1. Introduction
In an in vivo disease, helicobacter pylori (HP) microaerophilic gram-negative bacteria reside in the human stomach. HP infections are associated with gastric carcinoma (1,2,3), gastric ulcers (4), and carcinoma of the liver (5). Since many people worldwide are infected with HP bacteria (6), diagnostic HP assay is important in evaluating early-stage infections. Existing analytical discriminations have been dependent on DNA-amplified polymerase chain reaction (PCR), biosensing enzyme reaction and other detection systems, such as multiplex polymerase chain reaction (PCR) (7), the second-generation ELISA (Cobas Core, anti-H. pylori EIA Roche, Basle, Switzerland) (8), enzyme-linked immunosorbent assay (9), radioactive polymerase chain reaction (10), analysis of Helicobacter pylori infection in amouse model (11) and PCR double-strain DNA-binding method (12). In contrast, PCR methods are dependent on chromatographic separation, electrophoresis isolation, and other detection systems. Recently, electrochemical stripping accumulation techniques have been applied to diagnostic bioassay, which is simple, fast (13), and capable of sensitive signal amplification. Specialized modified working electrode techniques have also been widely applied to clinical detections, such as infrared photo diode electrode (14), the carbon nanotube paste electrode (15,16), the mercury-modified carbon electrode (17,18), and other sensors. Nevertheless, electrochemical HP assays are rarely examined, including electrochemical (3,4-DHS) and (2,5-DHS) probes (19), an amperometricimmunoreactor with rotation incorporated into an FI analytical system (20), and a screen-printed immunosensor (21). These methods use specific properties that are still complicated and unattainable for in vivo direct assay. In this study, a novel fluorine doped on a carbon nanotube (CNT) sensor (22) was used for HP recognition. Its carbon nanotube structure had a large surface area (23), a wide range of potential applications (24), and electrocatalytic reaction (–[Cx–(H–F)n]n+M0↔M+) (22,25,26), making it applicable to biological recognition and HP diagnosis.
However, these technologies are time-consuming and complex. It also has the disadvantage of having a high detection limit, and materials CNT can be useable to the human body and can be directly diagnosed within the body muscles for in vivo or in vitro. For these purpose, Carbon nanotubes are stable to acids and bases. Additionally, the electron transfer speed is fast and it is not toxic to the human body. Amplifies the redox reaction of viral or cellular ions. Also, CNT atoms react sensitively to electrons. When using a mixture of bismuth and carbon nanotubes for this catalytic effect is sensitive detection limits can be reached (27). Carbon nanotubes (CNTs) coupled with bismuth have a very large surface area. It is also electrically conductive. The physical properties of CNTs vary depending on the number of bonds of carbon atoms forming the wall. A single-wall nanotube is a tube with one wall made of carbon atoms. It has excellent electrical and thermal conductivity. Therefore, it is suitable as a biological ion diagnostic material. (28) However, double-wall nanotubes with two walls have excellent electrical conductivity and mechanical properties. So a multi-wall nanotube is a tube with multiple walls made of carbon atoms. They have superior mechanical properties than electrical and thermal properties. It is easy to manufacture and has a wide range of applications. As for mechanical properties, it is a material with strong tensile strength and elastic modulus. This is due to the sp² covalent bonds formed between carbon atoms.Multi-walled carbon nanotubes have a tensile strength of 63 to 100 gigapascals (GPa) or more. In single-wall experiments, carbon nanotubes exhibit very high tensile modulus in the range of 640 GPa to 1 TPa. It also has a high tensile strength of 150 to 180 GPa. Therefore, the lifespan of the ion diagnostic sensor is extended (29). However, the mechanical properties of carbon nanotubes weaken under compression conditions. Due to the high aspect ratio of CNTs, their ductility increases when subjected to compressive, torsional, or bending stresses. And it has a bending nature. The Young’s modulus of CNTs in the radial direction is measured in GPa. Therefore, carbon nanotubes become very soft and linear in the radial direction. Also, in textile applications, specific strength is a measure of the mechanical effects of the fiber. Specific strength of the material (force per unit area at breakage) divided by density. SI units are expressed as Pa·m³/kg or N·m/kg. Biotic fibers with low density show high specific strength. The density of carbon nanotubes is 1.3 to 1.4 g/c. However, the density of carbon steel is 7.85 g/cm. The specific strength of carbon steel is 154 kN·m·kg-1. The specific strength of carbon nanotubes is 48,000 kN·m·kg-1. Therefore, CNTs exhibit high specific strength. And CNTs show the best specific strength among materials with known specific gravity. The mechanical properties of carbon nanotubes are stronger than iron and aluminum, and are lighter than aluminum (2.0g/cm³), the lightest metal among metals. Therefore, it is convenient to process and can be applied to diagnostic sensors. Carbon fiber has the disadvantage that only 1% of its molecular structure is modified. However, carbon nanotubes can withstand even 15% deformation. Carbon nanotubes can be used in a wider range of human body materials than steel, diamond, copper, and fiber. Carbon nanotubes exhibit metallic or semiconducting properties depending on the chirality of the coiled axis. Therefore, a diagnostic detection circuit can be configured. Metallic carbon nanotubes transmit electrons in the longitudinal direction without being scattered. Single-walled carbon nanotubes exhibit large acoustic phonons. It has a high thermal conductivity of 6000 W/(m·K). When electricity is passed through carbon nanotubes, they emit light that is more than 100 times more efficient than LEDs (light-emitting diodes). Therefore, it can be used in photoelectric sensors. The thermal conductivity of carbon nanotubes is the same as that of diamond, which is the highest in nature. Therefore, thermoelectric sensor analysis can be used. Tensile strength exceeds diamond. Carbon nanotubes have the same level of electrical conductivity as copper. Therefore, it is possible to synthesize it with conductive resin. (30) For the above reasons, it can be used as a virus diagnostic sensor material. (31).
