1. Introduction
Solar ultraviolet (UV) radiation is among the most significant ecological factors influencing life on Earth’s surface. It is classified into several types with UV-A (400-315 nm) and UV-B (315-280 nm) being most environmentally relevant [
1,
2]. Absorption of the more energetic UV-B, unlike UV-A, is highly dependent on the amount of atmospheric ozone [
3,
4], which is one of the motivations for studying the biological effects separately for UV-A and UV-B. Direct influence of UV on terrestrial ecosystems is obvious, but both UV-A and UV-B can also penetrate into the upper layers of aquatic ecosystems. The water transparency for UV greatly varies, but in the waterbodies with the lowest concentrations of dissolved organic matter 10 % reduction of UV intensity is found down to depths of at least 30 m for UV-A and 15 m for UV-B [
5,
6,
7].
The roles of UV radiation in aquatic ecosystems are tightly bound to a multitude of other factors in complex ways, and both higher or lower UV levels reaching certain depths can shift the animal communities by favoring different species [
7,
8]. This fact requires thorough understanding of UV adaptation mechanisms and their past evolutionary changes in various taxonomic groups in order to improve predictions and management of changes in aquatic ecosystems. UV adaptation strategies generally include avoidance, protection with UV-screening compounds such as melanin or carotenoids, and coping with UV effects on the cellular level if the first two options are ineffective or impossible [
9]. The outcomes of UV exposure for animal cells include direct DNA damage and enhanced production of reactive oxygen species (ROS) that may trigger a counteracting increase in activities of enzymatic and non-enzymatic antioxidants [
9,
10].
Ancient Lake Baikal located deep in Eurasia provides unique opportunities for studying the evolution of UV adaptation mechanisms. Its water is one the most transparent among all lakes in the world [
11]. Most importantly, Baikal is the birthplace to the only deep-water fauna arisen in freshwater, which inhabits the lake down to its maximal depths of over 1640 m [
12,
13]. In the case of highly diverse endemic amphipods (Amphipoda, Crustacea) descending from shallow-water
Gammarus-like species [
14], the species diversification involved adaptations to high UV levels in littoral zone of the lake as well as gradual colonization and adaptation to the expanding deep-water zone with no UV at all [
15]. Thus, we can hypothesize that modern Baikal littoral amphipods should be well adapted to UV, while deep-water species could have lost their systems for UV protection. Such loss of energetically expensive protection systems was found, for example, in the case of response to rising temperature in Antarctic notothenioid fish [
16]. Since deep-water amphipods of Lake Baikal emerged independently from their oceanic counterparts [
14], the evolutionary changes in their adaptation mechanisms, particularly those related to UV protection, hold significant value for understanding both the evolution of UV defense and the processes that lead to the loss of adaptive traits when selective pressures diminish.
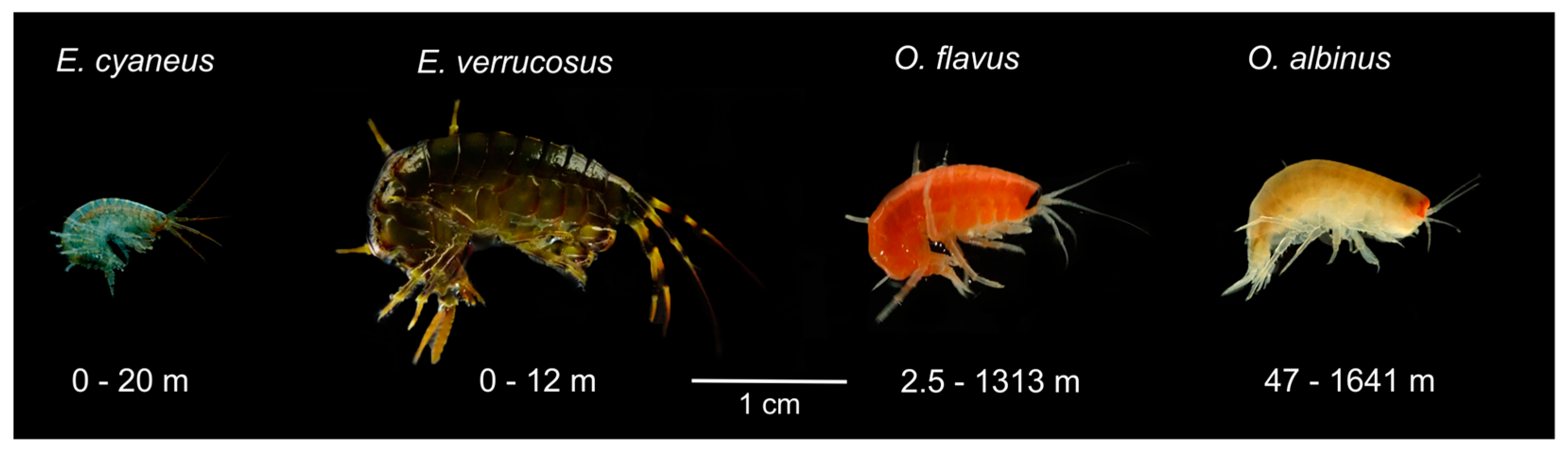
The littoral zone of Lake Baikal is inhabited by a plethora of species, many of which belong to the genus
Eulimnogammarus [
17]. Among the most widespread species of the genus,
E. cyaneus (Dybowsky, 1874) is one of the smallest and
E. verrucosus (Gerstfeldt, 1858) is one of the largest (
Figure 1) and, thus, may demonstrate different sensitivities to UV. The diversity of amphipods in the dark deep-water pf Baikal zone is also high. The representatives of the deep-water genus
Ommatogammarus are particularly useful as a model for comparative studies, as these nektobenthic scavengers [
18] are easy to collect using traps with rotten fish bait and are similar in size to the littoral species (
Figure 1). Recently we showed that
O. flavus (Dybowsky, 1874) and
O. albinus (Dybowsky, 1874) inhabiting wide ranges of depths can both be used in experiments under atmospheric pressure [
19], which is essential for comparisons with littoral species.
