3.1. The Gross Anatomy of the Human and Murine Atrioventricular Junctions
Removing the parietal walls of the right-sided chambers provides an en-face view of the septal atrioventricular junctions, and the septal surfaces of the morphologically right atrium and right ventricle (
Figure 1A). A view of an episcopic dataset from a neonatal mouse prepared in comparable fashion shows the similarities when the murine heart is positioned so as to parallel the human heart as viewed in attitudinally appropriate fashion (
Figure 1B).
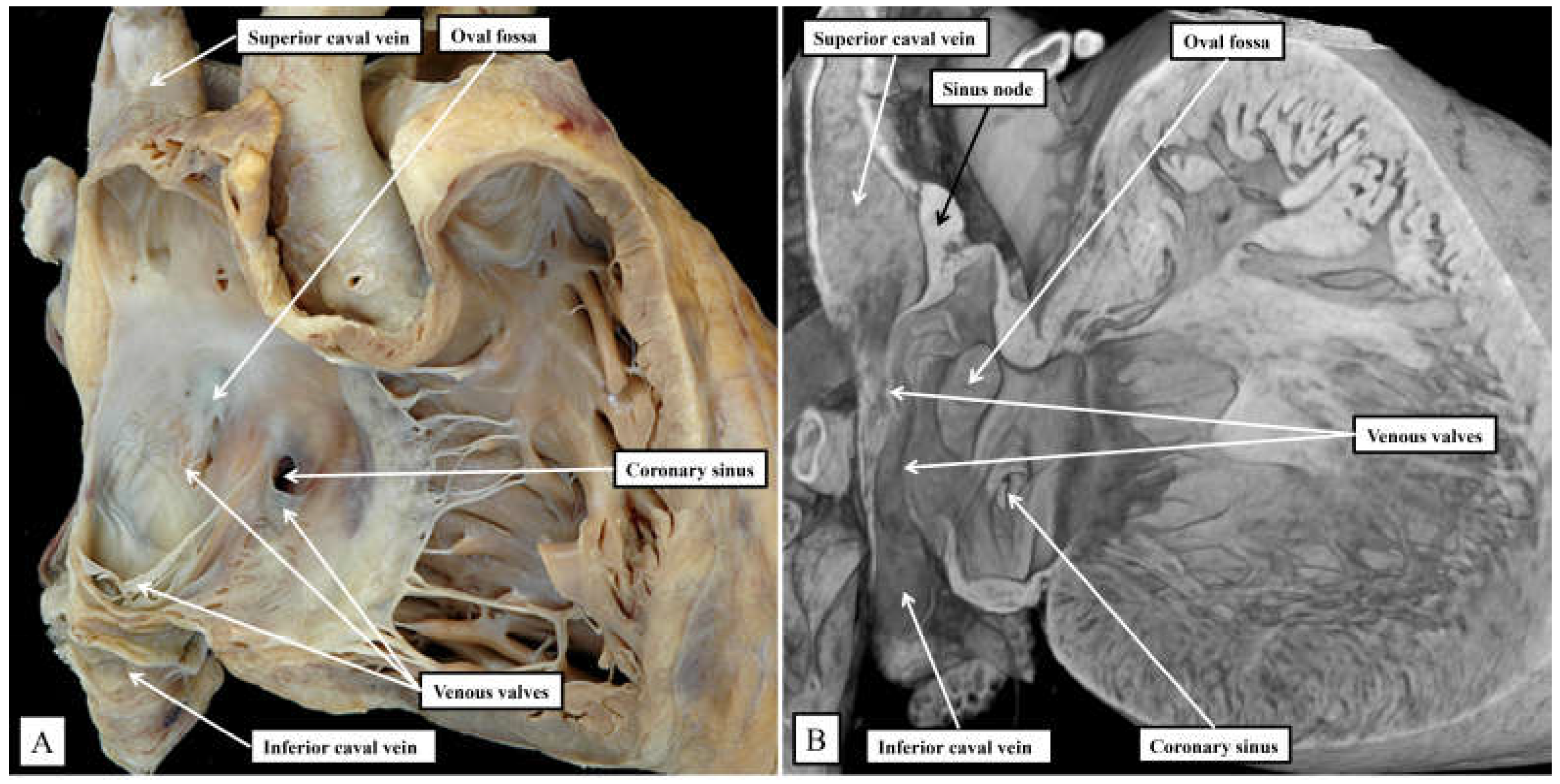
As is shown in the photographs, when viewed attitudinally, the features are comparable. There are, however, subtle differences which impact on the location of the components of the atrioventricular conduction axis. Perhaps the most significant difference is the presence of a persistent left superior caval vein in the murine heart. This channel opens inferiorly into the vestibular region of the right atrium through an enlarged orifice of the coronary sinus (
Figure 1B). In the human heart, the coronary sinus opens much more superiorly within the cavity of the right atrium (
Figure 1A). The superior and inferior caval veins enter the systemic venous sinus in comparable fashion in both species, but the opening of the venous sinus to the morphogically right atrium is more dorsal in the murine heart (
Figure 1B). The junction between the venous sinus and the remainder of the atrium is then much more obvious in the murine heart, since the venous valves are much better preserved, with obvious right and left venous valves coming together at a superior commissure attached within the atrial appendage by the prominent pectinate muscle known as the septum spurium (
Figure 1B). In the human heart, it is usually only parts of the right venous valve that persist. These remnants are seen as the Eustachian valve, which partially guards the entrance of the inferior caval vein, and the Thebesian valve, which guards the opening of the coronary sinus (
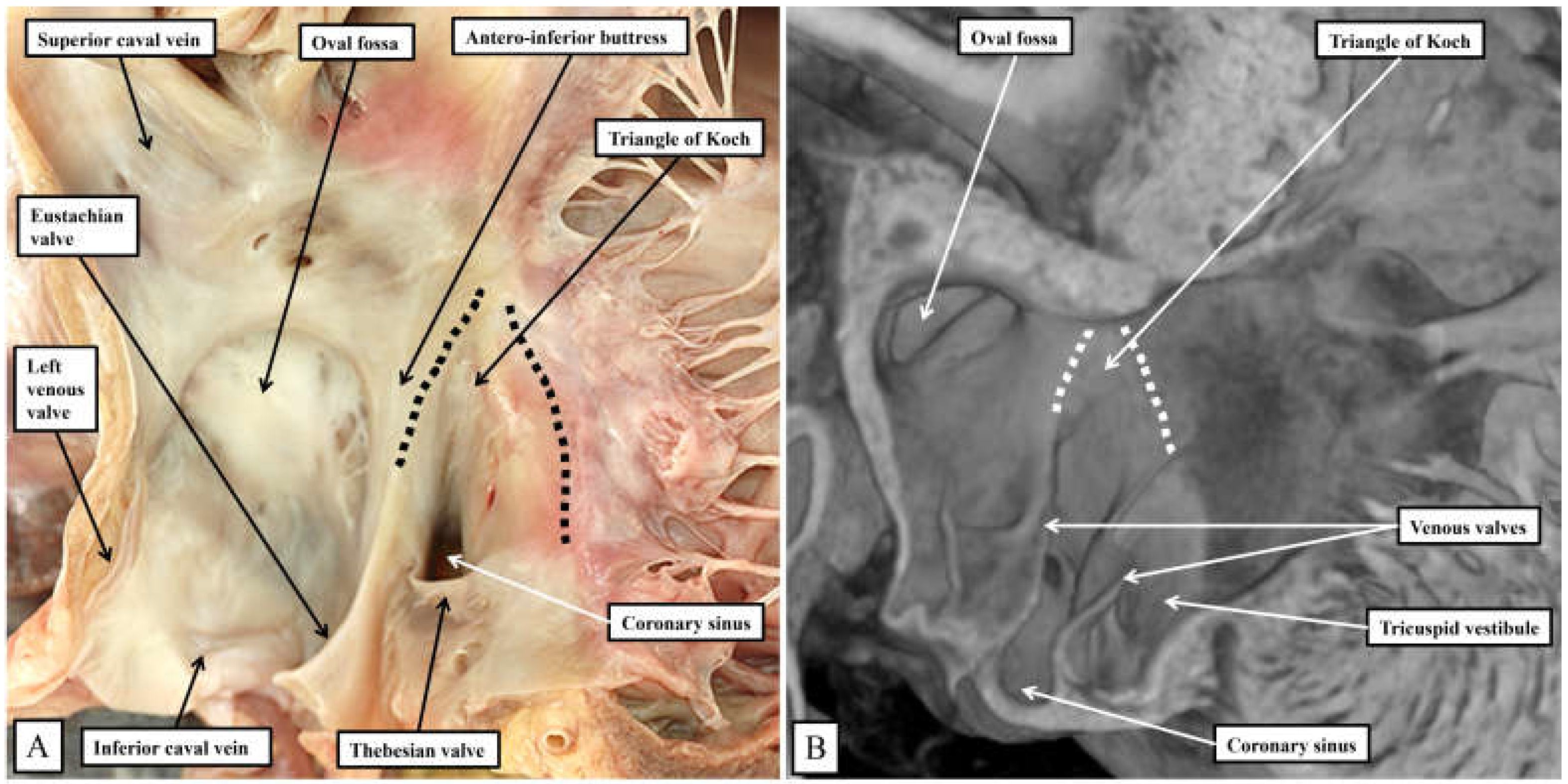
