1. Introduction
Endometriosis is a chronic gynecological disorder that is characterized by the presence of endometrial-like tissue outside the uterine cavity. It can be found on the ovaries, fallopian tubes, and other pelvic organs [
1]. It affects approximately 10% of women of reproductive age worldwide. This indicates that it affects millions of women globally [
1]. Even though it has a high prevalence, endometriosis remains an underdiagnosed disease among all. It can be largely due to the variability in symptoms and the invasive nature of definitive diagnosis via laparoscopy. This ectopic endometrial tissue undergoes cyclical changes. These changes are in response to the hormonal fluctuations [
3]. This can lead to chronic inflammation, pain, and the formation of scar tissue. Some clinical signs and symptoms of endometriosis include severe dysmenorrhea, chronic pelvic pain and dyspareunia. It also includes infertility in many affected individuals. The pathogenesis of endometriosis is complex. It is affected by many factors. These include genetic, hormonal, and immunological factors, but they still remain not fully understood. This condition significantly impacts women's physical and emotional well-being. This further contributes to pelvic pain and subfertility [
4]
Endometriosis is usually with a range of signs and symptoms that can significantly impact a woman's quality of life. The most common symptom is dysmenorrhea. It can be characterized by severe and debilitating menstrual cramps which can often worsen over time. Chronic pelvic pain is another vital symptom of the condition. It continues beyond menstrual periods. It can sometimes be increased by physical activity or intercourse, leading to dyspareunia. Many women with endometriosis also report lower back and abdominal pain. This pain can be intermittent or constant. In addition to pain, endometriosis is a major cause of infertility. It affects up to 50% of women with the disorder. This is attributed to the effect on the pelvic anatomy along with inflammation. It can also be due to the presence of adhesions, which can impair the function of the ovaries, fallopian tubes, and uterus. Other symptoms also include menorrhagia or heavy menstrual bleeding. Further irregular menstrual cycles are also part of it. Gastrointestinal symptoms, such as diarrhea and constipation are also present prevalently [
5]
The bloating, and nausea, are also frequently reported side by side. All of these are flared up during menstrual periods. This is due to the proximity of endometrial implants to the intestines. Urinary symptoms, including dysuria and hematuria. It may show when endometrial lesions involve the bladder. Fatigue is a less specific but common symptom observed among patients. It is more likely to result from chronic pain and the body’s inflammatory response. The variance and intermingling of these symptoms with other conditions make it difficult to diagnose. Such conditions involve irritable bowel syndrome (IBS) and pelvic inflammatory disease (PID). These can contribute to the present diagnostic challenges associated with endometriosis. Thus, the range of symptoms in endometriosis is very broad. It can significantly interfere with the daily activities of women. It can lead to emotional distress and reduced overall quality of life [
6] .
The exact etiology is still not fully understood. These are several theories proposed in the literature. The most accepted hypothesis is retrograde menstruation [
7]. This means that the menstrual blood flows backward through the fallopian tubes into the pelvic cavity. Other theories include coelomic metaplasia (transformation of peritoneal cells into endometrial cells) which is also of great interest [
8] .The embryonic rest theory and altered immune responses are also considered. Genetic factors also play a major role as endometriosis tends to run in families. Hormonal influences are significant as well for the cause. The estrogen promotion of the growth and maintenance of endometrial lesions can be a contributory factor [
9] .
Endometriosis not only affects physical health but also has a substantial socioeconomic burden on the patients. The chronic pain associated with the condition can lead to significant emotional stress. Infertility can lead to depression and anxiety. Women with endometriosis often have delays in the diagnosis. It can be diagnosed in 7 to 10 years. This delay exacerbates the suffering of the patients and frustration. The economic impact includes direct costs related to healthcare utilization. Ans the indirect costs from loss of productivity and work deficits. The effective management of endometriosis is essential. It can improve individual health outcomes as well as alleviate broader societal burdens [
10].
