea, insomnia, appetite loss, constipation, pain in the mouth, social eating, social contact, sexuality, problems opening mouth, dry mouth, felt ill, weight loss (direct correlation);
PSQI sleep latency with global health status, physical functioning, role functioning, emotional functioning, cognitive functioning, weight gain (inverse correlation), fatigue, pain, dyspnea, insomnia, appetite loss, constipation, diarrhoea, social contact, sexuality, dry mouth (direct correlation);
PSQI sleep duration with weight gain (inverse correlation), insomnia (direct correlation);
PSQI habitual sleep efficiency with weight gain (inverse correlation), insomnia, diarrhoea, social eating, social contact, sexuality, sticky saliva (direct correlation);
PSQI sleep disturbances with global health status, physical functioning, emotional functioning, cognitive functioning (inverse correlation), fatigue, pain, dyspnea, insomnia, appetite loss, constipation, diarrhoea, financial difficulties, pain in the mouth, swallowing, sexuality, problems opening mouth, dry mouth, sticky saliva, cough, felt ill, weight loss (direct correlation);
PSQI use of sleeping medications with physical functioning, emotional functioning, (inverse correlation), dyspnea, financial difficulties (direct correlation);
PSQI daytime dysfunction with global health status, physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning (inverse correlation), fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, pain in the mouth, swallowing, social contact, problems opening mouth, dry mouth, sticky saliva, felt ill, weight loss (direct correlation).
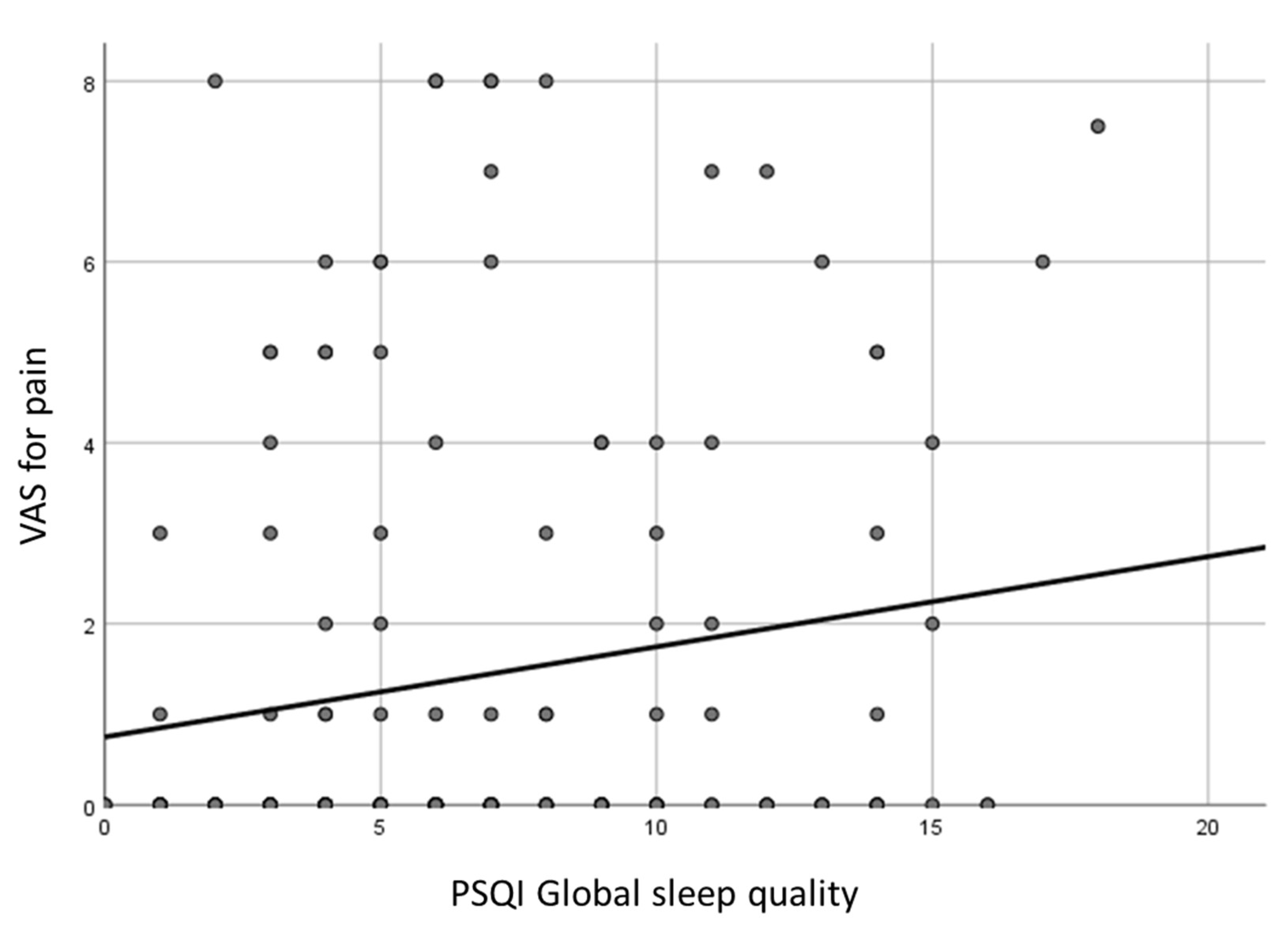
The VAS score for pain was significantly related to the following PSQI scores in a direct relationship: global score, sleep quality, sleep disturbances, daytime dysfunction (p<0.05).