O. albinus has greater preferred depths than
O. flavus and paler coloration, which potentially indicates the higher sensitivity of the former to UV.
Here we aimed at estimating the tolerance of the deep-water Baikal amphipods under environmentally relevant UV levels in comparison to the littoral species and exploring the biochemical mechanisms underlying their differences. We observed a decrease in UV tolerance with increasing preferred depth in the two studied Ommatogammarus species and found a link to the differences in the levels of carotenoids and carotenoid-binding proteins in these amphipods. Additionally, we tested the potential antagonistic interactions between the deep-water species and the littoral amphipods in the absence of UV radiation.
2. Materials and Methods
2.1. UV Measurements
Intensity if the visible light was measured with a Megeon-21170 luxmeter (Megeon, Russia), while the intensities of the UV radiation at the surface were measured separately for UV-A, UV-B and UV-C using TKA-PKM(13) UV radiometer (TKA, Russia). For underwater UV measurements (combined UV-A and UV-B) we used a Center 532 UV radiometer (Center, China) fixed inside a glass container (IKEA, Sweden) mounted on a metal frame to keep the UV probe horizontally under water.
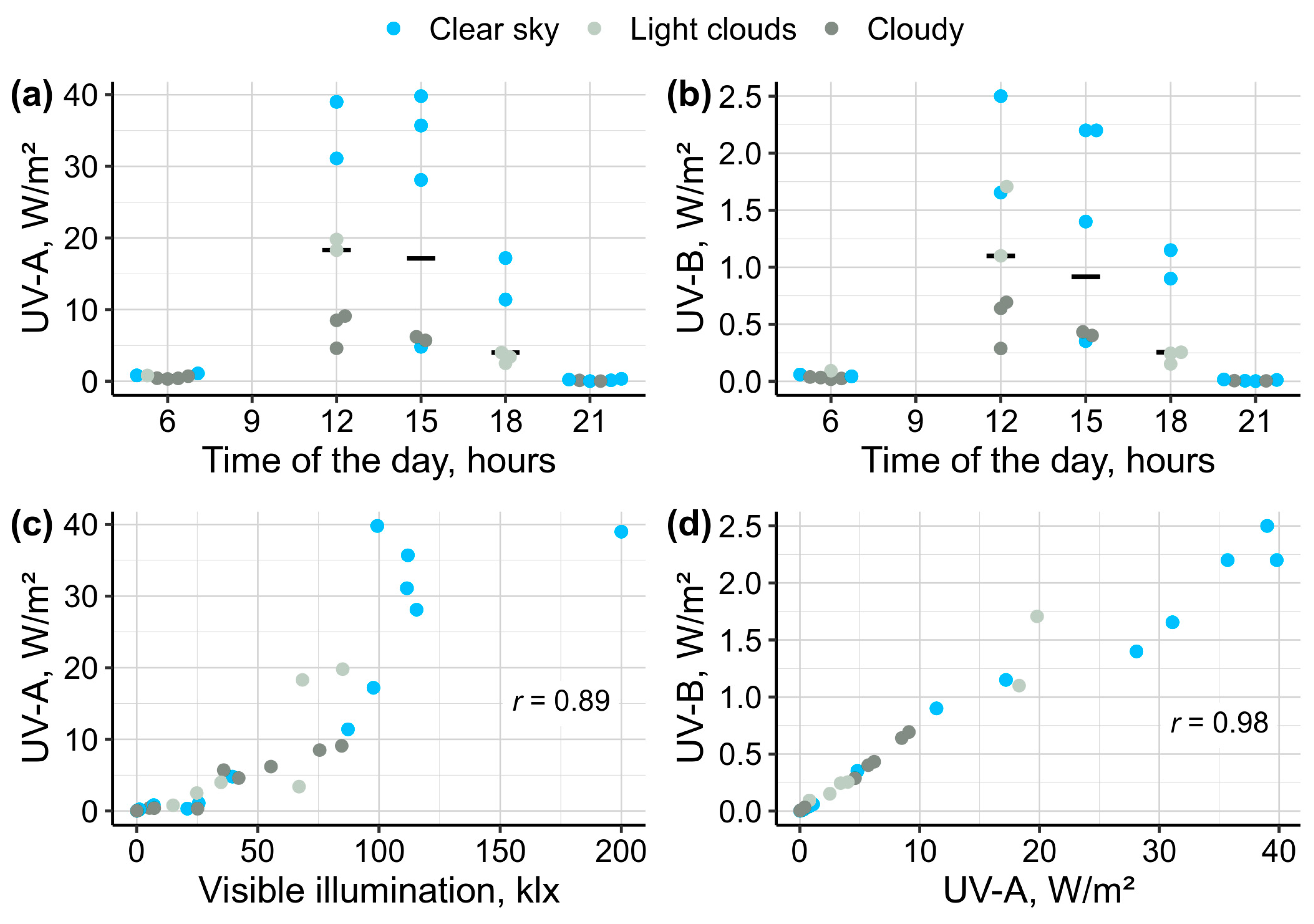
We monitored the intensities of solar UV radiation and visible illumination near the shoreline of Lake Baikal on 4th-10th July 2024 in the Bolshie Koty village (51.90325° N 105.06981° E) at 6 am, 12 am, 3 pm, 6 pm and 9 pm. The probes were always directed to the sun during the measurements. Underwater UV measurements at 1 m depth were performed at the same location on 9th July 2022 at about 2 pm when the sky was totally clear.
Visible and UV output of all light sources used for the experiments was estimated on the distance of 25 cm from the lamps, which is the distance between the lamps and the bottom of aquaria in the used experimental setup.
2.2. Animal Sampling and Maintenance
All experimental procedures with amphipods were conducted in accordance with the EU Directive 2010/63/EU for animal experiments and were approved by the Animal Subjects Research Committee of the Institute of Biology at Irkutsk State University (the protocol #009). All studied species are neither endangered nor protected. The species identification was conducted using the taxonomic keys of Bazikalova [
20].
