Submitted:
02 October 2024
Posted:
04 October 2024
You are already at the latest version
Abstract
Keywords:
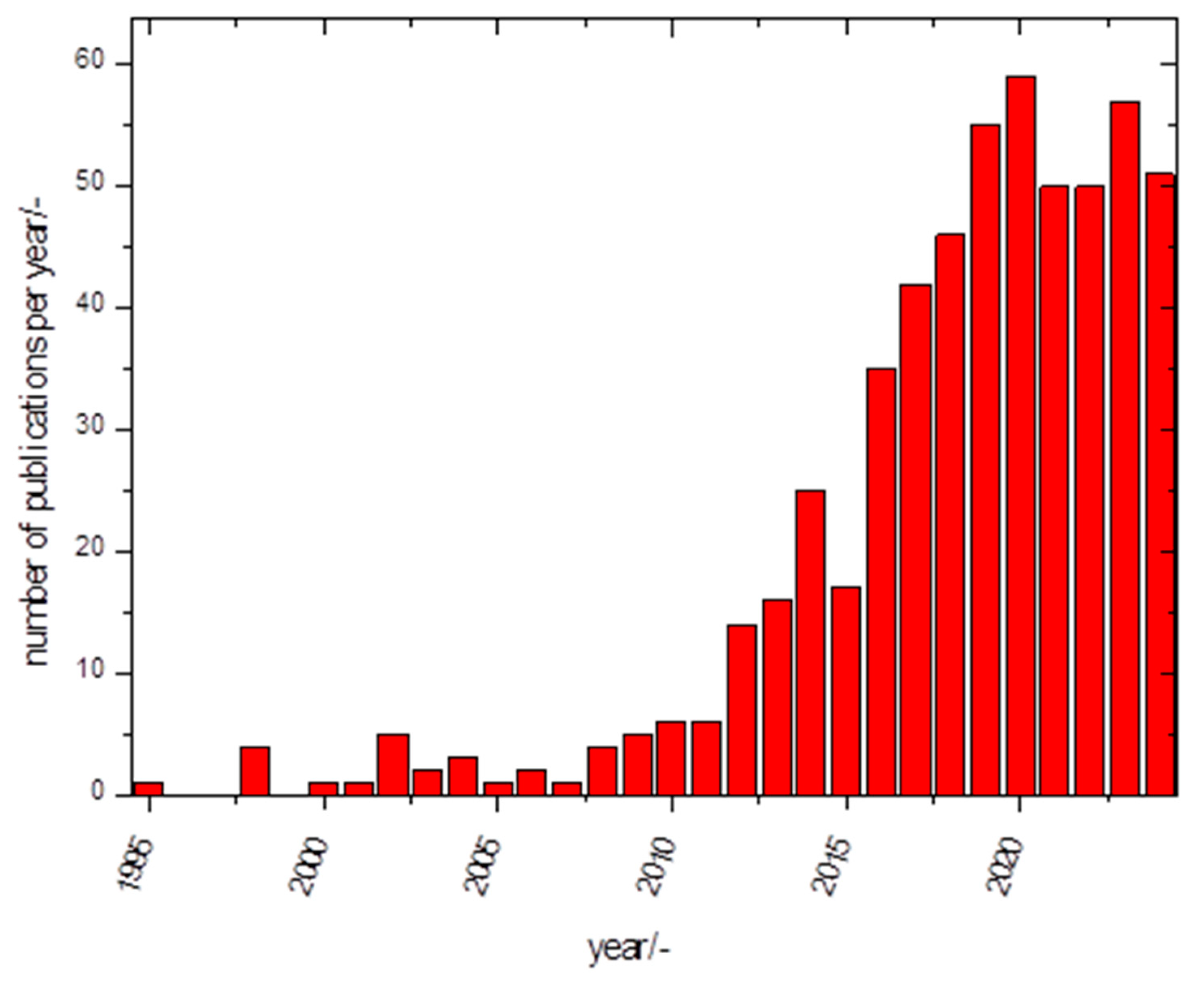
1. Introduction
2. Fundamentals: Ionically Conducting Polymers
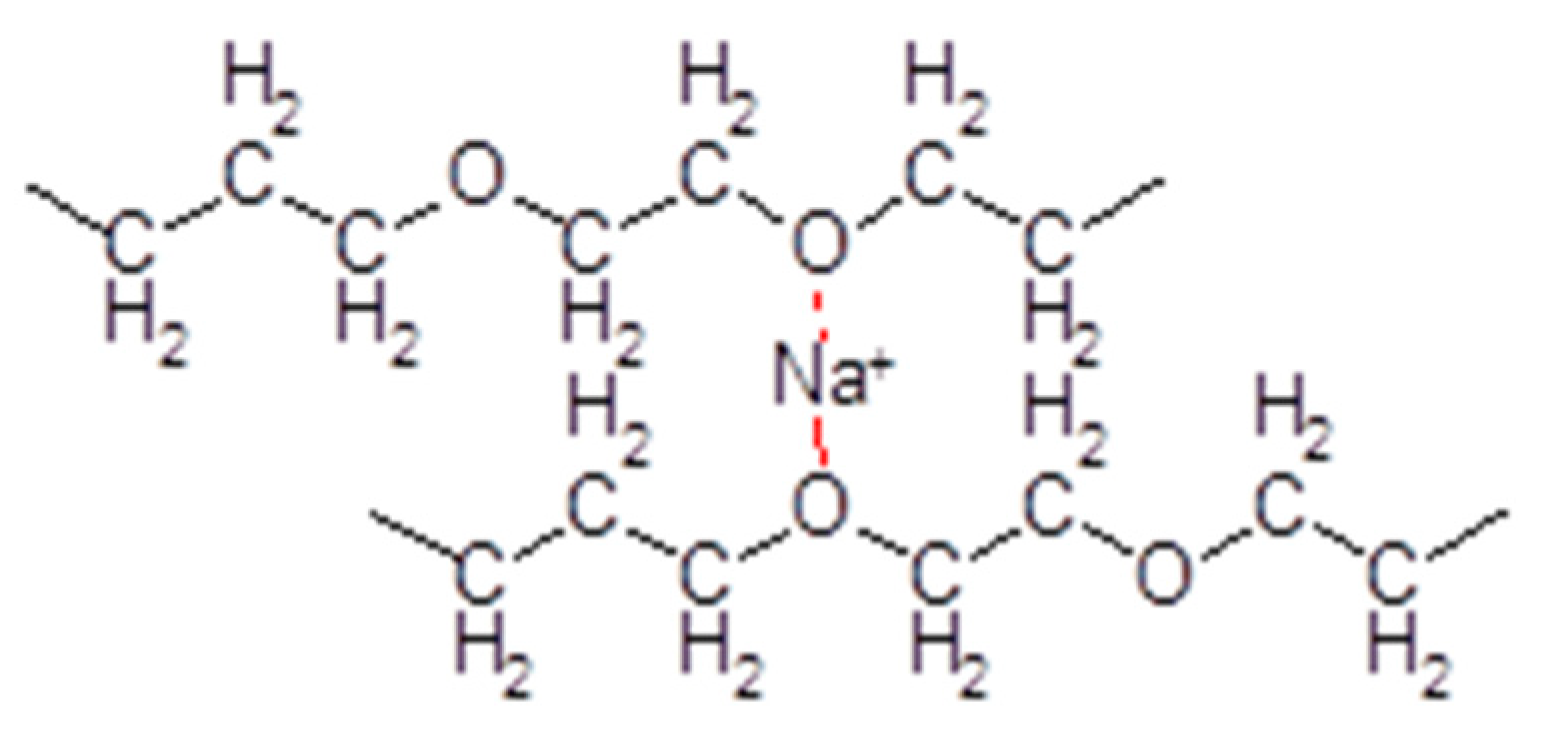
- Wide available electrode potential window
- high ionic conductivity sufficient chemical and electrochemical stability
- thermal stability
- compatibility with electrode and separator materials
- environmental compatibility
- low price
- sustainable resources
3. Solid Polymer Electrolytes in Supercapacitors
3.1. Plain SPEs
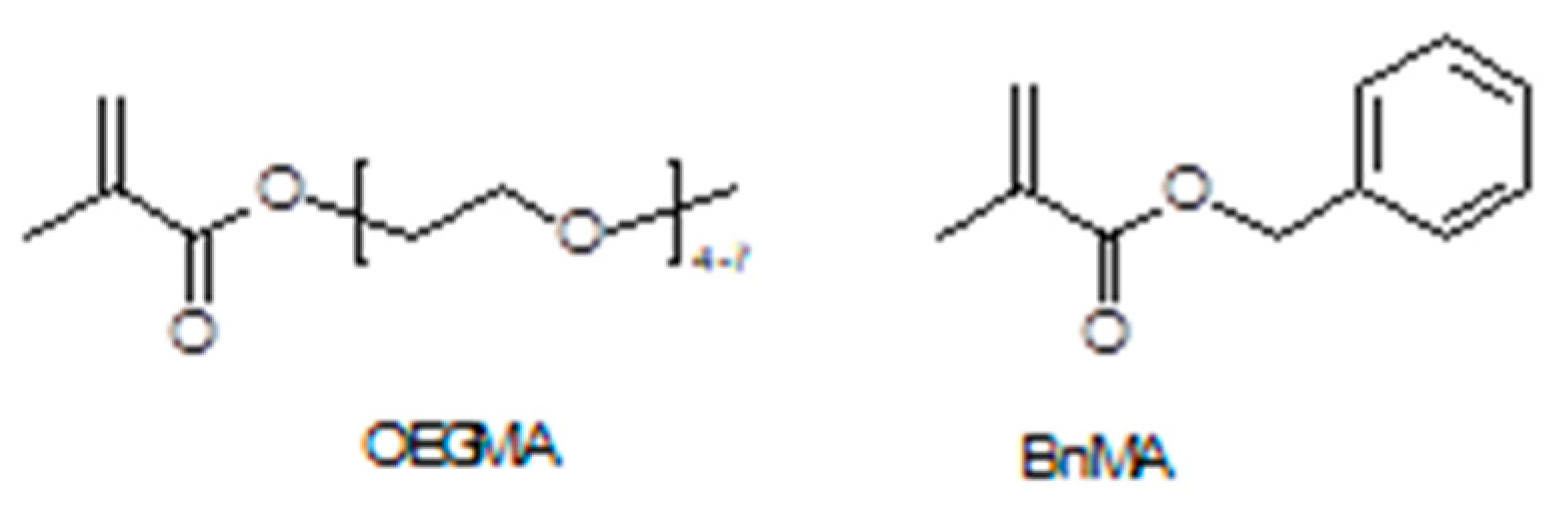
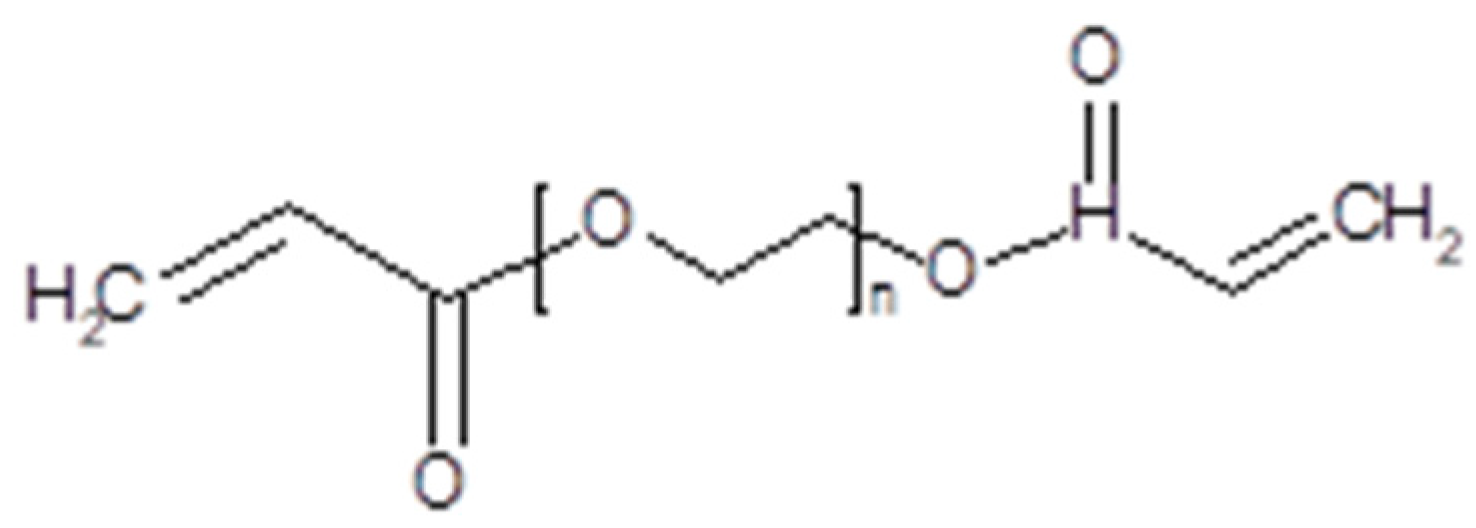
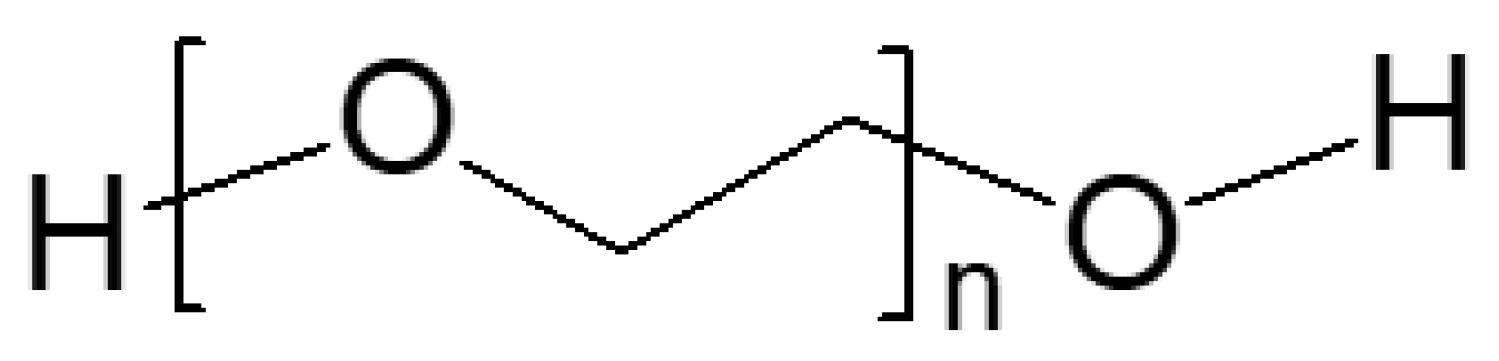
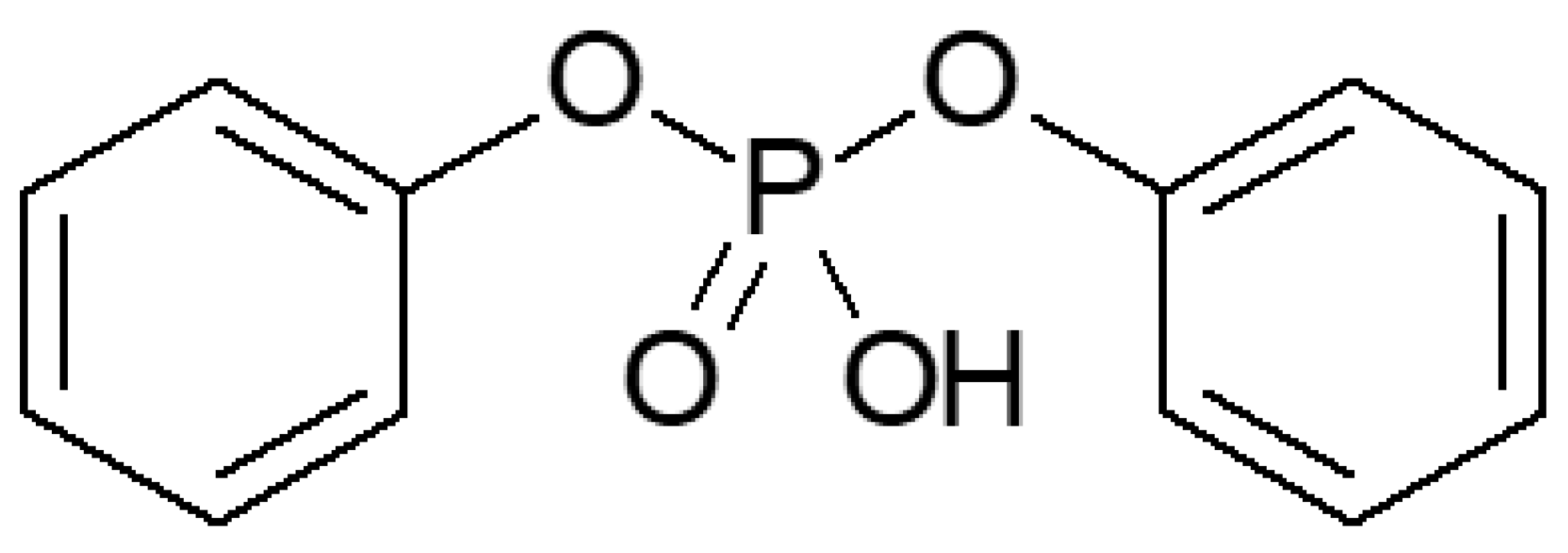
3.2. Plasticized10 Polymer Electrolytes in Supercapacitors
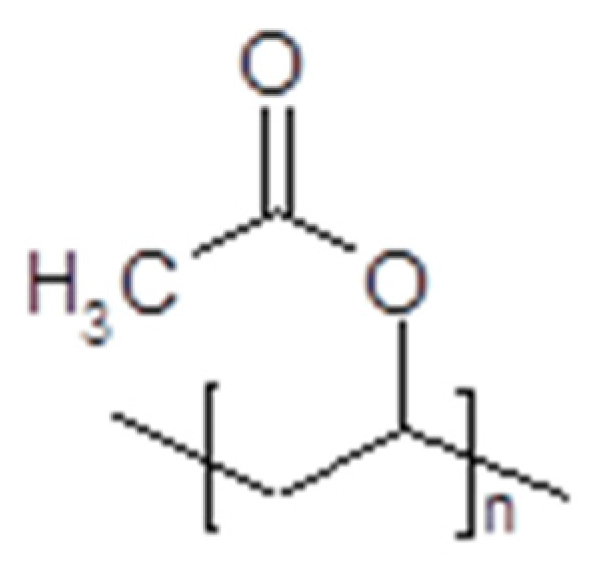
- PVDF-HFP and IL
- PVDF-HFP and IL and electrolyte salt
- PVDF-HFP and IL and salt and plasticizer
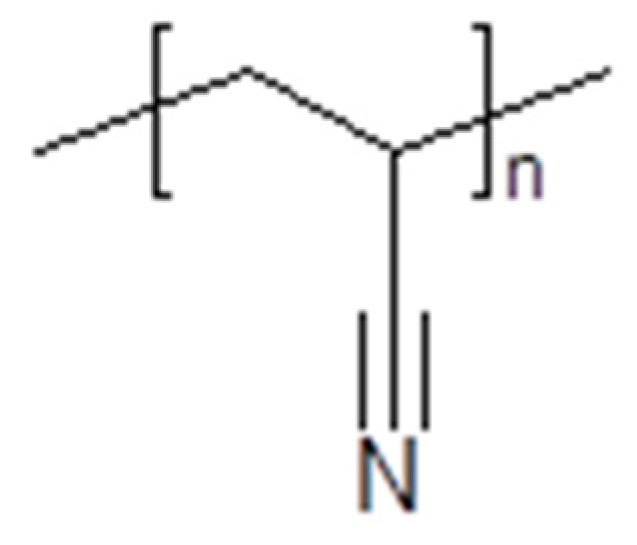
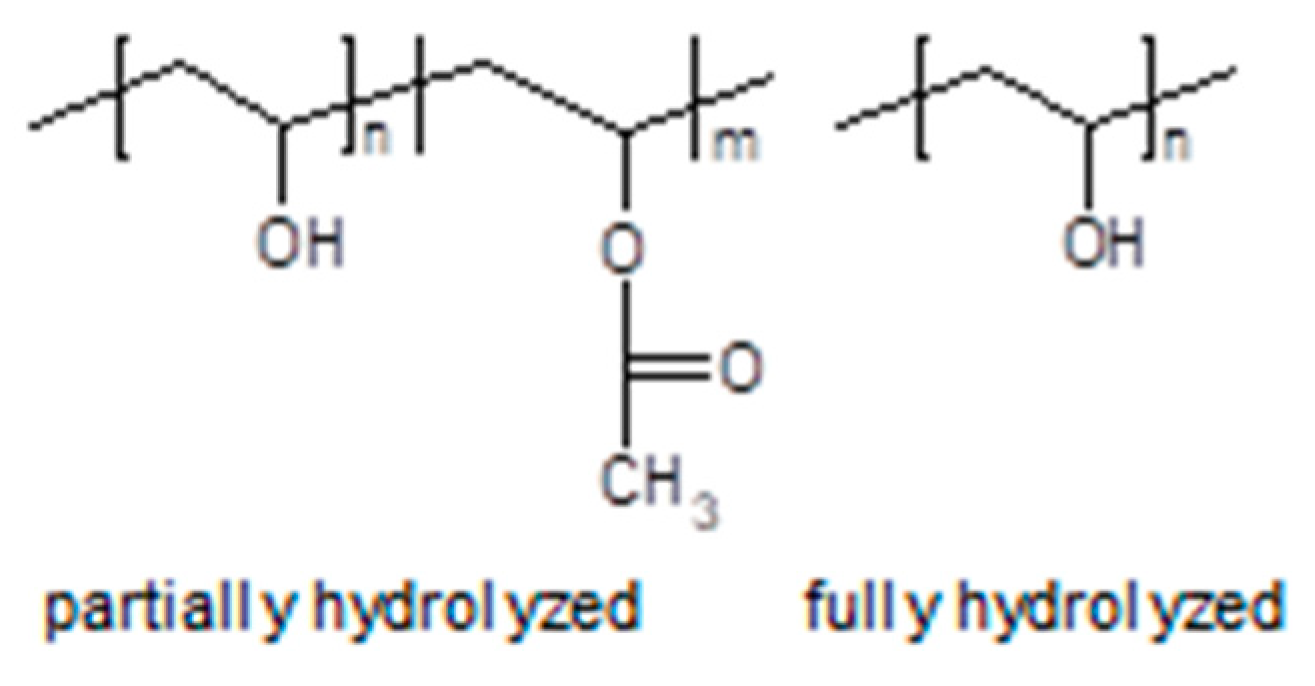
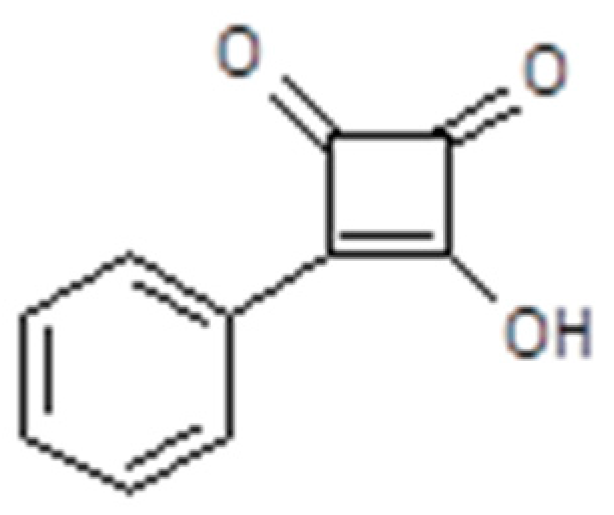
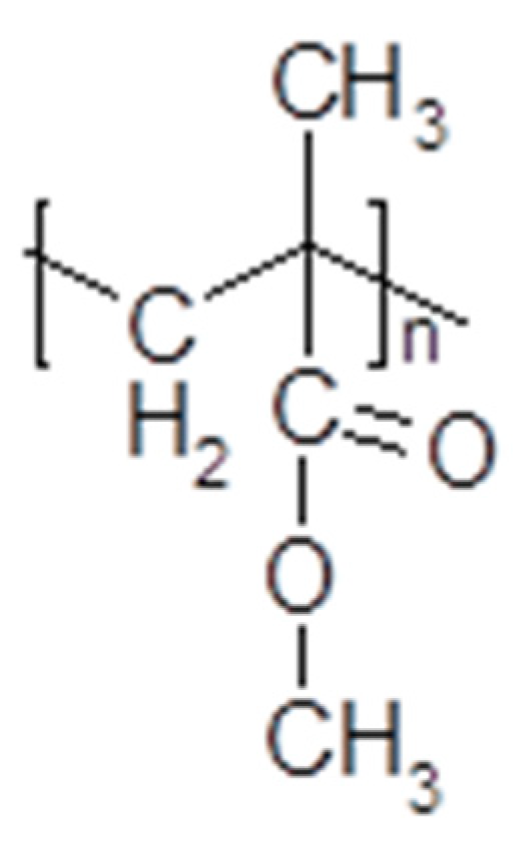
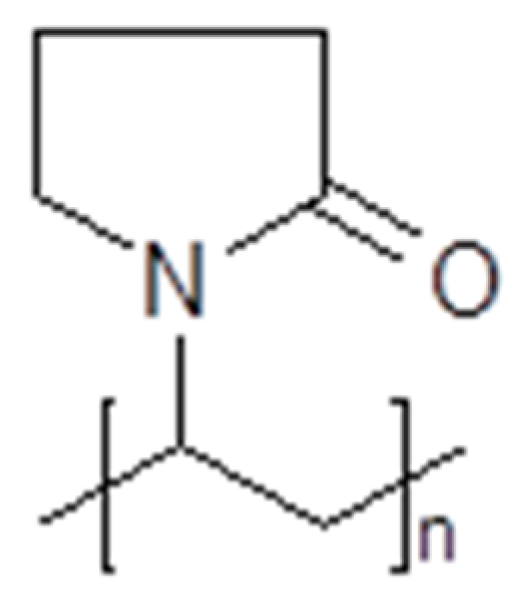
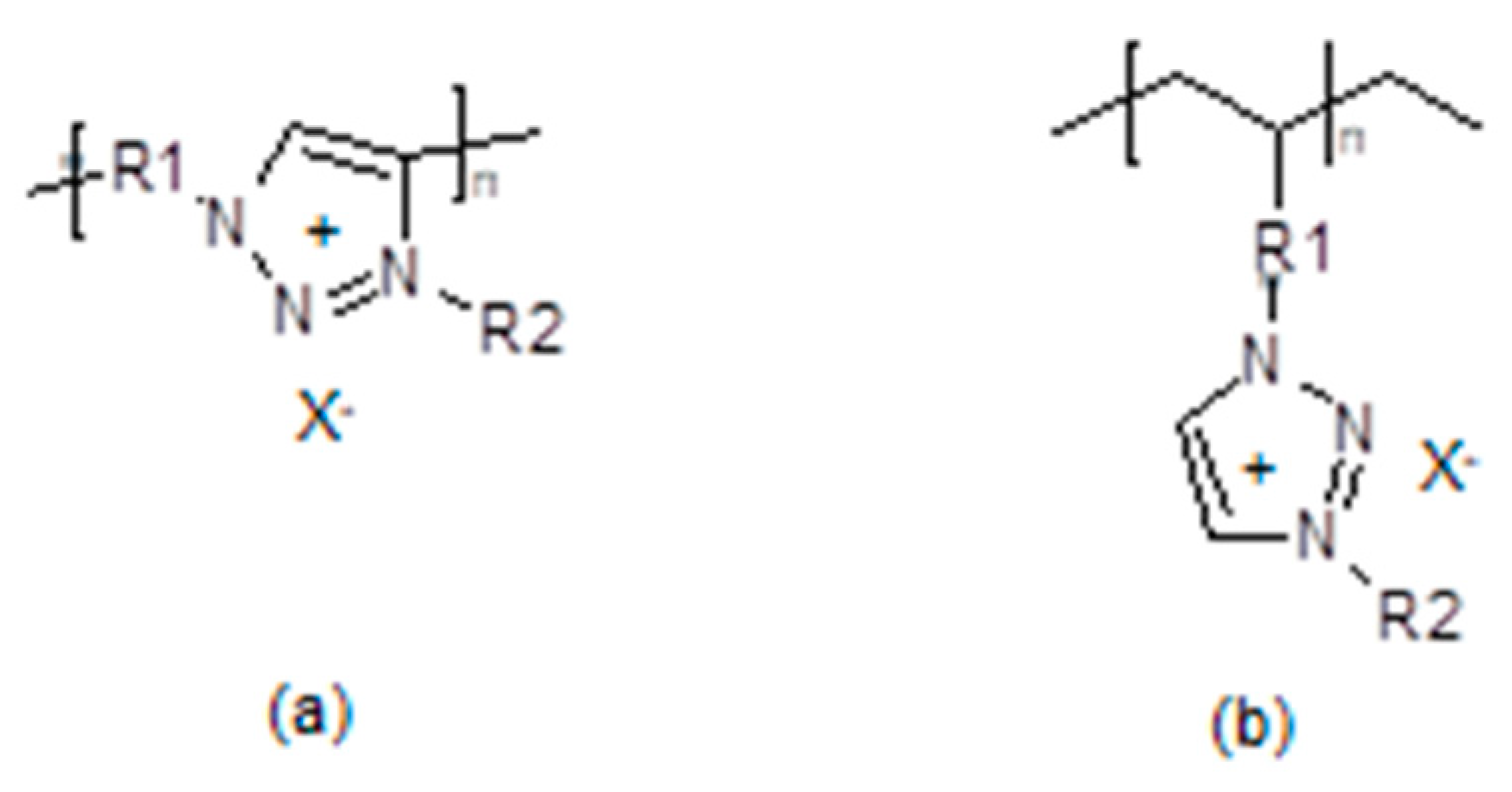
3.4. Polymerized Ionic Liquids
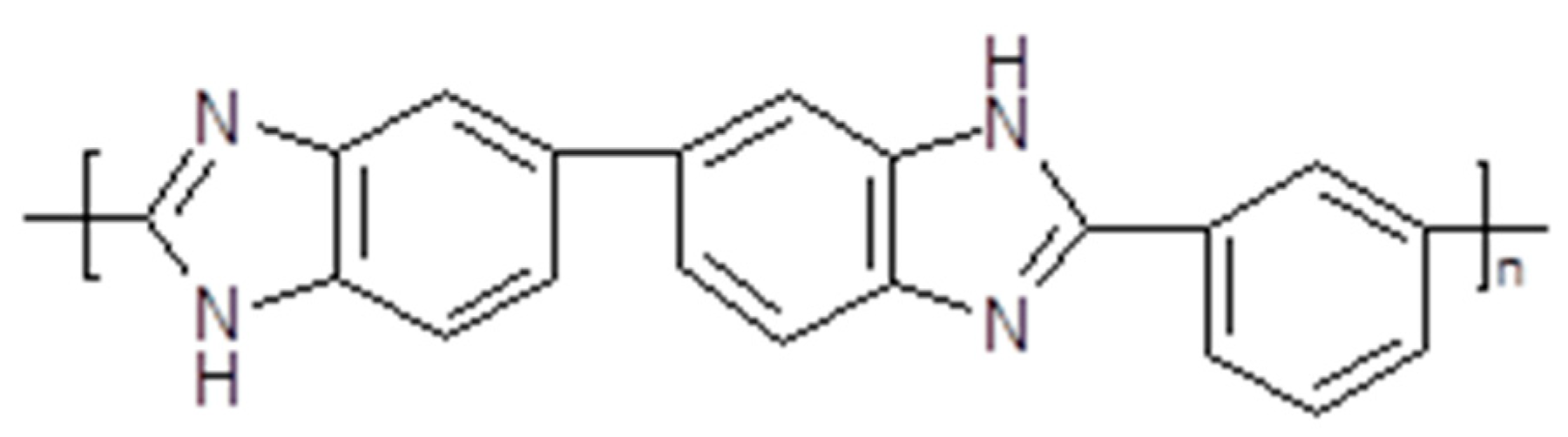
3.3. Ion Exchange Polymers as Electrolytes in Supercapacitors
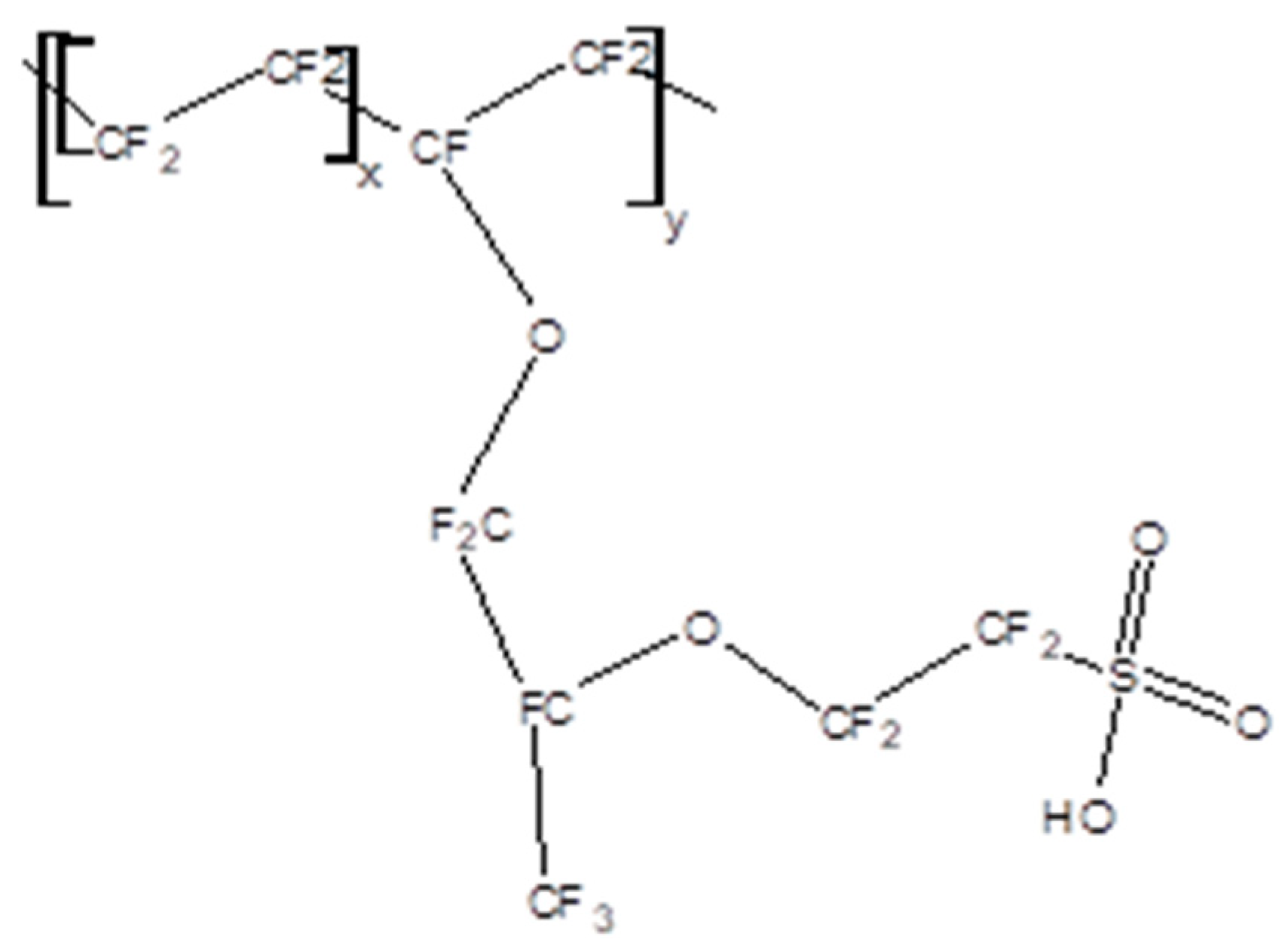
3.5. Approaches towards Improved Electrolyte/Electrolyte Interfaces
- The dissolved electrolyte (polymer(s), plasticizer, electrolyte salt) is coated onto the porous electrode, before/after drying electrodes are assembled with/without additional separator. Typical examples: copolymer electrolyte [119], polymer-ionic liquid mixtures [253]. Or – the other way around – the electrodes are soaked in the still liquid SPE, for typical examples see [323,354].
