1. Introduction
Obesity is a major driver of metabolic dysfunctions, including Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). The global prevalence of MASLD is around 30% of the global population [
1]. MASLD encompasses a range of liver diseases, such as non-alcoholic steatohepatitis (NASH), characterized by inflammation and liver damage that can lead to scarring [
2]. A key factor for the development of these conditions is oxidative stress, which propels the accumulation of membrane lipid peroxides in cellular membranes[
3]. Lipid droplets (LDs) within the hepatocyte play a protective role against preventing lipoperoxidation and protecting plasma membrane from the harmful effects of oxidation of polyunsaturated fatty acids (PUFAs). When there is buildup of lipid peroxides, the cell initiates ferroptosis, an iron-dependent programmed cell death is mostly caused by the process of membrane lipoperoxidation [
4]. In ferroptosis, iron serves as a catalyst, and cells reduced mitochondrial volume, but the cell membrane remains intact (the particularities of ferroptosis will be further discussed below). Importantly, in liver injury and MASLD, liver cells are more susceptible to ferroptosis[
5].
The renaming of NAFLD to MASLD reflects an evolving understanding of the disease etiology, emphasizing the centrality of metabolic dysfunction, particularly insulin resistance (IR). Exercise, recognized as one of the most effective treatments for MASLD, reduces fatty tissue and liver fat infiltration, helping to mitigate oxidative damage, inflammation, and even improve fibrosis scores associated with disease progression[
6]. During exercise, myokines are produced by skeletal muscle cells and play a key role in metabolic health and inter-organ crosstalk. They influence physiological processes such as insulin sensitivity, aging, glucose metabolism, and pancreatic beta cell function, impacting various organs [
7,
8]. This review provides a complementary perspective on the liver-protective effects of muscle derived myokines, emphasizing their role in preventing lipoperoxidation and ferroptosis in MASLD.
2. Insulin Resistance as a Central Driver in MASLD
A hallmark of metabolic syndrome is IR, which also plays a significant role in the development of MASLD. IR occurs when cells in muscle, and adipose tissue become less responsive to insulin, a hormone essential for maintaining glucose homeostasis[
9,
10]. In response, the pancreas produces more insulin, as higher levels are needed to regulate blood glucose effectively. In the liver, IR leads to increase
de novo lipogenesis and reduces fatty acid oxidation, causing an accumulation of triglycerides inside the hepatocytes[
11]. Lipid overload is one of the major factors of hepatic steatosis, a hallmark of MASLD. The progression from hepatic steatosis to more severe forms of liver diseases, such as non-alcoholic steatohepatitis NASH, involves several factors, with IR playing a central role[
12]. IR leads to hyperglycemia and hyperinsulinemia, both of which contribute to increase oxidative stress and inflammation[
13,
14]. This harmful environment triggers the peroxidation of LDs, resulting in the formation of reactive aldehydes like malondialdehyde (MDA). These toxic byproducts cause damage to cellular components, leading to inflammatory responses and fibrosis.
The role of ferroptosis adds further complexity to this scenario. IR is often accompanied by low-grade inflammation, which raises levels of inflammatory cytokines. These cytokines may trap iron within hepatic stellate cells (HSC), leading to an imbalance where iron overload in HSCs and iron deficiency in hepatocytes are key features of both steatohepatitis and NAFLD[
15,
16]. This imbalance triggers ROS-dependent activating the iron-induced fibrogenic processes in HCS [
17]. Additionally, research suggest that chronic iron overload associated with IR may worsen it by promoting hepatic ferroptosis via inhibition of JAK2/STAT3/SLC7A11 pathway [
8]. Treatment with the iron chelator deferasirox has been shown to improve hepatic IR caused by iron overload [
8].
In the context of IR, the liver's antioxidant defenses, including GSH and GPX4, are insufficient to counteract redox imbalance[
18,
19]. This imbalance leads to the accumulation of lipid peroxides, which triggers necroptotic cell death and exacerbates liver inflammation and damage[
20]. Aerobic exercise primarily relies on oxidative metabolism and stimulates type I (slow-twitch) muscle fibers, which are rich in mitochondria and well-suited for endurance activities. In contrast, resistance exercise activates glycolytic metabolism and engages type II (fast-twitch) muscle fibers, specialized for shorth bursts of power and strength[
21,
22]. Notably, resistance exercise has been found to improve NAFLD more effectively than aerobic exercise, likely due to the release of myokines that may positively influence lipid metabolism in the liver [
23].
3. Mechanisms of Lipid Peroxidation
Lipoperoxidation is a process under which oxidants attack lipids, mostly PUFAs, in a cell membrane. This reaction can propagate through additional membranes in a chain reaction, which can only be halted by an antioxidant.
3.1. Lipoperoxidation Process
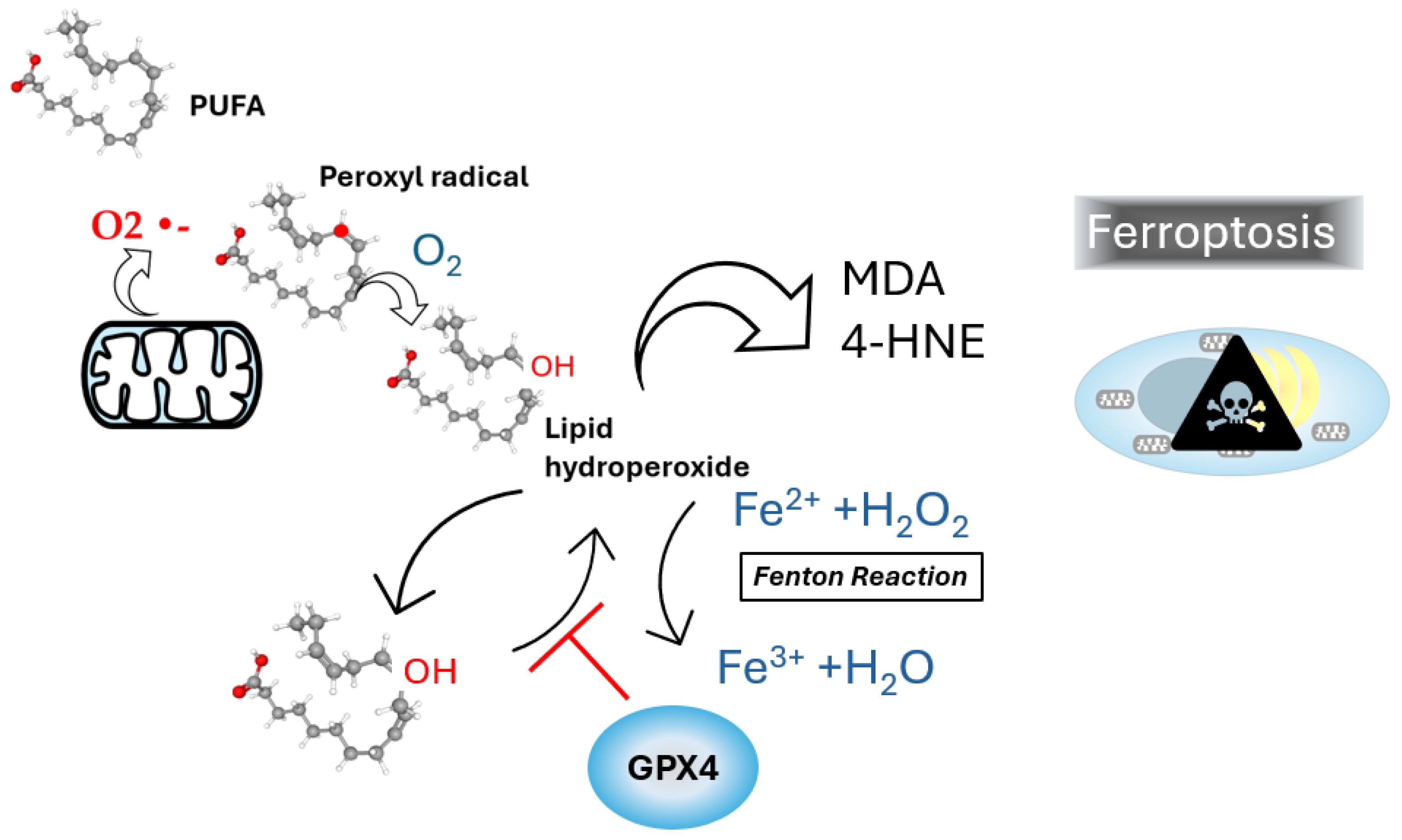
Lipoperoxidation is one of the main molecular mechanisms involved in oxidative damage to cellular membranes by reactive oxygen species (ROS), targeting lipids with carbon-carbon double bonds, particularly PUFAs[
24]. Due to their multiple bonds, PUFAs are especially susceptible to oxidative attack by ROS, such as superoxide anion (O2
•-) and hydrogen peroxide (H
2O
2), which are often produced in excess during mitochondrial dysfunction and inflammatory responses. During lipoperoxidation, radical formed react with oxygen, generating lipid peroxyl radicals that propagate the chain reaction, ultimately leading to the formation of lipid hydroperoxides. The Fenton reaction, catalyzed by iron, further exacerbates lipid peroxidation by generation hydroxyl radicals (OH•) through the interaction of ferrous iron (Fe
2+) hydrogen peroxide, driving the initiation and propagation of lipid peroxidation[
25] as shown in
figure 1.
