Submitted:
01 October 2024
Posted:
04 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
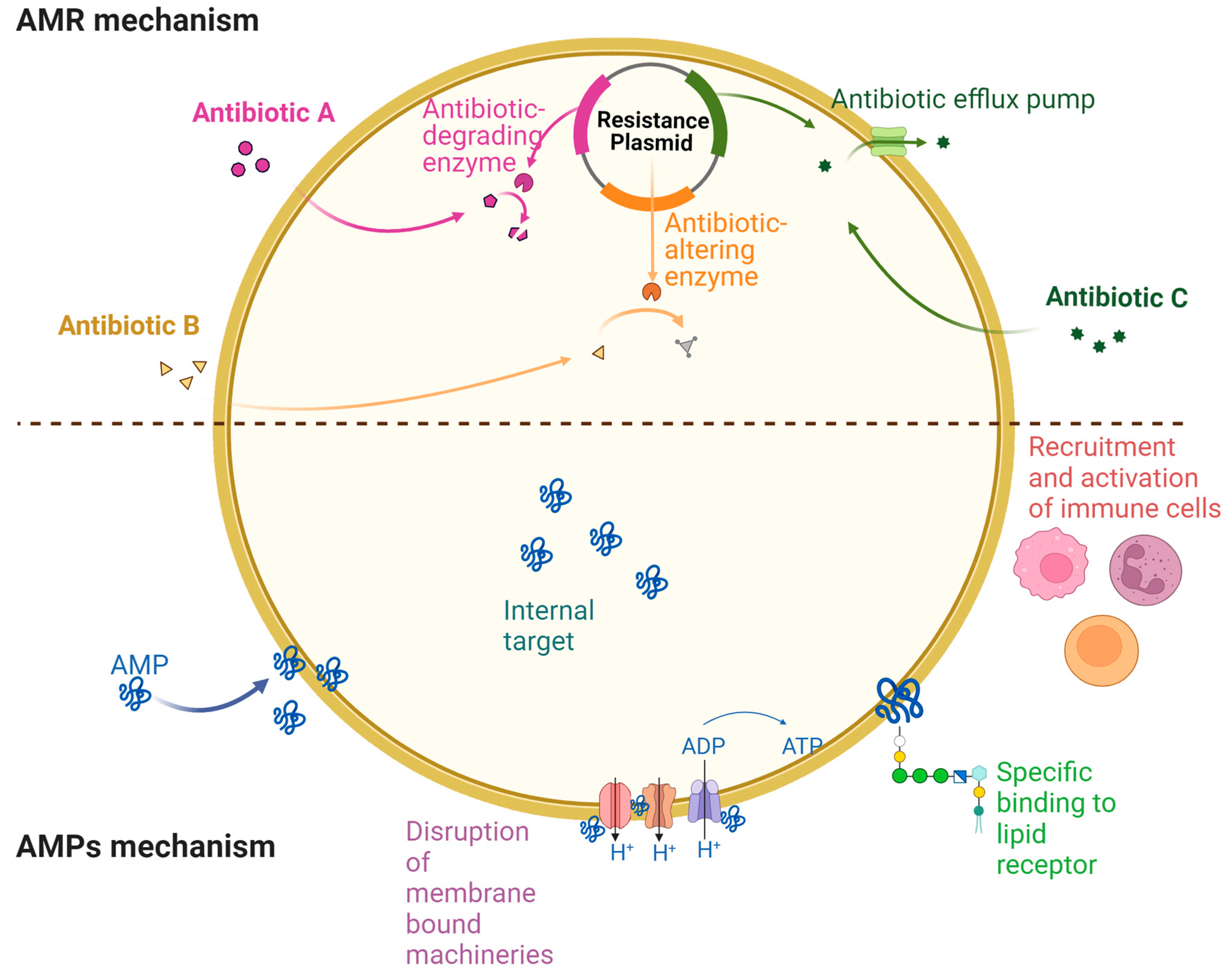
2. Antibiotic Resistance Drivers and Mechanisms
3. AMP Mechanisms of Action in Treating Drug-Resistant Bacteria
3.1. AMPs Mediated Membrane Disruption
3.2. AMPs Mediated Intracellular Targeting
3.3. Immune Activity Regulation by AMPs
3.4. AMPs as Role Players in Eradicating Biofilm-Mediated Drug Resistance
4. AMPs Approved for Clinical Use or under Clinical Trials
5. Synergistic Action of AMPs with Conventional Antibiotics
6. Challenges with AMPs for Therapeutic Applications against Superbugs
7. Conclusions and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthc. 2023, 11, 1–20. [Google Scholar] [CrossRef]
- Lee Ventola, M.C. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Compr. Biochem. 1963, 11, 181–224. [Google Scholar] [CrossRef]
- Matthyssen, T.; Li, W.; Holden, J.A.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. The Potential of Modified and Multimeric Antimicrobial Peptide Materials as Superbug Killers. Front. Chem. 2022, 9, 1–23. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Walsh, C. Where will new antibiotics come from? Pharm. Eng. 2017, 37, 72. [Google Scholar] [CrossRef]
- Nagarajan, D.; Roy, N.; Kulkarni, O.; Nanajkar, N.; Datey, A.; Ravichandran, S.; Thakur, C.; Sandeep, T.; Aprameya, I.V.; Sarma, S.P.; et al. W76: A designed antimicrobial peptide to combat carbapenem- And tigecycline-resistant Acinetobacter baumannii. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Chen, S.P.; Chen, E.H.L.; Yang, S.Y.; Kuo, P.S.; Jan, H.M.; Yang, T.C.; Hsieh, M.Y.; Lee, K.T.; Lin, C.H.; Chen, R.P.Y. A Systematic Study of the Stability, Safety, and Efficacy of the de novo Designed Antimicrobial Peptide PepD2 and Its Modified Derivatives Against Acinetobacter baumannii. Front. Microbiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Mohan, N.M.; Zorgani, A.; Jalowicki, G.; Kerr, A.; Khaldi, N.; Martins, M. Unlocking NuriPep 1653 From Common Pea Protein: A Potent Antimicrobial Peptide to Tackle a Pan-Drug Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Dadgostar, P.; Ng, W.J.; Hing, C.L.; Loo, C.B.; Hoh, E.K.; Loke, I.L.; Ee, K.Y. Ginger-Enriched Honey Attenuates Antibiotic Resistant Pseudomonas aeruginosa Quorum Sensing Virulence Factors and Biofilm Formation. Antibiotics 2023, 12, 1123. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Han, M.; Zhu, Y.; Creek, D.J.; Lin, Y.; Anderson, D.; Shen, H.; Tsuji, B.; Gutu, A.D.; Moskowitz, S.M.; Velkov, T.; et al. Alterations of Metabolic and Lipid Profiles in Polymyxin-. Antimicrob. Agents Chemother. 2018, 62, 1–14. [Google Scholar] [CrossRef]
- Hankins, J.V.; Madsen, J.A.; Giles, D.K.; Brodbelt, J.S.; Trent, M.S. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 8722–8727. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 1687–1692. [Google Scholar] [CrossRef]
- Verkleij, A.J.; Zwaal, R.F.; Roelofsen, B.; Comfurius, P.; Kastelijn, D.; Van Deenen, L.L. The asymmetric distribution of phospholipids in the human red cell membrane. Bba 1973, 21, 1154–1157. [Google Scholar]
- Yount, M.R.Y. and N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 453–499. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Biopolym.-Pept. Sci. Sect. 2008, 90, 369–383. [Google Scholar] [CrossRef]
- Paulmann, M.; Arnold, T.; Linke, D.; Özdirekcan, S.; Kopp, A.; Gutsmann, T.; Kalbacher, H.; Wanke, I.; Schuenemann, V.J.; Habeck, M.; et al. Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J. Biol. Chem. 2012, 287, 8434–8443. [Google Scholar] [CrossRef]
- Cytryńska, M.; Zdybicka-Barabas, A. Defense peptides: Recent developments. Biomol. Concepts 2015, 6, 237–251. [Google Scholar] [CrossRef]
- Bellomio, A.; Vincent, P.A.; De Arcuri, B.F.; Farias, R.N.; Morero, R.D. Microcin J25 has dual and independent mechanisms of action in Escherichia coli: RNA polymerase inhibition and increased superoxide production. J. Bacteriol. 2007, 189, 4180–4186. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Ofek, I.; Cohen, S.; Rahmani, R.; Kabha, K.; Tamarkin, D.; Herzig, Y.; Rubinstein, E. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob. Agents Chemother. 1994, 38, 374–377. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Le, C.F. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, 1–16. [Google Scholar] [CrossRef]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS J. 2009. [CrossRef]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef]
- Selsted, M.E.; Novotny, M.J.; Morris, W.L.; Tang, Y.Q.; Smith, W.; Cullor, J.S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 1992, 267, 4292–4295. [Google Scholar] [CrossRef]
- Falla, T.J.; Nedra Karunaratne, D.; Hancock, R.E.W. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996, 271, 19298–19303. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Krishnakumari, V.; Nagaraj, R.; Sitaram, N. Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett. 1996, 395, 48–52. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chen, C.; Jou, M.L.; Lee, A.Y.L.; Lin, Y.C.; Yu, Y.P.; Huang, W.T.; Wu, S.H. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: Evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005, 33, 4053–4064. [Google Scholar] [CrossRef]
- Ghosh, A.; Kar, R.K.; Jana, J.; Saha, A.; Jana, B.; Krishnamoorthy, J.; Kumar, D.; Ghosh, S.; Chatterjee, S.; Bhunia, A. Indolicidin targets duplex DNA: Structural and mechanistic insight through a combination of spectroscopy and microscopy. ChemMedChem 2014, 9, 2052–2058. [Google Scholar] [CrossRef]
- Adelman, K.; Yuzenkova, J.; La Porta, A.; Zenkin, N.; Lee, J.; Lis, J.T.; Borukhov, S.; Wang, M.D.; Severinov, K. Molecular mechanism of transcription inhibition by peptide antibiotic Microcin J25. Mol. Cell 2004, 14, 753–762. [Google Scholar] [CrossRef]
- Ho, Y.H.; Sung, T.C.; Chen, C.S. Lactoferricin B inhibits the phosphorylation of the two-component system response regulators BasR and CreB. Mol. Cell. Proteomics 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Tu, Y.H.; Ho, Y.H.; Chuang, Y.C.; Chen, P.C.; Chen, C.S. Identification of lactoferricin B intracellular targets using an escherichia coli proteome chip. PLoS ONE 2011, 6, e0028197. [Google Scholar] [CrossRef]