2. Materials and Method
2.1. Technical Systems
Voltammetric measurements were carried out using the second-versions of Bioelectronics-2 system from the authors’ institution. The electric circuitry had very small computerized systems, a 3.0 V potential range, a 2 mA current range, and a 10 pA measuring current. It was as compact as a cellular phone, measuring 3”x2”x1”. It used a rechargeable battery with a USB power source and was capable of USB port data telecommunication with a PC. It can be used for bioassay, microorganism recognition, and sensor techniques for individual and laboratory conditions.
2.2. Sensor Preparation
The graphite pencil (PE) working electrode was prepared with 5H or 2B pencil lead (2 mm in diameter). The fluorine immobilized on a carbon nanotube (HFCNT) working sensor was prepared with a mixing paste of 40% carbon nanotube powder (Nanotech Co., Ltd., Choongnam, South Korea, 330-816), 40% HF (sigma standard, concentrated solution), and 20% mineral oil. The mixture was homogenized in a mortar for 30 minutes. The paste was inserted into a 5cm- glass (parafilm coated) needle-type capillary tube 1.5 mm in diameter, and a copper wire 0.5 mm in diameter was connected to the electrochemical measurement system. The DNACNT (double-stranded DNA and carbon nanotube powder mixed paste) was prepared with the same method using 40% DNA (double-stranded and prepared from calf thymus sigma), 40% carbon nanotube graphite powder, and 20% mineral oil. The HGCNT (metal mercury and carbon nanotube mixed paste) was made using 40% Hg (1,000mg/L mercury stranded from sigma), 40% carbon nanotube graphite powder, and 20% mineral oil. The HFCNT (conc fluorine and carbon nanotube mixed paste) was made using 40% Hf (cocn–Hf solution stranded from sigma), 40% carbon nanotube graphite powder, and 20% mineral oil. Here, the Hf, DNA, and Hg were immobilized on a carbon nanotube paste surface using a cyclic scan with a -2.0V initial potential, a 2.0V switching potential, and a 0.5V scan rate for 20 repetitions in a 0.1M NH4H2PO4 electrolyte solution. The three sensors were rinsed with water to remove any regent solution residue, and then dried using an air gun. The three modified CNT sensors were subsequently transferred to a cell that contained 0.1M NH4H2PO4, and their SW stripping voltammograms were recorded. The reference electrode used was Ag/AgCl, and the auxiliary electrode, a 0.2mm-diameter platinum wire. A three-electrode cell was used to monitor the voltammetric signal.
3. Results
3.1. Voltammetric Procedure and the Electron Microscope
All the analytical solutions were used in 18M ohm cm
-1 double-distilled water. Analytical grades of a 0.1M NH
4H
2PO
4 electrolyte solution with a pH level of 4.75 and an experimental reagent from Aldrich Chemical Co. were prepared. The voltammograms were measured in dissolved oxygen, and each measurement did not require an electrode cleaning time. The phosphoric acid solution was found to be the most suitable medium. The common parameters for the CV were a scan rate of 500 mVs
-1, an initial potential of +2.0 V, and a switching potential of -2.0 V. The stripping voltammograms were performed using the
Figure 3A parameters.
The HP-cultured structure was scanned with a field-emission-scanning electron microscope (FE-SEM, JSM-6700F JEOL, Ltd.).
3.2. HP Culture and Tissue Collection
The HP culture was grown at 37 °C on Columbia agar that contained 7% horse blood. The plates were incubated from 72 to 140 h in a circulate under microaerophilic conditions of 5 % CO2, 4 % O2, 86 % N2, and 5 % H2 using gas packs. The HP was washed in phosphate-buffered saline. The suspension was acidified with concentrated hydrochloric acid. It was disrupted with an ultrasonic probe at mid-power under ice cooling. Gastric tissues (0.25 g) were collected from 12 patients and 12 healthy persons, including 4 males and 20 females, ages 30 to 55. Tissues were obtained between 9 am and 5 pm at the general hospital where one of the authors worked. An aliquot of gastric tissue was placed in a glass vial that contained disinfected 5mL saline and stored at -10°C. This was allowed to thaw at room temperature and was used directly.
The PCR DNA was isolated from the 100-ml tissue solution using a Core-OneTMGenomic DNA isolation kit (
www.corebio.com), with Cat. No. TD-100 manufacturer’s guide. This was used for the proteinase K-lysis method. The DNA was eluted under a 10-mM Tris-HCl buffer and at pH 8.5, and was used for diagnostic analysis.