The accumulation of lipid hydroperoxides plays a central role in ferroptosis, as these compounds can decompose into toxic aldehydes such as MDA and 4-hydroxynonenal (4-HNE). These aldehydes are highly reactive and can crosslink proteins, nucleic acids, and other cellular components, resulting in cell death[
26,
27]. LDs, organelles responsible for storing neutral lipids and sterols esters, show a significant increase in number and in MASLD. LDs, along with other ROS-producing organelles are closely physically associated. For example, NADPH oxidases in mitochondrial membranes and complexes I and III in lysosomes are potential ROS sources in proximity to LDs[
28]. LDs act as antioxidant organelles by storing PUFAs in their neutral lipid core, preventing their oxidation[
29,
30]. Mitochondria associated with LDs, known as peridroplet mitochondria, are abundant in steatotic liver, likely as a compensatory mechanism to fatty acid overload. This interaction has been linked to the progression of MASLD[
31]. Inside LDs, PUFAs are particularly susceptible to peroxidation, and lipoperoxidation inside the LDs has been observed in steatotic hepatocytes[
32]. Since LDs primarily consists of triacylglycerols and sterol esters, they lack a hydrophilic environment for antioxidant enzyme. Consequently, the only defense against lipid peroxidation withing LDs involves lipid-soluble antioxidant molecules such as tocopherol and retinyl esters[
33,
34]. The assessment of lipid peroxidation in LDs can be achieved using fluorescent probes such as BODIPY-C11, which shifts fluorescence from ∼ 590 nm a ∼ 510 nm upon oxidation. This tool provides valuable insights into lipid peroxidation dynamics in live cells [
35].
4. Protective Antioxidant Responses in Hepatocytes Against Lipid Peroxidation
The cellular defense against lipid peroxidation involves a variety of antioxidant systems, with, Kelch-like ECH-associated protein 1 (Keap1) playing a pivotal role in regulating the antioxidant response. Keap1 controls the activity of nuclear factor erythroid 2–related factor 2 (Nrf2), a key transcription factor. Under conditions of oxidative stress, Keap1 undergoes conformational changes that allow Nrf2 to translocate into the nucleus, where it activates the expression of antioxidant genes[
36]. Among these genes are those encoding enzymes such glutamate-cysteine ligase (GCL), the rate-limiting enzyme in the synthesis of GSH[
37]. Glutathione is a critical antioxidant that reduces lipid hydroperoxides through the action of GPX4. GPX4 is essential for detoxifying lipid hydroperoxides, preventing their accumulation and subsequent initiation of ferroptosis. In the absence of sufficient GPX4 activity or adequate GSH levels, lipid hydroperoxides accumulate, leading to the triggering of ferroptosis [
38]. This pathway underscores the importance of maintaining redox homeostasis in preventing lipid peroxidation and the associated risk of cell death. Interestingly an increased proportion of GPX4 positive nuclei was found present in MASLD patients compared to controls[
39].
5. Ferroptosis and Its Implications in MASLD
Oxidative stress plays a central role in the pathophysiology of MASLD, driving lipid peroxidation of cellular membranes, including LDs, and leading to the formation of reactive aldehydes such as MDA, which can cause cell damage[
40]. In high fat diet (HFD) fed mice, GSH levels are significantly reduced, along with the activity of antioxidant enzymes such as superoxide dismutase (SOD), and catalase (CAT). This imbalance results in increased lipid peroxidation[
41]. As mentioned above, GPX4 is a regulator of ferroptosis because of its ability to neutralize lipoperoxidation. Strategies that activate the Nrf2/HO-1/GPX4 pathway can reverse the ferroptosis process in MASLD[
42,
43]. When GSH is depleted, GPX4 activity also decreases, leading to a rise in lipid peroxides. Furthermore, a dysfunctional cystine/glutamate antiporter system xc− (System Xc-) disrupts antioxidant defenses by limiting cystine uptake, exacerbating oxidative stress[
44].
A hallmark of MASLD is lipid accumulation within hepatocytes, increasing the availability of fatty acids susceptible to lipid peroxidation[
45]. One of the organelles most affected by this lipid overload is the endoplasmic reticulum (ER). Lipid accumulation induces ER stress, which is triggered by the activation of one of the three transducers: inositol-requiring enzyme 1a (IRE1a), PKR-like ER kinase (PERK), and activating transcription factor 6a (ATF6a), culminating in the activation of the unfolded protein response (UPR)[
46]. Although LDs offer a protective role, they are insufficient to counterbalance the additional ER stress, leading to UPR activation [
47]. Excess intracellular lipids form de
novo lipogenesis contribute to an increase in endogenous saturated fatty acids (SFAs), such as palmitate, which can further promote UPR activation through alterations in phosphatidylcholine species [
48]. Elevated levels of SFAs and sterols can stretch the ER membrane, increasing stiffness and causing oligomerization of IRE1 and PERK activating the UPR[
49,
50]. This stress disrupts normal LD biogenesis.
6. The Role of Myokines on Skeletal Muscle Physiology
Skeletal muscle, a major site of myokine production, plays a crucial role in metabolic regulation and overall health. It contains various cell types, including satellite cells, which are pivotal for muscle growth, repair, and regeneration. Upon muscle contraction, satellite cells are activated, promoting muscle adaptation and hypertrophy, processes that are partly mediated by myokines[
51,
52]. Key myokines, such as irisin and interleukin-6 (IL-6) enhance glucose uptake and improve insulin sensitivity in skeletal muscle, contributing to metabolic health. These interactions help maintain blood glucose homeostasis and may offer protection against metabolic disorders such as IR and type 2 diabetes. Here we will discuss the aberrant expression of myokines, their unique characteristics, and the critical role they play in the pathophysiology of MASLD, shedding light on their potential implications for disease progression.
