1. Introduction
Pain is a complex phenomenon that goes beyond neural nociception. It is characterized by an unpleasant sensory and emotional experience associated with the actual or potential tissue damage [
1]. The experience of pain results from complex brain interpretation, not limited to the nociceptive input [
2]. Chronic pain is defined by persistent pain lasting more than 3 months, even after treatment onset [
3]. The prevalence of chronic pain is ~20.5% of the US population [
4]. In Brazil, the prevalence is approximately 45.3% [
5]. Its etiology is multifaceted, arising from a spectrum of conditions including musculoskeletal injuries, chronic illnesses, and neuropathies [
1,
5].
Chronic pain is characterized by some features, including the intensity variability ranging from mild to severe, and the usual oscillating nature, with periods of remission alternating with symptoms’ occurrence [
1,
2,
3,
4,
5,
6]. Chronic pain disrupts the individuals' quality of life, impairing their ability to engage in routine activities such as work, sleep, and social interactions [
1,
2,
3,
4,
5,
6,
7]. Previous studies indicate that psychological factors significantly influence the way people react to painful stimuli and that there is a direct relationship between socioeconomic level and the perception of pain intensity [
8,
9]. Many of those studies highlight the family income as an explanatory variable, based on the principle that families struggling to meet their basic needs face a significant accumulation of stress [
9]. Individuals trapped in this stress-pain-anxiety cycle tend to have higher prevalence of chronic pain, experiencing greater intensity and frequency of pain [
5,
8,
9].
Evidence indicates that chronic pain constitutes a significant burden worldwide, accounting for substantial health expenditures [
10]. An analysis of the overall US healthcare expenditures in 2016 revealed that low back and neck pain incurred the highest costs, totaling US
$134.5 billion [
10]. Additionally, other musculoskeletal disorders represented the second largest expense, amounting to US
$129.8 billion [
9,
10,
11]. Moreover, the management of chronic pain is still a challenge, often defying conventional therapeutic paradigms, and demanding comprehensive, tailored interventions [
12]. In this context, transcutaneous spinal direct current stimulation (tsDCS) emerges as a non-invasive method that shows promising effects on neurophysiological processing, particularly for chronic pain management [13,15].
The basic tsDCS principle involves applying electrical current between two electrodes. An anodal electrode is often placed on the thoracic vertebrae (T10-T12), while the cathode is placed on a referenced area, such as the somatosensory cortex [
13,
15] or cervical vertebrae (C7). Low-intensity current flows through the skin, bones, cerebrospinal fluid and nervous tissue [
13,
16]. Typically, a direct current of 1-2.5 milliamps (mA) is used [
17]. Electrodes positioning creates an electrical gradient that can alter the metabolic activity of underlying neural tissues [
14,
17].
The tsDCS has demonstrated the ability to induce neurophysiological changes both locally at the site of the stimulation along the spine, as well as distally in supraspinal and even cortical regions [
17,
18]. Two non-mutually exclusive synaptic mechanisms of cathodic polarization have been postulated for tsDCS, ultimately resulting in facilitation of spinal impulses: 1. inhibition of the γ-aminobutyric acid (GABA)ergic system; and 2. direct overexcitation of postsynaptic neurons, likely stemming from enhanced glutamate release at the spinal level [
17,
19]. Recent evidence has confirmed a synaptic role from cathodic stimulation on spinal interneurons in humans [
20].
Several tsDCS studies have demonstrated notable clinical effects on chronic pain perception, including a significant reduction in Visual Analogue Scale (VAS) scores, along with progressive improvements in quality of life and alleviation of associated symptoms [
13,
14,
21,
22].
However, based on the literature review, no studies were found that examined the immediate effects of tsDCS in patients with chronic pain using distinct intensities of 0.5 mA and 2.0 mA. The aim of the current study was to determine whether there is a difference in the magnitude of pain perception as self-reported by the Visual Analog Scale (VAS) and measured by the pressure algometry. Therefore, the present study aimed to analyze the immediate pain responses to tsDCS application at different intensities (2.0 and 0.5 mA).