Figure 1A). The two valves come together at the area of the atrial wall separating the orifice of the coronary sinus from the oval fossa. Usually described as the sinus septum, the area is a fold between the walls of the inferior caval vein and the coronary sinus. The area of union between the valves then continues as a tendinous structure that runs within the antero-inferior buttress of the oval fossa. As we will describe, this fibrous entity, known as the tendon of Todaro, forms a boundary of the triangle of Koch, itself providing a landmark to the site of the atrial components of the conduction axis. The situation is more obvious in the murine heart, since venous valves marking both the right and left borders of the systemic venous sinus remain subsequent to birth (
Figure 1B). In the human heart, nonetheless, with careful examination, it is sometimes possible to recognise small remnants of the left venous valve plastered on to the septal surface of the oval fossa and its surrounds. In the murine heart, the right and left venous valves form a tunnel that directs the flow from the inferior caval vein towards the oval fossa. The superior extension of both valves into the area between the coronary sinus and the oval fossa is also much more marked, with a triangular area such as described initially by Koch also to be found in the mouse heart (see below). There are also subtle differences in the arrangement of the oval fossa between the species. In the human heart, the superior rim of the fossa is an obvious infolding between the attachments of the superior caval vein to the right atrium, and the right pulmonary veins to the left atrium. This arrangement is not found in the murine heart, since the pulmonary veins open to the morphologically left atrium through a common orifice located inferiorly rather than superiorly, albeit that a fold is present in the dorsal wall. The flap valve of the oval fossa in the murine heart, in consequence, closes against the roof of the atrium (
Figure 2A). , rather than directly against the superior interatrial fold as is the case in the human heart.
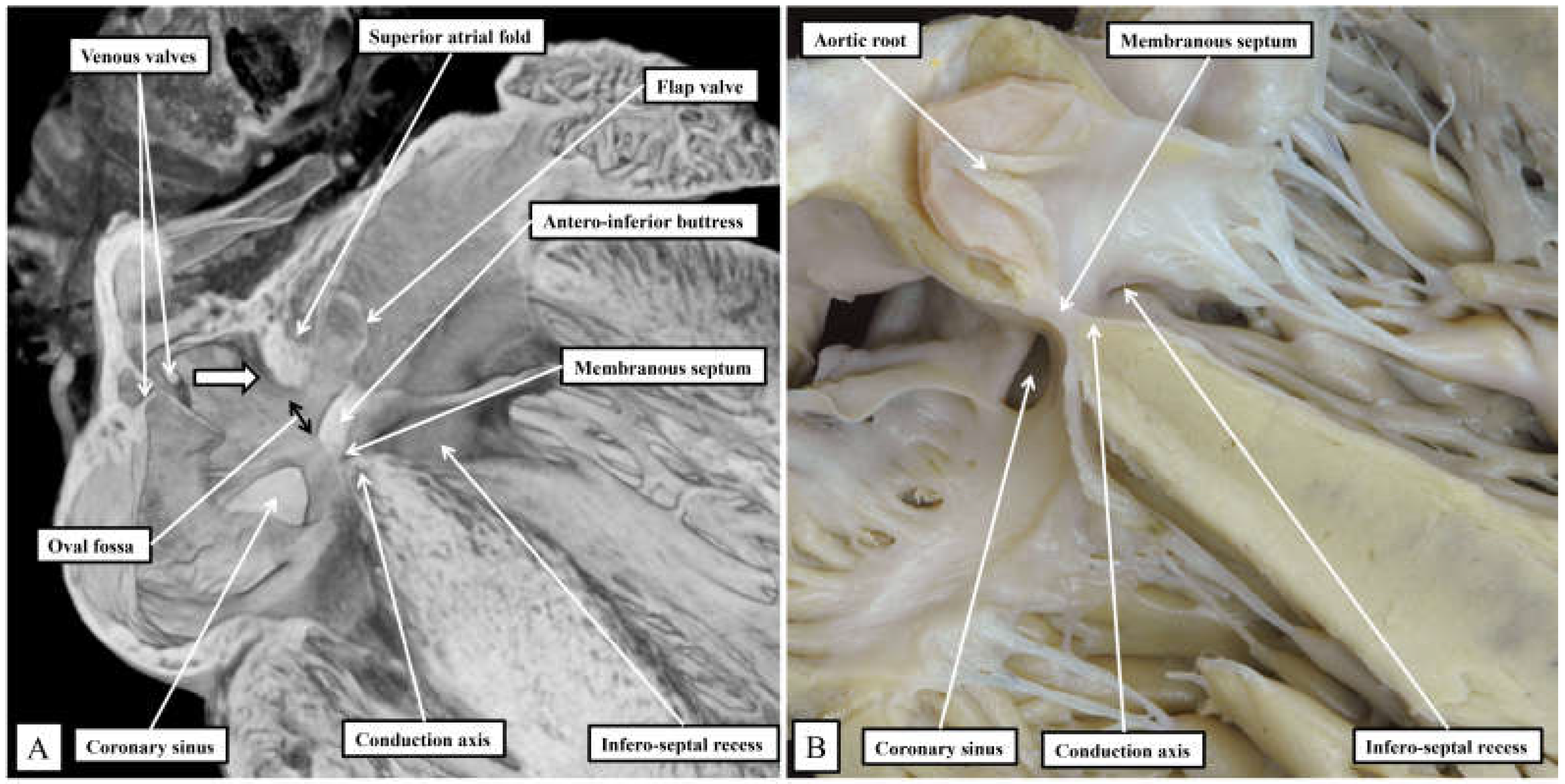
The superior fold is often incorrectly identified as the “septum secundum”. In both species, however, the true second atrial septum can be identified as the antero-inferior buttress of the oval fossa, well seen in the murine heart when frontal sections are taken through the fossa (
Figure 2A).