- The electrodes are soaked with a solvent, possible the same also used in preparation of the electrolyte; the wet electrodes are joined with the electrolyte. Transfer of ions from the electrolyte into the solvent filling the porous electrode body proceeds. Typical example: dimethylacetamide [115].
- The electrodes are soaked with monomers (mostly in suitable solvents) of the electrolyte polymer, these mono- or oligomers can even act as binders for the active electrode material. Upon assembly of the electrodes with the polymer electrolyte a continuous transition from the polymer in the electrolyte to the monomer in the electrolyte is established. Typical example: RuO2 and Nafion® [474].
- In a similar approach with GPEs the porous electrodes are immersed into the still rather liquid electrolyte mixture. Typical example: PVA with Li2SO4 in an EDLC-de vice [366].
3.6. Gel22 Polymer Electrolytes
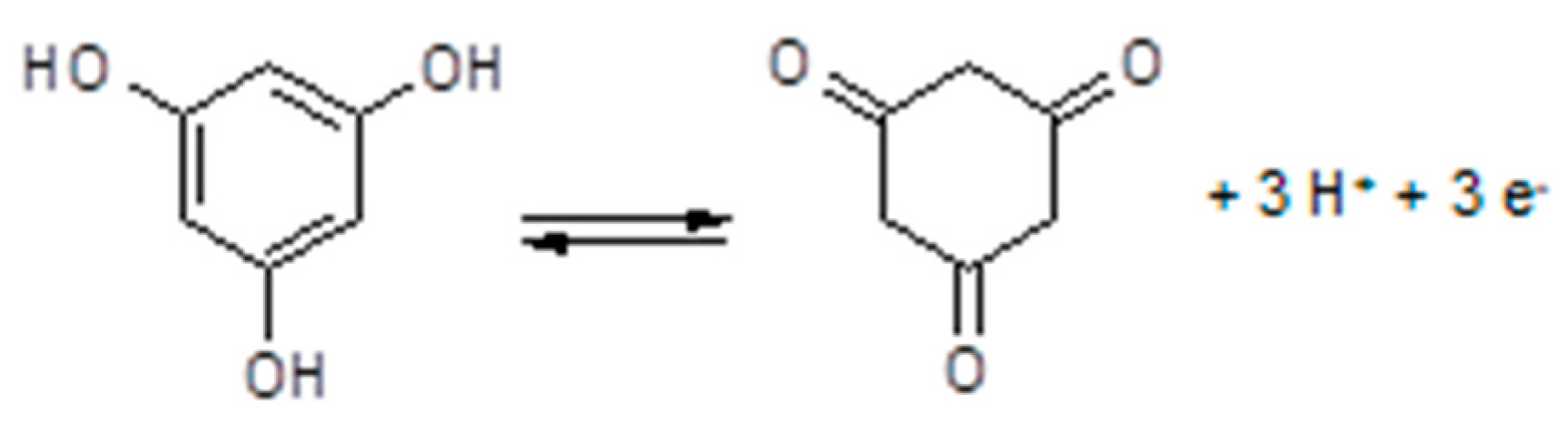
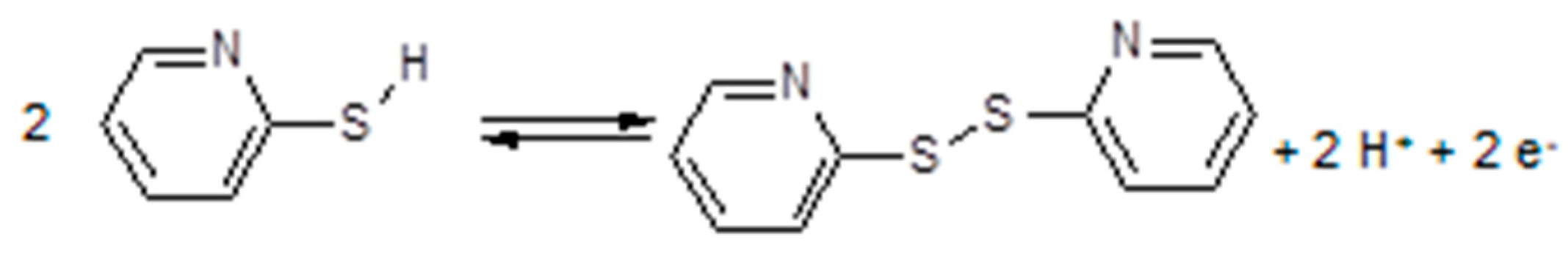
3.7. Polymer Electrolytes with Added Redox Systems
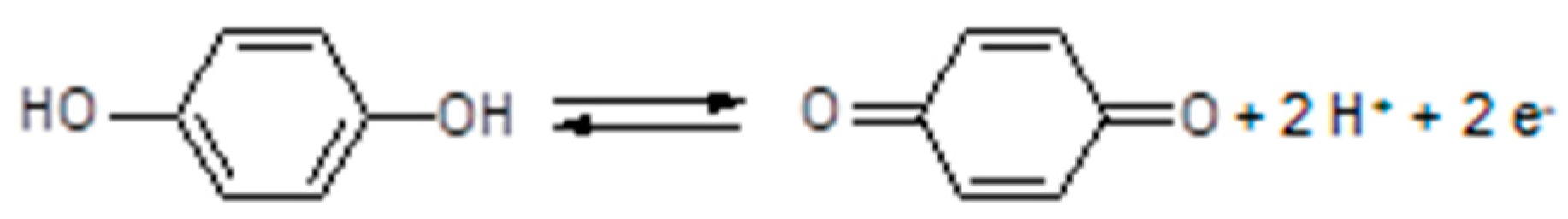
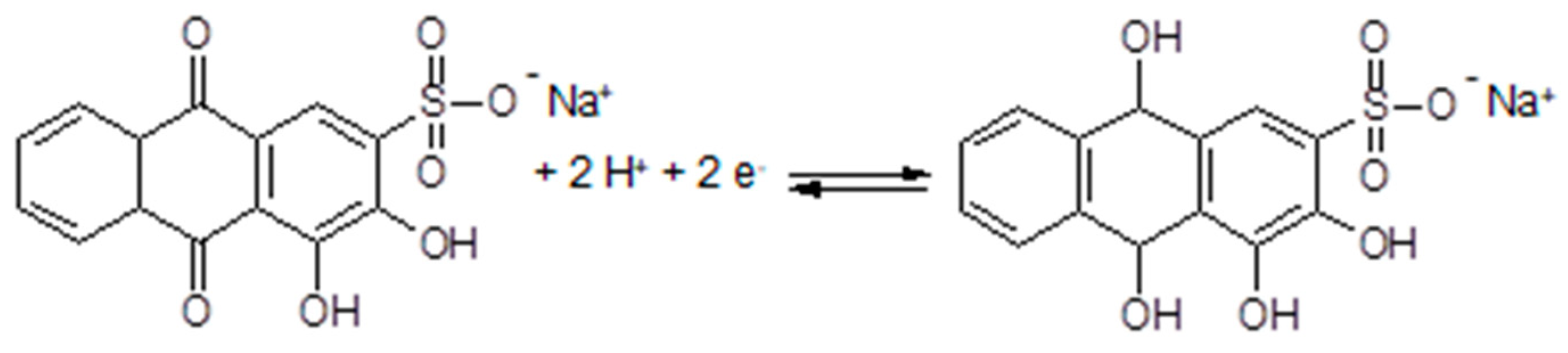
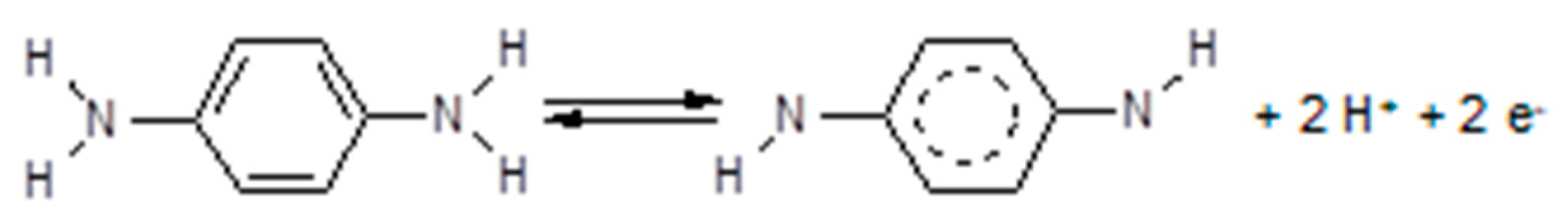
3.8. Polymer Electrolytes and Device Properties
4. Conclusions, Outlook and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
| 1 | Possibly the authors also know somebody who is quasi-dead. |
| 2 | This acronym is used throughout this report without repeating the imprecise definition again and again. |
| 3 | TFSI is the common acronym designating the IL-anion bis(trifluoromethanesulfonyl)imide |
| 4 | A reference provided in this report turns out to be completely unrelated and useless. |
| 5 | To call the device a magnesium capacitor apparently only because a magnesium salt was added seems to be a far stretch of the facts. |
| 6 | Assuming the acronym CMC nowhere explained in this report has this meaning. |
| 7 | Different from the author’s opinion this is the name of the plant, not of any chemical compound! |
| 8 | Presumably the term “cycle retention” means capacitance retention. |
| 9 | In this report the discussion quotes a non-existent report on ion transport attributed to Vehicle and Grotthus – a most memorable error in this also otherwise flawed report. |
| 10 | The synonym plastified is also found. |
| 11 | Tf or TF are common acronyms specifying the IL-anion trifluoromethanesulfonate |
| 12 | This acronym is not exactly systematic, it misses the -co- between the two constituents, it also misses the P with HFP. Never¬theless it appears to be firmly established, and different from some other authors (see e.g. the systematically speaking more reasonable P(VDF-HFP) in [271]) no attempt is made to create a new and finally only confusing further acronym. The acronym PVDF(HFP) as used in [239,276] definitely makes no sense. Sometimes the authors apparently also accepted this fact, elsewhere in the report they call the copolymer PVDF-HFP. The acronym PVdF(HFP) also lacks logic [218]. |
| 13 | Why this SPE has been called SN-based is mysterious at least. |
| 14 | For mysterious reasons these authors coined the rather non-logical acronym HEP. |
| 15 | Only one of numerous inconsistencies in this report. |
| 16 | The report is hard to understand and lacks many relevant details. |
| 17 | The authors of this study conveniently ignore completely an earlier study with exactly this electrolyte system in [33]. |
| 18 | Why this material is a biopolymer remains as unclear as the biodegradability remains questionable given the IL-content. |
| 19 | Why this SPE can be reasonably called “ionic liquid based” remains mysterious. |
| 20 | MWNT appears to be a rather uncommon acronym used only by these authors. |
| 21 | Certainly this material does not contain CF3 chains as claimed by the authors in [463]. |
| 22 | The terms gel and gelled are sometimes mixed up, confused and taken as synonyms – although the former is a noun, the latter an adjective. Gel’s can be obtained by gelling of a liquid by adding a gelling agent [2], they can also be obtained by treating a solid, e.g. a polymer, with a suitable liquid. This process is also called plastification because it yields a more „plastic“ material. Both materials may behave similar and may have similar properties, accordingly the distinction is sometimes difficult. In this report assignments and claims of the authors are used as points of reference. |
| 23 | To assign the acronym CNF, which is used to name carbon nanofibers in the rest of the world, to this material is a most inspired idea to mislead readers. |
| 24 | The electrolyte itself is hardly redox-active as claimed in the report’s title. |
| 25 | The material is certainly not LiI-based as claimed in the title of the report. |
| 26 | Certainly this is not lithium perchloride as stated in the report. |
| 27 | Whether this shall be called outstanding seems to be a matter of further debate. |
| 28 | The scientific community calls this compound hydroquinone. |
| 29 | Solubility of this oligomer is not addressed in the report. In case it is really completely insoluble this system is not properly placed in this section. |
| 30 | Close examination of supplementary information revealed it as PVDF-HFP dissolved in DMF; the obtained membrane was soaked in a solution of NaPF6 in ethylene carbonate and dimethyl carbonate. |
References
- Pandya, D.J.; Pandian, P.M.; Kumar, I.; Parmar, A.; Sravanthi; Singh, N.; Al-Saheb, A.J.A.; Arun, V. Supercapacitors: Review of materials and fabrication methods. Mater. Today: Proc. 2023. [CrossRef]
- Holze, R. Between the Electrodes of a Supercapacitor: An Update on Electrolytes. Adv. Mater. Sci. Technol. 2024, 6, 0627771. [Google Scholar] [CrossRef]
- Dhanda, M.; Arora, R.; Ahlawat, S.; Nehra, S.; Lata, S. Electrolyte as a panacea to contemporary scientific world of super-capacitive energy: A condense report. J. Energy Storage 2022, 52. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Bhardwaj, P.; Grace, A.N.; Stephan, A.M. Role of Polymers in Enhancing the Performance of Electrochemical Supercapacitors: A Review. Batter. Supercaps 2021, 4, 571–584. [Google Scholar] [CrossRef]
- Subrahmanya, S.V.; Yethadka, S.N.; K, N.G. Electronic Booster PEDOT:PSS-Enriched Guar Gum as Eco-Friendly Gel Electrolyte for Supercapacitor. ACS Omega 2024, 9, 24610–24615. [Google Scholar] [CrossRef]
- Beenarani, B.B.; Sugumaran, C.P. The Electrochemical Performance of Simple, Flexible and Highly Thermally Stable PVA-TiO2 Nanocomposite in an All-Solid-State Supercapacitor. IEEE Trans. Nanotechnol. 2021, 20, 215–223. [Google Scholar] [CrossRef]
- Wu, Y.; Holze, R. Battery and/or supercapacitor?—On the merger of two electrochemical storage system families. Energy Storage Convers. 2024, 2, 491. [Google Scholar] [CrossRef]
- Ye, T.; Li, L.; Zhang, Y. Recent Progress in Solid Electrolytes for Energy Storage Devices. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Pan, F.; He, Q.; Zhang, Q. Solid polymer electrolytes reinforced with porous polypropylene separators for all-solid-state supercapacitors. RSC Adv. 2023, 13, 34652–34659. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhang, F.; Liu, Q.; Shao, W.; Song, C.; Liu, S.; Zhang, S.; Li, N.; Jian, X.; et al. Novel polymer electrolyte derived from diazonaphthone monomers for an aqueous supercapacitor with high cell potential and superior safety. Electrochimica Acta 2022, 410, 139995. [Google Scholar] [CrossRef]
- Yang, C.; Bai, Y.; Xu, H.; Li, M.; Cong, Z.; Li, H.; Chen, W.; Zhao, B.; Han, X. Porosity Tunable Poly(Lactic Acid)-Based Composite Gel Polymer Electrolyte with High Electrolyte Uptake for Quasi-Solid-State Supercapacitors. Polymers 2022, 14, 1881. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Geng, C.-L.; Wang, Y.-L.; Sun, S.-J.; Huang, Y.-F.; Wu, J.-H. Design of a redox-active “water-in-salt” hydrogel polymer electrolyte for superior-performance quasi-solid-state supercapacitors. New J. Chem. 2020, 44, 17070–17078. [Google Scholar] [CrossRef]
- Khan, M.S.; Shakya, P.; Bhardwaj, N.; Jhankal, D.; Sharma, A.K.; Banerjee, M.K.; Sachdev, K. Chemical vapor deposited graphene-based quasi-solid-state ultrathin and flexible sodium-ion supercapacitor. J. Electrochem. Sci. Technol. 2022, 12, 799–813. [Google Scholar] [CrossRef]
- Amaral, M.M.; Venâncio, R.; Peterlevitz, A.C.; Zanin, H. Recent advances on quasi-solid-state electrolytes for supercapacitors. J. Energy Chem. 2022, 67, 697–717. [Google Scholar] [CrossRef]
- Kizling, M.; Biedul, P.; Zabost, D.; Stolarczyk, K.; Bilewicz, R. Application of Hydroxyethyl Methacrylate and Ethylene Glycol Methacrylate Phosphate Copolymer as Hydrogel Electrolyte in Enzymatic Fuel Cell. Electroanalysis 2016, 28, 2444–2451. [Google Scholar] [CrossRef]
- Singh, M.K.; Sharma, A.K.; Chaurasia, S.K. Enhanced energy density of quasi-solid-state supercapacitor based on activated carbon electrode derived from honeycomb and gel polymer electrolyte with redox-additive methylene blue. Energy Storage 2023, 6. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, M.K.; Yadav, N.; Hashmi, S.A. High performance quasi-solid-state supercapacitors with peanut-shell- derived porous carbon. J. Power Sources 2018, 402, 133–146. [Google Scholar] [CrossRef]
- Zhong, J.; Fan, L.Q.; Wu, X.; Wu, J.H.; . Liu, G.J.; Lin, J.M.; Huang, M.L.; Wei, Y.L. Improved energy density of quasi-solid-state supercapacitors using sandwich-type redox-active gel polymer electrolytes. Electrochim. Acta 2015, 166, 150–156. [Google Scholar] [CrossRef]
- Lee, D.; Yang, M.; Choi, U.H.; Kim, J. Bioinspired Synaptic Branched Network within Quasi-Solid Polymer Electrolyte for High-Performance Microsupercapacitors. Small 2024, e2308821. [Google Scholar] [CrossRef]
- Lim, B.H.; Kim, J.M.; Nguyen, V.-T.; Kim, H.; Park, C.W.; Lee, J.K.; Lee, C.-H.; Yoo, J.; Min, B.K.; Kim, S.K. Functionalized methyl cellulose/LiClO4 composite as an environmentally friendly quasi-solid polymer electrolyte for solid-state electrochromic devices and cellulose-based supercapacitors. Mater. Today Energy 2023, 33. [Google Scholar] [CrossRef]
- Singh, M.K.; Chaurasia, S.K. Performance of ionic liquid–based quasi-solid-state hybrid battery supercapacitor fabricated with porous carbon capacitive cathode and proton battery anode. Energy Storage 2022, 4, e310. [Google Scholar] [CrossRef]
- Samui, A.B.; Sivaraman, P. Solid polymer electrolytes for supercapacitors. in: Polymer Electrolytes: Fundamentals and Applications (C. Sequeira, D. Santos Eds.) Woodhead Publishing, Cambridge 2010, p. 431-470.
- Ainiya, L. The recent advances on potential solid electrolytes for all-solid-state supercapacitors: A short review. J. Physics: Conf. Ser. 2019, 1417. [Google Scholar] [CrossRef]
- Kovalska, E.; Kocabas, C. Organic electrolytes for graphene-based supercapacitor: Liquid, gel or solid. Mater. Today Commun. 2016, 7, 155–160. [Google Scholar] [CrossRef]
- Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2021, 14, 4000. [Google Scholar] [CrossRef]
- Fang, X.; Yao, D. An overview of solid-like electrolytes for supercapacitors. ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE). 2013, 6A, 1-10.
- Zaman, W.; Hortance, N.; Dixit, M.B.; De Andrade, V.; Hatzell, K.B. Visualizing percolation and ion transport in hybrid solid electrolytes for Li–metal batteries. J. Mater. Chem. A 2019, 7, 23914–23921. [Google Scholar] [CrossRef]
- Mallela, V.S.; Ilankumaran, V.; Rao, N. Trends in Cardiac Pacemaker Batteries. Ind. Pac. Electrophysiol. J. 2004, 4, 201–212. [Google Scholar]
- Flory, P.J. Introductory lecture. Faraday Discuss. Chem. Soc. 1974, 57, 7–18. [Google Scholar] [CrossRef]
- Stephan, A.M.; Thomas, S. Electrolytes: Gel. in: Encyclopedia of Electrochemical Power Sources Vol. 1 (J. Garche, C.K. Dyer, P.T. Moseley, Z. Ogumi, D.A.J. Rand, B. Scrosati Ed.) Elsevier, Amsterdam, The Netherlands, 2009, p. 140-152.
- Wu, Y.; Holze, R. Electrochemical energy conversion and storage. WILEY-VCH, Weinheim, Germany, 2022.
- Wu, Y.; Holze, R. Self-discharge in supercapacitors: Causes, effects and therapies: An overview. Electrochem. Energy Technol. 2021, 7, 1–37. [Google Scholar]
- Wada, H.; Yoshikawa, K.; Nohara, S.; Furukawa, N.; Inoue, H.; Sugoh, N.; Iwasaki, H.; Iwakura, C. Electrochemical characteristics of new electric double layer capacitor with acidic polymer hydrogel electrolyte. J. Power Sources 2006, 159, 1464–1467. [Google Scholar] [CrossRef]
- Gray, F.M. Solid Polymer Electrolytes. VCH, Weinheim, Germany, 1991.