2. Materials and Methods
2.1. Participants
Fifty-Five individuals (18-85 years) from both sexes participated in the study. The sample calculation was performed using the G-Power software [
18,
19]. The a priori 2-tailed biserial model sample size calculation was performed using the G-power software (version 3.1, Franz Faul, University of Kiel) considering the effect size of 0.602 obtained from a previous, similar study [
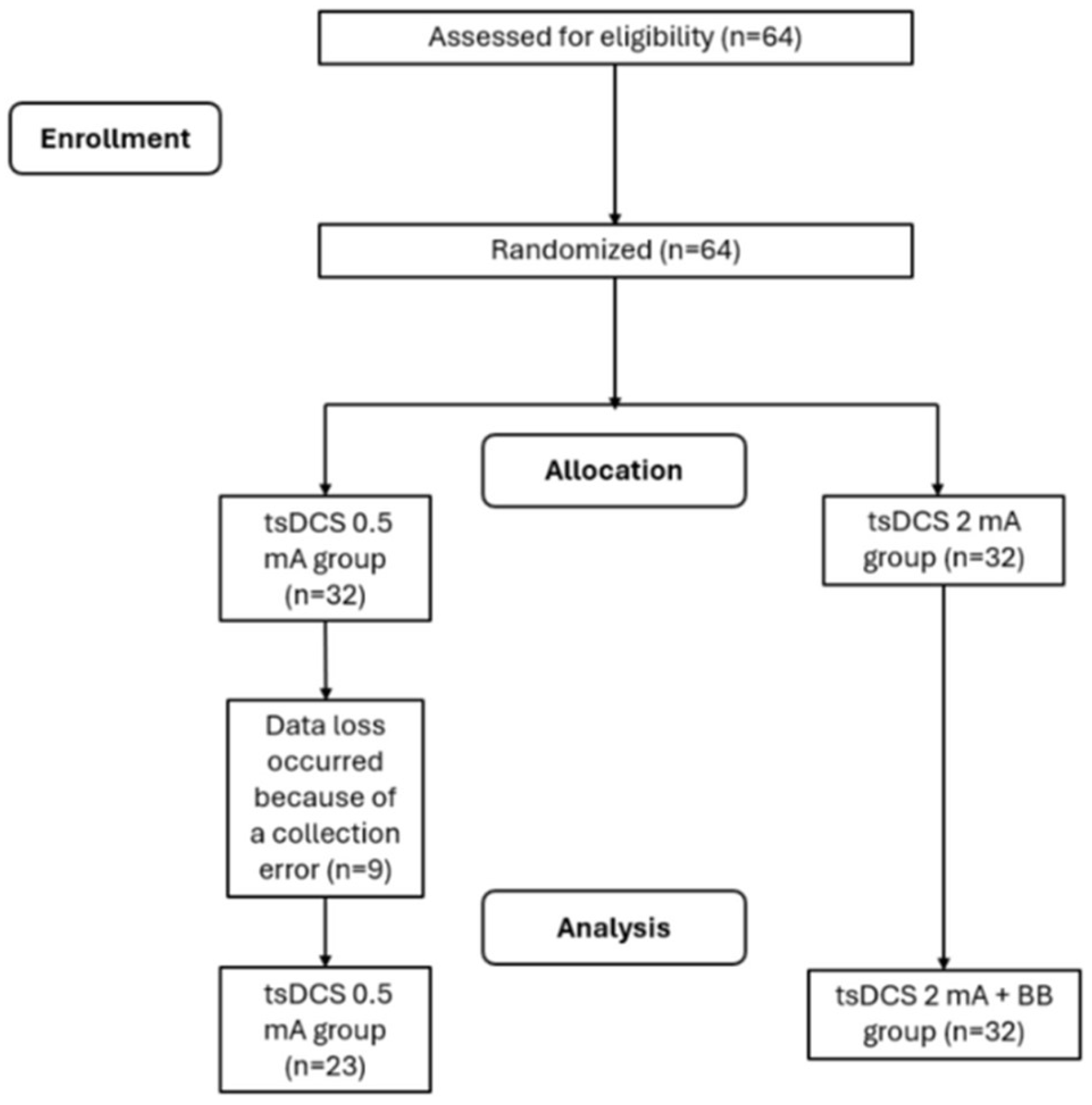
22]. Using the within-group comparison (experimental group), with an alpha of 5% and a minimum sampling power (1 − β) of 95%. A sample size of 38 participants was returned with an actual power of 0.951. Considering a 40% of drop-out, the final sample was constituted by 64 participants. Inclusion criteria were: adults aged between 18 and 85 years, with normal hearing, no history of neuropsychiatric disorders or self-reported use of psychoactive substances, and suffering from chronic pain (lasting more than 3 months). The exclusion criteria were self-reported absence of the following conditions: cardiac pacemakers, pregnancy, injuries, metal in or near the electrode position on the spine. (e.g., aneurysm clips or coils, firearm projectile fragments). Participants were included or excluded according to the presented criteria and were randomly in 2 groups of 32 people each: tsDCS 2 mA group and tsDCS 0.5 mA group (
Figure 1). The randomization sequence was performed using the
http://www.randomizer.org website, considering 64 participants, an input of 2 groups, and the uniqueness of each position in the randomization ranking. The allocation concealment was preserved by informing the therapist of the participant’s group assignment only after their enrollment in the research. This randomized clinical trial was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Federal