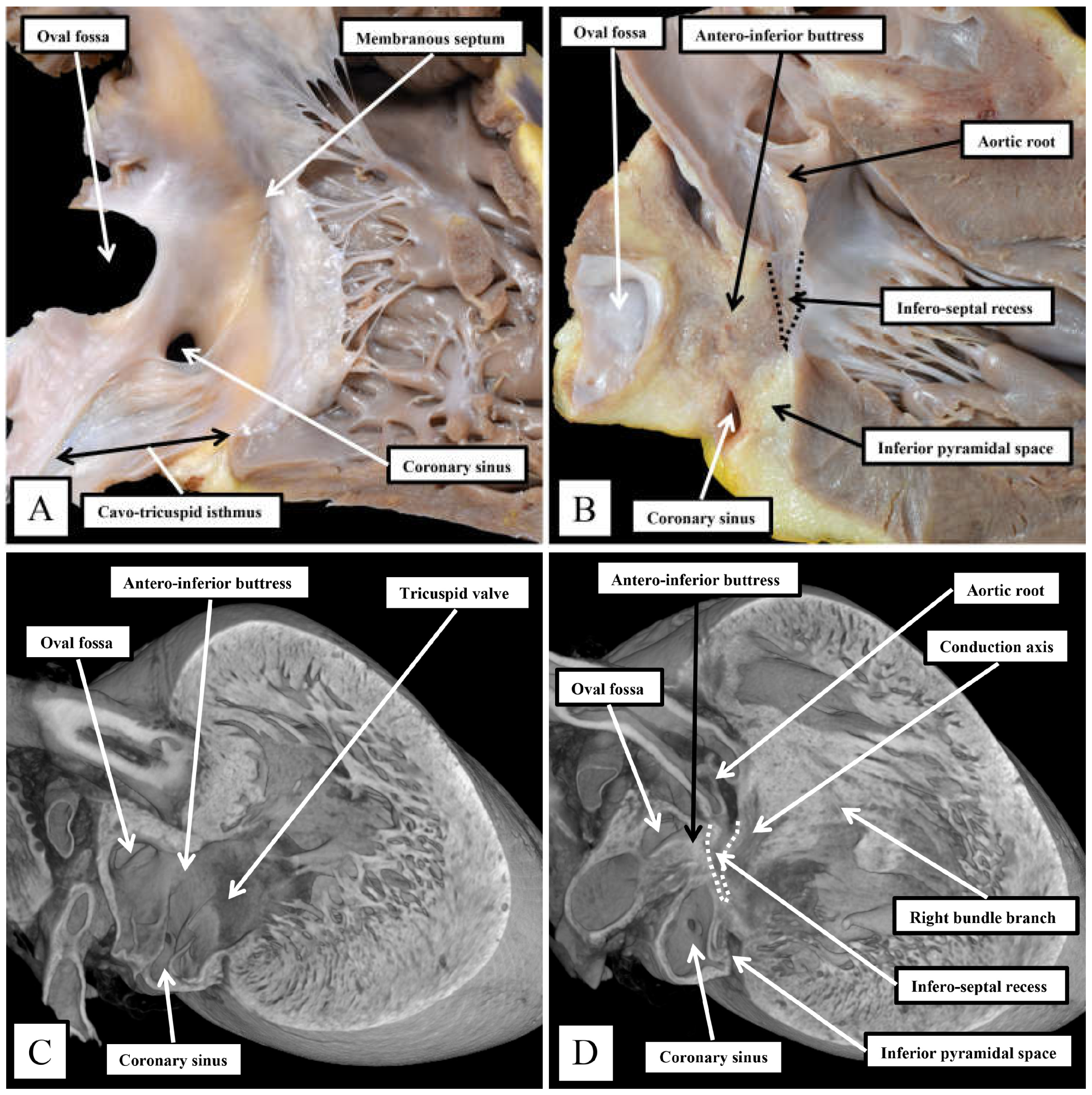
Figure 2A then serves to emphasise another major, but subtle, difference between the species that achieves significance when considering the location of the conduction axis. This is the presence of a much reduced membranous septum in the murine heart, with the septum itself located inferior to the antero-inferior buttress (
Figure 2A). A comparable section through the membranous septum in the human heart cuts through the aortic root, rather than the oval fossa. The fossa in the human heart is located inferiorly and posteriorly relative to the membranous septum, and is not seen in cuts made in the frontal plane (
Figure 2B). The difference in the location of the membranous septum between the species can be related, in part, to the morphology of the coronary sinus.
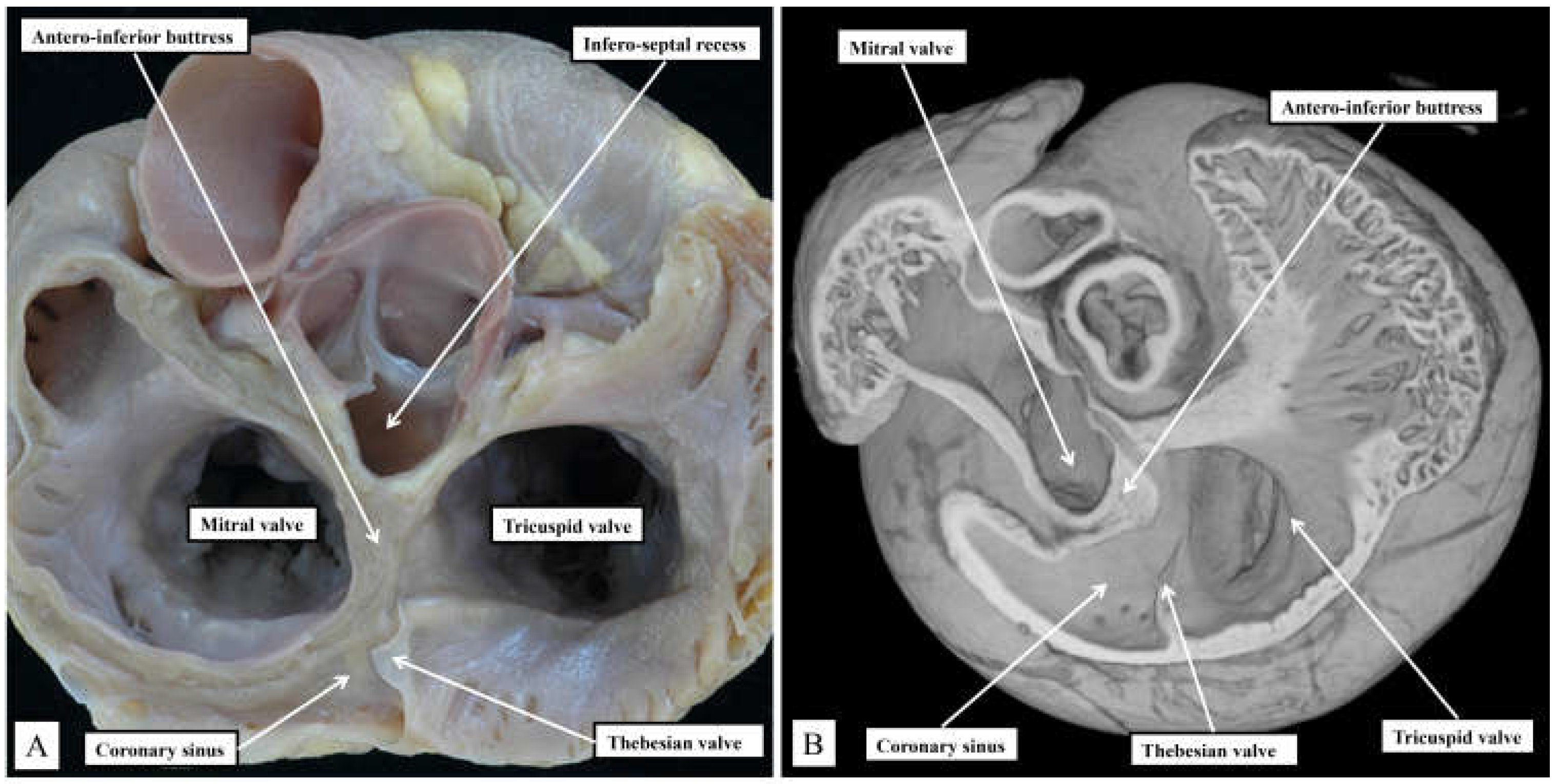
In the human heart, the coronary sinus serves only as the conduit for return of the greater part of the venous drainage of the heart, since the left superior caval vein has usually regressed in the human heart, with its walls persisting only as the ligament of Marshall. In the murine heart, as already explained, it is the norm for the left superior caval vein to persist, and to open to the right atrium through the orifice of the coronary sinus. This produces a marked difference in the relationship of the orifices of the right and left atrioventricular junctions, guarded by the tricuspid and mitral valves (
Figure 3).
