1. Introduction
The
Yucca L. (Asparagaceae) genus is composed of 40 to 50 succulent plant species, including shrubs and evergreen trees native to North and Central America [
1]. Mexico is home to approximately 30 species, 53% of which are native plants [
2].
This genus is commonly known as yuca, and different civilizations have benefited from its exceptional characteristics. Its versatility has enabled the production of food, makeup, and medicine [
3]. Yuca was fundamental for the native population of North America. They used the fibers from the leaves to make strings, baskets, and clothes. Archeologists have found evidence that yuca was used in Arizona more than two thousand years ago [
4]. Medicinal use is an important value of yuca [
5]. This genus is currently considered a source of saponins and polyphenols, which have a particular chemical structure and biological activity [
6]. Nevertheless, in the Potosino-Zacatecano region, yuca is not fully exploited and currently only its fruits are consumed. This situation is partially the consequence of the classification of some species (such as
Y. grandiflora, Y. lacandonica, and
Y. queretaroensis) as “subjected to special protection” under the NOM-059-SEMARNAT-2010 Mexican official standard [
7].
An alternative use of this species is the production of flour for commercial products. However, the multidimensional flour properties of this species are unknown. These properties play a key role in the prediction of the texture of final products, preventing possible defects that reduce their quality. The objective of this study was to characterize the multidimensional properties of the flour produced from leaves and stems of Yucca decipiens Trel. used to produce food and natural, semisynthetic, or synthetic biomaterials. This study aims to characterize the multidimensional properties of yuca stem and leaf flours, to develop new materials and products within the scope of product engineering, processes and industrial design. The characterization of these flours will allow to evaluate their viability and performance in the production of food and a wide range of biomaterials, whether natural, semi-synthetic or synthetic, contributing to the development of innovations in the design of new products that promote sustainability.
2. Results
2.1. Physical Properties
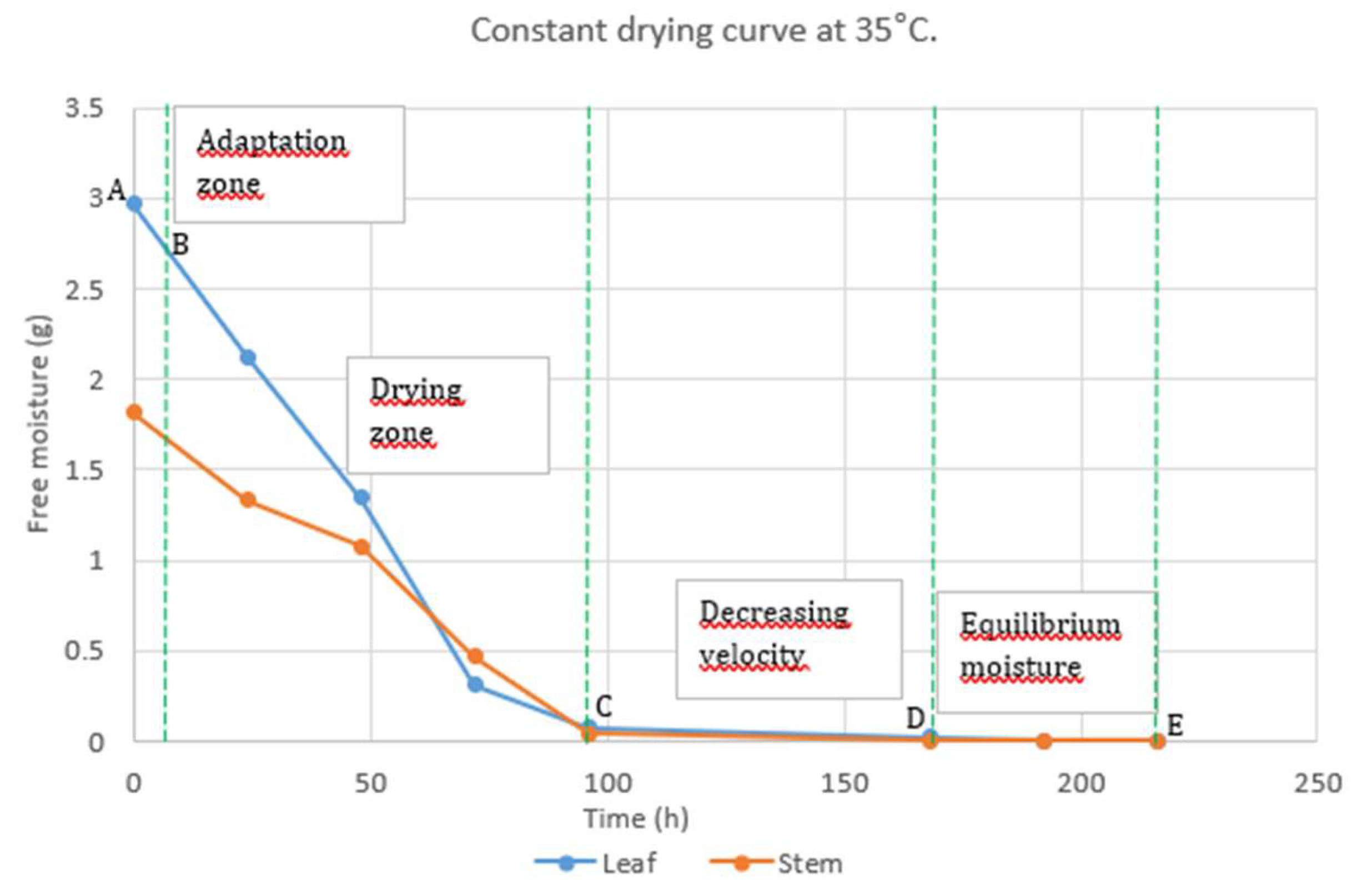
2.1.1. Drying
In the drying zone, the leaves lost water at a rate of 30 g h
-1, while the stems lost water at a rate of 18 g h
-1. After 72 h, the stems and the leaves lost 75 and 90% of their weight, respectively (
Figure 2). A constant weight was recorded 150 h after the drying process began.
