1. Introduction
Lymphadenomegaly, or the enlargement of lymph nodes, can occur in dogs suffering from various conditions, including neoplasms [
1,
2,
3], infectious diseases, or idiopathic ailments [
4,
5], as well as inflammatory disorders [
6,
7]. In the realm of veterinary literature, the presence of enlarged lymph nodes frequently captures attention in oncological studies, particularly those focusing on lymphoma or lymph node metastasis [
8,
9,
10,
11]. Notably, neoplastic diseases have been found to be the most prevalent diagnosis in both dogs and cats presenting with radiologically enlarged lymph nodes [
12]. As a result, lymph nodes are often targeted in oncological assessments, including procedures like sentinel lymph node biopsies [
13]. In both veterinary and human medical literature [
14], inflammatory disorders are also acknowledged as differential diagnoses for cases involving lymph node enlargement. However, the significance of such enlargement in the context of evaluating inflammatory conditions in dogs has been discussed rather infrequently. This lack of discourse may stem from the reality that during examinations of enlarged lymph nodes in canines, inflammation can coexist with neoplasia, making it challenging to differentiate between inflammatory and neoplastic disorders without observing significant lymphadenopathy. Therefore, there is a pressing need for thorough investigations focused on lymph node size in dogs that have exclusively non-neoplastic disorders. Such studies are essential to clarify the intricate relationship between lymphadenomegaly and inflammation, particularly in the context of systemic inflammation. Nonetheless, to the authors' knowledge, no previous investigations addressing this specific issue have been reported in the veterinary literature.
Dogs with neoplastic disorders may represent a valuable target population for research focused on lymphadenomegaly and systemic inflammation. However, this raises an important question regarding the most effective methods for evaluating lymph nodes in this group. Typically, lymph node enlargement is assessed clinically through the palpation of superficial lymph nodes, but this approach is inherently qualitative and does not provide insight into deeper lymph nodes, which may be more sensitive to systemic inflammation due to their anatomical positioning.
Ultrasonography has been proposed as a method for assessing deep lymph nodes in dogs. Although this technique has been utilized in oncological studies, it has proven to be somewhat problematic for diagnostics, often lacking the ability to effectively differentiate between malignant and benign deep lymph nodes based solely on hypoechogenicity and other imaging findings [
15]. Additionally, factors like gas-related distortion of sound waves can hinder accurate ultrasonographic visualization of these deeper structures [
16].
As a potentially superior alternative, computed tomography (CT) offers a useful, non-invasive imaging modality for evaluating deeper lymph nodes. Unlike ultrasonography, CT provides robust visualization that is unaffected by respiratory gases. It also delivers high-resolution images quickly and accurately identifies lymph node locations [
16]. The advantages of CT for deep lymph assessments have been demonstrated in various oncological investigations. For instance, it has enabled the differentiation between malignant and benign tumors based on characteristic imaging of sternal lymph nodes, and it has shown that a tracheobronchial lymph node diameter greater than 12 mm significantly increases the likelihood of metastatic tumors [
17]. Moreover, CT facilitates accurate measurement of geometric parameters, including the diameters of deep lymph nodes, thereby generating quantitative data that can enhance statistical evaluations of neoplastic systemic inflammation and improve diagnostic accuracy for neoplastic disorders. This combination of factors underscores the potential for CT to play a pivotal role in the assessment and management of lymphadenomegaly in dogs with neoplastic diseases.
An evaluation of lymphadenomegaly in inflammatory disorders would benefit from evidence on associations between deep lymph node geometry and relevant biomarkers of systemic inflammation. In both veterinary and human medicine, C reactive protein (CRP) is widely used as one such marker. This acute phrase protein also has utility in assessments of tumors and for post-surgical monitoring [
19,
20,
21], and it is rapidly and markedly elevated at the onset of inflammation. CRP is a very sensitive, albeit not selective, marker of inflammation. Establishing associations between deep lymph node enlargement and plasma CRP concentration would thus provide useful evidence on the sensitivity of the former in inflammatory conditions, but there is a paucity of reported evidence on any such association in dogs. We speculated that there would be a relationship between serum CRP levels and the maximal diameters of deep lymph nodes.
Accordingly, in this study, we aimed to investigate associations between lymphadenomegaly and levels of the inflammatory marker CRP, using CT images of deep lymph nodes (sternal, cranial mediastinal, and iliac) in small dogs with non-neoplastic disorders.
2. Materials and Methods
2.1. Study Population
For evaluation in this retrospective study, we targeted 369 dogs which underwent CT examinations and plasma CRP measurements on the same day, while receiving medical care at Kagoshima University Veterinary Teaching Hospital (Kagoshima, Japan) between January and December 2022. The inclusion criteria were a body weight not exceeding 10 kg, so as to minimize any bias in body weight or size (set in reference to a previous report [
22]), and the presence of tomographically measurable sternal, cranial mediastinal, and/or internal iliac lymph nodes. Dogs were excluded from the study when their cases corresponded to any of the following conditions: diagnosis of a neoplasm, previous treatment for a neoplasm, and any evidence in their medical history that could give rise to suspicion of cancer and/or metastasis. These exclusion criteria were set to ensure that no cases of lymphadenomegaly due to lymph node metastasis or inflammatory reactive hyperplasia associated with the cancer were included in this study. Ethics approval was waived for this retrospective study, because it did not involve any procedures with experimental animals. It was conducted in accordance with the research ethics bylaws of Kagoshima University. The owner of each dog evaluated in this study had given consent to the use of the relevant data in research at the time of medical examinations.
