Submitted:
03 October 2024
Posted:
03 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
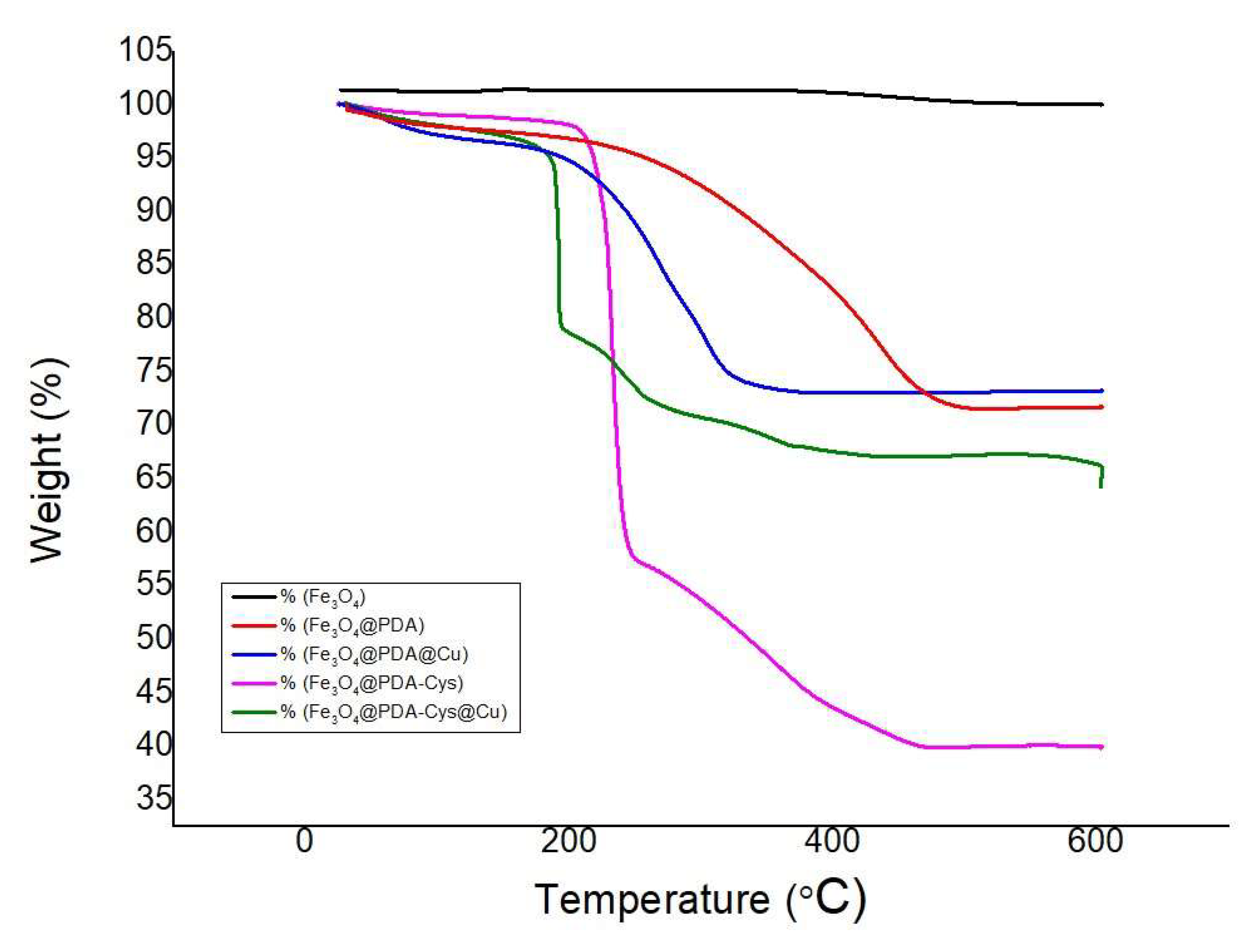
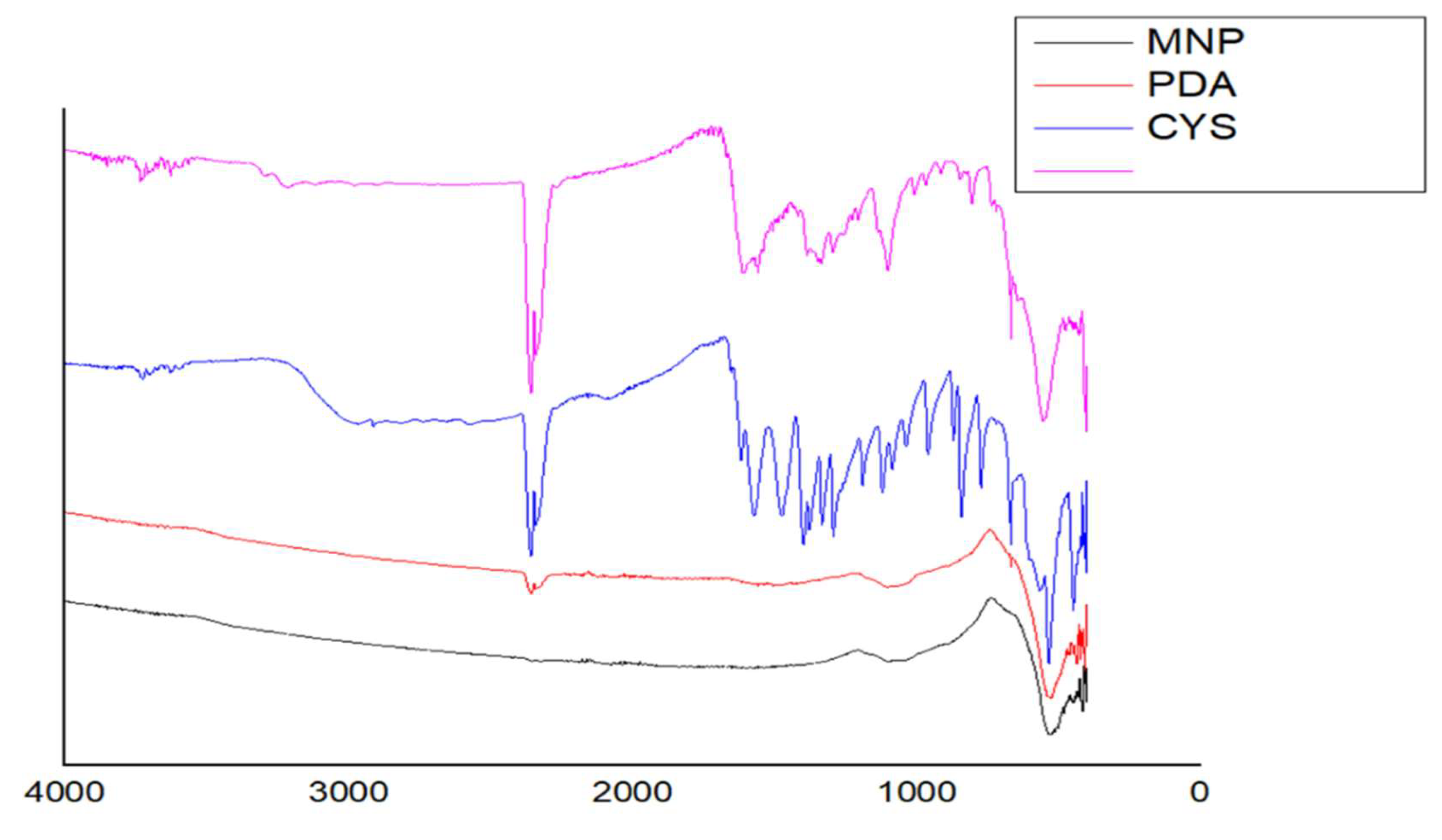
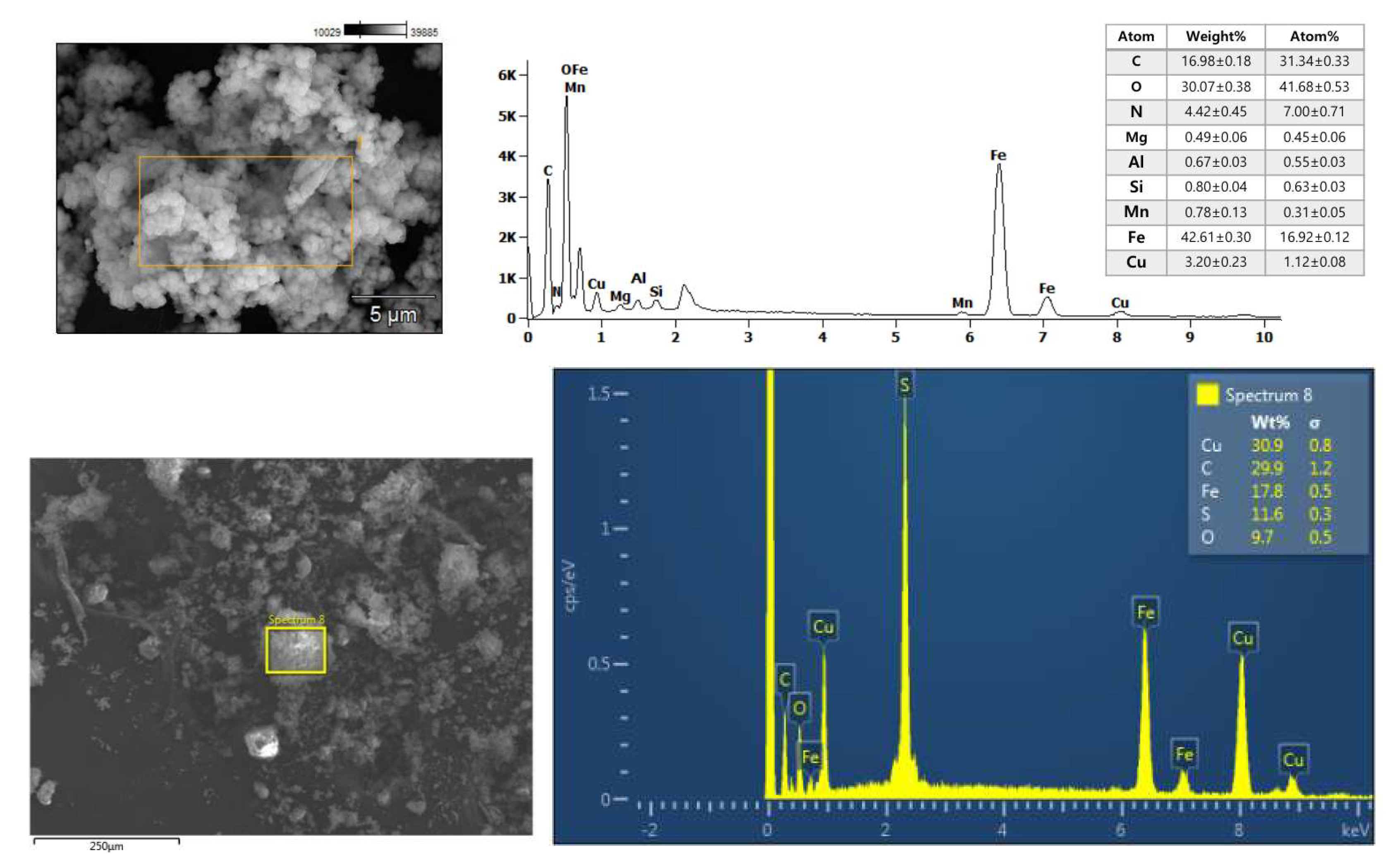
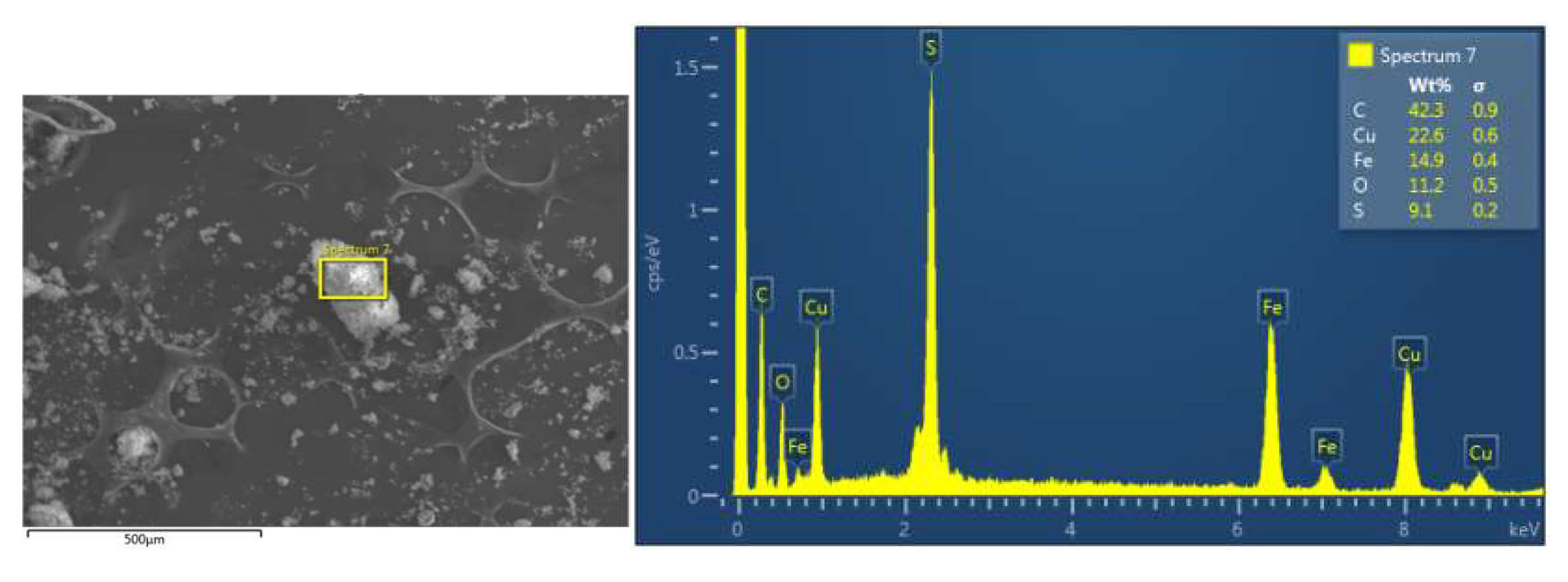
2.1. Characterization
2.2. Application to Organic Reactions
 | ||||||||
| Recycling | 0 | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| Yielda | 98% (5 h) | 97% (7 h) | 72% (7 h) | 51% (7 h) | - | - | - | - |
| Yieldb | 99% | 99% | 96% | 96% | 95% | 95% | 95% | 90% |
3. Conclusion
4. Materials and Methods
4.1. Procedure for Fe3O4@PDA
4.2. Procedure for Fe3O4@PDA-Cys
4.3. Procedure for Fe3O4@PDA-Cys@Cu
4.4. General Procedure for Sonogashira Coupling
4.5. General Procedure for Click Reaction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: New York, USA, 1997. [Google Scholar]
- Akhtar, R.; Zahoor, A.F.; Parveen, B.; Suleman, M. Development of environmental friendly synthetic strategies for Sonogashira cross coupling reaction: An update. Synth Commun. 2019, 4, 167–192. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Cassar, L. Synthesis of aryl- and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes. J. Organomet. Chem. 1975, 93, 253–257. [Google Scholar] [CrossRef]
- Dieck, H.A.; Heck, R.F. Palladium catalyzed synthesis of aryl, heterocyclic and vinylic acetylene derivatives. J. Organomet. Chem. 1975, 93, 259–263. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Heuze, K.; Mery, D.; Gauss, D.; Astruc, D. Copper-free, recoverable dendritic Pd catalysts for the Sonogashira reaction. Chem. Commun. 2003, 2274–2275. [Google Scholar] [CrossRef]