College of Juiz de Fora (number 69441023.5.0000.5147). Additionally, the study was registered in the Brazilian clinical trials registry (number RBR-9252kwm). The participants were briefed on the assessment and intervention protocols conducted throughout the study, and each one provided written consent after being fully informed about the procedures.
2.2. Transcutaneous Spinal Direct Current Stimulation (tsDCS)
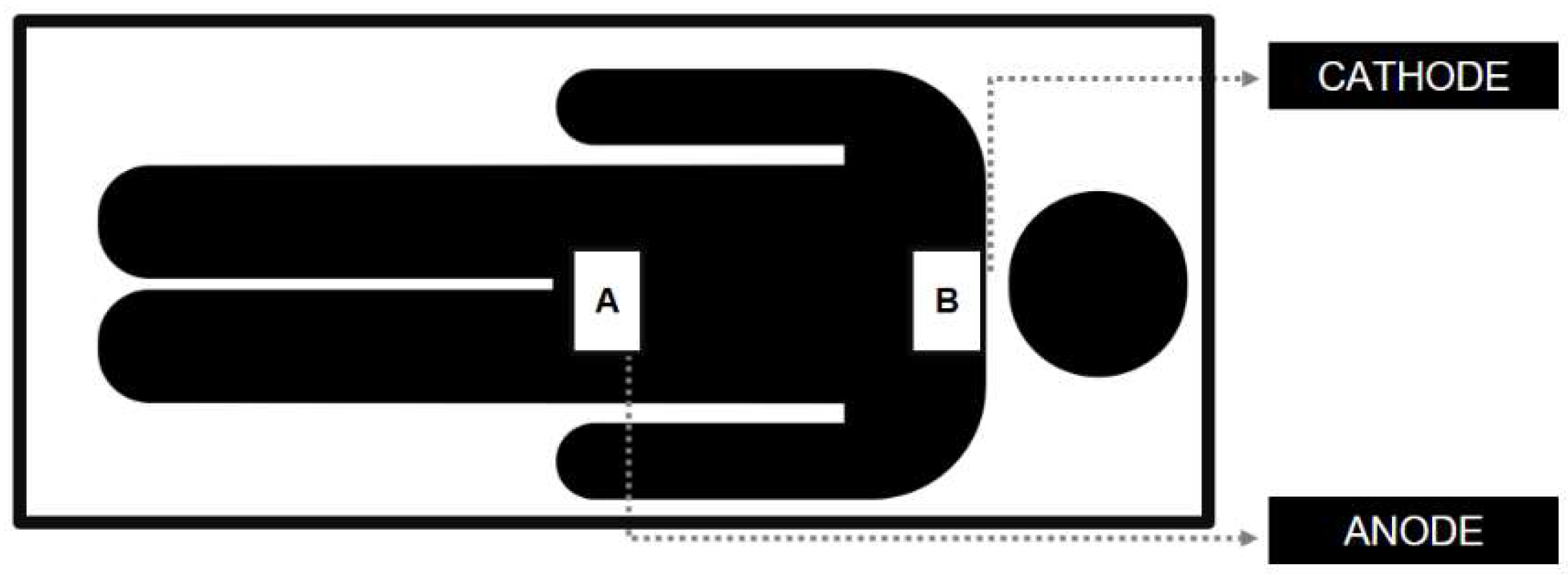
Electrical stimulation was applied using an analog equipment (Neuroeletrical, São Paulo, Brazil) with a 4-mA maximum current. Applied currents intensity were 2 mA or 0.5 mA, for 20 minutes. Two 50 cm2 (10 × 5 cm) rubber electrodes wrapped in a saline solution-soaked sponge were used. The anode was placed over the 12th thoracic vertebra, while the cathode was positioned over the 7th cervical vertebra (
Figure 2). A 30-s ramp-up current initiated the procedure and a 30-s ramp-down finished the stimulation period.
2.3. Experimental Protocol
The study collected pre- and post-experimental pain levels from each participant utilizing the Visual Analogue Scale (VAS) [
23] to gauge subjective perception and pressure algometry to measure the pressure-pain threshold [
24]. For the VAS, participants were seated and instructed to indicate their current pain level by marking a 10-centimeter line with a pen. Pressure algometry with algometer (MED.DOR Ltd., Brazil; maximum compression = 50 kgf, precision = 0.1 kgf, 3-digit display) involved participants sitting in a chair with their feet flat on the floor, hands resting on their thighs, and their torso upright. While a trained evaluator performed three consecutive repetitions on the skin surface of the participant who had the most pronounced self-reported pain24. The location indicated by the participant received progressive pressure of 1 kg/s controlled by a metronome until the participant felt pain, which in turn was indicated by raising the hand.