The management approaches to endometriosis involve both medical and surgical strategies. These approaches aim to reduce pain in patients. It also targets to slow the progression of the disease. It further aims to improve fertility in many cases. Medical treatments primarily focus on hormonal therapies. These therapies are designed to suppress ovarian function and menstruation. This reduces the cyclical nature of endometrial lesion growth. This reduction decreases the associated pain. Common hormonal treatments include oral contraceptives, progestins, and gonadotropin-releasing hormone (GnRH) agonists. These treatments can be effective in managing symptoms but they often come with side effects. These side effects include weight gain and mood changes along with decreased bone density. These treatments are not considered as a permanent cure [
11] .
Surgical management is primarily performed via laparoscopy. It involves the excision or ablation of endometrial lesions as well as the adhesions. Excision surgery is generally preferred in the course of treatment. The reason is because it aims to remove the disease completely. It may result in better pain relief and lower recurrence rates compared to ablation. Surgery is often recommended for women who do not respond to medical treatments and have severe pain. It is also recommended for women attempting to conceive. However, surgery is not without risks, including potential damage to surrounding organs, and the repeated occurrence of endometriosis remains a significant issue [
12] .
The impact of endometriosis on obstetric outcomes is a critical area of research. Women with endometriosis are at an elevated risk of several pregnancy-related complications. It includes miscarriage, preterm birth, placenta previa, and pregnancy-induced hypertension. These complications necessitate careful monitoring and management during pregnancy. The relationship between endometriosis and adverse obstetric outcomes may be influenced by the location of the disease. It is also affected by the severity of the disease and the type of treatment received [
13] .
This research paper aims to explore the obstetric outcomes associated with endometriosis treatment or surgery. By reviewing the existing studies, this paper seeks to determine the impact of various treatments on obstetric outcomes mainly conceive, pregnancy, and childbirth outcomes. This paper will help to inform clinical practices and improve management strategies for women with endometriosis who are planning to conceive or are already pregnant.
2. Materials and Methods
Data Searching Strategy