6.1. Dysregulation of Myokines Levels Is Associated with MASLD
As discussed, a key hallmark of MASLD is lipid accumulation inside the hepatocytes which leads to fibrosis and inflammation. Dysregulation of myokine levels significantly contributes to the etiology and progression of MASLD. Myokines, regulates metabolic processes, including lipid and glucose metabolism. Conditions physical inactivity or disabilities can worsen metabolic dysfunction in liver disease[
53,
54]. However, certain myokines produced during chronic exercise promote β oxidation and glucose uptake, counteracting intrahepatic lipid accumulation. Here we will present an overview of the most extensively studied myokines and their association with the liver health will be shown.
6.2. Myostatin (MSTN)
Myostatin is a dimeric protein from the transforming growth factor (TGF-β) superfamily, composed of two identical 12.4 kDa subunits connected by a disulfide bond. It signals through the activin type II receptors, ActRIIB and ActRIIA, which are expressed in skeletal muscle, heart tissue, and adipose tissue. These receptors trigger intracellular signaling, activating the Smad3/4 transcription factor complex to modulate gene expression[
55]. Myostatin is a crucial regulator of muscle mass and metabolic processes, inhibiting muscle growth and promoting adipogenesis. In mice, overexpression of MSTN leads to increased epididymal fat accumulation and reduced cardiac and muscular mass [
56]. Conversely, MSTN depletion mitigates age-related increases in adipose tissue, obesity, and diabetes and reduces fat accumulation when consuming a high-calorie diet. Mechanistically, MSTN depletion enhances fatty acid oxidation, lipolysis and promotes brown fat production in white adipose tissue[
57].
Skeletal muscle production of myostatin enables its release to the bloodstream, potentially impacting tissues expressing ActRIIA or ActRIIB, including liver. In the liver, myostatin stimulates HSCs, inducing gene expression program related to fibrosis via activation of the c-Jun N-terminal kinase (JNK) pathway[
58]. Additionally, myostatin has an antioxidant effect, as its inhibition enhances AMP-activated protein kinase (AMPK) signaling, which activates glucose-6-phosphate dehydrogenase (G6PD) and the pentose phosphate pathway. This leads to increased NADPH production, higher glutathione reductase activity, and improved antioxidant capacity[
59]. MSTN also negatively regulates liver growth hormone by reducing insulin growth factor 1 (IGF-1)levels[
60].
It is important to mention that there is conflicting information regarding myostatin levels in liver diseases. On the one hand, myostatin levels are decreased in patients with cirrhosis, and this reduction has been proposed as a predictive biomarker of acute-on-chronic liver failure[
61]. On the other hand, elevated serum myostatin levels have been associated with poor prognosis in patients with liver cirrhosis, where increased collagen synthesis is driven by high myostatin levels [
62].
6.3. Interleukin 6
Inteleukin-6 (IL-6) is a cytokine composed of four α-helices, playing a crucial role in immune signaling. It binds to the IL-6 receptor alpha (IL-6Rα), forming a complex with the signal-transducing receptor gp130 [
63]. When gp130 is dimerized by the IL-6 and IL-6R α complex, it activates the constitutively bound tyrosine kinase JAK1. In hepatocytes, this pathway induces the expression of factors responsible for hepatic acute-phase protein induction, such as hepcidin[
64]. Interestingly, while IL6 is a classic biomarker of acute inflammation, exercise also increases plasma IL-6 levels. During sepsis, there is a marked and acute rise of circulating tumor necrosis factor alpha (TNFα), followed by an increase in IL-6 levels. Studies consistently show that plasma IL-6 is not preceded by a rise in TNFα. It has been consistently shown that plasma IL-6 levels rise during muscular exercise, accompanied by increases in IL-1 receptor antagonist (IL-1ra) and the anti-inflammatory cytokine IL-10. Additionally, concentrations of chemokines like interleukin 8 (IL-8), macrophage inflammatory protein-1alpha (MIP-1α) and beta (MIP-1β) increase, while TNF-α remains unchanged[
65]. Notably, chlorogenic acid, a dietary polyphenol, has been reported to reduce hepcidin production by inhibiting the IL-6/JAK2/STAT3 pathway in the liver[
66]. This suggests that exercise-derived IL-6 may protect the liver against ferroptosis by inhibiting this pathway. However, MASDL is characterized by high levels of TNFα, which exacerbate the ferroptosis process. In this context, maresin 1 has been shown to inhibit ferroptosis-induced liver injury by suppressing the release of TNF-α and IL-6[
42]. Consistent with this, exercise is a potent stimulus for increasing maresin synthesis[
67].
6.4. Irisin
Irisin is a peptide consisting of 112 amino acid residues, primarily produced by the cleavage of the fibronectin type III domain containing 5 (FNDC5). It exerts it effects through the integrin αVβ5 receptor, with eHsp90α acting as its cofactor [
4]. Due to its antioxidant properties, irisin is considered a hepatoprotective peptide. It regulates crucial cellular processes such as ferroptosis, inflammasome activation, autophagy, mitochondrial fission and fusion, ER stress, and cell death [
68]. Irisin promotes the browning of white adipose tissue (WAT) and enhancer thermogenesis by binding to its receptor and activating a downstream signaling pathways, including the AMP-activated protein kinase (AMPK) and p38 MAPK [
69].
Irisin shows potential as an anti-pyroptosis/apoptosis agent in septic liver injury, as it inhibits apoptosis, NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome activation, and nuclear factor-κB (NF-κB) signaling, thereby reducing lipopolysaccharide (LPS) induced liver damage. In LPS-challenged mice, irisin treatment was found to decrease inflammatory responses, ROS generation, and cell death[
70]. Additionally, sepsis patients have been shown to exhibit lower serum irisin levels[
71]. In septic mice, irisin treatment improved mitochondrial function, increased ATP production, decreased inflammation and ROS levels, and protected against ferroptosis. The protective effects of irisin were associated with GPX4, and these effects were mitigated when irisin's receptor was blocked[
72]. According to this study, exercise reduces hepatic steatosis and fibrosis in NAFLD by increasing irisin levels. Irisin helps limit inflammation by competitively binding to myeloid differentiation protein 2 (MD2) and inhibiting the MD2-Tolk -like receptor 4 (TLR4) pathway. The protective effects of irisin on inflammation, fibrosis, and lipid metabolism suggest that exercise may be an effective treatment for MASLD [
73].