Adults of the littoral amphipods
E. cyaneus and
E. verrucosus were collected with a hand net near the shoreline of Lake Baikal at the Listvyanka village (51.87058° N 104.82827° E) from depths of 0–1 m during summer and early autumn (if not stated otherwise). The deep-water scavengers
O. flavus and
O. albinus were sampled using 5-l plastic traps with rotten fish in net bags as the bait. The traps were installed from ice in March and placed at the bottom in the bay of Bolshie Koty village at depths of approximately 300, 600 and 700 m as described previously [
19].
The amphipods were sorted by species under temperature-controlled conditions, transported to the laboratory in insulated boxes and kept separately by the species in 2-l tanks (high-density polypropylene of food-grade quality or glass) filled with continuously aerated water from Lake Baikal. The littoral
Eulimnogammarus species were kept at 6 °C (average temperature in the littoral zone of Lake Baikal [
22]) and acclimated to laboratory conditions for at least 4 days, while deep-water
Ommatogammarus species were kept at 4 °C (except for the comet assay, when the experiment temperature was also 6 °C due to technical restrictions) and acclimated for at least 7 days. Water exchanges with the following feeding were performed every 3 days. For littoral species the feed was the dried and ground mixture of algae and amphipods from the sampling location [
23], while in the case of deep-water scavengers we used pieces of white fish meat [
19].
2.3. Experimental Setup for UV Exposures
Amphipods were subjected to two types of UV radiation separately using two lamps TL44D25/09N (Philips) per aquarium for UV-A exposures or two lamps TB218-12 combined with one lamp TB218-10 (Simple Zoo) per aquarium for UV-B exposures. All used UV lamps also have a substantial visible output, and control groups of amphipods were subjected to visible illumination without UV using either one daylight luminescent lamp (Philips) for control to UV-A or two lamps TB218-02 (Simple Zoo) for control to UV-B. The control lamps were wrapped into dark plastic films of different transparency to roughly match the illuminance of the respective experimental lamps and to completely remove the remaining UV. During the experiments all lamps gradually lose their intensities, which we noticed only at the end of the study. At the beginning of the study the