4. Discussion
4.1. Electrode and Voltammetric Concentration Effects
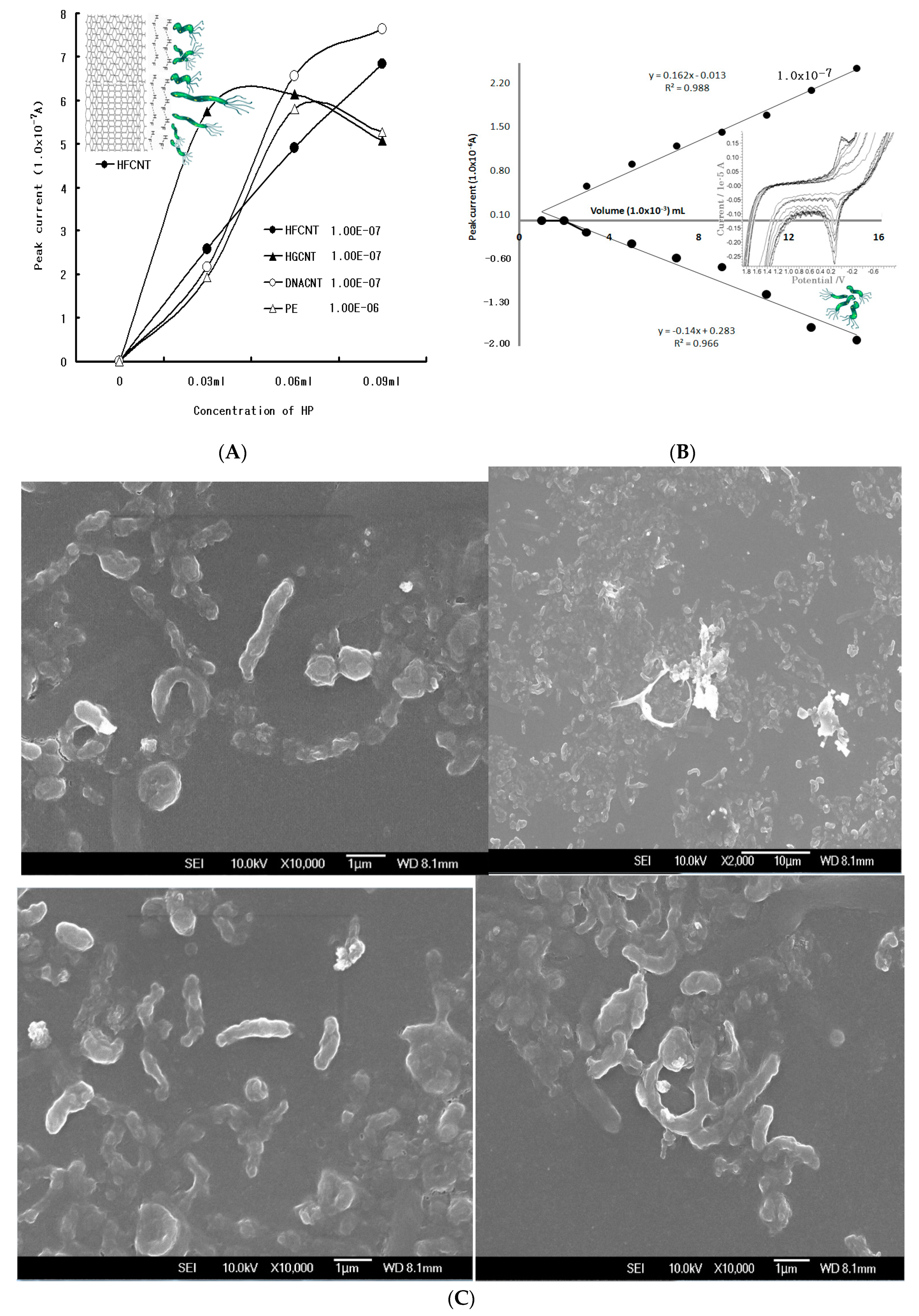
Since electrode properties depend on their chemical structure, common and specialized sensors were used to compare the PE, HFCNT, HGCNT, and DNACNT.
Figure 1A shows a 0-0.09 mL HP concentration in SW anodic stripping voltammetry using three electrodes at optimum parameters. A 0.0 V anodic peak potential was obtained in all the sensors. While the HGCNT was sensitive and its peak width was sharp, its linear working range was very short. The DNACNT and PE were not sufficient. Since the HFCNT was linear and its peak width was sensitive, it can be used in HP assay. Thus, the CV voltammograms determined the analytical anodic and cathodic peak potentials with an HP concentration with the addition of eight points.
Figure 1B shows low voltammograms of the 0.003-0.024 mL concentrations, but the oxidation of the 0.0 V peak currents increased from 0.183x10
-6 A to 2.06x10
-6 A, whereas the reduction currents varied from 0.554x10
-7 A to 2.37x10
-7 A. The reduction peak was poor, the oxidation was more hole-sensitive than the reduction current, and the peak width was sharp. Thus, more sensitive peak signals were detected via SW stripping optimization.