6.5. Fibroblast Growth Factor 21 (FGF21)
FGF21 is a member of the FGFs with endocrine, paracrine, and autocrine functions. It is primarily expressed in the liver, adipose tissue, and skeletal muscle, where its main role is regulating glucose homeostasis and lipid degradation during fasting conditions[
74]. FGF21 binds to FGFR1c or FGFR3c receptors, along with the transmembrane co-receptor Klotho, forming a highly regulated protein complex [
75]. In hepatocytes, the FGF21/Klotho complex stimulates fatty acid beta-oxidation, gluconeogenesis, and ketogenesis[
76]. In adipose tissue, FGF21 promotes lipolysis and glucose [
76]. In skeletal muscle, FGF21 plays a crucial role in glucose uptake, muscle remodeling, activating atrophy programs, and mitophagy [
77,
78]. Acute exercise induces FGF21 expression in the liver but not in skeletal muscle or adipose tissue [
79]. ER stress is one of the triggers of FGF21 overexpression, mediated through the IRE1α /XBP1 and activating transcription factor 4 (ATF4) pathways, or cyclic AMP-responsive element-binding protein H (CREBH) cleavage pathway[
80]. These signals activate Nrf2, a critical antioxidant transcription factor, suggesting that FGF21 may serve as a protective myokine, enhancing the antioxidant response in MASLD. Additionally, exercise increases FGF21 sensitivity in adipose tissue by upregulating the gene expression of FGFR1 and KLB, improving metabolic biomarkers such as plasma insulin and triglycerides levels in HFD-fed mice[
81]. Overexpression of FGF21 in hepatocytes upregulates Nrf2 and
Gpx4, Slac7a11, and
Flt gene expression, improving the cells’ antioxidant capacity. This leads to increase GSH synthesis, and reduced lipid peroxidation, highlighting FGF21 protective role against ferroptosis[
82].
6.6. Insulin Like Growth Factor
IGF-1 is a peptide hormone with 70 amino acids, primarily produced by the liver. Its expression is stimulated by growth hormone. IGF-1 binds to IGF-1 receptors in skeletal muscle, bone, kidney, and cardiac tissues, with its most well-known functions involving protein synthesis, differentiation, and cell survival[
83,
84]. The IGF-1/mTOR/ p70S6K-1 axis drives an anabolic program within cells[
85]. Exercise has been shown to increase IGF-1 gene expression following resistance training in rats[
86]. While similar result are observed in humans; a meta-analysis revealed that IGF-1 levels only increases after resistance exercise in individuals over 60 years old, but not in younger adults[
87]. Additionally, IGF-1 has been reported to play protective role against liver damage associated with iron overload in a cirrhotic rat model, where IGF-1 treatment prevented increases in MDA, iron stores, and ROS[
88].
6.7. Brain-Derived Neurotrophic Factor (BDNF)
BDNF is a peptide growth factor classified as a neurotrophin, consisting of 247 amino acids derived from cleavage of proBDNF. Its receptor, tyrosine receptor kinase B (TrkB), is expressed in both neuronal and non-neural tissues, including skeletal muscle, adipose tissue and cardiac tissue. The BDNF/TrkB complex activates signaling pathways that regulate synaptic plasticity, energy metabolism, cellular survival and proliferation[
89,
90]. Interestingly, BDNF is detectable in human plasma and has been proposed as a biomarker of some neurological diseases[
91,
92]. Low plasma BDNF levels have been found in individuals with obesity and type 2 diabetes[
90], but elevated levels are observed in patients with NAFLD, with the increase being proportional to the severity of the condition [
93]. Exercise is one of the stimuli known to increase BDNF plasma levels. For instance, long-term running in mice, has been shown to elevate mature BDNF and stimulates neurogenesis in the hippocampus[
94]. Another factor that modulates BDNF gene expression is β-hydroxybutyrate, a ketone body synthesized by the liver during fasting or exercise. β-hydroxybutyrate is transported via the bloodstream to various tissues, where it stimulates BDNF gene expression in the brain [
95]. BDNF also has beneficial effects on the liver, promoting activation β oxidation of free fatty acids and inhibiting gluconeogenesis though the activation of AMPK, in mouse hepatocytes[
96]. Some studies suggest that BDNF exerts antioxidant effects, particularly through the activation of Nrf2 in astrocytes. Although inadequate Nrf2 activation in astrocytes may lead to ferroptosis-like cell death in neurons, there is no evidence to establish a direct or indirect antioxidant function in neurons[
97].
7. Exercise Against Ferroptosis in MASLD
Exercise offers significant benefits for reversing MASLD. Primarily, it increases calorie expenditure and activates processes like beta-oxidation and gluconeogenesis. Certain aerobic exercise protocols have been shown to alter the architecture of lipid droplets, reducing their size and increasing their contact surface with mitochondria [
98]. Regular aerobic exercise is recommended to improve vascular and metabolic co-morbidities associated with MASLD[
99]. However, there is a gap in the evidence regarding the direct impact of specific exercise protocols on reducing liver fat infiltration. For example, a protocol involving 60 minutes of aerobic exercise per day, 5 days per week, for 15 weeks, significantly improved insulin sensitivity, lipid profile, and the histological score of hepatic steatosis and inflammation in HFD mice. This protocol also enhanced the degradation of hepatic lipid droplets in lysosomes[
100]. Similarly, such exercise protocols have been found to reduce lipoperoxidation markers and increase the protein levels of Nrf2 and GPX4 in HFD fed mice, indicating improved antioxidant defense [
101].
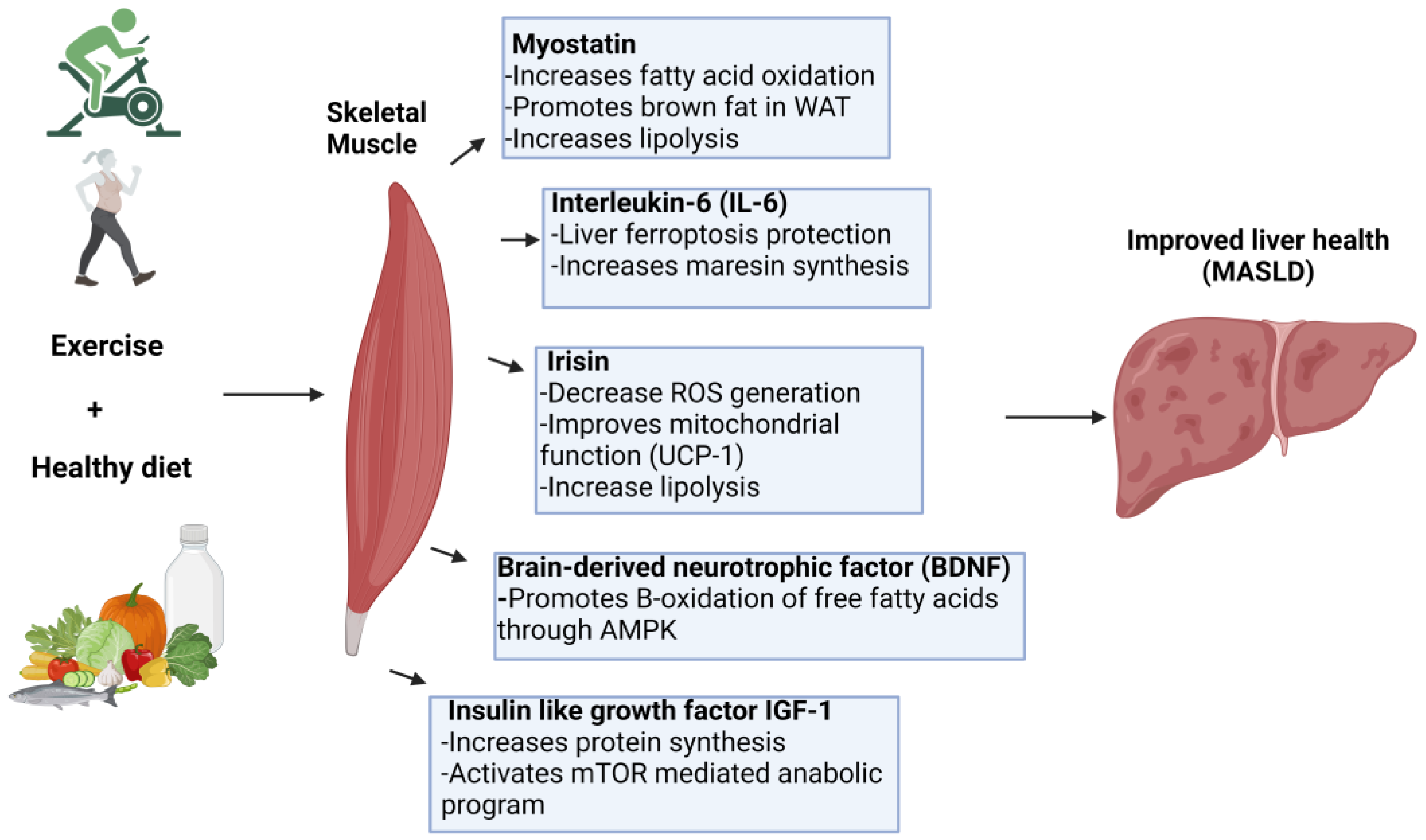
Figure 2.