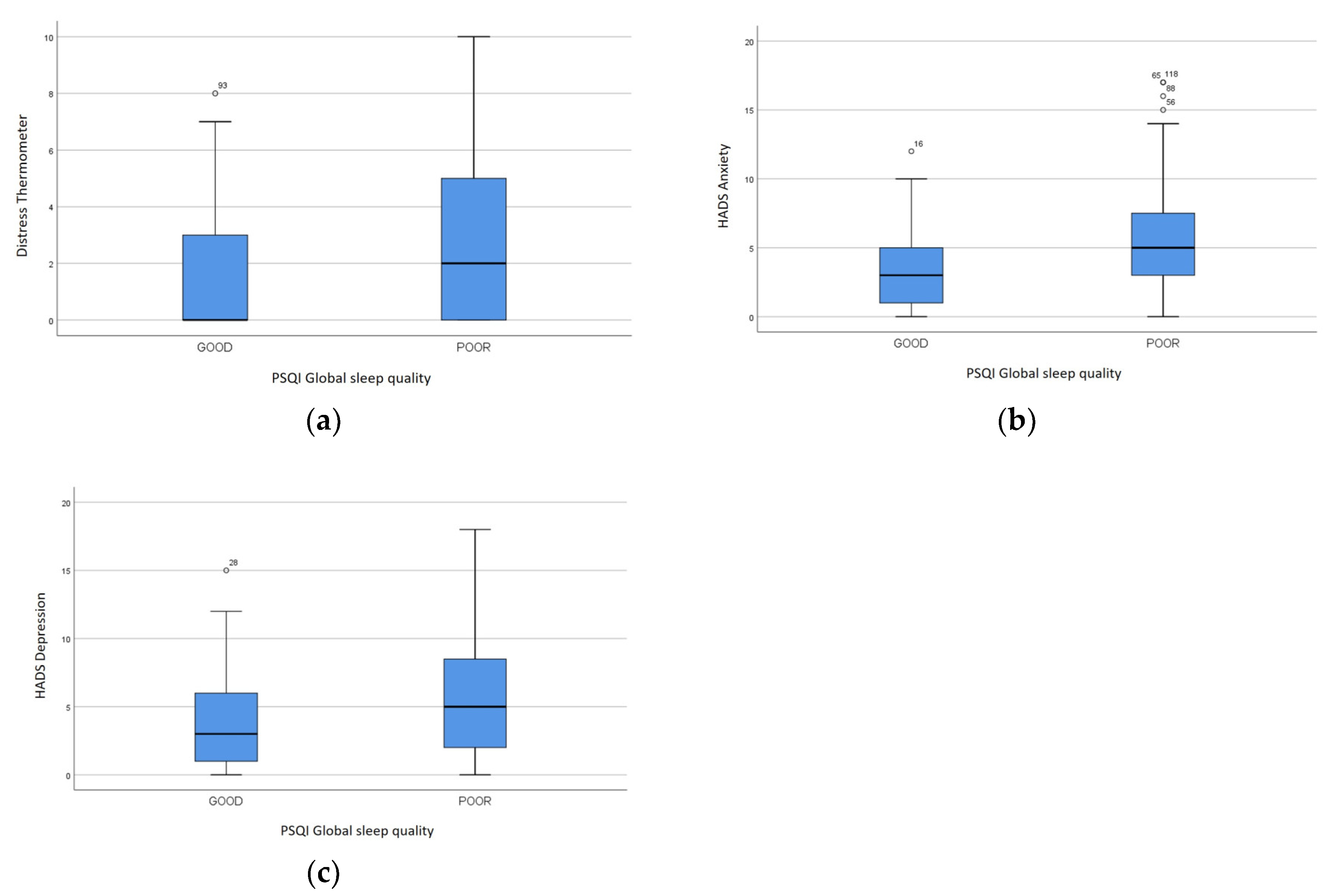
The DT and the HADS anxiety score demonstrated a direct relationship with PSQI global score, sleep quality, sleep latency, sleep disturbances, use of sleeping medications, daytime dysfunction (p<0.05). The HADS depression score had a direct association with the same PSQI scores (p<0.05), except for use of sleeping medications. In particular, the DT was 0 (IQR 3) and 2 (IQR 5) in subjects with good or poor sleep quality, respectively (p=0.006). The HADS anxiety score was 3 (IQR 4) and 5 (IQR 5) in patients with good or poor sleep quality, respectively (p=0.001), while the HADS depression score was 3 (IQR 5) and 5 (IQR 7), respectively (p=0.011) (
Figure 2).
Figure 4.
DT, HADS anxiety and depression scores Psychological distress in subjects with good and poor sleep quality: (a) DT (p<0.05), (b) HADS anxiety (p<0.05), (c) HADS depression (p<0.05).
Figure 4.
DT, HADS anxiety and depression scores Psychological distress in subjects with good and poor sleep quality: (a) DT (p<0.05), (b) HADS anxiety (p<0.05), (c) HADS depression (p<0.05).
The ESS score was directly associated with VAS score for pain and EORTC fatigue and inversely related to EORTC cognitive and social functioning, PSQI sleep disturbances and daytime dysfunction (p<0.05). It did not impact on DT and HADS scores (p>0.05).
No significant correlation was observed between risk of sleep apnea and EORTC scores (p>0.05). The PSQI sleep disturbances score was significantly associated to STOP-BANG category (p=0.001), being slightly higher in subjects with high risk of sleep apnea (mean value 1.48), compared to those with intermediate (mean value 1.29) and low risk (mean value 1.05). The STOP-BANG category did not negatively affect DT and HADS scores (p>0.05).