- Schnapp, D.; Kemp, G.D.; Smith, V.J. Purification and characterization of a proline-rich antibacterial peptide, pdf. 1996, 539, 532–539. [Google Scholar]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The host antimicrobial peptide Bac71-35 binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Le, C.F.; Yusof, M.Y.M.; Hassan, H.; Sekaran, S.D. In vitro properties of designed antimicrobial peptides that exhibit potent antipneumococcal activity and produces synergism in combination with penicillin. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Le, C.F.; Gudimella, R.; Razali, R.; Manikam, R.; Sekaran, S.D. Transcriptome analysis of Streptococcus pneumoniae treated with the designed antimicrobial peptides, DM3. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1–14. [Google Scholar] [CrossRef]
- Hiemstra, P.S. Defensins and cathelicidins in inflammatory lung disease: Beyond antimicrobial activity. Biochem. Soc. Trans. 2006, 34, 276–278. [Google Scholar] [CrossRef]
- Tan, B.H.; Meinken, C.; Bastian, M.; Bruns, H.; Legaspi, A.; Ochoa, M.T.; Krutzik, S.R.; Bloom, B.R.; Ganz, T.; Modlin, R.L.; et al. Macrophages Acquire Neutrophil Granules for Antimicrobial Activity against Intracellular Pathogens. J. Immunol. 2006. [Google Scholar] [CrossRef]
- Zheng, Y.; Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human α-defensins from neutrophils. Br. J. Dermatol. 2007. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Hubert, P.; Delvenne, P.; Herfs, M. Defensins: “Simple” antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev. 2015, 26, 361–370. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000, 68, 9–14. [Google Scholar] [CrossRef]
- García, J.R.C.; Jaumann, F.; Schulz, S.; Krause, A.; Rodríguez-Jiménez, J.; Forssmann, U.; Adermann, K.; Klüver, E.; Vogelmeier, C.; Becker, D.; et al. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity: Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001, 306, 257–264. [Google Scholar] [CrossRef]
- Röhrl, J.; Yang, D.; Oppenheim, J.J.; Hehlgans, T. Human β-Defensin 2 and 3 and Their Mouse Orthologs Induce Chemotaxis through Interaction with CCR2. J. Immunol. 2010, 184, 6688–6694. [Google Scholar] [CrossRef]
- Lim, H.K.; O’Neill, H.C. Identification of Stromal Cells in Spleen Which Support Myelopoiesis. Front. Cell Dev. Biol. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Engelmayer, J.; Blezinger, P.; Varadhachary, A. Talactoferrin Stimulates Wound Healing With Modulation of Inflammation. J. Surg. Res. 2008, 149, 278–286. [Google Scholar] [CrossRef]
- Gaio, V.; Cerca, N. Cells released from S. epidermidis biofilms present increased antibiotic tolerance to multiple antibiotics. PeerJ 2019, 2019. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial biofilm destruction: A focused review on the recent use of phage-based strategies with other antibiofilm agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Heal. 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol. 2011, 11, 114. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef]
- Hell, É.; Giske, C.G.; Nelson, A.; Römling, U.; Marchini, G. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett. Appl. Microbiol. 2010, 50, 211–215. [Google Scholar] [CrossRef]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Shiba, K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling 2010, 26, 103–110. [Google Scholar] [CrossRef]
- Gopal, R.; Kim, Y.G.; Lee, J.H.; Lee, S.K.; Chae, J.D.; Son, B.K.; Seo, C.H.; Park, Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2014, 58, 1622–1629. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Mermer, S.; Turhan, T.; Bolat, E.; Aydemir, S.; Yamazhan, T.; Pullukcu, H.; Arda, B.; Sipahi, H.; Ulusoy, S.; Sipahi, O.R. Ceftaroline versus vancomycin in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) in an experimental MRSA meningitis model. J. Glob. Antimicrob. Resist. 2020, 22, 147–151. [Google Scholar] [CrossRef]
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and its use in the treatment of infected wounds. Nature 1944, 154, 703. [Google Scholar] [CrossRef]
- UMBREIT, W.W. Mechanisms of antibacterial action. Pharmacol. Rev. 1953, 5, 275–284. [Google Scholar] [CrossRef]
- Sahoo, S.; Mohanty, J.N.; Routray, S.P.; Khandia, R.; Das, J.; Shah, S.; Swarnkar, T. Colistin the last resort drug in 21st century antibiotics to combat Multidrug resistance superbugs. J. Exp. Biol. Agric. Sci. 2023, 11, 919–929. [Google Scholar] [CrossRef]
- Ye, Y.; Xia, Z.; Zhang, D.; Sheng, Z.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Multifunctional pharmaceutical effects of the antibiotic daptomycin. Biomed Res. Int. 2019, 2019, 8609218. [Google Scholar] [CrossRef]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68. [Google Scholar] [CrossRef]
- Baindara, P.; Dinata, R.; Mandal, S.M. Marine Bacteriocins: An Evolutionary Gold Mine to Payoff Antibiotic Resistance. Mar. Drugs 2024, 22. [Google Scholar] [CrossRef]
- Kalita, A.; Verma, I.; Khuller, G.K. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J. Infect. Dis. 2004, 190, 1476–1480. [Google Scholar] [CrossRef]
- Baindara, P.; Singh, N.; Ranjan, M.; Nallabelli, N.; Chaudhry, V.; Pathania, G.L.; Sharma, N.; Kumar, A.; Patil, P.B.; Korpole, S. Laterosporulin10: A novel defensin like class iid bacteriocin from brevibacillus sp. strain SKDU10 with inhibitory activity against microbial pathogens. Microbiol. (United Kingdom) 2016, 162, 1286–1299. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Le Lay, C.; Baah, J.; Drider, D. Antibiotic and antimicrobial peptide combinations: Synergistic inhibition of Pseudomonas fluorescens and antibiotic-resistant variants. Res. Microbiol. 2012, 163, 101–108. [Google Scholar] [CrossRef]
- Brumfitt, W.; Salton, M.R.J.; Hamilton-Miller, J.M.T. Nisin, alone and combined with peptidoglycan-modulating antibiotics: Activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 2002, 50, 731–734. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Fortuna, M.; Scalise, G. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1999, 44, 641–645. [Google Scholar] [CrossRef]
- Lebel, G.; Piché, F.; Frenette, M.; Gottschalk, M.; Grenier, D. Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides 2013, 50, 19–23. [Google Scholar] [CrossRef]
