Submitted:
02 October 2024
Posted:
04 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background
1.1.1. Minor Closed Head Injury
1.1.2. Pathophysiology
1.1.3. Shear Injury in TBI
1.1.4. Blood Brain Barrier Dysfunction in TBI Acute And Chronic
1.2. Structural Imaging in mTBI
1.3. Physiologic Imaging
1.3.1. Diffusion Tensor Imaging
1.3.2. Dynamic Contrast Enhanced (DCE) MRI

1.3.3 18-Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)
1.3.4. Arterial Spin Labeling MRI
1.3.6 Summary of techniques
2. Conclusion
Acronyms Dictionary
| TBI- mild traumatic brain injury |
| TJ protein ZO-1-tight junction protein Zona Occludens 1 |
| C3a- complement C3a protein |
| C3aR- complement C3a receptor protein |
| pTau- hyperphosphorylated Tau |
| TDP-43- Transactive response DNA binding protein- 43 |
| FLAIR- fluid attenuated inversion recovery |
| SWI- Susceptibility Weighted Images |
| ASL MRI-Arterial Spin Labeling MRI |
| PLD- Post labeling delay |
| DTI MRI- Diffusion Tensor Imaging |
| DCE MRI- Dynamic Contrast Enhanced MRI |
| PET- Positron Emission Tomography |
| S/N- Signal to Noise |
| SKAT 6- Sport concussion Assessment Tool |
| VOMS- Vestibular Ocular Motor Screening |
| SCOAT 6- Sports Concussion Office Assessment Tool |
| CTE- chronic traumatic encephalopathy |
| cMTT- mean capillary transit time |
| GF- glymphatic flow |
| BBB- Blood brain barrier |
| IGG- Immunoglobulin G |
| NVU- the neurovascular unit |
| ECM- extracellular matrix |
| IL1 β- interleukin 1 beta |
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Forrest, R.H.J.; Henry, J.D.; McGarry, P.J.; Marshall, R.N. Mild traumatic brain injury in New Zealand: factors influencing post-concussion symptom recovery time in a specialised concussion service. J. Prim. Heal. Care 2018, 10, 159–166. [Google Scholar] [CrossRef]
- Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. CDC NIH Rep to Congr 1–74.
- Reith, Florence CM, Hester F. Lingams, Belinda J. Gabe, Fiona E. Lecky, Ian Roberts, and Andrew IR Maas. "Differential effects of the Glasgow Coma Scale Score and its Components: An analysis of 54,069 patients with traumatic brain injury." Injury 48, no. 9 (2017): 1932-1943. [CrossRef]
- Dessy, A.M.; Yuk, F.J.; Maniya, A.Y.; Gometz, A.; Rasouli, J.J.; Lovell, M.R.; Choudhri, T.F. Review of Assessment Scales for Diagnosing and Monitoring Sports-related Concussion. Cureus 2017, 9, e1922. [Google Scholar] [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; Von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Williams, R.M.; Puetz, T.W.; Giza, C.C.; Broglio, S.P. Concussion Recovery Time Among High School and Collegiate Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 893–903. [Google Scholar] [CrossRef]
- Williams, R.M.; Puetz, T.W.; Giza, C.C.; Broglio, S.P. Concussion Recovery Time Among High School and Collegiate Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 893–903. [Google Scholar] [CrossRef]
- Wintermark, M.; Sanelli, P.C.; Anzai, Y.; Tsiouris, A.J.; Whitlow, C.T.; Druzgal, T.J.; Gean, A.D.; Lui, Y.W.; Norbash, A.M.; Raji, C.; et al. Imaging Evidence and Recommendations for Traumatic Brain Injury: Conventional Neuroimaging Techniques. J. Am. Coll. Radiol. 2015, 12, e1–e14. [Google Scholar] [CrossRef]
- Eierud, C.; Craddock, R.C.; Fletcher, S.; Aulakh, M.; King-Casas, B.; Kuehl, D.; LaConte, S.M. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage: Clin. 2014, 4, 283–294. [Google Scholar] [CrossRef]
- Roberts, M.A.; Manshadi, F.F.; Bushnell, D.L.; Hines, M.E. Neurobehavioural dysfunction following mild traumatic brain injury in childhood: A case report with positive findings on positron emission tomography (PET). Brain Inj. 1995, 9, 427–436. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Brett, B.L.; Broglio, S.; A Davis, G.; Giza, C.C.; Guskiewicz, K.M.; Harmon, K.G.; Herring, S.; Howell, D.R.; Master, C.L.; et al. Introducing the Sport Concussion Assessment Tool 6 (SCAT6). Br. J. Sports Med. 2023, 57, 619–621. [Google Scholar] [CrossRef]
- Patricios, Jon, Geoff M. Schneider, Jacqueline van Ierssel, Laura K. Purcell, Gavin A. Davis, Ruben J. Echemendia, Pierre Frémont et al. "Sport concussion office assessment tool 6." British journal of sports medicine 57, no. 11 (2023): 651-667. [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; Von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Meier, T.B.; Bellgowan, P.S.F.; Singh, R.; Kuplicki, R.; Polanski, D.W.; Mayer, A.R. Recovery of Cerebral Blood Flow Following Sports-Related Concussion. JAMA Neurol. 2015, 72, 530–538. [Google Scholar] [CrossRef]
- Roberts, M.A.; Manshadi, F.F.; Bushnell, D.L.; Hines, M.E. Neurobehavioural dysfunction following mild traumatic brain injury in childhood: A case report with positive findings on positron emission tomography (PET). Brain Inj. 1995, 9, 427–436. [Google Scholar] [CrossRef]
