Background
The first histogenetic concepts favored dural origin of meningioma which was based on its dural attachment, Bright in 1831 noticed the histological similarities between meningioma cells and arachnoid villi cells [
1]. In 1864 Cleland, proposed that meningioma is derived from arachnoid cells, this was further confirmed by Yamashima et al, whom found similarities in ultrastructure cell adhesion mechanisms and extracellular matrix components in 1996.
Although meningioma is thought to arise from arachnoidal cap cells (meningiothelial) which arises embryologically from neural crest and mesoderm rather than neural ectoderm, however, the histogenesis has not been completely resolved, and the meningothelial phenotype has both ectodermal characteristics, frequently expressing epithelial membrane antigen (EMA)and forming desmosomes, and mesenchymal characteristics capable of synthesizing collagen [
2]. Taking tumor constitution, tumor are made of tumor cells and tumor microenvironment and this microenvironment which contains cancerous stem cell, is called cellular environment, it is essential for tumor survival. In addition, there is a complex interaction between CSCs and their microenvironment which further regulate CSC growth. These cellular environments include the extra cellular matrix, mesenchymal stem cells, endothelial cells and signal molecules like growth factors and cytokines. Moreover, there is special microenvironment that surrounding the cancerous stem cell which is called niches, this niche controls and governs the fate of the cancerous stem cell [
3].Nevertheless, Cancerous stem cell (CSC) is capable of initiating tumor growth and metastasis. This cell (CSC) which is a small subpopulation of cells that form cancer cells in an unregulated manner and resulting in carcinogenesis, recently it is known to drive the formation, development and progression of tumor [
4]. On the other hand, , Stem cell which has been discovered currently by scientists, has capabilities to self-renew, grow indefinitely, and differentiate or develop into multiple types of cells and tissues [
5].
Along with that, many researches were held and have been shown different types of stem cells exist but they all are found in very small populations in the human body, one stem cell may be found in 100,000 cells in circulating) blood, and the number of mesenchymal stem cell is approximately I in 10
8 peripheral blood mononuclear cell [
6].
Furthermore, they have different types of receptors that differ in their structure and affinity for the signaling molecules, physiologically the cell use these receptor and signal molecule which bind to them as mood of communications with other cell and thus do its normal function in the body [
7].And by taking the biological properties of these receptors, the so called stem cell marker emerges. These markers are known to be genes and their protein products used by scientists to isolate and identify certain stem cell. [
8]Nevertheless, stem cells can also be identified by technology of functional assays which are considered the gold standard for the identification and therapeutic purposes in the future.
Many experiments are conducted to detect a single surface marker expressed equally by all mesenchymal stem cell, however, there is no unique marker found in all stem cells [
9].
And Currently, Arbab et al. reported isolation of tumor stem-like cells from human meningiom among Sudanese patients ( published data)
Objectives
1/ To describe the RNA (cDNA) of CD 44, CD 73 and CD 105 genes as stem cell markers in meningioma among Sudanese patients
2/ To isolate cancerous like-stem cell from meningioma tissue samples and implant the isolated cancerous stem cells into albino-western rat.
Material and Methods
This is a prospective cross-sectional experimental study done at the National Center of Neurological Sciences (NCNS) Khartoum, from 2019 to 2021. The study included 23 cerebral tumors that were radio- logically diagnosed preoperatively as meningioma. Ethical approval was taken from Institute of Endemic disease, Khartoum University (certificate number 53/2018), and written consent was obtained from each patient.
The tumor specimens were obtained immediately from the operating room, before surgery was completed, and sent to the research laboratory in the center within 2-5 minutes. At arrival to the laboratory, small portions of each tumor were selected for RNA extraction. Eight tissue samples were processed for stem cell isolation.
Method
1/ RNA and PCR
RNA isolation was done Using Innu PREP RNA – MiniKit (Analytic Jenam cDNA was done By using Maxime – RT PreMix Kit (intron biotechnology)) and eventually PCR was done using Maxime PCR premix Kit ( i-taq) entro-biotechnology, 2µLof cDNAwas added to 1 µl of ( CD44, CD73, CD105, and β-actine house keeper gene, forward and reversed )
Stem Cell Isolation and Animal Experiment
Stem cell was isolated from 8 tumors using the National center of neurological sciences protocol for isolation of stem cell from adipose tissue.