Mechanisms by which myokines improves liver health in MASLD. Myokines released by exercise including myostatin, IL-6, irisin, BDNF, and IGF-1—promote liver health in MASLD. These myokines enhance fatty acid oxidation, improve mitochondrial function, reduce oxidative stress, and protect against ferroptosis in the liver. Together, these molecular pathways contribute to improved metabolic function and overall liver health in MASLD.
Figure 2.
Mechanisms by which myokines improves liver health in MASLD. Myokines released by exercise including myostatin, IL-6, irisin, BDNF, and IGF-1—promote liver health in MASLD. These myokines enhance fatty acid oxidation, improve mitochondrial function, reduce oxidative stress, and protect against ferroptosis in the liver. Together, these molecular pathways contribute to improved metabolic function and overall liver health in MASLD.
8. Conclusions
Obesity continues to be a significant global public health issue, with metabolic dysfunction-associated steatotic liver disease (MASLD) as one of its primary consequences. MASLD, defined by hepatic fat buildup without evident symptoms, currently has limited therapeutic approaches. Nonetheless, lifestyle alterations, especially physical activity, have demonstrated encouraging outcomes in slowing, and even reversing, the progression of liver damage due to MASLD. Specifically, we provided evidence of how physical activity inhibits lipid droplet formation and subsequent oxidation in the liver as well as the impact of muscle-derived myokines on the liver (figure 2).
We provided evidence, from preclinical and clinical research, that myokines improve cellular processes such as ER stress, mitochondrial dysfunction, and glucose mismanagement in the liver. This slows down the activation of fibrotic programs associated with the progression of liver damage and can facilitate the reversal of MASLD. However, more research is needed as the literature is confined to a specific subset of myokines. Despite the limited number of myokines that have been investigated, they have provided significant insights into the intricate biological mechanisms that slow and reverse MASLD progression.
Conflicts of Interest
Evan M. Paules is a Balchem Postdoctoral Fellow. Alejandra Espinosa, Concurso CIDI 2023 N°20, DEXE 142 del 2024
References
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients With Type 2 Diabetes. Clin Gastroenterol Hepatol 2024, S1542-3565(24)00287-8. [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr 2023, 32, 197–213. [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem Biophys Res Commun 2017, 482, 419–425. [CrossRef]
- Feng, G.; Byrne, C.D.; Targher, G.; Wang, F.; Zheng, M.-H. Ferroptosis and Metabolic Dysfunction-Associated Fatty Liver Disease: Is There a Link? Liver Int 2022, 42, 1496–1502. [CrossRef]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The Multifaceted Role of Ferroptosis in Liver Disease. Cell Death Differ 2022, 29, 467–480. [CrossRef]
- Oh, S.; Tanaka, K.; Warabi, E.; Shoda, J. Exercise Reduces Inflammation and Oxidative Stress in Obesity-Related Liver Diseases. Med Sci Sports Exerc 2013, 45, 2214–2222. [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and Role of Irisin in Glucose Homeostasis. Nat Rev Endocrinol 2017, 13, 324–337. [CrossRef]
- Mo, M.; Pan, L.; Deng, L.; Liang, M.; Xia, N.; Liang, Y. Iron Overload Induces Hepatic Ferroptosis and Insulin Resistance by Inhibiting the Jak2/Stat3/Slc7a11 Signaling Pathway. Cell Biochem Biophys 2024. [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev 2018, 98, 2133–2223. [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res 2020, 2020, 3920196. [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin Resistance Drives Hepatic de Novo Lipogenesis in Nonalcoholic Fatty Liver Disease. J Clin Invest 2020, 130, 1453–1460. [CrossRef]
- Horn, P.; Tacke, F. Metabolic Reprogramming in Liver Fibrosis. Cell Metab 2024, 36, 1439–1455. [CrossRef]
- Ling, P.-R.; Smith, R.J.; Bistrian, B.R. Acute Effects of Hyperglycemia and Hyperinsulinemia on Hepatic Oxidative Stress and the Systemic Inflammatory Response in Rats. Crit Care Med 2007, 35, 555–560. [CrossRef]
- Perkins, J.M.; Joy, N.G.; Tate, D.B.; Davis, S.N. Acute Effects of Hyperinsulinemia and Hyperglycemia on Vascular Inflammatory Biomarkers and Endothelial Function in Overweight and Obese Humans. Am J Physiol Endocrinol Metab 2015, 309, E168-176. [CrossRef]
- Tsukamoto, H. Iron Regulation of Hepatic Macrophage TNFalpha Expression. Free Radic Biol Med 2002, 32, 309–313. [CrossRef]
- Yuan, Q.; Zhang, Z.; Hu, X.; Liao, J.; Kuang, J. miR-374a/Myc Axis Modulates Iron Overload-Induced Production of ROS and the Activation of Hepatic Stellate Cells via TGF-Β1 and IL-6. Biochem Biophys Res Commun 2019, 515, 499–504. [CrossRef]
- Gao, H.; Jin, Z.; Bandyopadhyay, G.; Wang, G.; Zhang, D.; Rocha, K.C.E.; Liu, X.; Zhao, H.; Kisseleva, T.; Brenner, D.A.; et al. Aberrant Iron Distribution via Hepatocyte-Stellate Cell Axis Drives Liver Lipogenesis and Fibrosis. Cell Metab 2022, 34, 1201-1213.e5. [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 Emission and Cellular Redox State Link Excess Fat Intake to Insulin Resistance in Both Rodents and Humans. J. Clin. Invest. 2009, 119, 573–581. [CrossRef]
- Polavarapu, R.; Spitz, D.R.; Sim, J.E.; Follansbee, M.H.; Oberley, L.W.; Rahemtulla, A.; Nanji, A.A. Increased Lipid Peroxidation and Impaired Antioxidant Enzyme Function Is Associated with Pathological Liver Injury in Experimental Alcoholic Liver Disease in Rats Fed Diets High in Corn Oil and Fish Oil. Hepatology 1998, 27, 1317–1323. [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase Prevents Oxidative Stress-Related Muscle Atrophy and Weakness. Redox Biology 2019, 20, 68–74. [CrossRef]
- Zierath, J.R.; Hawley, J.A. Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties. PLoS Biol 2004, 2, e348. [CrossRef]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. Resistance Exercise in Non-Alcoholic Fatty Liver Disease: A Systematic Review. J Hepatol 2017, 66, 142–152. [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev 2014, 2014, 360438. [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci 2022, 24, 449. [CrossRef]
- Milkovic, L.; Zarkovic, N.; Marusic, Z.; Zarkovic, K.; Jaganjac, M. The 4-Hydroxynonenal-Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets? Antioxidants (Basel) 2023, 12, 856. [CrossRef]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis Driven by Radical Oxidation of N-6 Polyunsaturated Fatty Acids Mediates Acetaminophen-Induced Acute Liver Failure. Cell Death Dis 2020, 11, 144. [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat Rev Mol Cell Biol 2019, 20, 137–155. [CrossRef]
- Bailey, A.P.; Koster, G.; Guillermier, C.; Hirst, E.M.A.; MacRae, J.I.; Lechene, C.P.; Postle, A.D.; Gould, A.P. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell 2015, 163, 340–353. [CrossRef]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J Biol Med 2019, 92, 435–452.