2.2. Evaluation of Lymph Node Images
The dogs had undergone CT imaging with a dedicated 16-herical sliced CT scanner (Aquilion TSX-201A, Toshiba Medical Systems Corporation, Tochigi, Japan), with or without anesthesia. These CT images were acquired with reading and measurement under two conditions (soft tissue condition WL50:WW350; lung condition WL:-50WW:1500). Slice thickness was 1 mm. Blood was collected from each dog on the same day as the CT imagining, for standard blood chemistry examinations, which included measurement of CRP concentration in plasma with a clinical chemistry analyzer (Fuji DRI-CHEM NX500, Fujifilm Corporation, Japan).
In this study, the CT images for each dog were retrieved, for a determination of lymph node diameters in three target regions. All images were acquired in DICOM format and were read and measured using the DICOM viewer OsiriX Ver. 5.9. As target regions, those for the sternal and cranial mediastinal lymph nodes were selected as corresponding to the most commonly identified nodes by CT within the thoracic cavity [
16], and that for the internal iliac lymph nodes was then selected as these nodes are adjacent to the abdominal aorta and are among the most easily assessable nodes in the abdominal cavity.
Transverse CT images of the thoracic and abdominal cavities of each dog were examined for the target lymph nodes. The sternal, cranial mediastinal, and internal iliac lymph nodes were identified on the relevant CT image, as structures of the expected appearance (spherical or oval in shape, with distinct margins) at their expected anatomical locations.
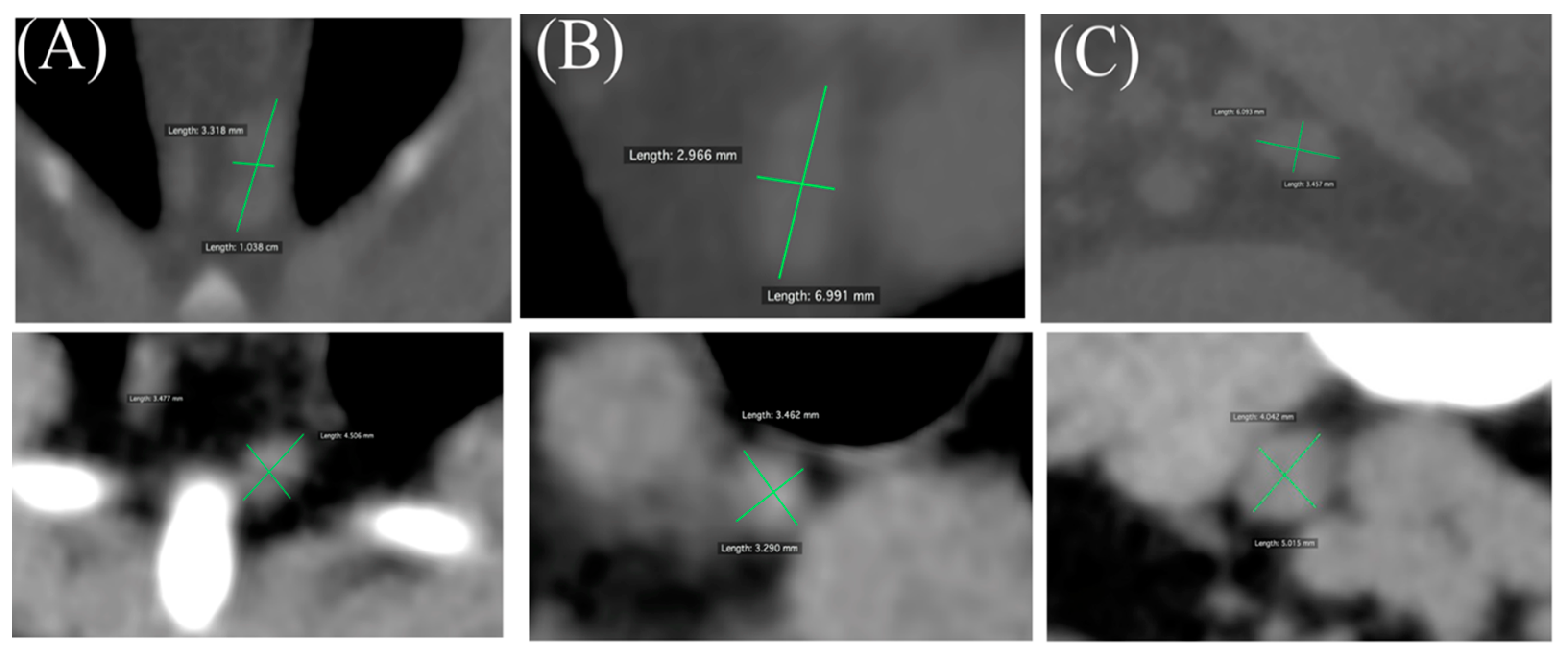
When target lymph nodes were identified, two notional, perpendicular lines were plotted through the center of the relevant lymph line extending to its border, and the longer of the two lines was regarded as its maximal diameter. Its measured length was read by the DICOM viewer scale function to the nearest mm and adopted as the value for evaluation (
Figure 1). In cases of multiple lymph nodes at a given location, the maximal diameter of the largest node was adopted as the measurement result. Lymph nodes were considered unmeasurable and excluded from the analysis when CT images did not show a structure of the expected appearance at the expected anatomical location, an occupying lesion was detected there, or there was evidence of pleural fluid or ascites. Measurements were performed by a single veterinarian (YI) and confirmed by a second veterinarian (NM) engaged at the Diagnostic Imaging Department of Kagoshima University Veterinary Teaching Hospital.
A measurable lymph node was regarded as a spherical or oval marginal structure with a well-defined boundary at an anatomically expected location. Two notional lines were drawn perpendicular to the boundary line, the longer of the two lines was considered the largest diameter, and the measured length was taken as the value for evaluation.