- Ilvesmäki, T.; Luoto, T.M.; Hakulinen, U.; Brander, A.; Ryymin, P.; Eskola, H.; Iverson, G.L.; Öhman, J. Acute mild traumatic brain injury is not associated with white matter change on diffusion tensor imaging. Brain 2014, 137, 1876–1882. [Google Scholar] [CrossRef]
- Lee, H.; Wintermark, M.; Gean, A.D.; Ghajar, J.; Manley, G.T.; Mukherjee, P. Focal Lesions in Acute Mild Traumatic Brain Injury and Neurocognitive Outcome: CT versus 3T MRI. J. Neurotrauma 2008, 25, 1049–1056. [Google Scholar] [CrossRef]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic Brain Injury and Mitochondrial Dysfunction. Am. J. Med Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef]
- Vagnozzi R, Tavazzi B, Signoretti S, Amorini AM, Belli A, Cimatti M, et al. Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment part I. Neurosurgery. 2007; 61:379–89. [CrossRef]
- Bergsneider, M.; Hovda, D.A.; Shalmon, E.; Kelly, D.F.; Vespa, P.M.; Martin, N.A.; Phelps, M.E.; McArthur, D.L.; Caron, M.J.; Kraus, J.F.; et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997, 86, 241–251. [Google Scholar] [CrossRef]
- Hay, Jennifer R., Victoria E. Johnson, Adam MH Young, Douglas H. Smith, and William Stewart. "Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans." Journal of neuropathology and experimental neurology 74, no. 1: 12 (2015), 2015. [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef]
- Christman, C.W.; Grady, M.S.; Walker, S.A.; Holloway, K.L.; Povlishock, J.T. Ultrastructural Studies of Diffuse Axonal Injury in Humans. J. Neurotrauma 1994, 11, 173–186. [Google Scholar] [CrossRef]
- El Sayed, Tamer, Alejandro Mota, Fernando Fraternali, and Michael Ortiz. "Biomechanics of traumatic brain injury." Computer Methods in Applied Mechanics and Engineering 197, no. 51-52 (2008): 4692-4701.
- Meier, T.B.; Bellgowan, P.S.F.; Singh, R.; Kuplicki, R.; Polanski, D.W.; Mayer, A.R. Recovery of Cerebral Blood Flow Following Sports-Related Concussion. JAMA Neurol. 2015, 72, 530–538. [Google Scholar] [CrossRef]
- Roberts, M.A.; Manshadi, F.F.; Bushnell, D.L.; Hines, M.E. Neurobehavioural dysfunction following mild traumatic brain injury in childhood: A case report with positive findings on positron emission tomography (PET). Brain Inj. 1995, 9, 427–436. [Google Scholar] [CrossRef]
- Meaney, D.F.; Smith, D.H. Biomechanics of Concussion. Clin. Sports Med. 2011, 30, 19–31. [Google Scholar] [CrossRef]
- Donat, C.K.; Lopez, M.Y.; Sastre, M.; Baxan, N.; Goldfinger, M.; Seeamber, R.; Müller, F.; Davies, P.; Hellyer, P.; Siegkas, P.; et al. From biomechanics to pathology: predicting axonal injury from patterns of strain after traumatic brain injury. Brain 2021, 144, 70–91. [Google Scholar] [CrossRef]
- Glushakova, Olena Y., Danny Johnson, and Ronald L. Hayes. "Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood–brain barrier disruption, and progressive white matter damage." Journal of neurotrauma 31, no. 13 (2014): 1180-1193. [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef]
- Haider, M.N.; Leddy, J.J.; Hinds, A.L.; Aronoff, N.; Rein, D.; Poulsen, D.; Willer, B.S. Intracranial pressure changes after mild traumatic brain injury: a systematic review. Brain Inj. 2018, 32, 809–815. [Google Scholar] [CrossRef]
- Barzó, P.; Marmarou, A.; Fatouros, P.; Corwin, F.; Dunbar, J. Magnetic resonance imaging—monitored acute blood-brain barrier changes in experimental traumatic brain injury. J. Neurosurg. 1996, 85, 1113–1121. [Google Scholar] [CrossRef]
- Morganti-Kossmann, Maria Cristina, E. Yan, and Nicole Bye. "Animal models of traumatic brain injury: is there an optimal model to reproduce human brain injury in the laboratory?" Injury 41 (2010): S10-S13. [CrossRef]
- Johnson, Victoria E., David F. Meaney, D. Kacy Cullen, and Douglas H. Smith. "Animal models of traumatic brain injury." Handbook of clinical neurology 127 (2015): 115-128. [CrossRef]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock (Augusta, Ga). 001; 16:165–77. [CrossRef]
- Doherty, C.P.; O’keefe, E.; Wallace, E.; Loftus, T.; Keaney, J.; Kealy, J.; Humphries, M.M.; Molloy, M.G.; Meaney, J.F.; Farrell, M.; et al. Blood–Brain Barrier Dysfunction as a Hallmark Pathology in Chronic Traumatic Encephalopathy. J. Neuropathol. Exp. Neurol. 2016, 75, 656–662. [Google Scholar] [CrossRef]
- Hattori, N.; Huang, S.-C.; Wu, H.-M.; Liao, W.; Glenn, T.C.; Vespa, P.M.; E Phelps, M.; A Hovda, D.; Bergsneider, M. Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. . 2004, 45, 775–83. [Google Scholar]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of Pro- and Anti-Inflammatory Cytokines and Chemokines in the Pathophysiology of Traumatic Brain Injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef]
- Woodcock T, Morganti-Kossmann C. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013; 4:18. [CrossRef]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of Pro- and Anti-Inflammatory Cytokines and Chemokines in the Pathophysiology of Traumatic Brain Injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef]