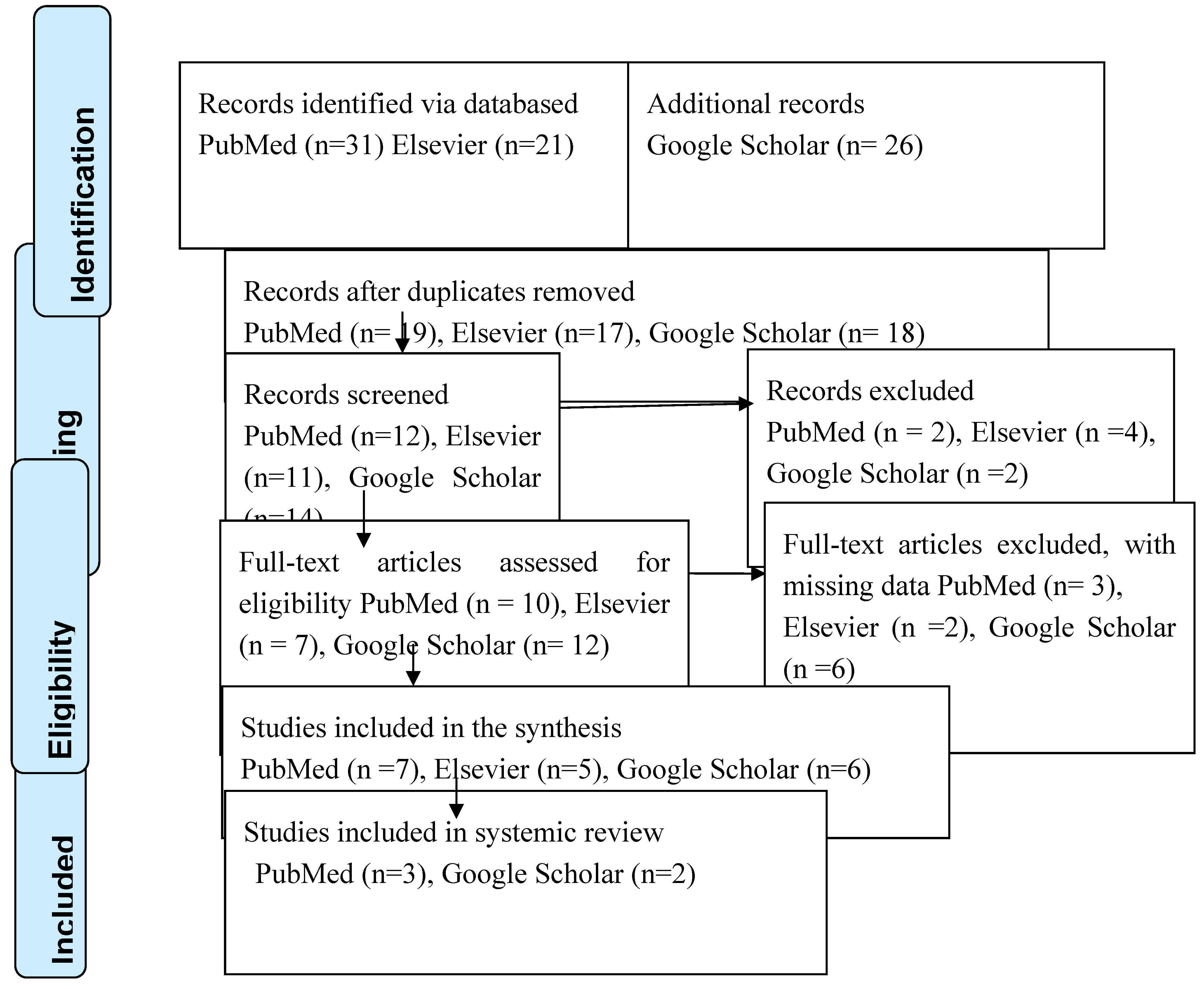
A systematic and comprehensive search was conducted to identify studies relevant to the obstetric outcomes of endometriosis treatment. There were electronic databases were searched: PubMed, Google Scholar, Scopus, and Elsevier. To ensure the research was conducted effectively, the study adhered to PRISMA standards (PRISMA Statement). The search strategy used a combination of various keywords and Medical Subject Headings (MeSH) terms associated with obstetric outcomes, endometriosis, treatment, and surgery. Boolean operators (AND, OR) were utilized to associate search terms optimally. Filters and limits were applied to refine the search results based on publication type, study design, and language. The search was restricted to papers published in English. Additionally, the reference lists present in the relevant articles and systematic reviews were manually examined to identify further studies meeting the inclusion criteria.
Table 1.
Keywords and MeSH phrases utilized in the optimization method.
Table 1.
Keywords and MeSH phrases utilized in the optimization method.
| Category |
MeSH Terms/ Keywords |
| Obstetric Outcomes |
Pregnancy, conceive, fertility, ovulation |
| Endometriosis |
Endometria, endometrial implants, endometrial lesions |
| Treatment |
Hormonal therapy, oral contraceptives, progestins |
| Surgery |
Laparoscopy, ablation, surgical procedure |
Inclusion Criteria
Studies that met the following criteria were included:
Addressed the treatments of endometriosis in women
Included original research articles, systematic reviews
Published in journals
Written in English language
Exclusion Criteria
Studies mentioned below were excluded:
Did not focus on the treatment strategies with respect to obstetric outcomes in endometriosis
Case reports and narrative reviews
Lack of English translation of the publication
Data Extraction and Synthesis
The review method involved a systematic approach where reviewers assessed selected articles to determine their eligibility for inclusion. Predetermined criteria were used to select research. The full-text publications were also evaluated. The data was then extracted from the articles. Reviewers decide whether to accept or reject research proposals based on these criteria. The data management system greatly facilitated article selection. This inquiry used a systematic approach to identify and evaluate relevant papers. It was done by adhering to PRISMA guidelines for quality work. The careful selection and organization of appropriate clinical trials ensure the reliability and accuracy of the outcomes throughout the study process.