Representative examples of clinically enlarged (upper) and clinically unremarkable (lower) lymph nodes are shown for sternal (A), cranial mediastinal (B), and internal iliac (C) lymph nodes.
2.3. Other Data Retrieved
The medical records for each dog with measurable lymph nodes were retrieved, and demographic information (age, breed, sex, and body weight), and plasma CRP concentration and white blood cell count (WBC) were collated for evaluation in this study. Each medical record was also checked for any findings of superficial lymph node enlargement on palpation.
2.4. Associations between Lymph Node Enlargement and CRP Level
Lymph nodes were regarded as enlarged when their diameter exceeded a threshold value, set in reference to the literature (sternal lymph node: 7 mm; cranial mediastinal and internal iliac lymph nodes: 6 mm) [
24,
25,
26,
27].Plasma CRP concentrations >0.7mg/dL were regarded as clinically elevated, based on the reference range at our institution. Lymph node diameters were compared between dogs with clinically elevated and clinically unremarkable plasma CRP, using Welch's t test.
2.5. Statistics Analysis
We evaluated the correlation between lymph node diameter and plasma CRP concentration in a single regression analysis, but the model showed poorness of fit. Thus, we also evaluated these parameters in multiple regression analyses, with WBC and the presence of intra-abdominal disease as independent variables, to obtain an adjusted R
2 value derived from correlation coefficients for evaluation (intra-abdominal disease was regarded as a condition developing in an organ or structure within the abdominal cavity, based on the individual dog’s medical record). All analyses were performed using a statistical software package (EZR version 1.64, Saitama Medical Center, Jichi Medical University, Japan) [
27]. P values < 0.05 were regarded as statistically significant.
3. Results
Demographic and clinical characteristics of the study population
A total of 40 dogs were included in the study population (mean age: 14.2 years; mean body weight 4.9 kg), of which 15 were neutered males, four were intact males, 14 were neutered females, and seven were intact females. The dog breeds encompassed in this study (in descending order of magnitude) were Miniature Dachshund (n = 11), Toy poodle (n = 9), Chihuahua (n = 7), Shih Tzu (n = 4), Papillon (n = 2), Yorkshire terrier (n = 2), French bulldog (n = 1), Miniature schnauzer (n = 1), Miniature pinscher (n = 1), Shiba Inu (n = 1), and mixed breed (n = 1). The diagnosed conditions for this study population (as determined by the relevant original attending veterinarian) encompassed Cushing’s syndrome (n=4); single or multiple cervical dish herniation (n = 3); cystoliths, herniated disc, gallbladder mucocele, intestinal or small intestinal foreign body, and tracheal collapse (n = 2 each); and suspected pancreatitis, lesional adherence to the radial-ulnar ligament, immune-mediated neutropenia, chronic bronchitis, spinal osteoarthritis, rhinitis, encephalitis, idiopathic polyarthritis, cholelithiasis, aortic thrombus, polyarthritis, choledocholithiasis, duodenal polyposis, syncope, uterine cysticercosis, eosinophilic enteritis, laryngeal paralysis, megaesophagus, acute pancreatitis, hepatitis, diaphragmatic hernia, inflammatory polyps, and lymphocytic plasmatic rhinitis, and epilepsy (n = 1 each). Demographic and diagnostic data are presented in
Table 1.
For these 40 dogs, the sternal, cranial mediastinal, and internal iliac lymph nodes were measurable in 34, 34, and 39 cases, respectively. Imaged lymph nodes measured were mostly of predefined morphology and enhanced shade. In some cases, low-absorption areas were visible within the lymph nodes, but they did not affect determination of nodal morphology or diameter. Representative CT images of measurable lymph nodes are shown in
Figure 1.
Based on the measured diameter, the subpopulations with enlarged lymph nodes were as below. A total of 2/34 dogs (5.8%) showed enlarged sternal lymph nodes (7.4 or 8.1 mm). Both of these dogs were neutered females, one was a Miniature Dachshund (age: 1 year 5 months, body weight: 9.3 kg) diagnosed with an intestinal foreign body, and the other was a Toy Poodle (age: 10 months, body weight: 2.94 kg) diagnosed with diaphragmatic hernia.
A total of 4/34 dogs (11.7%) showed enlarged cranial mediastinal lymph nodes (range: 6.2 mm to 9.3 mm). These dogs comprised one intact and three neutered males (total: n=4; age: 2 years 6 months to 11 years 6 months), two of which were Toy Poodles, one was a Chihuahua, and one was a Miniature Pinscher. The diagnosed conditions for these dogs were duodenal intussusception, Cushing's syndrome, immune-mediated neutropenia, and cervical disc herniation (n = 1 each).
A total of 9/39 dogs (23.0%) showed enlarged internal iliac lymph nodes (range: 6.0 to 8.1 mm). These dogs comprised two intact and six neutered males and one neutered female (age: 2 years 4 months to 15 years 7 months). The diagnosed conditions for these dogs were epilepsy, syncope, gallbladder mucocele, cholelithiasis, Cushing syndrome, immune-mediated neutropenia, duodenal intussusception, and cervical disc herniation (n=1, each).
Blood chemistry revealed that 18/40 dogs (45%) had clinically elevated plasma CRP. Furthermore, 12/40 dogs (30%) showed elevated WBC counts (
Table 1).
Palpably enlarged superficial lymph nodes were only noted in one case (an instance of enlarged mandibular lymph nodes).