4. Discussion
This study focused on investigating the quality of life, sleep disorders, and their associated factors in patients treated for HNC. Among the most prevalent sleep disturbances in this population were insomnia and OSA, both extensively reported in the literature as the most frequent sleep-related problems affecting individuals with HNC [
2]. Similarly, our study confirmed that these two conditions were the primary sleep disorders impacting the patients’ outcomes, contributing significantly to their reduced quality of life.
Our study population of 151 patients consisted primarily of male patients (69.5%), with a median age of 66 years. As expected, a substantial proportion of patients (68.2%) were former smokers. Additionally, 21.2% of patients reported regular alcohol consumption. The demographics and lifestyle factors of our patient cohort were in line with the epidemiology of this type of cancer and other similar studies, further affirming the validity of our sample as representative of the HNC population [
6,
18,
19,
20].
When examining the tumor sites in our study, 30.5% of patients diagnosed with laryngeal and hypopharyngeal tumors, 39.1% had tumors in the oral cavity, 19.9% had oropharyngeal tumors, and the remaining 10.6% were affected by tumors in other sites, such as the salivary glands and nasopharynx. Most patients had an early stage of the disease (50.5%). In terms of treatment modalities, our study revealed that 43.7% of patients underwent surgery as their primary and only form of treatment. In contrast, 17.9% of patients received chemoradiotherapy alone. The remaining 38.4% of patients received a combination of both surgical intervention and chemoradiotherapy.