- Woodcock T, Morganti-Kossmann C. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013; 4:18. [CrossRef]
- Engelhardt, B.; Liebner, S. Novel insights into the development and maintenance of the blood–brain barrier. Cell Tissue Res. 2014, 355, 687–699. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Bhowmick, S.; D'Mello, V.; Caruso, D.; Wallerstein, A.; Abdul-Muneer, P. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019, 317, 260–270. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Andjelkovic, Anuska V., et al. "Blood-brain barrier dysfunction in normal aging and neurodegeneration: mechanisms, impact, and treatments." Stroke 54.3 (2023): 661-672. [CrossRef]
- Kadry, Hossam, Behnam Noorani, and Luca Cucullo. "A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity." Fluids and Barriers of the CNS 17. 1: (2020), 2020. [CrossRef]
- Zhao, L.; Tannenbaum, A.; Bakker, E.N.T.P.; Benveniste, H. Physiology of Glymphatic Solute Transport and Waste Clearance from the Brain. Physiology 2022, 37, 349–362. [Google Scholar] [CrossRef]
- Preston, Jane E., N. Joan Abbott, and David J. Begley. "Transcytosis of macromolecules at the blood–brain barrier." Advances in pharmacology 71 (2014): 147-163. [CrossRef]
- van Leeuwen, E.; Hampton, M.B.; Smyth, L.C. Redox signalling and regulation of the blood-brain barrier. Int. J. Biochem. Cell Biol. 2020, 125, 105794. [Google Scholar] [CrossRef]
- Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood–brain barrier formation and stroke. Dev Neurobiol 71:1018–1039. [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Hudson, Natalie, and Matthew Campbell. "Tight junctions of the neurovascular unit. 7: Frontiers in Molecular Neuroscience 14 (2021), 2021. [CrossRef]
- Sun, X.-C.; Cheng, C.-J.; Zhou, C.; Chen, H.; Zheng, J.-F.; Guo, Z.-D.; Huang, Z.-J.; Wu, Y.; Zhong, J.-J. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen. Res. 2020, 15, 2318–2326. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: a general review. Int. J. Neurosci. 2016, 127, 624–633. [Google Scholar] [CrossRef]
- Zhang J, Puvenna V, Janigro D. Biomarkers of traumatic brain injury and their relationship to pathology. Translational research in traumatic brain injury: CRC Press/Taylor and Francis Group; 2016.
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Joseph, C.R. Progressive Age-Associated Blood–Brain Barrier Leak/Dysfunction-Nexus of Neurodegenerative Disease Using MRI Markers to Identify Preclinical Disease and Potential New Targets for Future Treatments. Diagnostics 2024, 14, 726. [Google Scholar] [CrossRef]
- Propson, N.E.; Roy, E.R.; Litvinchuk, A.; Köhl, J.; Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Nakajima, Y.; Horiuchi, Y.; Kamata, H.; Yukawa, M.; Kuwabara, M.; Tsubokawa, T. Distinct Time Courses of Secondary Brain Damage in the Hippocampus Following Brain Concussion and Contusion in Rats. Tohoku J. Exp. Med. 2010, 221, 229–235. [Google Scholar] [CrossRef]
- Ryan LM, Warden DL. Post concussion syndrome. Int Rev Psychiatry. 2003; 15:310–6.
- Prins, M.L.; Alexander, D.; Giza, C.C.; Hovda, D.A. Repeated Mild Traumatic Brain Injury: Mechanisms of Cerebral Vulnerability. J. Neurotrauma 2013, 30, 30–38. [Google Scholar] [CrossRef]
- Laurer HL, Bareyre FM, Lee VM, Trojanowski JQ, Longhi L, Hoover R, et al. Mild head injury increasing the brain's vulnerability to a second concussive impact. J Neurosurg. 2001; 95:859–70. [CrossRef]
- Sorby-Adams, A.J.; Marcoionni, A.M.; Dempsey, E.R.; Woenig, J.A.; Turner, R.J. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int. J. Mol. Sci. 2017, 18, 1788. [Google Scholar] [CrossRef]
- Andjelkovic, Anuska V., et al. "Blood-brain barrier dysfunction in normal aging and neurodegeneration: mechanisms, impact, and treatments." Stroke 54.3 (2023): 661-672. [CrossRef]
- Farace E, Alves WM. Do women fare worse? A metanalysis of gender differences in outcome after traumatic brain injury. Neurosurg Focus. 2000; 8:1–8.
- Bennett ER, Reuter-Rice K, Laskowitz DT. Genetic influences in traumatic brain injury. Transl Res Traumatic Brain Inj. 2016.
- McKee, A.C.; Stein, T.D.; Nowinski, C.J.; Stern, R.A.; Daneshvar, D.H.; Alvarez, V.E.; Lee, H.-S.; Hall, G.; Wojtowicz, S.M.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2012, 136, 43–64. [Google Scholar] [CrossRef]
- Ayubcha, Cyrus, Mona-Elisabeth Revheim, Andrew Newberg, Mateen Moghbel, Chaitanya Rojulpote, Thomas J. Werner, and Abass Alavi. "A critical review of radiotracers in the positron emission tomography imaging of traumatic brain injury: FDG, tau, and amyloid imaging in mild traumatic brain injury and chronic traumatic encephalopathy." European Journal of Nuclear Medicine and Molecular Imaging 48 (2021): 623-641.