Deep lymph node evaluation in dogs with elevated plasma CRP
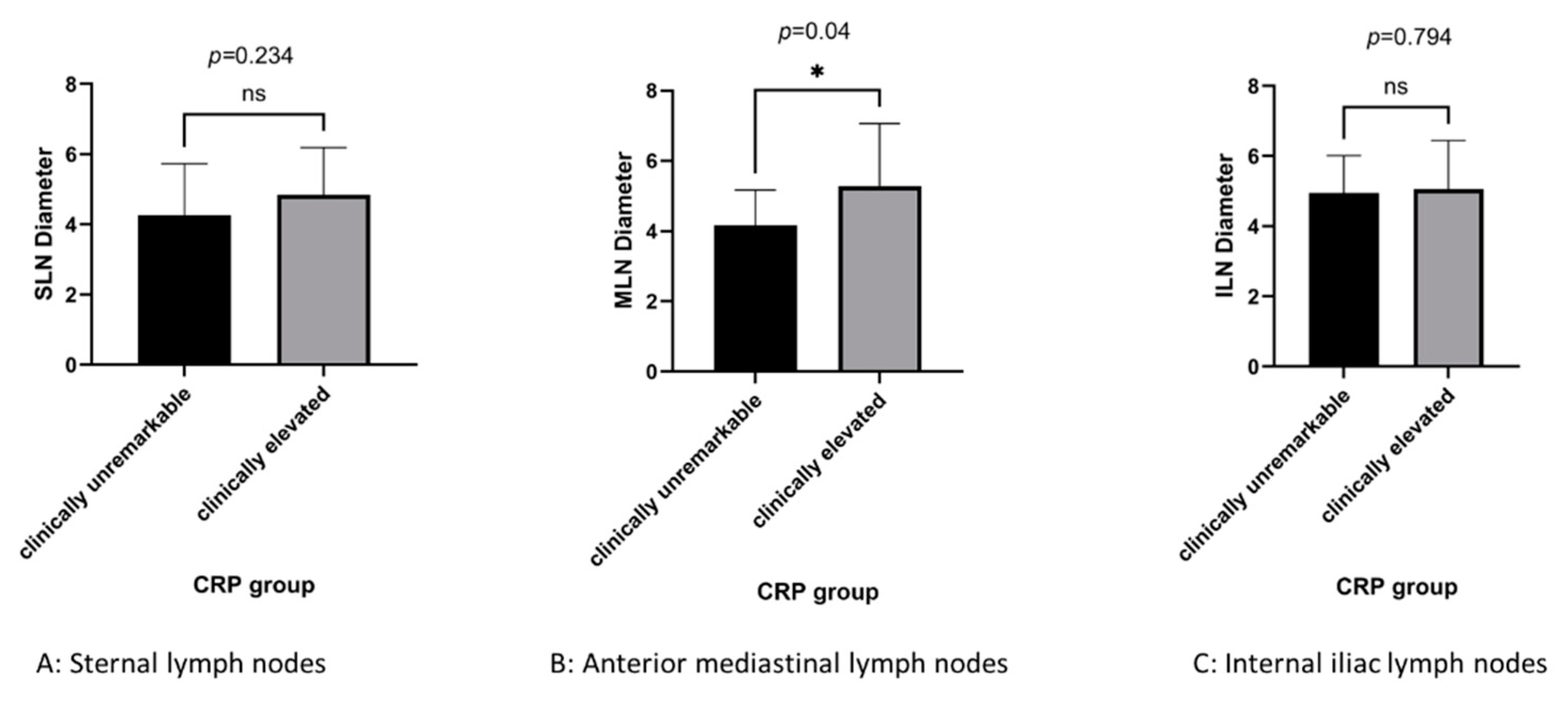
Lymph node diameters were compared between dogs with clinically elevated and clinically unremarkable plasma CRP concentrations. Dogs with clinically elevated plasma CRP showed significantly greater cranial mediastinal lymph node diameter than dogs with clinically unremarkable plasma CRP concentration (5.28 vs. 4.17 mm; p = 0.04), but no significant differences for the sternal (4.84 vs. 4.25 mm; p = 0.247) or internal iliac (5.05 vs. 4.95 mm; p = 0.799) lymph node (
Figure 2).
Lymph node diameters (mm) in dogs with clinically elevated or clinically unremarkable plasma CRP levels. Based on the reference values testing companies provided, CRP was categorized as clinically unremarkable at concentrations <0.7mg/dL, or elevated at concentrations of 0.7-7.0< mg/dL.
Associations between lymph node diameter and plasma CRP concentration in simple and multiple regression analyses.