outputs of the lamps were the following: 5.2 W/m2 of UV-A, 0.16 W/m2 of UV-B and 0.07 W/m2 of UV-C for the UV-A lamps, and to ~0.4 W/m2 of UV-B, ~1 W/m2 of UV-A and 0.02 W/m2 of UV-C for the UV-B lamps. After completion of the study the outputs decreased to: 1.6 W/m2 of UV-A, 0.05 W/m2 of UV-B and 0.02 W/m2 of UV-C for the UV-A lamps, and to 0.1 W/m2 of UV-B, 0.4 W/m2 of UV-A and 0.02 W/m2 of UV-C for the UV-B lamps. The visible illuminance of experimental and control lamps respectively at the end of the study was ~300 lx and ~500 lx for the UV-A experiments, as well as ~1.2 klx and ~1.6 klx for the UV-B experiments. The distance from the lamps to the bottom of tanks, where the animals prefer to stay, was 25 cm.
All UV exposures consisted of 24-h cycles including 12 h of illumination and 12 h of darkness. Each experiment included four aquaria (each is considered an independent replicate) located below two sets of lamps of the specific type; i.e., exposure of one species under UV-A included four aquaria under the UV-A lamps and also four aquaria under control lamps with only visible illumination. The height of the water column was always 12 cm, and no stones were provided in the aquaria for the UV exposures.
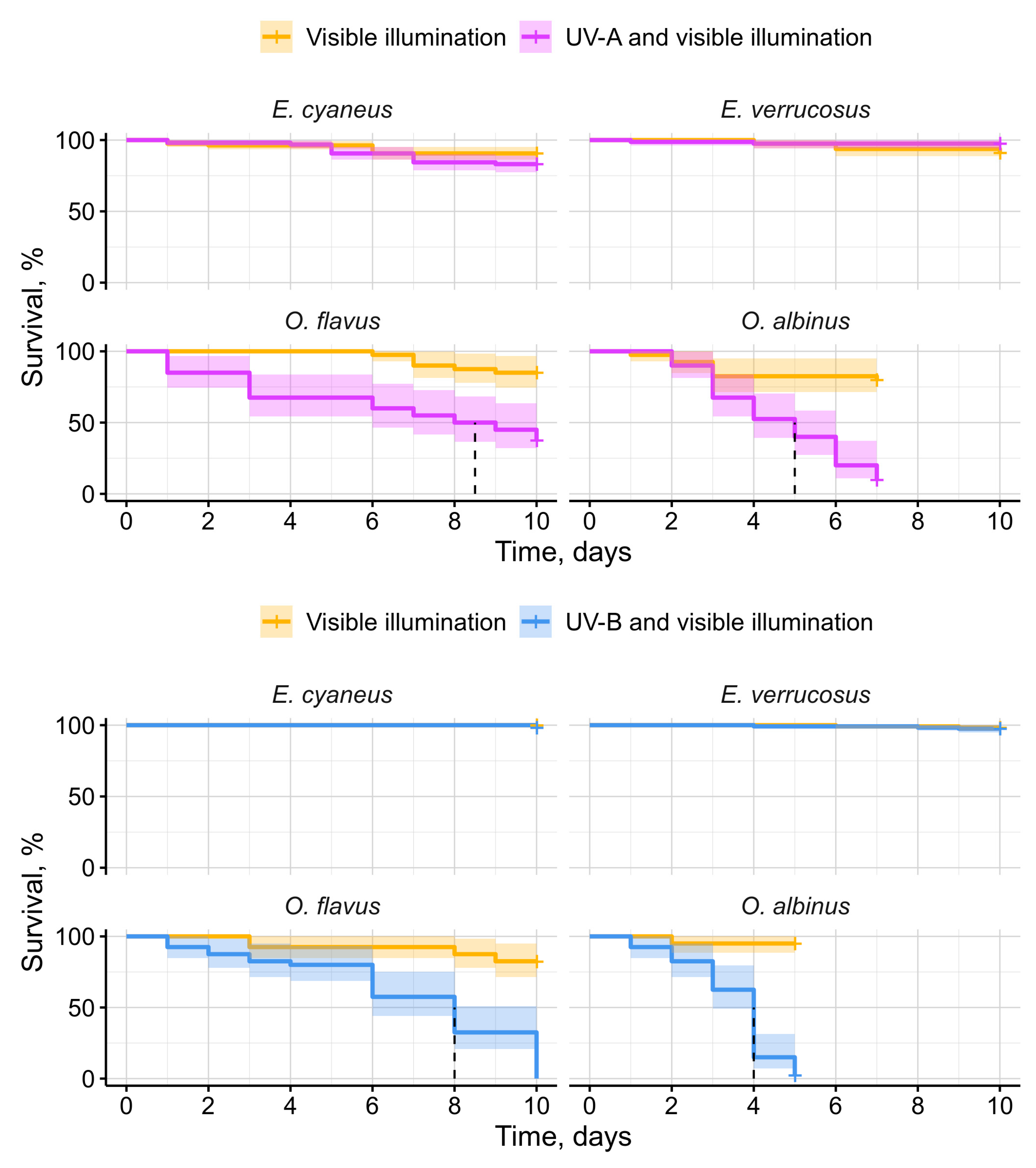
During the experiments for estimation of survival each aquarium contained 15 individuals of E. verrucosus, 20 individuals of E. cyaneus, 10 individuals of O. flavus or 10 individuals of O. albinus. Mortality of amphipods was checked once a day. In the survival experiments on Ommatogammarus species, we also included an additional parallel control group that experienced darkness during the whole experiment. Since there were no statistically significant differences in survival (see below) between the main control groups with visible illumination and the additional control groups with no illumination for both species and both types of control lamps, the latter groups are not depicted.
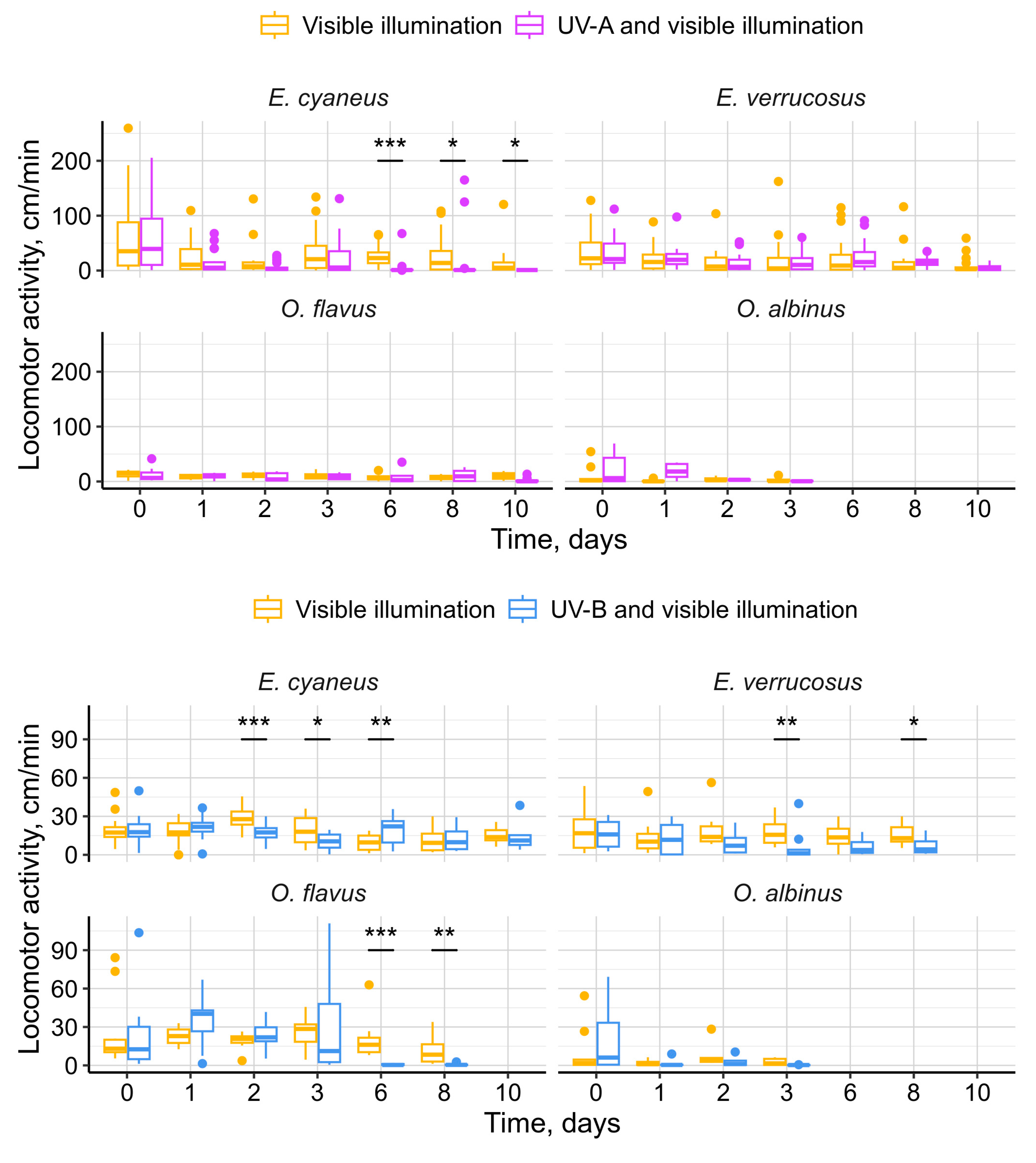
2.4. Estimation of Locomotor Activity
The horizontal locomotor activity of amphipods during 5 min at each time point of UV exposures was recorded using a Tough TG-5 camera (Olympus, China). The first point (day 0) was a few hours after placing animals into experimental conditions. Amphipod’s locomotor activity during 1 min at each time point was then estimated in ImageJ [
24] using the MTrackJ plugin.