- Sun, X.; Yu, Q.; Qi, Y.; Kang, B.; Zhao, X.; Liu, L.; Wang, P.; Cong, M.; Liu, T. Peridroplet Mitochondria Are Associated with the Severity of MASLD and the Prevention of MASLD by Diethyldithiocarbamate. J Lipid Res 2024, 65, 100590. [CrossRef]
- Nocetti, D.; Espinosa, A.; Pino-De la Fuente, F.; Sacristán, C.; Bucarey, J.L.; Ruiz, P.; Valenzuela, R.; Chouinard-Watkins, R.; Pepper, I.; Troncoso, R.; et al. Lipid Droplets Are Both Highly Oxidized and Plin2-Covered in Hepatocytes of Diet-Induced Obese Mice. Appl Physiol Nutr Metab 2020, 45, 1368–1376. [CrossRef]
- Hara, M.; Wu, W.; Malechka, V.V.; Takahashi, Y.; Ma, J.-X.; Moiseyev, G. PNPLA2 Mobilizes Retinyl Esters from Retinosomes and Promotes the Generation of 11-Cis-Retinal in the Visual Cycle. Cell Rep 2023, 42, 112091. [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid Droplet Functions beyond Energy Storage. Biochim Biophys Acta Mol Cell Biol Lipids 2017, 1862, 1260–1272. [CrossRef]
- Maturana, G.; Segovia, J.; Olea-Azar, C.; Uribe-Oporto, E.; Espinosa, A.; Zúñiga-López, M.C. Evaluation of the Effects of Chia (Salvia Hispanica L.) Leaves Ethanolic Extracts Supplementation on Biochemical and Hepatic Markers on Diet-Induced Obese Mice. Antioxidants 2023, 12, 1108. [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol 2020, 40, e00099-20. [CrossRef]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, Function, and Post-Translational Regulation of the Catalytic and Modifier Subunits of Glutamate Cysteine Ligase. Mol Aspects Med 2009, 30, 86–98. [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res 2021, 31, 107–125. [CrossRef]
- Peleman, C.; Hellemans, S.; Veeckmans, G.; Arras, W.; Zheng, H.; Koeken, I.; Van San, E.; Hassannia, B.; Walravens, M.; Kayirangwa, E.; et al. Ferroptosis Is a Targetable Detrimental Factor in Metabolic Dysfunction-Associated Steatotic Liver Disease. Cell Death Differ 2024, 31, 1113–1126. [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative Stress, Cardiolipin and Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease. World J Gastroenterol 2014, 20, 14205–14218. [CrossRef]
- Saha, M.; Das, S.; Manna, K.; Saha, K.D. Melatonin Targets Ferroptosis through Bimodal Alteration of Redox Environment and Cellular Pathways in NAFLD Model. Biosci Rep 2023, 43, BSR20230128. [CrossRef]
- Yang, W.; Wang, Y.; Zhang, C.; Huang, Y.; Yu, J.; Shi, L.; Zhang, P.; Yin, Y.; Li, R.; Tao, K. Maresin1 Protect Against Ferroptosis-Induced Liver Injury Through ROS Inhibition and Nrf2/HO-1/GPX4 Activation. Front Pharmacol 2022, 13, 865689. [CrossRef]
- Li, J.-J.; Dai, W.-Q.; Mo, W.-H.; Xu, W.-Q.; Li, Y.-Y.; Guo, C.-Y.; Xu, X.-F. Fucoidan Ameliorates Ferroptosis in Ischemia-Reperfusion-Induced Liver Injury through Nrf2/HO-1/GPX4 Activation. J Clin Transl Hepatol 2023, 11, 1341–1354. [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular Basis for Redox Control by the Human Cystine/Glutamate Antiporter System Xc. Nat Commun 2021, 12, 7147. [CrossRef]
- Berardo, C.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: Current Issues and Future Perspectives in Preclinical and Clinical Research. Int J Mol Sci 2020, 21, 9646. [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M.U. UPR Proteins IRE1 and PERK Switch BiP from Chaperone to ER Stress Sensor. Nat Struct Mol Biol 2019, 26, 1053–1062. [CrossRef]
- Venkatesan, N.; Doskey, L.C.; Malhi, H. The Role of Endoplasmic Reticulum in Lipotoxicity during Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Pathogenesis. Am J Pathol 2023, 193, 1887–1899. [CrossRef]
- Pineau, L.; Colas, J.; Dupont, S.; Beney, L.; Fleurat-Lessard, P.; Berjeaud, J.-M.; Bergès, T.; Ferreira, T. Lipid-Induced ER Stress: Synergistic Effects of Sterols and Saturated Fatty Acids. Traffic 2009, 10, 673–690. [CrossRef]
- L’homme, L.; Sermikli, B.P.; Staels, B.; Piette, J.; Legrand-Poels, S.; Dombrowicz, D. Saturated Fatty Acids Promote GDF15 Expression in Human Macrophages through the PERK/eIF2/CHOP Signaling Pathway. Nutrients 2020, 12, 3771. [CrossRef]
- Cao, J.; Dai, D.-L.; Yao, L.; Yu, H.-H.; Ning, B.; Zhang, Q.; Chen, J.; Cheng, W.-H.; Shen, W.; Yang, Z.-X. Saturated Fatty Acid Induction of Endoplasmic Reticulum Stress and Apoptosis in Human Liver Cells via the PERK/ATF4/CHOP Signaling Pathway. Mol Cell Biochem 2012, 364, 115–129. [CrossRef]
- Li, Y.; Chen, W.; Ogawa, K.; Koide, M.; Takahashi, T.; Hagiwara, Y.; Itoi, E.; Aizawa, T.; Tsuchiya, M.; Izumi, R.; et al. Feeder-Supported in Vitro Exercise Model Using Human Satellite Cells from Patients with Sporadic Inclusion Body Myositis. Sci Rep 2022, 12, 1082. [CrossRef]
- Thomas, A.C.Q.; Brown, A.; Hatt, A.A.; Manta, K.; Costa-Parke, A.; Kamal, M.; Joanisse, S.; McGlory, C.; Phillips, S.M.; Kumbhare, D.; et al. Short-Term Aerobic Conditioning Prior to Resistance Training Augments Muscle Hypertrophy and Satellite Cell Content in Healthy Young Men and Women. FASEB J 2022, 36, e22500. [CrossRef]
- Long, M.T.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Esliger, D.W.; Vasan, R.S.; Fox, C.S.; Murabito, J.M. Hepatic Steatosis Is Associated with Lower Levels of Physical Activity Measured via Accelerometry. Obesity (Silver Spring) 2015, 23, 1259–1266. [CrossRef]
- von Loeffelholz, C.; Roth, J.; Coldewey, S.M.; Birkenfeld, A.L. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines 2021, 9, 1853. [CrossRef]
- Rodgers, B.D.; Ward, C.W. Myostatin/Activin Receptor Ligands in Muscle and the Development Status of Attenuating Drugs. Endocrine Reviews 2022, 43, 329–365. [CrossRef]