When the isolated cells confluence reached >80% in the second and third passage, the immunophyenotype of isolated stem cells for CD13, CD45 and HLA-DR was performed using (PE, PECY5 and FITC) flurochromes and then the reaction events were identified using flow-cytometery.
The tumori-genicity of the isolated stem cells was tested using 8 albinos Wister rats which were obtained from the Experimental Animals Center, Faculty of Veterinary Medicine, University of Khartoum, Sudan. These rats were nominated for intracranial application of stem cells on the frontal lobe through burr-holes that done under general anesthesia. To assess the tumori-genicity of isolated cells (MSLC), the brain rats were injected with 6X106 candidates mesenchymal like stem cells (MSLC) that obtained from our cell line, using insulin syringe. The rats were observed over three months. The experimented rats were then sacrificed and its brains were carefully removed and stored in 4% formaldehyde for histopathological verification. (H&E and immune-staining).
Results
All tumors (23) that were excised in the operation room were verified histologically as meningioma. As shown in the table below.
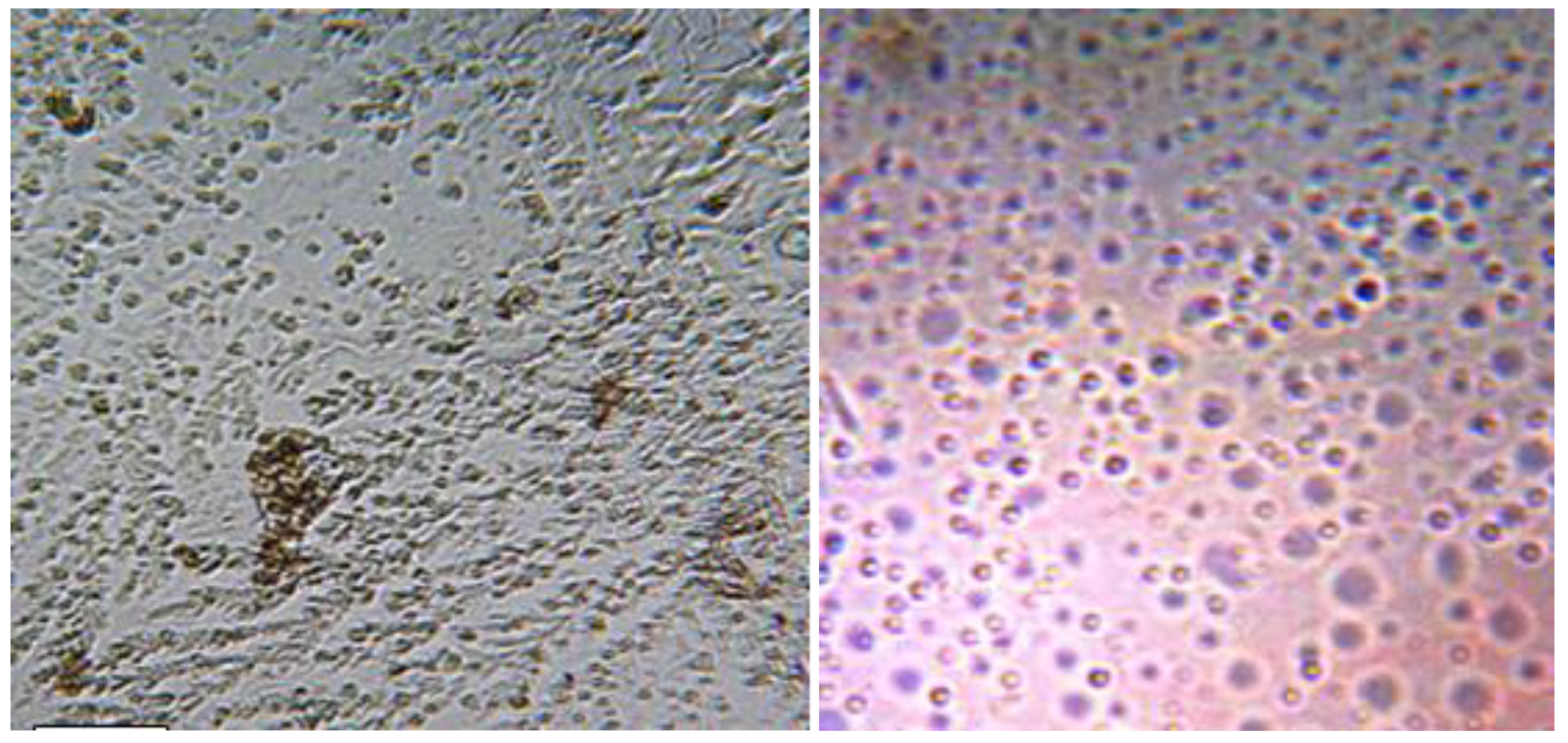
All the 8 tumors that were excised in the operation room and were verified histologically as meningioma,( 4 were fibrous,2 fibrous recurrence, one meningiothelial, one atypical) their photos of culture plates displayed >80% growing cells and expressed the main features of mesenchymallike-stem cells (MLSCs) which included plastic adherent, shape morphology was shown in the figure below. The findings of flow cytometery, provided through immunophenotyping, were negative for CD13, CD45 and HLA-DR.