The overall quality of life in our sample was good with a median score of 83 at the EORTC-QLQ30 Questionnaire. Crucially, the highest score in all the different symptoms affecting quality of life was given to insomnia, signaling that sleep quality was one of the most important factors affecting the perceived quality of life. Pain and fatigue related to treatments are the most probable cause of insomnia, while also emotional distress and anxiety related to the disease can be an aggravating factor in impairing the sleep quality of our patients. This was true in every cohort of the study, but it was crucial for patients who underwent radiotherapy as primary or adjuvant treatment. Indeed, the most common symptom reported in the H&N35 was xerostomia, which is strongly related to radiation therapy. Xerostomia is a long-term collateral effect that can impair quality of life and sleep quality for many years after the end of the treatment. This was in agreement with literature findings which analyzed sleep quality, with a particular focus on insomnia and OSA, and the perceived quality of life in HNC patients treated with radiotherapy [
18,
21].
In our study, more than half of the patients (55.6%) reported experiencing poor sleep quality, as indicated by their responses on the PSQI questionnaire. At the same time, the median ESS score was 1, suggesting that, although no significant daytime hypersomnia, the patients perceived their overall sleep quality to be quite poor. Despite the relatively low ESS score, we observed direct correlation between ESS score and the VAS score for pain and EORTC fatigue. Moreover, it was inversely related to cognitive and social functioning. Additionally, a correlation between ESS and PSQI scores revealed that those with higher ESS scores experienced more disturbances during sleep and reported lower energy levels during the day. Furthermore, we found multiple statistically significant correlations between numerous items of the PSQI and the EORTC-QLQ30 and H&N35 scores, confirming once more the fundamental link between good sleep and good quality of life. The correlation was further confirmed by the direct relationships between VAS score for pain and PSQI scores. Everything suggested that even though hypersomnia was not a major concern, it was still closely related to sleep quality in our sample, and in turn, this poorer sleep quality was found to have a negative impact on overall quality of life. These findings were consistent with previous literature, that also reported a strong relationship among sleep disturbances, hypersomnia, and overall quality of life in similar patient populations [
9,
22].
OSA was assessed in our patient population using the STOP-BANG questionnaire. The results revealed that 16.6% of the patients were categorized as being at high risk for OSA, while an additional 34.4% were identified as having a moderate risk. These findings are consistent with existing literature, which reports that the prevalence of OSA among HNC patients ranged between 52% and 92%, depending on various factors, such as the stage, the treatment and the specific location of the tumor [
22,
23]. This high variability in OSA prevalence is largely attributed to the different anatomical changes that occur in patients undergoing surgery or radiotherapy depending on the specific site of the tumor. These treatments often result in alterations to the airway, such as tissue scarring or structural shifts, which can lead to significant airway obstruction. As a result, these physical changes can exacerbate pre-existing OSA or even induce the condition in patients who were previously unaffected. Our findings highlighted the importance of monitoring for OSA in this patient population, as it could significantly impact not only their sleep quality but also their overall health and recovery [
24].
Our study found a statistically significant difference in perceived sleep quality between patients at high risk of OSA and those at lower risk, based on the correlation between STOP-BANG and PSQI scores. Specifically, patients with a higher risk of OSA reported worse sleep quality compared to those in the low-risk group. This finding suggests that OSA, that is more prevalent among HNC individuals [
25], has a profound negative impact on sleep quality in this population.
Direct correlation between the psychological scores (DT and HADS) and the PSQI global score was also observed. Poor sleep was strictly connected to anxiety, depression and emotional distress in our cohort. These psychological factors are known to exacerbate sleep disturbances, as both depression and anxiety are closely linked to insomnia, further compounding the problem of sleep disruption in these patients [
26]. This finding stresses the importance of good psychological support for HNC patients as a mean not only to improve mental health but also the sleep quality. Indeed, a disturbed sleep quality can lead to an even worse quality of life.