- Polymer Electrolytes (T. Winie, A.K. Arof, S. Thomas Eds.) WILEY-VCH, Weinheim, Germany, 2020.
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Tien, C.-P.; Teng, H. Efficient ion transport in activated carbon capacitors assembled with gelled polymer electrolytes based on poly(ethylene oxide) cured with poly(propylene oxide) diamines. J. Taiwan Inst. Chem. Eng. 2009, 40, 452–456. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to measure and report the capacity of electrochemical double layers, supercapacitors, and their electrode materials. J. Solid State Electr. 2020, 24, 3215–3230. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Arof, A.K. Characterization of ionic liquid added poly(vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int. J. Hydrogen Energy 2015, 40, 852–862. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Wang, S.; Huang, Y.; Wang, J.; Liu, H.; Dou, S.; Chou, S. The Emerging Electrochemical Activation Tactic for Aqueous Energy Storage: Fundamentals, Applications, and Future. Adv. Funct. Mater. 2022, 32, 2111720. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J. Phys. D: Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Yi, J.; Huo, Z.; Asiri, A.M.; Alamry, K.A.; Li, J. Development and Application of Electrolytes in Supercapacitors. Prog. Chem. 2018, 30, 1624–1633. [Google Scholar]
- Xun, Z.y.; Hou, P.; Liu, Y.; Ni, S.p.; Huo, P.F. Research progress of polymer electrolytes in supercapacitors. J. Mater. Engin. 2019, 47, 71–83. [Google Scholar]
- Taneja, N.; Kumar, A.; Gupta, P.; Gupta, M.; Singh, P.; Bharti; Agrawal, N.; Bocchetta, P.; Kumar, Y. Advancements in liquid and solid electrolytes for their utilization in electrochemical systems. J. Energy Storage 2022, 56. [Google Scholar] [CrossRef]
- Vineeth, S.K.; Sreeram, P.; Vlad, A.; Joy, R.; Raghavan, P.; Pullanchiyodan, A. Polymer blend nanocomposite electrolytes for advanced energy storage applications in: Polymer blend nanocomposite electrolytes for advanced energy storage (S. Thomas, A.R. Ajitha, M. Jaroszewskie, Eds.) Elsevier, Amsterdam, The Netherlands, 2023, 203-238.
- Samui, A.B.; Sivaraman, P. Solid polymer electrolytes for supercapacitors. in: Polymer Electrolytes: Fundamentals and Applications (C. Sequeira, D. Santos Eds.) Woodhead Publishing, Cambridge, United Kingdom, 2010, p. 431-470.
- Sharma, S.; Pathak, D.; Dhiman, N.; Kumar, R.; Kumar, M. FTIR, thermal and ionic conductivity studies of nanocomposite polymer electrolytes. Surf. Innov. 2019, 7, 51–58. [Google Scholar] [CrossRef]
- Ingram, M.D.; Pappin, A.J.; Delalande, F.; Poupard, D.; Terzulli, G. Development of electrochemical capacitors incorporating processable polymer gel electrolytes. Electrochimica Acta 1998, 43, 1601–1605. [Google Scholar] [CrossRef]
- Li, J.; Qiao, J.; Lian, K. Hydroxide ion conducting polymer electrolytes and their applications in solid supercapacitors: A review. Energy Storage Mater. 2020, 24, 6–21. [Google Scholar] [CrossRef]
- Berg, S.; Kelly, T.; Porat, I.; Moradi-Ghadi, B.; Ardebili, H. Mechanical deformation effects on ion conduction in stretchable polymer electrolytes. Appl. Phys. Lett. 2018, 113, 083903. [Google Scholar] [CrossRef]
- Yu, D.; Li, X.; Xu, J. Safety regulation of gel electrolytes in electrochemical energy storage devices. Sci. China Mater. 2019, 62, 1556–1573. [Google Scholar] [CrossRef]
- Singh, A.; Bhardwaj, R.; Mishra, R.K.; Sundramoorthy, A.K.; Gupta, V.; Arya, S. Potential of functional gel polymers as electrolytes for supercapacitors. Ionics 2023, 29, 3831–3851. [Google Scholar] [CrossRef]
- Boonen, L.; Kitzler, P.; Kasum, J. Processing of aqueous polymer electrolytes for supercapacitors via different industrial application methods. Prog. Org. Coatings 2018, 115, 107–114. [Google Scholar] [CrossRef]
- Seol, M.-L.; Nam, I.; Sadatian, E.; Dutta, N.; Han, J.-W.; Meyyappan, M. Printable Gel Polymer Electrolytes for Solid-State Printed Supercapacitors. Materials 2021, 14, 316. [Google Scholar] [CrossRef]
- Jeong, H.T.; Du, J.F.; Kim, Y.R. Development of Flexible Energy Storage Device by Using Polymer Electrolyte Based on Ionic Liquid. ChemistrySelect 2017, 2, 6057–6061. [Google Scholar] [CrossRef]
- Khan, H.A.; Tawalbeh, M.; Aljawrneh, B.; Abuwatfa, W.; Al-Othman, A.; Sadeghifar, H.; Olabi, A.G. A comprehensive review on supercapacitors: Their promise to flexibility, high temperature, materials, design, and challenges. Energy 2024, 295. [Google Scholar] [CrossRef]
- Badi, N.; Theodore, A.M.; Alghamdi, S.A.; Al-Aoh, H.A.; Lakhouit, A.; Roy, A.S.; Alatawi, A.S.; Ignatiev, A. Fabrication and Characterization of Flexible Solid Polymers Electrolytes for Supercapacitor Application. Polymers 2022, 14, 3837. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Liu, Z.; Zhi, C. Evaluating Flexibility and Wearability of Flexible Energy Storage Devices. Joule 2019, 3, 613–619. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Yang, S.; Kim, I.-J.; Oh, W.-C. Recent advances in 2D MXene and solid state electrolyte for energy storage applications: Comprehensive review. Chem. Eng. J. 2023, 472. [Google Scholar] [CrossRef]
- Cho, D.H.; Cho, K.G.; An, S.; Kim, M.S.; Oh, H.W.; Yeo, J.; Yoo, W.C.; Hong, K.; Kim, M.; Lee, K.H. Self-healable, stretchable, and nonvolatile solid polymer electrolytes for sustainable energy storage and sensing applications. Energy Storage Mater. 2022, 45, 323–331. [Google Scholar] [CrossRef]
- Lassègues, J.; Grondin, J.; Hernandez, M.; Marée, B. Proton conducting polymer blends and hybrid organic inorganic materials. Solid State Ionics 2001, 145, 37–45. [Google Scholar] [CrossRef]
- González-Gil, R.M.; Borràs, M.; Chbani, A.; Abitbol, T.; Fall, A.; Aulin, C.; Aucher, C.; Martínez-Crespiera, S. Sustainable and Printable Nanocellulose-Based Ionogels as Gel Polymer Electrolytes for Supercapacitors. Nanomaterials 2022, 12, 273. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Prog. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Wang, F.; Kim, H.-J.; Park, S.; Kee, C.-D.; Kim, S.-J.; Oh, I.-K. Bendable and flexible supercapacitor based on polypyrrole-coated bacterial cellulose core-shell composite network. Compos. Sci. Technol. 2016, 128, 33–40. [Google Scholar] [CrossRef]
- Mokhtarnejad, M.; Ribeiro, E.L.; Mukherjee, D.; Khomami, B. 3D printed interdigitated supercapacitor using reduced graphene oxide-MnOx/Mn3O4 based electrodes. RSC Adv. 2022, 12, 17321–17329. [Google Scholar] [CrossRef]
- Lo, H.-J.; Huang, M.-C.; Lai, Y.-H.; Chen, H.-Y. Towards bi-functional all-solid-state supercapacitor based on nickel hydroxide-reduced graphene oxide composite electrodes. Mater. Chem. Phys. 2021, 262, 124306. [Google Scholar] [CrossRef]
- Seol, M.-L.; Nam, I.; Ribeiro, E.L.; Segel, B.; Lee, D.; Palma, T.; Wu, H.; Mukherjee, D.; Khomami, B.; Hill, C.; et al. All-Printed In-Plane Supercapacitors by Sequential Additive Manufacturing Process. ACS Appl. Energy Mater. 2020, 3, 4965–4973. [Google Scholar] [CrossRef]
- Weerasinghe, W.; Vidanapathirana, K.; Perera, K. Performance evaluation of polyaniline-based redox capacitors with respect to polymerization current density. AIMS Energy 2018, 6, 593–606. [Google Scholar] [CrossRef]
- Weerasinghe, W.; Vidanapathirana, K.; Perera, K.; Bandaranayake, C. Effect of polymerisation current density of electrodes on the performance of polypyrrole based redox-capacitor. J. Natl. Sci. Found. Sri Lanka 2017, 45, 73–77. [Google Scholar] [CrossRef]
- Majumdar, S.; Ray, R.; Sen, P. Anomalous intra diffusive behavior of chitosan/PVDF solid polymer electrolytes and the enhancement of effective specific capacitance with nanostructured spinel MnCoFeO4 electrode in solid-state supercapacitors. Electrochimica Acta 2021, 385, 138295. [Google Scholar] [CrossRef]
- Lee, S.J.; Yang, H.M.; Cho, K.G.; Seol, K.H.; Kim, S.; Hong, K.; Lee, K.H. Highly conductive and mechanically robust nanocomposite polymer electrolytes for solid-state electrochemical thin-film devices. Org. Electron. 2019, 65, 426–433. [Google Scholar] [CrossRef]
- Ortega, P.F.; Trigueiro, J.P.C.; Silva, G.G.; Lavall, R.L. Improving supercapacitor capacitance by using a novel gel nanocomposite polymer electrolyte based on nanostructured SiO2, PVDF and imidazolium ionic liquid. Electrochimica Acta 2016, 188, 809–817. [Google Scholar] [CrossRef]
- Latham, R.; Rowlands, S.; Schlindwein, W. Supercapacitors using polymer electrolytes based on poly(urethane). Solid State Ionics 2002, 147, 243–248. [Google Scholar] [CrossRef]
- Handayani, P.L.; Kim, T.; Song, Y.H.; Park, J.S.; Yang, S.J.; Choi, U.H. Tailoring molecular interaction in heteronetwork polymer electrolytes for stretchable, high-voltage fiber supercapacitors. Chem. Eng. J. 2023, 452, 139432. [Google Scholar] [CrossRef]
- Luo, N.; Wang, J.; Zhang, D.; Zhao, Y.; Wei, Y.; Liu, Y.; Zhang, Y.; Han, S.; Kong, X.; Huo, P. Inorganic nanoparticle-enhanced double-network hydrogel electrolytes for supercapacitor with superior low-temperature adaptability. Chem. Eng. J. 2024, 479. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Tao, F.; Pan, Q. Rationally Designed Self-Healing Hydrogel Electrolyte toward a Smart and Sustainable Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 27745–27753. [Google Scholar] [CrossRef]
- Ko, J.M.; Nam, J.H.; Won, J.H.; Kim, K.M. Supercapacitive properties of electrodeposited polyaniline electrode in acrylic gel polymer electrolytes. Synth. Met. 2014, 189, 152–156. [Google Scholar] [CrossRef]
- Schroeder, M.; Isken, P.; Winter, M.; Passerini, S.; Lex-Balducci, A.; Balducci, A. An Investigation on the Use of a Methacrylate-Based Gel Polymer Electrolyte in High Power Devices. J. Electrochem. Soc. 2013, 160, A1753–A1758. [Google Scholar] [CrossRef]
- Isken, P.; Winter, M.; Passerini, S.; Lex-Balducci, A. Methacrylate based gel polymer electrolyte for lithium-ion batteries. J. Power Sources 2013, 225, 157–162. [Google Scholar] [CrossRef]
- Chaudoy, V.; Van, F.T.; Deschamps, M.; Ghamouss, F. Ionic liquids in a poly ethylene oxide cross-linked gel polymer as an electrolyte for electrical double layer capacitor. J. Power Sources 2017, 342, 872–878. [Google Scholar] [CrossRef]
- Kim, M.; Gu, M.G.; Jeong, H.; Song, E.; Jeon, J.W.; Huh, K.-M.; Kang, P.; Kim, S.-K.; Kim, B.G. Laser Scribing of Fluorinated Polyimide Films to Generate Microporous Structures for High-Performance Micro-supercapacitor Electrodes. ACS Appl. Energy Mater. 2021, 4, 208–214. [Google Scholar] [CrossRef]
- Lee, D.; Park, G.; Kim, Y.; Choi, J.; Choi, U.H.; Kim, J. Tailoring ion dynamics in energy storage conductors for ultra-stable, high-performance solid-state microsupercapacitor array. Chem. Eng. J. 2023, 472. [Google Scholar] [CrossRef]
- Trivedi, M.; Kyu, T. Solid-state polymer magnesium supercapacitor. Solid State Ionics 2023, 394. [Google Scholar] [CrossRef]
- Khatmullina, K.G.; Slesarenko, N.A.; Chernyak, A.V.; Baymuratova, G.R.; Yudina, A.V.; Berezin, M.P.; Tulibaeva, G.Z.; Slesarenko, A.A.; Shestakov, A.F.; Yarmolenko, O.V. New Network Polymer Electrolytes Based on Ionic Liquid and SiO2 Nanoparticles for Energy Storage Systems. Membranes 2023, 13, 548. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. LiClO4-doped plasticized chitosan and poly(ethylene glycol) blend as biodegradable polymer electrolyte for supercapacitors. Ionics 2013, 19, 277–285. [Google Scholar] [CrossRef]
- Reiter, J.; Vondrák, J.; Michálek, J.; Mička, Z. Ternary polymer electrolytes with 1-methylimidazole based ionic liquids and aprotic solvents. Electrochimica Acta 2006, 52, 1398–1408. [Google Scholar] [CrossRef]
- Łatoszyńska, A.A.; Taberna, P.-L.; Simon, P.; Wieczorek, W. Proton conducting Gel Polymer Electrolytes for supercapacitor applications. Electrochimica Acta 2017, 242, 31–37. [Google Scholar] [CrossRef]
- Lassègues, J.-C.; Grondin, J.; Becker, T.; Servant, L.; Hernandez, M. Supercapacitor using a proton conducting polymer electrolyte. Solid State Ionics 1995, 77, 311–317. [Google Scholar] [CrossRef]
- Grondin, J.; Rodriguez, D.; Lasségues, J.C. Proton conducting polymer electrolyte-The Nylon 6-10/H3PO4 blends. Solid State Ionics 1995, 77, 70–75. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, T.; Dhapola, P.S.; Kumar, S.; Singh, D.; Nath, G.; Singh, V.; A Alheety, M.; Kakroo, S.; Singh, P.K. Ionic liquid doped solid polymer electrolyte: Synthesis, characterization and applications ICSEM-2021. High Perform. Polym. 2022, 34, 645–651. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Cao, L.; Kong, W.; Sun, Z.; Cheng, H.; Lu, Z.; Pan, H.; Xu, B. Cross-linking of polymer and ionic liquid as high-performance gel electrolyte for flexible solid-state supercapacitors. Electrochimica Acta 2017, 244, 112–118. [Google Scholar] [CrossRef]
- Lewandowski, A.; Zajder, M.; Frackowiak, E.; Béguin, F. Supercapacitor based on activated carbon and polyethylene oxide-KOH-H2O polymer electrolyte. Electrochim. Acta 2001, 46, 2777–2780. [Google Scholar] [CrossRef]
- Westover, A.S.; Shabab, F.N.; Tian, J.W.; Bernath, S.; Oakes, L.; Erwin, W.R.; Carter, R.; Bardhan, R.; Pint, C.L. Stretching Ion Conducting Polymer Electrolytes: In-Situ Correlation of Mechanical, Ionic Transport, and Optical Properties. J. Electrochem. Soc. 2014, 161, E112–E117. [Google Scholar] [CrossRef]
- Li, M.; Westover, A.S.; Carter, R.E.; Oakes, L.; Muralidharan, N.; Boire, T.C.; Sung, H.-J.; Pint, C.L. Noncovalent Pi–Pi Stacking at the Carbon–Electrolyte Interface: Controlling the Voltage Window of Electrochemical Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 19558–19566. [Google Scholar] [CrossRef]
- Sivaraman, P.; Thakur, A.; Kushwaha, R.K.; Ratna, D.; Samui, A.B. Poly(3-methyl thiophene)-Activated Carbon Hybrid Supercapacitor Based on Gel Polymer Electrolyte. Electrochem. Solid-state Lett. 2006, 9, A435–A438. [Google Scholar] [CrossRef]
- Sivaraman, P.; Shashidhara, K.; Thakur, A.P.; Samui, A.B.; Bhattacharyya, A.R. Nanocomposite solid polymer electrolytes based on polyethylene oxide, modified nanoclay, and tetraethylammonium tetrafluoroborate for application in solid-state supercapacitor. Polym. Eng. Sci. 2015, 55, 1536–1545. [Google Scholar] [CrossRef]
- Makino, S.; Yamamoto, R.; Sugimoto, S.; Sugimoto, W. Room temperature performance of 4 V aqueous hybrid supercapacitor using multi-layered lithium-doped carbon negative electrode. J. Power Sources 2016, 326, 711–716. [Google Scholar] [CrossRef]
- Raghu, S.; Devendrappa, H.; Ganesh, S.; Matteppanavar, S. Modification of PEO-based polymer electrolytes by electron beam irradiation for energy storage applications. Polym. Bull. 2023, 80, 381–394. [Google Scholar] [CrossRef]
- Kamboj, V.; Arya, A.; Tanwar, S.; Kumar, V.; Sharma, A.L. Nanofiller-assisted Na+-conducting polymer nanocomposite for ultracapacitor: structural, dielectric and electrochemical properties. J. Mater. Sci. 2021, 56, 6167–6187. [Google Scholar] [CrossRef]
- Negi, S.S.; Rawat, S.; Singh, P.K.; Savilov, S.V.; Yadav, T.; Yahya, M.Z.A.; Singh, R.C. Conducting Carbon Black Nano-Filler Doped Polymer Electrolyte for Electrochemical Application. ChemistrySelect 2024, 9. [Google Scholar] [CrossRef]
- Patel, V.K.; Sengwa, R.J. Development of flexible and stretchable PEO/PVP/LiTFSI/BaTiO3 electrolytes for lithium ion-conducting device technologies: exploration of nanofiller effect on the promising properties. J. Mater. Sci. Mater. Electron. 2023, 34, 1–18. [Google Scholar] [CrossRef]
- Jinisha, B.; Femy, A.F.; Ashima, M.S.; Jayalekshmi, S. Polyethylene oxide (PEO)/polyvinyl alcohol (PVA) complexed with lithium perchlorate (LiClO4) as a prospective material for making solid polymer electrolyte films. Mater. Today Proc. 2018, 5, 21189–21194. [Google Scholar] [CrossRef]
- Hassan, M.; Gondal, M.; Cevik, E.; Qahtan, T.; Bozkurt, A.; Dastageer, M. High performance pliable supercapacitor fabricated using activated carbon nanospheres intercalated into boron nitride nanoplates by pulsed laser ablation technique. Arab. J. Chem. 2020, 13, 6696–6707. [Google Scholar] [CrossRef]
- Virya, A.; Lian, K. Li2SO4-polyacrylamide polymer electrolytes for 2. 0 V solid symmetric supercapacitors. Electrochem. Commun. 2017, 81, 52–55. [Google Scholar]
- Sivaraman, P.; Kushwaha, R.; Shashidhara, K.; Hande, V.; Thakur, A.; Samui, A.; Khandpekar, M. All solid supercapacitor based on polyaniline and crosslinked sulfonated poly[ether ether ketone]. Electrochimica Acta 2010, 55, 2451–2456. [Google Scholar] [CrossRef]
- Khandpekar, M.M.; Kushwaha, R.K.; Pati, S.P. Design, fabrication, and evaluation of a 5 F-5 V prototype of solid-state PANI based supercapacitor. Solid-State Electr. 2011, 62, 156–160. [Google Scholar] [CrossRef]
- Cevik, E.; Günday, S.T.; Yusuf, A.; Almessiere, M.A.; Bozkurt, A. Boron-incorporated Sulfonated polysulfone/polyphosphoric acid electrolytes for supercapacitor application. Soft Mater. 2019, 17, 203–211. [Google Scholar] [CrossRef]
- Holze, R. Copolymers—A refined way to tailor intrinsically conducting polymers. Electrochimica Acta 2011, 58, 10479–10492. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, J.Y.; Suh, D.H.; Hong, Y.T.; Kim, T.-H. Electrode-Impregnable and Cross-Linkable Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Polymer Electrolytes with High Ionic Conductivity and a Large Voltage Window for Flexible Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 33913–33924. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, P.; Zhang, P.; Duan, N.; Liu, Q.; Qin, C. Cross-Linked Polyacrylic-Based Hydrogel Polymer Electrolytes for Flexible Supercapacitors. Polymers 2024, 16, 800. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Park, J.T.; Lee, J.M.; Kim, J.H. Polymethacrylate-comb-copolymer electrolyte for solid-state energy storage devices. Mater. Des. 2018, 149, 25–33. [Google Scholar] [CrossRef]
- Popall, M.; Andrei, M.; Kappel, J.; Kron, J.; Olma, K.; Olsowski, B. ORMOCERs as inorganic–organic electrolytes for new solid state lithium batteries and supercapacitors. Electrochimica Acta 1998, 43, 1155–1161. [Google Scholar] [CrossRef]
- Lee, K.T.S.; Wu, N.L. Manganese oxide supercapacitors with aqueous hydrogel electrolytes. ECS Trans. 2009, 16, 197–200. [Google Scholar] [CrossRef]
- Na, R.; Huo, P.; Zhang, X.; Zhang, S.; Du, Y.; Zhu, K.; Lu, Y.; Zhang, M.; Luan, J.; Wang, G. A flexible solid-state supercapacitor based on a poly(aryl ether ketone)–poly(ethylene glycol) copolymer solid polymer electrolyte for high temperature applications. RSC Adv. 2016, 6, 65186–65195. [Google Scholar] [CrossRef]
- Liang, N.; Ji, Y.; Zuo, D.; Zhang, H.; Xu, J. Improved performance of carbon-based supercapacitors with sulfonated poly(ether ether ketone)/poly(vinyl alcohol) composite membranes as separators. Polym. Int. 2019, 68, 120–124. [Google Scholar] [CrossRef]
- Huo, P.; Xun, Z.; Ni, S.; Liu, Y.; Wang, G.; Gu, J. Crosslinked quaternized poly(arylene ether sulfone) copolymer membrane applied in an electric double-layer capacitor for high energy density. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, P.; Xi, B.; Lin, W.; Liu, Y.; Wang, G.; Huo, P. A high energy density flexible solid-state supercapacitor based on poly (arylene ether sulfone) copolymers with polyether side chains for Li+ conducting polymer electrolytes. Mater. Chem. Phys. 2021, 267, 124623. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, J.K.; Lee, J.M.; Ryu, D.Y.; Kim, J.H. An amphiphilic block-graft copolymer electrolyte: synthesis, nanostructure, and use in solid-state flexible supercapacitors. J. Mater. Chem. A 2016, 4, 7848–7858. [Google Scholar] [CrossRef]
- Łatoszyńska, A.A.; Żukowska, G.Z.; Rutkowska, I.A.; Taberna, P.-L.; Simon, P.; Kulesza, P.J.; Wieczorek, W. Non-aqueous gel polymer electrolyte with phosphoric acid ester and its application for quasi solid-state supercapacitors. J. Power Sources 2015, 274, 1147–1154. [Google Scholar] [CrossRef]
- Anothumakkool, B.; T., A.T.A.; Veeliyath, S.; Vijayakumar, V.; Badiger, M.V.; Kurungot, S. High-Performance Flexible Solid-State Supercapacitor with an Extended Nanoregime Interface through in Situ Polymer Electrolyte Generation. ACS Appl. Mater. Interfaces 2016, 8, 1233–1241. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Ghosh, M.; T., A.T.A.; K., N.C.M.; Nair, S.B.; Badiger, M.V.; Kurungot, S. Water-in-Acid Gel Polymer Electrolyte Realized through a Phosphoric Acid-Enriched Polyelectrolyte Matrix toward Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 12630–12640. [Google Scholar] [CrossRef]
- Yong, H.; Park, H.; Jung, J.; Jung, C. A fundamental approach to design of injectable high-content gel polymer electrolyte for activated carbon electrode supercapacitors. J. Ind. Eng. Chem. 2019, 76, 429–436. [Google Scholar] [CrossRef]
- Yong, H.; Park, H.; Jung, C. Quasi-solid-state gel polymer electrolyte for a wide temperature range application of acetonitrile-based supercapacitors. J. Power Sources 2020, 447, 227390. [Google Scholar] [CrossRef]
- Moon, S.J.; Min, H.J.; Lee, C.S.; Kang, D.R.; Kim, J.H. Adhesive, free-standing, partially fluorinated comb copolymer electrolyte films for solid flexible supercapacitors. Chem. Eng. J. 2022, 429, 132240. [Google Scholar] [CrossRef]
- Mun, W.J.; Kim, B.; Moon, S.J.; Kim, J.H. Multifunctional, bicontinuous, flexible comb copolymer electrolyte for solid-state supercapacitors. Chem. Eng. J. 2023, 454. [Google Scholar] [CrossRef]
- Ghasemi, M.; Fahimi, Z.; Moradlou, O.; Sovizi, M.R. Porous gel polymer electrolyte for the solid state metal oxide supercapacitor with a wide potential window. J. Taiwan Inst. Chem. Eng. 2021, 118, 223–231. [Google Scholar] [CrossRef]
- Karaman, B.; Çevik, E.; Bozkurt, A. Novel flexible Li-doped PEO/copolymer electrolytes for supercapacitor application. Ionics 2019, 25, 1773–1781. [Google Scholar] [CrossRef]
- Hu, F.; Liu, Y.; Shao, W.; Zhang, T.; Liu, S.; Liu, D.; Zhang, S.; Jian, X. Novel poly(arylene ether ketone)/poly(ethylene glycol)-grafted poly(arylene ether ketone) composite microporous polymer electrolyte for electrical double-layer capacitors with efficient ionic transport. RSC Adv. 2021, 11, 14814–14823. [Google Scholar] [CrossRef]
- Lee, K.-T.; Lee, J.-F.; Wu, N.-L. Electrochemical characterizations on MnO2 supercapacitors with potassium polyacrylate and potassium polyacrylate-co-polyacrylamide gel polymer electrolytes. Electrochimica Acta 2009, 54, 6148–6153. [Google Scholar] [CrossRef]
- Handayani, P.L.; Nulandaya, L.; Cheon, J.Y.; Kim, T.; Yoo, S.I.; Choi, U.H. Self-assembled block copolymer electrolyte membranes with silica network-derived nanochannels for all-solid-state supercapacitors. Chem. Eng. J. 2022, 429, 132273. [Google Scholar] [CrossRef]
- Je, J.H.; Choi, U.H. Triple-network hydrogel polymer electrolytes: enabling flexible and robust supercapacitors for extreme conditions. Chem. Eng. J. 2024, 483. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, S.M.; Jung, H.Y. A super-thermostable, flexible supercapacitor for ultralight and high performance devices. J. Mater. Chem. A 2020, 8, 532–542. [Google Scholar] [CrossRef]
- Wang, J.A.; Ma, C.C.M.; Hu, C.C. Constructing a high-performance quasi-solid-state asymmetric supercapacitor: NaxMnO2@CNT/WPU-PAAK-Na2SO4/AC-CNT. Electrochim. Acta 2020, 334, 135576. [Google Scholar] [CrossRef]
- Hou, P.; Gao, C.; Wang, J.; Zhang, J.; Liu, Y.; Gu, J.; Huo, P. A semi-transparent polyurethane/porous wood composite gel polymer electrolyte for solid-state supercapacitor with high energy density and cycling stability. Chem. Eng. J. 2023, 454, 139954. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, B.; Li, H.; Liu, H. A flexible polycation-type anion-dominated conducting polymer as potential all-solid-state supercapacitor film electrolyte. Chem. Eng. J. 2017, 330, 753–756. [Google Scholar] [CrossRef]
- Wang, Y.; Auad, M.L.; Beckingham, B.S. 3D printing flexible supercapacitors based on crosslinked poly (acrylic acid-co-vinylimidazole). Eng. Rep. 2023, 5. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Yang, J.; Sun, W.; Ban, Q.; Gai, L.; Gong, Y.; Xu, Z.; Liu, L. Flame-Retardant, Highly Conductive, and Low-Temperature-Resistant Organic Gel Electrolyte for High-Performance All-Solid Supercapacitors. ChemSusChem 2021, 14, 2056–2066. [Google Scholar] [CrossRef]