2.5. Enzymatic Assays
After exposures under UV radiation, a group of amphipods was shock frozen in liquid nitrogen for measuring the activities of antioxidant enzymes peroxidase, catalase and glutathione-S-transferase using standard spectrophotometric methods [
25]. The extraction of enzymes was carried out in 0.1 M sodium phosphate (NaP) buffer with pH 6.5 using 1:4 w:v sample to buffer ratio. Enzyme activities were measured at 25 °C using Carry 50 UV/visible spectrophotometer (Varian, USA). The total peroxidase activity in the soluble fraction was measured using 4.4 mM guaiacol as the substrate in NaP buffer with pH 5 at 436 nm [
26]. Catalase activity was measured with 2.25 mM hydrogen peroxide as the substrate in NaP buffer with pH 7 at 240 nm [
27]. Glutathione-S-transferase activity was measured with 0.97 mM 1-chloro-2,4-dinitrobenzene as the substrate in NaP buffer with pH 6.5 at 340 nm [
28]. Enzyme activities were normalized per concentration of total protein measured using the method of Bradford [
29].
2.6. Comet Assay
We also estimated the amount of double- and single-stranded breaks in hemocyte DNA with comet assay. Immediately after UV exposures, hemolymph was sampled by the puncture of amphipod exoskeleton between the sixth and seventh segments as described previously [
30] without mixing the extracted hemolymph with an anticoagulant buffer. Comet assay was performed according to the protocol adapted for
Drosophila with electrophoresis in alkaline buffer cooled to 4 °C [
31]. DNA was visualized after staining with ethidium bromide in the RFP channel of an inverted fluorescent microscope Celena S (Logos Biosystems, the Republic of Korea), and the obtained images were further analyzed using specialized software CaspLab v1.2.3b2 comparing the ratio between damaged and undamaged DNA in each cell. Each biological replicate included at least 140 cells.
2.7. Estimation of Carotenoids and Carotenoid-Binding Protein
Adult individuals of
E. verrucosus,
O. flavus and
O. albinus were sampled in early spring of the same year for estimating the concentrations of a carotenoid-binding protein in hemolymph and carotenoids in the rest of the body. Amphipods were acclimated in laboratory, their hemolymph was extracted as described above (
Section 2.6) and they were immediately frozen in liquid nitrogen. Total carotenoid content in amphipods was assessed with a spectrophotometry-based method as described previously [
32] using a Carry 50 UV/visible spectrophotometer (Varian, USA).
The extracted hemolymph was immediately mixed 1:1 v:v with the anticoagulant buffer [
30], followed by 1:1 v:v dilution with the 2× Laemmli application buffer [
33] and incubation at 95 °C for 5 min before further storage at -20 °C. 10 μl of diluted hemolymph samples (i.e., containing 2.5 μl of native hemolymph) from all three species were subjected to one-dimensional polyacrylamide gel (10 %) electrophoresis and stained with Coomassie Brilliant Blue. The procedure was performed as described previously [
30] except for the number of animals used per hemolymph sample: one individual of
E. verrucosus, one individual of
O. albinus and two-three animals for
O. flavus. Stained gels were scanned jointly, and the bands corresponding to the 25-kDa carotenoid-binding protein [
32] were quantified in ImageJ [
24] after converting the image to gray values and calibrating the pixel values to optical density using built-in function (option “Uncalibrated OD”). Optical density was calculated both for original images and the “background” images obtained using the function “Subtract background” (with option “Create background”), and the latter values were subtracted from the former in order to get the final concentrations of the carotenoid-binding protein in arbitrary units.
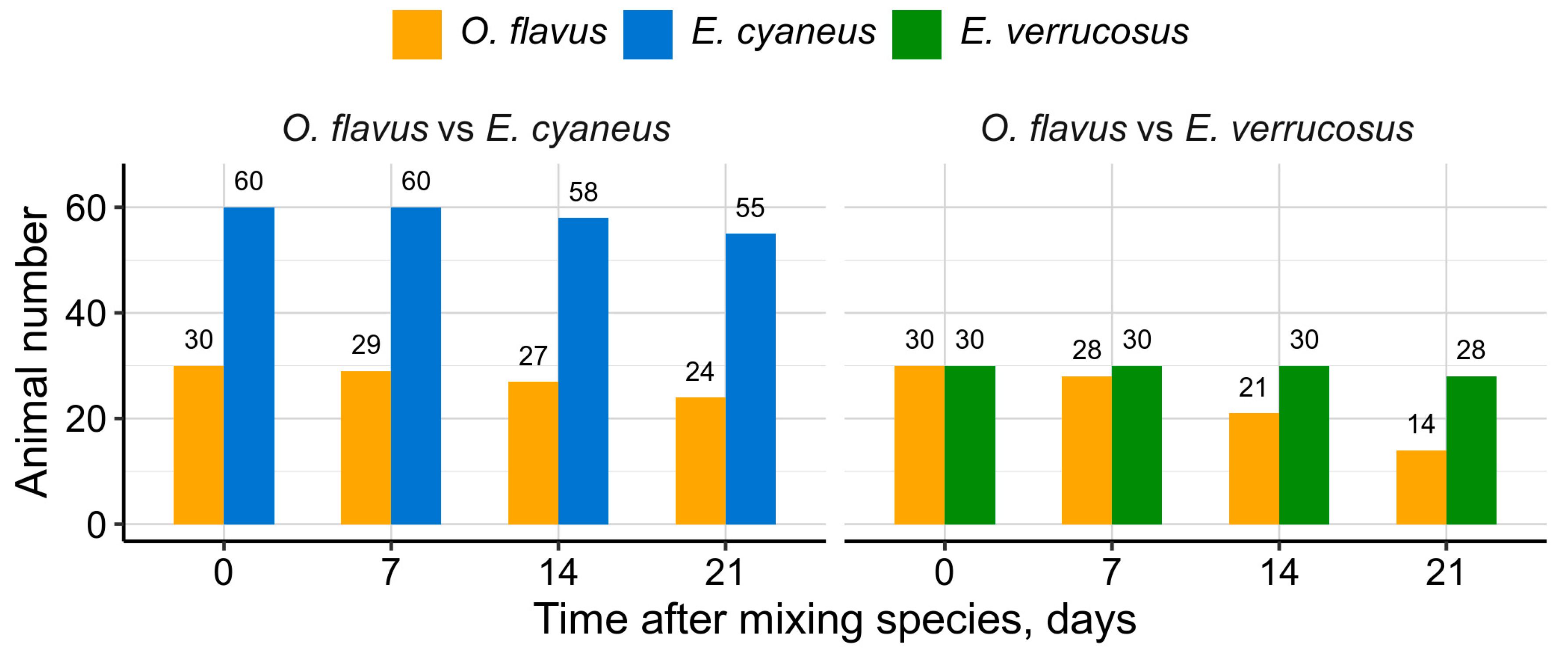
2.8. Cohabitation Experiments with Littoral and Deep-Water Amphipods
To estimate the predatory or competitive activity between the littoral and deep-water amphipods, we kept 30 individuals of
O. flavus with either 60 individuals of
E. cyaneus or 30 individuals of
E. verrucosus for three weeks at 6 °C after samplings in early spring. The 2:1 density ratio of