The strength of this study was the comprehensive analysis of sleep quality in relationship to quality of life and psychological distress. The main limitation was the absence of an objective diagnosis of OSA. Indeed, we evaluated only the sleep apnea risk by means of STOP-BANG questionnaire. Further studies should include objective tools, such as polysomnography.
5. Conclusions
Sleep disturbances, particularly OSA and insomnia, are frequent in HNC patients, and significantly impact their quality of life and psychological well-being. Our findings are consistent with existing literature, which highlights the complex relationship among sleep quality, cancer-related symptoms, and treatment-related toxicities. Given the effect of sleep on overall well-being, addressing sleep disorders should be a priority in the care of HNC patients. Future research should continue to explore the underlying mechanisms of sleep disturbances in this population and to develop targeted interventions to improve sleep quality and, ultimately, patient outcomes.
Author Contributions
Conceptualization, G.R.; methodology, G.R.; formal analysis, S.M. and G.R.; investigation, S.M., D.G., M.B., E.C., E.S.B.; data curation, S.M., D.G., M.B., E.C., E.S.B.; writing—original draft preparation, S.M., D.G. and G.R.; writing—review and editing, G.P.; visualization, S.M.; supervision, G.P.; project administration, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of A.O.U. Città della Salute e della Scienza di Torino - A.O. Ordine Mauriziano - A.S.L. Città di Torino (protocol code 0021433, date of approval 26 February 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, J.; Jolly, S. Obstructive sleep apnea and fatigue in head and neck cancer patients. Am. J. Clin. Oncol. 2015, 38, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.M.M.; Jansen, F.; de Vries, R.; Leemans, C.R.; van Straten, A.; Verdonck-de Leeuw, I.M. Prevalence of sleep disturbances among head and neck cancer patients: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 47, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Seifen, C.; Huppertz, T.; Matthias, C.; Gouveris, H. Obstructive Sleep Apnea in Patients with Head and Neck Cancer-More than Just a Comorbidity? Medicina (Kaunas). 2021, 57. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Campo, F.; Angeletti, D.; Allegra, E.; Minni, A.; Polimeni, A.; Greco, A.; de Vincentiis, M. Obstructive Sleep Apnoea in Patients Treated for Head and Neck Cancer: A Systematic Review of the Literature. Medicina (Kaunas). 2020, 56, 1–9. [Google Scholar] [CrossRef]
- Chen, D.; Yin, Z.; Fang, B. Measurements and status of sleep quality in patients with cancers. Support. Care Cancer 2018, 26, 405–414. [Google Scholar] [CrossRef]
- Gavidia, R.; Dunietz, G.L.; O’Brien, L.M.; Schütz, S.G.; Spector, M.E.; Swiecicki, P.L.; Chervin, R.D. Risk of obstructive sleep apnea after treatment of head and neck squamous cell carcinoma: a cross-sectional study. J. Clin. Sleep Med. 2022, 18, 1681–1686. [Google Scholar] [CrossRef]
- Kiss, B.; Neagos, C.M.; Jimborean, G.; Sárközi, H.K.; Szathmary, M.; Neagos, A. Comorbidities and Laryngeal Cancer in Patients with Obstructive Sleep Apnea: A Review. Medicina (Kaunas). 2023, 59. [Google Scholar] [CrossRef]
- Lin, H.C.; Friedman, M.; Chang, H.W.; Fang, F.M.; Lin, M.C.; Su, M.C. Impact of head and neck radiotherapy for patients with nasopharyngeal carcinoma on sleep-related breathing disorders. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 1166–1172. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Robbins, K.T.; Rao, K.; Malone, J.; Seiz, A.; Reminger, S.; Markwell, S.J.; Burra, V. Factors associated with fatigue, sleep, and cognitive function among patients with head and neck cancer. Head Neck 2008, 30, 1310–1317. [Google Scholar] [CrossRef]