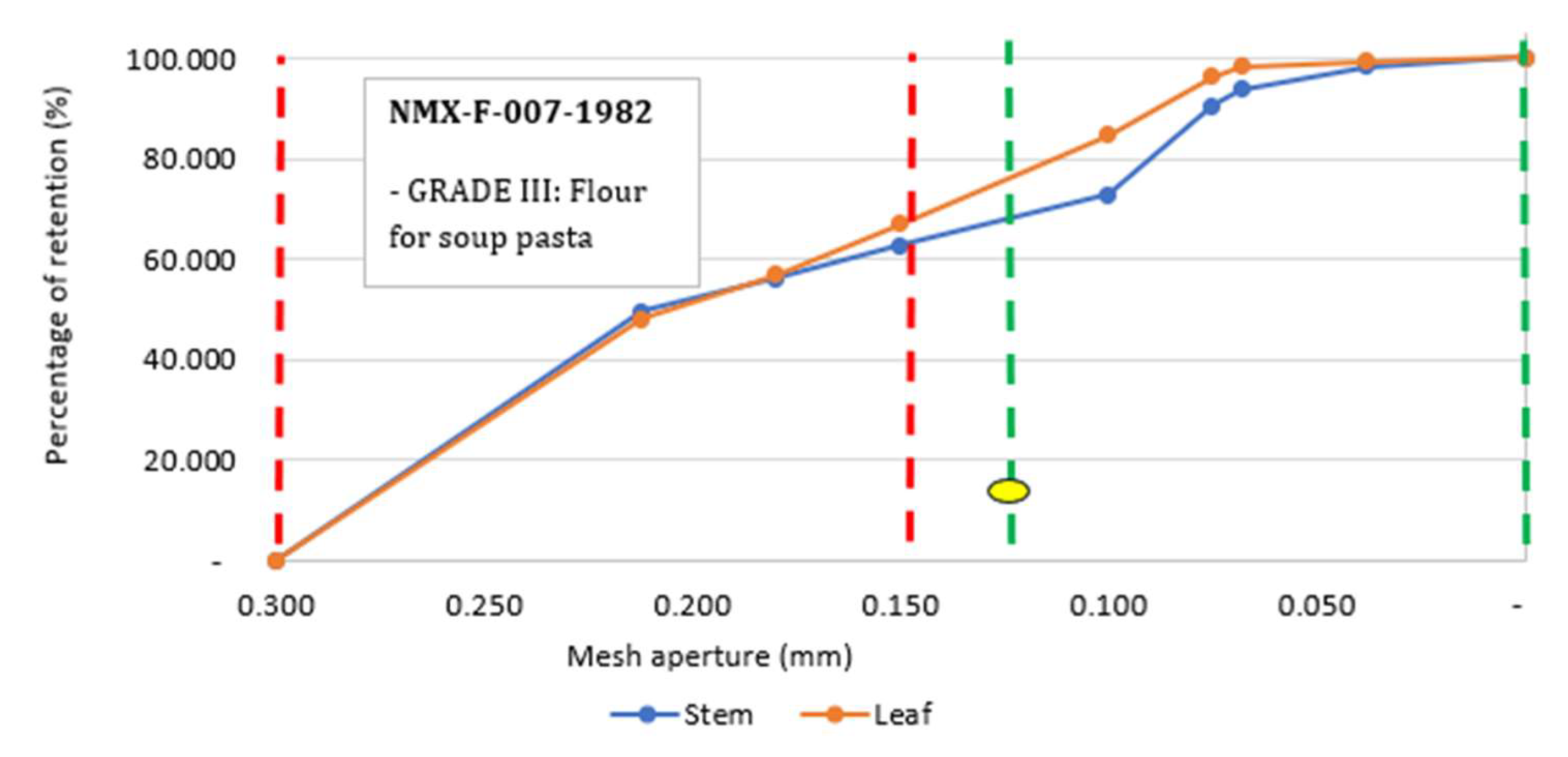
2.1.2. Granulometric Analysis
The sieving results show that flour made from the leaves and stem of yuca have a greater retention percentage in the 0.150 to 0.212 mm size interval. The red lines (Figure 3) show the classification limit of the grade III flour, according to the NMX-F-007-1982 Official Mexican Standard while green lines show the classification of the finest flours (grade I). However, this classification indicates that sieving with a 0.125 mm mesh should result in ≤10% retention; therefore, these flours do not fulfill the requirements of the NMX-F-007-1982.
2.1.3. Particle Size Index (PSI)
The particle size index of the leaf flour (124.69 ±15.29) was finer than the stem flour (144.08 ±20.16) and consequently can be considered more cohesive. Although the granulometry of yuca flours has been subjected to few studies, our results show that they have a low particle size index and therefore could be mixed with other flours, including corn flour.
2.1.4. Particles Size
According to the microscopic observations of yuca flours, stem flours would have a finer structure than leaf flours (
Table 1). Despite their great similarity, the difference seems to arise from the fibers found in the leaves and their resistance, since size depends on particle orientation.
2.2. Functional Properties
One of the most remarkable functional properties of organ flours include the greater expansion percentage of stem flour over leaf flour. The flour expansion test is related to the presence of gluten, because its properties facilitate the swelling of the dough during fermentation. Meanwhile, the remaining properties are similar (
Table 2).