In the murine heart, furthermore, it is often difficult to find a third leaflet guarding the right atrioventricular junction (
Figure 3B), whereas in the human heart the right atrioventricular valve usually closes in trifoliate fashion, even though it may be difficult to find a zone of apposition supported by a discrete papillary muscles between the inferior and the antero-superior leaflets. The other major difference to be noted in the junctional arrangements, however, is the much deeper “wedging” of the aortic root between the leaflets of the mitral valve and the septum (
Figure 3A). The combination of the enlarged orifice of the membranous septum and the lack of aortic wedging in the murine heart means that, although there is an infero-septal recess between the aortic leaflet of the mitral valve and the septum, it does not extend beneath the antero-inferior buttress of the atrial septum to the same extent as is seen in the human heart [
16]. The inferior pyramidal space, furthermore, does not extend as far superiorly in the murine heart as in the human heart. This, again, reflects the differing relationships between the orifices of the right and left atrioventricular junctions (
Figure 3), and the presence in the murine heart of the persistent left superior caval vein (
Figure 3B). In the human heart, the inferior pyramidal space extends beneath the antero-inferior buttress of the atrial septum, being confined by the diverging walls of the right and left atrial vestibules [
16]. In the murine heart, the vestibule of the right atrioventricular junction itself extends much more inferiorly, having to receive the enlarged orifice of the coronary sinus (
Figure 3B). In the human heart, it is the extensive superior extent of the inferior pyramidal space that serves to place the septal wall of the right atrial vestibule adjacent to the inferior extent of the infero-septal recess of the left ventricular outflow tract [
16]. These relationships, as we will explain, then determine the equally subtle differences in the location of the atrioventricular conduction axis relative to the septal landmarks in the human heart as opposed to the murine heart. The differences can be demonstrated by making a cut that removes the right atrial walls of the human heart, leaving behind the rims and floor of the oval fossa, but extending through the antero-inferior buttress of the atrial septum so as to cut into the infero-septal recess (
Figure 4A). The part of the heart reveald having removd the larger parts of the right atrial and right ventricular components then shows how the apex of the inferior pyramidal space, extending superiorly, meets the inferiorly directed apex of the infero-septal recess (
Figure 4B).
It is the septal surfaces of the right atrium, however, that provide the key landmarks to the location of the atrial components of the atrioventricular conduction axis. And here again, the arrangements between the species are similar, albeit with subtle differences. These differences again reflect, for the larger part, the presence in the murine heart, of the persistent left superior caval vein and the enlarged orifice of the coronary sinus. This means, as already discussed, that the orifice of the coronary sinus, guarded by the Thebesian valve, is positioned more superiorly in the human heart when compared with the arrangement seen in the murine heart. In both species, nonetheless, it remains possible to recognise how the Eustachian and Thebesian valves come together, extending to run as the tendon of Todaro within the atrial walls interposing between the orifice of the coronary sinus and the oval fossa. The arrangement is the more obvious in the murine heart, since the orifice of the coronary sinus itself extends to occupy the inferior component of the chamber when viewed in attitudinally appropriate arrangement for the human heart (
Figure 5).
In both species, therefore, it is possible to recognise the important triangle as initially described by Koch [
17]. The apex of the triangle, in the human heart, is adjacent to the infero-septal recess of the left ventricular outflow tract, albeit that the spaces are separated by the atrioventricular septal structures (
Figure 4B). In the murine heart, in contrast, the aortic root is located more superiorly when viewed in attitudinally appropriate fashion, reflecting the lack of wedging when compared to the human heart (
Figure 3). The greater inferior extent of the orifice of the coronary sinus in the murine heart (
Figure 5B) then produces a major difference in the structure of the cavo-tricuspid isthmus. In the human heart, the isthmus incorporates an extensive sub-Thebesian sinus (
Figure 5A). In the murine heart, again when considered attitudinally, the vestibular sinus is anterior, rather than inferior, when assessed relative to the Thebesian valve (
Figure 5B). All of these subtle changes between the human and murine hearts in terms of the gross anatomy are then significant when set against the location of the atrioventricular conduction axis in the two species.
3.2. The Specifics of the Atrioventricular Conduction Axis of the Human Heart
It was the stellar investigation of Tawara that clarified the very existence of the atrioventricular conduction axis [
18]. In his monumental monograph, Tawara described the findings in several animal species, albeit not paying any attention to the murine heart. Our own findings in the human heart [
14] are very much an endorsement of his observations. As can be inferred from the illustrations provided by Tawara, when the triangle of Koch is considered in attitudinally appropriate fashion, and when serial sections are assessed moving inferiorly to superior across the inferior pyramidal space, it is possible to recognise how the atrial working myocardium of the atrial vestibules give rises to the right and left inferior extensions of the atrioventricular node, with the rightward extension extending significantly inferiorly to pass within the septal isthmus between the orifice of the coronary sinus and the hinge of the septal leaflet of the tricuspid valve [
13]. It is these inferior extensions that constitute the slow pathways into the node [
19]. In
Figure 7, we are showing comparable serial sections, taken in short axis from the same dataset as shown in
Figure 6, but placed so as to run superiorly from the base of the ventricular cone (
Figure 6A) towards the ventricular apex (
Figure 6E). The right inferior nodal extensions is thus seen in the deepest section (
Figure 6E).