- Krebber, A.M.H.; Jansen, F.; Cuijpers, P.; Leemans, C.R.; Verdonck-de Leeuw, I.M. Screening for psychological distress in follow-up care to identify head and neck cancer patients with untreated distress. Support. Care Cancer 2016, 24, 2541–2548. [Google Scholar] [CrossRef]
- Pilz, M.J.; Gamper, E.M.; Efficace, F.; Arraras, J.I.; Nolte, S.; Liegl, G.; Rose, M.; Giesinger, J.M. EORTC QLQ-C30 general population normative data for Italy by sex, age and health condition: an analysis of 1,036 individuals. BMC Public Health 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, K.; Hammerlid, E.; Ahlner-Elmqvist, M.; De Graeff, A.; Boysen, M.; Evensen, J.F.; Biörklund, A.; De Leeuw, J.R.J.; Fayers, P.M.; Jannert, M.; et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J. Clin. Oncol. 1999, 17, 1008–1019. [Google Scholar] [PubMed]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Chen, P.Y.; Chuang, L.P.; Chen, N.H.; Tu, Y.K.; Hsieh, Y.J.; Wang, Y.C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Gonçalves, M.T.; Malafaia, S.; Moutinho dos Santos, J.; Roth, T.; Marques, D.R. Epworth sleepiness scale: A meta-analytic study on the internal consistency. Sleep Med. 2023, 109, 261–269. [Google Scholar] [CrossRef]
- Ownby, K.K. Use of the Distress Thermometer in Clinical Practice. J. Adv. Pract. Oncol. 2019, 10, 175. [Google Scholar]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual. Life Outcomes 2003, 1. [Google Scholar] [CrossRef]
- Iovoli, A.J.; Smith, K.; Yu, H.; Kluczynski, M.A.; Jungquist, C.R.; Ray, A.D.; Farrugia, M.K.; Gu, F.; Singh, A.K. Association of Insomnia and Obstructive Sleep Apnea with Worse Oral Mucositis and Quality of Life in Head and Neck Cancer Patients Undergoing Radiation Therapy. Cancers (Basel). 2024, 16. [Google Scholar] [CrossRef]
- Gil, O.; Fenske, B.; Bremert, T.; Vollmer, M.; Scharf, C.; Busch, C.J.; Blaurock, M. Prevalence of Obstructive Sleep Apnea in Head and Neck Squamous Cell Carcinoma Patients before and after Treatment. Medicina (Kaunas). 2024, 60. [Google Scholar] [CrossRef]
- Mehanna, H.; Paleri, V.; West, C.M.L.; Nutting, C. Head and neck cancer--Part 1: Epidemiology, presentation, and prevention. BMJ 2010, 341, 663–666. [Google Scholar] [CrossRef]
- Yoshikawa, F.; Nozaki-Taguchi, N.; Yamamoto, A.; Tanaka, N.; Tanzawa, A.; Uzawa, K.; Isono, S. Preoperative sleep-disordered breathing and craniofacial abnormalities are risk factors for postoperative sleep-disordered breathing in patients undergoing skin-flap oropharyngeal reconstruction surgery for oral cavity cancer: a prospective case-control study. Sleep Breath. 2024, 28, 797–806. [Google Scholar] [PubMed]
- Faiz, S.A.; Balachandran, D.; Hessel, A.C.; Lei, X.; Beadle, B.M.; William, W.N.; Bashoura, L. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist 2014, 19, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Landsberg, R.; Pryor, S.; Syed, Z.; Ibrahim, H.; Caldarelli, D.D. The occurrence of sleep-disordered breathing among patients with head and neck cancer. Laryngoscope 2001, 111, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Graefe, H.; Gehrking, E.; König, I.R.; Wollenberg, B. Sleep apnoea in patients after treatment of head neck cancer. Acta Otolaryngol. 2009, 129, 1300–1305. [Google Scholar] [CrossRef]
- Loth, A.; Michel, J.; Giorgi, R.; Santini, L.; Rey, M.; Elbaum, J.M.; Roux, N.; Giovanni, A.; Dessi, P.; Fakhry, N. Prevalence of obstructive sleep apnoea syndrome following oropharyngeal cancer treatment: A prospective cohort study. Clin. Otolaryngol. 2017, 42, 1281–1288. [Google Scholar] [CrossRef]
- Li, N.; Otomaru, T.; Taniguchi, H. Sleep quality in long-term survivors of head and neck cancer: preliminary findings. Support. Care Cancer 2017, 25, 3741–3748. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).