Figure 1 presents the PRISMA flow diagram used to identify the research.
| Study |
Country |
Study design |
Sample size |
Treatment/Surgery |
Outcome |
| Marcello Ceccaroni et al. (2021) |
Italy |
Randomized control trial |
146 |
GnRH treatment (Triptorelin/ Leuprorelin 3.75mg) |
Assisted reproductive technology was used to make conception possible in patients. |
| Guo et al. (2022) |
China |
Prospective Randomized control trial |
300 |
GnRH treatment |
Assisted reproductive technology was used to make conception possible in patients. |
| Elisabet Rodr´ıguezTarrega et al. (2020) |
Italy |
Randomized control trial |
200 |
GnRH treatment (Triptorelin 3.75mg) |
Ovarian stimulation was reported to be longer compared with dosage of gonadotropins. It was also evaluated that GnRH treatment for three before IVF in women with endometriosis was not able to improve the pregnancy rate among patients. |
| Guler et al. (2017) |
Turkey |
Retrospective cohort |
78 |
Laparoscopic surgery of peritoneal endometriosis |
Dominant follicles decreased in the surgical group. The transferred embryos and infertility duration were lower |
| Cakiroglu et al. (2017) |
Turkey |
Retrospective cohort |
110 |
Laparoscopic surgery |
Clinical pregnancy rates increased after the endometrial ovarian surgery |
A single-blind and placebo-controlled trial done clinically involved 200 women suffering from infertility associated with endometriosis. The efficacy of a three-month treatment of gonadotrophin-releasing hormone agonist (GnRHa) before in vitro fertilization (IVF) was assessed. The research participants were assigned to either a GnRHa treatment group or a placebo group. The primary ending point was the pregnancy rate clinically. The study reported no significant difference in clinical pregnancy rates among the two groups. The rates of 25.3% in the GnRHa group and 33.7% in the placebo group (P = 0.212) were reported. The cumulative live birth rate reported also not significantly different, reported at 22.0% in the GnRHa group and 33.7% in the control group (p = 0.077). Notably, ovarian stimulation duration and the total gonadotrophin dosage were significantly higher in the GnRHa group (both P < 0.001), and serum oestradiol levels on the HCG day were less (P = 0.001). Additionally, the GnRHa group experienced a higher cancellation rate (P = 0.042) and fewer cleavage-stage embryos (P = 0.023). In a subset of the 40 participants of this research, analysis of follicular fluid revealed no significant statistical differences in the levels of oestradiol, testosterone, and androstenedione between groups, except for significantly lower testosterone levels in the GnRHa group (P < 0.001). These findings indicate that a three-month GnRHa treatment before IVF did not enhance pregnancy rates clinically in women with endometriosis [
14] .
The study compared the medroxyprogesterone acetate (MPA) and human menopausal gonadotrophin (HMG) protocol versus the ultra-long gonadotropin-releasing hormone (GnRH) antagonist protocol in 300 participants of the research. These patients were with progressed ovarian endometriosis with IVF. The participants were sub-grouped in two groups: one getting the MPA + HMG protocol and the other the 1-month ultra-long GnRH agonist protocol. Results indicated that the MPA + HMG group required a decreased dose of hMG and had a shorter medication duration compared to the GnRH agonist group. A significant difference was observed in the Follicle-to-Oocyte Index among the two groups (P<0.001). However, differences of significant consideration were absent in ovarian response or the number of mature oocytes. The fertilized oocytes and viable embryos also did not report any difference. Additionally, pregnancy-induced clinical and live birth rates were the same among the groups, regardless of whether fresh or frozen embryo transfers were used in the GnRH agonist group. No major differences in the time to embryo transfer were reported in the findings. It also includes medical costs or negative effects. The findings suggest that the MPA + HMG protocol yields comparable oocyte retrieval numbers and pregnancy outcomes to the ultra-long GnRH agonist protocol, indicating that the MPA + HMG protocol could be a viable alternative for IVF treatment in patients with ovarian endometriosis [
15] .