The H&E microscopic examination of the rat brain slices that were subjected to MSCs application revealed numerous spindle shaped cells, neutrophilia, slight lymphocytosis and RBCs suggestive of meningioma. Positive immune-reactivity of EMA and , PR
Likewise, All sample expressed CD44, but CD73 and CD 105 expression was variable, not on the different subtype, but within the same type.
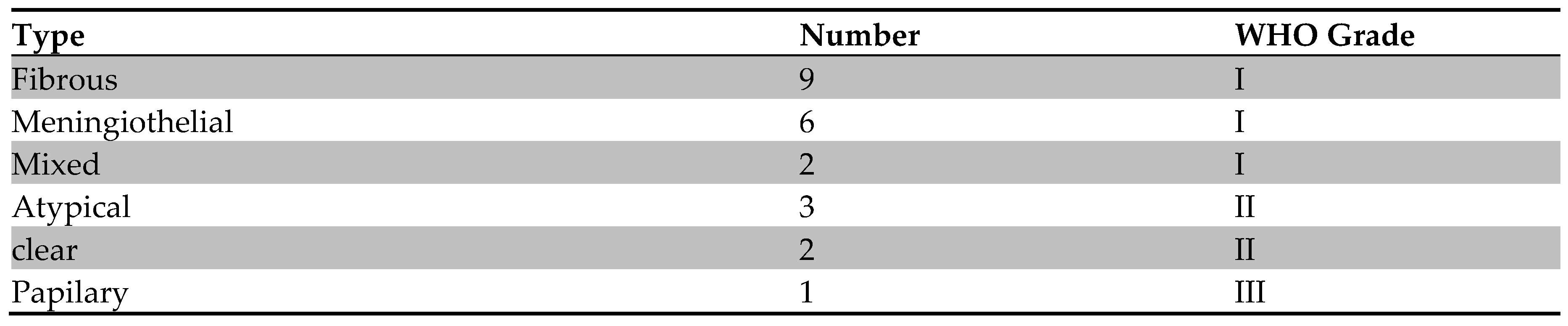
Table| shows the frequency of meningioma subtypes and WHO grade
Figure | photo slides of cultured cells expressing the main features of mesenchymal stem cells.
Discussion
Cancers stem cell (CSC) concept is supported by an increasing number of studies demonstrating the presence of subpopulations of CSCs expressing stem cell markers. Expression of such markers using PCR supported identification of such cell types to be cancerous stem cell. However, the function of these markers in the context of their sub cellular localization is an unexplored area of research. Identification of cancerous stem cell remains vague, and cancerous stem cell distribution, heterogeneity, and relationship with tumor grade, remain uncertain. Many recent studies have addressed heterogeneity of glioma brain tumor using challenging approaches [
10]. Though, very few studies were documented for meningioma heterogeneity, one of these studies showed hetero-regional expression in all meningioma grades,[
11] and concluded that,this heterogeneity might be explained by means of cancerous stem cell hypothesis, where CSCs acquire new changes in the early development of disease and maintain new changes with disease progression.
Meningioma represents unique model for exploring tumor progression among other central nervous system neoplasm, as they include tumors grades with a variety of aggressiveness and grades. Furthermore, most meningioma is benign in histology, however, they have tendency to be aggressive in behavior and difficult to treat, especially those which arise in eloquent areas of the brain and offer challenges for surgical resection. In the present study
, CD44, as stem cell markers was expressed in all tissue samples however,
CD73 and
CD105 expression was inconsistent m this variation might be explain by the different biological behavior of this tumor not only within different subtype but within the same grade [
12].