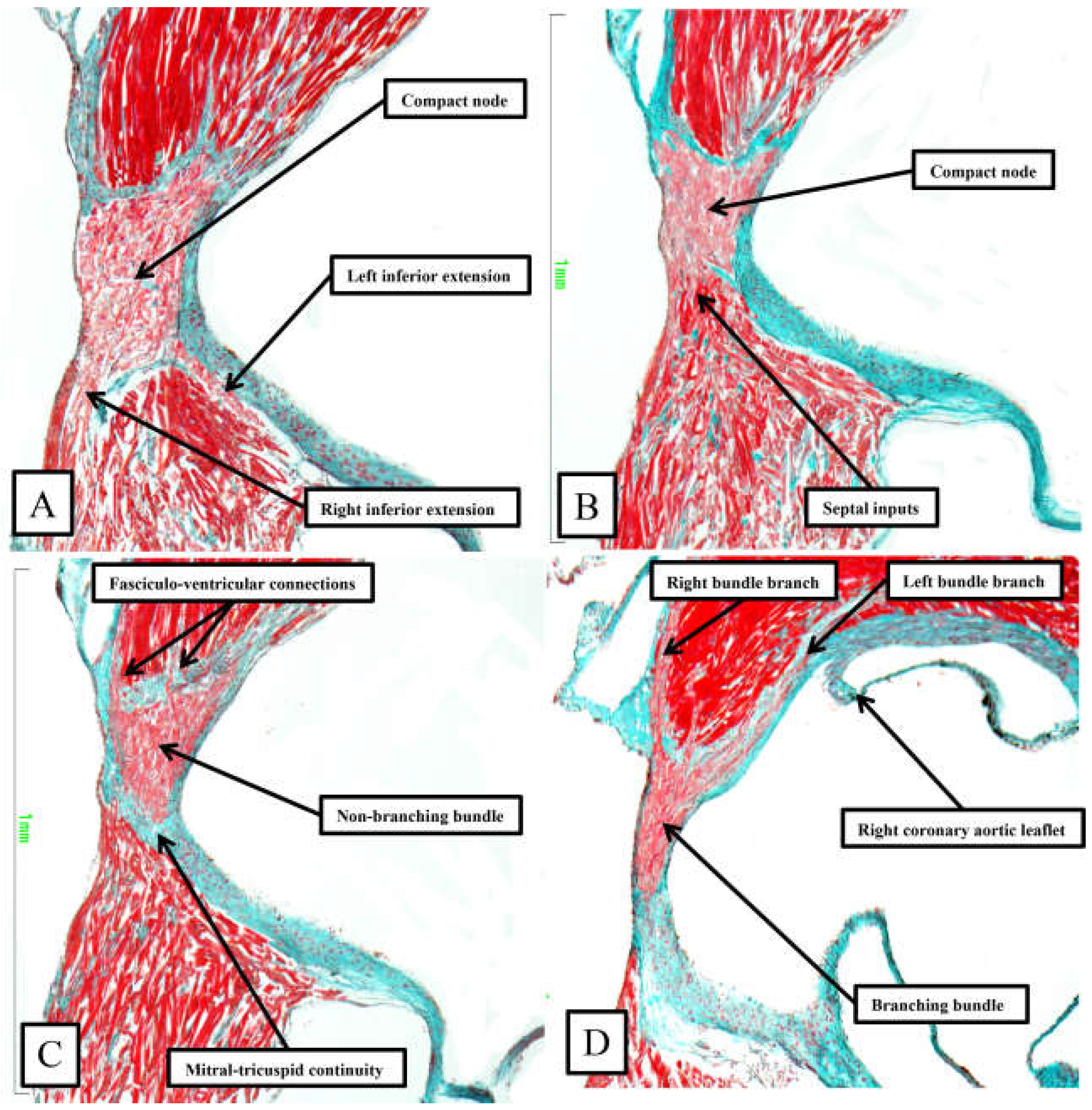
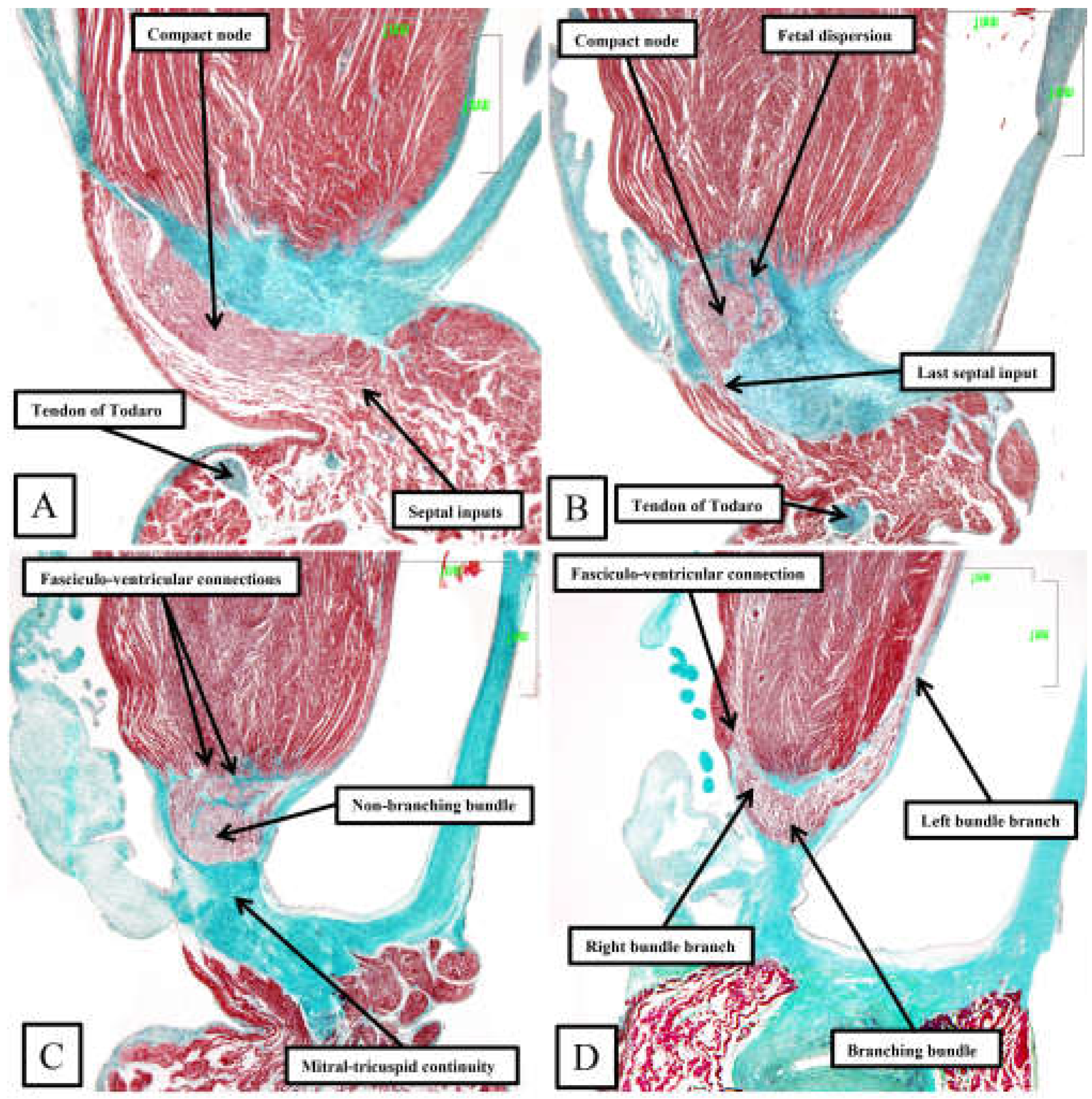
Taken together, the sections show how the inferior extensions of the node, merging with the working myocardium of the atrial vestibules, come together to form the compact atrioventricular node. In the human heart, the compact node is set as a half-oval against the prominent fibrous tissues of the atrioventricular junction (
Figure 7A,B). The compact node then receives direct inputs from the antero-inferior buttress of the atrial septum (
Figure 7C,D). It is the last input from the atrial septum that forms the fast pathway into the node [
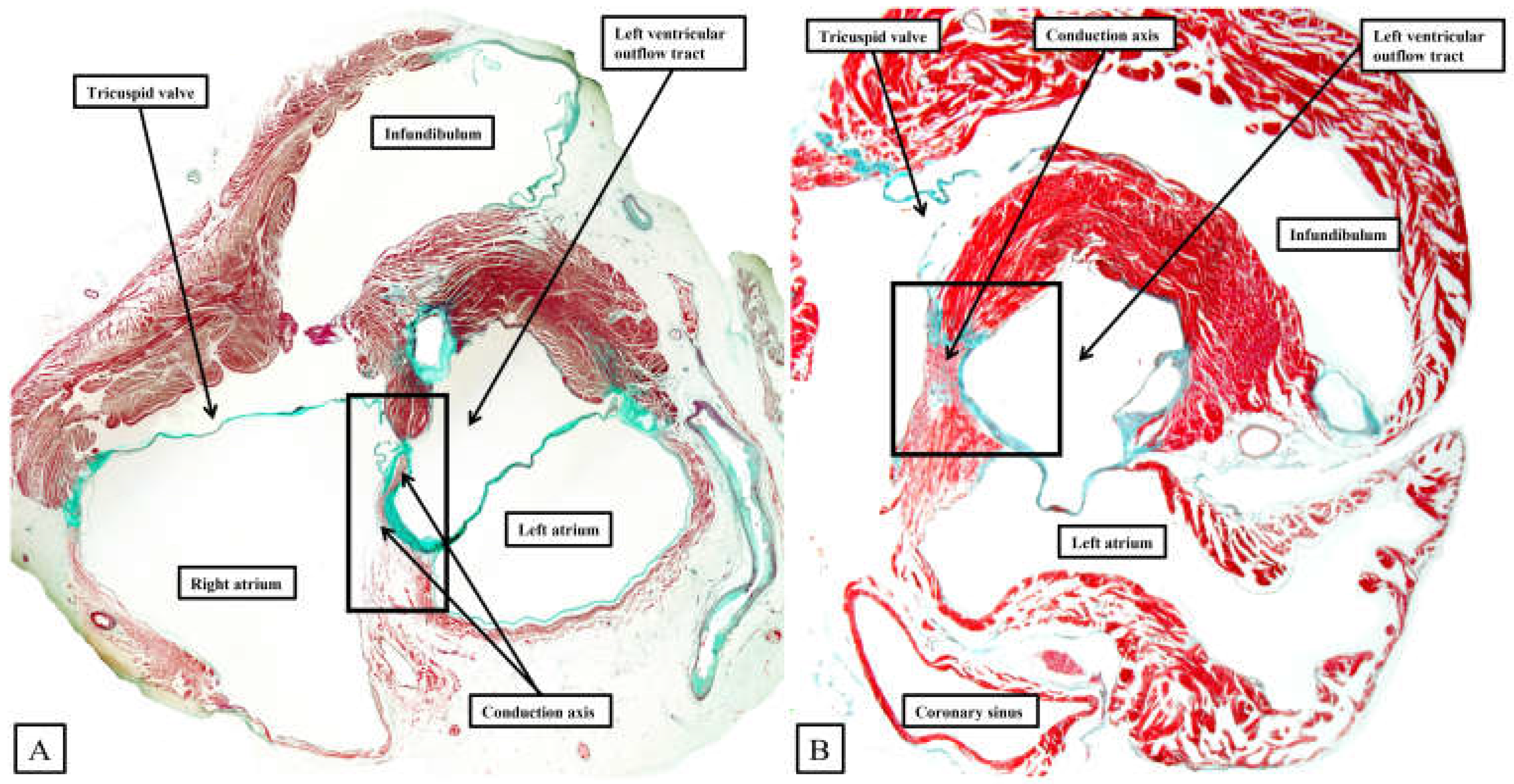
19]. The transition from the atrial to the ventricular components of the conduction axis, along with the arrangement of the ventricular components, is better seen when sections are cut in the frontal plane (
Figure 8).
The frontal sections show well how the compact node is formed as a half-oval of histologically specialised cells supported on the so-called central fibrous body. The fibrous tissue is derived from the atrioventricular cushions that, in the developing heart, divide the atrioventricular canal into its right and left parts. As was described initially by Keith and Flack [