- Mataraci, E.; Dosler, S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2012, 56, 6366–6371. [Google Scholar] [CrossRef]
- De Gier, M.G.; Bauke Albada, H.; Josten, M.; Willems, R.; Leavis, H.; Van Mansveld, R.; Paganelli, F.L.; Dekker, B.; Lammers, J.W.J.; Sahl, H.G.; et al. Synergistic activity of a short lipidated antimicrobial peptide (lipoAMP) and colistin or tobramycin against Pseudomonas aeruginosa from cystic fibrosis patients. Medchemcomm 2016, 7, 148–156. [Google Scholar] [CrossRef]
- Duong, L.; Gross, S.P.; Siryaporn, A. Developing Antimicrobial Synergy With AMPs. Front. Med. Technol. 2021, 3, 640981. [Google Scholar] [CrossRef]
- Bowdish, D.; Davidson, D.; Hancock, R. A Re-evaluation of the Role of Host Defence Peptides in Mammalian Immunity. Curr. Protein Pept. Sci. 2005, 6, 35–51. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Jorelle, A.B.J.; Sarra, S.; Viktorovna, P.I.; Davares, A.K.L.; Ingrid, N.K.C.; Steve, A.A.F.; Andreevna, S.L.; Vyacheslavovna, Y.N.; Carime, B.Z. Short review on the potential alternatives to antibiotics in the era of antibiotic resistance. J. Appl. Pharm. Sci. 2022, 12, 029–040. [Google Scholar] [CrossRef]
- Miller, W.R.; Bayer, A.S.; Arias, C.A. Mechanism of action and resistance to daptomycin in Staphylococcus aureus and enterococci. Cold Spring Harb. Perspect. Med. 2016, 6, a026997. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef]
- Prince, A.; Sandhu, P.; Kumar, P.; Dash, E.; Sharma, S.; Arakha, M.; Jha, S.; Akhter, Y.; Saleem, M. Lipid-II Independent Antimicrobial Mechanism of Nisin Depends on Its Crowding and Degree of Oligomerization. Sci. Rep. 2016, 6, 37908. [Google Scholar] [CrossRef]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van Der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.A.; et al. Peptidomimetic antibiotics target outer-membrane biogenesis in pseudomonas aeruginosa. Science (80-. ). 2010, 327, 1010–1013. [Google Scholar] [CrossRef]
- Landa, A.; Jiménez, L.; Willms, K.; Jiménez-García, L.F.; Lara-Martínez, R.; Robert, L.; Cirioni, O.; Barańska-Rybak, W.; Kamysz, W. Antimicrobial peptides (Temporin A and Iseganan IB-367): Effect on the cysticerci of Taenia crassiceps. Mol. Biochem. Parasitol. 2009, 164, 126–130. [Google Scholar] [CrossRef]
- Alam, M.Z.; Wu, X.; Mascio, C.; Chesnel, L.; Hurdle, J.G. Mode of action and bactericidal properties of surotomycin against growing and nongrowing clostridium difficile. Antimicrob. Agents Chemother. 2015, 59, 5165–5170. [Google Scholar] [CrossRef]
- Rubinchik, E.; Dugourd, D.; Algara, T.; Pasetka, C.; Friedland, H.D. Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int. J. Antimicrob. Agents 2009, 34, 457–461. [Google Scholar] [CrossRef]
- Crowther, G.S.; Baines, S.D.; Todhunter, S.L.; Freeman, J.; Chilton, C.H.; Wilcox, M.H. Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J. Antimicrob. Chemother. 2013, 68, 168–176. [Google Scholar] [CrossRef]
- Malanovic, N.; Leber, R.; Schmuck, M.; Kriechbaum, M.; Cordfunke, R.A.; Drijfhout, J.W.; De Breij, A.; Nibbering, P.H.; Kolb, D.; Lohner, K. Phospholipid-driven differences determine the action of the synthetic antimicrobial peptide OP-145 on Gram-positive bacterial and mammalian membrane model systems. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 2437–2447. [Google Scholar] [CrossRef]
- Sivertsen, A.; Isaksson, J.; Leiros, H.K.S.; Svenson, J.; Svendsen, J.S.; Brandsdal, B.O. Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct. Biol. 2014, 14, 1–14. [Google Scholar] [CrossRef]
- Van Groenendael, R.; Kox, M.; Van Eijk, L.T.; Pickkers, P. Immunomodulatory and kidney-protective effects of the human chorionic gonadotropin derivate EA-230. Nephron 2018, 140, 148–151. [Google Scholar] [CrossRef]
- PJM Brouwer, C. Structure-Activity Relationship Study of Synthetic Variants Derived from the Highly Potent Human Antimicrobial Peptide hLF(1-11). Cohesive J. Microbiol. Infect. Dis. 2018, 1, 1–19. [Google Scholar] [CrossRef]
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W.; et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef]
- Fulco, P.; Wenzel, R.P. Ramoplanin: A topical lipoglycodepsipeptide antibacterial agent. Expert Rev. Anti. Infect. Ther. 2006, 4, 939–945. [Google Scholar] [CrossRef]
- Bulger, E.M.; Maier, R.V.; Sperry, J.; Joshi, M.; Henry, S.; Moore, F.A.; Moldawer, L.L.; Demetriades, D.; Talving, P.; Schreiber, M.; et al. A novel drug for treatment of necrotizing soft-tissue infections: A randomized clinical trial. JAMA Surg. 2014, 149, 528–536. [Google Scholar] [CrossRef]
- Muchintala, D.; Suresh, V.; Raju, D.; Sashidhar, R.B. Synthesis and characterization of cecropin peptide-based silver nanocomposites: Its antibacterial activity and mode of action. Mater. Sci. Eng. C 2020, 110, 110712. [Google Scholar] [CrossRef]
- Leeds, J.A.; Sachdeva, M.; Mullin, S.; Dzink-Fox, J.; LaMarche, M.J. Mechanism of action of and mechanism of reduced susceptibility to the novel anti-Clostridium difficile compound LFF571. Antimicrob. Agents Chemother. 2012, 56, 4463–4465. [Google Scholar] [CrossRef]
- Peyrusson, F.; Butler, D.; Tulkens, P.M.; Van Bambeke, F. Cellular pharmacokinetics and intracellular activity of the novel peptide deformylase inhibitor GSK1322322 against Staphylococcus aureus laboratory and clinical strains with various resistance phenotypes: Studies with human THP-1 monocytes and J774 murine. Antimicrob. Agents Chemother. 2015, 59, 5747–5760. [Google Scholar] [CrossRef]
- Mensa, B.; Howell, G.L.; Scott, R.; DeGrado, W.F. Comparative mechanistic studies of brilacidin, daptomycin, and the antimicrobial peptide LL16. Antimicrob. Agents Chemother. 2014, 58, 5136–5145. [Google Scholar] [CrossRef]
- Ooi, N.; Miller, K.; Hobbs, J.; Rhys-Williams, W.; Love, W.; Chopra, I. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J. Antimicrob. Chemother. 2009, 64, 735–740. [Google Scholar] [CrossRef]
- Itoh, H.; Tokumoto, K.; Kaji, T.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Total Synthesis and Biological Mode of Action of WAP-8294A2: A Menaquinone-Targeting Antibiotic. J. Org. Chem. 2018, 83, 6924–6935. [Google Scholar] [CrossRef]
- Miyake, O.; Ochiai, A.; Hashimoto, W.; Murata, K. Origin and Diversity of Alginate Lyases of Families PL-5 and -7 in Sphingomonas sp. Strain A1. J. Bacteriol. 2004, 186, 2891–2896. [Google Scholar] [CrossRef]