- Thathagar, M.B.; Beckers, J.; Rothenberg, G. Palladium-free and ligand-free Sonogashira cross-coupling. Green Chem. 2004, 6, 215–218. [Google Scholar] [CrossRef]
- Ghabdian, M.; Nasseri, M.A.; Allahresani, A.; Motavallizadehkakhky, A. Heterogenized Cu (II) salen complex grafted on graphene oxide nanosheets as a precursing catalyst for the Pd-free Sonogashira coupling. Appl. Organomet. Chem. 2018, 32, e4545. [Google Scholar] [CrossRef]
- Ma, D.; Liu, F. CuI-catalyzed coupling reaction of aryl halides with terminal alkynes in the absence of palladium and phosphine. Chem. Commun. 2004, 1934–1935. [Google Scholar] [CrossRef]
- Lina, C.X.; Zhua, J.F.; Lia, Q.S.; Aoa, L.H.; Jina, Y.J.; Xua, F.B.; Hua, F.Z.; Yuana, Y.F. 2,5-Bis(2-(diphenylphosphino)phenyl)-1,3,4-oxadiazole ligands and their Cu(I) complexes for Sonogashira coupling reaction. Appl. Organomet. Chem. 2014, 28, 298–303. [Google Scholar] [CrossRef]
- Okuro, K.; Furuune, M.; Enna, M.; Miura, M.; Nomura, M. Synthesis of aryl- and vinylacetylene derivatives by copper-catalyzed reaction of aryl and vinyl iodides with terminal alkynes. J. Org. Chem. 1993, 58, 4716–4721. [Google Scholar] [CrossRef]
- Saejueng, P.; Bates, C.G.; Venkataraman, D. Copper(I)-Catalyzed Coupling of Terminal Acetylenes with Aryl or Vinyl Halides. Synthesis, 1712. [Google Scholar]