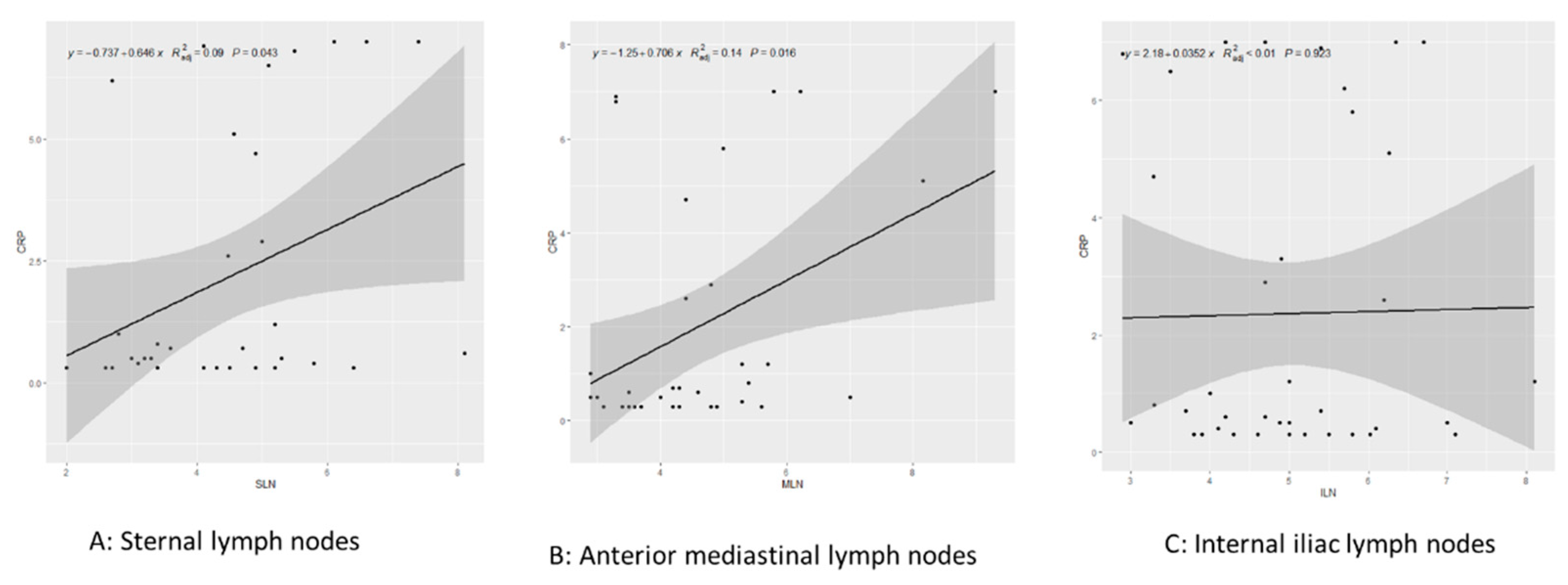
Single regression analysis revealed that cranial mediastinal node diameter was significantly correlated with plasma CRP concentration (adjusted R
2=0.15, p=0.01>a), but we found no significant correlations for the sternal (adjusted R
2=0.09, p=0.122) or internal iliac (adjusted R
2=−0.005, p=0.43) lymph node (
Figure 3).
In multiple regression analysis, neither WBC count nor the presence/absence of intraabdominal disease was significantly correlated with plasma CRP concentration. The variance inflation factor (VIF) was < 2 for all variables in the regression model (
Table 2).
Figure 3.
Single regression analysis targeting lymph node size and CRP concentration.
Figure 3.
Single regression analysis targeting lymph node size and CRP concentration.
A: sternal lymph nodes; B: anterior mediastinal lymph nodes; C: internal iliac lymph nodes.
Each graph shows the relevant regression line, with the gray area representing the 95% confidence interval.
Table 2.
Multiple regression analysis for WBC, abdominal disease, and CRP by lymph node.
Table 2.
Multiple regression analysis for WBC, abdominal disease, and CRP by lymph node.
| |
β |
t |
p |
95%CI |
| Sternal lymph nodes |
|
|
|
|
| CRP(mg/dL) |
0.13 |
1.39 |
0.17 |
-0.06-0.34 |
| WBC(k/μL) |
0.01 |
0.72 |
0.47 |
-0.03-0.06 |
Abdominal disease
(Yes=1, No=0) |
0.57 |
1.14 |
0.26 |
-0.45-1.6 |
| |
|
|
|
|
| Cranial mediastinal lymph nodes |
|
|
|
|
| CRP(mg/dL) |
0.33 |
3.01 |
<0.01 * |
0.10-0.56 |
| WBC(k/μL) |
-0.03 |
-1.35 |
0.18 |
-0.08-0.02 |
Abdominal disease
(Yes=1, No=0) |
-0.44 |
-0.87 |
0.38 |
-1.49-0.6 |
| |
|
|
|
|
| Internal iliac lymph nodes |
|
|
|
|
| CRP(mg/dL) |
-0.06 |
-0.72 |
0.475 |
-0.24-0.11 |
| WBC(k/μL) |
0.0032 |
1.65 |
0.107 |
-0.01-0.07 |
Abdominal disease
(Yes=1, No=0) |
0.026 |
0.06 |
0.949 |
-0.81-0.86 |
4. Discussion
In this study, we investigated associations between CT-measured canine lymphadenomegaly and elevated CRP in dogs without neoplastic disorders, targeting deep lymph nodes (cranial mediastinal, sternal, and internal iliac lymph nodes), based on our speculation that serum CRP levels would show associations with deep lymph node diameter in this population. Although this acute phase protein may not totally reflect an inflammatory condition, we consider that its utility for quantitative assessments make it a biologically relevant parameter for this study. To the authors’ knowledge, this is the first report on such an association in the canine medical literature.
As a key finding, somewhat surprisingly, only the cranial mediastinal lymph node showed an association with CRP. Cranial mediastinal lymph node diameter was significantly correlated with plasma CRP concentration, and dogs with clinically elevated CRP showed a significantly greater cranial mediastinal lymph node diameter than dogs with clinically unremarkable CRP. Previously, mediastinal lymphadenomegaly has largely been reported in dogs with neoplastic diseases or heart failure [
28,
29,
30], and in the human literature, it has also been associated with cystic fibrosis, cardiac disorders, and COVID-19 [
32,
33,
34]. The dogs with enlarged mediastinal lymph nodes in this study had been diagnosed with a range of conditions (polyarthritis, idiopathic polyarthritis, disk herniation, and gallbladder mucocele; n=1 for each). Systemic inflammatory responses have been reported in dogs with idiopathic polyarthritis, disc herniation, and gallbladder mucocele. Since elevated serum CRP levels accompanying systemic inflammatory response have been reported in gallbladder mucocele and polyarthritis, [
35,
36,
37], we consider that the cranial mediastinal lymph node enlargement in the corresponding cases in this study may well reflect systemic inflammation. On the other hand, more caution is required in interpreting the case of disk herniation in this study, since there are reports of no elevated serum CRP levels for this condition [
38,
39], and the cause of the elevated serum CRP levels in the relevant dog is unclear. Furthermore, the anatomical position of the cranial mediastinal lymph node lymph nodes [
40,
41], embedded in fat around central blood vessels such as the vena cava and aortic arch, indicates that these lymph nodes could be readily sensitive to systemic inflammatory responses. Our findings are thus not inconsistent with a phenomenon reflecting systemic inflammation. It is also noteworthy that our findings on cranial mediastinal lymph node enlargement came in a population with almost no palpably enlarged superficial lymph nodes (a single instance noted in 40 dogs).