2.4. Data Extraction
For the VAS, the raters measured the vertical marking using a caliper and subsequently stored it in an online Excel spreadsheet. For algometry the data were immediately were extracted and organized into digital format, specifically an online Excel spreadsheet. For responsiveness, the data were categorized into RESPONSIVE (1) and NON-RESPONSIVE (2).
2.5. Statistical analysis
The Shapiro-Wilk and the Levene tests were used to assess data normality and homogeneity, respectively. After a logarithmic transformation, the Shapiro-Wilk test were performed to assess the sample’s normality. The factorial ANOVA with repeated measures was conducted to assess between- and within-group differences considering the time and time*group interactions. The chi-square test for association was used to assess the frequencies. Significance was established at p < 0.05. All assessments were conducted using the Jamovi software (Jamovi project, version 0.9 2020).
3. Results
Participants’ characteristics are shown in
Table 1. The findings revealed a significant temporal effect for both VAS (F = 21.057; p < 0.001) and pressure algometry (F = 4.430; p = 0.04). However, there were no significant between-group differences for the time*group interaction for VAS (F = 0.539; p = 0.466) nor for the pressure algometry (F = 0.07; p = 0.78). No significant differences were observed for between-group frequencies (gl= 6; p= 0.477).
4. Discussion
The present findings showed immediate differences on pain perception and on pressure pain thresholds after both 2 and 0.5 mA intensities. However, no between-group (2 mA vs 0.5 mA) differences were observed after tsDCS.