E. cyaneus to
O. flavus was used due to the smaller size of the former species (
Figure 1). We also also used
E. verrucosus with length of 1-1.5 cm in order to roughly match the size of
O. flavus. For this experiment we used two large aquaria with bottom sized 45×36 cm for each mixed group and covered the bottom with stones of various sizes to let the animals hide. The amphipods experienced dim light regime without UV, and their mortality was checked every five days during the whole experiment.
2.9. Data Analysis and Statistics
All data were analyzed in R v4.3.1 [
34] mostly with built-in functions and visualized using the ggplot2 v3.4.4 [
35] and ggbeeswarm v0.7.2 packages. Visualization of survival curves, their statistical comparisons and estimation of median lethal times (LT
50) were performed with the packages survival v3.5-7 [
36] and survminer v0.4.9 [
37]. Statistical significance of differences in measured parameters under UV exposures was estimated in comparison to respective parallel or initial (only in the case of comet assay) control groups using Mann-Whitney’s test with Holm’s correction for multiple comparisons within each figure panel. All possible comparisons within panel were performed only in the case of contents of carotenoids and carotenoid-binding protein in three species.
4. Discussion
In this study we aimed at assessing the UV sensitivity of adult endemic amphipods from littoral and deep-water habitats of Lake Baikal (
Figure 1). Our research showed species- and habitat-specific differences in the survival, locomotion and DNA damage under UV irradiation, as well as in the levels of the potential UV-protective components like carotenoids and an antioxidant enzyme. One limitation of this study was the gradual decrease in UV intensities of the used lamps, which was not immediately detected and led to declining levels of UV exposures throughout the study. While this limitation complicates the exact determination of the UV dose received by experimental animals, the applied ranges of UV intensities are well below the median intensities of solar ultraviolet on the water surface during the day (
Figure 2) and, thus, are environmentally relevant for Baikal littoral zone.
Our estimates of the UV intensity at 1-m depth of Baikal water showed that approximately 64 % of incoming UV radiation reached this depth. Assuming that most of UV radiation measured at 1-m depth was UV-A and considering the median UV-A intensity measured at the surface during the sunny part of the day (
Figure 2a), the applied UV-A levels at the beginning and the end of our study corresponded to ~3-m and ~5-m water depth, respectively. In case of applied UV-B intensities we could not estimate the corresponding depths.
We first conducted the exposures to estimate survival, locomotor, and enzymatic activities on
E. cyaneus and
E. verrucosus and only after that started the same experiments for
O. flavus and
O. albinus. Importantly, both deep-water species were always processed for each parameter at the same time, which allows for direct comparison of their mortality. The experiments for the comet assay were the latest and performed for all four species in parallel. Since two littoral species experienced higher UV output than the two deep-water species, the observed differences in mortality between them emphasize considerably higher sensitivity of the deep-water species to UV radiation (
Figure 3).
Overall, we found no UV intolerance in
E. cyaneus and
E. verrucosus (
Figure 3) that dwell from the shoreline and down to over 10 m depth. In contrast
, O. flavus and
O. albinus were more vulnerable to UV radiation (
Figure 3), with their sensitivity increasing in correlation with the greater preferred depths (100-200 m and 300-500 m for
O. flavus and
O. albinus, respectively [
19]). Notably,
O. flavus was occasionally reported from 2.5 m depth, whereas the pale
O. albinus was not found above 47 m depth [
20]. These findings indicate that UV radiation might be a contributing factor in limiting the vertical distribution of the deep-water Baikal amphipods, particularly of the more UV sensitive