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; Alosco, M.L.; Solomon, T.M.; Nowinski, C.J.; McHale, L.; et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017, 318, 360–370. [Google Scholar] [CrossRef]
- Lenihan, M.W.; Jordan, B.D. The Clinical Presentation of Chronic Traumatic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Karton, Clara, and T. Blaine Hoshizaki. "Concussive and subconcussive brain trauma: the complexity of impact biomechanics and injury risk in contact sport." Handbook of clinical neurology 158 (2018): 39-49.78.
- Bang SA, Song YS, Moon BS, Lee BC, Lee H-y, Kim J-M, et al. Neuropsychological, metabolic, and GABAA receptor studies in subjects with repetitive traumatic brain injury. J Neurotrauma. 2016; 33:1005–14. [CrossRef]
- Wooten, D.W.; Ortiz-Terán, L.; Zubcevik, N.; Zhang, X.; Huang, C.; Sepulcre, J.; Atassi, N.; Johnson, K.A.; Zafonte, R.D.; El Fakhri, G. Multi-Modal Signatures of Tau Pathology, Neuronal Fiber Integrity, and Functional Connectivity in Traumatic Brain Injury. J. Neurotrauma 2019, 36, 3233–3243. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging links between cerebrovascular and neurodegenerative diseases—a special role for pericytes. Embo Rep. 2019, 20, e48070. [Google Scholar] [CrossRef]
- Searson, Peter Charles, et al. "The influence of physiological and pathological perturbations on blood-brain barrier function." Frontiers in Neuroscience 17 (2023): 1289894. [CrossRef]
- Elschot, E.P.M.; Backes, W.H.; Postma, A.A.; van Oostenbrugge, R.J.; Staals, J.; Rouhl, R.P.; Jansen, J.F. A Comprehensive View on MRI Techniques for Imaging Blood-Brain Barrier Integrity. Investig. Radiol. 2020, 56, 10–19. [Google Scholar] [CrossRef]
- Eierud, C.; Craddock, R.C.; Fletcher, S.; Aulakh, M.; King-Casas, B.; Kuehl, D.; LaConte, S.M. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage: Clin. 2014, 4, 283–294. [Google Scholar] [CrossRef]
- Sparks, P.; Lawrence, T.; Hinze, S. Neuroimaging in the Diagnosis of Chronic Traumatic Encephalopathy: A Systematic Review. Am. J. Ther. 2020, 30, S1–S10. [Google Scholar] [CrossRef]
- Ranzenberger LR, Das JM, Snyder T. Diffusion Tensor Imaging. 2023 Nov 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed]
- Kim, E.; Yoo, R.-E.; Seong, M.Y.; Oh, B.-M. A systematic review and data synthesis of longitudinal changes in white matter integrity after mild traumatic brain injury assessed by diffusion tensor imaging in adults. Eur. J. Radiol. 2021, 147, 110117. [Google Scholar] [CrossRef]