The current findings are aligned with Guidetti et al. (2021), which employed tsDCS in chronic pain participants. However, some differences from the current study must be addressed [
14]. The T12 anodal and C7 cathodal positioning in the present study contrasts with the previous one that has chosen the T12 anodal and somatosensory area cathodal montage. The current study also employed the 2.0 mA intensity, while Guidetti et al. (2021) applied 2.5 mA. Additionally, a 0.5 mA comparison group was included in the present set-up, whereas the previous study employed a simulated therapy, so the current would not exert any significant influence for the assessed outcomes. However, in another study by Guidetti et al. (2023), who delves into the modeling of electrical fields in tsDCS, observed that the relationship between electrical dose and clinical response remains unclear in tsDCS, requiring further investigation [
16]. A non-linear pattern is often expected considering the current intensity and the physiological outcome, suggesting that higher field intensities do not inherently correlate with increased effects. The present study had similar results as the 0.5 mA dose yielded similar outcomes than 2 mA.
Notably, Guidetti et al. (2021) observed significant pain score differences after one week of intervention [
14]. However, the result is not representative due to the small sample size. Regarding the sample characteristics, a robust sample size calculation was carried out for the present study, with a diverse set of participants considering diagnosis and age in comparison with other studies.
Choi et al.'s (2019) pilot investigation examined the immediate response to chronic neuropathic pain following spinal cord injury through tsDCS application with T12 anodal and CZ cathodal positioning [
22]. The assessment was performed right after the current, with additional 1-hour, and 2-hour post-session assessments. Despite no differences were noted immediately significant differences were not observed, the post hoc analysis revealed a significant difference from baseline at the 1-hour mark. This finding potentially supports the hypothesis that tsDCS effects occur cumulatively over an extended period of treatment.
The findings reported by Leonor et al. (2018), examining the effects of tsDCS in 8 healthy female participants, indicate that tsDCS attenuates the nociceptive stimuli, and significantly influences the LEP-N2 wave elicited by nociceptive laser stimulation applied to the lower limbs [
25]. The authors used a temperature-controlled CO2 laser to activate Aδ and C fiber thermonociceptors, providing brief pulses of radiant heat to the hands and feet with pre-established settings for activation thresholds of Aδ and C fiber thermonociceptors [
25].The author proposes that the observed selective neuromodulatory response may be plausibly attributed to anodal blockade of axonal conduction within the spinal cord. Furthermore, it is suggested that the post-stimulation effects of low thoracic tsDCS could be associated with synaptic modulation of local processing and/or transmission of nociceptive stimuli at the dorsal horn level.
Another study conducted by Bocci et al. (2015) proposes that tsDCS modulates inhibitory GABA(A)ergic drive, as evidenced in a small sample of 10 healthy participants [
18]. Likewise, the authors opted for cathodal stimulation on the shoulder and anodal stimulation at T11, administering an intensity of 2.5 mA for 20 minutes in a single session. Despite the absence of immediate effects, electrophysiological assessment revealed some molecular-level alterations. Nonetheless, caution is suggested when interpreting those effects, mainly due to sample size limitations and the absence of sample size calculation.
The current study has some limitations that must be addressed. The sample heterogeneity concerning the diagnosis and age, as well as the heterogeneity of pain duration. However, the results followed the same pattern for all participants, suggesting that such heterogeneity was not a cofactor to change the current analysis. Thus, the chronic pain seemed to drive the observed effects regardless the diagnosis or age. Further research is important to clarify the tsDCS dynamics in chronic pain in short- and long-term protocols featuring increased number of sessions. Another limitation was the lack of a sham group. As distinct intensities have shown similar effects, it is advisable to integrate a simulated therapy group for further studies.