- Yu, T.; Xue, P.; Ma, S.; Gu, Y.; Wang, Y.; Xu, X. Thermal Self-Protection Behavior of Energy Storage Devices Using a Thermally Responsive Smart Polymer Electrolyte. ChemistrySelect 2022, 7, e202104499. [Google Scholar] [CrossRef]
- Hsueh, M.-F.; Huang, C.-W.; Wu, C.-A.; Kuo, P.-L.; Teng, H. The Synergistic Effect of Nitrile and Ether Functionalities for Gel Electrolytes Used in Supercapacitors. J. Phys. Chem. C 2013, 117, 16751–16758. [Google Scholar] [CrossRef]
- Oliveira da Silva, L.C.; Soares, B.G. New all solid-state polymer electrolyte based on epoxy resin and ionic liquid for high temperature applications. J. Appl. Polym. Sci. 2018, 135, 45838. [Google Scholar] [CrossRef]
- Lee, S.; Choi, U.H. High Ion Conducting Dobule Network Crosslinked Gel Polymer Electrolytes for High-Performance Supercapacitors. Macromol. Chem. Phys. 2023, 224. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, T.; Jung, B.M.; Lee, S.B.; Choi, U.H. Multifunctional Epoxy-Based Solid Polymer Electrolytes for Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 35108–35117. [Google Scholar] [CrossRef]
- Lee, D.; Song, Y.H.; Choi, U.H.; Kim, J. Highly Flexible and Stable Solid-State Supercapacitors Based on a Homogeneous Thin Ion Gel Polymer Electrolyte Using a Poly(dimethylsiloxane) Stamp. ACS Appl. Mater. Interfaces 2019, 11, 42221–42232. [Google Scholar] [CrossRef]
- Han, Y.K.; Kwon, S.J.; Choi, J.R.; Jung, B.M. Fiber supercapacitor using epoxy-based gel polymer electrolyte with high ionic conductivity and mechanical flexibility. Funct. Compos. Struct. 2021, 3, 035005. [Google Scholar] [CrossRef]
- Song, Y.H.; Kim, T.; Choi, U.H. Tuning Morphology and Properties of Epoxy-Based Solid-State Polymer Electrolytes by Molecular Interaction for Flexible All-Solid-State Supercapacitors. Chem. Mater. 2020, 32, 3879–3892. [Google Scholar] [CrossRef]
- Adak, N.C.; Lim, S.; Lee, G.-H.; Lee, W. Epoxy-based multifunctional solid polymer electrolytes for structural batteries and supercapacitors. a short review. Front. Chem. 2024, 12, 1330655. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kwon, O.; Kim, D.; Park, Y.-B.; Park, H.W. Multifunctional enhancement of woven carbon fiber/ZnO nanotube-based structural supercapacitor and polyester resin-domain solid-polymer electrolytes. Chem. Eng. J. 2017, 325, 672–680. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Kim, N.; Jeong, H.E.; Park, Y.-B.; Park, H.W. Bimetallic copper cobalt selenide nanowire-anchored woven carbon fiber-based structural supercapacitors. Chem. Eng. J. 2019, 355, 551–559. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Cho, M.-S.; Nam, J.-D. New Strategy and Easy Fabrication of Solid-State Supercapacitor Based on Polypyrrole and Nitrile Rubber. J. Nanosci. Nanotechnol. 2008, 8, 4722–4725. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoon, Y.; Ryu, J.H.; Kim, J.H.; Ji, S.; Song, W.; Myung, S.; Lim, J.; Jung, H.K.; Lee, S.S.; Lee, J.; An, K.S. Recyclable High-Performance Polymer Electrolyte Based on a Modified Methyl Cellulose-Lithium Trifluoromethanesulfonate Salt Composite for Sustainable Energy Systems. ChemSusChem 2020, 13, 376–384. [Google Scholar] [CrossRef]
- Shamsuri, N.; Hamsan, M.; Shukur, M.; Alias, Y.; Halim, S.; Aziz, S.; Jahidin, A.; Sulaiman, M.; Yuwana, L.; Siong, S.O.J.; et al. Enhancing EDLC applications with [BMIM]BF4-integrated cellulose gel electrolyte for sustainable energy storage. J. Energy Storage 2024, 75. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Mohammed, P.A.; Rashid, S.O.; Karim, W.O.; Al-Asbahi, B.A.; Ahmed, A.A.; Kadir, M. Steps towards the ideal CV and GCD results with biodegradable polymer electrolytes: Plasticized MC based green electrolyte for EDLC application. J. Energy Storage 2024, 76. [Google Scholar] [CrossRef]
- Manfo, T.A. Development and Characterization of a New Solid Polymer Electrolyte for Supercapacitor Device. Int. J. Electrochem. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Sharma, A.K.; Singh, P.K.; Lu, L.; Ni, J.; Savilov, S.V.; Kuznetsov, A.; Polu, A.R.; Singh, A.; Singh, M.K. Structural, thermal, and electrochemical studies of biodegradable gel polymer electrolyte for electric double layer capacitor. High Perform. Polym. 2022, 34, 673–682. [Google Scholar] [CrossRef]
- Reddygunta, K.K.R.; Beresford, R.; Šiller, L.; Berlouis, L.; Ivaturi, A. Activated Carbon Utilization from Corn Derivatives for High-Energy-Density Flexible Supercapacitors. Energy Fuels 2023, 37, 19248–19265. [Google Scholar] [CrossRef]
- Kadam, S.L.; Ingole, R.S.; Tiwari, N.G.; Nakate, U.T.; Nakate, Y.T.; Kamat, R.K.; Ok, J.G.; Kulkarni, S.B. Facile synthesis of nanourchin like manganese oxide electrode material for high performance symmetric supercapacitor. Surfaces Interfaces 2023, 42. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Bozkurt, A.; Iqbal, A.; Asiri, S.M.; Alqarni, A.N.; Almofleh, A. Scalable, Quasi-Solid-State Bio-polymer Hydrogel Electrolytes for High-Performance Supercapacitor Applications. ACS Sustain. Chem. Eng. 2022, 10, 10839–10848. [Google Scholar] [CrossRef]
- Qiu, F.; Huang, Y.; Luo, C.; Li, X.; Wang, M.; Cao, H. An Acid-Resistant Gel Polymer Electrolyte Based on Lignocellulose of Natural Biomass for Supercapacitors. Energy Technol. 2020, 8. [Google Scholar] [CrossRef]
- Qiu, F.; Huang, Y.; Hu, X.; Li, B.; Zhang, X.; Luo, C.; Li, X.; Wang, M.; Wu, Y.; Cao, H. An Ecofriendly Gel Polymer Electrolyte Based on Natural Lignocellulose with Ultrahigh Electrolyte Uptake and Excellent Ionic Conductivity for Alkaline Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 6031–6042. [Google Scholar] [CrossRef]
- Selvakumar, M.; Bhat, D.K. LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J. Appl. Polym. Sci. 2008, 110, 594–602. [Google Scholar] [CrossRef]
- Jorn-Am, T.; Supchocksoonthorn, P.; Pholauyphon, W.; Manyam, J.; Chanthad, C.; Paoprasert, P. Quasi-Solid, Bio-Renewable Supercapacitors Based on Cassava Peel and Cassava Starch and the Use of Carbon Dots as Performance Enhancers. Energy Fuels 2022, 36, 7865–7877. [Google Scholar] [CrossRef]
- Sudhakar, Y.; Selvakumar, M. Lithium perchlorate doped plasticized chitosan and starch blend as biodegradable polymer electrolyte for supercapacitors. Electrochimica Acta 2012, 78, 398–405. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Aziz, D.M.; Mohammed, P.A.; Karim, W.O.; Abdullah, R.M.; Woo, H.J.; Halim, N.A.; Hamsan, M.H.; Kadir, M.F. Study of dielectric and interfacial properties of functional biopolymer-based electrolyte with enhanced conductivity for energy storage application. Mater. Chem. Phys. 2024, 322. [Google Scholar] [CrossRef]
- Vorobiov, V.K.; Smirnov, M.A.; Bobrova, N.V.; Sokolova, M.P. Chitosan-supported deep eutectic solvent as bio-based electrolyte for flexible supercapacitor. Mater. Lett. 2021, 283, 128889. [Google Scholar] [CrossRef]
- Majumdar, S.; Sen, P.; Ray, R. High-performance graphene oxide-grafted chitosan-starch solid biopolymer electrolytes for flexible hybrid supercapacitors. J. Solid State Electrochem. 2022, 26, 527–547. [Google Scholar] [CrossRef]
- Rai, K.J.; Saini, D.S.; Shahi, P.; Khan, M.; Farid, A.; Kumar, M. Conductivity and electrochemical behaviour of CoFe2O4 dispersed potato starch-based solid biopolymer electrolyte for energy application. Ionics 2024, 30, 819–831. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M. Ionic conductivity studies and dielectric studies of Poly(styrene sulphonic acid)/starch blend polymer electrolyte containing LiClO4. J. Appl. Electrochem. 2013, 43, 21–29. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, Y.R.; Jeong, H.T. All-solid-state supercapacitor composed of reduced graphene oxide (rGO)/activated carbon (AC) composite and polymer electrolyte. Carbon Lett. 2020, 30, 107–113. [Google Scholar] [CrossRef]
- Sudhakar, Y.; Selvakumar, M.; Bhat, D.K. Tubular array, dielectric, conductivity and electrochemical properties of biodegradable gel polymer electrolyte. Mater. Sci. Eng. B 2014, 180, 12–19. [Google Scholar] [CrossRef]
- Sumana, V.S.; Sudhakar, Y.N.; Nagaraja, G.K.; B, S.M.; P, P. Exploring the potential of poly (caprolactone) and guar gum biodegradable blend film: an investigation for supercapacitor. Mater. Res. Express 2024, 11, 055303. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Zhou, X.; Lin, X.; Cai, Y.; Shen, M.; Huang, X.; Liu, H.; Xu, X. Self-adhesive, freeze-tolerant, and strong hydrogel electrolyte containing xanthan gum enables the high-performance of zinc-ion hybrid supercapacitors. Int. J. Biol. Macromol. 2024, 265, 131143. [Google Scholar] [CrossRef]
- Chupp, J.; Shellikeri, A.; Palui, G.; Chatterjee, J. Chitosan-based gel film electrolytes containing ionic liquid and lithium salt for energy storage applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Ren, W.; Zhao, L.; Li, X.; Wang, M.; Lin, Y. Natural Biomass Hydrogel Based on Cotton Fibers/PVA for Acid Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 9144–9153. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Zheng, H.; Zhao, L.; Liu, J.; Zou, C.; Dong, Z.; Li, X.; Wang, M.; Lin, Y. Cotton Fiber/PVA-Based Neutral Hydrogel with Al3+ as an Electrolyte Additive for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 644–656. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Qiu, F.; Ren, W.; Zou, C.; Li, X.; Wang, M.; Lin, Y. A new environmentally friendly gel polymer electrolyte based on cotton-PVA composited membrane for alkaline supercapacitors with increased operating voltage. J. Mater. Sci. 2021, 56, 11027–11043. [Google Scholar] [CrossRef]
- Torres, F.G.; De-La-Torre, G.E.; Gonzales, K.N.; Troncoso, O.P. Bacterial-Polymer-Based Electrolytes: Recent Progress and Applications. ACS Appl. Energy Mater. 2020, 3, 11500–11515. [Google Scholar] [CrossRef]
- Torres, F.G.; De-La-Torre, G.E. Algal-based polysaccharides as polymer electrolytes in modern electrochemical energy conversion and storage systems: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Huo, P.; Ni, S.; Hou, P.; Xun, Z.; Liu, Y.; Gu, J. A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor. Polymers 2019, 11, 863. [Google Scholar] [CrossRef]
- Gao, C.; Duan, Y.; Liu, Y.; Gu, J.; Guo, Z.; Huo, P. A high energy density supercapacitor fabricated with aqueous polymer electrolyte based on soybean protein isolate grafted by polyacrylic acid. J. Power Sources 2022, 541. [Google Scholar] [CrossRef]
- Duan, Y.; Long, J.; Li, Y.; Tian, X.; Li, J.; Fang, Z.; Wang, J.; Huo, P. Lignin/soy protein isolate-based hydrogel polymer electrolytes for flexible solid-state supercapacitors with low temperature resistance. J. Solid State Electrochem. 2023, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Xun, Z.; Zhao, C.; Liu, Y.; Gu, J.; Huo, P. Converting soy protein isolate into biomass-based polymer electrolyte by grafting modification for high-performance supercapacitors. Int. J. Biol. Macromol. 2022, 209, 268–278. [Google Scholar] [CrossRef]
- Xun, Z.; Ni, S.; Gao, Z.; Zhang, Y.; Gu, J.; Huo, P. Construction of Polymer Electrolyte Based on Soybean Protein Isolate and Hydroxyethyl Cellulose for a Flexible Solid-State Supercapacitor. Polymers 2019, 11, 1895. [Google Scholar] [CrossRef]
- Premalatha, M.; Mathavan, T.; Selvasekarapandian, S.; Selvalakshmi, S. Structural and Electrical Characterization of Tamarind Seed Polysaccharide (TSP) doped with NH4HCO2. 62ND DAE Solid State Physics Symposium 2018, 1942, 070005.
- Kim, I.; San, S.T.; Mendhe, A.C.; Dhas, S.D.; Jeon, S.-B.; Kim, D. Rheological and Electrochemical Properties of Biodegradable Chia Mucilage Gel Electrolyte Applied to Supercapacitor. Batteries 2023, 9, 512. [Google Scholar] [CrossRef]
- Ye, T.; Li, D.; Liu, H.; She, X.; Xia, Y.; Zhang, S.; Zhang, H.; Yang, D. Seaweed Biomass-Derived Flame-Retardant Gel Electrolyte Membrane for Safe Solid-State Supercapacitors. Macromolecules 2018, 51, 9360–9367. [Google Scholar] [CrossRef]
- Fuzlin, A.F.; Samsudin, A.S. Studies on favorable ionic conduction and structural properties of biopolymer electrolytes system-based alginate. Polym. Bull. 2020, 78, 2155–2175. [Google Scholar] [CrossRef]
- Park, S.M.; Choi, U.H. Highly stretchable and conductive hybrid gel polymer electrolytes enabled by a dual cross-linking approach. Macromol. Res. 2023, 31, 499–509. [Google Scholar] [CrossRef]
- Jansi, R.; Vinay, B.; Revathy, M.S.; Sasikumar, P.; Marasamy, L.; Janani, A.; Haldhar, R.; Kim, S.-C.; Almarhoon, Z.M.; Hossain, M.K. Synergistic Blends of Sodium Alginate and Pectin Biopolymer Hosts as Conducting Electrolytes for Electrochemical Applications. ACS Omega 2024, 9, 13906–13916. [Google Scholar] [CrossRef]
- Najafloo, M.; Naji, L. Resilient 3D porous self-healable triple network hydrogels reinforced with graphene oxide for high-performance flexible supercapacitors. J. Alloy. Compd. 2024, 1002. [Google Scholar] [CrossRef]
- Harikumar, M.; Batabyal, S.K. Biopolymer pectin with calcium ion crosslinker as biocompatiable electrolyte for energy storage applications. Electrochimica Acta 2024, 489. [Google Scholar] [CrossRef]
- Na, R.; Wang, X.; Lu, N.; Huo, G.; Lin, H.; Wang, G. Novel egg white gel polymer electrolyte and a green solid-state supercapacitor derived from the egg and rice waste. Electrochimica Acta 2018, 274, 316–325. [Google Scholar] [CrossRef]
- Verma, K.D.; Sinha, P.; Ghorai, M.K.; Kar, K.K. Mesoporous electrode from human hair and bio-based gel polymer electrolyte for high-performance supercapacitor. Diam. Relat. Mater. 2022, 123. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Y.; Wang, H.; Wang, T.; Wu, H.; Li, J.; Li, Y.; Wang, M.; Zhang, J. Lignin Isolated from Poplar Wood for Porous Carbons as Electrode for High-Energy Renewable Supercapacitor Driven by Lignin/Deep Eutectic Solvent Composite Gel Polymer Electrolyte. ACS Appl. Energy Mater. 2022, 5, 6393–6400. [Google Scholar] [CrossRef]
- Poojari, V.; Devadiga, D.; Hegde, N.; Sangeetha, D.N.; Santosh, M.S.; Selvakumar, M. Conductivity and Electrochemical Behavior of Plasticized Polymer Electrolyte for Dye-Sensitized Solar Cell Integrated Supercapacitor. J. Electrochem. Energy Convers. Storage 2020, 17, 1–19. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yin, J.L.; Meng, X.L.; Ma, P. Investigations of electrochemical double-layer capacitors using activated carbon electrodes and gel polymer electrolytes. J. Harbin Eng. Univ. 2007, 28, 705–710. [Google Scholar]
- Bandara, T.M.W.J.; Gunasekara, L.B.E.; Gunathilake, S.M.S.; Mellander, B.-E. Transport parameters of charge carriers in PEO-LiTf-based, plasticized, composite, and plasticized-composite electrolytes intended for Li-ion batteries. Ionics 2022, 28, 2701–2714. [Google Scholar] [CrossRef]
- Shenbagavalli, S.; Muthuvinayagam, M.; Revathy, M.S.; Sasikumar, P. Ionic conductivity and dielectric studies on PVP/PEO/(NH4)2Ce(NO3)6 based solid polymer-blend electrolytes. Bull. Mater. Sci. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Rajeevan, S.; John, S.; George, S.C. Polyvinylidene fluoride: A multifunctional polymer in supercapacitor applications. J. Power Sources 2021, 504, 230037. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Lei, G.; Liu, H. Ionic liquid-gelled polyvinylidene fluoride/polyvinyl acetate polymer electrolyte for solid supercapacitor. Chem. Eng. J. 2014, 258, 320–326. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A. Ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate-based gel polymer electrolyte for elec¬trochemical capacitors. J. Mater. Chem. A 2013, 1, 3372–3378. [Google Scholar] [CrossRef]
- Satheesh, A.; Navaneeth, P.; Suneesh, P.V.; C, S.; Kandasamy, E. Synthesis, characterization and study of electrochemical applicability of novel asymmetrically substituted 1,3-dialkyl-1,2,3-benzotriazolium salts for supercapacitor fabrication. RSC Adv. 2023, 13, 14737–14746. [Google Scholar] [CrossRef]
- Satheesh, A.; Kandasamy, E. Dibutyl benzotriazolium tetrafluoroborate doped PANI as an electrode material for energy storage. J. Energy Storage 2024, 88. [Google Scholar] [CrossRef]
- Hor, A.A.; Hashmi, S. Optimization of hierarchical porous carbon derived from a biomass pollen-cone as high-performance electrodes for supercapacitors. Electrochimica Acta 2020, 356, 136826. [Google Scholar] [CrossRef]
- Muchakayala, R.; Song, S.; Wang, J.; Fan, Y.; Bengeppagari, M.; Chen, J.; Tan, M. Development and supercapacitor application of ionic liquid-incorporated gel polymer electrolyte films. J. Ind. Eng. Chem. 2018, 59, 79–89. [Google Scholar] [CrossRef]
- Obeidat, A.M.; Gharaibeh, M.A.; Obaidat, M. Solid-state supercapacitors with ionic liquid gel polymer electrolyte and polypyrrole electrodes for electrical energy storage. J. Energy Storage 2017, 13, 123–128. [Google Scholar] [CrossRef]
- Pendashteh, A.; Senokos, E.; Palma, J.; Anderson, M.; Vilatela, J.J.; Marcilla, R. Manganese dioxide decoration of macroscopic carbon nanotube fibers: From high-performance liquid-based to all-solid-state supercapacitors. J. Power Sources 2017, 372, 64–73. [Google Scholar] [CrossRef]
- Chen, W.; Xing, Z.; Wei, Y.; Zhang, X.; Zhang, Q. High thermal safety and conductivity gel polymer electrolyte composed of ionic liquid [EMIM][BF4] and PVDF-HFP for EDLCs. Polymer 2023, 268. [Google Scholar] [CrossRef]
- Pandey, G.P.; Rastogi, A.C.; Westgate, C.R. All-solid-state supercapacitors with poly(3,4-ethylene-dioxythio¬phene)-coated carbon fiber paper electrodes and ionic liquid gel polymer electrolyte. J. Power Sources 2014, 245, 857–865. [Google Scholar] [CrossRef]
- Ujjain, S.K.; Ahuja, P.; Bhatia, R.; Attri, P. Printable multi-walled carbon nanotubes thin film for high performance all solid state flexible supercapacitors. Mater. Res. Bull. 2016, 83, 167–171. [Google Scholar] [CrossRef]
- Tuhania, P.; Singh, P.K.; Bhattacharya, B.; Dhapola, P.S.; Yadav, S.; Shukla, P.; Gupta, M. PVDF-HFP and 1-ethyl-3-methylimidazolium thiocyanate–doped polymer electrolyte for efficient supercapacitors. High Perform. Polym. 2018, 30, 911–917. [Google Scholar] [CrossRef]
- Senokos, E.; Reguero, V.; Cabana, L.; Palma, J.; Marcilla, R.; Vilatela, J.J. Large-Area, All-Solid, and Flexible Electric Double Layer Capacitors Based on CNT Fiber Electrodes and Polymer Electrolytes. Adv. Mater. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Obeidat, A.; Gharaibeh, M.A. Electrochemical Performance of MnO2 for Energy Storage Supercapacitors in Solid-State Design. Int. J. Renew. Energy Res. 2018, 8, 1229–1235. [Google Scholar] [CrossRef]
- Iglesias, D.; Senokos, E.; Alemán, B.; Cabana, L.; Navío, C.; Marcilla, R.; Prato, M.; Vilatela, J.J.; Marchesan, S. Gas-Phase Functionalization of Macroscopic Carbon Nanotube Fiber Assemblies: Reaction Control, Electrochemical Properties, and Use for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 5760–5770. [Google Scholar] [CrossRef]
- Mohit, N.; Yadav, N.; Hashmi, S. High energy density solid-state supercapacitors based on porous carbon electrodes derived from pre-treated bio-waste precursor sugarcane bagasse. J. Energy Storage 2022, 55. [Google Scholar] [CrossRef]
- Sellam; Hashmi, S.A. Quasi-solid-state pseudocapacitors using proton-conducting gel polymer electrolyte and poly(3-methyl thiophene)–ruthenium oxide composite electrodes. J. Solid State Electrochem. 2013, 18, 465–475. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, M.K.; Yadav, N.; Hashmi, S.A. High performance quasi-solid-state supercapacitors with peanut-shell- derived porous carbon. J. Power Sources 2018, 402, 133–146. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, A.; Tripathi, S. Structural and electrochemical studies of bromide derived ionic liquid-based gel polymer electrolyte for energy storage application. J. Energy Storage 2020, 32, 101723. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, R.; Kaur, A. Novel hierarchical porous carbon derived from biomass Citrus limetta pulp for high-performance quasi-solid-state supercapacitor electrodes. J. Energy Storage 2023, 71. [Google Scholar] [CrossRef]
- Suleman, M.; Othman, M.; Hashmi, S.; Kumar, Y.; Deraman, M.; Omar, R.; Jasni, M. Activated graphene oxide/reduced graphene oxide electrodes and low viscous sulfonium cation based ionic liquid incorporated flexible gel polymer electrolyte for high rate supercapacitors. J. Alloy. Compd. 2017, 695, 3376–3392. [Google Scholar] [CrossRef]
- Osaka, T.; Liu, X.; Nojima, M.; Momma, T. An Electrochemical Double Layer Capacitor Using an Activated Carbon Electrode with Gel Electrolyte Binder. J. Electrochem. Soc. 1999, 146, 1724–1729. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chung, I.J. An All-Solid-State Electrochemical Supercapacitor Based on Poly3-(4-fluorophenylthiophene) Composite Electrodes. J. Electrochem. Soc. 2002, 149, A1376–A1380. [Google Scholar] [CrossRef]
- Yadav, N.; Mishra, K.; Hashmi, S. Optimization of porous polymer electrolyte for quasi-solid-state electrical double layer supercapacitor. Electrochimica Acta 2017, 235, 570–582. [Google Scholar] [CrossRef]
- Suleman, M.; Deraman, M.; Hashmi, S.A.; Othman, M.A.R.; Kumar, Y.; Rajouria, S.K.; Jasni, M.R.M. Accommodating succino¬nitrile rotators in micro-pores of 3D nano-structured cactus carbon for assisting micro-crystallite organization, ion transport and surplus pseudo-capacitance: An extreme temperature supercapacitor behavior,Electrochim. Acta 2020, 333, 135547. [Google Scholar]
- Masouras, A.; Giannopoulos, D.; Hasa, B.; Katsaounis, A.; Kostopoulos, V. Hybrid graphene nanoplatelet/manganese oxide electrodes for solid-state supercapacitors and application to carbon fiber composite multifunctional materials. J. Energy Storage 2019, 23, 515–525. [Google Scholar] [CrossRef]
- Pandey, G.P.; Liu, T.; Hancock, C.; Li, Y.; Sun, X.S.; Li, J. Thermostable gel polymer electrolyte based on succinonitrile and ionic liquid for high-performance solid-state supercapacitors. J. Power Sources 2016, 328, 510–519. [Google Scholar] [CrossRef]
- Bhat, Y.; Yadav, N.; Hashmi, S. A high performance flexible gel polymer electrolyte incorporated with suberonitrile as additive for quasi-solid carbon supercapacitor. Mater. Sci. Eng. B 2020, 262, 114721. [Google Scholar] [CrossRef]
- Ahmed, A.; Rafat, M.; Ahmed, S. Activated carbon derived from custard apple shell for efficient supercapacitor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035013. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Performance of chitosan derived activated carbon in supercapacitor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025003. [Google Scholar] [CrossRef]
- Ahmed, S.; Rafat, M.; Singh, M.K.; A Hashmi, S. A free-standing, flexible PEDOT:PSS film and its nanocomposites with graphene nanoplatelets as electrodes for quasi-solid-state supercapacitors. Nanotechnology 2018, 29, 395401. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Yuan, X.; Mo, J.; Li, C.; Fu, L.; Zhu, Y.; Wu, X.; Wu, Y. A quasi-solid-state Li-ion capacitor with high energy density based on Li3VO4/carbon nanofibers and electrochemically-exfoliated graphene sheets, J. Mater. Chem. A 2017, 5, 14922–14929. [Google Scholar] [CrossRef]
- Ahmed, S.; Parvaz, M.; Johari, R.; Rafat, M. Studies on activated carbon derived from neem (azadirachta indica) bio-waste, and its application as supercapacitor electrode. Mater. Res. Express 2018, 5, 045601. [Google Scholar] [CrossRef]
- Issar, S.; Jhajhria, D.; Adalati, R.; Kumar, P.; Kodan, S.; Chandra, R. High-voltage (> 3 V) energy storage device based on sputter-grown TiCrN microelectrodes towards miniaturized applications. J. Energy Storage 2024, 95. [Google Scholar] [CrossRef]
- Yadav, N.; Ritu; Promila; Hashmi, S.A. Hierarchical porous carbon derived from eucalyptus-bark as a sustainable electrode for high-performance solid-state supercapacitors. Sustain. Energy Fuels 2020, 4, 1730–1746. [Google Scholar] [CrossRef]
- Gnanakan, S.R.P.; Murugananthem, N.; Subramania, A. Organic acid doped polythiophene nanoparticles as electrode material for redox supercapacitors. Polym. Adv. Technol. 2011, 22, 788–793. [Google Scholar] [CrossRef]