In this study, we were unable to establish an association between sternal lymph node enlargement and systemic inflammation. The diameter of this lymph node did not differ significantly between dogs with clinically elevated CRP and those with clinically unremarkable CRP. Furthermore, sternal lymph node diameter and plasma CRP were not significantly correlated in multiple regression analysis, although we did note such a correlation in preliminary single regression analysis (which is not regarded as the major endpoint in this evaluation due to poorness of fit). Considering this preliminary finding, and the small number of dogs with enlarged sternal lymph nodes (n=2) in this study, we cannot exclude a potential association with CRP, which might emerge in analyses with a larger population. In previous reports in dogs, sternal lymphadenomegaly has been linked with lymphoma and metastatic tumors [
12,
22,
42,
43]; however, the dogs with enlarged sternal lymph nodes in this study were diagnosed with diaphragmatic hernia and an intestinal foreign body (n=1, each), and the authors are unaware of any reports on sternal lymphadenomegaly arising with these conditions in dogs.
This study provided no evidence that internal iliac lymph node enlargement reflects systemic inflammation, as we found no significant correlation with CRP in multiple regression analysis, or significant differences between dogs with clinically elevated and unremarkable CRP. The internal iliac lymph nodes are located within the hypogastric lymphosome, which occupies a relatively small region at the caudal end of the abdominopelvic cavity, indicating that these lymph nodes are potentially less sensitive to systemic inflammatory responses than the nodes in centers we investigated in the thoracic cavity.
Although lymph nodes are always likely to reflect localized inflammation, our findings suggest an interesting possibility that effects of systemic inflammation may vary in magnitude between lymph nodes at different anatomical locations in dogs. Some caution is required in interpreting these results though, because of the possibility that localized inflammation-induced lymph node enlargement may have occurred without CRP elevation in some cases, which might have affected the statistical evaluation. Anatomically, sternal lymph nodes are drain by lymphatic vessels to the sternal skin, mammary glands, chest wall, and abdominal wall, whereas mediastinal lymph nodes are drain the neck and chest regions [
44]. Accordingly, inflammation in these regions could be expected to cause reactive enlargement of these lymph nodes. Nevertheless, we speculate that lymph nodes such as the mediastinal lymph nodes, which are closer to the major blood vessels and thoracic ducts, may be more sensitive to systemic inflammation. Interestingly, almost no superficial lymph enlargement was reported in the dogs in this study, indicating potentially greater sensitivity for these deep lymph nodes and that images of deep lymph node enlargement may provide more useful clinical information on the lymphatic system than findings solely based on palpation.
Even with the caveats related to the interpretation of our findings, we suggest that CT findings of cranial mediastinal lymph node enlargement in dogs should prompt clinicians to consider inflammatory disorders, and this may illustrate one of the advantages of CT imaging. It is difficult to evaluate lymph node size using palpation or other imaging modalities such as ultrasonography. With CT, clinicians can accurately elucidate changes in lymph node size in a way that might otherwise not be possible. CT imaging has been proposed as a useful complementary technique for oncological evaluations [
18,
41], and we suggest it may have a similar potential utility for assessments of inflammation.
This study presents several limitations, partly due to its design as a hypothesis-generating investigation. Being a retrospective study, we could not confirm the pathological status of the lymph nodes directly; for superficial lymph nodes, we relied solely on information recorded in the medical records, which indicated whether they were enlarged. Additionally, our data were collected from dogs at a single time point—specifically, upon referral to our hospital—preventing us from exploring any temporal aspects of the relationship between CRP levels and lymph node diameter. Understanding this relationship over time would be valuable, particularly since CRP functions as an acute phase protein. Another limitation is the wide range of diagnoses represented among the dogs in this study, which complicates our ability to draw meaningful conclusions from a heterogeneous population. The hypotheses we are formulating regarding the potential utility of lymph node enlargement in evaluating inflammatory conditions will necessitate evidence from future studies conducted over extended periods, ideally within a more uniform population Given that CRP has a relatively short half-life in the bloodstream, it is more likely to reflect inflammation close to its onset. Thus, it would be beneficial to establish the time course of systemic inflammation-related lymph node enlargement and compare it to the timeline of CRP elevation. Furthermore, our analysis was limited to one biomarker (CRP). Expanding this investigation to include a broader array of biomarkers could yield additional valuable insights into the associations between lymph node size and systemic inflammation. Such an approach would enhance our understanding of the dynamics of inflammation in dogs and could inform better clinical practices.
5. Conclusions
We demonstrated that CT-measured maximal cranial mediastinal lymph node diameter may have some potential utility in evaluations of systemic inflammation, based on its association with measured plasma CRP concentration, in small dogs free of neoplastic disease. We suggest that CT determinations of cranial mediastinal lymphadenomegaly may prove useful in holistic assessments of dogs with inflammatory conditions. Such determinations should give rise to clinical consideration of inflammatory disorders when noted, and may inform clinical decisions on further diagnostic testing.