Figure 1C shows the scanning electron micrograph images of the HP cells. The white images are the concentrated cell surfaces, which are used for the voltammetric standard solutions.
Table 1 is shown appeared as straight line. However, the other electrode appeared in the form of short curves
4.2. SW Parameters and Statistics
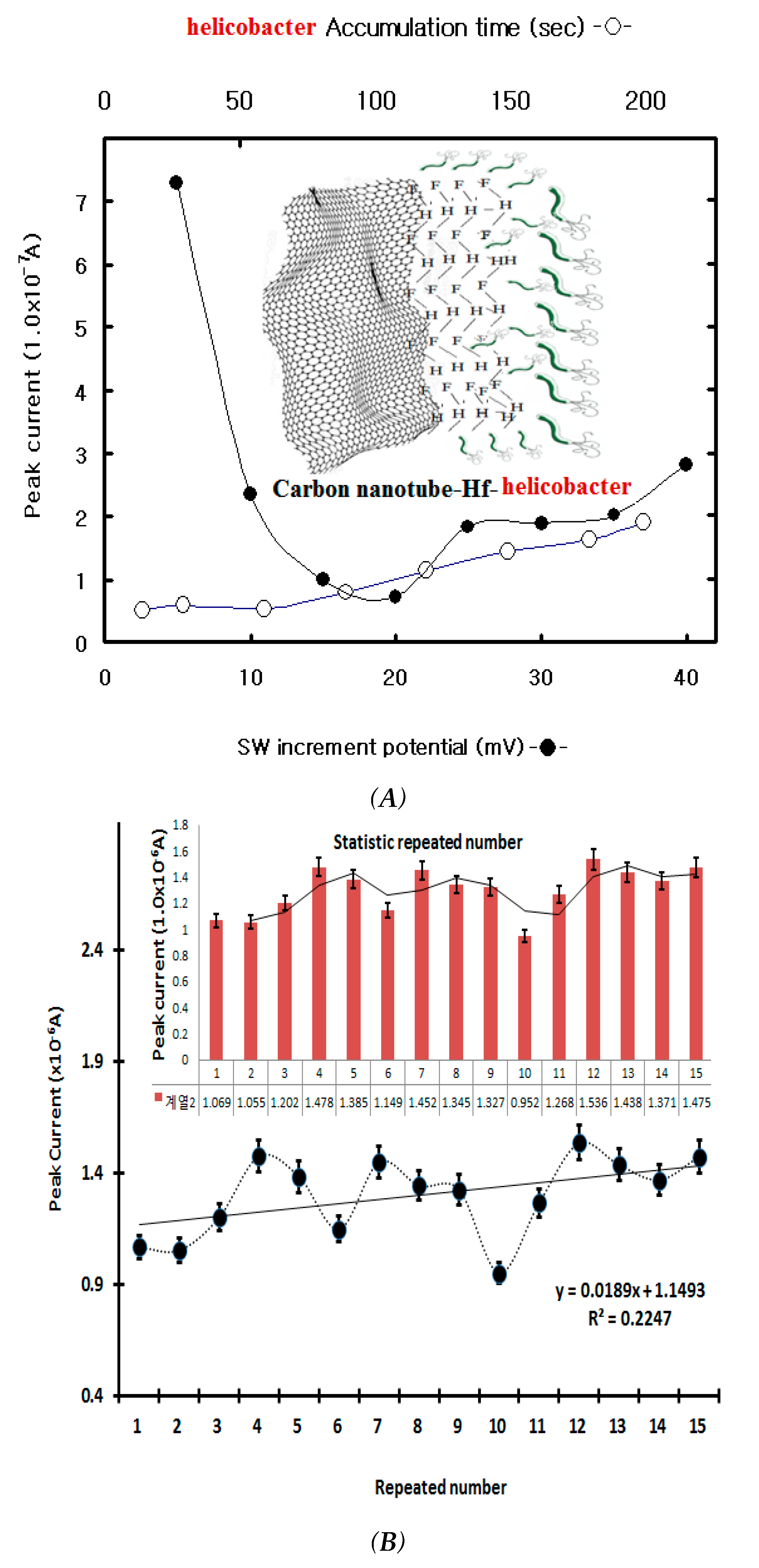
In SW stripping voltammetry, time variation influences signal amplification. In this study, the SW stripping accumulation time was first examined using eight-point variations. The results are shown in
Figure 2A. The peak current increased from 0.53x10
-7 to 1.90x10
-7, but the peak width was wide and sensitive at 200 sec. Thus, the accumulation time was fixed at 200 sec. Under these conditions, the SW incremental potentials were examined using eight-point variations. They decreased quickly from 7.93x10
-7 A to 0.459x10
-7 A; the peak width was broad and expanded; and the current was high at 5 mV and then decreased. Thus, the accumulation time was 200 s, and the incremental potential was fixed at 5 mV. Under these conditions, other parameters were examined using the initial potential, the SW frequency, and the amplitude variation. The final results were -1.0 V initial potential, a 20 Hz SW frequency, a 50 mV amplitude, a 200-s accumulation time, a 5 mV incremental potential, and a 0.1M NH
4H
2PO
4 (4.7 pH) strength. Under these conditions, electrode stability was examined with 0.01mL HP added and 100-s accumulation time. The first five points were varied from 0.41x10
-6 A to 0.92x10
-6 A then increased (not shown here) before they were linearized and stabilized. The errors are shown in
Figure 2B. The minimum current was 0.927x10
-6 A and the maximum current was 1.594x10
-6 A. The static relative standard deviation was R
2=0.08376, and then usable working ranges were examined using SWSV.