2.2.1. Pelshenke Value
According to the fermentation test, the yuca stem flour floats for a shorter period than the flour made from the leaves (
Table 3); wheat flours record similar values, flotation for 1:31 min and disintegrating after 46:03 min. Meanwhile, both stem and leaf flours recorded a similar disintegration time.
2.2.2. Gravimetric Properties
The particle density of the flour produced from yuca stems (0.42 g mL
-1) was greater than the flour from the leaves (0.29 g mL
-1) (
Table 4).
The porosity of yuca flours ranged from 38 to 44%. These values are like the porosity of Amaranthus sp. (35%) and wheat (65%) flour
2.3. Frictional Properties
2.3.1. Internal Friction
Leaf and stem yuca flours had a 0.92 ±0.09 and 0.85 ±0.10 internal friction coefficients, respectively. Nevertheless, various characteristics also influence internal friction, including size, shape, volume, density, grain surface, moisture content, and the particle orientation.
2.3.2. External Friction
The flours made from both organs recorded a higher external friction coefficient on wood, while the lowest external friction value was recorded on ceramic tiles (
Table 5).
2.3.3. Texture Properties
The texture profile analysis recorder that stems flour was harder than leaf flour. The same behavior was recorded for the other variables from this flour (
Table 6).
The texture profile of doughs is important for finished products. In this case, the values of all the texture characteristics were lower in leaf dough than in stem dough, except for resilience, which recorded the same trend in both (stem dough: 0.66).
2.4. Rheological Properties
2.4.1. Viscosity
The viscosity reported at 20 °C was different for both leaf and stem flour. Stem and leaf flours exposed to 20 °C and with a fine particle size (0.116 and 0.147 mm, respectively) recorded low viscosity values (
Table 7). Therefore, these values where lower than those reported in other research with various flour types, as consequence of the greater thermal-mechanical damage.
2.4.2. Electrical Conductivity and pH
The higher value of electrical conductivity was reached at 35 °C, whereas the pH of de leaf flour was significantly greater than the stem flour pH (
Table 8). Electrical conductivity and processing time have a remarkable relationship. As the sample reaches higher temperatures in a shorter time, less damage to the starch and other compounds should be recorded, resulting in a lower gelling. This effect is especially clear when cooking and viscosity of the product are analyzed.
3. Discussion
3.1. Physical Properties
3.1.1. Drying
Regarding drying, the phenomenon of decreased water loss rate has been attributed to the compaction of matter caused by the progressive elimination of free water, which results in an increase in the concentration of solute in the tissue, complicating water loss [
8].
3.1.2. Granulometric Analysis
The Mexican Standard NMX-F-007-1982 ( [
9], also mentions grade II flours; however, it does not specify particle size, since it is classified as cookie flour and cookies are prepared with various types of mixtures, depending on their type. According to their sieving, the flours meet the granulometry conditions established by the NMX-F-007-1982 (SCFI, 1982) [
9] They can be classified as grade II and grade III flours, considering that the 0.297 mm (50 ASTM) and 0.149 mm (100 ASTM) mesh fractions have a minimum particle retention of 73%. The #50 mesh used in this analysis has slightly wider openings (0.300 mm) than the 50 ASTM. According to the Mexican standard for wheat flour, these flours are classified and designated as grade III and II flours, which can be used to make cookies and soup pasta. Currently, there is scarce information about the sieving of flours from yuca organs.
The
Particle size index (PSI) is higher in flours from yuca organs than flours produced from Triticea hybrids (Poaceae) with a particle size index of 26.00 [
10]; these flours have a fine particle size with relatively low values for damaged starch [
11]. Authors like [
12] determined that ground blue corn flours (
Zea mays L.) have a fine particle ratio (particle index: 83-94); these results are closer to the particle size index of flours produced from yuca organs. According to [
13], finely sieved corn samples produce extruded fragments with greater expansion than the samples from medium and coarse sieving (low PSI), under certain processing conditions.
3.2. Functional Properties
Dough from yuca flours requires a similar water volume than wheat flours [
14,
15]. The water absorption of flours is fundamental to achieve a dough with appropriate consistency; likewise, it affects viscosity, volume, texture, and the cooking process of food products [
16]. However, factors such as pH, ionic strength, and temperature impact the water retention capacity of the proteins, while polar amino acids (e.g., lysine, threonine, and tryptophan) impact their swelling power and water retention [
17,
18,
19]. Finally, yuca has a lower oil absorption capacity than other sources, including cowpeas [
20]. This capacity can be impacted by other factors, such as surface structure and porosity [
21]. Regarding foam formation, both yuca stem and leaf flours recorded a high yield, likely because of the amphipolar characteristics of proteins that function as tensioactive agents [
22].
Regarding the Pelshenke value [
23] mention that gluten seems to capture the CO
2 molecules generated during the fermentation process, facilitating a faster flotation than gluten-free flour. In this sense, the results coincide with the parameters defined by the 56-60 Physicochemical Test Methods of the Cereals and Grain Association [
24], which would classify
Y. decipiens stem and leaf flours as equivalent to soft, soft wheat.
3.3. Gravimetric Properties
The values obtained are closer to the density of corn flour (0.420-0.435) [
25], while their high density suggests that these flours have a fine granulometric class [
26]. The porosity of cassava flours ranged from 38 to 44%. These values are similar to the porosity of
Amaranthus sp. flour (35%) and wheat flour (65%) [
27], and according to [
28], porosity is related to the formation of small air bubbles that retain CO
2 during formulation (
Table 4)
3.3.1. Internal Friction
Regarding internal friction, our values exceed those reported by [
29] for tuna flours (
Opuntia ficus-indica) but are similar to the values reported by [
30] for green bean (
Phaseolus vulgaris L.) and pea (
Pisum sativum L.) flours. Authors such as [
31] recorded similar values in flours made from
Cucurbita sp. seeds, with shell (0.81) and without shell (0.56). In both cases, these values —which are like those of flours made from seeds with shell— have been attributed to the presence of fibers [
32]. According to [
33] the values resulting from this research suggest that these flours have a limited flow.