- Nath, G.; Singh, P.K.; Dhapola, P.S.; Dohare, S.; Noor, I.M.; Sharma, T.; Singh, A. Fabrication of cornstarch biopolymer-derived nano porous carbon as electrode material for supercapacitor application. Biomass- Convers. Biorefinery 2022, 14, 7635–7642. [Google Scholar] [CrossRef]
- Pandey, G.; Hashmi, S. Solid-state supercapacitors with ionic liquid based gel polymer electrolyte: Effect of lithium salt addition. J. Power Sources 2013, 243, 211–218. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Jain, A.; Gupta, A.; Mishra, M. Electrical and electrochemical studies on magnesium ion-based polymer gel electrolytes. J. Solid State Electrochem. 2012, 16, 1799–1806. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S.K. Converting eucalyptus leaves into mesoporous carbon for its application in quasi solid-state supercapacitors. J. Solid State Electrochem. 2013, 17, 2545–2550. [Google Scholar] [CrossRef]
- Jain, A.; Michalska, M.; Zaszczyńska, A.; Denis, P. Surface modification of activated carbon with silver nanoparticles for electrochemical double layer capacitors. J. Energy Storage 2022, 54. [Google Scholar] [CrossRef]
- Jain, A.; Michalska, M. Enhanced electrochemical properties of multiwalled carbon nanotubes modified with silver nanoparticles for energy storage application. Mater. Chem. Phys. 2024, 317. [Google Scholar] [CrossRef]
- Witecka, A.; Pietrzyk-Thel, P.; Krajewski, M.; Sobczak, K.; Wolska, A.; Jain, A. Preparation of activated carbon/iron oxide/chitosan electrodes for symmetric supercapacitor using electrophoretic deposition: A facile, fast and sustainable approach. J. Alloy. Compd. 2024, 985. [Google Scholar] [CrossRef]
- Michalska, M.; Pietrzyk-Thel, P.; Sobczak, K.; Janssen, M.; Jain, A. Carbon framework modification; an interesting strategy to improve the energy storage and dye adsorption. Energy Adv. 2024, 3, 1354–1366. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, A.; Tripathi, S.K. Structural, electrical and electrochemical studies of ionic liquid-based polymer gel electrolyte using magnesium salt for supercapacitor application. J. Polym. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S.K.; Gupta, A.; Kumari, M. Fabrication and characterization of electrochemical double layer capacitors using ionic liquid-based gel polymer electrolyte with chemically treated activated charcoal electrodes. J. Solid State Electrochem. 2012, 17, 713–726. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S.K. Experimental studies on high-performance supercapacitor based on nanogel polymer electrolyte with treated activated charcoal. Ionics 2012, 19, 549–557. [Google Scholar] [CrossRef]
- Shanmugaraj, P.; Swaminathan, A.; Ravi, R.K.; Dasaiah, M.; Kumar, P.S.; Sakunthala, A. Preparation and characterization of porous PVdF-HFP/graphene oxide composite membranes by solution casting technique. J. Mater. Sci. Mater. Electr. 2019, 30, 20079–20087. [Google Scholar] [CrossRef]
- Prasadini, K.W.; Perera, K.S.; Vidanapathirana, K.P. Preliminary study on the performance of a redox capacitor with the use of ionic liquid-based gel polymer electrolyte and polypyrrole electrodes. J. Mater. Sci. Mater. Electron. 2021, 32, 17629–17636. [Google Scholar] [CrossRef]
- Redda, H.G.; Nikodimos, Y.; Su, W.-N.; Chen, R.-S.; Jiang, S.-K.; Abrha, L.H.; Hagos, T.M.; Bezabh, H.K.; Weldeyohannes, H.H.; Hwang, B.J. Enhancing the electrochemical performance of a flexible solid-state supercapacitor using a gel polymer electrolyte. Mater. Today Commun. 2021, 26. [Google Scholar] [CrossRef]
- Murphy, J.N.; Schneider, C.M.; Hawboldt, K.; Kerton, F.M. Hard to Soft: Biogenic Absorbent Sponge-like Material from Waste Mussel Shells. Matter 2020, 3, 2029–2041. [Google Scholar] [CrossRef]
- Murphy, J.N.; Mendes, T.; Kerton, F.M.; MacFarlane, D.R. Biorenewable Calcite as an Inorganic Filler in Ionic Liquid Gel Polymer Electrolytes for Supercapacitors. ACS Omega 2023, 8, 21418–21424. [Google Scholar] [CrossRef]
- Sharma, S.; Pathak, D.; Dhiman, N.; Kumar, R. Characterization of PVdF-HFP-based nanocomposite plasticized polymer electrolytes. Surf. Innov. 2017, 5, 251–256. [Google Scholar] [CrossRef]
- Pandey, G.P.; Rastogi, A.C. Solid-State Supercapacitors Based on Pulse Polymerized Poly(3,4-ethylenedioxythiophene) Electrodes and Ionic Liquid Gel Polymer Electrolyte. J. Electrochem. Soc. 2012, 159, A1664–A1671. [Google Scholar] [CrossRef]
- Pandey, G.; Hashmi, S. Performance of solid-state supercapacitors with ionic liquid 1-ethyl-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate based gel polymer electrolyte and modified MWCNT electrodes. Electrochimica Acta 2013, 105, 333–341. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.; Zhang, X.; Sun, X.; Li, C.; Ge, X.; Ma, Y. Flexible Solid-State Supercapacitors with Enhanced Performance from Hierarchically Graphene Nanocomposite Electrodes and Ionic Liquid Incorporated Gel Polymer Electrolyte. Adv. Funct. Mater. 2017, 28. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, D.; Singh, A.; Srivastava, M.; Kumar, S.; Singh, R.; Yadav, T.; Alheety, M.A.; Singh, P.K. Waste peanut shells derived activated carbon for dual electrochemical applications. Energy Storage 2024, 6. [Google Scholar] [CrossRef]
- Shi, M.-J.; Kou, S.-Z.; Shen, B.-S.; Lang, J.-W.; Yang, Z.; Yan, X.-B. Improving the performance of all-solid-state supercapacitors by modifying ionic liquid gel electrolytes with graphene nanosheets prepared by arc-discharge. Chin. Chem. Lett. 2014, 25, 859–864. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, J.-P.; Zhuang, Q.-Q.; Zhao, X.-Y.; Zhou, Z.; Wei, Y.-L.; Zhao, M.; Bai, H.-C. Biomass-derived three-dimensional hierarchical porous carbon network for symmetric supercapacitors with ultra-high energy density in ionic liquid electrolyte. Electrochimica Acta 2021, 371, 137825. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, F.; Zhang, T.; Huang, Y.; Chen, Y. A high-performance all-solid-state supercapacitor with graphene-doped carbon material electrodes and a graphene oxide-doped ion gel electrolyte. Carbon 2014, 72, 381–386. [Google Scholar] [CrossRef]
- Pandey, G.P.; Klankowski, S.A.; Liu, T.; Wu, J.; Li, J. Toward highly stable solid-state unconventional thin-film battery-supercapacitor hybrid devices: Interfacing vertical core-shell array electrodes with a gel polymer electrolyte. J. Power Sources 2017, 342, 1006–1016. [Google Scholar] [CrossRef]
- Singh, M.K.; Hashmi, S.A. Performance of solid-state hybrid supercapacitor with LiFePO4/AC composite cathode and Li4Ti5O12 as anode. Ionics 2017, 23, 2931–2942. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Wang, G.; Zhang, J.; Zhang, S. Sandwich-like NiO/rGO nanoarchitectures for 4 V solid-state asymmetric-supercapacitors with high energy density. Electrochimica Acta 2018, 283, 1401–1410. [Google Scholar] [CrossRef]
- Poochai, C.; Sriprachuabwong, C.; Sodtipinta, J.; Lohitkarn, J.; Pasakon, P.; Primpray, V.; Maeboonruan, N.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. Alpha-MnO2 nanofibers/nitrogen and sulfur-co-doped reduced graphene oxide for 4.5 V quasi-solid state supercapacitors using ionic liquid-based polymer electrolyte. J. Colloid Interface Sci. 2021, 583, 734–745. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y. A High-Performance Graphene Oxide-Doped Ion Gel as Gel Polymer Electrolyte for All-Solid-State Supercapacitor Applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, P.K.; Agarwal, D.; Singh Dhapola, P.; Sharma, T.; Savilov, S.V.; Arkhipova, E.A.; Singh, M.K.; Singh, A. Structure, Dielectric, and Electrochemical Studies on Poly(Vinylidene Fluoride-Co-Hexafluoropropylene)/IonicLiquid 1-Ethyl¬-3-Methylimidazolium Tricyanomethanide-Based Polymer Electrolytes. Phys. Status Solidi A 2022, 219, 2100711. [Google Scholar] [CrossRef]
- Liu, J.; Ahmed, S.; Wang, T.; Song, S. Flexible thermotolerant Zn-ion hybrid supercapacitors enabled by heat-resistant polymer electrolyte. Chem. Eng. J. 2022, 451. [Google Scholar] [CrossRef]
- Dhawan, R.; Singh, A.; Kumar, S.; Dhapola, P.S.; Alheety, M.A.; Yahya, M.Z.A.; Serguei, S. Futuristic Approach Towards Replacement of Aqueous Electrolyte with Solid Polymer Electrolyte for Supercapacitor Applications. J. Electron. Mater. 2023, 52, 4295–4301. [Google Scholar] [CrossRef]
- Yohans, M.; Singh, M.; Singh, R.C.; Shukla, P.K.; Singh, V.; Singh, P.K. Poly(vinylidine fluoride-co-hexa-fluoropropylene)-doped zinc acetate polymer electrolyte for supercapacitor application. High Perform. Polym. 2020, 32, 151–157. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, Y.J.; Park, N.-G.; Kang, M.G.; Chang, S.H. Redox supercapacitor using polyaniline doped with Li salt as electrode. Solid State Ionics 2002, 152-153, 861–866. [Google Scholar] [CrossRef]
- Ryu, K.S.; Wu, X.; Lee, Y.; Chang, S.H. Electrochemical capacitor composed of doped polyaniline and polymer electrolyte membrane. J. Appl. Polym. Sci. 2003, 89, 1300–1304. [Google Scholar] [CrossRef]
- Hong, K.; Yuk, J.; Kim, H.J.; Lee, J.Y.; Kim, S.; Lee, J.-L.; Lee, K.H. Electrospun polymer electrolyte nanocomposites for solid-state energy storage. Compos. Part B: Eng. 2018, 152, 275–281. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Highly efficient gel polymer electrolytes for all solid-state electrochemical charge storage devices. Electrochimica Acta 2018, 278, 137–148. [Google Scholar] [CrossRef]
- Park, Y.J.; Bae, J. Novel P(VDF-TrFE) Polymer Electrolytes: Their Use in High-Efficiency, All-Solid-State Electrochemical Capacitors Using ZnO Nanowires. J. Electrochem. Sci. Technol. 2018, 9, 126–132. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, C.; Yuan, B.; Li, H.; Chen, W.; Yin, H.; Zhao, B.; Shen,F.;. Han, X. A UV cross-linked gel polymer electrolyte enabling high-rate and high voltage window for quasi-solid-state supercapacitors. J. Energy Chem. 2023, 76, 41-50.
- Suleman, M.; Kumar, Y.; Hashmi, S.A. Structural and Electrochemical Properties of Succinonitrile-Based Gel Polymer Electrolytes: Role of Ionic Liquid Addition. J. Phys. Chem. B 2013, 117, 7436–7443. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S. Fabrication and characterization of energy storing supercapacitor devices using coconut shell based activated charcoal electrode. Mater. Sci. Eng. B 2014, 183, 54–60. [Google Scholar] [CrossRef]
- Tamilarasan, P.; Ramaprabhu, S. Stretchable supercapacitors based on highly stretchable ionic liquid incorporated polymer electrolyte. Mater. Chem. Phys. 2014, 148, 48–56. [Google Scholar] [CrossRef]
- Zakariya’u, I.; Gultekin, B.; Singh, V.; Singh, P.K. Electrochemical double-layer supercapacitor using poly(methyl methacrylate) solid polymer electrolyte. High Perform. Polym. 2020, 32, 201–207. [Google Scholar] [CrossRef]
- Sivaraman, P.; Thakur, A.; Kushwaha, R.K.; Ratna, D.; Samui, A.B. Poly(3-methyl thiophene)-Activated Carbon Hybrid Supercapacitor Based on Gel Polymer Electrolyte. Electrochem. Solid-state Lett. 2006, 9, A435–A438. [Google Scholar] [CrossRef]
- Deshagani, S.; Naskar, I.; Padval, G.G.; Ghosal, P.; Deepa, M. Electrical Conduction in CoWO4 Flanked by Carbon and ZnFe2O4 Nanoparticulate Assembly and a Poly(ethylene oxide) Gel for Enhanced Electrochemical Activity. ACS Appl. Energy Mater. 2022, 5, 13520–13534. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, J.Y.; Suh, D.H.; Hong, Y.T.; Kim, T.-H. Electrode-Impregnable and Cross-Linkable Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Polymer Electrolytes with High Ionic Conductivity and a Large Voltage Window for Flexible Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 33913–33924. [Google Scholar] [CrossRef]
- Karaman, B.; Çevik, E.; Bozkurt, A. Novel flexible Li-doped PEO/copolymer electrolytes for supercapacitor application. Ionics 2019, 25, 1773–1781. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Sahoo, S.; Manoharan, S.; Kim, S.-J. Electrospinning: A High Efficacy Self-Charging MoSe2 Solid-State Supercapacitor Using Electrospun Nanofibrous Piezoelectric Separator with Ionogel Electrolyte (Adv. Mater. Interfaces 12/2018). Adv. Mater. Interfaces 2018, 5. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Ahmed, S.; Wang, H.; Wang, T.; Song, S. A study of low-temperature solid-state supercapacitors based on Al-ion conducting polymer electrolyte and graphene electrodes. J. Power Sources 2021, 488. [Google Scholar] [CrossRef]
- Gu, M.G.; Song, E.; Kim, S.-K. Robust and Highly Ion-Conducting Gel Polymer Electrolytes with Semi-Interpenetrating Polymer Network Structure. Macromol. Res. 2021, 29, 211–216. [Google Scholar] [CrossRef]
- Mitra, S.; Shukla, A.; Sampath, S. Electrochemical capacitors with plasticized gel-polymer electrolytes. J. Power Sources 2001, 101, 213–218. [Google Scholar] [CrossRef]
- Prasad, K.R.; Munichandraiah, N. Electrochemical studies of polyaniline in a gel polymer electrolyte - High energy and high power characteristics of a solid-state redox supercapacitor. Electrochem.Solid State Lett. 2002, 5, A271–A274. [Google Scholar] [CrossRef]
- Fahimi, Z.; Ghasemi, M.; Alavijeh, F.K.; Moradlou, O. Electrochemical investigations of the various electrolytes for high energy density metal oxide supercapacitor. J. Solid State Electrochem. 2022, 26, 2389–2399. [Google Scholar] [CrossRef]
- Chen, H.-L.; Jiao, X.-N.; Zhou, J.-T. The research progress of polyhedral oligomeric silsesquioxane (POSS) applied to electrical energy storage elements. Funct. Mater. Lett. 2017, 10. [Google Scholar] [CrossRef]
- Ghasemi, M.; Fahimi, Z.; Moradlou, O.; Sovizi, M.R. Porous gel polymer electrolyte for the solid state metal oxide supercapacitor with a wide potential window. J. Taiwan Inst. Chem. Eng. 2021, 118, 223–231. [Google Scholar] [CrossRef]
- Shin, C.; Yao, L.; Lin, H.; Liu, P.; Ng, T.N. Photothermal Supercapacitors with Gel Polymer Electrolytes for Wide Temperature Range Operation. ACS Energy Lett. 2023, 8, 1911–1918. [Google Scholar] [CrossRef]
- Liu, X.; Osaka, T. Properties of Electric Double-Layer Capacitors with Various Polymer Gel Electrolytes. J. Electrochem. Soc. 1997, 144, 3066–3071. [Google Scholar] [CrossRef]
- Huang, C.; Wu, C.; Hou, S.; Kuo, P.; Hsieh, C.; Teng, H. Gel Electrolyte Derived from Poly(ethylene glycol) Blending Poly(acrylonitrile) Applicable to Roll-to-Roll Assembly of Electric Double Layer Capacitors. Adv. Funct. Mater. 2012, 22, 4677–4685. [Google Scholar] [CrossRef]
- Lee, D.; Yang, M.; Choi, U.H.; Kim, J. Bioinspired Synaptic Branched Network within Quasi-Solid Polymer Electrolyte for High-Performance Microsupercapacitors. Small 2024, e2308821. [Google Scholar] [CrossRef]
- Park, H.; Yong, H.; Jung, J.; Jung, C. Selective Effect of Gel Polymer Electrolytes on Suppressing Decomposition and Evaporation of Electrolyte in Acetonitrile-Based Supercapacitors at Elevated Temperature. ChemElectroChem 2019, 6, 4418–4428. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K. Fabrication and performance studies of a cable-type flexible asymmetric supercapacitor. Phys. Chem. Chem. Phys. 2014, 16, 15692–15698. [Google Scholar] [CrossRef]
- Chatterjee, J.; Liu, T.; Wang, B.; Zheng, J.P. Structural Investigation of Polyvinyl Alcohol Gel Electrolyte by Small Angle X-ray Scattering. ECS Trans. 2011, 33, 17–25. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Aziz, S.B.; Murad, A.R. Diffusion and ion carrier mobility studies in binary SPEs based on PVA integrated with K+ ion provider salt: structural and electrical insights. Ionics 2022, 28, 5153–5169. [Google Scholar] [CrossRef]
- Hedayati, D.P.; Singh, G.; Kucher, M.; Keene, T.D.; Böhm, R. Physicochemical Modeling of Electrochemical Impedance in Solid-State Supercapacitors. Materials 2023, 16, 1232. [Google Scholar] [CrossRef]
- Dong, X.; Guo, Z.; Song, Y.; Hou, M.; Wang, J.; Wang, Y.; Xia, Y. Flexible and Wire-Shaped Micro-Supercapacitor Based on Ni(OH)2-Nanowire and Ordered Mesoporous Carbon Electrodes. Adv. Funct. Mater. 2014, 24, 3405–3412. [Google Scholar] [CrossRef]
- Ahmad, A.; Gondal, M.A.; Hassan, M.; Iqbal, R.; Ullah, S.; Alzahrani, A.S.; Memon, W.A.; Mabood, F.; Melhi, S. Preparation and Characterization of Physically Activated Carbon and Its Energetic Application for All-Solid-State Supercapacitors: A Case Study. ACS Omega 2023, 8, 21653–21663. [Google Scholar] [CrossRef]
- Hou, G.-M.; Huang, Y.-F.; Ruan, W.-H.; Zhang, M.-Q.; Rong, M.-Z. Hypergrafted nano-silica modified polymer gel electrolyte for high-performance solid-state supercapacitor. J. Solid State Electrochem. 2015, 20, 1903–1911. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Huang, N.M. Electrochemical properties of free-standing polypyrrole/graphene oxide/zinc oxide flexible supercapacitor. Int. J. Energy Res. 2014, 39, 111–119. [Google Scholar] [CrossRef]
- Yang, C.; Dong, L.; Chen, Z.; Lu, H. High-Performance All-Solid-State Supercapacitor Based on the Assembly of Graphene and Manganese(II) Phosphate Nanosheets. J. Phys. Chem. C 2014, 118, 18884–18891. [Google Scholar] [CrossRef]
- Kim, S.I.; Kang, J.H.; Kim, S.W.; Jang, J.H. A new approach to high-performance flexible supercapacitors: Mesoporous three¬¬-di¬mensional Ni-electrodes. Nano Energy 2017, 39, 639–646. [Google Scholar] [CrossRef]
- Ye, X.; Zhu, Y.; Tang, Z.; Wan, Z.; Jia, C. In-situ chemical reduction produced graphene paper for flexible supercapacitors with impressive capacitive performance. J. Power Sources 2017, 360, 48–58. [Google Scholar] [CrossRef]
- Anand, S.; Choudhury, A. MnMoS4 anchored at carbon nanofiber as a flexible electrode for solid-state asymmetric supercapacitor device. Mater. Chem. Phys. 2023, 299. [Google Scholar] [CrossRef]
- Yang, C.; Dong, L.; Chen, Z.; Lu, H. High-Performance All-Solid-State Supercapacitor Based on the Assembly of Graphene and Manganese(II) Phosphate Nanosheets. J. Phys. Chem. C 2014, 118, 18884–18891. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Q.; Li, C.; Zhu, L.; Huang, Y.; Zhu, X.; Zhu, X.; Sun, Y. Hydrogel-derived nitrogen-doped porous carbon framework with vanadium nitride decoration for supercapacitors with superior cycling performance. J. Mater. Sci. Technol. 2023, 155, 167–174. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Stabilized TiN Nanowire Arrays for High-Performance and Flexible Supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef]
- Bai, X.; Cao, D.; Zhang, H. Constructing ZnCo2O4@LDH Core–Shell hierarchical structure for high performance supercapacitor electrodes. Ceram. Int. 2019, 45, 14943–14952. [Google Scholar] [CrossRef]
- Quan, B.; Meng, Y.; Li, L.; Yao, Z.; Liu, Z.; Wang, K.; Wei, Z.; Gu, C.; Li, J. Vertical few-layer graphene/metalized Si-nanocone arrays as 3D electrodes for solid-state supercapacitors with large areal capacitance and superior rate capability. Appl. Surf. Sci. 2017, 404, 238–245. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, D.H.; Kim, W.; Jang, J. Highly ordered, polypyrrole-coated Co(OH)2 architectures for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 6603–6609. [Google Scholar] [CrossRef]
- Hu, X.; Fan, L.; Qin, G.; Shen, Z.; Chen, J.; Wang, M.; Yang, J.; Chen, Q. Flexible and low temperature resistant double network alkaline gel polymer electrolyte with dual-role KOH for supercapacitor. J. Power Sources 2019, 414, 201–209. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, D.K.; Bhattacharyya, T.K. Flexible High-Energy-Density 4.3 V Supercapacitor Based on a Trimetallic Sulfide Cathode and a Bimetallic Sulfide-Polypyrrole Anode with an Ionic Liquid Gel Polymer Electrolyte. ACS Appl. Electron. Mater. 2023, 5, 6362–6383. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Rao, G.R. A high energy flexible symmetric supercapacitor fabricated using N-doped activated carbon derived from palm flowers. Nanoscale Adv. 2021, 3, 5417–5429. [Google Scholar] [CrossRef]
- Ju, G.; Khan, M.A.; Zheng, H.; An, Z.; Wu, M.; Zhao, H.; Xu, J.; Zhang, L.; Bilal, S.; Zhang, J. Honeycomb-like polyaniline for flexible and folding all-solid-state supercapacitors. Front. Mater. Sci. 2019, 13, 133–144. [Google Scholar] [CrossRef]
- Beenarani, B.B.; Sugumaran, C.P. A flexible, cost-effective, and eco-friendly solid state supercapacitor based on PVA/KCl/ Carbon black nanocomposite. Ionics 2020, 26, 1465–1473. [Google Scholar] [CrossRef]
- Wang, M.; Duong, L.D.; Mai, N.T.; Kim, S.; Kim, Y.; Seo, H.; Kim, Y.C.; Jang, W.; Lee, Y.; Suhr, J.; et al. All-Solid-State Reduced Graphene Oxide Supercapacitor with Large Volumetric Capacitance and Ultralong Stability Prepared by Electrophoretic Deposition Method. ACS Appl. Mater. Interfaces 2014, 7, 1348–1354. [Google Scholar] [CrossRef]
- Hong, S.; Kim, H.; Gao, S.; Lavall, R.L.; Jung, H.Y.; Jung, Y.J. Reconfigurable solid-state electrolytes for high performance flexible supercapacitor. J. Power Sources 2019, 432, 16–23. [Google Scholar] [CrossRef]
- Otrokhov, G.; Pankratov, D.; Shumakovich, G.; Khlupova, M.; Zeifman, Y.; Vasil’eva, I.; Morozova, O.; Yaropolov, A. Enzymatic synthesis of polyaniline/multi-walled carbon nanotube composite with core shell structure and its electrochemical characterization for supercapacitor application. Electrochimica Acta 2014, 123, 151–157. [Google Scholar] [CrossRef]
- N., R.; A., K.; H.M., M.; M.R., N.; Mondal, D.; Nataraj, S.K.; Ghosh, D. Binder free self-standing high performance supercapacitive electrode based on graphene/titanium carbide composite aerogel. Appl. Surf. Sci. 2019, 481, 892–899. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Qi, F.; Zhang, S.; Wen, J.; Shu, W.; Luo, P.; Zhang, R.; Hu, S.; Liu, Q. Designing high electrochemical surface area between polyaniline and hydrogel polymer electrolyte for flexible supercapacitors. Appl. Surf. Sci. 2019, 507, 145135. [Google Scholar] [CrossRef]
- Manikandan, R.; Raj, C.J.; Moulton, S.E.; Todorov, T.S.; Yu, K.H.; Kim, B.C. High Energy Density Heteroatom (O, N and S) Enriched Activated Carbon for Rational Design of Symmetric Supercapacitors. Chem. – A Eur. J. 2020, 27, 669–682. [Google Scholar] [CrossRef]
- Raj, C.J.; Manikandan, R.; Cho, W.-J.; Yu, K.H.; Kim, B.C. High-performance flexible and wearable planar supercapacitor of manganese dioxide nanoflowers on carbon fiber cloth. Ceram. Int. 2020, 46, 21736–21743. [Google Scholar] [CrossRef]
- Kannagi, K. Super critically synthesized V2O5 spheres based supercapacitors using polymer electrolyte. Appl. Surf. Sci. 2018, 456, 13–18. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, V.C.; Patil, U.M.; Shinde, P.A.; Lokhande, C.D. High Performance All-Solid-State Asymmetric Supercapacitor Device Based on 3D Nanospheres of beta-MnO2 and Nanoflowers of O-SnS. ACS Sustainable Chem.Eng. 2018, 6, 787–802. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Peng, J.; Cao, P.; Cai, X.; Li, J.; Zhai, M. A Flexible Ionic Liquid Gelled PVA-Li2SO4 Polymer Electrolyte for Semi-Solid-State Supercapacitors. Adv. Mater.Interfaces 2015, 2, 1500267. [Google Scholar] [CrossRef]
- Lee, H.; Lee, G.; Yun, J.; Keum, K.; Hong, S.Y.; Song, C.; Kim, J.W.; Lee, J.H.; Oh, S.Y.; Kim, D.S.; et al. Facile fabrication of a fully biodegradable and stretchable serpentine-shaped wire supercapacitor. Chem. Eng. J. 2019, 366, 62–71. [Google Scholar] [CrossRef]
- Rafique, A.; Massa, A.; Fontana, M.; Bianco, S.; Chiodoni, A.; Pirri, C.F.; Hernández, S.; Lamberti, A. Highly Uniform Anodically Deposited Film of MnO2 Nanoflakes on Carbon Fibers for Flexible and Wearable Fiber-Shaped Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 28386–28393. [Google Scholar] [CrossRef]
- Tombolesi, S.; Zanieri, N.; Bargnesi, L.; Mernini, M.; Lacarbonara, G.; Arbizzani, C. A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices. Polymers 2023, 15, 3087. [Google Scholar] [CrossRef]
- Pulikkottil, M.; Thomas, A.; Neelanchery, M.M.; Elavumkal, V.G.; Ansari, S. Highly Efficient Solid-State Supercapacitor with Porous Electrode Material. Energy Technol. 2023, 11. [Google Scholar] [CrossRef]