- Yu, H.B.; Kielczewska, A.; Rozek, A.; Takenaka, S.; Li, Y.; Thorson, L.; Hancock, R.E.W.; Guarna, M.M.; North, J.R.; Foster, L.J.; et al. Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J. Biol. Chem. 2009, 284, 36007–36011. [Google Scholar] [CrossRef]
- Kruszewska, D.; Sahl, H.G.; Bierbaum, G.; Pag, U.; Hynes, S.O.; Ljungh, Å. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J. Antimicrob. Chemother. 2004, 54, 648–653. [Google Scholar] [CrossRef]
- Ekkelenkamp, M.B.; Hanssen, M.; Hsu, S.T.D.; De Jong, A.; Milatovic, D.; Verhoef, J.; Van Nuland, N.A.J. Isolation and structural characterization of epilancin 15X, a novel lantibiotic from a clinical strain of Staphylococcus epidermidis. FEBS Lett. 2005, 579, 1917–1922. [Google Scholar] [CrossRef]
- Mota-Meira, M.; Morency, H.; Lavoie, M.C. In vivo activity of mutacin B-Ny266. J. Antimicrob. Chemother. 2005, 56, 869–871. [Google Scholar] [CrossRef]
- Halliwell, S.; Warn, P.; Sattar, A.; Derrick, J.P.; Upton, M. A single dose of epidermicin NI01 is sufficient to eradicate MRSA from the nares of cotton rats. J. Antimicrob. Chemother. 2017, 72, 778–781. [Google Scholar] [CrossRef]
- Kokai-Kun, J.F.; Walsh, S.M.; Chanturiya, T.; Mond, J.J. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 2003, 47, 1589–1597. [Google Scholar] [CrossRef]
- Kers, J.A.; Sharp, R.E.; Defusco, A.W.; Park, J.H.; Xu, J.; Pulse, M.E.; Weiss, W.J.; Handfield, M. Mutacin 1140 lantibiotic variants are efficacious against Clostridium difficile infection. Front. Microbiol. 2018, 9, 415. [Google Scholar] [CrossRef]
- Kers, J.A.; DeFusco, A.W.; Park, J.H.; Xu, J.; Pulse, M.E.; Weiss, W.J.; Handfield, M. OG716: Designing a fit-for-purpose lantibiotic for the treatment of Clostridium difficile infections. PLoS ONE 2018, 13, e0197467. [Google Scholar] [CrossRef]
- Pulse, M.E.; Weiss, W.J.; Kers, J.A.; DeFusco, A.W.; Park, J.H.; Handfield, M. Pharmacological, toxicological, and dose range assessment of OG716, a novel lantibiotic for the treatment of clostridium difficile-associated infection. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Boakes, S.; Ayala, T.; Herman, M.; Appleyard, A.N.; Dawson, M.J.; Cortés, J. Generation of an actagardine A variant library through saturation mutagenesis. Appl. Microbiol. Biotechnol. 2012, 95, 1509–1517. [Google Scholar] [CrossRef]
- Netz, D.J.A.; Pohl, R.; Beck-Sickinger, A.G.; Selmer, T.; Pierik, A.J.; Bastos, M.D.C.D.F.; Sahl, H.G. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J. Mol. Biol. 2002, 319, 745–756. [Google Scholar] [CrossRef]
- Vassiliadis, G.; Destoumieux-Garzón, D.; Lombard, C.; Rebuffat, S.; Peduzzi, J. Isolation and characterization of two members of the siderophore-microcin family, microcins M and H47. Antimicrob. Agents Chemother. 2010, 54, 288–297. [Google Scholar] [CrossRef]
- Gavrish, E.; Sit, C.S.; Cao, S.; Kandror, O.; Spoering, A.; Peoples, A.; Ling, L.; Fetterman, A.; Hughes, D.; Bissell, A.; et al. Lassomycin, a ribosomally synthesized cyclic peptide, kills mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2. Chem. Biol. 2014, 21, 509–518. [Google Scholar] [CrossRef]
- Aguilar-Pérez, C.; Gracia, B.; Rodrigues, L.; Vitoria, A.; Cebrián, R.; Deboosère, N.; Song, O.; Brodin, P.; Maqueda, M.; Aínsaa, J.A. Synergy between circular bacteriocin AS-48 and ethambutol against mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Hanchi, H.; Hammami, R.; Gingras, H.; Kourda, R.; Bergeron, M.G.; Ben Hamida, J.; Ouellette, M.; Fliss, I. Inhibition of MRSA and of Clostridium difficile by durancin 61A: Synergy with bacteriocins and antibiotics. Future Microbiol. 2017, 12, 205–212. [Google Scholar] [CrossRef]
- Balty, C.; Guillot, A.; Fradale, L.; Brewee, C.; Boulay, M.; Kubiak, X.; Benjdia, A.; Berteau, O. Ruminococcin C, an anti-clostridial sactipeptide produced by a prominent member of the human microbiota Ruminococcus gnavus. J. Biol. Chem. 2019, 294, 14512–14525. [Google Scholar] [CrossRef]
- Gebhart, D.; Lok, S.; Clare, S.; Tomas, M.; Stares, M.; Scholl, D.; Donskey, C.J.; Lawley, T.D.; Govoni, G.R. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. MBio 2015, 6. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B.; Umetsu, Y.; Peigneur, S.; Li, P.; Ohki, S.; Tytgat, J. Adaptively evolved human oral actinomyces-sourced defensins show therapeutic potential. EMBO Mol. Med. 2022, 14. [Google Scholar] [CrossRef]
- Shen, B.; Song, J.; Zhao, Y.; Zhang, Y.; Liu, G.; Li, X.; Guo, X.; Li, W.; Cao, Z.; Wu, Y. Triintsin, a human pathogenic fungus-derived defensin with broad-spectrum antimicrobial activity. Peptides 2018, 107, 61–67. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wu, C.; Moreira, R.; Dorantes, D.; Pappas, T.; Sundararajan, A.; Lin, H.; Pamer, E.G.; van der Donk, W.A. Activity of Gut-Derived Nisin-like Lantibiotics against Human Gut Pathogens and Commensals. ACS Chem. Biol. 2024, 19, 357–369. [Google Scholar] [CrossRef]
- Baindara, P.; Chaudhry, V.; Mittal, G.; Liao, L.M.; Matos, C.O.; Khatri, N.; Franco, O.L.; Patil, P.B.; Korpole, S. Characterization of the antimicrobial peptide penisin, a class Ia novel lantibiotic from Paenibacillus sp. strain A3. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef]
- Baindara, P.; Roy, D.; Mandal, S.M. CycP: A Novel Self-Assembled Vesicle-Forming Cyclic Antimicrobial Peptide to Control Drug-Resistant S. aureus. Bioengineering 2024, 11, 855. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B.; Harvey, P.J.; Craik, D.J. Dermatophytic defensin with antiinfective potential. Proc. Natl. Acad. Sci. USA 2012, 109, 8495–8500. [Google Scholar] [CrossRef]
- Dobson, A.; O’Connor, P.M.; Cotter, P.D.; Ross, R.P.; Hill, C. Impact of the broad-spectrum antimicrobial peptide, lacticin 3147, on Streptococcus mutans growing in a biofilm and in human saliva. J. Appl. Microbiol. 2011, 111, 1515–1523. [Google Scholar] [CrossRef]
- Bolocan, A.S.; Pennone, V.; O’Connor, P.M.; Coffey, A.; Nicolau, A.I.; McAuliffe, O.; Jordan, K. Inhibition of Listeria monocytogenes biofilms by bacteriocin-producing bacteria isolated from mushroom substrate. J. Appl. Microbiol. 2017, 122, 279–293. [Google Scholar] [CrossRef]