Endometriosis has been confirmed to negatively affect the ovaries. The toxic substances, such as free iron, and oxidative species such as reactive oxygen (ROS) and proteolytic enzymes include inflammatory factors too [
16] .These factors are released within endometriomas and can lead to elevated oxidative stress, decreasing maturation of follicles, and impairing fertilization. Evidence suggested that a 3-month ultra-long GnRH agonist protocol might produce a more suitable surrounding for oocyte maturation [
17]. It can result in better quality of oocyte as well as the embryo with improved fertilization rates. However, MPA treatment in IVF/ICSI can similarly improve this environment remains controversial. MPA is commonly utilized to decrease pain and increase the general comfort in majority of the women suffering from endometriosis [
18] .The mechanisms of MPA and other progestins are not fully understood, but their efficacy is thought to stem from pituitary inhibition. It also includes the atrophy of endometriotic lesions. Additionally, MPA may reduce inflammation at the sites of implants of endometriosis. Elongated treatment with MPA for eight days has been shown to decrease luciferase activity by around 36% and reduce CCL5/RANTES protein production by 50%, while shorter treatments of two or four days did not have significant effects on CCL5/RANTES protein production [
19] . Furthermore, eight-day treatment by MPA elevated the expression of progesterone receptors. There were no significant differences observed in the outcomes among patients who underwent fresh embryo transfer (ET) or frozen embryo transfer (FET) using both protocols, which may be due to the effect of multiple factors on clinical outcomes. However, the rates of fertilization in the MPA + HMG group were higher. This improvement is associated to decreased oxidative stress as well as inflammation, as well as a developed environment for the development of follicles in endometriosis 20 .
A retrospective study was conducted on records as well as the pregnancy outcomes of one-year postoperative patients. There were 335 patients suffering from endometriosis-associated infertility who endured laparoscopic surgery between 2018 and 2020. The pregnancy rate through the one year post-surgery was reported to be 57.3%, with the high rates found three to six months duration after the surgery. Many associated factors such as the Body Mass Index (BMI) with p = 0.515 and the occurrence of dysmenorrhea were included. It also includes a history of pelvic surgery with p = 0.247 and a kind of pathology for endometriosis with p reported to be 0.893. Additionally, preoperative serum carbohydrate antigen 125 (CA125) levels did not significantly affect postoperative pregnancy rates. Comparatively, the period of infertility (P = 0.029) and the presence of adenomyosis (P = 0.042) was also assessed. The surgery duration (P = 0.015) and blood loss intraoperative (P = 0.050) had a significant effect on postoperative pregnancy rates. Notably, participants with mild (Stage I-II) endometriosis had increased pregnancy rates compared to those with moderate to severe (Stage III-IV) disease (P = 0.009). Factors influencing the postoperative live birth rate included blood loss (P = 0.010), age (P = 0.002), endometriosis stage (P = 0.018), and the occurrence of adenomyosis (P = 0.022). The findings showed that, for patients with endometriosis-associated difficulty in fertilization undergoing laparoscopic surgery, adverse factors for pregnancy within the first postoperative year include age over 35 years, infertility duration over three years, surgery time of two hours or more, intraoperative blood loss of 50 ml or more, concurrent adenomyosis, severe endometriosis, and low preoperative AMH levels. However, BMI, history of pelvic surgery, dysmenorrhea, types of endometriosis pathology, and preoperative CA125 levels did not show statistically significant effects on pregnancy rates postoperatively. For postoperative live birth rates, adverse factors were age over 35 years, advanced endometriosis, and intraoperative blood loss of 50 ml or more [
21]
This systematic review is one of the first to determine the effect of treatment outcomes on endometriosis. These include obstetric outcomes without considering the need to focus on one specific endometriosis type. This approach is specifically relevant as endometriosis is a heterogeneous disease pathologically. It is characterized by a wide range of lesions, the majority of the time coexisting. Our study, unlike previous research, takes consideration of all the endometriosis phenotypes and it offers an extensive overview of the disease. It can help clinicians in making their daily therapeutic choices undergoing infertile endometriosis patients presenting various types of lesions.