- Mevada, C.; Mukhopadhyay, M. Enhancement of electrochemical properties of hydrous ruthenium oxide nanoparticles coated on chemically activated carbon cloth for solid-state symmetrical supercapacitor application. Mater. Chem. Phys. 2020, 245, 122784. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Brza, M.A.; Ahmed, M.B.; Murad, A.R.; Tahir, H.B.; Abdullah, R.M.; Hadi, J.M.; Hussen, S.A. Exploring the sustainable frontier by investigating structural, electrochemical and ion transport properties of potassium salt-doped PVA-based polymer electrolyte for supercapacitor application. J. Energy Storage 2023, 71. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Dennis, J.O.; Shukur, M.F.B.A.; Nasef, M.M.E.; Usman, F. Preparation and characterization of gel polymer electrolyte based on PVA-K2CO3. Polym. Plast. Technol. Mater. 2020, 59, 1679–1697. [Google Scholar] [CrossRef]
- Anjum, N.; Grota, M.; Li, D.; Shen, C. Laminate composite-based highly durable and flexible supercapacitors for wearable energy storage. J. Energy Storage 2020, 29, 101460. [Google Scholar] [CrossRef]
- Huffstutler, J.D.; Wasala, M.; Richie, J.; Barron, J.; Winchester, A.; Ghosh, S.; Yang, C.; Xu, W.; Song, L.; Kar, S.; et al. High Performance Graphene-Based Electrochemical Double Layer Capacitors Using 1-Butyl-1-methylpyrrolidinium tris (pentafluoroethyl) trifluorophosphate Ionic Liquid as an Electrolyte. Electronics 2018, 7, 229. [Google Scholar] [CrossRef]
- Stempien, Z.; Khalid, M.; Kozicki, M.; Kozanecki, M.; Varela, H.; Filipczak, P.; Pawlak, R.; .Korzeniewska, E.; Sąsiadek, E. In-situ deposition of reduced graphene oxide layers on textile surfaces by the reactive inkjet printing technique and their use in supercapacitor applications. Synth. Met. 2019, 256. [Google Scholar] [CrossRef]
- Mitra, S.; Katiyar, M. Integration of a Flexible, Stretchable, Environment-Benign, and Highly Conductive PVA/H3PO4 Hydrogel as a Quasi Solid-State Electrolyte in Reduced Graphene Oxide Supercapacitors. ACS Appl. Polym. Mater. 2023, 5, 9825–9835. [Google Scholar] [CrossRef]
- Pu, J.; Wang, X.; Zhang, T.; Li, S.; Liu, J.; Komvopoulos, K. High-energy-density, all-solid-state microsupercapacitors with three-dimensional interdigital electrodes of carbon/polymer electrolyte composite. Nanotechnology 2015, 27, 045701–045701. [Google Scholar] [CrossRef]
- Jung, H.Y.; Karimi, M.B.; Hahm, M.G.; Ajayan, P.M.; Jung, Y.J. Transparent, flexible supercapacitors from nano-engineered carbon films. Sci. Rep. 2012, 2, 773. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Y.; Li, T.; Huang, Y.; Zhang, A.; Miao, M. High Performance Carbon Nanotube Yarn Supercapacitors with a Surface-Oxidized Copper Current Collector. ACS Appl. Mater. Interfaces 2015, 7, 25835–25842. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Gorkin III, R.; Beirne, S.; Shu, K.; Wallace, G.G. Three dimensional (3D) printed electrodes for interdigitated supercapacitors. Electrochem. Commun. 2014, 41, 20–23. [Google Scholar] [CrossRef]
- Sharma, N.; Koshy, A.M.; Kandregula, G.R.; Ramanujam, K.; Ray, D.; Swaminathan, P. Printed Silver Nanowire-PEDOT:PSS Composite Electrodes for Flexible Transparent Microsupercapacitor Applications. ACS Appl. Energy Mater. 2023, 7, 363–372. [Google Scholar] [CrossRef]
- Anjum, N.; Joyal, N.; Iroegbu, J.; Li, D.; Shen, C. Humidity-modulated properties of hydrogel polymer electrolytes for flexible supercapacitors. J. Power Sources 2021, 499. [Google Scholar] [CrossRef]
- Jeong, H.T.; Du, J.F.; Kim, Y.R.; Raj, C.J.; Kim, B.C. Electrochemical performances of highly stretchable polyurethane (PU) supercapacitors based on nanocarbon materials composites. J. Alloy. Compd. 2018, 777, 67–72. [Google Scholar] [CrossRef]
- Akbulut, S.; Yilmaz, M.; Raina, S.; Hsu, S.H.; Kang, W.P. Solid-state supercapacitor cell based on 3D nanostructured MnO2/CNT microelectrode array on graphite and H3PO4/PVA electrolyte. Diamond Related Mater. 2017, 74, 222–228. [Google Scholar] [CrossRef]
- Fornasini, L.; Scaravonati, S.; Magnani, G.; Morenghi, A.; Sidoli, M.; Bersani, D.; Bertoni, G.; Aversa, L.; Verucchi, R.; Riccò, M.; et al. In situ decoration of laser-scribed graphene with TiO2 nanoparticles for scalable high-performance micro-supercapacitors. Carbon 2021, 176, 296–306. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lo, C.-T. Crucial structural parameters affecting electrochemical properties of activated electrospun carbon fibers as solid-state supercapacitor electrodes. J. Mater. Sci. 2023, 58, 15144–15161. [Google Scholar] [CrossRef]
- Jung, H.Y.; Karimi, M.B.; Hahm, M.G.; Ajayan, P.M.; Jung, Y.J. Transparent, flexible supercapacitors from nano-engineered carbon films. Sci. Rep. 2012, 2, 773. [Google Scholar] [CrossRef]
- Khan, M.S.; Murtaza, I.; Shuja, A.; Khan, H.R.; Abid, R.; Nunez, C.G.; Fahad, S.; Tariq, H.; Naveed, A. Tailored NiO-pBOA-GNP ternary nanocomposite: Advances in flexible supercapacitors and practical applications for wearable technology and environmental monitoring. J. Energy Storage 2024, 86, 111128. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, J.; Chen, C.; Lu, Y.; Ge, Y.; Zhang, X. Poly(vinyl Alcohol) Borate Gel Polymer Electrolytes Prepared by Electrodeposition and Their Application in Electrochemical Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3473–3481. [Google Scholar] [CrossRef]
- Jha, P.K.; Kashyap, V.; Gupta, K.; Kumar, V.; Debnath, A.K.; Roy, D.; Rana, S.; Kurungot, S.; Ballav, N. In-situ generated Mn3O4-reduced graphene oxide nanocomposite for oxygen reduction reaction and isolated reduced graphene oxide for supercapacitor applications. Carbon 2019, 154, 285–291. [Google Scholar] [CrossRef]
- Anothumakkool, B.; T., A.T.A.; Bhange, S.N.; Unni, S.M.; Badiger, M.V.; Kurungot, S. Design of a High Performance Thin All-Solid-State Supercapacitor Mimicking the Active Interface of Its Liquid-State Counterpart. ACS Appl. Mater. Interfaces 2013, 5, 13397–13404. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Chen, S.; Pan, W.; Zhang, J. A flexible solid-state supercapacitor based on graphene/polyaniline paper electrodes. J. Energy Chem. 2018, 32, 166–173. [Google Scholar] [CrossRef]
- Du, H.; Wu, Z.; Xu, Y.; Liu, S.; Yang, H. Poly(3,4-ethylenedioxythiophene) Based Solid-State Polymer Supercapacitor with Ionic Liquid Gel Polymer Electrolyte. Polymers 2020, 12, 297. [Google Scholar] [CrossRef]
- Mevada, C.; Chandran, P.S.; Mukhopadhyay, M. Room-temperature synthesis of tin oxide nanoparticles using gallic acid monohydrate for symmetrical supercapacitor application. J. Energy Storage 2020, 28, 101197. [Google Scholar] [CrossRef]
- Yao, Z.; Quan, B.; Yang, T.; Li, J.; Gu, C. Flexible supercapacitors based on vertical graphene/carbon fabric with high rate performance. Appl. Surf. Sci. 2022, 610. [Google Scholar] [CrossRef]
- Kadam, S.L.; Mane, S.M.; Ingole, R.S.; Dhasade, S.S.; Shin, J.C.; Kulkarni, S.B. Time-intended effect on electrochemical performance of hydrothermally reduced graphene oxide nanosheets: Design and study of solid-state symmetric supercapacitor. J. Mater. Sci. Mater. Electron. 2021, 32, 14901–14918. [Google Scholar] [CrossRef]
- Rajaputra, S.S.; Pennada, N.; Yerramilli, A.; Kummara, N.M. Graphene based sulfonated polyvinyl alcohol hydrogel nanocomposite for flexible supercapacitors. J. Electrochem. Sci. Eng. 2021, 11, 197–207. [Google Scholar] [CrossRef]
- Alipoori, S.; Aboutalebi, S.H.; Barsbay, M. Enhancing the performance of solid-state supercapacitors: Optimizing the molecular interactions in flexible gel polymer electrolytes. J. Solid State Electrochem. 2024, 28, 2643–2657. [Google Scholar] [CrossRef]
- Lal, M.S.; Ramaprabhu, S. High Areal Capacitance of Flexible Supercapacitors Fabricated with Carbon Cloth-Carbon Fiber-TiO2 Electrodes and Different Hydrogel Polymer Electrolytes. J. Electrochem. Soc. 2022, 169, 020514. [Google Scholar] [CrossRef]
- Aljafari, B.; Takshi, A. Gel Electrolyte Based Supercapacitors with Higher Capacitances and Lower Resistances than Devices with a Liquid Electrolyte. MRS Adv. 2018, 3, 1261–1267. [Google Scholar] [CrossRef]
- Chen, X.; Holze, R. Surfactants as Performance-Enhancing Additives in Supercapacitor Electrolyte Solutions—An Overview. Batteries 2023, 10, 4. [Google Scholar] [CrossRef]
- Prakash, A.; De, S.; Holla, S.R.; Nayak, R.; Selvaraj, S. Investigation of surfactant micelles on the performance of a gel-polymer electrolyte. Int. J. Hydrogen Energy 2024, 73, 191–202. [Google Scholar] [CrossRef]
- Le, P.-A.; Nguyen, V.-T.; Sahoo, S.K.; Tseng, T.Y.; Wei, K.-H. Porous carbon materials derived from areca palm leaves for high performance symmetrical solid-state supercapacitors. J. Mater. Sci. 2020, 55, 10751–10764. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, J.; Song, C.; Li, X.; Wang, Z.; Yang, J.; Cheng, J.; Li, H.; Wang, B. 2.2V high performance symmetrical fiber-shaped aqueous supercapacitors enabled by “water-in-salt” gel electrolyte and N-Doped graphene fiber. Energy Storage Mater. 2019, 24, 495–503. [Google Scholar] [CrossRef]
- Karaman, B.; Bozkurt, A. Enhanced performance of supercapacitor based on boric acid doped PVA-H2SO4 gel polymer electrolyte system. Int. J. Hydrogen Energy 2018, 43, 6229–6237. [Google Scholar] [CrossRef]
- Qin, G.; Wang, M.; Fan, L.; Fang, X.; Zhang, D.; Liu, J.; Qin, J.; Shi, J.; Yang, J.; Chen, Q. Multifunctional supramolecular gel polymer electrolyte for self-healable and cold-resistant supercapacitor. J. Power Sources 2020, 474, 228602. [Google Scholar] [CrossRef]
- Tian, S.; Wu, S.; Cui, S.; Tian, Y.; Balkus, K.J.; Zhou, L.; Xiong, G. High-performance solid-state supercapacitors integrated with thermal management systems based on phase change materials: All in one. Chem. Eng. J. 2022, 446. [Google Scholar] [CrossRef]
- Hu, J.; Xie, K.; Liu, X.; Guo, S.; Shen, C.; Liu, X.; Li, X.; Wang, J.-G.; Wei, B. Dramatically Enhanced Ion Conductivity of Gel Polymer Electrolyte for Supercapacitor via h-BN Nanosheets Doping. Electrochimica Acta 2017, 227, 455–461. [Google Scholar] [CrossRef]
- Rosi, M.; Iskandar, F.; Abdullah, M. ; Khairurrijal Hydrogel-Polymer Electrolytes Based on Polyvinyl Alcohol and Hydroxyethylcellulose for Supercapacitor Applications. Int. J. Electrochem. Sci. 2014, 9, 4251–4256. [Google Scholar] [CrossRef]
- Anneser, K.; Reichstein, J.; Braxmeier, S.; Reichenauer, G. Carbon xerogel based electric double layer capacitors with polymer gel electrolytes – Improving the performance by adjusting the type of electrolyte and its processing. Electrochimica Acta 2018, 278, 196–203. [Google Scholar] [CrossRef]
- Chen, P.; Chen, H.; Qiu, J.; Zhou, C. Inkjet printing of single-walled carbon nanotube/RuO2 nanowire supercapacitors on cloth fabrics and flexible substrates. Nano Res. 2010, 3, 594–603. [Google Scholar] [CrossRef]
- Hashmi, S.; Upadhyaya, H. Polypyrrole and poly(3-methyl thiophene)-based solid state redox supercapacitors using ion conducting polymer electrolyte. Solid State Ionics 2002, 152-153, 883–889. [Google Scholar] [CrossRef]
- Hashim, M.A.; Yatim, N.M; Mahmud, N.A.C.; Sazali, N.E.S.; Hamdan, E.; Yahya, M.A.; Ngah, C.E.Z.C.W. ; Suhaimi; S. Hybrid Solid Polymer Electrolyte From Diapers As Separator For Electrochemical Double Layer Capacitor (EDLC). AIP Conf. Proc. 2018, 1972, 020001-1. [Google Scholar]
- Wan, J.; Lv, T.; Liu, Y.; Wang, X.; Yang, Y.; Chen, Z.; Qi, Y.; Cao, S.; Chen, T. Flexible Asymmetric Supercapacitors with Extremely Slow Self-Discharge Rate Enabled by a Bilayer Heterostructure Polymer Electrolyte. Adv. Funct. Mater. 2021, 32, 2108794. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, K.; Lv, T.; Chen, Z.; Cao, S.; Lu, Q.; Zheng, F.; Chen, T. Revealing the Mechanism of Bilayer Heterogeneous Polyelectrolytes to Suppress the Self-Discharge of Symmetric Supercapacitors. Small Struct. 2023, 4. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, H.; Lv, T.; Wan, J.; Dong, K.; Chen, Z.; Chen, T. Regulating the Self-Discharge of Flexible All-Solid-State Supercapacitors by a Heterogeneous Polymer Electrolyte. Small 2021, 17, 2102054. [Google Scholar] [CrossRef]
- Le, P.A.; Le, V.Q.; Nguyen, N.T.; Phung, V.B.T. Food seasoning-derived gel polymer electrolyte and pulse-plasma exfoliated graphene nanosheet electrodes for symmetrical solid-state supercapacitors. RSC Adv. 2022, 12, 1515–1526. [Google Scholar] [CrossRef]
- Peng, S.; Jiang, X.; Xiang, X.; Chen, K.; Chen, G.; Jiang, X.; Hou, L. High-performance and flexible solid-state supercapacitorsbased on high toughness and thermoplastic poly(vinylalcohol)/NaCl/glycerol supramolecular gel polymer. Electrochim. Acta 2019, 324, 134874. [Google Scholar] [CrossRef]
- Shuaibu, A.D.; Shah, S.S.; Alzahrani, A.S.; Aziz, A. Development and assessment of an innovative gel electrolyte using polyvinyl alcohol, lithium sulfate, and 1-butyl-3-methylimidazolium trifluoromethanesulfonate for advanced supercapacitor performance. J. Energy Storage 2024, 92. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Peng, J.; Cao, P.; Cai, X.; Li, J.; Zhai, M. A Flexible Ionic Liquid Gelled PVA-Li2SO4 Polymer Electrolyte for Semi-Solid-State Supercapacitors. Adv. Mater. Interfaces 2015, 2, 1500267. [Google Scholar] [CrossRef]
- Rag, S.A.; Selvakumar, M.; Bhat, S.; Chidangil, S.; De, S. Synthesis and Characterization of Reduced Graphene Oxide for Supercapacitor Application with a Biodegradable Electrolyte. J. Electron. Mater. 2019, 49, 985–994. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Arof, A. Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties. Int. J. Hydrogen Energy 2014, 39, 2953–2963. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdulwahid, R.T.; Halim, N.A.; Hassan, J.; Abdulrahman, A.F.; Al-Saeedi, S.I.; Hadi, J.M.; Kadir, M.F.Z.; Hamad, S.M.; et al. Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics. Green Process. Synth. 2024, 13. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, H.T. Development and optimization of ionic liquid based gel polymer electrolyte for all solid-state supercapacitor. J. Energy Storage 2021, 42. [Google Scholar] [CrossRef]
- Aziz, M.F.; Shamsuri, N.A.; Hamsan, M.H.; Yusof, Y.M.; Azam, M.A.; Aziz, S.B.; Rusdi, H.; Kadir, M.F.Z.; Shukur, M.F. An elucidation of symmetric supercapacitor with crustacean-based polymer blend electrolyte. J. Appl. Polym. Sci. 2023, 141. [Google Scholar] [CrossRef]
- Jang, H.S.; Raj, C.J.; Lee, W.G.; Kim, B.C.; Yu, K.H. Enhanced supercapacitive performances of functionalized activated carbon in novel gel polymer electrolytes with ionic liquid redox-mediated poly(vinyl alcohol)/phosphoric acid. RSC Adv. 2016, 6, 75376–75383. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Muchakayala, R.; Song, S. High-performance Mg-ion conducting poly(vinyl alcohol) membranes: Preparation, characterization and application in supercapacitors. J. Membr. Sci. 2018, 555, 280–289. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Song, S. Na-ion conducting gel polymer membrane for flexible supercapacitor application. Electrochimica Acta 2019, 330, 135322. [Google Scholar] [CrossRef]
- Khanam, Z.; Liu, J.; Song, S. Flexible graphene paper electrode prepared via polyvinyl alcohol-assisted shear-exfoliation for all-solid-state polymer supercapacitor application. Electrochimica Acta 2020, 363, 137208. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Zheng, Y.; Koh, X.; Lim, Q.F.; Sharma, M.; Fam, D.W.H. Elucidating the relationship between mechanical properties and ionic conductivity in a highly conductive gel polymer electrolyte. Mater. Lett. 2021, 294, 129789. [Google Scholar] [CrossRef]
- Satheesh, A.; Sushil, S.; Bharathan, A.; Navaneeth, P.; Suneesh, P.V.; Kandasamy, E. Development of dialkyl-triazolium ionogels for printed supercapacitor. Mater. Lett. 2024, 358. [Google Scholar] [CrossRef]
- Rai, N.; Singh, C.P.; Ranjta, L.; Yahya, M.Z.A. XRD, DSC, and Dielectric Studies of MWNT-Doped Polymer Electrolytes for Supercapacitor Application. J. Electron. Mater. 2023, 52, 4269–4278. [Google Scholar] [CrossRef]
- Shan, Q.; Ding, Q.; Wang, X.; Wu, W. Electrochemical Preparation of Hydroxylated Boron Nitride Nanosheets for Solid–State Flexible Supercapacitors Using Deep Eutectic Solvent and Water Mixture as Electrolytes. Langmuir 2022, 38, 8169–8178. [Google Scholar] [CrossRef]
- Gao, H.; Lian, K. A H5BW12O40-polyvinyl alcohol polymer electrolyte and its application in solid supercapacitors. J.Mater.Chem.A 2016, 4, 9585–9592. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, B.K. Carbon nanotube–zinc oxide electrode and gel polymer electrolyte for electrochemical supercapacitors. J. Alloy. Compd. 2009, 480, L17–L19. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, J.; Chen, C.; Lu, Y.; Pampal, E.S.; Luo, L.; Zhu, P.; Zhang, X. Superior high-voltage aqueous carbon/carbon supercapacitors operating with in situ electrodeposited polyvinyl alcohol borate gel polymer electrolytes. J. Mater. Chem. A 2016, 4, 16588–16596. [Google Scholar] [CrossRef]
- Yan, T.; Zou, Y.; Zhang, X.; Li, D.; Guo, X.; Yang, D. Hydrogen Bond Interpenetrated Agarose/PVA Network: A Highly Ionic Conductive and Flame-Retardant Gel Polymer Electrolyte. ACS Appl. Mater. Interfaces 2021, 13, 9856–9864. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, S.; Yan, Y.; Wang, L.; Xu, G.; Yarlagadda, S.; Chou, T.-W. High-Performance Structural Supercapacitors Based on Aligned Discontinuous Carbon Fiber Electrodes and Solid Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 11774–11782. [Google Scholar] [CrossRef]
- Zihong, S.; Anbao, Y. Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte. Chin.J.Chem.Eng. 2009, 17, 150–155. [Google Scholar]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2019, 27, 101072. [Google Scholar] [CrossRef]
- Dennis, J.O.; Shukur, M.F.; Aldaghri, O.A.; Ibnaouf, K.H.; Adam, A.A.; Usman, F.; Hassan, Y.M.; Alsadig, A.; Danbature, W.L.; Abdulkadir, B.A. A Review of Current Trends on Polyvinyl Alcohol (PVA)-Based Solid Polymer Electrolytes. Molecules 2023, 28, 1781. [Google Scholar] [CrossRef]
- Vondrák, J.; Reiter, J.; Velická, J.; Sedlařı́ková, M. PMMA-based aprotic gel electrolytes. Solid State Ionics 2004, 170, 79–82. [Google Scholar] [CrossRef]
- Latif, F.A.; Zailani, N.A.M.; Al Shukaili, Z.S.M.; Zamri, S.F.M.; Kasim, N.A.M.; Rani, M.S.A.; Norrrahim, M.N.F. Review of poly (methyl methacrylate) based polymer electrolytes in solid-state supercapacitors. Int. J. Electrochem. Sci. 2022, 17, 22013. [Google Scholar] [CrossRef]
- Navarrete-Astorga, E.; Rodríguez-Moreno, J.; Dalchiele, E.A.; Schrebler, R.; Leyton, P.; Ramos-Barrado, J.R.; Martín, F. A transparent solid-state ion gel for supercapacitor device applications. J. Solid State Electrochem. 2017, 21, 1431–1444. [Google Scholar] [CrossRef]
- Latif, F.A.; Zailani, N.A.M.; Al Shukaili, Z.S.M.; Zamri, S.F.M.; Kasim, N.A.M.; Rani, M.S.A.; Norrrahim, M.N.F. Review of poly (methyl methacrylate) based polymer electrolytes in solid-state supercapacitors. Int. J. Electrochem. Sci. 2022, 17, 22013. [Google Scholar] [CrossRef]
- Lee, G.; Kim, J.W.; Park, H.; Lee, J.Y.; Lee, H.; Song, C.; Jin, S.W.; Keum, K.; Lee, C.H.; Ha, J.S. Skin-Like, Dynamically Stretchable, Planar Supercapacitors with Buckled Carbon Nanotube/Mn-Mo Mixed Oxide Electrodes and Air-Stable Organic Electrolyte. ACS Nano 2019, 13, 855–866. [Google Scholar] [CrossRef]
- Miltenburg, M.B.; An, S.Y.; Obhi, N.K.; Grignon, E.; McAllister, B.T.; Seferos, D.S. Ambipolar Poly(3,4-ethylene¬dioxy¬thio¬phene)-Pendant Tetrachlorinated Perylene Diimide for Symmetric Supercapacitors. ACS Appl. Polym. Mater. 2020, 2, 5574–5580. [Google Scholar] [CrossRef]
- Zaki, N.H.M.; Mahmud, Z.S.; Hassan, O.H.; Yahya, M.Z.A.; Ali, A.M.M. A Symmetric Supercapacitor Based On 30% Poly (Methyl Methacrylate) Grafted Natural Rubber (MG30) Polymer and Activated Carbon Electrodes. AIP Conf. Proc. 2017, 1875, 020016. [Google Scholar]
- Cai, C.; Yuan, P.; Tang, J.; Guo, Y.; Ma, X. Ultrathin paper-like boron-doped carbon nanosheet electrodes combined with boron-enriched gel polymer electrolytes for high-performance energy storage. J. Mater. Chem. A 2016, 4, 15589–15596. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, P.; Cai, C.; Fu, Y.; Ma, X. Combining Nature-Inspired, Graphene-Wrapped Flexible Electrodes with Nanocomposite Polymer Electrolyte for Asymmetric Capacitive Energy Storage. Adv. Energy Mater. 2016, 6, 1600813. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Anothumakkool, B.; T., A.T.A.; Nair, S.B.; Badiger, M.V.; Kurungot, S. An all-solid-state-supercapacitor possessing a non-aqueous gel polymer electrolyte prepared using a UV-assisted in situ polymerization strategy. J. Mater. Chem. A 2017, 5, 8461–8476. [Google Scholar] [CrossRef]
- Lee, D.; Song, Y.; Song, Y.; Oh, S.J.; Choi, U.H.; Kim, J. Multi-Foldable and Environmentally-Stable All-Solid-State Supercapacitor Based on Hierarchical Nano-Canyon Structured Ionic-Gel Polymer Electrolyte. Adv. Funct. Mater. 2022, 32, 2109907. [Google Scholar] [CrossRef]
- Lu, H.; Wang, P.; Ma, Y.; Liu, M.; Chang, L.; Feng, R.; Luo, S.; Zhang, Z.; Wang, Y.; Yuan, Y. In situ-fabricated quasi-solid polymer electrolytes incorporating an ionic liquid for flexible supercapacitors. Sustain. Energy Fuels 2023, 8, 358–368. [Google Scholar] [CrossRef]
- Jin, J.; Mu, H.; Wang, W.; Li, X.; Cheng, Q.; Wang, G. Long-life flexible supercapacitors based on nitrogen-doped porous graphene@π-conjugated polymer film electrodes and porous quasi-solid-state polymer electrolyte. Electrochimica Acta 2019, 317, 250–260. [Google Scholar] [CrossRef]
- Mitra, S.; Shukla, A.K.; Sampath, S. Electrochemical Capacitors Based on Sol-Gel Derived, Ionically Conducting Composite Solid Electrolytes. Electrochem. Solid-state Lett. 2003, 6, A149–A153. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, B.; Li, H.; Liu, H. A flexible polycation-type anion-dominated conducting polymer as potential all-solid-state supercapacitor film electrolyte. Chem. Eng. J. 2017, 330, 753–756. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Mohan, A.M.V. Wearable Energy Storage Devices. deGruyter, Berlin 2021.
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, C.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Zhang, L.; Hu, W.; Wilkinson, D.P.; Zhang, J. Opportunities of Flexible and Portable Electrochemical Devices for Energy Storage: Expanding the Spotlight onto Semi-solid/Solid Electrolytes. Chem. Rev. 2022, 122, 17155–17239. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. Performance of solid state supercapacitors based on polymer electrolytes containing different ionic liquids. J. Power Sources 2016, 326, 560–568. [Google Scholar] [CrossRef]
- Moon, S.J.; Min, H.J.; Lee, C.S.; Kang, D.R.; Kim, J.H. Adhesive, free-standing, partially fluorinated comb copolymer electrolyte films for solid flexible supercapacitors. Chem. Eng. J. 2021, 429, 132240. [Google Scholar] [CrossRef]
- Mun, W.J.; Kim, B.; Moon, S.J.; Kim, J.H. Multifunctional, bicontinuous, flexible comb copolymer electrolyte for solid-state supercapacitors. Chem. Eng. J. 2022, 454. [Google Scholar] [CrossRef]
- B. Kim, Y. Ko, W.J. Mun, K.C. Kim, J.H. Kim Synthesis and self-assembly of bottlebrush block copolymer electrolytes for solid-state supercapacitors. Chem. Eng. J. 2024, 489, 151400.
- Na, R.; Huo, P.; Zhang, X.; Zhang, S.; Du, Y.; Zhu, K.; Lu, Y.; Zhang, M.; Luan, J.; Wang, G. A flexible solid-state supercapacitor based on a poly(aryl ether ketone)-poly(ethylene glycol) copolymer solid polymer electrolyte for high temperature applications RSC Adv. 2016, 6, 65186-65195.
- Vineeth, S.K.; Sreeram, P.; Vlad, A.; Joy, R.; Raghavan, P.; Pullanchiyodan, A. Polymer blend nanocomposite electrolytes for advanced energy storage applications in: Polymer blend nanocomposites for energy storage applications. (S. Thomas, A.R. Ajitha, M. Jaroszewskie, Eds.), Elsevier, Amsterdam 2023, p. 203-238.
- Ye, C.; Qin, Q.; Liu, J.; Mao, W.; Yan, J.; Wang, Y.; Cui, J.; Zhang, Q.; Yang, L.; Wu, Y. Coordination derived stable Ni–Co MOFs for foldable all-solid-state supercapacitors with high specific energy. J. Mater. Chem. A 2019, 7, 4998–5008. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, N.; Liu, C.; Yan, Li, C.C.;Cui, J.; Liu, J.; Hu, X.; Wu, Y. Al doped Ni-Co layered double hydroxides with surface-sulphuration for highly stable flexible supercapacitors. J. Colloid Interf. Sci. 2022, 615, 173-183.