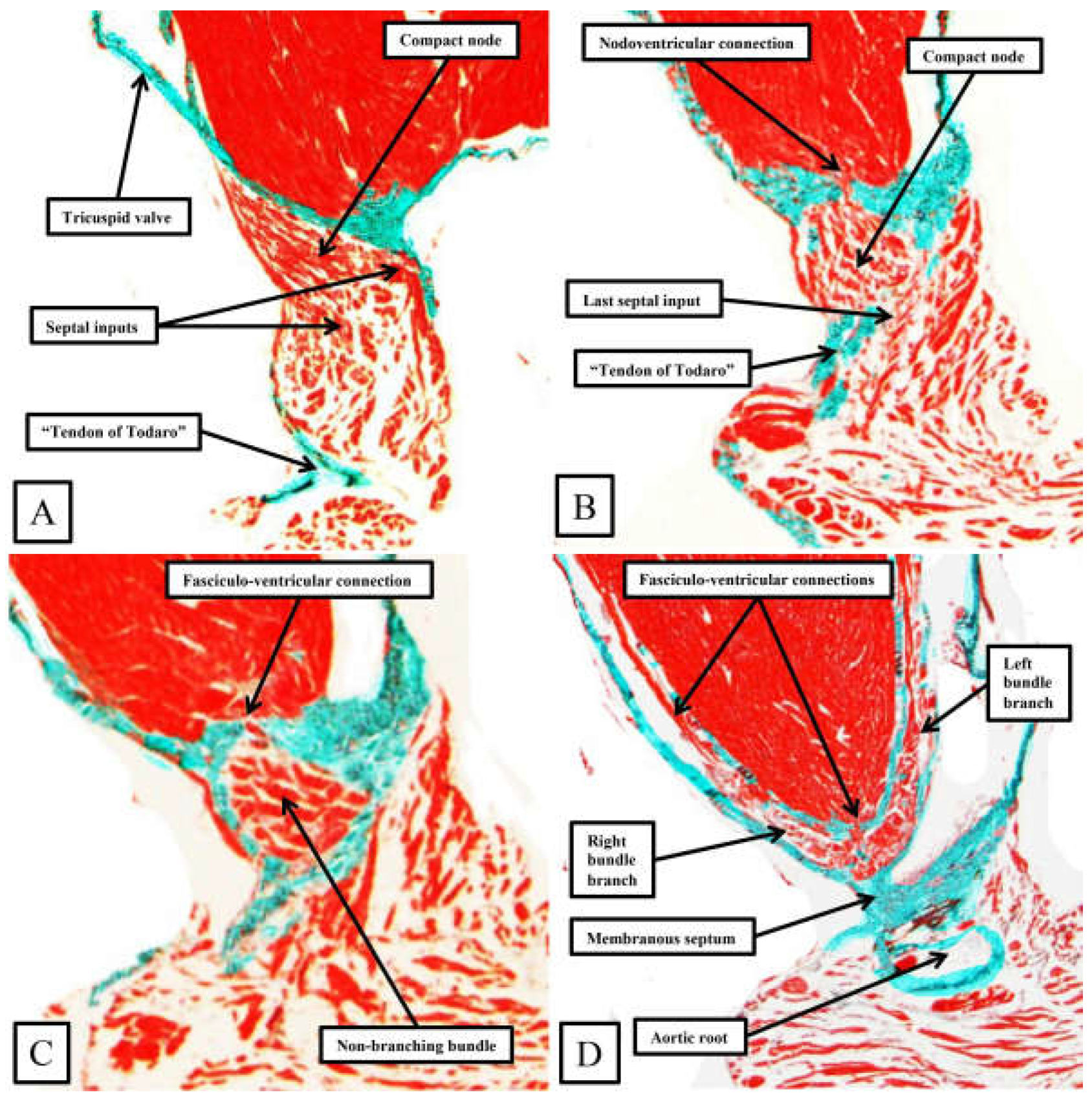
20], the insulation of the axis from the atrial myocardium is provided by union of the fibrous tissues of the cushions that develop into the leaflets of the tricuspid and mitral valves. This is well seen in
Figure 8B and C, with the atrial myocardium still joining the node in
Figure 8B, but insulated from the axis in
Figure 8C. It was this feature of insulation of the axis from the atrial myocardium that Tawara [
18] used as the criterion for distinction between atrioventricular node (
Figure 8B) and the non-branching atrioventricular bundle (
Figure 8C). As can also be seen in
Figure 8C, although the axis is insulated from the atrial myocardium, connections still exist between the non-branching bundle and the crest of the muscular ventricular septum. These are the fasciculo-ventricular connections, or superior septal pathways, that were identified by Mahaim as the paraspecific system for atrioventricular conduction [