Throughout the study, participants experienced some adverse effects, including skin burns at the electrode application site (impacting ~20% of the sample) and headaches during the session. These effects, previously documented in literature [
26], were mitigated by diluting the conductive saline fluid in distilled water, yielding a 0.6% solution instead of 0.9%.
During the study, 9 participants were lost: 4 chose not to undergo immediate reassessment, and 5 felt discomfort in the posture selected for the application.
5. Conclusions
The present results suggest that a single 20-minute session of 2 and 0.5 mA tsDCS improves chronic pain perception equally for both intensities.
Author Contributions
Conceptualization, Kariny Realino do Rosário Ferreira, Maria de Cássia Souza Macedo, Alexandre Wesley de Carvalho Barbosa, Bianca Rossi Botim and Michelle Cristina Sales Almeida Barbosa; methodology, Kariny Realino do Rosário Ferreira, Maria de Cássia Souza Macedo, Alexandre Wesley de Carvalho Barbosa, Bianca Rossi Botim and Michelle Cristina Sales Almeida Barbosa; formal analysis, Kariny Realino do Rosário Ferreira, Alexandre Wesley de carvalho Barbosa and Gabriela Lopes Gama; investigation, Kariny Realino do Rosário Ferreira, Maria de Cassia Souza Macedo, Ana Luiza Guimarães Alves, Arthur Ferreira Esquirio, Bianca Rossi Botim, Gabrielly Souza Jacob, Mayra Evelise Cunha dos Santos, Gabriela Lopes Gama, Michelle Cristina Sales Almeida Barbosa, Alexandre Wesley Carvalho Barbosa; resources, Kariny Realino do Rosário Ferreira, Bianca Rossi Botim, Alexandre Wesley Carvalho Barbosa; data curation, Kariny Realino do Rosário Ferreira, Bianca Rossi Botim, Alexandre Wesley Carvalho Barbosa; writing—original draft preparation, Alexandre Wesley Carvalho Barbosa; writing—review and editing, Alexandre Wesley Carvalho Barbosa.
Funding
Throughout the duration of this study, the principal investigator received financial support the Coordination for the Improvement of Higher Education Personnel (CAPES) and from the Research Support Foundation of the State of Minas Gerais (FAPEMIG).
Institutional Review Board Statement
Ethics Committee of the Federal College of Juiz de Fora (number 69441023.5.0000.5147). Additionally, the study was registered in the Brazilian clinical trials registry (number RBR-9252kwm).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data from this study will be made available upon request.
Conflicts of Interest
The authors reported no conflict of interest.
References
- St John Smith, E. Advances in understanding nociception and neuropathic pain. Journal of Neurology. 2018, 265, 231–8. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors : the sensors of the pain pathway Review series Nociceptors : the sensors of the pain pathway. J Clin Invest. 2010, 120, 3760–72. [Google Scholar] [CrossRef] [PubMed]
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain Among Adults — United States, 2019–2021. MMWR Morbidity and Mortality Weekly Report. 2023, 72, 379–85. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. PAIN. 2022, 163. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.V.M.; Oliveira ABG de Silva GMR da Silva M de F da Bergamo, P. E.; Parise, M.; et al. Prevalence of chronic pain in Brazil: A systematic review and meta-analysis. Clinics. 2023, 78, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; et al. Morbidity and Mortality Weekly Report Centers for Disease Control and Prevention MMWR Editorial and Production Staff (Weekly) MMWR Editorial Board. Rep. 2018, 67. [Google Scholar]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: a review of its epidemiology and associated factors in population-based studies. British Journal of Anaesthesia. 2019, 123, e273–83. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.P.; Souza CP de, Q.; Barbosa, W.J.M.; Santos-Júnior, F.F.U. ; de Oliveira AS, Prevalence of chronic pain in Brazil: systematic review. Brazilian Journal Of Pain. 2021, 4, 257–67. [Google Scholar]
- Kosminsky, M.; do Nascimento, M.G.; de Oliveira, G.N.S. Financial stress and pain, what follows an economic crisis? Integrative review. Brazilian Journal Of Pain. 2020, 3, 280–4. [Google Scholar] [CrossRef]
- Lam, C.M.; Sanderson, M.; Vu, D.T.; Sayed, D.; Latif, U.; Chadwick, A.L.; et al. Musculoskeletal and Neuropathic Pain in COVID-19. Diagnostics. 2024, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; et al. US Health Care Spending by Payer and Health Condition, 1996-2016. JAMA.