- Qin, Q.; Du, X.; Xu, C.; Huang, S.; Wang, W.; Zhang, Y.; Yan, J.; Liu, J.; Wu, Y. Flexible Supercapacitors Based on Solid Ion Conducting Polymer with High Mechanical Strength. J. Electrochem. Soc. 2017, 164, A1952–A1957. [Google Scholar] [CrossRef]
- Mao, T.; Wang, S.; Wang, X.; Liu, F.; Li, J.; Chen, H.; Wang, D.; Liu, G.; Xu, J.; Wang, Z. High-Temperature and All-Solid-State Flexible Supercapacitors with Excellent Long-Term Stability Based on Porous Polybenzimidazole/Functional Ionic Liquid Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 17742–17750. [Google Scholar] [CrossRef]
- Xu, C.; Yan, J.; Qin, Q.; Deng, Y.; Cheng, J.; Zhang, Y.; Wu, Y. All solid supercapacitors based on an anion conducting polymer electrolyte. RSC Adv. 2016, 6, 19826–19832. [Google Scholar] [CrossRef]
- Qin, Q.; Ou, D.; Ye, C.; Chen, L.; Lan, B.; Yan, J.; Wu, Y. Systematic study on hybrid supercapacitor of Ni-Co layered double hydroxide//activated carbons. Electrochimica Acta 2019, 305, 403–415. [Google Scholar] [CrossRef]
- Rathod, D.; Vijay, M.; Islam, N.; Kannan, R.; Kharul, U.; Kurungot, S.; Pillai, V. Design of an “all solid-state” supercapacitor based on phosphoric acid doped polybenzimidazole (PBI) electrolyte. J. Appl. Electrochem. 2009, 39, 1097–1103. [Google Scholar] [CrossRef]
- Mao, T.; Wang, S.; Wang, X.; Liu, F.; Li, J.; Chen, H.; Wang, D.; Liu, G.; Xu, J.; Wang, Z. High-Temperature and All-Solid-State Flexible Supercapacitors with Excellent Long-Term Stability Based on Porous Polybenzimidazole/Functional Ionic Liquid Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 17742–17750. [Google Scholar] [CrossRef]
- Na, R.; Huo, G.; Zhang, S.; Huo, P.; Du, Y.; Luan, J.; Zhu, K.; Wang, G. A novel poly(ethylene glycol)–grafted poly(arylene ether ketone) blend micro-porous polymer electrolyte for solid-state electric double layer capacitors formed by incorporating a chitosan-based LiClO4 gel electrolyte. J. Mater. Chem. A 2016, 4, 18116–18127. [Google Scholar] [CrossRef]
- Torres, F.G.; De-La-Torre, G.E. Algal-based polysaccharides as polymer electrolytes in modern electrochemical energy conversion and storage systems: A review. Carbohydr. Polym. Technol. Appl. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Qiu, F.; Huang, Y.; Hu, X.; Li, B.; Zhang, X.; Luo, C.; Li, X.; Wang, M.; Wu, Y.; Cao, H. An Ecofriendly Gel Polymer Electrolyte Based on Natural Lignocellulose with Ultrahigh Electrolyte Uptake and Excellent Ionic Conductivity for Alkaline Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 6031–6042. [Google Scholar] [CrossRef]
- Nath, G.; Dhapola, P.S.; Sahoo, N.; Singh, S.; Singh, V.; Singh, P.K. Polyvinylpyrrolidone with ammonium iodide and plasticizer ethylene carbonate solid polymer electrolyte for supercapacitor application. J. Thermoplast. Compos. Mater. 2020, 35, 879–890. [Google Scholar] [CrossRef]
- Sellam; Hashmi, S.A. High Rate Performance of Flexible Pseudocapacitors fabricated using Ionic-Liquid-Based Proton Conducting Polymer Electrolyte with Poly(3, 4-ethylenedioxythiophene):Poly(styrene sulfonate) and Its Hydrous Ruthenium Oxide Composite Electrodes. ACS Appl. Mater. Interfaces 2013, 5, 3875–3883. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K.; Senthil, S. Kumar Reduced graphene oxide derived from used cell graphite and its green fabrication as an eco-friendly supercapacitor. RSC Adv. 2014, 4, 60039–60051. [Google Scholar] [CrossRef]
- Wang, M.; Fan, L.; Qin, G.; Hu, X.; Wang, Y.; Wang, C.; Yang, J.; Chen, Q. Flexible and low temperature resistant semi-IPN network gel polymer electrolyte membrane and its application in supercapacitor. J. Membr. Sci. 2020, 597, 117740. [Google Scholar] [CrossRef]
- Sangeetha, D.N.; Hegde, N.; Poojari, V.; Devadiga, D.; Sudhakar, Y.N.; Santosh, M.S.; Selvakumar, M. Conductivity/Electrochemical Study of Polyvinyl pyrrolidone-Poly(vinyl alcohol)/I3- Thin Film Electrolyte for Integrated Dye-Sensitized Solar Cells and Supercapacitors. J. Electron. Mater. 2020, 49, 6325–6335. [Google Scholar] [CrossRef]
- Krishnan, K.; Karuthapandi, S.; Vijayaraghavan, S. Ionic transport kinetics and enhanced energy storage in the electrode/poly(N-vinyl imidazole) interface for micro-supercapacitors. RSC Adv. 2020, 10, 45019–45027. [Google Scholar] [CrossRef]
- Yang, J.; Guo, M.; Feng, L.; Hao, J.; Guo, Y.; Li, Z.; Liu, S.; Qin, G.; Sun, G.; Chen, Q. Swelling-resistant microgel-reinforced hydrogel polymer electrolytes for flexible all-in-one supercapacitors with high performances. J. Mater. Chem. C 2023, 11, 7419–7426. [Google Scholar] [CrossRef]
- Genovese, M.; Wu, H.; Virya, A.; Li, J.; Shen, P.; Lian, K. Ultrathin all-solid-state supercapacitor devices based on chitosan activated carbon electrodes and polymer electrolytes. Electrochimica Acta 2018, 273, 392–401. [Google Scholar] [CrossRef]
- Rafidi, N.; Bashir, S.; Hina, M.; Gunalan, S.; Ramesh, S.; Ramesh, K. Renewable and soft dynamic supercapacitors based on poly (acrylamide) hydrogel electrolytes and porous carbon electrodes. Polym. Bull. 2022, 80, 1285–1302. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, S.; Yan, T.; Cai, R.; Zou, Y. In Situ Polymerization of Xanthan/Acrylamide for Highly Ionic Conductive Gel Polymer Electrolytes with Unique Interpenetrating Network. ACS Appl. Polym. Mater. 2022, 4, 9241–9249. [Google Scholar] [CrossRef]
- Diao, W.; Wu, L.; Ma, X.; Wang, L.; Bu, X.; Ni, W.; Yang, X.; Fang, Y. Reversibly highly stretchable and self-healable zwitterion-containing polyelectrolyte hydrogel with high ionic conductivity for high-performance flexible and cold-resistant supercapacitor. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Wang, P.-H.; Tseng, L.-H.; Li, W.-C.; Lin, C.-H.; Wen, T.-C. Zwitterionic semi-IPN electrolyte with high ionic conductivity and high modulus achieving flexible 2.4 V aqueous supercapacitors. J. Taiwan Inst. Chem. Eng. 2021, 126, 58–66. [Google Scholar] [CrossRef]
- Shamsuri, N.A.; Majid, S.R.; Hamsan, M.H.; Halim, S.N.A.; Manan, N.S.A.; Sulaiman, M.; Jahidin, A.H.; Halim, N.A.; Aziz, S.B.; Kadir, M.F.Z. Enhancing ionic conductivity and flexibility in solid polymer electrolytes with rainforest honey as a green plasticizer. J. Appl. Polym. Sci. 2024, 141. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, L.-M. Rheological characteristics of chitin/ionic liquid gels and electrochemical properties of regenerated chitin hydrogels. Colloids Surfaces A: Physicochem. Eng. Asp. 2019, 586, 124220. [Google Scholar] [CrossRef]
- Kasprzak, D.; Galiński, M. Biopolymer-based gel electrolytes with an ionic liquid for high-voltage electrochemical capacitors. Electrochem. Commun. 2022, 138. [Google Scholar] [CrossRef]
- Hamsan, M.; Aziz, S.B.; Azha, M.; Azli, A.; Shukur, M.; Yusof, Y.; Muzakir, S.; Manan, N.S.; Kadir, M. Solid-state double layer capacitors and protonic cell fabricated with dextran from Leuconostoc mesenteroides based green polymer electrolyte. Mater. Chem. Phys. 2020, 241. [Google Scholar] [CrossRef]
- Tseng, L.-H.; Wang, P.-H.; Li, W.-C.; Lin, C.-H.; Wen, T.-C. Enhancing the ionic conductivity and mechanical properties of zwitterionic polymer electrolytes by betaine-functionalized graphene oxide for high-performance and flexible supercapacitors. J. Power Sources 2021, 516, 230624. [Google Scholar] [CrossRef]
- Gao, H.; Lian, K. Proton-conducting polymer electrolytes and their applications in solid supercapacitors: a review. RSC Adv. 2014, 4, 33091–33113. [Google Scholar] [CrossRef]
- Stradins, J. [Theodore von Grotthuss, 1785-1822]. 1975, 32, 322–8. [Google Scholar]
- Staiti, P.; Lufrano, F. Nafion® and Fumapem® polymer electrolytes for the development of advanced solid-state supercapacitors. Electrochimica Acta 2016, 206, 432–439. [Google Scholar] [CrossRef]
- Yamada, A.; Goodenough, J.B. Keggin-Type Heteropolyacids as Electrode Materials for Electrochemical Supercapacitors. J. Electrochem. Soc. 1998, 145, 737–743. [Google Scholar] [CrossRef]
- Paleo, A.; Staiti, P.; Rocha, A.; Squadrito, G.; Lufrano, F. Lifetime assessment of solid-state hybrid supercapacitors based on cotton fabric electrodes. J. Power Sources 2019, 434, 226735. [Google Scholar] [CrossRef]
- Thomas, M.; Cannilla, C.; Brigandì, A.; Nicotera, I.; Lufrano, F. Nanoarchitectonics of high-performance supercapacitors based on mesoporous carbon and MnO2 electrodes using Aquivion electrolyte membrane. J. Alloy. Compd. 2023, 960. [Google Scholar] [CrossRef]
- Thomas, M.; Veleva, S.; Karamanova, B.; Brigandì, A.; Rey-Raap, N.; Arenillas, A.; Stoyanova, A.; Lufrano, F. Highly stable and reliable asymmetric solid-state supercapacitors with low self-discharge rates. Sustain. Mater. Technol. 2023, 38. [Google Scholar] [CrossRef]
- Karamanova, B.; Mladenova, E.; Thomas, M.; Rey-Raap, N.; Arenillas, A.; Lufrano, F.; Stoyanova, A. Electrochemical Performance of Symmetric Solid-State Supercapacitors Based on Carbon Xerogel Electrodes and Solid Polymer Electrolytes. Gels 2023, 9, 983. [Google Scholar] [CrossRef]
- Subramaniam, C.K.; Boopalan, G. Study of storage capacity in various carbon/graphene-based solid-state supercapacitors. Appl. Phys. A 2014, 116, 887–891. [Google Scholar] [CrossRef]
- Lee, P.-C.; Hyun, J.E.; Jeoung, S.K.; Nam, J.-D.; Hwang, T.; Kim, K.J.; Solasa, K.C. Ionic polymer metal composites for use as an organic electrolyte supercapacitor. Smart Mater. Struct. 2019, 28, 054003. [Google Scholar] [CrossRef]
- Choi, B.G.; Hong, J.; Hong, W.H.; Hammond, P.T.; Park, H. Facilitated Ion Transport in All-Solid-State Flexible Supercapacitors. ACS Nano 2011, 5, 7205–7213. [Google Scholar] [CrossRef]
- Huang, C.; Grant, P.S. One-step spray processing of high power all-solid-state supercapacitors. Sci. Rep. 2013, 3, srep02393. [Google Scholar] [CrossRef]
- Slade, S.; Campbell, S.A.; Ralph, T.R.; Walsh, F.C. Ionic Conductivity of an Extruded Nafion 1100 EW Series of Membranes. J. Electrochem. Soc. 2002, 149, A1556–A1564. [Google Scholar] [CrossRef]
- Sumner, J.J.; Creager, S.E.; Ma, J.J.; DesMarteau, D.D. Proton Conductivity in Nafion® 117 and in a Novel Bis[(perfluoroalkyl)sulfonyl]imide Ionomer Membrane. J. Electrochem. Soc. 1998, 145, 107–110. [Google Scholar] [CrossRef]
- Samui, A.B.; Sivaraman, P. Solid polymer electrolytes for supercapacitors. in: Polymer Electrolytes: Fundamentals and Applications (C. Sequeira, D. Santos Eds.) Woodhead Publishing, Cambridge 2010, p. 431-470.
- Li, J.; Qiao, J.; Lian, K. Hydroxide ion conducting polymer electrolytes and their applications in solid supercapacitors: A review. Energy Storage Mater. 2019, 24, 6–21. [Google Scholar] [CrossRef]
- Park, K.-W.; Ahn, H.-J.; Sung, Y.-E. All-solid-state supercapacitor using a Nafion® polymer membrane and its hybridization with a direct methanol fuel cell. J. Power Sources 2002, 109, 500–506. [Google Scholar] [CrossRef]
- Lufrano, F.; Staiti, P. Conductivity and Capacitance Properties of a Supercapacitor Based on Nafion Electrolyte in a Nonaqueous System. Electrochem. Solid-state Lett. 2004, 7, A447–A450. [Google Scholar] [CrossRef]
- Lufrano, F.; Staiti, P. Performance improvement of Nafion based solid state electrochemical supercapacitor. Electrochimica Acta 2004, 49, 2683–2689. [Google Scholar] [CrossRef]
- Staiti, P.; Lufrano, F. Design, Fabrication, and Evaluation of a 1.5 F and 5 V Prototype of Solid-State Electrochemical Supercapacitor. J. Electrochem. Soc. 2005, 152, A617–A621. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Flores-Lopez, S.L.; dos Santos-Gomez, L.; Brigandi, A.; Thomas, M.; Stoyanova, A.E.; Lufrano, F.; Arenillas, A. Graphene Doped Carbon-Gels and MnO2 for Next Generation of Solid-State Asymmetric Supercapacitors. ChemElectroChem 2023, 10, e202300161. [Google Scholar] [CrossRef]
- Staiti, P.; Lufrano, F. Investigation of polymer electrolyte hybrid supercapacitor based on manganese oxide–carbon electrodes. Electrochimica Acta 2010, 55, 7436–7442. [Google Scholar] [CrossRef]
- Chen, H.; Gwee, L.; Choi, J.H.; De La Cruz, D.S.; Winey, K.I.; Elabd, Y.A. Ion conduction in polymerized ionic liquids and ionic liquid-polymer mixtures. 237th Nat.Mtg.ACS, 22.-26.03. 2009, Book of Abstracts 2009.
- Sultana, S.; Ahmed, K.; Jiwanti, P.K.; Wardhana, B.Y.; Shiblee, N.I. Ionic Liquid-Based Gels for Applications in Electrochemical Energy Storage and Conversion Devices: A Review of Recent Progress and Future Prospects. Gels 2021, 8, 2. [Google Scholar] [CrossRef]
- Singh, P.K.; Sabin, K.C.; Chen, X. Ionic liquid–solid polymer electrolyte blends for supercapacitor applications. Polym. Bull. 2015, 73, 255–263. [Google Scholar] [CrossRef]
- Pandey, G.P.; Kumar, Y.; Hashmi, S.A. Ionic liquid incorporated polymer electrolytes for supercapacitor application. Ind. J. Chem. A 2010, 49, 743–751. [Google Scholar]
- Kalinova, R.; Dimitrov, I.; Novakov, C.; Veleva, S.; Stoyanova, A. Modular Platform for Synthesis of Poly(Ionic Liquid) Electrolytes for Electrochemical Applications in Supercapacitors. ChemistrySelect 2021, 6, 3795–3801. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Ambrogi, M.; Tiruye, G.A.; Cordella, D.; Fernandes, A.M.; Grygiel, K.; Isik, M.; Patil, N.; Porcarelli, L.; Rocasalbas, G.; et al. Innovative polyelectrolytes/poly(ionic liquid)s for energy and the environment. Polym. Int. 2017, 66, 1119–1128. [Google Scholar] [CrossRef]
- Yusuf, S.N.F.; Yahya, R.; Arof, A.K. Ionic Liquid Enhancement of Polymer Electrolyte Conductivity and their Effects on the Performance of Electrochemical Devices. Progress and devlopments in ionic liquids 2017 157-183.
- Obadia, M.M.; Drockenmuller, E. Poly(1,2,3-triazolium)s: a new class of functional polymer electrolytes. Chem. Commun. 2015, 52, 2433–2450. [Google Scholar] [CrossRef]
- Karlsson, C.; Jannasch, P. Highly Conductive Nonstoichiometric Protic Poly(ionic liquid) Electrolytes. ACS Appl. Energy Mater. 2019, 2, 6841–6850. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Feng, H.; Diaz, M.; Ortiz, A.; Ortiz, I.; Knapik-Kowalczuk, J.; Vilas, M.; Verdía, P.; Tojo, E.; Saito, T.; et al. Revealing the Charge Transport Mechanism in Polymerized Ionic Liquids: Insight from High Pressure Conductivity Studies. Chem. Mater. 2017, 29, 8082–8092. [Google Scholar] [CrossRef]
- Alexandre, S.A.; Silva, G.G.; Santamaría, R.; Trigueiro, J.P.C.; Lavall, R.L. A highly adhesive PIL/IL gel polymer electrolyte for use in flexible solid state supercapacitors. Electrochimica Acta 2019, 299, 789–799. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. Performance of solid state supercapacitors based on polymer electrolytes containing different ionic liquids. J. Power Sources 2016, 326, 560–568. [Google Scholar] [CrossRef]
- de Oliveira, P.S.; Alexandre, S.A.; Silva, G.G.; Trigueiro, J.P.C.; Lavall, R.L. PIL/IL gel polymer electrolytes: The influence of the IL ions on the properties of solid-state supercapacitors. Eur. Polym. J. 2018, 108, 452–460. [Google Scholar] [CrossRef]
- Dhukia, S.; Singh, G.; Singh, V.; Singh, V. Efficient self healable blended cationic and anionic poly(ionic liquids) under ambient conditions. J. Appl. Polym. Sci. 2024, 141, e55432. [Google Scholar] [CrossRef]
- Shi, M.; Lin, T.; Wang, Y.; Hu, Y.; Peng, J.; Li, J.; Zhai, M. One-step radiation synthesis of novel star-shaped polymeric ionic liquid-POSS gel electrolytes with high ionic conductivity and mechanical properties for supercapacitor. J. Mater. Sci. 2020, 55, 16347–16359. [Google Scholar] [CrossRef]
- Wen, X.; Dong, T.; Liu, A.; Zheng, S.; Chen, S.; Han, Y.; Zhang, S. A new solid-state electrolyte based on polymeric ionic liquid for high-performance supercapacitor. Ionics 2018, 25, 241–251. [Google Scholar] [CrossRef]
- Jamil, R.; Silvester, D.S. Ionic liquid gel polymer electrolytes for flexible supercapacitors: Challenges and prospects. Curr. Opin. Electrochem. 2022, 35. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, J.; Liu, Y.; Chen, W.; Qi, W.; Zhao, L. Radiation synthesis strategy of poly(ionic liquid)/MXene gel polymer for supercapacitor electrolyte. Ionics 2023, 29, 2865–2875. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. All-solid state supercapacitors operating at 3.5 V by using ionic liquid based polymer electrolytes. J. Power Sources 2015, 279, 472–480. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Q.X.; Yunsheng, Y.; Xue, Y.; Wang, Y.; Peng, H.Y.; Xie, X.L.; Mai, Y.-W. Constructing desirable ion-conducting channels within ionic liquid-based composite polymer electrolytes by using polymeric ionic liquid-functionalized 2D mesoporous silica nanoplates. Nano Energy 2017, 33, 110–123. [Google Scholar] [CrossRef]
- Yan, C.; Jin, M.; Pan, X.; Ma, L.; Ma, X. A flexible polyelectrolyte-based gel polymer electrolyte for high-performance all-solid-state supercapacitor application. RSC Adv. 2020, 10, 9299–9308. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Ghosh, M.; Kurian, M.; Torris, A.; Dilwale, S.; Badiger, M.V.; Winter, M.; Nair, J.R.; Kurungot, S. An In Situ Cross-Linked Nonaqueous Polymer Electrolyte for Zinc-Metal Polymer Batteries and Hybrid Supercapacitors. Small 2020, 16, e2002528. [Google Scholar] [CrossRef]
- Senokos, E.; Rana, M.; Vila, M.; Fernandez-Cestau, J.; Costa, R.D.; Marcilla, R.; Vilatela, J.J. Transparent and flexible high-power supercapacitors based on carbon nanotube fibre aerogels. Nanoscale 2020, 12, 16980–16986. [Google Scholar] [CrossRef]
- Li, L.; Lu, N.; Jiang, D.; Chen, Z.; Zhang, W.; Zheng, W.; Zhu, X.; Wang, G. A universal strategy to improve interfacial kinetics of solid supercapacitors used in high temperature. J. Colloid Interface Sci. 2020, 586, 110–119. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Ahmed, S.; Wang, H.; Wang, T.; Song, S. A study of low-temperature solid-state supercapacitors based on Al-ion conducting polymer electrolyte and graphene electrodes. J. Power Sources 2021, 488. [Google Scholar] [CrossRef]
- Yong, H.; Park, H.; Jung, C. Quasi-solid-state gel polymer electrolyte for a wide temperature range application of acetonitrile-based supercapacitors. J. Power Sources 2019, 447, 227390. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, J.; Snaith, H.J.; Grant, P.S. Engineering the Membrane/Electrode Interface To Improve the Performance of Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 20756–20765. [Google Scholar] [CrossRef]
- Moon, W.G.; Kim, G.-P.; Lee, M.; Song, H.D.; Yi, J. A Biodegradable Gel Electrolyte for Use in High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3503–3511. [Google Scholar] [CrossRef]
- Lv, L.; Hui, B.; Zhang, X.; Zou, Y.; Yang, D. Lamellar agarose/graphene oxide gel polymer electrolyte network for all-solid-state supercapacitor. Chem. Eng. J. 2022, 452. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, D.; Wu, C.; Wei, G.; Wei, J.; Tai, Z.; Xi, S.; Shen, S.; Wang, Q.; Chen, Y. Diffusion-determined assembly of all-climate supercapacitors via bioinspired aligned gels. J. Mater. Chem. A 2019, 7, 19753–19760. [Google Scholar] [CrossRef]
- Park, H.; Chung, J.; Yong, H.; Jung, J.; Jung, C. Roles of gel polymer electrolytes for high-power activated carbon supercapacitors: ion reservoir and binder-like effects. RSC Adv. 2020, 10, 4690–4697. [Google Scholar] [CrossRef]
- Xu, M.; Yue, W.; Zhang, L.; Chen, K.; Li, S.; Xu, Y.; Xu, Q.; Huang, J.; Xie, H. Engineering chitosan into a recyclable and flame-resistant gel electrolyte via a dual cross-linking strategy for flexible supercapacitors. Green Chem. 2023, 26, 918–926. [Google Scholar] [CrossRef]
- Xue, X.; Wan, L.; Li, W.; Tan, X.; Du, X.; Tong, Y. A Self-Healing Gel Polymer Electrolyte, Based on a Macromolecule Cross-Linked Chitosan for Flexible Supercapacitors. Gels 2022, 9, 8. [Google Scholar] [CrossRef]
- Landi, G.; La Notte, L.; Granata, V.; Avallone, G.; Barone, C.; Carapella, G.; Pagano, S.; Palma, A.L.; Sdringola, P.; Puglisi, G. Impact of Acetate-Based Hydrogel Electrolyte on Electrical Performance and Stability of Eco-Friendly Supercapacitors. ChemElectroChem 2023, 10. [Google Scholar] [CrossRef]
- Kadir, M.F.Z. Non-Faradaic-based supercapacitor fabricated with fish skin gelatin biopolymer electrolyte. Ionics 2021, 27, 2219–2229. [Google Scholar] [CrossRef]
- Landi, G.; La Notte, L.; Palma, A.L.; Puglisi, G. Electrochemical Performance of Biopolymer-Based Hydrogel Electrolyte for Supercapacitors with Eco-Friendly Binders. Polymers 2022, 14, 4445. [Google Scholar] [CrossRef]
- Alam Zaidi, S.F.; Saeed, A.; Ho, V.-C.; Heo, J.H.; Cho, H.H.; Sarwar, N.; Lee, N.-E.; Mun, J.; Lee, J.H. Chitosan-reinforced gelatin composite hydrogel as a tough, anti-freezing, and flame-retardant gel polymer electrolyte for flexible supercapacitors. Int. J. Biol. Macromol. 2023, 234, 123725. [Google Scholar] [CrossRef]
- Chauhan, J.K.; Kumar, M.; Yadav, M.; Tiwari, T.; Srivastava, N. Effect of NaClO4 concentration on electrolytic behaviour of corn starch film for supercapacitor application. Ionics 2017, 23, 2943–2949. [Google Scholar] [CrossRef]
- Ong, A.C.W.; Shamsuri, N.A.; Zaine, S.N.A.; Panuh, D.; Shukur, M.F. Nanocomposite polymer electrolytes comprising starch-lithium acetate and titania for all-solid-state supercapacitor. Ionics 2021, 27, 853–865. [Google Scholar] [CrossRef]
- Ahuja, H.; Dhapola, P.S.; Rahul; Sahoo, N.G.; Singh, V.; Singh, P.K. Ionic liquid (1-hexyl-3-methylimidazolium iodide)-incorporated biopolymer electrolyte for efficient supercapacitor. High Perform. Polym. 2020, 32, 220–225. [Google Scholar] [CrossRef]
- Konwar, S.; Singh, D.; Strzałkowski, K.; Bin Masri, M.N.; Yahya, M.Z.A.; Diantoro, M.; Savilov, S.V.; Singh, P.K. Stable and Efficient Dye-Sensitized Solar Cells and Supercapacitors Developed Using Ionic-Liquid-Doped Biopolymer Electrolytes. Molecules 2023, 28, 5099. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Comparing Triflate and Hexafluorophosphate Anions of Ionic Liquids in Polymer Electrolytes for Supercapacitor Applications. Materials 2014, 7, 4019–4033. [Google Scholar] [CrossRef]
- Hou, M.; Xu, M.; Hu, Y.; Li, B. Nanocellulose incorporated graphene/polypyrrole film with a sandwich-like architecture for preparing flexible supercapacitor electrodes. Electrochimica Acta 2019, 313, 245–254. [Google Scholar] [CrossRef]
- Mitta, S.B.; Kim, J.; Rana, H.H.; Kokkiligadda, S.; Lim, Y.T.; Bhang, S.H.; Park, H.S.; Um, S.H. A biospecies-derived genomic DNA hybrid gel electrolyte for electrochemical energy storage. PNAS Nexus 2024, 3, pgae213. [Google Scholar] [CrossRef]
- Pal, B.; Yasin, A.; Kunwar, R.; Yang, S.; Yusoff, M.M.; Jose, R. Polymer versus Cation of Gel Polymer Electrolytes in the Charge Storage of Asymmetric Supercapacitors. Ind. Eng. Chem. Res. 2018, 58, 654–664. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J. Capacitance performance of carbon paper supercapacitor using redox-mediated gel polymer electrolyte. J. Sol-Gel Sci. Technol. 2018, 86, 760–772. [Google Scholar] [CrossRef]
- Geng, C.-L.; Fan, L.-Q.; Wang, C.-Y.; Wang, Y.-L.; Sun, S.-J.; Song, Z.-Y.; Liu, N.; Wu, J.-H. High energy density and high working voltage of a quasi-solid-state supercapacitor with a redox-active ionic liquid added gel polymer electrolyte. New J. Chem. 2019, 43, 18935–18942. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Tu, Q.-M.; Geng, C.-L.; Huang, J.-L.; Gu, Y.; Lin, J.-M.; Huang, Y.-F.; Wu, J.-H. High energy density and low self-discharge of a quasi-solid-state supercapacitor with carbon nanotubes incorporated redox-active ionic liquid-based gel polymer electrolyte. Electrochimica Acta 2019, 331, 135425. [Google Scholar] [CrossRef]
- Hor, A.A.; Yadav, N.; Hashmi, S.A. High-Energy-Density 3. 5 V Carbon Supercapacitor Fabricated with Ionic-Li¬quid¬Incorporated Redox-Active Gel Polymer Electrolyte. ACS Appl. Energy Mater. 2022, 5, 7627–7641. [Google Scholar]