3. Conclusion
Endometriosis is a prevalent and complex gynecological condition that significantly impacts women's reproductive health, often leading to infertility and chronic pain. This systematic review aimed to evaluate the obstetric outcomes associated with various treatments for endometriosis, focusing on medical and surgical interventions Our review highlighted that endometriosis treatment, particularly using GnRH agonists and laparoscopic surgery, has varied effects on fertility and pregnancy outcomes. GnRH agonist treatment, although effective in reducing pain and lesion growth, did not significantly enhance clinical pregnancy rates in women undergoing IVF. The duration of ovarian stimulation and total gonadotrophin dosage was higher in the GnRH group, with no improvement in cumulative live birth rates, indicating that while GnRH treatments are useful for managing symptoms, their direct impact on enhancing fertility remains limited. Conversely, laparoscopic surgery demonstrated a more favorable impact on fertility outcomes. The pregnancy rates post-surgery were notably higher, especially within the first six months post-operation. Factors such as age, duration of infertility, presence of adenomyosis, and severity of endometriosis were significant determinants of successful postoperative pregnancies. The surgery's ability to directly remove endometrial lesions and adhesions may account for these improved outcomes. Despite these findings, endometriosis is still a challenging disease to manage due to its heterogeneous nature. This review underscores the necessity for personalized treatment plans, considering the patient's specific endometriosis phenotype, severity, and individual reproductive goals. Future research should continue to refine these strategies and explore novel therapeutic options to enhance obstetric outcomes for women with endometriosis. Both medical and surgical treatments for endometriosis offer benefits, but their impact on obstetric outcomes varies. Laparoscopic surgery appears to offer more substantial improvements in fertility, especially in cases of mild to moderate endometriosis. Effective management of endometriosis requires a multidisciplinary approach and individualized treatment plans to optimize reproductive health and overall quality of life for affected women.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Maria Giovanna Vastarella. And Gorizio Pieretti, methodology; Diego Domenico Fasulo, software; Gennaro Gaudino, validation; Feiciano Ciccarelli and Maria Giovanna Vastarella, formal analysis; Vincenzo Vastarella, investigation; Paolino Mauro, resources; Diago Fasulo, data curation; Maria Giovanna Vastarella, writing—original draft preparation
Funding
“This research received no external funding”
Institutional Review Board Statement
“Ethical review and approval were waived for this study due to REVIEW RETROSPECTIVE PAPER
Informed Consent Statement
“Informed consent was obtained from all subjects involved in the study.”
Data Availability Statement
Data employed in this paper were recovered from Pubmed, Sciencedirect, WHO register, National birth registers
Conflicts of Interest
“The authors declare no conflicts of interest.”
References
- Ronsini, C.; Iavarone, I.; Braca, E.; Vastarella, M.G.; De Franciscis, P.; Torella, M. The Efficiency of Sclerotherapy for the Management of Endometrioma: A Systematic Review and Meta-Analysis of Clinical and Fertility Outcomes. Medicina 2023, 59, 1643. [Google Scholar] [CrossRef] [PubMed]
- Endometriosis. (2023). https://www.who.int/news-room/fact-sheets/detail/endometriosis#:~:text=Key%20facts,age%20women%20and%20girls%20globally.