- Yoen, H.; Yoo, R.-E.; Choi, S.H.; Kim, E.; Oh, B.-M.; Yang, D.; Hwang, I.; Kang, K.M.; Yun, T.J.; Kim, J.-H.; et al. Blood-Brain Barrier Disruption in Mild Traumatic Brain Injury Patients with Post-Concussion Syndrome: Evaluation with Region-Based Quantification of Dynamic Contrast-Enhanced MR Imaging Parameters Using Automatic Whole-Brain Segmentation. Korean J. Radiol. 2021, 22, 118–130. [Google Scholar] [CrossRef]
- Ware, J.B.; Sinha, S.; Morrison, J.; Walter, A.E.; Gugger, J.J.; Schneider, A.L.; Dabrowski, C.; Zamore, H.; Wesley, L.; Magdamo, B.; et al. Dynamic contrast enhanced MRI for characterization of blood-brain-barrier dysfunction after traumatic brain injury. NeuroImage: Clin. 2022, 36, 103236. [Google Scholar] [CrossRef]
- Oh, Sung Suk, Eun-Hee Lee, Jong-Hoon Kim, Young Beom Seo, Yoo Jin Choo, Juyoung Park, and Min Cheol Chang. "The use of dynamic contrast-enhanced magnetic resonance imaging for the evaluation of blood-brain barrier disruption in traumatic brain injury: what is the evidence?" Brain Sciences 11, no. 6 (2021): 775. [CrossRef]
- Iyad, N.; S. Ahmad, M.; Alkhatib, S.G.; Hjouj, M. Gadolinium contrast agents- challenges and opportunities of a multidisciplinary approach: Literature review. Eur. J. Radiol. Open 2023, 11, 100503. [Google Scholar] [CrossRef]
- Rasschaert, Marlène, Roy O. Weller, Josef A. Schroeder, Christoph Brochhausen, and Jean-Marc Idée. "Retention of gadolinium in brain parenchyma: pathways for speciation, access, and distribution. A critical review." Journal of Magnetic Resonance Imaging 52, no. 5 (2020): 1293-1305. [CrossRef]
- Stanescu, A.L.; Shaw, D.W.; Murata, N.; Murata, K.; Rutledge, J.C.; Maloney, E.; Maravilla, K.R. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr. Radiol. 2020, 50, 388–396. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: a critical review. Front. Neuroenergetics 2014, 5, 13. [Google Scholar] [CrossRef]
- Gandy, S.; DeKosky, S.T. [18F]-T807 tauopathy PET imaging in chronic traumatic encephalopathy. F1000Research 2014, 3, 229. [Google Scholar] [CrossRef]
- Selwyn, R.; Hockenbury, N.; Jaiswal, S.; Mathur, S.; Armstrong, R.C.; Byrnes, K.R. Mild Traumatic Brain Injury Results in Depressed Cerebral Glucose Uptake: An 18FDG PET Study. J. Neurotrauma 2013, 30, 1943–1953. [Google Scholar] [CrossRef]
- Wu, H.-M.; Huang, S.-C.; Hattori, N.; Glenn, T.C.; Vespa, P.M.; Yu, C.-L.; Hovda, D.A.; Phelps, M.E.; Bergsneider, M. Selective Metabolic Reduction in Gray Matter Acutely following Human Traumatic Brain Injury. J. Neurotrauma 2004, 21, 149–161. [Google Scholar] [CrossRef]
- Hattori, N.; Huang, S.-C.; Wu, H.-M.; Liao, W.; Glenn, T.C.; Vespa, P.M.; E Phelps, M.; A Hovda, D.; Bergsneider, M. Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. . 2004, 45, 775–83. [Google Scholar]