4]. In the human heart, having taken a short course along the crest of the muscular venticular septum, the axis then branches to give rise to the right and left bundles (
Figure 8D). Additional fasciculo-ventricular connections have been observed in almost all our datasets, as seen also in
Figure 8D, between the rightward margin of the branching bundle and the crest of the muscular ventricular septum [
5].
3.3. The Specifics of the Atrioventricular Conduction Axis of the Murine Heart
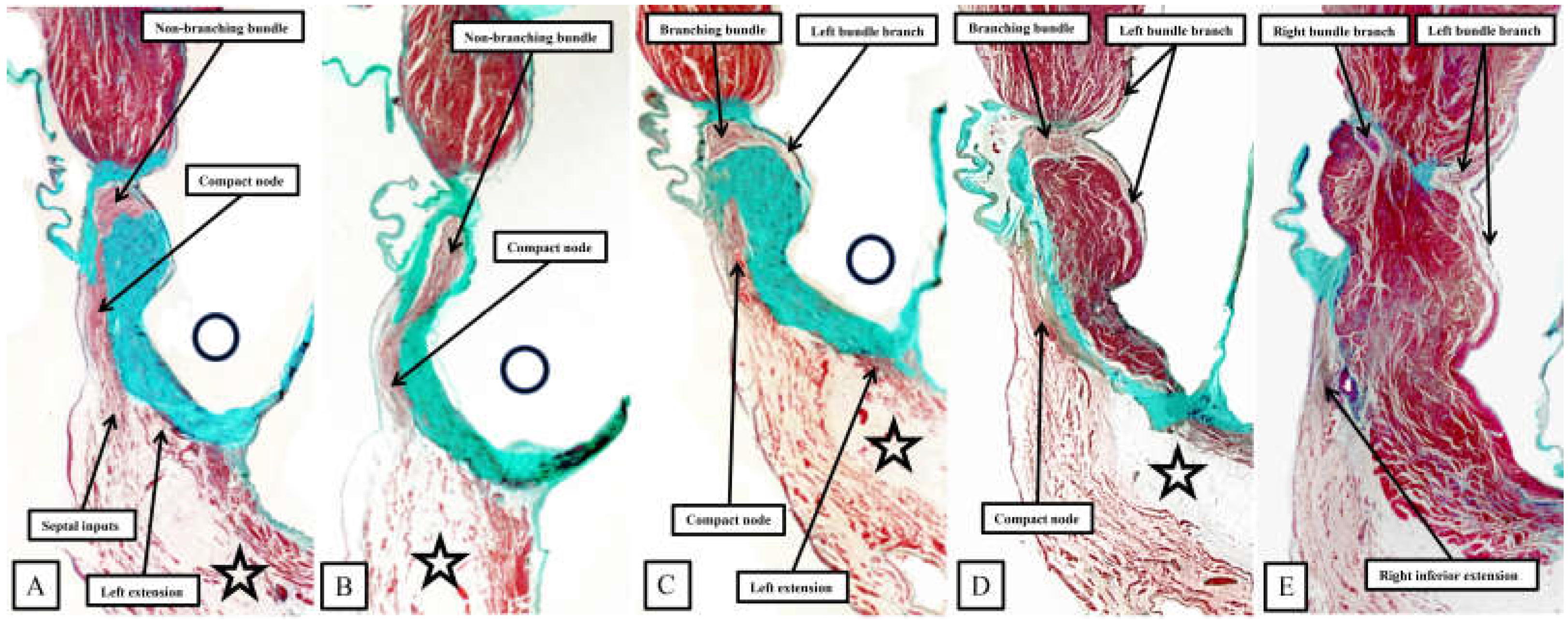
Although having the same basic arrangement as shown in
Figure 7 and
Figure 8 for the human heart, there are important differences in the murine heart (
Figure 9).
Figure 9.
The images, taken from murine heart sectioned in the short axis of the ventricular cone (see square in
Figure 6), show the basic arrangement of the atrioventricular conduction axis in the murine heart.
Figure 9.
The images, taken from murine heart sectioned in the short axis of the ventricular cone (see square in
Figure 6), show the basic arrangement of the atrioventricular conduction axis in the murine heart.