Author Contributions
Conceptualization, Y.I. and N.M.; methodology, Y.I.; validation, Y.I., Y.F.,T.S.,K.T.,T.K.,A.N. and M.T.; formal analysis, Y.I. and N.M..; investigation, Y.I., Y.F.,T.S.,K.T.,T.K.,A.N. and M.T.; data curation, Y.I., Y.F.,T.S.,K.T.,T.K.,A.N. and M.T.; writing—original draft preparation, Y.I.; writing—review and editing, N.M.; visualization, Y.I.; supervision, N.M.; project administration, Y.I. and N.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it did not involve any procedures with experimental animals. It was conducted in accordance with the research ethics bylaws of Kagoshima University. The owner of each dog evaluated in this study had given consent to the use of data in research at the time of medical examinations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank all our colleagues at Kagoshima University Veterinary Teaching Hospital for their help in collecting CT images and providing medical care to our patients, our fellow researcher, Nobuhiro Nozaki, for helping with statistical analysis, and Henry Smith (Co-chair of the Veterinary Special Interest Group in the European Medical Writers Association) for helping with the English editing of a draft of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zandvliet M. Canine lymphoma: a review. Vet Q. 2016;36(2):76-104. [CrossRef]
- Hisasue M, Nishimura T, Neo S, et al. A dog with acute myelomonocytic leukemia. J Vet Med Sci. 2008;70(6):619-621. [CrossRef]
- Sapierzyński R, Czopowicz M, Jagielski D. Metastatic lymphadenomegaly in dogs - cytological study. Pol J Vet Sci. 2017;20(4):731-736. [CrossRef]
- Golly E, Breitschwerdt EB, Balakrishnan N, Moore D, Bizikova P. Bartonella henselae, Bartonella koehlerae and Rickettsia rickettsii seroconversion and seroreversion in a dog with acute-onset fever, lameness, and lymphadenopathy followed by a protracted disease course. Vet Parasitol Reg Stud Reports. 2017;7:19-24. [CrossRef]
- Dor C, Gajanayake I, Kortum A, et al. Characterisation and outcome of idiopathic pyogranulomatous lymphadenitis in 64 English springer spaniel dogs. J Small Anim Pract. 2019;60(9):551-558. [CrossRef]
- Ribas Latre A, McPartland A, Cain D, et al. Canine sterile steroid-responsive lymphadenitis in 49 dogs. J Small Anim Pract. 2019;60(5):280-290. [CrossRef]
- Sapierzyński R, Micuń J. Lymphadenomegaly in dogs--cytological study. Pol J Vet Sci. 2009;12(2):263-268.
- Menghini TL, Schwarz T, Dancer S, Gray C, MacGillivray T, Blacklock KLB. Contrast-enhanced CT predictors of lymph nodal metastasis in dogs with oral melanoma. Vet Radiol Ultrasound. 2023;64(4):694-705. [CrossRef]
- Skinner OT, Boston SE, Giglio RF, Whitley EM, Colee JC, Porter EG. Diagnostic accuracy of contrast-enhanced computed tomography for assessment of mandibular and medial retropharyngeal lymph node metastasis in dogs with oral and nasal cancer. Vet Comp Oncol. 2018;16(4):562-570. [CrossRef]
- Cotter B, Zwicker LA, Waldner C, et al. Inter- and intraobserver agreement for CT measurement of mandibular and medial retropharyngeal lymph nodes is excellent in dogs with histologically confirmed oral melanoma. Vet Radiol Ultrasound. 2022;63(1):73-81. [CrossRef]
- Burgess HJ, MacDonald Dickinson V, Kerr M, Bienzle D. Marginal zone lymphoma in a dog. Vet Clin Pathol. 2020;49(2):312-318. [CrossRef]
- Smith K, O'Brien R. Radiographic characterization of enlarged sternal lymph nodes in 71 dogs and 13 cats. J Am Anim Hosp Assoc. 2012;48(3):176-181. [CrossRef]
- Suami H, Yamashita S, Soto-Miranda MA, Chang DW. Lymphatic territories (lymphosomes) in a canine: an animal model for investigation of postoperative lymphatic alterations. PLoS One. 2013;8(7):e69222. Published 2013 Jul 24. [CrossRef]
- Gaddey HL, Riegel AM. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am Fam Physician. 2016;94(11):896-903.