Table 2: shown for the counts in statistical plot in
Figure 2B. The error range of 15th repeated measurements under the same concentratation conditions is shen in the table
4.3. Working Range and Application in Patients’ Stomach Tissue
Using optimum parameters, the linear working ranges were sought with the HFCNT set in optimum SW voltammetry.
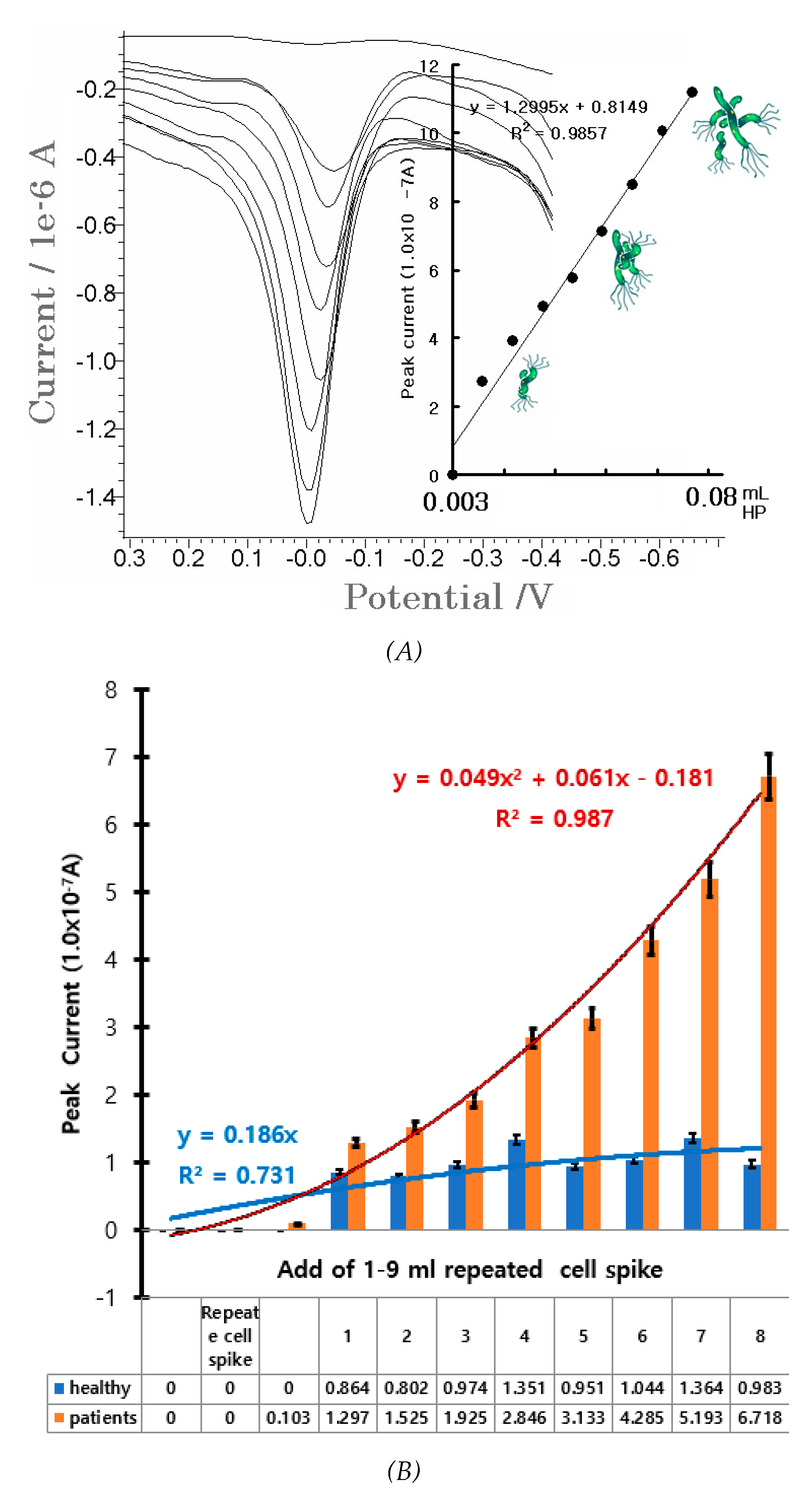
Figure 3A shows a variation from 0.01 to 1.0x10
-9 mL HP. The first curve refers to the electrolyte blank solution. It was simple and did not manifest any signal, whereas the other voltammograms sharply increased from 2.2x10
-7 A to 11.6x10
-7 A with nine points, and linear equations was obtained at △x/△y=1.2995x+0.8149. The slop was sensitive and precision was R
2 = 0.9857. Detection time was faster and more sensitive than that of other common analytical methods. Moreover, the SW peak current was sensitive then the CV peak. These indicate feasible use in the recognition of HP infection and gastric carcinoma. To validate, an analytical application was carried out on certain patients using healthy stomach tissue. About 0.25 g of human stomach cells were extracted from a healthy patient and flushed with water and diluted in one drop of 0.1M HCl. The sample was then further diluted in 10 mL distilled water. The 0.1 mL solutions were directly used in the SW stripping voltammetry. At first, the two additions did not exhibit any peak current. Thus, continued additions were performed with 1-9 mL concentrations.