- Ronsini, C.; Reino, A.; Molitierno, R.; Vastarella, M.G.; La Mantia, E.; De Franciscis, P. Critical Overview of Serous Endometrial Intraepithelial Cancer Treatment: Systematic Review of Adjuvant Options. Life 2023, 13, 1429. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; He, L.; Ma, Y.; Zhang, Y.; Zheng, W. Systematic review and meta-analysis on the effect of adjuvant gonadotropin-releasing hormone agonist (GnRH-a) on pregnancy outcomes in women with endometriosis following conservative surgery. BMC Pregnancy Childbirth 2024, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lian, N.; Guo, S.; Xie, X. Analysis of factors affecting pregnancy rate after laparoscopic surgery for infertility associated with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 297, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Gazzo, I.; Crosa, M.; Rosato, F.P.; Barra, F.; Maggiore, U.L.R. Impact of surgery for endometriosis on the outcomes of in vitro fertilization. Best Pr. Res. Clin. Obstet. Gynaecol. 2024, 95, 102496. [Google Scholar] [CrossRef] [PubMed]
- D’hooghe, T.M.; Debrock, S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum. Reprod. Updat. 2002, 8, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Dietze, R.; Kudipudi, P.K.; Horné, F.; Meinhold-Heerlein, I. Endometriosis in MRKH cases as a proof for the coelomic metaplasia hypothesis? Reproduction 2019, 158, R41–R47. [Google Scholar] [CrossRef] [PubMed]
- Czyzyk, A.; Podfigurna, A.; Szeliga, A.; Meczekalski, B. Update on endometriosis pathogenesis. Minerva Obstet. Gynecol. 2017, 69, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.S.; Ross, V.; Stern, C.; Rogers, P.A.W.; Healey, M. Obstetric Outcome After Surgical Treatment of Endometriosis: A Review of the Literature. Front. Reprod. Heal. 2021, 3, 750750. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroni, M.; Clarizia, R.; Liverani, S.; Donati, A.; Ceccarello, M.; Manzone, M.; Roviglione, G.; Ferrero, S. Dienogest vs GnRH agonists as postoperative therapy after laparoscopic eradication of deep infiltrating endometriosis with bowel and parametrial surgery: a randomized controlled trial. Gynecol. Endocrinol. 2021, 37, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.; Ravanbakhsh, R.; Parsanezhad, M.E.; Alborzi, M.; Alborzi, S.; Dehbashi, S. A comparison of follicular response of ovaries to ovulation induction after laparoscopic ovarian cystectomy or fenestration and coagulation versus normal ovaries in patients with endometrioma. Fertil. Steril. 2007, 88, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawahara, N.; Ogawa, K.; Yoshimoto, C. A Relationship Between Endometriosis and Obstetric Complications. Reprod. Sci. 2020, 27, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tárrega, E.; Monzo, A.M.; Quiroga, R.; Polo-Sánchez, P.; Fernández-Colom, P.; Monterde-Estrada, M.; Novella-Maestre, E.; Pellicer, A. Effect of GnRH agonist before IVF on outcomes in infertile endometriosis patients: a randomized controlled trial. Reprod. Biomed. Online 2020, 41, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Du, T.; Gao, H.; Xi, Q.; Wu, L.; Lyu, Q.; Zhu, Q. The comparison of two different protocols ultra-long versus medroxyprogesterone acetate in women with ovarian endometriosis: a prospective randomized controlled trial. Reprod. Heal. 2022, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Viganò, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Updat. 2013, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ghulmiyyah, J.; Sharma, R.; Halabi, J.; Agarwal, A. Power of Proteomics in Linking Oxidative Stress and Female Infertility. BioMed Res. Int. 2014, 2014, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; Manganaro, L.; Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pr. Res. Clin. Obstet. Gynaecol. 2014, 28, 655–681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lebovic, D.I.; Taylor, R.N. Long-Term Progestin Treatment Inhibits RANTES (Regulated on Activation, Normal T Cell Expressed and Secreted) Gene Expression in Human Endometrial Stromal Cells. J. Clin. Endocrinol. Metab. 2002, 87, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Du, T.; Gao, H.; Xi, Q.; Wu, L.; Lyu, Q.; Zhu, Q. The comparison of two different protocols ultra-long versus medroxyprogesterone acetate in women with ovarian endometriosis: a prospective randomized controlled trial. Reprod. Heal. 2022, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lian, N.; Guo, S.; Xie, X. Analysis of factors affecting pregnancy rate after laparoscopic surgery for infertility associated with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 297, 214–220. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).