O. albinus.
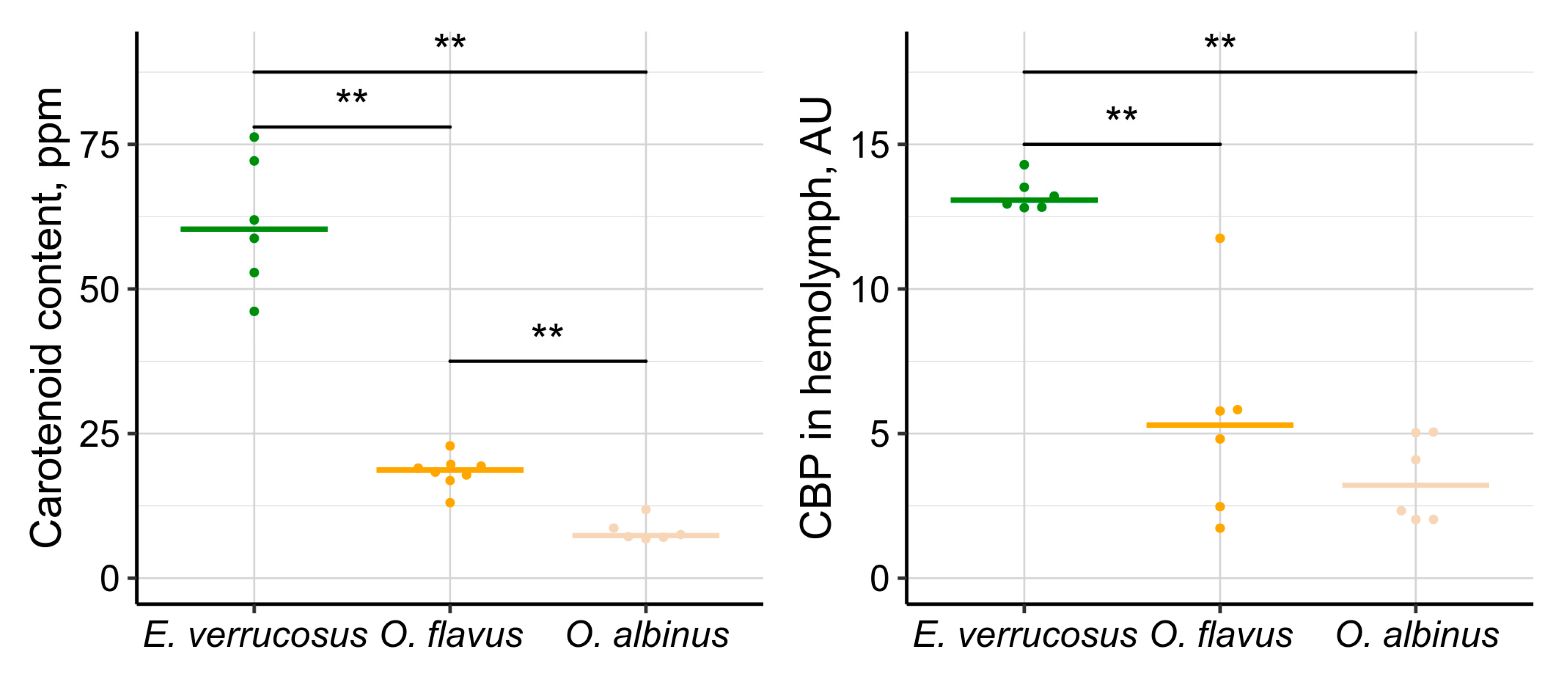
The species-specific differences in amounts of carotenoids and the carotenoid-binding protein (CBP) correlated with the observed differences in survival of
E. verrucosus,
O. flavus and
O. albinus under UV radiation (
Figure 7). These findings are consistent with the well-established role of carotenoids as protective pigments against UV radiation [
9]. However, the two estimated parameters correlated between each other and here we could not distinguish the effects of free and protein-bound carotenoids for UV protection. The species-specific differences in the carotenoid system may suggest an evolutionary loss of UV protectors in response to the long-term absence of solar UV radiation, particularly in
O. albinus. However, the exact biochemical and genetic mechanisms driving this loss, along with the potential for ontogenetic plasticity, remain to be explored.
Our motivation for monitoring amphipod locomotor activity under UV was the search for avoidance reaction and elevated activity. On the contrary, we mostly observed negative effects of UV irradiation on the locomotor activity of Baikal amphipods (
Figure 4), suggesting UV-induced behavioral stress. Interestingly,
E. cyaneus exhibited a decrease in activity under UV-A irradiation, whereas
E. verrucosus did not, which may indicate higher UV sensitivity in
E. cyaneus, potentially due to its smaller size. While
O. flavus and
O. albinus were generally less active than littoral species,
O. flavus, along with
E. cyaneus and
E. verrucosus, showed a reduction in locomotor activity under UV-B irradiation. The ecological significance of these locomotor disturbances remains unclear, but they could potentially impair the ability of littoral amphipods to distribute for long distances without a shelter protecting them from solar UV. This may contribute to geographic isolation of genetic lines within littoral species by sandy parts of Baikal shoreline [
38].
Measurements of the antioxidant enzymes peroxidase and catalase did not indicate oxidative stress under UV irradiation in any of the four amphipod species studied. Glutathione-S-transferase (GST) activity similarly showed no increase in the UV-exposed littoral species. However, interestingly, in both deep-water species exposed to UV-B, we observed a notable increase in GST activity (
Figure 5). GST is a multifunctional enzyme that plays an important role in detoxification of exogenous and endogenous toxic compounds [
39,
40]. This suggests that the selected UV levels do not induce significant ROS production in the studied amphipod species but specifically UV-B may cause accumulation of some toxic compounds in the less UV-tolerant deep-water species. However, considering that UV-A penetrates to greater depths than UV-B [