- Bergsneider, M.; Hovda, D.A.; Shalmon, E.; Kelly, D.F.; Vespa, P.M.; Martin, N.A.; Phelps, M.E.; McArthur, D.L.; Caron, M.J.; Kraus, J.F.; et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997, 86, 241–251. [Google Scholar] [CrossRef]
- Worley, G.; Hoffman, J.M.; Paine, S.S.; Kalman, S.L.; Claerhout, S.J.; Boyko, O.B.; Kandt, R.S.; Santos, C.C.; Hanson, M.W.; Oakes, W.J.; et al. 18-FLUORODEOXYGLUCOSE POSITRON EMISSION TOMOGRAPHY IN CHILDREN AND ADOLESCENTS WITH TRAUMATIC BRAIN INJURY. Dev. Med. Child Neurol. 1995, 37, 213–220. [Google Scholar] [CrossRef]
- Bergsneider, M.; Hovda, D.A.; Lee, S.M.; Kelly, D.F.; McARTHUR, D.L.; Vespa, P.M.; Lee, J.H.; Huang, S.-C.; Martin, N.A.; Phelps, M.E.; et al. Dissociation of Cerebral Glucose Metabolism and Level of Consciousness During the Period of Metabolic Depression Following Human Traumatic Brain Injury. J. Neurotrauma 2000, 17, 389–401. [Google Scholar] [CrossRef]
- level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma. 2000; 17:389–401.
- Buchsbaum MS, Simmons AN, DeCastro A, Farid N, Matthews SC. Clusters of low 18F-fluorodeoxyglucose uptake voxels in combat veterans with traumatic brain injury and post-traumatic stress disorder. J Neurotrauma. 2015; 32:1736–50. [CrossRef]
- Komura, A.; Kawasaki, T.; Yamada, Y.; Uzuyama, S.; Asano, Y.; Shinoda, J. Cerebral Glucose Metabolism in Patients with Chronic Mental and Cognitive Sequelae after a Single Blunt Mild Traumatic Brain Injury without Visible Brain Lesions. J. Neurotrauma 2019, 36, 641–649. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Shivamurthy, V.K.N.; Tahari, A.K.; Marcus, C.; Subramaniam, R.M. Brain FDG PET and the Diagnosis of Dementia. Am. J. Roentgenol. 2015, 204, W76–W85. [Google Scholar] [CrossRef]
- Moghbel MC, Saboury B, Basu S, Metzler SD, Torigian DA, Långström B, et al. Amyloid-β imaging with PET in Alzheimer’s disease: is it feasible with current radiotracers and technologies? Springer; 2012.
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Barrio JR, Small GW, Wong K-P, Huang S-C, Liu J, Merrill DA, et al. In vivo characterization of chronic traumatic encephalopathy using [F-18] FDDNP PET brain imaging. Proc Natl Acad Sci. E: 2015;112, 2015. [CrossRef]
- Harada, R.; Okamura, N.; Furumoto, S.; Tago, T.; Yanai, K.; Arai, H.; Kudo, Y. Characteristics of Tau and Its Ligands in PET Imaging. Biomolecules 2016, 6, 7. [Google Scholar] [CrossRef]
- Choi SR, Schneider JA, Bennett DA, Beach TG, Bedell BJ, Zehntner SP, et al. Correlation of amyloid PET ligand florbetapir F 18 binding with Aβ aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis Assoc Disord.2012;26:8–16. [CrossRef]