- De Swarte M, Alexander K, Rannou B, D'Anjou MA, Blond L, Beauchamp G. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet Radiol Ultrasound. 2011 Jul-Aug;52(4):451-6. Epub 2011 Mar 7. PMID: 21382121. [CrossRef]
- Kayanuma H, Yamada K, Maruo T, Kanai E. Computed tomography of thoracic lymph nodes in 100 dogs with no abnormalities in the dominated area. J Vet Med Sci. 2020;82(3):279-285. [CrossRef]
- Nakamura M, Takahashi M, Ohno K, et al. C-reactive protein concentration in dogs with various diseases. J Vet Med Sci. 2008;70(2):127-131. [CrossRef]
- Malin K, Witkowska-Piłaszewicz O. C-Reactive Protein as a Diagnostic Marker in Dogs: A Review. Animals (Basel). 2022;12(20):2888. Published 2022 Oct 21. [CrossRef]
- Covin MA, Steiner JM. Measurement and clinical applications of C-reactive protein in gastrointestinal diseases of dogs. Vet Clin Pathol. 2022;50 Suppl 1(Suppl 1):29-36. [CrossRef]
- Indzhova V, Czopowicz M, Kilpatrick S, Gutierrez-Quintana R, Brocal J. Signalment and C-reactive protein values in dogs with immune-mediated polyarthritis and steroid responsive meningitis arteritis. Front Vet Sci. 2023;10:1091318. Published 2023 Feb 14. [CrossRef]
- Nyman HT, O'Brien RT. The sonographic evaluation of lymph nodes. Clin Tech Small Anim Pract. 2007;22(3):128-137. [CrossRef]
- Cordella A, Saunders J, Stock E. Sternal lymphadenopathy in dogs with malignancy in different localizations: A CT retrospective study of 60 cases. Front Vet Sci. 2022;9:1019196. Published 2022 Oct 21. [CrossRef]
- Stehlík L, Vitulová H, Simeoni F, Proks P, Vignoli M. Computed tomography measurements of presumptively normal canine sternal lymph nodes. BMC Vet Res. 2020;16(1):269. Published 2020 Aug 3. [CrossRef]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. [CrossRef]
- Iwasaki R, Mori T, Ito Y, Kawabe M, Murakmi M, Maruo K. Computed Tomographic Evaluation of Presumptively Normal Canine Sternal Lymph Nodes. J Am Anim Hosp Assoc. 2016;52(6):371-377. [CrossRef]
- Milovancev M, Nemanic S, Bobe G. Computed tomographic assessment of sternal lymph node dimensions and attenuation in healthy dogs. Am J Vet Res. 2017;78(3):289-294. [CrossRef]
- Beukers M, Grosso FV, Voorhout G. Computed tomographic characteristics of presumed normal canine abdominal lymph nodes. Vet Radiol Ultrasound. 2013;54(6):610-617. [CrossRef]
- Teodori S, Aste G, Tamburro R, Morselli-Labate AM, Simeoni F, Vignoli M. Computed Tomography Evaluation of Normal Canine Abdominal Lymph Nodes: Retrospective Study of Size and Morphology According to Body Weight and Age in 45 Dogs. Vet Sci. 2021;8(3):44. Published 2021 Mar 7. [CrossRef]
- Reeve EJ, Mapletoft EK, Schiborra F, Maddox TW, Lamb CR, Warren-Smith CMR. Mediastinal lymphoma in dogs is homogeneous compared to thymic epithelial neoplasia and is more likely to envelop the cranial vena cava in CT images. Vet Radiol Ultrasound. 2020;61(1):25-32. [CrossRef]
- Bae H, Kim SK, Yu D. Comparative analysis of the aberrant immunophenotype and clinical characteristics in dogs with lymphoma: a study of 27 cases. Front Vet Sci. 2023;10:1254458. Published 2023 Oct 16. [CrossRef]
- Taweesedt PT, Surani S. Mediastinal lymphadenopathy in COVID-19: A review of literature. World J Clin Cases. 2021;9(12):2703-2710. [CrossRef]
- Ngom A, Dumont P, Diot P, Lemarié E. Benign mediastinal lymphadenopathy in congestive heart failure. Chest. 2001;119(2):653-656. [CrossRef]
- Chow BJ, McKim DA, Shennib H, Dales RE. Superior vena cava obstruction secondary to mediastinal lymphadenopathy in a patient with cystic fibrosis. Chest. 1997;112(5):1438-1441. [CrossRef]
- Ullal TV, Jaffey JA, Kreisler R, et al. Increasing age and severe intraoperative hypotension associated with nonsurvival in dogs with gallbladder mucocele undergoing cholecystectomy. J Am Vet Med Assoc. 2023;261(12):1-9. Published 2023 Sep 4. [CrossRef]
- Jaffey JA, Graham A, VanEerde E, et al. Gallbladder Mucocele: Variables Associated with Outcome and the Utility of Ultrasonography to Identify Gallbladder Rupture in 219 Dogs (2007-2016) [published correction
appears in J Vet Intern Med. 2018 May;32(3):1290. https://doi.org/10.1111/jvim.15115]. J Vet Intern Med.
2018;32(1):195-200. [CrossRef]
- Ohno K, Yokoyama Y, Nakashima K, Setoguchi A, Fujino Y, Tsujimoto H. C-reactive protein concentration in canine idiopathic polyarthritis. J Vet Med Sci. 2006;68(12):1275-1279. [CrossRef]
- Foreman M, Vettorato E, Caine A, Monti P, Cherubini GB, Eminaga S. Serum C-reactive protein in dogs with paraplegia secondary to acute intervertebral disc extrusion. J Vet Intern Med. 2021;35(4):1857-1864. [CrossRef]
- Bathen-Noethen A, Carlson R, Menzel D, Mischke R, Tipold A. Concentrations of acute-phase proteins in dogs with steroid responsive meningitis-arteritis. J Vet Intern Med. 2008;22(5):1149-1156. [CrossRef]
- Baum H. The Lymphatic System of the Dog. April 1, 1918. Accessed September 4, 2024. https://openpress.usask.ca/k9lymphaticsystem/chapter/sternal-lymph-nodes/.
- Iwasaki R, Murakami M, Kawabe M, Heishima K, Sakai H, Mori T. Metastatic diagnosis of canine sternal lymph nodes using computed tomography characteristics: A retrospective cross-sectional study. Vet Comp Oncol. 2018;16(1):140-147. [CrossRef]
- Howard E, Alexander L Miller’s Anatomy of the Dog, 4th edition. Saunders 2012 535-562.
- Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987-2001). J Am Vet Med Assoc. 2003;222(9):1234-1236. [CrossRef]
- Rossi F, Körner M, Suárez J, et al. Computed tomographic-lymphography as a complementary technique for lymph node staging in dogs with malignant tumors of various sites. Vet Radiol Ultrasound. 2018;59(2):155-162. [CrossRef]
- Ullal TV, Jaffey JA, Kreisler R, et al. Increasing age and severe intraoperative hypotension associated with nonsurvival in dogs with gallbladder mucocele undergoing cholecystectomy. J Am Vet Med Assoc. 2023;261(12):1-9. Published 2023 Sep 4. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).