- Gujadhur, R.K.; Bates, C.G.; Venkataraman, D. Formation of Aryl−Nitrogen, Aryl−Oxygen, and Aryl−Carbon Bonds Using Well-Defined Copper(I)-Based Catalysts. Org. Lett. 2001, 3, 4315–4317. [Google Scholar] [CrossRef]
- Li, J.H.; Li, J.L.; Wang, D.P.; Pi, S.F.; Xie, Y.X.; Zhang, M.B.; Hu, X.C. CuI-Catalyzed Suzuki−Miyaura and Sonogashira Cross-Coupling Reactions Using DABCO as Ligand. J. Org. Chem. 2007, 72, 2053–2057. [Google Scholar] [CrossRef]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent advances and perspectives in copper-catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Petrignet, J.; Ngi, S.I.; Abarbri, M.; Thibonnet, J. Short and convenient synthesis of two natural phthalides by a copper(I) catalysed Sonogashira/oxacyclisation copper(I) process. Tetrahedron Lett. 2014, 55, 982–984. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Mohammadsaleh, F. Sonogashira reactions catalyzed by a new and efficient copper(I) catalyst incorporating N-benzyl DABCO chloride. Tetrahedron Lett. 2014, 55, 3459–3462. [Google Scholar] [CrossRef]
- Kodicherla, B.; Perumgani, C.P.; Mandapati, M.R. A reusable polystyrene-supported copper(II) catalytic system for N-arylation of indoles and Sonogashira coupling reactions in water. Appl. Catal. A: General, 2014, 483, 110–115. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, B.; Wu, Y.; Cai, M. MCM-41-immobilized Schiff base-pyridine bidentate copper(I) complex as a highly efficient and recyclable catalyst for the Sonogashira reaction. J. Organomet. Chem. 2015, 797, 21–28. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C–C, C–N, and C–O Ullmann-Type Coupling Reactions. Angew. Chem. Int. Ed. 2009, 49, 6954–6971. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Sun, H.; Zhu, Z.; Zhang, G.; Zhang, W.; Gao, Z. Triazine-Triazole Conjugates as Potent Ligands for Cu–Catalyzed Sonogashira Reaction. ChemistrySelect, 2016, 3, 391–395. [Google Scholar] [CrossRef]
- Carril, M.; Correa, A.; Bolm, C. Iron-Catalyzed Sonogashira Reactions. Angew. Chem. Int. Ed. 2008, 47, 4862–4865. [Google Scholar] [CrossRef]
- Sindhu, K.S.; Thankachan, A.P.; Thomas, A.M.; Anilkumar, G. Iron-Catalyzed Sonogashira Type Cross-Coupling Reaction of Aryl Iodides with Terminal Alkynes in Water under Aerobic Conditions. ChemistrySelect, 2016, 3, 556–559. [Google Scholar] [CrossRef]
- Volla, C.M.R.; Vogel, P. Iron/copper-catalyzed C–C cross-coupling of aryl iodides with terminal alkynes. Tetrahedron Lett. 2008, 49, 5961–5964. [Google Scholar] [CrossRef]
- Thankachan, A.P.; Sindhu, K.; Krishnan, K.K.; Anilkumar, G. An efficient zinc-catalyzed cross-coupling reaction of aryl iodides with terminal aromatic alkynes. Tetrahedron Lett. 2015, 56, 5525–5528. [Google Scholar] [CrossRef]
- Feng, L.; Liu, F.; Sun, P.; Bao, J. Sonogashira reaction of aryl halides with terminal alkynes catalyzed by cobalt hollow nanospheres. Synlett, 2008, 9, 1415–1417. [Google Scholar] [CrossRef]