- Saising, J.; Dube, L.; Ziebandt, A.K.; Voravuthikunchai, S.P.; Nega, M.; Götz, F. Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2012, 56, 5804–5810. [Google Scholar] [CrossRef]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021, 22. [Google Scholar] [CrossRef]
- Zhu, Y.; Johnson, T.J.; Myers, A.A.; Kanost, M.R. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem. Mol. Biol. 2003, 33, 541–559. [Google Scholar] [CrossRef]
- Jayamani, E.; Rajamuthiah, R.; Larkins-Ford, J.; Fuchs, B.B.; Conery, A.L.; Vilcinskas, A.; Ausubel, F.M.; Mylonakisa, E. Insect-derived cecropins display activity against Acinetobacter baumannii in a whole-animal high-throughput Caenorhabditis elegans model. Antimicrob. Agents Chemother. 2015, 59, 1728–1737. [Google Scholar] [CrossRef]
- Qi, J.; Gao, R.; Liu, C.; Shan, B.; Gao, F.; He, J.; Yuan, M.; Xie, H.; Jin, S.; Ma, Y. Potential role of the antimicrobial peptide tachyplesin III against multidrug-resistant P. aeruginosa and A. baumannii coinfection in an animal model. Infect. Drug Resist. 2019, 12, 2865–2874. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Aleshina, G.M.; Balandin, S.V.; Krasnosdembskaya, A.D.; Markelov, M.L.; Frolova, E.I.; Leonova, Y.F.; Tagaev, A.A.; Krasnodembsky, E.G.; Kokryakov, V.N. Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett. 2004, 577, 209–214. [Google Scholar] [CrossRef]
- Kim, M.K.; Kang, N.; Ko, S.J.; Park, J.; Park, E.; Shin, D.W.; Kim, S.H.; Lee, S.A.; Lee, J.I.; Lee, S.H.; et al. Antibacterial and antibiofilm activity and mode of action of magainin 2 against drug-resistant acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 3041. [Google Scholar] [CrossRef]
- Aleinein, R.A.; Hamoud, R.; Schäfer, H.; Wink, M. Molecular cloning and expression of ranalexin, a bioactive antimicrobial peptide from Rana catesbeiana in Escherichia coli and assessments of its biological activities. Appl. Microbiol. Biotechnol. 2013, 97, 3535–3543. [Google Scholar] [CrossRef]
- Subasinghage, A.P.; Conlon, J.M.; Hewage, C.M. Development of potent anti-infective agents from Silurana tropicalis: Conformational analysis of the amphipathic, alpha-helical antimicrobial peptide XT-7 and its non-haemolytic analogue [G4K]XT-7. Biochim. Biophys. Acta-Proteins Proteomics 2010, 1804, 1020–1028. [Google Scholar] [CrossRef]
- Conlon, J.M.; Al-Ghaferi, N.; Abraham, B.; Sonnevend, A.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Iwamuro, S. Antimicrobial peptides from the skin of the Tsushima brown frog Rana tsushimensis. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2006, 143, 42–49. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Zhang, Y.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Burrows, J.F.; Chen, T. Identification of novel Amurin-2 variants from the skin secretion of Rana amurensis, and the design of cationicity-enhanced analogues. Biochem. Biophys. Res. Commun. 2018, 497, 943–949. [Google Scholar] [CrossRef]
- Mishra, B.; Wang, X.; Lushnikova, T.; Zhang, Y.; Golla, R.M.; Narayana, J.L.; Wang, C.; McGuire, T.R.; Wang, G. Antibacterial, antifungal, anticancer activities and structural bioinformatics analysis of six naturally occurring temporins. Peptides 2018, 106, 9–20. [Google Scholar] [CrossRef]
- Conlon, J.M.; Abraham, B.; Sonnevend, A.; Jouenne, T.; Cosette, P.; Leprince, J.; Vaudry, H.; Bevier, C.R. Purification and characterization of antimicrobial peptides from the skin secretions of the carpenter frog Rana virgatipes (Ranidae, Aquarana). Regul. Pept. 2005, 131, 38–45. [Google Scholar] [CrossRef]
- Abbassi, F.; Lequin, O.; Piesse, C.; Goasdoué, N.; Foulon, T.; Nicolas, P.; Ladram, A. Temporin-SHf, a new type of Phe-rich and hydrophobic ultrashort antimicrobial peptide. J. Biol. Chem. 2010, 285, 16880–16892. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Prajeep, M.; Sonnevend, A.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; King, J.D. Host-defense peptides in skin secretions of the tetraploid frog Silurana epitropicalis with potent activity against methicillin-resistant Staphylococcus aureus (MRSA). Peptides 2012, 37, 113–119. [Google Scholar] [CrossRef]
- Yuan, Y.; Zai, Y.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Shaw, C.; Chen, T. A novel membrane-disruptive antimicrobial peptide from frog skin secretion against cystic fibrosis isolates and evaluation of anti-MRSA effect using Galleria mellonella model. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 849–856. [Google Scholar] [CrossRef]
- Portelinha, J.; Angeles-Boza, A.M. The Antimicrobial Peptide Gad-1 Clears Pseudomonas aeruginosa Biofilms under Cystic Fibrosis Conditions. ChemBioChem 2021, 22, 1646–1655. [Google Scholar] [CrossRef]
- Menousek, J.; Mishra, B.; Hanke, M.L.; Heim, C.E.; Kielian, T.; Wang, G. Database screening and in vivo efficacy of antimicrobial peptides against methicillin-resistant Staphylococcus aureus USA300. Int. J. Antimicrob. Agents 2012, 39, 402–406. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Dai, C.; Zhao, R.; Li, S.R.; Wu, Y.; Cao, Z.; Li, W. Imcroporin, a new cationic antimicrobial peptide from the venom of the scorpion Isometrus maculates. Antimicrob. Agents Chemother. 2009, 53, 3472–3477. [Google Scholar] [CrossRef]
- De Melo, E.T.; Estrela, A.B.; Santos, E.C.G.; Machado, P.R.L.; Farias, K.J.S.; Torres, T.M.; Carvalho, E.; Lima, J.P.M.S.; Silva-Júnior, A.A.; Barbosa, E.G.; et al. Structural characterization of a novel peptide with antimicrobial activity from the venom gland of the scorpion Tityus stigmurus: Stigmurin. Peptides 2015, 68, 3–10. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.; Xi, X.; Bininda-Emonds, O.R.P.; Shaw, C.; Chen, T.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018, 14, 599–607. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, Y.; Bommineni, Y.R.; Fernando, S.C.; Prakash, O.; Gilliland, S.E.; Zhang, G. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 2006, 281, 2858–2867. [Google Scholar] [CrossRef]
- van Dijk, A.; Molhoek, E.M.; Veldhuizen, E.J.A.; van Bokhoven, J.L.M.T.; Wagendorp, E.; Bikker, F.; Haagsman, H.P. Identification of chicken cathelicidin-2 core elements involved in antibacterial and immunomodulatory activities. Mol. Immunol. 2009, 46, 2465–2473. [Google Scholar] [CrossRef]