- Lister-James, J.; Pontecorvo, M.J.; Clark, C.; Joshi, A.D.; Mintun, M.A.; Zhang, W.; Lim, N.; Zhuang, Z.; Golding, G.; Choi, S.R.; et al. Florbetapir F-18: A Histopathologically Validated Beta-Amyloid Positron Emission Tomography Imaging Agent. Semin. Nucl. Med. 2011, 41, 300–304. [Google Scholar] [CrossRef]
- Baker, S.L.; Harrison, T.M.; Maass, A.; La Joie, R.; Jagust, W.J. Effect of Off-Target Binding on 18F-Flortaucipir Variability in Healthy Controls Across the Life Span. J. Nucl. Med. 2019, 60, 1444–1451. [Google Scholar] [CrossRef]
- Dani, M.; Brooks, D.J.; Edison, P. Tau imaging in neurodegenerative diseases. Eur. J. Nucl. Med. 2015, 43, 1139–1150. [Google Scholar] [CrossRef]
- Kantarci K, Lowe VJ, Boeve BF, Senjem ML, Tosakulwong N, Lesnick TG, et al. AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol. 2017; 81:58–67. [CrossRef]
- Joseph, C.R.; Benhatzel, C.M.; Stern, L.J.; Hopper, O.M.; Lockwood, M.D. Pilot study utilizing MRI 3D TGSE PASL (arterial spin labeling) differentiating clearance rates of labeled protons in the CNS of patients with early Alzheimer disease from normal subjects. Magma: Magn. Reson. Mater. Physics, Biol. Med. 2020, 33, 559–568. [Google Scholar] [CrossRef]
- Joseph, C.R.; Lim, J.K.; Grohol, B.N.; Zivcevska, M.; Lencke, J.; Rich, E.D.; Arrasmith, C.J.; Dorman, I.S.; Clark, B.W.; Love, K.; et al. Identifying delay in glymphatic clearance of labeled protons post-acute head trauma utilizing 3D ASL MRI (arterial spin labeling): a pilot study. Sci. Rep. 2024, 14, 1–10. [Google Scholar] [CrossRef]
- Zhao, Moss Y., Audrey P. Fan, David Yen-Ting Chen, Magdalena J. Sokolska, Jia Guo, Yosuke Ishii, David D. Shin et al. "Cerebrovascular reactivity measurements using simultaneous 15O-water PET and ASL MRI: Impacts of arterial transit time, labeling efficiency, and hematocrit." Neuroimage 233 (2021): 117955. [CrossRef]
- Elschot, E.P.M.; Backes, W.H.; Postma, A.A.; van Oostenbrugge, R.J.; Staals, J.; Rouhl, R.P.; Jansen, J.F. A Comprehensive View on MRI Techniques for Imaging Blood-Brain Barrier Integrity. Investig. Radiol. 2020, 56, 10–19. [Google Scholar] [CrossRef]
- Joseph, C.R.; Kreilach, A.; Reyna, V.A.; Kepler, T.A.; Taylor, B.V.; Kang, J.; McCorkle, D.; Rider, N.L. Utilizing Reduced Labeled Proton Clearance to Identify Preclinical Alzheimer Disease with 3D ASL MRI. Case Rep. Neurol. 2023, 15, 177–186. [Google Scholar] [CrossRef]
- Takahata, K.; Kimura, Y.; Sahara, N.; Koga, S.; Shimada, H.; Ichise, M.; Saito, F.; Moriguchi, S.; Kitamura, S.; Kubota, M.; et al. PET-detectable tau pathology correlates with long-term neuropsychiatric outcomes in patients with traumatic brain injury. Brain 2019, 142, 3265–3279. [Google Scholar] [CrossRef]