- Amrutha, S.; Radhika, S.; Anilkumar, G. Recent developments and trends in the iron- and cobalt-catalyzed Sonogashira reactions. Beilstein J. Org. Chem. 2022, 18, 262–285. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-catalysed reactions of alkynes: recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Huang, Y. A general synthesis of ynones from aldehydes via oxidative C–C bond cleavage under aerobic conditions. J. Am. Chem. Soc. 2014, 136, 12233–12236. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Koo, D.H.; Chang, S. Use of ruthenium/alumina as a convenient catalyst for copper-free sonogashira coupling reactions. Adv. Synth. Catal. 2004, 346, 1638–1640. [Google Scholar] [CrossRef]
- Garbacia, S.; Touzani, R.; Lavastre, O. Image analysis as a quantitative screening test in combinatorial catalysis: discovery of an unexpected ruthenium-based catalyst for the Sonogashira reaction. J. Comb. Chem. 2004, 6, 297–300. [Google Scholar] [CrossRef]
- Corma, A.; Juárez, R.; Boronat, M.; Sánchez, F.; Iglesias, M.; García, H. Gold catalyzes the Sonogashira coupling reaction without the requirement of palladium impurities. Chem. Commun. 2001, 47, 1446–1448. [Google Scholar] [CrossRef]
- Arundhathi, K.; Vaishnavi, P.; Aneeja, T.; Anilkumar, G. Copper-catalyzed Sonogashira reactions: advances and perspectives since 2014. RSC Adv. 2023, 13, 4823–4834. [Google Scholar] [CrossRef]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent advances and perspectives in copper catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Mohjer, F.; Mofatehnia, P.; Rangraz, Y.; Heravi, M.M. Pd-free, Sonogashira cross-coupling reaction. An update. J. Organomet. Chem. 2021, 936, 121712. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Rezazadeh, Z.; Kazemnejadi, M.; Allahresani, A. Magnetic Cu–Schiff base complex with an ionic tail as a recyclable bifunctional catalyst for base/Pd-free Sonogashira coupling reaction. J. Iran. Chem. Soc. 2019, 16, 2693–2705. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rezaei, F.; Khorsandi, Z. Pd/Cu-free Heck and Sonogashira cross–coupling reaction by Co nanoparticles immobilized on magnetic chitosan as reusable catalyst. Green Chem. 2017, 19, 1353–1361. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Rezazadeh, Z.; Kazemnejadi, M.; Allahresani, A. A Co–Cu bimetallic magnetic nanocatalyst with synergistic and bifunctional performance for the base-free Suzuki, Sonogashira, and C–N cross-coupling reactions in water. Dalton Trans. 2020, 49, 10645–10660. [Google Scholar] [CrossRef]
- Jung, D.Y.; Park, S.Y.; Kim, S.H. A facile protocol for copper-free palladium-catalyzed Sonogashira coupling in aqueous media. Bull. Korean Chem. Soc. 2022, 43, 110–116. [Google Scholar] [CrossRef]
- Shevate, R.; Kumar, M.; Karunakaran, M.; Hedhili, M.; Peinemann, K.V. Polydopamine/cysteine surface modified isoporous membranes with self-cleaning properties. J. Membr. Sci. 2017, 529, 185–194. [Google Scholar] [CrossRef]
- Zhao, Y.; Yeh, Y.W.; Liu, R.; You, J.M.; Qu, F.L. Facile deposition of gold nanoparticles on core-shell Fe3O4@polydopamine as recyclable nanocatalyst. Solid State Sci. 2015, 45, 9–14. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Jiang, S. Thioetherimide-modified cyanate ester resin with better molding performance for glass fiber reinforced composites. Polymers 2019, 11, 1458. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, D.; Xu, Q.; Jian, S. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by L-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Pazoki, F.; Salamatmanesh, A.; Bagheri, S.; Heydari, A. Synthesis and Characterization of Copper(I)-Cysteine Complex Supported on Magnetic Layered Double Hydroxide as an Efficient and Recyclable Catalyst System for Click Chemistry Using Choline Azide as Reagent and Reaction Medium. Catal. Lett. 2020, 150, 1186–1195. [Google Scholar] [CrossRef]
- Veisi, H.; Hemmati, S.; Safarimehr, P. (2018) In situ immobilized palladium nanoparticles on surface of poly-methyldopa coated-magnetic nanoparticles (Fe3O4@PMDA/Pd): A magnetically recyclable nanocatalyst for cyanation of aryl halides with K4[Fe(CN)6]. J. Catal. 2018, 365, 204–212. [Google Scholar] [CrossRef]
- Wu, J.; Ko, S.P.; Liu, H.; Kim, S.; Ju, J.; Kim, Y.K. Sub 5 nm magnetite nanoparticles: Synthesis, microstructure, and magnetic properties. Mater. Lett. 2007, 61, 3124–3129. [Google Scholar] [CrossRef]
- Kwak, S.J.; Oh, S.S.; Ahn, Y.H.; Kim, S.H. A Novel Heterogeneous Copper Catalyst Immobilized on Polydopamine-Coated Magnetite for Click Reaction. ChemistrySelect, 2023, 8, e20232515. [Google Scholar] [CrossRef]
- For references on the Fe₃O₄-based copper catalyst in click reactions, refer to our previous study (reference 49 above) and the references cited therein.