- Hee Lee, I.N.; Cho, Y.; Lehrer, R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997, 65, 2898–2903. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Gennaro, R.; Bagella, L.; Merluzzi, L.; Risso, A.; Zanettit, M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 1996, 271, 28375–28381. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Shimomoura, Y.; Yoshida, M.; Zheng, H.; Zhong, J.; Wright, S.C. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob. Agents Chemother. 1993, 37, 2534–2539. [Google Scholar] [CrossRef]
- Patil, A.A.; Ouellette, A.J.; Lu, W.; Zhang, G. Rattusin, an intestinal α-defensin-related peptide in rats with a unique cysteine spacing pattern and salt-insensitive antibacterial activities. Antimicrob. Agents Chemother. 2013, 57, 1823–1831. [Google Scholar] [CrossRef]
- Ouellette, A.J.; Hsieh, M.M.; Nosek, M.T.; Cano-Gauci, D.F.; Huttner, K.M.; Buick, R.N.; Selsted, M.E. Mouse Paneth cell defensins: Primary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 1994, 62, 5040–5047. [Google Scholar] [CrossRef]
- Blower, R.J.; Popov, S.G.; van Hoek, M.L. Cathelicidin peptide rescues G. mellonella infected with B. anthracis. Virulence 2018, 9, 287–293. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yuan, J.; Ösapay, G.; Ösapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science (80-. ). 1999, 286, 498–502. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Sigurdardóttir, T.; Schmidtchen, A.; Bodelsson, M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2005, 49, 2845–2850. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Greganti, G.; Quarta, M.; Scalise, G. In vitro activities of membrane-active peptides against gram-positive and gram-negative aerobic bacteria. Antimicrob. Agents Chemother. 1998, 42, 3320–3324. [Google Scholar] [CrossRef]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with L-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef]
- Alaiwa, M.H.A.; Reznikov, L.R.; Gansemer, N.D.; Sheets, K.A.; Horswill, A.R.; Stoltz, D.A.; Zabner, J.; Welsh, M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA 2014, 111, 18703–18708. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Elsawy, M.; Mattrasingh, D.; Klein, D.; Strehmel, J.; Beaulieu, C.; Wong, A.; Overhage, J. Synergy between Human Peptide LL-37 and Polymyxin B against Planktonic and Biofilm Cells of Escherichia coli and Pseudomonas aeruginosa. Antibiotics 2023, 12, 389. [Google Scholar] [CrossRef]
- Lin, L.; Nonejuie, P.; Munguia, J.; Hollands, A.; Olson, J.; Dam, Q.; Kumaraswamy, M.; Rivera, H.; Corriden, R.; Rohde, M.; et al. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity Against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine 2015, 2, 690–698. [Google Scholar] [CrossRef]
- Desbois, A.P.; Gemmell, C.G.; Coote, P.J. In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 2010, 35, 559–565. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, H.; Chen, R.; Liu, Q.; Jia, K.; Hu, D.L.; Chen, H.; Ye, C.; Peng, L.; Fang, R. Synergistic Antimicrobial Effect of Antimicrobial Peptides CATH-1, CATH-3, and PMAP-36 With Erythromycin Against Bacterial Pathogens. Front. Microbiol. 2022, 13, 953720. [Google Scholar] [CrossRef]
- Draper, L.A.; Cotter, P.D.; Hill, C.; Ross, R.P. The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol. 2013, 13, 212. [Google Scholar] [CrossRef]
- Mathur, H.; O’Connor, P.M.; Hill, C.; Cotter, P.D.; Ross, R.P. Analysis of anti-clostridium difficile activity of thuricin CD, vancomycin, metronidazole, ramoplanin, and actagardine, both singly and in paired combinations. Antimicrob. Agents Chemother. 2013, 57, 2882–2886. [Google Scholar] [CrossRef]
- Cavera, V.L.; Volski, A.; Chikindas, M.L. The Natural Antimicrobial Subtilosin A Synergizes with Lauramide Arginine Ethyl Ester (LAE), ε-Poly-l-lysine (Polylysine), Clindamycin Phosphate and Metronidazole, Against the Vaginal Pathogen Gardnerella vaginalis. Probiotics Antimicrob. Proteins 2015, 7, 164–171. [Google Scholar] [CrossRef]
- Lobos, O.; Padilla, A.; Padilla, C. In vitro antimicrobial effect of bacteriocin PsVP-10 in combination with chlorhexidine and triclosan against Streptococcus mutans and Streptococcus sobrinus strains. Arch. Oral Biol. 2009, 54, 230–234. [Google Scholar] [CrossRef]
- Herrmann, G.; Yang, L.; Wu, H.; Song, Z.; Wang, H.; Høiby, N.; Ulrich, M.; Molin, S.; Riethmüller, J.; Döring, G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010, 202, 1585–1592. [Google Scholar] [CrossRef]
- Rishi, P.; Preet, S.; Bharrhan, S.; Verma, I. In vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against Salmonella. Antimicrob. Agents Chemother. 2011, 55, 4176–4182. [Google Scholar] [CrossRef]
- Jahangiri, A.; Neshani, A.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Sedighian, H. Synergistic effect of two antimicrobial peptides, Nisin and P10 with conventional antibiotics against extensively drug-resistant Acinetobacter baumannii and colistin-resistant Pseudomonas aeruginosa isolates. Microb. Pathog. 2021, 150, 104700. [Google Scholar] [CrossRef]
- Nuding, S.; Frasch, T.; Schaller, M.; Stange, E.F.; Zabel, L.T. Synergistic effects of antimicrobial peptides and antibiotics against clostridium difficile. Antimicrob. Agents Chemother. 2014, 58, 5719–5725. [Google Scholar] [CrossRef]
| AMPs | Source/Type | Clinical Use | Mode of Action | Reference |
|---|---|---|---|---|
| Vancomycin | Streptococcus orientalis)/Tricyclic glycopeptide | Complicated infections caused by MRSA, C. difficile, and Gram-positive bacteria | Inhibition of cell wall biosynthesis | [88] |
| Bacitracin | B. licheniformis) | Pneumonia and empyema in infants | Cell wall interference and peptidoglycan synthesis interference | [72] |
| Daptomycin | Streptomyces roseosporus) | Skin bacterial infections | Membrane-lytic peptide, inhibition of DNA, RNA, and protein synthesis | [89] |
| Polymyxin B | Bacteria (Paenibacillus polymyxa) | Bacterial infections caused by Gram-negative bacteria | Targets membrane phospholipids (lipopolysaccharides and lipoproteins) | [90] |
| Gramicidin | Bacteria (B. brevis) | Dermatological and ophthalmological infections | Pore-forming peptide | [71] |
| Colistin | Bacteria (B. polymyxa) | Gram-negative bacterial infections, Pneumonia | Membrane-lytic peptide | [73] |
| AMPs | Type | Target | Clinical Trial ID | Phase | References |