- Reisz-Porszasz, S.; Bhasin, S.; Artaza, J.N.; Shen, R.; Sinha-Hikim, I.; Hogue, A.; Fielder, T.J.; Gonzalez-Cadavid, N.F. Lower Skeletal Muscle Mass in Male Transgenic Mice with Muscle-Specific Overexpression of Myostatin. Am J Physiol Endocrinol Metab 2003, 285, E876-888. [CrossRef]
- Burgess, K.; Xu, T.; Brown, R.; Han, B.; Welle, S. Effect of Myostatin Depletion on Weight Gain, Hyperglycemia, and Hepatic Steatosis during Five Months of High-Fat Feeding in Mice. PLoS One 2011, 6, e17090. [CrossRef]
- Delogu, W.; Caligiuri, A.; Provenzano, A.; Rosso, C.; Bugianesi, E.; Coratti, A.; Macias-Barragan, J.; Galastri, S.; Di Maira, G.; Marra, F. Myostatin Regulates the Fibrogenic Phenotype of Hepatic Stellate Cells via C-Jun N-Terminal Kinase Activation. Digestive and Liver Disease 2019, 51, 1400–1408. [CrossRef]
- Zhu, L.; Wang, X.; Wei, Z.; Yang, M.; Zhou, X.; Lei, J.; Bai, C.; Su, G.; Liu, X.; Yang, L.; et al. Myostatin Deficiency Enhances Antioxidant Capacity of Bovine Muscle via the SMAD-AMPK-G6PD Pathway. Oxid Med Cell Longev 2022, 2022, 3497644. [CrossRef]
- Czaja, W.; Nakamura, Y.K.; Li, N.; Eldridge, J.A.; DeAvila, D.M.; Thompson, T.B.; Rodgers, B.D. Myostatin Regulates Pituitary Development and Hepatic IGF1. Am J Physiol Endocrinol Metab 2019, 316, E1036–E1049. [CrossRef]
- Ruiz-Margáin, A.; Pohlmann, A.; Lanzerath, S.; Langheinrich, M.; Campos-Murguía, A.; Román-Calleja, B.M.; Schierwagen, R.; Klein, S.; Uschner, F.E.; Brol, M.J.; et al. Myostatin Is Associated with the Presence and Development of Acute-on-Chronic Liver Failure. JHEP Rep 2023, 5, 100761. [CrossRef]
- Yoshio, S.; Shimagaki, T.; Hashida, R.; Kawaguchi, T.; Tsutsui, Y.; Sakamoto, Y.; Yoshida, Y.; Kawai, H.; Yoshikawa, S.; Yamazoe, T.; et al. Myostatin as a Fibroblast-Activating Factor Impacts on Postoperative Outcome in Patients with Hepatocellular Carcinoma. Hepatol Res 2021, 51, 803–812. [CrossRef]
- Wilkinson, A.N.; Gartlan, K.H.; Kelly, G.; Samson, L.D.; Olver, S.D.; Avery, J.; Zomerdijk, N.; Tey, S.-K.; Lee, J.S.; Vuckovic, S.; et al. Granulocytes Are Unresponsive to IL-6 Due to an Absence of Gp130. J Immunol 2018, 200, 3547–3555. [CrossRef]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 Is the Major Regulator of Acute Phase Protein Synthesis in Adult Human Hepatocytes. FEBS Lett 1989, 242, 237–239. [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol Rev 2008, 88, 1379–1406. [CrossRef]
- Zhao, Y.; Wang, C.; Yang, T.; Wang, H.; Zhao, S.; Sun, N.; Chen, Y.; Zhang, H.; Fan, H. Chlorogenic Acid Alleviates Chronic Stress-Induced Duodenal Ferroptosis via the Inhibition of the IL-6/JAK2/STAT3 Signaling Pathway in Rats. J Agric Food Chem 2022, 70, 4353–4361. [CrossRef]
- Halade, G.V.; Upadhyay, G.; Marimuthu, M.; Wanling, X.; Kain, V. Exercise Reduces Pro-Inflammatory Lipids and Preserves Resolution Mediators That Calibrate Macrophage-Centric Immune Metabolism in Spleen and Heart Following Obesogenic Diet in Aging Mice. Journal of Molecular and Cellular Cardiology 2024, 188, 79–89. [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int J Mol Med 2019. [CrossRef]
- Shi, H.; Hao, X.; Sun, Y.; Zhao, Y.; Wang, Y.; Cao, X.; Gong, Z.; Ji, S.; Lu, J.; Yan, Y.; et al. Exercise-Inducible Circulating Extracellular Vesicle Irisin Promotes Browning and the Thermogenic Program in White Adipose Tissue. Acta Physiol (Oxf) 2024, 240, e14103. [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin Alleviates LPS-Induced Liver Injury and Inflammation through Inhibition of NLRP3 Inflammasome and NF-κB Signaling. J Recept Signal Transduct Res 2021, 41, 294–303. [CrossRef]
- Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Psallida, S.; Kounatidis, D.; Stratigou, T.; Marinou, I.; Vogiatzakis, E.; Dalamaga, M. Alterations of the Adipo-Myokine Irisin in Sepsis and Septic Shock: Diagnostic and Prognostic Implications. Biomolecules 2024, 14, 291. [CrossRef]
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum Irisin Levels Are Decreased in Patients with Sepsis, and Exogenous Irisin Suppresses Ferroptosis in the Liver of Septic Mice. Clin Transl Med 2020, 10, e173. [CrossRef]
- Zhu, W.; Sahar, N.E.; Javaid, H.M.A.; Pak, E.S.; Liang, G.; Wang, Y.; Ha, H.; Huh, J.Y. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 2021, 10, 3306. [CrossRef]
- Byun, S.; Seok, S.; Kim, Y.-C.; Zhang, Y.; Yau, P.; Iwamori, N.; Xu, H.E.; Ma, J.; Kemper, B.; Kemper, J.K. Fasting-Induced FGF21 Signaling Activates Hepatic Autophagy and Lipid Degradation via JMJD3 Histone Demethylase. Nat Commun 2020, 11, 807. [CrossRef]
- Kilkenny, D.M.; Rocheleau, J.V. The FGF21 Receptor Signaling Complex: Klothoβ, FGFR1c, and Other Regulatory Interactions. Vitam Horm 2016, 101, 17–58. [CrossRef]
- Donate-Correa, J.; Martín-Núñez, E.; Delgado, N.P.; de Fuentes, M.M.; Arduan, A.O.; Mora-Fernández, C.; Navarro González, J.F. Implications of Fibroblast Growth Factor/Klotho System in Glucose Metabolism and Diabetes. Cytokine Growth Factor Rev 2016, 28, 71–77. [CrossRef]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast Growth Factor 21 Controls Mitophagy and Muscle Mass. J Cachexia Sarcopenia Muscle 2019, 10, 630–642. [CrossRef]
- Mashili, F.L.; Austin, R.L.; Deshmukh, A.S.; Fritz, T.; Caidahl, K.; Bergdahl, K.; Zierath, J.R.; Chibalin, A.V.; Moller, D.E.; Kharitonenkov, A.; et al. Direct Effects of FGF21 on Glucose Uptake in Human Skeletal Muscle: Implications for Type 2 Diabetes and Obesity. Diabetes Metab Res Rev 2011, 27, 286–297. [CrossRef]