 | |||||||
| Entry | 1a | 2a | Cu-Platform | Solvent | Temp. | Time | Yielda |
| 1 | 3.6 mmol | 3.0 mmol | 60 mg | DMSO | 100 ℃ | 4 h | 84% |
| 2 | 3.6 mmol | 3.0 mmol | 80 mg | DMSO | 100 ℃ | 4 h | 99% |
| 3 | 3.6 mmol | 3.0 mmol | 80 mg | EtOH | reflux | 5 h | 99% |
| 4 | 3.6 mmol | 3.0 mmol | 80 mg | H2O | reflux | 5 h | 99% |
| 5 | 3.6 mmol | 3.0 mmol | 70 mg | EtOH | reflux | 5 h | 99% |
| 6 | 3.6 mmol | 3.0 mmol | 60 mg | EtOH | reflux | 5 h | 97% |
| 7 | 3.6 mmol | 3.0 mmol | 50 mg | EtOH | reflux | 5 h | 93% |
| 8 | 3.0 mmol | 3.0 mmol | 80 mg | EtOH | reflux | 4 h | 87% |
| 9 | 3.6 mmol | 3.0 mmol | 70 mg | EtOH | rt | 24 h | trace |
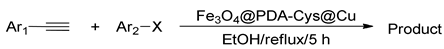 | ||||
| Entry | Ar1 | Ar2 | Product | Yielda |
| 1 |
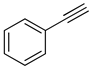 (1a) |
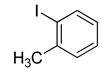 (2b) |
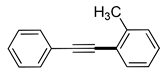 (3b) |
91% |
| 2 | (1a) |
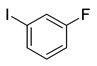 (2c) |
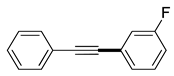 (3c) |
93% |
| 3 | (1a) |
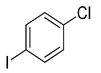 (2d) |
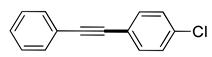 (3d) |
86% |
| 4 | (1a) |
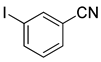 (2e) |
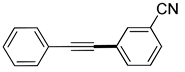 (3e) |
88% |
| 5 | (1a) |  (2f) (2f) |
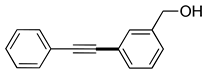 (3f) |
98% |
| 6 | 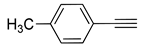 (1b) (1b) |
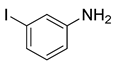 (2g) (2g) |
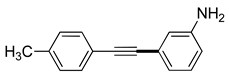 (3g) |
98% |
| 7 | (1b) | (2c) |
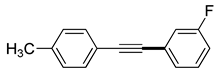 (3h) |
92% |
| 8 | 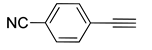 (1c) (1c) |
(2g) |
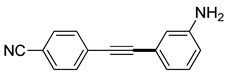 (3i) |
94% |
| 9 | 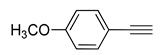 (1d) (1d) |
(2f) |
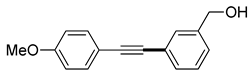 (3j) |
91% |
| 10 | 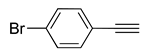 (1e) (1e) |
(2b) |
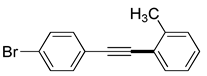 (3k) |
95% |
| 11 |
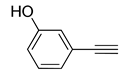 (1f) |
(2g) |
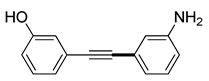 (3l) |
96% |
| 12 | (1a) | 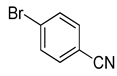 |
No reaction | - |
| 13 | (1a) | 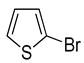 |
No reaction | - |
| 14 | (1a) | 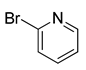 |
No reaction | - |
 | |||||||
| Recycle | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| Yielda | 98% | 98% | 96% | 97% | 95% | 94% | 90% |
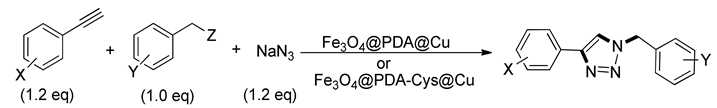 | ||||||
| Entry | X | Y | Z | Product | Resulta | Resultb |
| 1 | H | 4-Br | Br | 4b | 80% | 95% |
| 2 | H | 4-t-Bu | Br | 4c | 88% | 98% |
| 3 | H | 4-F | Cl | 4d | 90% | 90% |
| 4 | H | 4-CN | Cl | 4e | 85% | 90% |
| 5 | H | 2,4-Cl2 | Cl | 4f | 95% | 96% |
| 6 | 4-F | H | Br | 4g | 90% | 96% |
| 7 | 4-C4H9 | H | Br | 4h | 86% | 97% |
| 8 | 4-OCH3 | H | Br | 4i | 96% | 98% |
| 9 | 4-NO2 | H | Br | 4j | 90% | 91% |
| 10 | 3-OH | H | Br | 4k | 88% | 95% |
| 11 | 4-NO2 | 4-F | Cl | 4l | 88% | 92% |
| 12 | 4-F | 2,4-Cl2 | Cl | 4m | 80% | 92% |
| 13 | 3-CH3 | 4-t-Bu | Br | 4n | 96% | 97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