|---|---|---|---|---|---|
| Nisin | Lantibiotic | Gram-positive bacteria | NCT02928042 NCT02467972 | [91] | |
| Murepavadin (POL7080) | Derivative of protegrin | P. aeruginosa, K. pneumoniae | EUCTR2017-003933-27-EE | II | [92] |
| Iseganan (IB-367) | Derivative of protegrin | Pneumonia/oral mucositis | NCT00118781 NCT00022373 |
III | [93] |
| Surotomycin (CB-315) | Cyclic lipopeptide | C. difficile | NCT01597505 | III | [94] |
| Omiganan (MBI-226) | Derivative of indolicidin | Antisepsis/ catheter infection | NCT00231153 NCT00608959 | III | [95] |
| NVB-302 | Lantibiotic | C. difficile | ISRCTN40071144 | I | [96] |
| OP-145 | Derivative of LL-37 | Chronic middle ear infection | ISRCTN84220089 | II | [97] |
| LTX-109 | Synthetic tripeptide | MRSA, Impetigo | NCT01803035 NCT01158235 | II | [98] |
| EA-230 | Oligopeptide | Sepsis | NCT03145220 | II | [99] |
| hLF1-11 | Human lactoferrin derivative | Bacterial infections | NCT00430469 | II | [100] |
| C16G2 | Synthetic peptide | S. mutans | NCT03004365 | II | [101] |
| Ramoplanin (NTI851) | Glycolipodepsipeptide | C. difficile, VRE | NA | III | [102] |
| p2TA (AB103) | Synthetic peptide | Necrotic tissue infection | NA | III | [103] |
| D2A21 | Synthetic peptide | Burn wound infections | NA | III | [104] |
| LFF571 | Semisynthetic thiopeptide | C. difficile | NCT01232595 | II | [105] |
| GSK132232 (Lanopepde) | Synthetic hydrazide | Bacterial skin infection | NCT01209078 | II | [106] |
| PMX-30063 (Brilacidin) | Defensin mimetic | Acute bacterial skin infection | NCT01211470 NCT02052388 | II | [107] |
| XF-73 (Exeporfinim chloride) | Derivative of porphyrin | Staphylococal infection | NCT03915470 | II | [108] |
| Wap-8294A2 (Lotilibcin) | Naturally produced by Lysobacter species | Gram-positive bacteria | NA | II | [109] |
| PL-5 | Synthetic peptide | Skin infections | NA | I | [110] |
| IDR-1 | Bactenecin | Infection prevention | NA | I | [111] |
| Pexiganan | Analog of magainin isolated from the skin of the African clawed frog | Infections of diabetic foot ulcers, Gram positive and Gram negative bacteria | NCT01590758 | III |
| AMPs | Peptide Sequence | Source | Spectrum of Activity | References |
|---|---|---|---|---|
| Mersacidin | CTFTLPGGGGVCTLTSECIC | Bacillus sp. HIL Y-85 54728 | MRSA | [112] |
| Epilancin 15X | SASIVKTTIKASKKLCRGFTLTCGCHFTGKK | S. epidermidis 15X154 | MRSA, Gram-positive | [113] |
| Mutacin B-Ny266 | FKSWSFCTPGCAKTGSFNSYCC | S. mutans Ny266 | MRSA | [114] |
| Epidermicin NI01 | MAAFMKLIQFLATKGQKYVSLAWKHKGTILKWINAGQSFEWIYKQIKKLWA | S. epidermidis 224 | MRSA, Gram-positive | [115] |
| Actagardine A | SSGWVCTLTIECGTVICAC | A. garbadinensis ATCC 31049 | C. difficile, VRE, MRSA | [120] |
| Aureocin A53 | MSWLNFLKYIAKYGKKAVSAAWKYKGKVLEWLNVGPTLEWVWQKLKKIAGL | Staphylococcus aureus A53 | MRSA, Gram positive | [121] |
| LCI | AIKLVQSPNGNFAASFVLDGTKWIFKSKYYDSSKGYWVGIYEVWDRK | Bacillus subtilis strain A014 | MRSA, Gram positive and Gram-negative | |
| Microcin E492 | GETDPNTQLLNDLGNNMAWGAALGAPGGLGSAALGAAGGALQTVGQGLIDHGPVNVFIPVLIGPSWNGSGSGYNSATSSSG | K. pneumoniae RYC492 | K. pneumonia, and other Gram-negative bacteria | [122] |
| Lassomycin | GLRRLFADQLVGRRNI | Lentzea kentuckyensis | M. tuberculosis | [123] |
| Enterocin AS-48 | MAKEFGIPAAVAGTVINVVEAGGWVTTIVSILTAVGSGGLSLLAAAGRESIKAYLKKEIKKKGKRAVIAW | E. faecalis S-48 | MRSA, M. tuberculosis | [124] |
| Ruminococcin C | WGCVCSGSTAVANSHNAGPAYCVGYCGNNGVVTRNANANVAKTA | R. gnavus E1 | C. difficile and other MDR strains | [126] |
| Actinomycesin | GFGCPWNAYECDRHCVSKGYTGGNCRGKIRQTCHCY | Actinomyces sp. | MRSA, Gram-positive | [128] |
| Triintsin | GFGCPLNERECHSHCQSIGRKFGYCGGTLRLTCICGKE | Trichophyton interdigitale | MRSA, Gram-positive, and Gram-negative | [129] |
| Blauticin | ITSKSLCTPGCVTGILMTCPVQTATCGCQITGK | Blautia producta SCSK | MRSA, Gram-positive | [130] |
| Lan-Df | YKSKSVCTPGCPTGILMTCPLKTATCGCHITGK | Dorea formicigenerans | MRSA, Gram-positive | [130] |
| Penisin | NIGLFTSTCFSSQCFSSKCFTDTCFSSNCFTGRHQCGYTHGSC | Paenibacillus sp. A3 | MRSA, Gram-positive, and Gram-negative | [131] |
| Laterosporulin10 | ACVNQCPDAIDRFIVKDKGCHGVEKKYYKQVYVACMNGQHLYCRTEWGGPCQL | B. laterosporus SKDU10 | M. tuberculosis H37Rv, Gram-positive | [79] |
| CycP | CAWLWAPAWLWAC | Synthetic | Drug-resistant S. aureus | [132] |
| Plectasin | GFGCNGPWDEDDMQCHNHCKSIKGYKGGYCAKGGFVCKCY | Pseudoplectania nigrella | MRSA, Gram-positive | [133] |
| Micasin-1 | GFGCPFNENECHAHCLSIGRKFGFCAGPLRATCTCGKQ | Microsporum canis | MRSA, Gram-positive, and Gram-negative | [134] |
| Lacticin 3147 | CSTNTFSLSDYWGNNGAWCTLTHECMAWCK | Lactococcus lactis DPC3147 | S. mutans (Biofilm) | [135] |
| Subtilomycin | TWATIGKTIVQSVKKCRTFTCGCSLGSCSNCN | Bacillus subtilis MMA7 | L. monocytogenes (Biofilm) | [136] |
| Gallidermin | IASKFLCTPGCAKTGSFNSYCC | Staphylococcus gallinarum (F16/P57) | S. aureus, S. epidermidis (Biofilm) | [137] |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | Apis mellifera | A. baumannii, MRSA, P. aeruginosa, K. pneumonia, and Gram-positive bacteria | [138] |
| MS moricin | GKIPVKAIKQAGKVIGKGLRAINIAGTTHDVVSFFRPKKKKH | Manduca sexta | MRSA, Gram positive and Gram-negative | [139] |
| Cecropin A | RWKVFKKIEKVGRNIRDGVIKAAPAIEVLGQAKAL | Heliothis virescens | A. baumannii, P. aeruginosa, and other Gram-negative, and Gram-positive bacteria | [140] |
| Tachyplesin III | KWCFRVCYRGICYRKCR | Tachypleus gigas | P. aeruginosa, A. baumannii, biofilms, and Gram-positive bacteria | [141] |
| Arenicin-1 | RWCVYAYVRVRGVLVRYRRCW | Arenicola marina | MRSA, Gram-positive, and Gram-negative | [142] |
| Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | Xenopus laevis | K. pneumoniae, P. aeruginosa, A. baumannii, Anti-biofilm | [143] |
| Ranalexin | FLGGLIKIVPAMICAVTKKC | Rana catesbeiana (Bullfrog) | MRSA, Gram-positive, and Gram-negative | [144] |
| CPF-ST3 | GLLGPLLKIAAKVGSNLL | Frog | MRSA, Gram-positive, and Gram-negative | [145] |
| Brevinin-1TSa | FLGSIVGALASALPSLISKIRN | Frog | MRSA, Gram-positive, and Gram-negative | [146] |
| Brevinin-1DYa | FLSLALAALPKFLCLVFKKC | Frog | MRSA, Gram-positive, and Gram-negative | [147] |
| Brevinin-1DYb | FLSLALAALPKLFCLIFKKC | Frog | MRSA, Gram-positive, and Gram-negative | [147] |
| Temporin-1Oc | FLPLLASLFSRLF | Frog | MRSA, Gram-positive, | [148] |