- Kim, K.H.; Kim, S.H.; Min, Y.-K.; Yang, H.-M.; Lee, J.-B.; Lee, M.-S. Acute Exercise Induces FGF21 Expression in Mice and in Healthy Humans. PLoS One 2013, 8, e63517. [CrossRef]
- Warrier, M.; Paules, E.M.; Silva-Gomez, J.; Friday, W.B.; Bramlett, F.; Kim, H.; Zhang, K.; Trujillo-Gonzalez, I. Homocysteine-Induced Endoplasmic Reticulum Stress Activates FGF21 and Is Associated with Browning and Atrophy of White Adipose Tissue in Bhmt Knockout Mice. Heliyon 2023, 9, e13216. [CrossRef]
- Geng, L.; Liao, B.; Jin, L.; Huang, Z.; Triggle, C.R.; Ding, H.; Zhang, J.; Huang, Y.; Lin, Z.; Xu, A. Exercise Alleviates Obesity-Induced Metabolic Dysfunction via Enhancing FGF21 Sensitivity in Adipose Tissues. Cell Rep 2019, 26, 2738-2752.e4. [CrossRef]
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast Growth Factor 21 Attenuates Iron Overload-Induced Liver Injury and Fibrosis by Inhibiting Ferroptosis. Redox Biol 2021, 46, 102131. [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of Skeletal Muscle Growth by the IGF1-Akt/PKB Pathway: Insights from Genetic Models. Skelet Muscle 2011, 1, 4. [CrossRef]
- Bridgewater, D.J.; Ho, J.; Sauro, V.; Matsell, D.G. Insulin-like Growth Factors Inhibit Podocyte Apoptosis through the PI3 Kinase Pathway. Kidney Int 2005, 67, 1308–1314. [CrossRef]
- Song, Y.-H.; Godard, M.; Li, Y.; Richmond, S.R.; Rosenthal, N.; Delafontaine, P. Insulin-like Growth Factor I-Mediated Skeletal Muscle Hypertrophy Is Characterized by Increased mTOR-p70S6K Signaling without Increased Akt Phosphorylation. J Investig Med 2005, 53, 135–142. [CrossRef]
- Ribeiro, M.B.T.; Guzzoni, V.; Hord, J.M.; Lopes, G.N.; Marqueti, R. de C.; de Andrade, R.V.; Selistre-de-Araujo, H.S.; Durigan, J.L.Q. Resistance Training Regulates Gene Expression of Molecules Associated with Intramyocellular Lipids, Glucose Signaling and Fiber Size in Old Rats. Sci Rep 2017, 7, 8593. [CrossRef]
- Ye, G.; Xiao, Z.; Luo, Z.; Huang, X.; Abdelrahim, M.E.A.; Huang, W. Resistance Training Effect on Serum Insulin-like Growth Factor 1 in the Serum: A Meta-Analysis. Aging Male 2020, 23, 1471–1479. [CrossRef]
- García-Fernández, M.; Castilla-Cortázar, I.; Díaz-Sanchez, M.; Navarro, I.; Puche, J.E.; Castilla, A.; Casares, A.D.; Clavijo, E.; González-Barón, S. Antioxidant Effects of Insulin-like Growth Factor-I (IGF-I) in Rats with Advanced Liver Cirrhosis. BMC Gastroenterol 2005, 5, 7. [CrossRef]
- Esvald, E.-E.; Tuvikene, J.; Kiir, C.S.; Avarlaid, A.; Tamberg, L.; Sirp, A.; Shubina, A.; Cabrera-Cabrera, F.; Pihlak, A.; Koppel, I.; et al. Revisiting the Expression of BDNF and Its Receptors in Mammalian Development. Front Mol Neurosci 2023, 16, 1182499. [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of Exercise-Induced Brain-Derived Neurotrophic Factor Production in the Regulation of Energy Homeostasis in Mammals. Exp Physiol 2009, 94, 1153–1160. [CrossRef]
- Rahmani, F.; Saghazadeh, A.; Rahmani, M.; Teixeira, A.L.; Rezaei, N.; Aghamollaii, V.; Ardebili, H.E. Plasma Levels of Brain-Derived Neurotrophic Factor in Patients with Parkinson Disease: A Systematic Review and Meta-Analysis. Brain Res 2019, 1704, 127–136. [CrossRef]
- Hao, L.-S.; Du, Y.; Chen, L.; Jiao, Y.-G.; Cheng, Y. Brain-Derived Neurotrophic Factor as a Biomarker for Obsessive-Compulsive Disorder: A Meta-Analysis. J Psychiatr Res 2022, 151, 676–682. [CrossRef]
- Hattori, Y.; Yamada, H.; Munetsuna, E.; Ando, Y.; Mizuno, G.; Fujii, R.; Tsuboi, Y.; Ichino, N.; Osakabe, K.; Sugimoto, K.; et al. Increased Brain-Derived Neurotrophic Factor in the Serum of Persons with Nonalcoholic Fatty Liver Disease. Endocr J 2022, 69, 999–1006. [CrossRef]
- Marlatt, M.W.; Potter, M.C.; Lucassen, P.J.; van Praag, H. Running throughout Middle-Age Improves Memory Function, Hippocampal Neurogenesis, and BDNF Levels in Female C57BL/6J Mice. Dev Neurobiol 2012, 72, 943–952. [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. Elife 2016, 5, e15092. [CrossRef]
- Genzer, Y.; Chapnik, N.; Froy, O. Effect of Brain-Derived Neurotrophic Factor (BDNF) on Hepatocyte Metabolism. Int J Biochem Cell Biol 2017, 88, 69–74. [CrossRef]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian Control of BDNF-Mediated Nrf2 Activation in Astrocytes Protects Dopaminergic Neurons from Ferroptosis. Free Radic Biol Med 2019, 133, 169–178. [CrossRef]
- Bórquez, J.C.; Díaz-Castro, F.; La Fuente, F.P.; Espinoza, K.; Figueroa, A.M.; Martínez-Ruíz, I.; Hernández, V.; López-Soldado, I.; Ventura, R.; Domingo, J.C.; et al. Mitofusin-2 Induced by Exercise Modifies Lipid Droplet-Mitochondria Communication, Promoting Fatty Acid Oxidation in Male Mice with NAFLD. Metabolism 2023, 155765. [CrossRef]
- Keating, S.E.; George, J.; Johnson, N.A. The Benefits of Exercise for Patients with Non-Alcoholic Fatty Liver Disease. Expert Rev Gastroenterol Hepatol 2015, 9, 1247–1250. [CrossRef]
- Yang, Y.; Li, X.; Liu, Z.; Ruan, X.; Wang, H.; Zhang, Q.; Cao, L.; Song, L.; Chen, Y.; Sun, Y. Moderate Treadmill Exercise Alleviates NAFLD by Regulating the Biogenesis and Autophagy of Lipid Droplet. Nutrients 2022, 14, 4910. [CrossRef]
- Liu, Y.; Yang, W.; Yang, G.; Wang, H. Aerobic Exercise Relieves High-Fat Diet-Induced MAFLD by Activating Nrf2/GPX4 and Suppressing Ferroptosis in Mice a Concise and Informative Title: Exercise Relieves Mafld by Nrf2/Gpx4 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).