In this investigation, external friction recorded similar results to the findings of [
31] for flour made from shelled (0.59) and unshelled (0.81) seeds on glass and wood, respectively, as well as the results of [
34] for pea seeds on rubber (0.388-0.413), aluminum (0.292-0.351), stainless steel (0.270-0.311) and galvanized iron (0.360-0.409), therefore, our records are within the range reported by [
35] for barley seeds on galvanized tin (0.27-0.68), melamine (0.25-0.70) and stainless steel (0.27-0.71).
Regarding the texture properties, in both cases (leaf and stem) very low resilience was recorded, indicating a very high deformation and a very low time in which the dough returns to its original position after being pressed. These flours have a lower adhesive force than wheat flour (404.83 g) whose hardness is 4,543.85 g similar to the
Y. decipiens stem flour (3,135.48 g), although the deformation of our flours is lower compared to wheat flour (
Table 6) [
36].
3.4. Rheological Properties
3.4.1. Viscosity
Regarding viscosity, authors such as [
37] reported a maximum viscosity of 2,584 ±427 cP for nixtamal flour (corn), while, in general, extruded flours have viscosity values that vary from 665.8 ±6.6 to 2,403.5 ±34.6 cP, with large particles (1.3 mm). The highest value of electrical conductivity was reached at 35 °C, while the pH of the leaf flour was significantly higher than the pH of the stem flour (
Table 8). Regardless of the temperature recorded, a longer processing time implies higher cooking, which is reflected in both the viscosity and the gelation of the final product.
This result suggests that the heating ratio (i.e., the time required to reach the maximum temperature) may be the main factor influencing electrical conductivity. Therefore, samples processed at lower temperatures (15 °C) should record a lower degree of cooking than those processed at higher temperatures. On the other hand, cassava leaf flour has a pH of 9.38, which can be attributed to the presence or absence of some chemical compounds (e.g., proteins and saponins) whose solubility is associated with pH changes. According to [
38], when the pH decreases, the solubility of saponin increases.
This study promotes the sustainability and efficiency of an undervalued genetic resource with high potential for use in agro-industrial (assistant in fermentation processes) and food (processing and mixing for basic foods for daily consumption) purposes. This is important, considering that according to the Convention on Biological Diversity [
39], the genetic resources of a country are part of its heritage and must be seen as part of the strategies to face global challenges related to food security and industrial innovation.
4. Materials and Methods
The leaves and stems of Yucca decipiens Trel. were randomly collected in May 2023, from a plot located in Loma de La Carreta, Zacatecas, México (22°37.9190’ N and 101°52.799’ W). The plant material was processed in the Water-Soil-Plant Laboratory of the Campus San Luis Potosí of the Colegio de Postgraduados (22° 63’ 22” N and 101° 71’ 25” W) and analyzed in the Laboratory of the Coordinación Académica Región Altiplano Oeste (CARAO) of the Autonomous University of San Luis Potosí (22° 38’ 28.5” N and 101° 42’ 10.0” W).
4.1. Physical Properties
4.1.1. Drying
The leaves were removed by hand from the stem, which was cut into 3.0 cm thick slices. The samples of the leaves and stems (750 g each) were dried at 30 °C in a FELISA
® TE-HV30D oven. Afterwards, they were evenly distributed on previously washed aluminum trays until they reached a constant weight (1 h, at 105 °C). Subsequently, the samples were weighted in a Torrey gram scale every 24 h, until they reached constant weight [
40].
4.1.2. Milling
The dry leaves and stems were processed on a lab scale in a colloid mill with two stationary blades and a four cutting edge rotor (Thomas Scientific®, Wiley Mini-Mill 3383-L10, 115 V, 60 HZ, USA). The objective was to obtain flours with an even particle size <0.300 mm.
4.1.3. Granulometric Analysis
The flour was sieved according to the AOAC 965.22-1966 sieving method [
41], using a Ro-Tap
® sieve shaker (W. S. TylerTM, USA): 200 g of each flour were individually placed into a set of Alcón
® (Mexico) sieves of different sizes (50, 70, 80, 100, 150, 200, 250, and 400 ASTM) and shaken for 5 min. Finally, the fraction of flour retained on each sieve was weighted.
4.1.4. Particle Size Index
The particle size index was determined according to the method reported by [
25], using the equation:
Where: PSI = Particle size index; NMF = Number of mesh factor; and PSD = Particle size distribution (%). Each factor depended on the number of the set of sieves: 0.2 = mesh #20; 0.4 = mesh #40; 0.6 = mesh #60; 0.8 = mesh #80; 1.0 = mesh #100, and the bottom. Meanwhile, the retention percentage of each mesh was determined with the method described in the analysis of the particle size distribution.
4.1.5. Particle Size Distribution
The size and shape of flour particles were measured with digital image analysis. Measurement was carried out using the direct optical microscopy (OM), which allows the direct visualization of the particles. Measurement was determined using a MT315 digital microscope, with a 1200x chamber and a 7-inch HD dual lens (MUSTOOL®, China). The morphological variables were measured with the manual delimitation of the particle in the digital image, using the ImageJ 1.5.4 (64-bit) free software.