| Temporin-1Ga | SILPTIVSFLSKVF | Frog | MRSA, Gram-positive, | [148] |
| Temporin-1Vb | FLSIIAKVLGSLF | Frog | MRSA, Gram-positive | [149] |
| Temporin-SHd | FLPAALAGIGGILGKLF | Frog | MRSA, Gram-positive, and Gram-negative | [150] |
| CPF-SE1 | GFLGPLLKLGLKGVAKVIPHLIPSRQQ | Frog | MRSA, Gram-positive, and Gram-negative | [151] |
| CPF-SE2 | GFLGPLLKLGLKGAAKLLPQLLPSRQQ | Frog | MRSA, Gram-positive, and Gram-negative | [151] |
| Japonicin-2LF | FIVPSIFLLKKAFCIALKKC | Frog | MRSA, Gram-positive, and Gram-negative | [152] |
| Gaduscidin-1 | FIHHIIGWISHGVRAIHRAIH | Gadus morhua (Atlantic cod) | P. aeruginosa biofilms, Gram positive and Gram-negative | [153] |
| Piscidin 1 | FFHHIFRGIVHVGKTIHRLVTG | Fish | MRSA, Gram-positive, and Gram-negative | [154] |
| Pleurocidin | GWGSFFKKAAHVGKHVGKAALTHYL | Pleuronectes americanus | MRSA, Gram-positive, and Gram-negative | [155] |
| Imcroporin | FFSLLPSLIGGLVSAIK | Scorpions | MRSA, Gram-positive, | [156] |
| Stigmurin | FFSLIPSLVGGLISAFK | Scorpions | MRSA, Gram-positive, | [157] |
| MP-C | LNLKALLAVAKKIL | Insects | MRSA, Gram-positive, and Gram-negative | [158] |
| Chicken CATH-1 | RVKRVWPLVIRTVIAGYNLYRAIKKK | Gallus galllus | MRSA, Gram-positive, and Gram-negative | [159] |
| Chicken CATH-2 | RFGRFLRKIRRFRPKVTITIQGSARFG | Chicken | MRSA, Gram-positive, and Gram-negative | [160] |
| Clavanin A | VFQFLGKIIHHVGNFVHGFSHVF | Styela clava | MRSA, Gram-positive, | [161] |
| SMAP-29 | RGLRRLGRKIAHGVKKYGPTVLRIIRIAG | Sheep | MRSA, Gram-positive, and Gram-negative | [162] |
| BMAP-27 | GRFKRFRKKFKKLFKKLSPVIPLLHLG | Cattles | MRSA, Gram-positive, and Gram-negative | [163] |
| BMAP-28 | GGLRSLGRKILRAWKKYGPIIVPIIRIG | Cattles | MRSA, Gram-positive, and Gram-negative | [163] |
| Lactoferricin B | FKCRRWQWRMKKLGAPSITCVRRAF | Bos taurus | MRSA, Gram-positive, and Gram-negative | [164] |
| CAP18 | GLRKRLRKFRNKIKEKLKKIGQKIQGFVPKLAPRTDY | Oryctolagus cuniculus | MRSA, Gram-positive, and Gram-negative | [165] |
| Rattusin | LRVRRTLQCSCRRVCRNTCSCIRLSRSTYAS | Rattus norvegicus | MRSA, Gram-positive, and Gram-negative | [166] |
| Cryptdin-4 | LRGLLCYCRKGHCKRGERVRGTCGIRFLYCCPRR | Mus musculus | MRSA, Gram-positive, and Gram-negative | [167] |
| Protegrin 1 | RGGRLCYCRRRFCVCVGR | Sus scrofa | MRSA, A. baumannii, P. aeruginosa, Anti-biofilm, Gram positive | [168] |
| RTD-1 | GFCRCLCRRGVCRCICTR | Rhesus Macaque (Macaca mulatta) | MRSA, Gram-positive, and Gram-negative | [169] |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Human and other mammals | MRSA, P. aeruginosa, A. baumannii, Gram-positive, and Gram-negative | [170] |
| Indolicidin | ILPWKWPWWPWRR | Human and other mammals | MRSA, P. aeruginosa, Gram-positive, and Gram-negative | [171] |
| HNP-1 | ACYCRIPACIAGERRYGTCIYQGRLWAFCC | Homo sapiens | MRSA, C. difficile, Gram-positive, and Gram-negative | [172] |
| hBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | Homo sapiens | MRSA, Anti-biofilm, Gram-positive, and Gram-negative | [173] |
| AMPs | Antibiotics | Target pathogen | References |
|---|---|---|---|
| Nisin | Ramoplanin, Polymyxin E, Clarithromycin, Amoxicillin, Penicillin, Streptomycin, Ceftiofur, Tetracycline | MRSA, P. aeruginosa, S. suis | [81,82,83] |
| Nisin Z | Ampicillin, Chloramphenicol, Kanamycin, Lincomycin, Penicillin G, Rifampicin, Streptomycin, Tetracycline, Vancomycin | Multi-drug resistant P. fluorescens LRC-R73 | [80] |
| LL-37 | Polymyxin B, Azithromycin | Multi-drug resistant E. coli, P. Aeruginosa, A. baumannii, K. pneumoniae |
[174,175] |
| Ranalexin | Endopeptidase lysostaphin | MRSA | [176] |
| CATH-1, CATH-3, PMAP-36 | Erythromycin | Pathogenic strain of S. aureus, S. enteritidis, and E. coli | [177] |
| Lacticin 3147 | Polymyxin B | S. aureus 5247 | [178] |
| Actagardine | Ramoplanin, Metronidazole, Vancomycin | C. difficile | [179] |
| Thuricin CD | Ramoplanin, Vancomycin | C. difficile | [179] |
| Subtilosin A | Clindamycin phosphate, Metronidazole, Lauramide alginate, Ester poly-lysine | Vaginal pathogen G. vaginalis | [180] |
| PsVP-10 | Chlorhexidine | S. mutans, S. sobrinus | [181] |
| Colistin | Tobramycin, Azithromycin | P. aeruginosa, A. baumannii, K. pneumoniae | [175,182] |
| Cryptdin 2 | Ampicillin | S. typhimurium | [183] |
| Arenicin-1 | Erythromycin, Ampicillin, chloramphenicol | S. dermis, S. aureus, E. coli, and P. aeruginosa | [86] |
| Laterosporulin10 | Rifampicin | M. tuberculosis H37Rv | [79] |
| Gaduscidin-1 | Kanamycin, Ciprofloxacin | P. aeruginosa biofilms | [153] |
| Lactoferricin | Ciprofloxacin, Ceftazidim | P. aeruginosa | [184] |
| Human defensin 5 (HD5) | Meropenem | C. difficile | [185] |
| Human neutrophil peptide-1 (HNP1) | Rifampicin | M. tuberculosis H37Rv | [78] |
| Human β-defensin 3 (HBD3) |
Meropenem, Moxifloxacin, Piperacillin-Tazobactam, Tigecycline | C. difficile | [185] |
| Conventional antibiotics | AMPs |
|---|---|
| Not amenable to bioengineering | Highly amenable to bioengineering |
| Non-ribosomal synthesis | Ribosomal synthesis |
| Toxic | A good safety and tolerability profile |
| No immunomodulatory properties | Excellent immunomodulatory properties |
| High serum/ plasma stability (depends on the drug) | Low serum/ plasma stability |
| Usually high (depends on the drug) | Low half-life |
| Not degradable/persistent | Completely metabolized |
| Highly stable | Rapid clearance |
| Less diverse | Highly diverse (endless opportunities, especially in the case of bacterial AMPs) |
| Resistant to biofilms | Strong biofilm activities |
| Easy purification/ high yield | Complex purification/ low yield |
| Narrow spectrum/ Specific target | Broad spectrum/ multiple mechanisms of action |
| Highly prone to resistant development | Less prone to resistant development |
| High solubility | Low solubility |
| Good bioavailability | Depends on size |
| Not affected by digestive enzymes | Prone to digestive enzymes |
| High absorption | Poor absorption |
| Many side effects | Not identified |
| Narrow range for pH and temperature stability | Highly stable at a broad range of pH and temperature |
| Cost-effective | High cost of production |
| Many administration routs | Limited administration routs due to protein degradation issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