- Gorgoraptis, N.; Li, L.M.; Whittington, A.; Zimmerman, K.A.; Maclean, L.M.; McLeod, C.; Ross, E.; Heslegrave, A.; Zetterberg, H.; Passchier, J.; et al. In vivo detection of cerebral tau pathology in long-term survivors of traumatic brain injury. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Gandy, Sam, Milos D. Ikonomovic, Effie Mitsis, Gregory Elder, Stephen T. Ahlers, Jeffrey Barth, James R. Stone, and Steven T.DeKosky. "Chronic traumatic encephalopathy: clinical biomarker correlations and current concepts in pathogenesis." Molecular neurodegeneration 9 (2014): 1-22. [CrossRef]
- Ware, Jeffrey B., Saurabh Sinha, Justin Morrison, Alexa E. Walter, James J. Gugger, Andrea LC Schneider, Cian Dabrowski et al. "Dynamic contrast enhanced MRI for characterization of blood-brain-barrier dysfunction after traumatic brain injury." NeuroImage: Clinical 36 (2022): 103236. [CrossRef]
- Morgan, Richard, Jordon Prosapio, Sam Kara, Sreepadma Sonty, Pamela Youssef, and Kester Nedd. "Preliminary clinical diagnostic criteria for chronic traumatic encephalopathy: A case report and literature review." Interdisciplinary Neurosurgery 26 (2021): 101290. [CrossRef]
- Li, Ka-loh, Xiaoping Zhu, Nola Hylton, Geon-Ho Jahng, Michael W. Weiner, and Norbert Schuff. "Four-phase single-capillary stepwise model for kinetics in arterial spin labeling MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 53, no. 3 (2005): 511-518. [CrossRef]
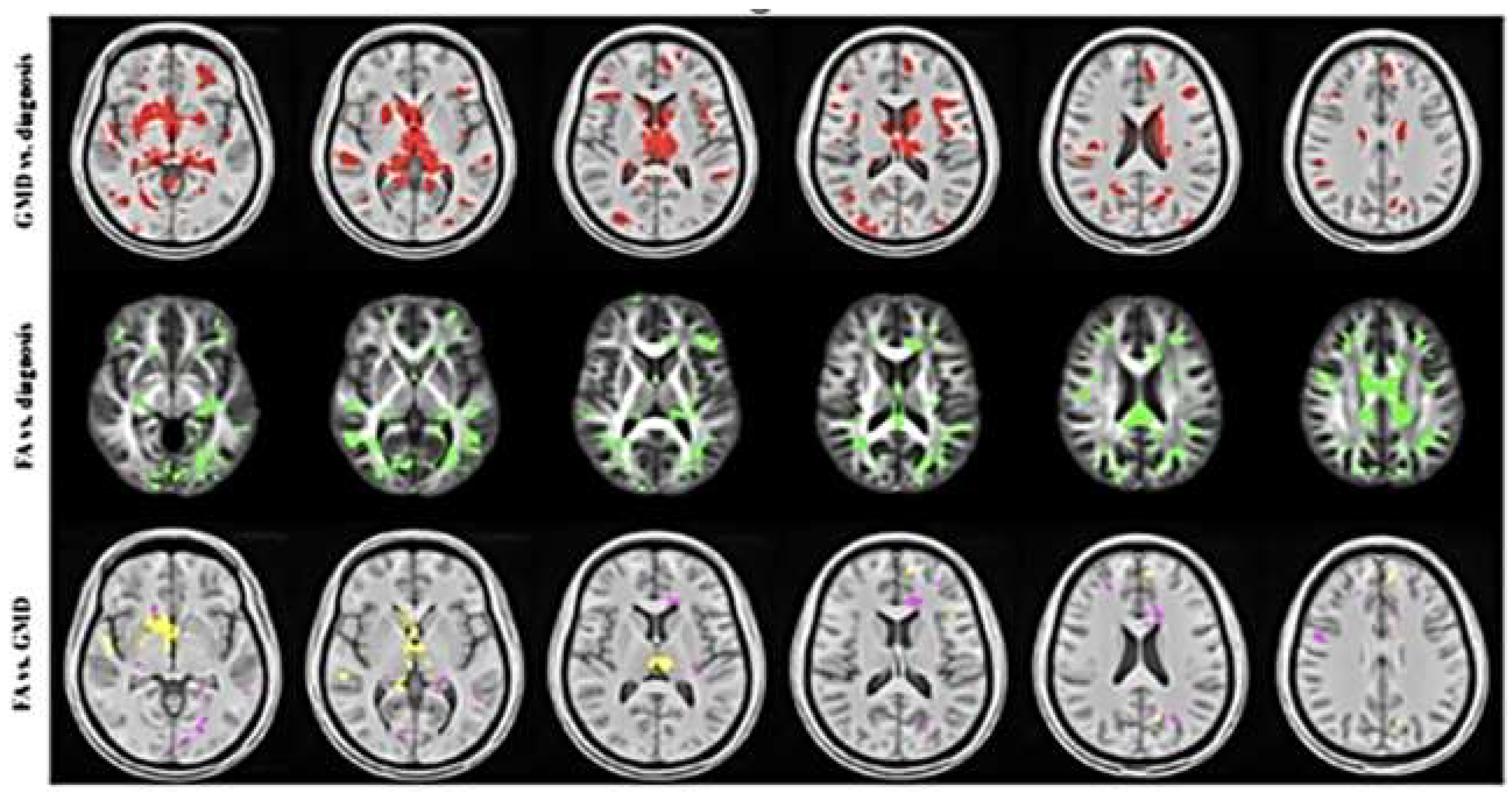
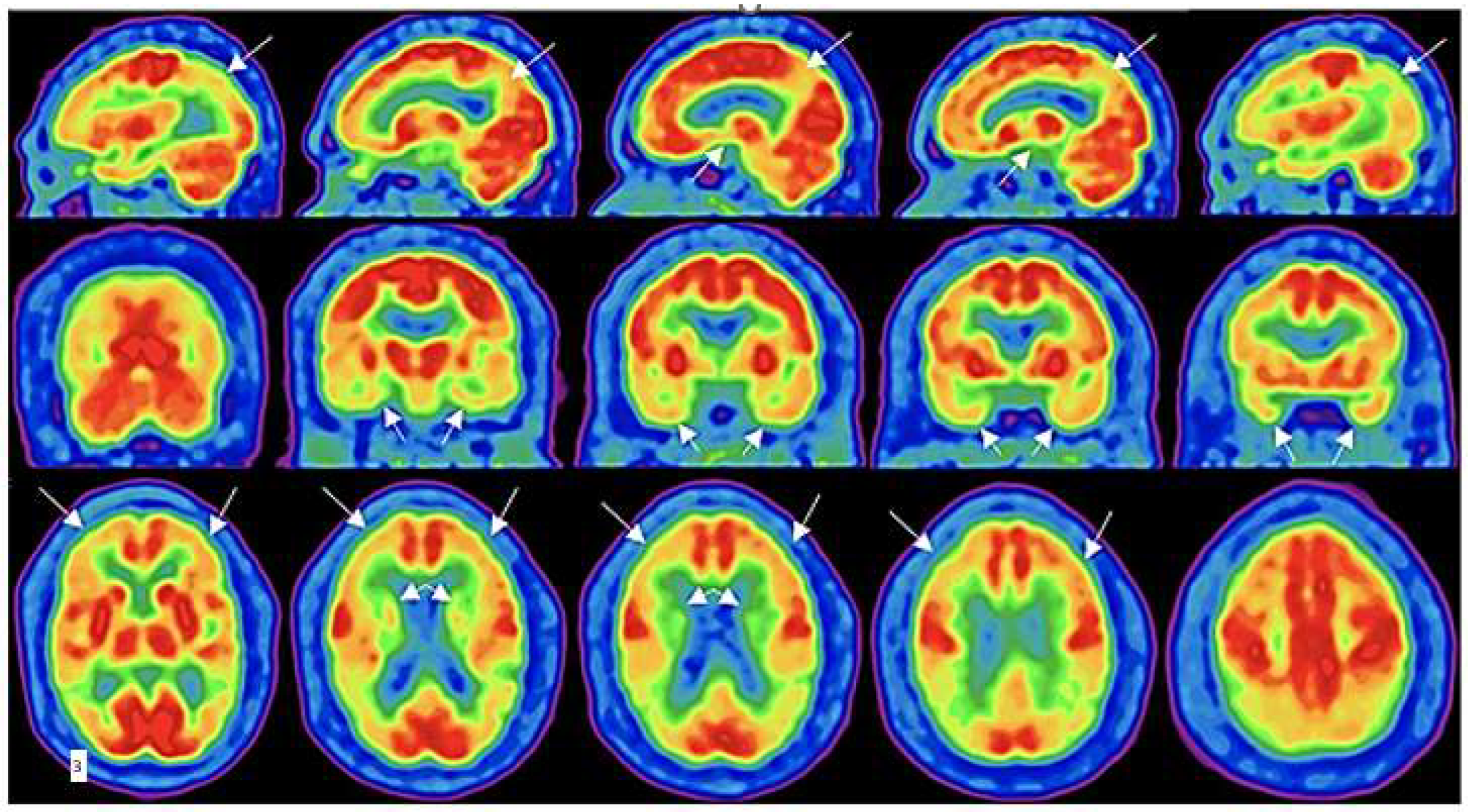
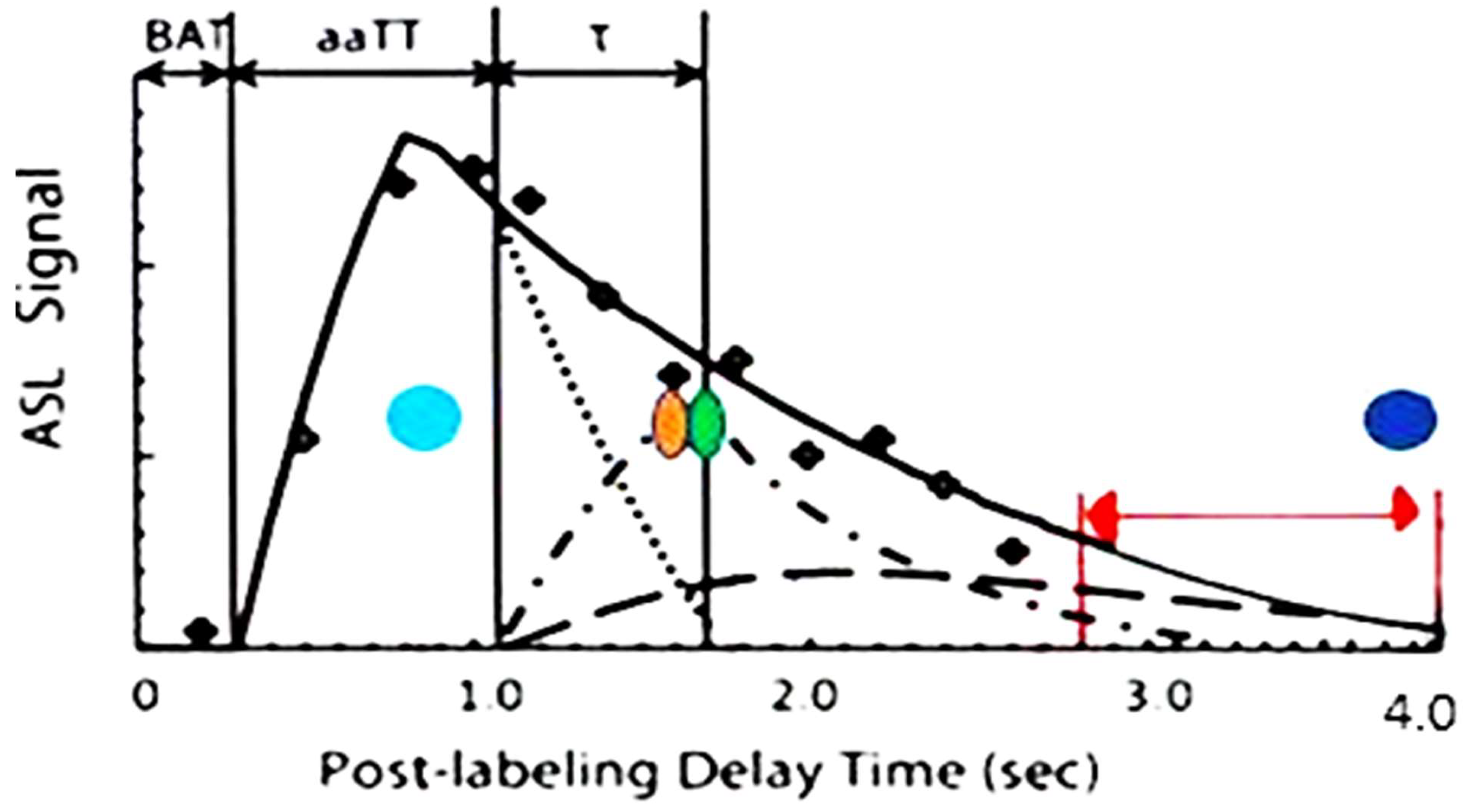
| Imaging procedure. | Target | Sensitivity | Limitations |
|---|---|---|---|
| Diffusion Tensor Imaging (DTI) MRI | Diffusion of water -white matter tracts | Not clear, older techniques problematic. New techniques in development | Technique specific sensitivity, scan time |
| Dynamic Contrast Enhanced (DCE) MRI | BBB leak of contrast | Sensitive for BBB leak | Requires gadolinium contrast, long scan times, not suitable for serial studies due to cerebral accumulation of GD+ |
| 18FDG-PET | Glucose utilization metabolic activity | Can identify the brief hypermetabolism acutely (animal studies) and chronic hypometabolism (human) chronically across severity spectrum | Cost, long scan times, availability of technology, |
| 3D ASL MRI | Perfusion/Diffusion of labeled protons | Highly sensitive in a small clinical trial in mTBI determining altered cMTT/BBB leak with delayed BBB clearance/recovery | Requires a 3T or greater field strength, Low signal, hand drawn ROI, requires a larger series for confirmation of sensitivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