4.2. Functional Properties
4.2.1. Water Absorption
Water absorption was determined following the method proposed by [
42]: 1 g of flour and 10 mL of water were placed into a centrifuge tube and shaken in a vortex for 30 s. Afterwards, the mixture was centrifuged at 2,500 rpm for 10 min. Subsequently, the supernatant was removed. Water absorption was the difference between the dough sample before and after the treatment. Absorption capacity was determined dividing the amount of retained water by the quantity of the sample, expressed as solids.
4.2.2. Oil Absorption
Was determined following a modified version of the method described by [
42]: 1.0 g of flour and 10 mL of commercial soy (
Glycine max L.) oil were placed into a centrifuge tube and shaken in a vortex at 20 °C for 30 s. Subsequently, the mixture was centrifuged at 2,500 rpm for 10 min and the supernatant was removed. Oil absorption was the difference between the dough sample weight before and after the treatment. Absorption capacity was determined dividing the amount of retained oil by the quantity of the sample, expressed as solids.
4.2.3. Swelling Capacity
The swelling capacity was determined using a modified version of the method proposed by [
43]: 1 g of flour was weighted in a test-tube and 10 mL of distilled water were added, gently stirring the mixture to spread the sample. The mixture was allowed to rest for 24 h at 18 °C. Afterwards, the final volume of the sample was measured. Swelling capacity was determined dividing the final sample volume by the weight of the sample, expressed as solids.
4.2.4. Foam Formation and Stability
These properties were determined following the methods of [
44]. To determine the foam capacity and stability of the samples, a 100 mL suspension of distilled water and 2.0 g of flour was prepared in a 100 mL beaker. Afterwards, the mixture was blended for 3.0 min and transferred to a 250 mL graduated cylinder to measure its volume. Foam formation capacity is the increase in volume after the foam was formed with respect to the initial volume expressed as a percentage.
4.2.5. Expansion Test
The fermentative properties of a given flour can be determined by the gas produced during dough fermentation. This property can be measured by adding yeast to the dough and incubating it at 30 °C. In order to perform this test, 1.0 g of salt and 2.0 g of sugar were crushed in a mortar; 25 g of flour were added to this mixture. In addition, 2.0 g of yeast were diluted in the volume of water used for the absorption test. Subsequently, the dough was kneaded. Once the dough was obtained, it was shaped into a small cylinder. Its height and base diameter were measured. Subsequently, the dough was placed in a volumetric cup and covered with tinfoil. In order to expand the dough, the cup was placed into a bath Marie, at 30 °C for 1 h. Finally, the height and diameter of the cylinder were measured again; if the dough cylinder lost its shape, the final volume was also measured.
4.2.6. Pelshenke Value
The aim of this test is to determine the stability of a dough during the fermentation process, measuring the time it takes to disintegrate under standardized conditions [
45]. The Pelshenke value test was carried out according to the 56-60 Physicochemical Test Methods of the Cereal & Grains Association [
46]. A 2.2 mL of a yeast suspension (10%) was previously prepared and added to 4.0 g of flour at 28 °C. The mixture was kneaded and divided into three even small balls. Afterwards, 120 mL of water were poured into a beaker. Water was kept at the same temperature (30 °C) during the whole test. The three small balls of dough were placed into the beaker and a chronometer was activated at the moment of immersion. Flotation and disintegration times were determined. Flotation is the time it takes for the small ball of dough to reach the water surface, while disintegration is the time it takes for the small ball of dough to lose its shape and start to fall apart.
4.3. Gravimetric Properties
4.3.1. Bulk Density
Bulk density (
ρb) was determined considering the 55-10.01 Physical Test Method (AACC Int, 2000) [
47], using the ratio between the dough mass (g) and a predefined volume of the sample (500 cm
3). The container suggested in the standard method was replaced by a beaker. Measurements were carried out in triplicate, placing 500 cm
3 of flour in a beaker, which had been previously weighted in a H-7294 analytical scale, with a 0.01 g accuracy (OHAUS, USA). Bulk density (
ρb) was calculated using the following equation:
Where: ρb is the bulk density (gcm-3); m is the weight of the sampling dough (g); and V is the total volume occupied by the sample (mL).
4.3.2. Particle Density
Particle density (
ρt) was determined following the method proposed by Bressani et al. (2001), measuring the compacted volume of 20 g of flour within a 25 mL cylinder. The results were expressed in g mL 1.
Where: ρt is the particle density (g cm-3); mg is the dough weight (g); and Vd is the volume of flour displacement (mL).
Porosity
The porosity percentage (ε) was calculated based on three replicates, according to the following equation [
48]:
Where: ε is the porosity; ρt is the particle density; and ρb is the bulk density.
4.4. Frictional Properties
4.4.1. Internal Friction
Internal friction (µi) was determined using a W-70945 plastic funnel (Weston
®, Mexico), with a removable internal cap, filled with the flours. Afterwards, the cap was removed, allowing the flours to reach their natural slope. Having previously measured the radius and height of the dough made with the flours, the angle of repose was calculated, using the following equation [
49]:
Where: is the internal friction; is the height of the resulting cone; and is the radius of the cone.
4.4.2. External Friction
The external friction (µe) of the flours was determined using sheets made up of different materials (wood 1 and 2, glass crystal, tile, plywood, polyethylene plastic, galvanized sheet, and stainless steel). Each sheet was gradually tilted until the 100 g flour samples completely slid. The tilt angle of the sheet was measured with a H-5648 plastic protractor (ULINE
®, Mexico), according to the following equation:
Where: µ_e is the external friction; and tan (α) is the tilt angle.
4.4.3. Texture
Was determined using a CT3 texture analyzer (Brookfield, China). A mixture of 4.5 g of flour and 2.5 mL of distilled water was prepared. The mixture was kneaded for 5 min. Afterwards, it was manually shaped into a ≈2 cm cube. The cube was analyzed with a texturometer, determining hardness (g), deformation depending on hardness (mm), percentage of deformation depending on hardness, finished hardness testing (mJ), deformation recovery (mm), work recovery (mJ), total work (mJ), adhesiveness strength (g), adhesiveness (mJ), resilience, and length of the sample (mm).