Figure 3B shows the results from the healthy (black) and diseased (white) stomach tissues. The “healthy” curve had no signal, and only noise was obtained at the range of 0.2x10
-7-0.95x10
-7 A. The “unhealthy” curve increased, however, from 0.5x10
-7 to 8.8x10
-7 A, and was 10 times more sensitive than the healthy tissues. These were subsequently used for HP analysis. Thus, the developed techniques can be applied in the diagnosis of HP infection. Expanded statistic applications were performed with 10 patients and 10 healthy persons, and a recognition rate of 70-80% was attained. The proposed methods can be used in patient diagnosis and in in vivo direct assay.
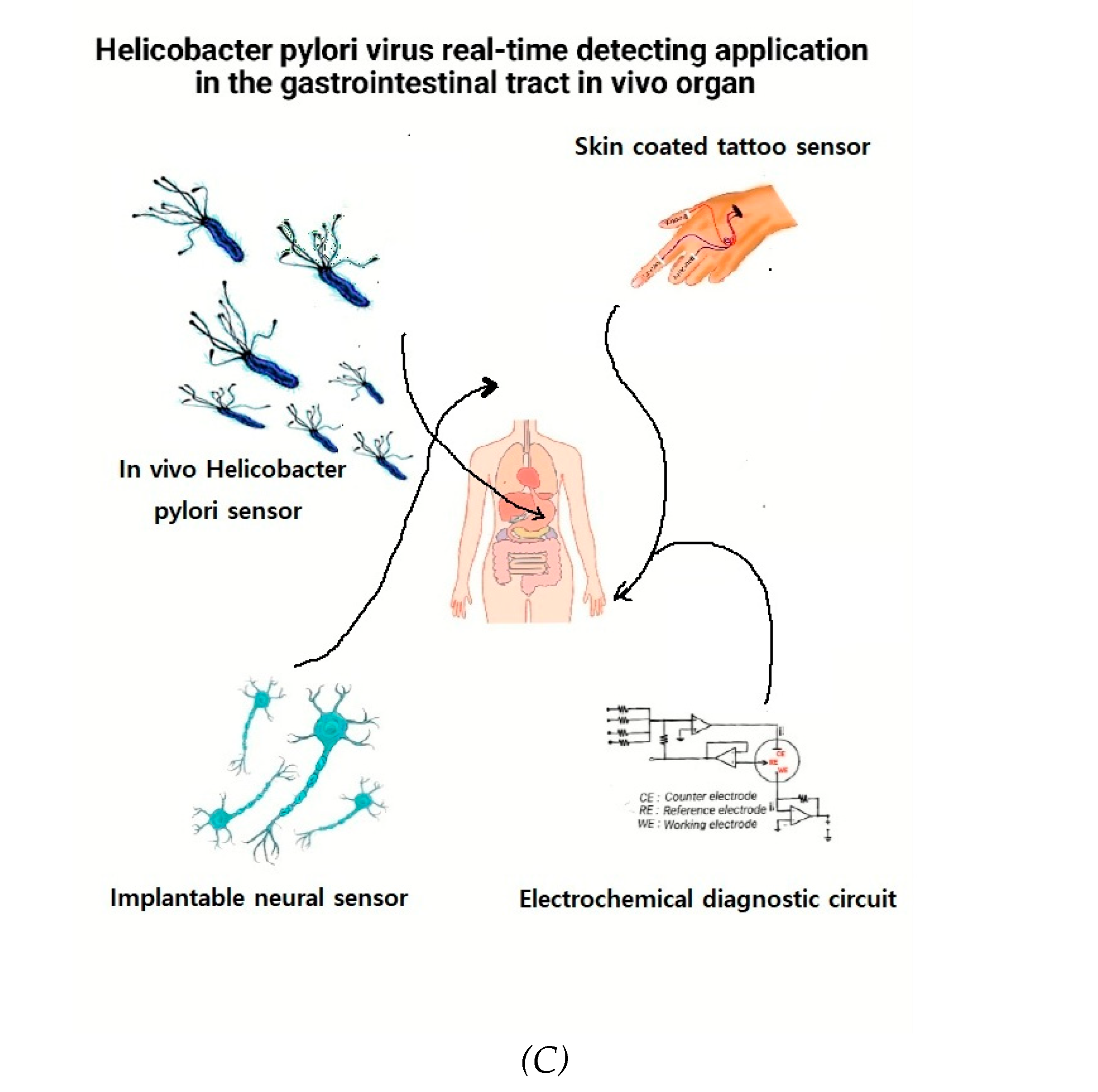
Figure 3C is shown such as simulation diagram being applied, shows real time diagnosis of Helicobacter pylori virus living in the stomach at human organ, also skin-coated tattoo sensor and diagnostic circuit, and pain transmission neurodiagnostic network, and 3-electrode systems of amplified operational voltammetric network.