5], exposure to UV-B radiation is likely ecologically irrelevant for
O. albinus and may be only partially relevant for
O. flavus.
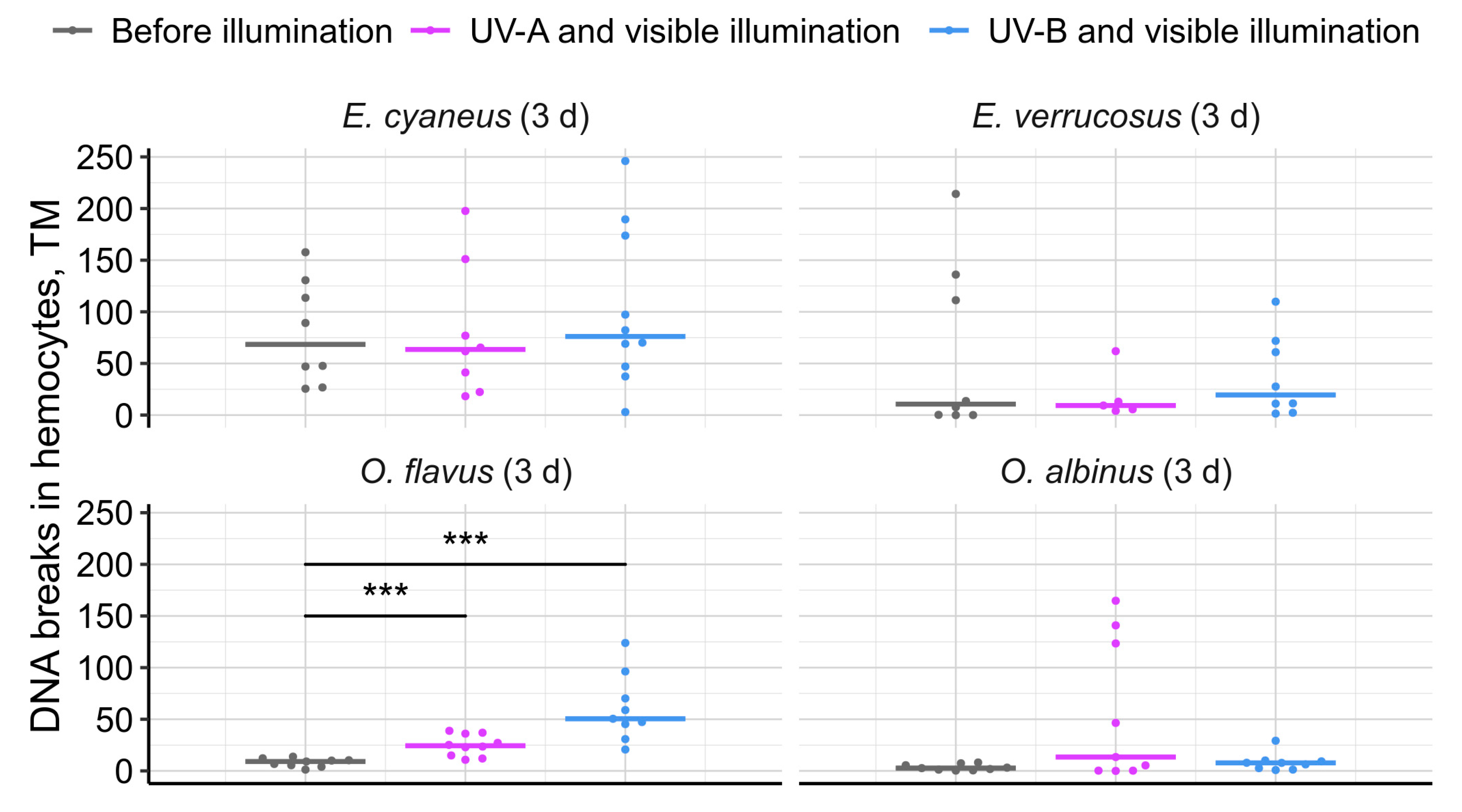
Interestingly, we observed hemocyte DNA damage only in
O. flavus, while no such damage was detected in
O. albinus or the littoral species (
Figure 6). In the comet assay experiments, all species were exposed to the same dose of UV radiation. The absence of a UV response in
O. albinus is challenging to explain, but we propose two possible hypotheses. First, hemocytes are primarily located deep within the organism, where they are shielded by outer tissues. In
O. albinus, this protection could be further enhanced by a higher lipid content [
41]. Additionally, since
O. albinus is less adapted to atmospheric pressure, it is plausible that immune functions were generally suppressed, leading to lower gene expression in hemocytes and more condensed chromatin, which might be less vulnerable to UV-induced DNA breaks. Lastly, we cannot rule out survivor bias, where individuals of
O. albinus with better DNA protection or repair mechanisms may have preferentially survived, reducing the observed DNA damage response.
Among the factors analyzed, UV-B appeared to have the most pronounced effects on the four amphipod species we studied. Nevertheless, there is a couple of caveats: (i) we could not fully exclude UV-A from the UV-B lamps and UV-B from the UV-A lamps, as well as even minor UV-C in both cases and (ii) the applied UV-B was slightly more intense than UV-A compared to their natural median levels, being 2.5 and 3.5 times lower than their observed medians during monitoring. Additionally, the cumulative UV dose plays a critical role in UV effects [
9], and in our study, the 12-hour exposures without hiding spots likely subjected the amphipods to a higher UV dose than they would naturally encounter in the littoral zone. Thus, we cannot rule out the possibility that UV exposures more closely mimicking the natural daily light cycle might result in slower mortality rates for the deep-water amphipods.
Overall, our findings suggest that solar UV radiation is one of the ecological factors limiting the distribution of deep-water amphipods to the littoral and possibly sublittoral zones of Lake Baikal. Observations from deep-water traps, where
Ommatogammarus scavengers were found feeding on other species (as indicated by their remains), prompted us to investigate their potential to prey on littoral species under atmospheric pressure and without UV exposure (
Figure 8). This experiment effectively modeled a scenario of reduced water transparency in the littoral zone, which could occur due to local eutrophication near the shoreline [[42.]. However,
O. flavus was unable to effectively cope with live
E. verrucosus under these conditions, highlighting the complex ecological differences between littoral and deep-water species.
Author Contributions
Conceptualization, M.T, E.K, A.G, Z.S. and I.S.; methodology, E.K., A.G., Z.S, and Y.R.; software, A.G. and Y.R; validation, A.G., E.K., Z.S, M.T. and K.V.; formal analysis, A.G., E.K. and Y.R.; investigation, E.K., Y.R., A.S., A.D., K.V. and P.D.; resources, M.T. and Z.S.; data curation E.K., Y.R., A.S., A.G. and K.V.; writing—original draft preparation, E.K. and A.G.; writing—review and editing, I.S., P.D., M.T., Y.R., Z.S., K.V., A.S. and A.D.; visualization, A.G., Y.R. and E.K.; supervision, M.T. and Z.S.; project administration, M.T., E.K., A.G., Z.S.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.