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: an update on burden, best practices, and new advances. The Lancet. 2021, 397, 2082–97. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. Journal of Clinical Medicine. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Ferrucci, R.; Vergari, M.; Aglieco, G.; Naci, A.; Versace, S. , et al. Effects of Transcutaneous Spinal Direct Current Stimulation (tsDCS) in Patients With Chronic Pain: A Clinical and Neurophysiological Study. Frontiers in Neurology, 1: 12(September).
- Mekhail, N.A.; Mathews, M.; Nageeb, F.; Guirguis, M.; Mekhail, M.N.; Cheng, J. Retrospective Review of 707 Cases of Spinal Cord Stimulation: Indications and Complications. Pain Practice. 2011, 11, 148–53. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Giannoni-Luza, S.; Bocci, T.; Pacheco-Barrios, K.; Bianchi, A.M.; Parazzini, M. , et al. Modeling Electric Fields in Transcutaneous Spinal Direct Current Stimulation: A Clinical Perspective. Biomedicines.
- Lenoir, C.; Jankovski, A.; Mouraux, A. Anodal Transcutaneous Spinal Direct Current Stimulation (tsDCS) Selectively Inhibits the Synaptic Efficacy of Nociceptive Transmission at Spinal Cord Level. Neuroscience, 1: 393.
- Bocci, T.; Barloscio, D.; Vergari, M.; Di Rollo, A.; Rossi, S.; Priori, A. , et al. Spinal Direct Current Stimulation Modulates Short Intracortical Inhibition. Neuromodulation. 2015, 18, 686–93. [Google Scholar] [CrossRef] [PubMed]
- Vergara, F.; Sardi, N.F.; Pescador, A.C.; Guaita, G.O.; Jark Stern, C.A.; Chichorro, J.G. , et al. Contribution of mesolimbic dopamine and kappa opioid systems to the transition from acute to chronic pain. Neuropharmacology, 1: 178(April), 1082. [Google Scholar]
- Thordstein, M.; Svantesson, M.; Rahin, H. Effect of transspinal direct current stimulation on afferent pain signalling in humans. Journal of Clinical Neuroscience, 1: 77.
- Akcay, G.; Nemutlu Samur, D.; Derin, N. Transcranial direct current stimulation alleviates nociceptive behavior in male rats with neuropathic pain by regulating oxidative stress and reducing neuroinflammation. Journal of Neuroscience Research. 2023, 101, 1457–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.A.; Kim, Y.; Shin, H.I. Pilot study of feasibility and effect of anodal transcutaneous spinal direct current stimulation on chronic neuropathic pain after spinal cord injury. Spinal Cord. 2019, 57, 461–70. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. International Journal of Rehabilitation Research. 2008, 31. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Mayorga, D.; dos Anjos, C.F.; de Cássia Macedo, M.; Fernandes, I.G.; Aedo-Muñoz, E.; Intelangelo, L. , et al. Instrumental validity and intra/inter-rater reliability of a novel low-cost digital pressure algometer. PeerJ, 1: 8.
- Lenoir, C.; Jankovski, A.; Mouraux, A. Anodal Transcutaneous Spinal Direct Current Stimulation (tsDCS) Selectively Inhibits the Synaptic Efficacy of Nociceptive Transmission at Spinal Cord Level. Neuroscience, 1: 393.
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R. , et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clinical Neurophysiology. 2017, 128, 1774–809. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).