As already shown in
Figure 5 and
Figure 6, the apex of the inferior pyramidal space in the murine heart does not overlap the infero-septal recess of the left ventricular outflow tract to the same extent in the murine as in the human heart. Because of this, there is less separation of the inputs to the compact atrioventricular node from the right and left atrial vestibules. The histologically specialised extensions, nonetheless, can still be distinguished from the working atrial myocardium on the basis of the staining characteristics (
Figure 9A). The compact node is more square in the murine heart, receiving extensive direct inputs from the antero-inferior buttress of the atrial septum (
Figure 9B). As in the human heart, nonetheless, the axis becomes insulated from the atrial myocardium as it passes beneath an area of fibrous continuity between the leaflets of the mitral and tricuspid valves (
Figure 9C). And, again as in the human heart, extensive fasciculo-ventricular connections, or superior septal pathways, can be identified between the non-branching bundle and the crest of the muscular ventricular septum (
Figure 9C). Following the arrangement in the human heart, albeit with a longer non-branching component, the axis then branches on the crest of the muscular septum. The similarities with the human heart are further seen in that the right bundle branch run as a narrow cord as it descends the muscular ventricular septum, whereas the left bundle branch takes a broader origin from the axis, ramifying in trifascicular fashion as its branches move towards the ventricular apex. As can be seen in
Figure 9D, however, the branches themselves are enclosed in well-formed fibrous sheaths in the murine heart, as in the human heart, as they descend the ventricular septum. There are, nonetheless, additional direct connections between the righward margin of the branching bundle and the septal crest before the right bundle itself becomes fully insulated. These connections produce additional superior septal pathways. These relationships can then be confirmed when murine hearts are cut in the frontal plane (
Figure 10).
When compared to the arrangement in the human heart, the antero-inferior buttress of the atrial septum can be seen to lie much more in an edge-to-edge fashion relative to the crest of the muscular septum, with a better formed central fibrous body in the human heart (Compare
Figure 8A and
Figure 10A). The last connection between the atrial myocardium and the axis, however, is still formed just prior to insulation of the axis by the fibrous continuity formed between the leaflets of the tricuspid and mitral valves, albeit with this insulation reinforced in the murine heart by a contibution from the tendon of Todaro (
Figure 10B and C). Connections are present in the murine heart between both the compact node (
Figure 10B) and the non-branching bundle (
Figure 10C) and the crest of the muscular ventricular septum. The non-branching bundle itself is then much longer in the murine heart, reflecting the greater distance between the poorly formed membranous septum and the aortic root. The axis does then branch on the crest of the septum, with the right and left fascicles well-insulated from the underlying septal myocardium (
Figure 10D). Additional direct connections can be identified, nonetheless, between the origin of the insulated cord-like right bundle branch and the working septal cardiomyocytes (
Figure 10D).
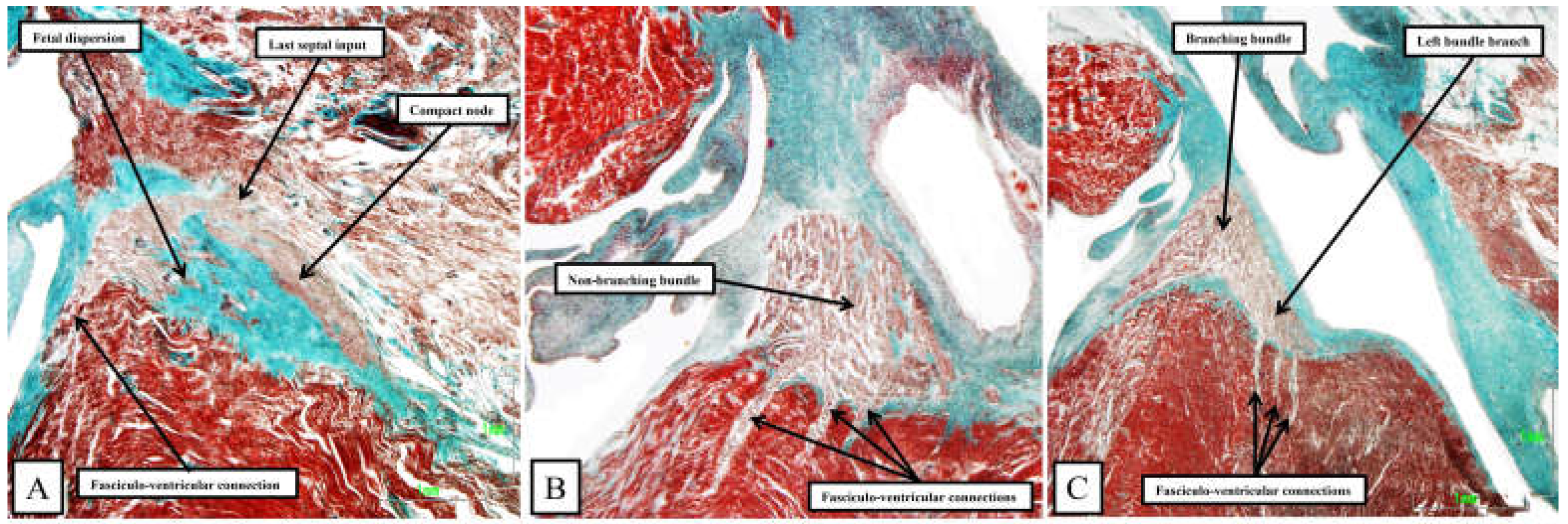
3.4. The Potential Significance of the Superior Septal Pathways
During early cardiac development, the ventricular walls are made up, in their larger part, by a meshwork of trabecular myocardium [
21]. At this initial stage, there is only a very thin compact component of the parietal ventricular walls. The septum, however, which forms concomitant with the “ballooning” of the ventricular apical components, is already forming by coalescence of the pre-existing trabeculations. With ongoing development, the surface layers of the septum specialise to form the ventricular bundle branches, with the trabeculations also coalescing to produce the papillary muscles of the atrioventricular valves, and the prominent septomarginal trabeculation of the right ventricle [
22]. The apical trabeculations also persist in the human heart, developing in such a way as to permit distinction of the morphologically right and morphologically left chambers, alsthough the difference in trabecular pattern is less well marked in the murine heart. The developing bundle branches themselves take their origin from a further specialised component that can be recognised as a ring surrounding the primary interventricular foramen [
22]. When first formed, there are extensive myocardial connections between the ring and the crest of the developing muscular ventricular septum. The ring is also continuous, initially, with the rightward margin of the atrioventricular canal myocardium. With expansion of the atrioventricular canal, and formation of the secondary interventricular foramen, the ventricular component of the ring becomes continuous with the atrioventricular canal myocardium at the crux of the heart, with the transition sandwiched between the atrioventricular cushions luminally and the tissues of the inferior atrioventricular groove epicardially. It is this location between the cushions, which become the leaflets of the atrioventricular valves and the central fibrous body, and the tissues of the atrioventricular groove which persists to provide the insulation of the atrioventricular node as it becomes the non-branching atrioventricular bundle. So as to function as a specialised conducting system, however, it is also necessary for the developing pathways to becomes insulated from the crest of the ventricular septum. Such insulation is not fully developed at the end of the embryonic period of development. Towards the end of the embryonic period, extensive connections remain between both the developing compact atrioventricular node and the non-branching bundle and the crest of the muscular ventricular septum. Throughout the earliest part of the fetal development, such connections can still be identified between the conduction axis and the crest of the muscular ventricular septum. These pathways initially form extensive nodoventricular and fasciculo-ventricular pathways. Towards the end of the fetal period, the nodoventricular pathways become less evident, being dispersed within the developing fibrous tissues of the central fibrous body, although discrete pathways can still be identified towards the end of the fetal period [
23]. The pathways between the non-branching bundle and the crest of the muscular ventricular septum, however, continue to remain visible as prominent structures (
Figure 11).