4.5. Rheological Properties
4.5.1. Viscosity
The fluid type was identified using the DV3T™ rheometer (Brookfield, China). A semiliquid dough was prepared with 1.0 g of each flour, diluted in 2.0 mL of water.
4.5.2. Electrical Conductivity
Was measured with a HI98129 Combo® meter (Hanna, Smithfield, RI, USA), with a mixture of 5% flour and 95% deionized water, at different temperatures (15 °C, 25 °C, and 35 °C), stabilized for two minutes.
4.5.3. Potential of Hydrogen (pH)
The pH was determined according to 943.02 potentiometric method of the AOAC [
41]. A sample of 10 g of flour were mixed with recently boiled 100 mL of water at 25 °C, in a 125 mL Erlenmeyer flask that was constantly stirred. After the flask was allowed to rest for 30 min, its content was filtered and the pH was measured with an Oakton™ digital potentiometer, calibrated with 4.0 and 7.0 pH buffer solutions.
4.6. Statistical Analysis
All the variables of the flours made from the leaves and stems of Y. decipiens were compared with Student’s t-test (α<0.05), using the R Project programming language (3.6.3-2022.02.0-443) under the RStudio® interface (both free software).
5. Conclusions
The multidimensional properties of yuca leaf and stem flours demonstrate that this plant resource has the appropriate characteristics to be used as a raw material to produce different food and non-food industrial products. Leaf flours are finer than stem flours, so they can be considered more cohesive. The flours from the evaluated yuca organs have a low particle size index and so they could be integrated into mixtures with other flours such as corn flour. The degree of fineness of the leaf and stem flours meets the standards required by NMX-007-1982, validating their suitability to produce various foods such as cookies, soup pastas, and other food products. These flours exhibit optimal characteristics, such as lower water and oil absorption, but greater expansion and foaming capacity, indicating their versatile use in the food industry.
Author Contributions
Conceptualization: SRMB and JCI; Formal analysis: GLA and SRMB; Investigation: SRMB and VMRV; Methodology: SRMB and CLP; Software GLA and SRMB; Supervision JCI and RMSH; Writing - original draft: SRMB, JCI GLA, VMRV, CLP and RMSH; Writing - review and editing: SRMB, JCI GLA, VMRV, CLP and RMSH.
Funding
This research was funded by the National Scholarship Program of the National Council of Science and Technology (CONAHCyT, Mexico; CVU 1079008).
Acknowledgments
To the Isidro Palacios Herbarium (SLPM) for their important collaboration in the analysis and precise identification of plant species, particularly in the taxonomic characterization of the yuca varieties investigated in this work. To the taxonomist Eleazar Carranza PhD for his contribution to the analysis of plant species samples, especially for his contribution to the precise identification of the Yucca decipiens species present in this study.
Conflicts of Interest
“The authors declare no conflicts of interest.”.
References
- Hochstätter, F. Yucca (Agavaceae). Band 3 México. Selbst Verlag. Portland, Oregon. 2004, p. 47 https://www.agavaceae.com/botanik/pflanzen/scans/gnr230/scan2/23950-1.pdf.
- Maiti, R.; Rodriguez, H.G.; The Native Yucca: a Boon to the Farmers of the Arid North-east Mexico. IJBSM. 2011, 2(Jun, 2), pp. 203–208. Available online: https://ojs.pphouse.org/index.php/IJBSM/article/view/137 (accessed on 28 jun 2024).
- Patel, S.; Goyal, A. Recent Developments in Mushrooms as Anti-Cancer Therapeutics: A Review. 3 Biotech. 2012, 2(2), 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pearlstein, T.; Steiner, M. Premenstrual dysphoric disorder: burden of illness and treatment update. J Psychiatry Neurosci. 2008, 33(4), 291–301. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Fu, Y.J.; Zu, Y.G.; Schwarz, G.; Konkimalla, V.S.; Wink, M. Molecular target-guided tumor therapy with natural products derived from traditional Chinese medicine. Curr Med Chem. 2007, 14(19), 2024–32. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules. 2021, 26(17), 5251. [Google Scholar] [CrossRef] [PubMed]
- DOF. Diario Oficial de la Federación. NOM-059-SEMARNAT-2010 (NORMA Oficial Mexicana NOM-059-SEMARNAT-2010). Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. [Environmental protection - Native species of wild flora and fauna in Mexico - Risk categories and specifications for their inclusion, exclusion or change - List of species at risk]. 2010. Available online: http://sitios1.dif.gob.mx/alimentacion/docs/NMX-F-007.
- Pineda-Castro, M.L.; Chacón-Villalobos, A.; Cordero-Gamboa, G. Efecto de las condiciones de secado sobre la cinética de deshidratación de las hojas de morera (Morus alba). Agronomía Mesoamericana. 2008, 20 (2), 275–283. [CrossRef]
- SCFI (Secretaría de Comercio y Fomento Industrial). Norma Mexicana NMX-F-007-1982. Harina de trigo [WHEAT FLOUR].1982. Available online: http://sitios1.dif.gob.mx/alimentacion/docs/NMX-F-007 (accessed on 12 Jun 2024).