- Patla, S.K.; Pal, P.; Ghosh, A. Enhancement of Specific Capacitance in Carbon Supercapacitors Using Dual Redox Additive-Based Gel Polymer Electrolytes. ACS Appl. Energy Mater. 2023, 6, 8989–9000. [Google Scholar] [CrossRef]
- Gan, F.; Xu, Y.; Yang, H.; Ren, Z.; Du, H. PEDOT solid-state polymer supercapacitor assembled with a KI-doped gel polymer electrolyte. J. Appl. Polym. Sci. 2019, 137. [Google Scholar] [CrossRef]
- Kanika; Gairola, Y.; Tomar, R.; Kumar, S.; Konwar, S.; Savilov, S.V.; Yadav, T.; Yahya, M.Z.A.; Singh, P.K. Supercapacitor using polyvinyl alcohol doped ionic liquid 1-butyl-1-methylpyrrolidinium hexafluorophosphate polymer electrolyte system. Energy Storage 2024, 6, e569. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Gao, H.; Liu, Q.; Zhu, J.; Wang, F.; Jiang, M. Constructing High-Energy-Density Aqueous Supercapacitors with Potassium Iodide-Doped Electrolytes by a Precharging Method. ACS Appl. Energy Mater. 2020, 3, 2674–2681. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, Y.; Mansour, A.N.; Zhou, X. Experimental Studies of Mediator-Enhanced Polymer Electrolyte Supercapacitors. Electrochem. Solid-state Lett. 2011, 14, A25–A28. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, J.; Cai, S.; Zhou, X.; Mansour, A.N. Development of all-solid-state mediator-enhanced supercapacitors with polyvinylidene fluoride/lithium trifluoromethanesulfonate separators. J. Power Sources 2011, 196, 10479–10483. [Google Scholar] [CrossRef]
- Tu, Q.-M.; Fan, L.-Q.; Pan, F.; Huang, J.-L.; Gu, Y.; Lin, J.-M.; Huang, M.-L.; Huang, Y.-F.; Wu, J.-H. Design of a novel redox-active gel polymer electrolyte with a dual-role ionic liquid for flexible supercapacitors. Electrochimica Acta 2018, 268, 562–568. [Google Scholar] [CrossRef]
- Nandhinilakshmi, M.; Vanitha, D.; Nallamuthu, N.; Sundaramahalingam, K.; Saranya, P.; A, S. Fabrication of high-performance symmetrical supercapacitor using lithium iodide-based biopolymer electrolyte. Ionics 2023, 30, 1031–1049. [Google Scholar] [CrossRef]
- Nandhinilakshmi, M.; Vanitha, D.; Nallamuthu, N.; Sundaramahalingam, K.; Saranya, P.; Samad, S.A. High Performance Lithium Ion-Conducting Plasticized Biopolymer Electrolyte for Supercapacitor Application. J. Polym. Environ. 2024, 32, 5157–5178. [Google Scholar] [CrossRef]
- Tang, X.; Lui, Y.H.; Merhi, A.R.; Chen, B.; Ding, S.; Zhang, B.; Hu, S. Redox-Active Hydrogel Polymer Electrolytes with Different pH Values for Enhancing the Energy Density of the Hybrid Solid-State Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 44429–44440. [Google Scholar] [CrossRef]
- Zhong, J.; Fan, L.-Q.; Wu, X.; Wu, J.-H.; Liu, G.-J.; Lin, J.-M.; Huang, M.-L.; Wei, Y.-L. Improved energy density of quasi-solid-state supercapacitors using sandwich-type redox-active gel polymer electrolytes. Electrochimica Acta 2015, 166, 150–156. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Zhong, J.; Zhang, C.-Y.; Wu, J.-H.; Wei, Y.-L. Improving the energy density of quasi-solid-state supercapacitors by assembling two redox-active gel electrolytes. Int. J. Hydrogen Energy 2016, 41, 5725–5732. [Google Scholar] [CrossRef]
- Karuppiah, A.; Natarajan, A.; Angamuthu, G.; Rengarajan, V. Cobalt complex-based redox mediator-assisted gel polymer electrolyte (PVA-H2SO4-[Co(en)3]Cl3) for high-performance supercapacitor. Ionics 2022, 28, 4779–4792. [Google Scholar] [CrossRef]
- Cevik, E.; Bozkurt, A. Redox active polymer metal chelates for use in flexible symmetrical supercapacitors: Cobalt-containing poly(acrylic acid) polymer electrolytes. J. Energy Chem. 2020, 55, 145–153. [Google Scholar] [CrossRef]
- Lee, S.; An, G.-H. Reversible faradaic reactions involving redox mediators and oxygen-containing groups on carbon fiber electrode for high-performance flexible fibrous supercapacitors. J. Energy Chem. 2022, 68, 1–11. [Google Scholar] [CrossRef]
- Sinha, P.; Yadav, A.; Tyagi, A.; Paik, P.; Yokoi, H.; Naskar, A.K.; Kuila, T.; Kar, K.K. Keratin-derived functional carbon with superior charge storage and transport for high-performance supercapacitors. Carbon 2020, 168, 419–438. [Google Scholar] [CrossRef]
- Qiao, X.; Yan, Z.; Zhang, C.; Wang, Y.; Akin, M.; Zhou, X.; Mansour, A.N.; Ko, J.K.; Waller, G.H.; Martin, C.A.; et al. Electrochemical and In Situ Spectroscopic Study of the Effect of Prussian Blue as a Mediator in a Solid-State Supercapacitor. J. Electrochem. Soc. 2021, 168, 106505. [Google Scholar] [CrossRef]
- Qin, G.; Wu, C.; Song, X.; He, W.; Yang, J.; Yu, X.; Chen, Q. Multifunctional enhanced energy density integrated supercapacitor based on self-healing redox-mediated gel polymer electrolyte. Fuel 2023, 357. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Y.; Zhang, W.; He, W.; Su, X.; Lv, Q.; Yu, X.; Chen, Q.; Yang, J. Integrated supercapacitor with self-healing, arbitrary deformability and anti-freezing based on gradient interface structure from electrode to electrolyte. J. Colloid Interface Sci. 2022, 635, 427–440. [Google Scholar] [CrossRef]
- Vuong, T.T.T.; Phan, H.T.; Vu Thi Thu, N.; Nguyen, P.L.; Nguyen, H.T.; Le, H.V.; Nguyen, N.T.; Phung, T.V.B.; Le, P.A. Friendly Environmental Strategies to Recycle Zinc-Carbon Batteries for Excellent Gel Polymer Electrolyte (PVA-ZnSO4-H2SO4) and Carbon Materials for Symmetrical Solid-State Supercapacitors. ACS Omega 2024, 9, 27710–27721. [Google Scholar] [CrossRef]
- Dutta, A.; Nayak, R.; Selvakumar, M.; Devadiga, D.; Selvaraj, P.; Kumar, S.S. Graphite/copper nanoparticle-based high-performance micro supercapacitor with porous wet paper-based PVA-PVP blend polymer electrolyte. Mater. Lett. 2021, 295, 129849. [Google Scholar] [CrossRef]
- Ma, J.; Xie, Y. Electrochemical performance of the homologous molybdenum(vi) redox-active gel polymer electrolyte system. New J. Chem. 2020, 45, 3418–3431. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y. Electrochemical performance of carbon paper supercapacitor using sodium molybdate gel polymer electrolyte and nickel molybdate electrode. J. Solid State Electrochem. 2019, 23, 1911–1927. [Google Scholar] [CrossRef]
- Cevik, E.; Bozkurt, A.; Hassan, M.; Gondal, M.A.; Qahtan, T.F. Redox-Mediated Poly(2-acrylamido-2-methyl-1-propanesulfonic acid)/Ammonium Molybdate Hydrogels for Highly Effective Flexible Supercapacitors. ChemElectroChem 2019, 6, 2876–2882. [Google Scholar] [CrossRef]
- Cevik, E.; Bozkurt, A.; Dirican, M.; Zhang, X. High performance flexible supercapacitors including redox active molybdate incorporated Poly(vinylphosphonic acid) hydrogels. Int. J. Hydrogen Energy 2020, 45, 2186–2194. [Google Scholar] [CrossRef]
- Nandi, P.; Subramaniam, C. Rationalizing the Role of Polyoxometalate-Based Gel-Polymer Electrolytes to Achieve Five-Fold Increase in the Specific Capacitance of Hard-Carbon-Based Supercapacitors. Adv. Energy Sustainability Res. 2024, 2024, 2300281. [Google Scholar] [CrossRef]
- Mantravadi, R.; Chinnam, P.R.; Dikin, D.A.; Wunder, S.L. High Conductivity, High Strength Solid Electrolytes Formed by in Situ Encapsulation of Ionic Liquids in Nanofibrillar Methyl Cellulose Networks. ACS Appl. Mater. Interfaces 2016, 8, 13426–13436. [Google Scholar] [CrossRef]
- Singh, K.; Kaur, A. Methyl-orange/reduced graphene oxide composite as the electrode material for the solid-state supercapacitor. Int. J. Chem. React. Eng. 2023, 22, 59–67. [Google Scholar] [CrossRef]
- Le, P.-A.; Nguyen, V.-T.; Yen, P.-J.; Tseng, T.-Y.; Wei, K.-H. A new redox phloroglucinol additive incorporated gel polymer electrolyte for flexible symmetrical solid-state supercapacitors. Sustain. Energy Fuels 2019, 3, 1536–1544. [Google Scholar] [CrossRef]
- Jinisha, B.; Anilkumar, K.M.; Manoj, M.; Ashraf, C.M.; Pradeep, V.S.; Jayalekshmi, S. Solid-state supercapacitor with impressive performance characteristics, assembled using redox-mediated gel polymer electrolyte. J. Solid State Electrochem. 2019, 23, 3343–3353. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Hashmi, S.A. Ionic liquid incorporated, redox-active blend polymer electrolyte for high energy density quasi-solid-state carbon supercapacitor. J. Power Sources 2020, 451, 227771. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Fan, L.; Lin, Y.; Xu, K.; Tang, Z.; Cheng, C.; Tang, S.; Lin, J.; Huang, M.; et al. A novel redox-mediated gel polymer electrolyte for high-performance supercapacitor. J. Power Sources 2012, 198, 402–407. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Hashmi, S.A. High-Energy-Density Carbon Supercapacitors Incorporating a Plastic-Crystal-Based Nonaqueous Redox-Active Gel Polymer Electrolyte. ACS Appl. Energy Mater. 2021, 4, 6635–6649. [Google Scholar] [CrossRef]
- Potphode, D.D.; Sinha, L.; Shirage, P.M. Redox additive enhanced capacitance: Multi-walled carbon nanotubes/polyaniline nanocomposite based symmetric supercapacitors for rapid charge storage. Appl. Surf. Sci. 2018, 469, 162–172. [Google Scholar] [CrossRef]
- Thongsai, N.; Jirawanichakun, N.; Jorn-Am, T.; Supchocksoonthorn, P.; Paoprasert, P. Hydroquinone-mediated, bio-renewable corn starch electrolyte assembled with corn leaf-derived activated carbon for a high-performance, sustainable supercapacitor. Biomass- Bioenergy 2024, 182. [Google Scholar] [CrossRef]
- Hashmi, S.; Suematsu, S.; Naoi, K. All solid-state redox supercapacitors based on supramolecular 1,5-diaminoanthraquinone oligomeric electrode and polymeric electrolytes. J. Power Sources 2004, 137, 145–151. [Google Scholar] [CrossRef]
- Sun, K.; Dong, M.; Feng, E.; Peng, H.; Ma, G.; Zhao, G.; Lei, Z. High performance solid state supercapacitor based on a 2-mercaptopyridine redox-mediated gel polymer. RSC Adv. 2015, 5, 22419–22425. [Google Scholar] [CrossRef]
- Hassan, N.; Holze, R. Surface enhanced Raman spectroscopy of self-assembled monolayers of 2-mercaptopyridine on a gold electrode. Russ. J. Electrochem. 2012, 48, 401–411. [Google Scholar] [CrossRef]
- Hassan, N.; Holze, R. A comparative electrochemical study of electrosorbed 2- and 4-mercaptopyridines and their application as corrosion inhibitors at C60 steel. J. Chem. Sci. 2009, 121, 693–701. [Google Scholar] [CrossRef]
- Naya, S.-I.; Teranishi, M.; Isobe, T.; Tada, H. Light wavelength-switchable photocatalytic reaction by gold nanoparticle-loaded titanium(iv) dioxide. Chem. Commun. 2010, 46, 815–817. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. Mesoporous silica functionalized with 2-mercaptopyridine: Synthesis, characterization and employment for Hg(II) adsorption. Microporous Mesoporous Mater. 2005, 89, 58–68. [Google Scholar] [CrossRef]
- Kuwamura, N.; Kitano, K.; Hirotsu, M.; Nishioka, T.; Teki, Y.; Santo, R.; Ichimura, A.; Hashimoto, H.; Wright, L.J.; Kinoshita, I. Redox-controlled, reversible rearrangement of a tris(2-pyridylthio)methyl ligand on nickel to an isomer with an "N,S-confused" 2-pyridylthiolate arm. Chem. Eur. J. 2011, 17, 10708–10715. [Google Scholar] [CrossRef]
- Yu, F.D.; Huang, M.L.; Qiu, Z.Y.; Li, Z.L.; Lin, Y.B.; Fan, L.Q.; Wu, J.H. A novel redox-active gel polymer electrolyte for supercapacitors. J. Funct. Mater. 2013, 44, 2791–2795. [Google Scholar]
- Ma, G.; Dong, M.; Sun, K.; Feng, E.; Peng, H.; Lei, Z. A redox mediator doped gel polymer as an electrolyte and separator for a high performance solid state supercapacitor. J. Mater. Chem. A 2015, 3, 4035–4041. [Google Scholar] [CrossRef]
- Sun, K.; Ran, F.; Zhao, G.; Zhu, Y.; Zheng, Y.; Ma, M.; Zheng, X.; Ma, G.; Lei, Z. High energy density of quasi-solid-state supercapacitor based on redox-mediated gel polymer electrolyte. RSC Adv. 2016, 6, 55225–55232. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, T.; Yan, W.; Huang, D.; Zhang, Y.; Wan, Q.; Yang, N. A high-performance flexible supercapacitor using dual alkaline redox electrolytes. Carbon 2022, 188, 315–324. [Google Scholar] [CrossRef]
- Ma, G.; Feng, E.; Sun, K.; Peng, H.; Li, J.; Lei, Z. A novel and high-effective redox-mediated gel polymer electrolyte for supercapacitor. Electrochimica Acta 2014, 135, 461–466. [Google Scholar] [CrossRef]
- Yu, F.; Huang, M.; Wu, J.; Qiu, Z.; Fan, L.; Lin, J.; Lin, Y. A redox-mediator-doped gel polymer electrolyte applied in quasi-solid-state supercapacitors. J.Appl.Polym.Sci. 2014, 131, 39784. [Google Scholar] [CrossRef]
- Xun, Z.; Liu, Y.; Gu, J.; Liu, L.; Huo, P. A Biomass-Based Redox Gel Polymer Electrolyte for Improving Energy Density of Flexible Supercapacitor. J. Electrochem. Soc. 2019, 166, A2300–A2312. [Google Scholar] [CrossRef]
- Yadav, N.; Hashmi, S.A. Energy enhancement of quasi-solid-state supercapacitors based on a non-aqueous gel polymer electrolyte via a synergistic effect of dual redox additives diphenylamine and potassium iodide. J. Mater. Chem. A 2020, 8, 18266–18279. [Google Scholar] [CrossRef]
- Gunday, S.T.; Qahtan, T.; Cevik, E.; Anil, I.; Alagha, O. ; Bozkurt; A. Highly Flexible and Tailorable Cobalt-Doped Cross-Linked Polyacrylamide-Based Electrolytes for Use in High-Performance Supercapacitors. Chem. Asian J. 2021, 16, 1438–1444. [Google Scholar]
- Yadav, N.; Yadav, N.; Singh, M.K.; Hashmi, S.A. Nonaqueous, Redox-Active Gel Polymer Electrolyte for High-Performance Supercapacitor. Energy Technol. 2019, 7, 1900132. [Google Scholar] [CrossRef]
- Cevik, E.; Bozkurt, A. Design of high-performance flexible symmetric supercapacitors energized by redox-mediated hydrogels including metal-doped acidic polyelectrolyte. Int. J. Energy Res. 2020, 44, 4309–4320. [Google Scholar] [CrossRef]
- Hwang, M.; Jeong, J.S.; Lee, J.-C.; Yu, S.; Jung, H.S.; Cho, B.-S.; Kim, K.-Y. Composite solid polymer electrolyte with silica filler for structural supercapacitor applications. Korean J. Chem. Eng. 2021, 38, 454–460. [Google Scholar] [CrossRef]
- Gienger, E.B.; Snyder, J.F.; Wetzel, E.D.; Xu, K. Multifunctional structural composite supercapacitor development and evaluation. International SAMPE Technical Conference 2012.
- Zhou, H.; Li, H.; Li, L.; Liu, T.; Chen, G.; Zhu, Y.; Zhou, L.; Huang, H. Structural composite energy storage devices d a review. Mater.Today Energy 2022, 24, 100924. [Google Scholar] [CrossRef]
- Javaid, A.; Ho, K.; Bismarck, A.; Steinke, J.; Shaffer, M.; Greenhalgh, E. Carbon fibre-reinforced poly(ethylene glycol) diglycidylether based multifunctional structural supercapacitor composites for electrical energy storage applications. J. Compos. Mater. 2015, 50, 2155–2163. [Google Scholar] [CrossRef]
- Kazari, H.; Farkhondehnia, M.; Maric, M.; Hubert, P.; Kazari, H.; Farkhondehnia, M.; Maric, M.; Hubert, P. Recyclable multiscale composite structural supercapacitor. Polym. Compos. 2024, 45, 12016–12032. [Google Scholar] [CrossRef]
- Yao, L.; Zheng, K.; Koripally, N.; Eedugurala, N.; Azoulay, J.D.; Zhang, X.; Ng, T.N. Structural pseudocapacitors with reinforced interfaces to increase multifunctional efficiency. Sci. Adv. 2023, 9, eadh0069. [Google Scholar] [CrossRef]
- Javaid, A.; Zafrullah, M.B.; Khan, F.U.H.; Bhatti, G.M. Improving the multifunctionality of structural supercapacitors by interleaving graphene nanoplatelets between carbon fibers and solid polymer electrolyte. J. Compos. Mater. 2018, 53, 1401–1409. [Google Scholar] [CrossRef]
- Javaid, A.; Ho, K.; Bismarck, A.; Steinke, J.; Shaffer, M.; Greenhalgh, E. Improving the multifunctional behaviour of structural supercapacitors by incorporating chemically activated carbon fibres and mesoporous silica particles as reinforcement. J. Compos. Mater. 2018, 52, 3085–3097. [Google Scholar] [CrossRef]
- Hudak, N.S.; Schlichting, A.D.; Eisenbeiser, K. Structural Supercapacitors with Enhanced Performance Using Carbon Nanotubes and Polyaniline. J. Electrochem. Soc. 2017, 164, A691–A700. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wu, C.-Y.; Hsueh, Y.-T.; Huang, H.-H. Electromechanical properties of embedded multifunctional energy storage composite with activated carbon fiber/PVDF gel electrolyte. J. Chin. Inst. Eng. 2021, 44, 252–260. [Google Scholar] [CrossRef]
- Senokos, E.; Ou, Y.; Torres, J.J.; Sket, F.; González, C.; Marcilla, R.; Vilatela, J.J. Energy storage in structural composites by introducing CNT fiber/polymer electrolyte interleaves. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Javaid, A.J.A.; Shahzaib, A.S.A.; Tahir, H.T.H.; Ali, M.A.M.; Younus, W.Y.A.W. Investigation of Mechanical and Electrochemical Performance of Multifunctional Carbon-Fiber Reinforced Polymer Composites for Electrical Energy Storage Applications. J. Chem. Soc. Pak. 2019, 41, 444–444. [Google Scholar] [CrossRef]
- Javaid, A.; Ho, K.; Bismarck, A.; Shaffer, M.; Steinke, J.; Greenhalgh, E. Multifunctional structural supercapacitors for electrical energy storage applications. J. Compos. Mater. 2013, 48, 1409–1416. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, S.; Yan, Y.; Wang, L.; Xu, G.; Yarlagadda, S.; Chou, T.-W. High-Performance Structural Supercapacitors Based on Aligned Discontinuous Carbon Fiber Electrodes and Solid Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 11774–11782. [Google Scholar] [CrossRef]
- Snyder, J.; Gienger, E.; Wetzel, E. Performance metrics for structural composites with electrochemical multifunctionality. J. Compos. Mater. 2015, 49, 1835–1848. [Google Scholar] [CrossRef]
- Demir, B.; Chan, K.-Y.; Searles, D.J. Structural Electrolytes Based on Epoxy Resins and Ionic Liquids: A Molecular-Level Investigation. Macromolecules 2020, 53, 7635–7649. [Google Scholar] [CrossRef]
- Shirshova, N.; Qian, H.; Shaffer, M.S.; Steinke, J.H.; Greenhalgh, E.S.; Curtis, P.T.; Kucernak, A.; Bismarck, A. Structural composite supercapacitors. Compos. Part A: Appl. Sci. Manuf. 2012, 46, 96–107. [Google Scholar] [CrossRef]
- Gienger, E.B.; Snyder, J.F.; Wetzel, E.D. Structural Composite Supercapacitors: Electrical and Mechanical Impact of Separators and Processing ConditionsArmy Research Laboratory, Aberdeen Proving Ground 2013.
- Snyder, J.F.; Gienger, E.; Wetzel, E.D.; Xu, K. Energy density and rate limitations in structural composite supercapacitors. Proceedings of SPIE - The International Society for Optical Engineering 2012, 8377, 837709. [Google Scholar]
- Gallagher, T.M.; Ciocanel, C.; Browder, C. Structural load bearing supercapacitors using a pegdge based solid polymer electro¬lyte matrix. ASME 2011 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, SMASIS 2011 2011, 1, 141-148.
- Artigas-Arnaudas, J.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Effect of electrode surface treatment on carbon fiber based structural supercapacitors: Electrochemical analysis, mechanical performance and proof-of-concept. J. Energy Storage 2023, 59. [Google Scholar] [CrossRef]
- Joyal, N.; Chang, Y.-C.; Shonar, M.; Chalivendra, V.; Shen, C. Solid polymer electrolytes with hydrates for structural supercapacitors. J. Energy Storage 2022, 51. [Google Scholar] [CrossRef]
- Sánchez-Romate, X.F.; Del Bosque, A.; Artigas-Arnaudas, J.; Muñoz, B.K.; Sánchez, M.; Ureña, A. A proof of concept of a structural supercapacitor made of graphene coated woven carbon fibers: EIS study and mechanical performance. Electrochimica Acta 2021, 370. [Google Scholar] [CrossRef]
- Bae, S.-H.; Jeon, C.; Oh, S.; Kim, C.-G.; Seo, M.; Oh, I.-K. Load-bearing supercapacitor based on bicontinuous PEO-b-P(S-co-DVB) structural electrolyte integrated with conductive nanowire-carbon fiber electrodes. Carbon 2018, 139, 10–20. [Google Scholar] [CrossRef]
- Cho, B.-S.; Choi, J.; Kim, K.-Y. Preparation and properties of solid polymer electrolyte based on imidazolium-based ionic liquids for structural capacitors. Fibers Polym. 2017, 18, 1452–1458. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Park, Y.-B.; Park, H.W. Recent development and challenges of multifunctional structural supercapacitors for automotive industries. Int. J. Energy Res. 2017, 41, 1397–1411. [Google Scholar] [CrossRef]
- Qian, H.; Kucernak, A.R.; Greenhalgh, E.S.; Bismarck, A.; Shaffer, M.S.P. Multifunctional Structural Supercapacitor Composites Based on Carbon Aerogel Modified High Performance Carbon Fiber Fabric. ACS Appl. Mater. Interfaces 2013, 5, 6113–6122. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Zhang, C.; Zhou, X. Development of structural supercapacitors with epoxy based adhesive polymer electrolyte. J. Energy Storage 2019, 26, 100968. [Google Scholar] [CrossRef]
- Lingappan, N.; Lim, S.; Lee, G.-H.; Tung, H.T.; Luan, V.H.; Lee, W. Recent advances on fiber-reinforced multifunctional composites for structural supercapacitors. Funct. Compos. Struct. 2022, 4, 012001. [Google Scholar] [CrossRef]
- Javaid, A.; Khalid, O.; Shakeel, A.; Noreen, S. Multifunctional structural supercapacitors based on polyaniline deposited carbon fiber reinforced epoxy composites. J. Energy Storage 2020, 33, 102168. [Google Scholar] [CrossRef]
- Qian, H.; Kucernak, A.R.; Greenhalgh, E.S.; Bismarck, A.; Shaffer, M.S.P. Multifunctional Structural Supercapacitor Composites Based on Carbon Aerogel Modified High Performance Carbon Fiber Fabric. ACS Appl. Mater. Interfaces 2013, 5, 6113–6122. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, K.-H.; Lee, S.-S.; Ahn, D.B.; Chun, J.; Kang, S.H.; Roh, K.C.; Lee, S.-Y. On-demand solid-state artistic ultrahigh areal energy density microsupercapacitors. Energy Storage Mater. 2022, 47, 569–578. [Google Scholar] [CrossRef]
- Flexible Supercapacitors (G. Shen, Z. Flexible Supercapacitors (G. Shen, Z.Lou, D. Chen, Eds.) WILEY, Hoboken, USA, 2022.
- Flexible and Wearable Supercapacitors: Constitutive Aspects and Future Perspectives. Encyc.Energy Stor.Vol.1-4 2022, 1-4, 351-360.
- Gopalakrishnan, A,; Badhulika, S. Flexible supercapacitors based on 2D materials. in: Fundamentals and Supercapacitor Applications of 2D Materials (C.S. Rout, D.J. Late Eds.) Elsevier, Amsterdam 2021, p. 253-310.
- Lewis, T.W.; Kim, B.C.; Spinks, G.M.; Wallace, G.G. Evaluation of solid polymer electrolytes for use in conducting polymer/nanotube actuators. Proc. SPIE 2000, 3987, 351–357. [Google Scholar]
- Kim, H.; Kong, S.M.; A Kim, J.; Yoon, G.; Na, Y.H.; Kim, S.K. Polyampholyte saturated with simulated-body-fluid as a flexible, stretchable and self-healing gel electrolyte for biocompatible energy storages and capacitive circuit elements. Chem. Eng. J. 2023, 477. [Google Scholar] [CrossRef]
- Barzegar, F.; Bello, A.; Dangbegnon, J.; Manyala, N.; Xia, X. Asymmetric Carbon Supercapacitor with Activated Expanded Graphite as Cathode and Pinecone Tree Activated Carbon as Anode Materials. Energy Procedia 2017, 105, 4098–4103. [Google Scholar] [CrossRef]
- Barzegar, F.; Dangbegnon, J.K.; Bello, A.; Momodu, D.Y.; Johnson Jr., A.T.C; Manyala, N. Effect of conductive additives to gel electrolytes on activated carbon-based supercapacitors. AIP Adv. 2015, 5, 097171.
- Wang, C.; Wang, F.; Liu, Z.; Zhao, Y.; Liu, Y.; Yue, Q.; Zhu, H.; Deng, Y.; Wu, Y.; Zhao, D. N-doped carbon hollow microspheres for metal-free quasi-solid-state full sodium-ion capacitors. Nano Energy 2017, 41, 674–680. [Google Scholar] [CrossRef]
- Dai, J.; Fu, K.; Palanisamy, R.; Gong, A.; Pastel, G.; Kornfeld, R.; Zhu, H.; Sanghadasa, M.; Bekyarova, E.; Hu, L. A solid state energy storage device with supercapacitor–battery hybrid design. J. Mater. Chem. A 2017, 5, 15266–15272. [Google Scholar] [CrossRef]
- Na, R.; Lu, N.; Zhang, S.; Huo, G.; Yang, Y.; Zhang, C.; Mu, Y.; Luo, Y.; Wang, G. Facile synthesis of a high-performance, fire-re¬tardant organic gel polymer electrolyte for flexible solid-state supercapacitors. Electrochim. Acta 2018, 290, 262–272. [Google Scholar] [CrossRef]
- Negre, L.; Daffos, B.; Turq, V.; Taberna, P.; Simon, P. Ionogel-based solid-state supercapacitor operating over a wide range of temperature. Electrochimica Acta 2016, 206, 490–495. [Google Scholar] [CrossRef]
- Huang, X.; Wu, W. Novel preparation of attapulgite-reduced graphene oxide hydrogel composite and its application in flexible solid-state supercapacitors. Nanotechnology 2022, 33, 205704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