Table 3 is shown results data in the statistical diagram with repeated in put of real normal and patien samples in
Figure 3A,B.
Figure 3.
(A) SWSV anodic 0.003-0.08 mL HP (5.613x103-3.92x104 CFU/ml HP) added to a 0.1M NH4H2PO4 solution. SW stripping: 50 mV amplitude, 20 Hz frequency, -1.0 V accumulation potential, and 200-s accumulation time were used with a pH of 4.75. (B) Addition of 1-9 ml of diluted stomach tissue from healthy patients via SWSV. (C) Simulation diagram being applied, shows real time diagnosis of Helicobacter pylori virus living in the stomach, skin-coated tattoo sensor and diagnostic circuit, pain transmission neurodiagnostic network, and 3-electrode systems of amplified operational voltammetric network.
Figure 3.
(A) SWSV anodic 0.003-0.08 mL HP (5.613x103-3.92x104 CFU/ml HP) added to a 0.1M NH4H2PO4 solution. SW stripping: 50 mV amplitude, 20 Hz frequency, -1.0 V accumulation potential, and 200-s accumulation time were used with a pH of 4.75. (B) Addition of 1-9 ml of diluted stomach tissue from healthy patients via SWSV. (C) Simulation diagram being applied, shows real time diagnosis of Helicobacter pylori virus living in the stomach, skin-coated tattoo sensor and diagnostic circuit, pain transmission neurodiagnostic network, and 3-electrode systems of amplified operational voltammetric network.
5. Conclusions
Biomolecules of HP were searched using handheld voltammetric circuits. Their analytical sensitivities were compared using common and novel HFCNT. The novel HFCNT was found to be more sensitive than common sensors. Optimum conditions comprising a 200-s accumulation time, a -1.0 V SW initial potential, and a 4.75-pH electrolyte strength were attained. The optimum conditions reached a lower detection limit of 2.5x102 CFU/ml HP (S/N=3). These results indicate that the sensor systems developed can be used in the detection of early-stage HP infection in the stomach tissue of human organic cells and in in vivo body fluid with a catheter-type sensor. They can also be applicable in any other field that requires real-time, in-organ direct assay.
Abbreviations
HP: Helicobacter pylori; HFCNT: fluorine immobilized on a carbon nanotube ; SW: square; SWSV: stripping voltammetry; PCR: polymerase chain reaction; PE: graphite pencil;HFCNT: fluorine immobilized on a carbon nanotube; DNACNT: double-stranded DNA; HGCNT: metal mercury and carbon nanotube mixed paste;
Author Contributions
All authors analyzed the data and wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This paper was supported by the Academic Research Fund of DrMyungki (MIKE) Hong in 2021
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article
Acknowledgments
We thank all authors
Competing interests
The authors declare no competing interests
References
- Stephanie AC, Robert JO 2008 Application of polymerase chain reaction-based assays for rapid identification and antibiotic resistance screening of Helicobacter pylori in gastric biopsies. DiagnMicr Infect Dis 6:67-71.
- Akira T, Yasuhiko K, Masaharu S, Atsunori S, Hiroshi T, et al. 2007 Basis of Decreased Risk of Gastric Cancer in Severe Atrophic Gastritis with Eradication of Helicobacter pylori. Dig Dis Sci 52:232-239.
- Masaharu T, Hiroyasu l, Shigeru O, Haruo T, Yoshiko Y 1993 The Association of Helicobacter pylori with Differentiated-Type Early Gastric Cancer. Cancer72:1841-1845.
- Elisabet C, Jonas A, Mark RE, Alan GM, Carol LN 2004 Chemical cross-linking of the urease complex from Helicobacter pylori and analysis by Fourier transform ion cyclotron resonance mass spectrometry and molecular modeling. Int J Mass Spectrom 234:137-144.
- Philippe A, Armelle M, Lurdes M, Lurdes M, Paulette BS, Charles B, et al. 2000 Detection of Helicobacter Species in the Liver of Patients with and without Primary Liver Carcinoma. Cancer 89:1431-1439.
- Nuvjeevan SD, Nicole AH, Sarah LJM 2007 Characterization of the Helicobacter pylori NikR-PureA DNA Interaction: Metal Ion Requirements and Sequence Specificity. Biochemistry-US 46:2520-2529.
- Stephanie AC, Robert JO 2008 Application of polymerase chain reaction-based assays for rapid identification and antibiotic resistance screening of Helicobacter pylori in gastric biopsies. DiagnMicrInfec Dis 61:67-71.
- Dulciene MMQ, Edilberto NM, Gifone AR, Andreia MRO, Celso AO, Paula PM, et al. 1998 cagA-positive helicobacter pylori and risk for developing gastric Carcinoma in Brazil. Int. J. Cancer 78:135-139.
- Pooria G, Mohsen A, Amir G, Leily S, Hossein AT, Ali K, et al. 2007 Detection of Helicobacter pylori by enzyme-linked immunosorbent assay of thermophilic helicase-dependent isothermal DNA amplification. Diagn Micro Infec Dis 59:243-249.