- Castaño, M.N.; Ferrari, E.D.; Picca, A.T.; Curti, M.I.; Ribotta, P.D.; León, A.E.; Paccapelo, H.A. Caracterización de harinas de tritíceas híbridas [Characterization of hybrid triticum flours]. Agriscientia, 2017, 34(1), 15-25. [CrossRef]
- Ramírez, A.; Pérez, G.T.; Ribotta, P.D.; León, A.E. The occurrence of friabilins in triticale and their relationship with grain hardness and baking quality. J. Agric. Food Chem. 2003, 51(24), 7161–7181. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Aburto, A.; Ponce-García, N.; Ramírez-Wong, B.; Figueroa, J.D.C. Efecto del tamaño de partícula y temperatura en la viscosidad de botanas extrudidas nixtamalizadas de maíz azul integral [Effect of particle size and temperature on the viscosity of extruded nixtamalized snacks made from whole blue corn]. Investigación y Desarrollo en Ciencia y Tecnología de Alimentos. 2019, 4, 56–65 http://www.fcb.uanl.mx/IDCyTA/files/volume4/4/1/8.pdf. [Google Scholar]
- Budâcan, I.; Pop, D.; Drocaş, I. Size distribution of maize milled particles obtained by using a hammer mill. Acta Technical Napocensis. 2013, 56(4). https://atnamam.utcluj.ro/index.php/Acta/article/view/108.
- Hernández, A. Microbiología Industrial. 1er ed.; Editorial Universidad Estatal a Distancia, San José, Costa Rica. 2003. https://www.academia.edu/6057657/microbiologia_industrial.
- González, M.C. Use of sweet potato (Ipomoea batatas) for the development of functional flours and their application in the production of gluten-reduced muffins. Tesis de Licenciatura. Universidad Autónoma de Puebla. 2016. https://repositorioinstitucional.buap.mx/items/a6624583-fd60-427e-b7e5-52360d2ce18e.
- Niba, L.L.; Bokonga, M.M.; Jackson, E.L.; Schlimme, D.S.; Li, B.W. Physicochemical properties and starch granular characteristics of flour from various Manihot esculenta (cassava) genotypes. J. Food Sci. 2001, 67(5), 1701– 1705. [CrossRef]
- Andrade-Mahecha, M.; Tapia-Blácido, D.R.; Menegalli, F.C. Physical–chemical, thermal, and functional properties of achira (Canna Indica l. ) flour and starch from different geographical origin. Starch – Starke. 2012, 64, 348–358. [Google Scholar] [CrossRef]
- Yusuf, A.; Ayedun, H.; Sanni, L. Chemical composition and functional properties of raw and roasted Nigerian benniseed (Sesamum indicum) and bambara groundnut (Vigna subterranean). Food Chemistry. 2008, 111, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Badui-Dergal, S. Química de los alimentos [Food chemistry]. Cuarta ed.; Publisher: Editorial Pearson educación, México, 2006; https://www.academia.edu/28233446/qu%c3%admica_de_los_alimentos_badui_4edi. [Google Scholar]
- Miquilena, E.; Higuera, A.; Rodríguez, B. Evaluación de propiedades funcionales de cuatro harinas de semillas de leguminosas comestibles cultivadas en Venezuela [Evaluation of functional properties of four seed flours from edible legumes grown in Venezuela]. Revista de la Facultad de Agronomía (Universidad de Zulia). 2016, 33(1), 58-75. https://produccioncientificaluz.org/index.php/agronomia/article/view/27193. 2719. [Google Scholar]
- Dobarganes, C.; Márquez-Ruiz, G.; Velasco, J. Interactions between fat and food during deep-frying. European Journal of Lipid Science and Technology. 2000, 102, 521–528. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Thiebaud, M.; Dumay, E. High Pressure — Low Temperature Processing of Foods: A Review. Winter, R., Advances in High Pressure Bioscience and Biotechnology II. Springer, Berlin, Heidelberg. 2003. [CrossRef]
- Sciarini, L.S.; Steffolani, M.E.; León, A.E. El rol del gluten en la panificación y el desafío de prescindir de su aporte en la elaboración de pan. Agriscientia, 2016, 33(2), 61-74.
- AACC (American Association of Cereal Chemists). Approved Methods of the AACC. 1976. Available online: https://www.cerealsgrains.org/resources/methods/Pages/default.aspx (accessed on 12 April 2024).
- Bedolla, S.; Rooney, L.W. Characteristics of US and Mexican instant maize flours for tortilla and snack preparation. Cereals Food World. 1984, 29(11), 732-735. https://europepmc.org/article/AGR/IND85022082.
- Bressani, R.; Turcios, J.C.; Reyes, L.; Mérida, R. Caracterización física y química de harinas industriales nixtamalizadas de maíz de consumo humano en América Central [Physical and chemical characterization of nixtamalized industrial corn flours for human consumption in Central America]. Archivos Latinoamericanos de Nutrición. 2001, 51(3), 309-313. https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222001000300015.