In our findings, expression of
CD44 explored new type of cells other than meningiothelial cells which is the meningioma cancerous stem cell. One of the known physiological functions of
CD44 is cellular adhesion, aggregation and migration[
13] On the other hand,
CD44 marker was used in this study, which is known as trans-membrane and extracellular glycoprotein, and was found in a wide variety of tissues including the central nervous system
Though, studies revealed that,
CD105 marker is considered as an important marker for mesenchymal stem cell, [
14] and the role of
CD 105 marker was observed in angiogenesis process, vascularization and regulation of cell migration in meningioma brain tumor.[
15] In this study,
CD73 was expressed in all our samples as well as
CD44 and
CD105. Numerous studies have established the use of
CD73 as a marker for mesenchymal stem cells and cancer [
16].
Moreover, meningioma deliberately harbors cancerous stem cell that deregulates stem cell expression profile, and may cause recurrence of the tumor [
17]. Targeting of this cell may predict definite treatment and better prognosis [
18].
In our study we tested the capacity of isolated cells to proliferate in-vivo and its tumori-genicity. Despite the copious investigations in the areas of cancerous stem cell, little is known about stem like cells existence in human meningioma and its tumori-genicity.
Conflicts of Interest
There is no conflict of interest.
References
- Peyre M, Salaud C, Clermont-Taranchon E, Niwa-Kawakita M, Goutagny S, Mawrin C, Giovannini M, Kalamarides M. PDGF activation in PGDS-positive arachnoid cells induces meningioma formation in mice promoting tumor progression in combination with Nf2 and Cdkn2ab loss. Oncotarget. 2015 Oct 20;6(32):32713.
- Perry A. (eds). Russell and Rubinstein’s Pathology of Tumors of the Nervous System. London: Hodder Arnold; 2006. pp. 427–474.
- Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells.Cell stem cell. 2015 Mar 5; 16(3):225-38.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells.nature. 2001 Nov;414(6859):105-11.
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche.Cell. 2005 Jul 29;122(2):289-301.
- Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. The Journal of cell biology. 2001 May 28;153(5):1133-40.
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007 Feb 15;109(4):1422-32.
- Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells.BMB reports. 2017 Jun;50(6):285.
- Lv, Feng-Juan, Rocky S. Tuan, Kenneth MC Cheung, and Victor YL Leung. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem cells2014; 32, no. 6 1408-1419.
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014 Jun 20;344(6190):1396-401.
- McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell.2017;168(4):613–628. [CrossRef]
- Alsadig Gassoum, M AArbab, Sawsan AH Aldeaf, Lamyaa A Elhassan and Ahmed M Elhassan.Ki 67 Antigen Immunohistochemistry in Intracranial Meningioma among Sudanese Patients.International Journal of Current Research.May 2014; Vol. 6, issue 05, pp. 6643-6646.
- Sneath RJ, Mangham DC. CD44 isoform expression in synovial sarcoma correlates with epitheliogenesis but not prognosis. Histopathology. 2000 Aug;37(2):166-74.
- Jin HJ, Park SK, Oh W, Yang YS, Kim SW, Choi SJ. Down-regulation of CD105 is associated with multi-lineage differentiation in human umbilical cord blood-derived mesenchymal stem cells. Biochemical and biophysical research communications. 2009 Apr 17;381(4):676-81.
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. The FASEB Journal. 2003 Jun;17(9):984-92.
- Lupia M, Angiolini F, Bertalot G, Freddi S, Sachsenmeier KF, Chisci E, Kutryb-Zajac B, Confalonieri S, Smolenski RT, Giovannoni R, Colombo N. CD73 regulates stemness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Reports. 2018 Apr 10;10(4):1412-25.
- Hueng DY, Sytwu HK, Huang SM, Chang C, Ma HI. Isolation and characterization of tumor stem-like cells from human meningiomas.Journal of neuro-oncology. 2011 Aug;104(1):45-53.
- Shivapathasundram G, Wickremesekera AC, Tan ST, Itinteang T. Tumour stem cells in meningioma: a review. Journal of Clinical Neuroscience. 2018 Jan 1;47:66-71.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).