- Liviu A.S, Pelayo C, Luis E.B, Barbara G.S 2003 Detection and typing of Helicobacter pylori cagA/vacA genes by radioactive, one-step polymerase chain reaction in stool samples from children. J Microbiol Meth 52:197-207.
- Lesley ES, Jo AC, J. Russell L, Paolo G, Phillip DS, Ken BW 2000 Quantitative analysis of Helicobacter pylori infection in a mouse model, J Immunol Methods 242:67–78.
- Agnes R, Bela M, Zsuzsa U, Zsolt T, Laszlo P 2001 Determination of Helicobacter pylori cagA,vacA genotypes with real-time PCR melting curve analysis. J Physiol-Paris 95:369-377.
- Suw YL, Young SJ, Myung HK, In kH, Woon WJ, Hyun SK 2004 Determination of Caffeine Using a Simple Graphite Pencil Electrodewith Square-Wave Anodic Stripping Voltammetry. Microchim. Acta 146:207-213.
- Suw YL, Yun KK 2006 Assay of glucose in urine and drinking water with voltammetric working sensors of infrared photo diode electrode. Sensor Actuat A-phys 127:41-48.
- Suw YL 2005 Detection of dopamine in the pharmacy with a carbon nanotube paste electrode using voltammetry. Bioelectrochemistry 68:232-236.
- Suw YL, Young SJ, Sung KK, Hyun KL 2007 Trace Analysis of Lead and Copper Ions in Fish Tissue Using Paste Electrodes. Anal Lett 40:2683-2692.
- Suw YL, Sang SS, Sung kK, Young SJ, Chang HL 2006 Determination of Ge(IV)in rice in a mercury coated glassy carbon electrode in the presence of catechol. Food Chem 96:337-343.
- Suw YL 2006 Real-time Voltammetric Assay of Cadmium Ions in Plant Tissue and Fish Brain Core, Bull.Korean Chem. Soc 27:1613-1617.
- Monica RP, Tania G, Encarnacion L, Felix P 2007 Comprehensive study of interactions between DNA and new electroactive Schiff base ligands Application to the detection of singly mismatched Helicobacter pylori sequences. BiosensBioelectron 22:2675-2681.
- German AM, Angel AJT, Irma EDV, Roberto A.O, Julio R 2005 Continuous flow/stopped flow system using an immunobiosensor for quantification of human serum IgG antibodies to Helicobacter pylori. Anal Biochem 337:195-202.
- German A.M, Irma E.DV, Julio R 2007 Screen printed immunosensor for quantification of human serum IgG antibodies to Helicobacter pylori. Sensor Actuat B-chem 128:23-30.
- Suw YL 2008 Diagnosis of copper ions in vascular tracts using a fluorine-doped carbon nanotube sensor. Talanta 74:1635-1641.
- Randhir P.D, Joseph W 2004 Electrochemical detection of carbohydrates at carbon-nanotube modified glassy-carbon electrodes. ElectrochemCommun 6: 284-287.
- Yanhong L, Xiaoying Y, Yanfeng M, Feng D, Zunfeng L, Yongsheng C 2006 Self-assembled branched nanostructures of single-walled carbon nanotubes with DNA as linkers. ChemPhys Lett 419: 390-393.
- Zoran M, Primozˇ B, 2005 Copper(I) hexafluoroantimonate– an example of a compound with CuI in a solely fluorine environment, Journal of Fluorine Chemistry 126: 803–808.
- 26. Tsuyoshi N, Vinay G, Yoshimi O, Henri G, Zoran M, Boris Ž 2004 Influence of cointercalated HF on the electrochemical behavior of highly fluorinated graphite, Journal of Power Sources 137: 80–87.
- Jongwan Choi, Jiwon Min, Jason Sahngwook Kim, Jung Hyun Park, SuwYoung Ly, Neurotransmitter Assay for In Vivo Nerve Signal Detection Using Bismuth Immobilized on a Carbon Nanotube Paste Electrode, Micromachines 2023, 14, 1899. [CrossRef]
- Huck Jun Hong. Suw Young Ly. Voltammetric Detection of Tetrodotoxin Real-time In Vivo of Mouse Organs using DNA-immobilized Carbon Nanotube Sensors. Current Analytical Chemistry, 2019, 15, 1-8.
- John S. Bulmer, Adarsh Kaniyoor, James A. Elliott. .A Meta Analysis of Conductive and Strong Carbon Nanotube Materials. Advanced Materials. 2021. 7. 19. [CrossRef]
- Donghoon Oh, Youngjin Kang, Hyuck Jung, Hyejin Song, Yousuk Cho. Dojin Kim. Electrical and Optical Property of Single-Wall Carbon Nanotubes Films. Kor. J. Mater. Res. 19, No. 9 (2009). [CrossRef]
- Suw Young Ly, Jung Eun Kim and Woo Yeon Moon. Diagnostic assay of carcinoembryonic antigen tumor markers using a fluorine immobilized biosensor with handmade voltammetric circuit. European Journal of Cancer Prevention 20. 58-62. 2011.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).