- Elías-Silupu, J.W.; García-Rivas, P.C.E.; Pérez-Salcedo, R.; Yauris-Silvera, C.R. Caracterización Fisicoquímica de Pan con Sustitución Parcial de Harina de Trigo por Harina de Quinua (Chenopodium quinoa willd) y Kiwicha (Amaranthus caudatus l.) Germinadas [Physicochemical Characterization of Bread with Partial Sustitución of wheat flour for Quinoa flour (Chenopodium quinoa willd) and Kiwicha (Amaranthus caudatus l.)] Germinated. SENDAS. 2021, 2(2), 69 - 83. [CrossRef]
- Dickinson, E. Food emulsions and foams: stabilization by particles. Current Opinion in Colloid & Interface Science. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Álvarez-Castillo, M.J.; Rössel-Kipping, E.D.; Ortiz-Laurel, H.; López-Martínez, L.A.; Amante-Orozco, A. Potential of the physical and chemical characteristics of prickly pear (Opuntia Albicarpa Seheinvar var. villanueva) seeds in agroindustrial processes: frutos de las cactáceas. Agro productividad. 2021, 6. [CrossRef]
- Martínez-Betancourt, S.R.; Rössel-Kipping, E.D.; López-Martínez, L.A.; Ortiz-Laurel, H.; Loera-Alvarado, G.; Amante-Orozco, A.; Ruiz-Vera, V.M. Potential use of physical characteristics of squash seeds (Cucurbita moschata), pea pods (Pisum sativum) and green bean (Phaseolus vulgaris) in Agroindustry 4.0. Agrociencia, 2022. [CrossRef]
- Rössel-Kipping, E.D.; Ortiz-Laurel, H.; Amante-Orozco, A.; Durán-García, H.M.; López- Martínez, L.A. Características físicas y químicas de la semilla de calabaza para mecanización y procesamiento [Physical and chemical characteristics of pumpkin seeds for machining and processing]. Nova scientia. 2018, 10(21), 61–77. [Google Scholar] [CrossRef]
- Ospina, M.J. Características físico-mecánicas y análisis de calidad de granos [Physical-mechanical characteristics and quality analysis of grains]. Bogotá. Universidad Nacional de Colombia. Departamento de Ingeniería Agrícola. 2001, pp. 225.https://books.google.com.ec/books?id=2DWmqb6xP3wC&printsec=frontcover&hl=es#v=onepage&q&f=false.
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Particle properties. In: food powders, physical properties, processing and funtionality. Food engineering series. 2005, 55-88. [CrossRef]
- Ganjloo, A.; Bimakr, M.; Zarringhalami, M.; Jalili-Safaryan, M.; Ghorbani, M. Moisture-dependent physical properties of green peas (Pisum sativum L.). International Food Research Journal. 2018, 25(3), 1246-1252. http://www.ifrj.upm.edu.my/.
- Sologubik, C.A.; Campañone, L.A.; Pagano, A.M.; Gely, M.C. Effect of moisture content on some physical properties of barley. Ind. Crops Prod. 2013, 43, 762–767. [Google Scholar] [CrossRef]
- Domínguez-Zarate, P.A.; García-Martínez, I.; Güemes-Vera, N.; Totosaus, A. Textura, color y aceptación sensorial de tortillas y pan producidos con harina de ramón (Brosimum alicastrum) para incrementar la fibra dietética total [Texture, colour and sensory acceptance of tortillas and bread produced with ramon flour (Brosimum alicastrum) to increase total dietary fibre.]. Ciencia Y Tecnología Agropecuaria. 2019, 20(3), 699–719. [CrossRef]
- Contreras-Jiménez, B.; Morales-Sánchez, E.; Reyes-Vega, M.L.; Gaytán-Martínez, M. Propiedades funcionales de harinas de maíz nixtamalizado obtenidas por extrusión a baja temperatura [Functional properties of nixtamalized corn flours obtained by low-temperature extrusion]. CyTA. Journal of Food. 2013, 12, 263 270. [Google Scholar] [CrossRef]
- Roddick, J. G. Steroidal Glycoalkaloid Alpha-Tomatine. Phytochemistry. 1974, 13(1), 9–25. [Google Scholar] [CrossRef]
- Convention on Biological Diversity (CDB). Elías-Silupu on Biological Diversity. United Nations Environment Programme. 1992. Available online: https://www.biodiversidad.gob.mx/planeta/internacional/cbd (accessed on 12 April 2024).
- AOAC (Association of official analytical chemists). Official Methods of Analysis of A.O.A.C. Internacional. (18ava ed.). 2005. Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 22 may 2024).
- AOAC (Association of official analytical chemists). Association of Official Analytical Chemists International Official Methods of Analysis. 16th edition. 1997. Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 22 may 2024).
- Beuchat, L. Functional and electrophoretic characteristics of succynalated peanut flour proteins. Journal Agricultural and Food Chemistr. 1977, 25, 258–263. [Google Scholar] [CrossRef]
- Robertson, J.A.; Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibaukt, F. Hydration properties of dietary fibre and resistant starch: a European collaborative study. Lebensmittel Wissenschaft und Technologie. 2000, 33(2), 72–79. [Google Scholar] [CrossRef]
- Coffman, C.; García, V. Functional properties and amino acid content of a protein isolate from mung bean flour. Journal Food Technology. 1977, 12, 473–487. [Google Scholar] [CrossRef]
- 56-60 Physicochemical Test Methods of the Cereal & Grains Association (1976). https://www.cerealsgrains.org/resources/Methods/Pages/56PhysicochemicalTests.aspx. (accessed on 24 April 2024).
- Serna, G.H. Gerencia estratégica. Teoría, metodología, alineamiento, implementación y mapas estratégicos. Índices de gestión [Strategic management. Theory, methodology, alignment, implementation and strategic maps. Management índices]. 9ª ed.; Bogotá, Colombia: 3R Editores. 2003.
- AACC (American Association of Cereal Chemists). Method 55-10.01. Approved Methods of the AACC. 2000. Available online: https://www.cerealsgrains.org/resources/methods/Pages/default.aspx (accessed on 12 April 2024).
- Mohsenin, N.N. Physical properties of plant and animal materials: Structure, physical characteristics, and mechanical properties. Gordon and Breach Science Publishers. 2nd Ed.; New York, NY, USA. 1986, p. 891. https://www.taylorfrancis.com/books/mono/10. 4324/9781003062325/physical-properties-plant-animal-materials-1-physical-characteristics-mechanical-properties-nuri-mohsenin.
- Dutta, S. K.; Nema, V.K.; Bharddwaj, R.K. Physical properties of gram. Journal of Agricultural Engineering Research. 1988, 39, 259–268. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).