Submitted:
03 October 2024
Posted:
04 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
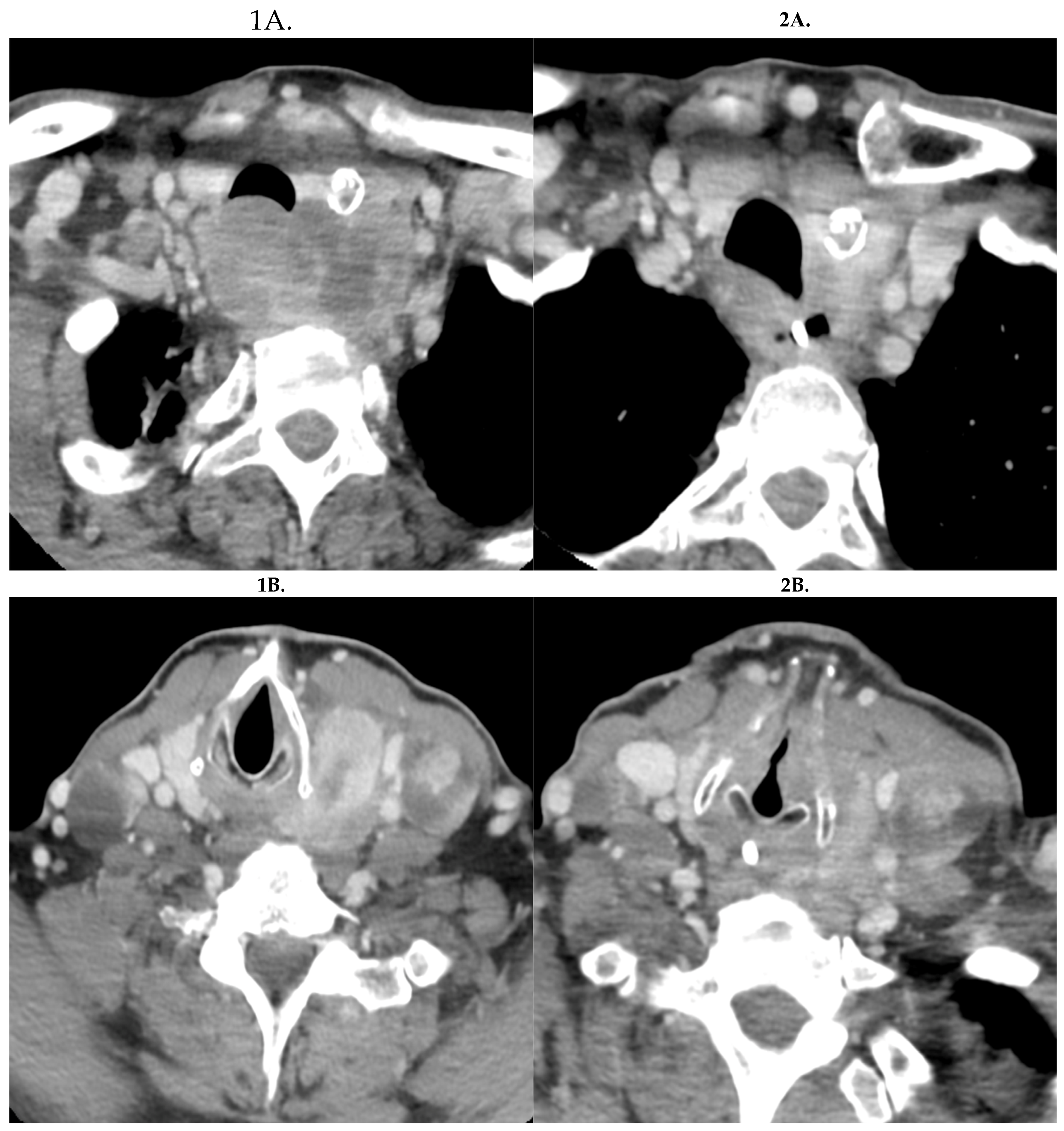
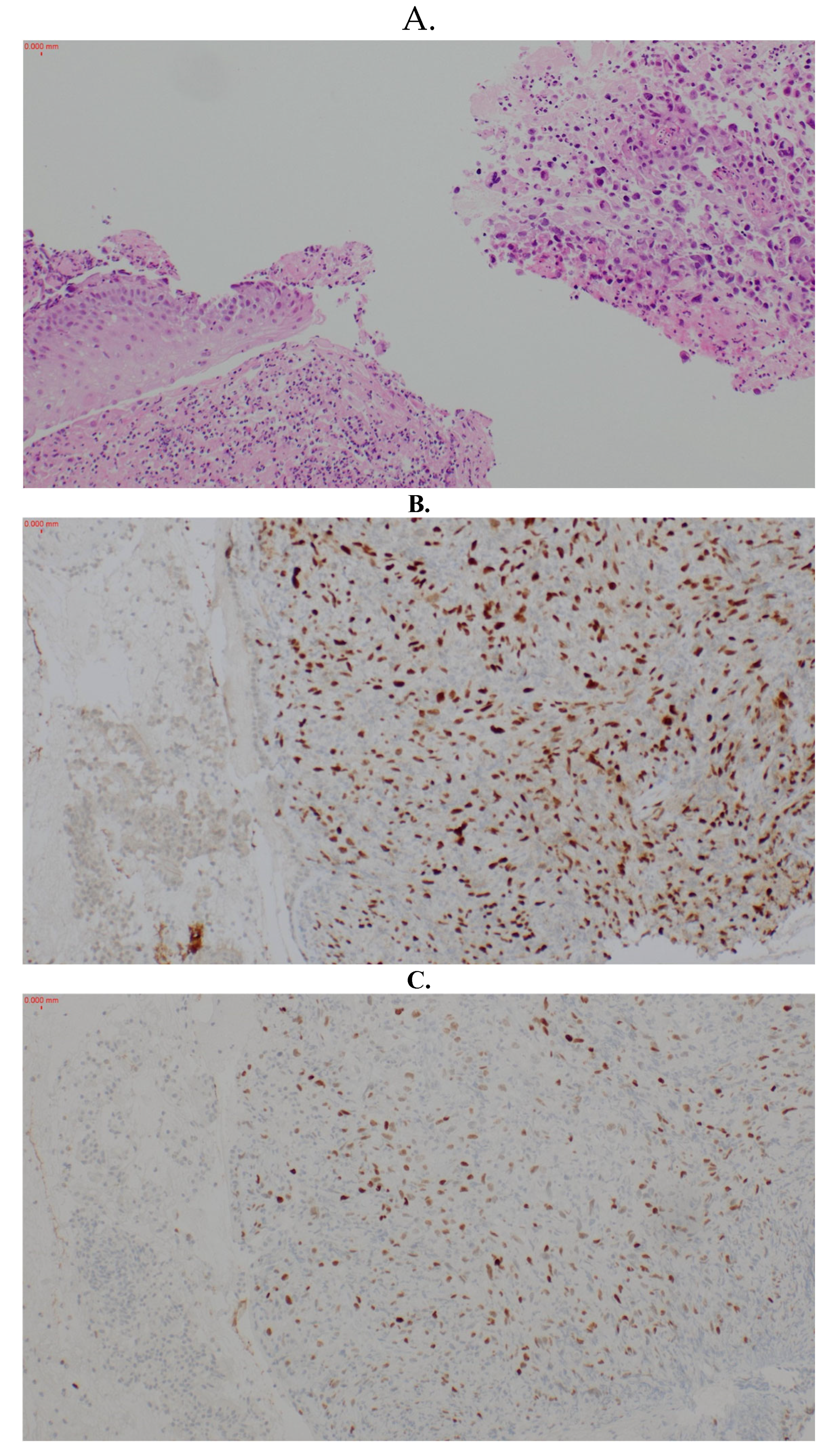
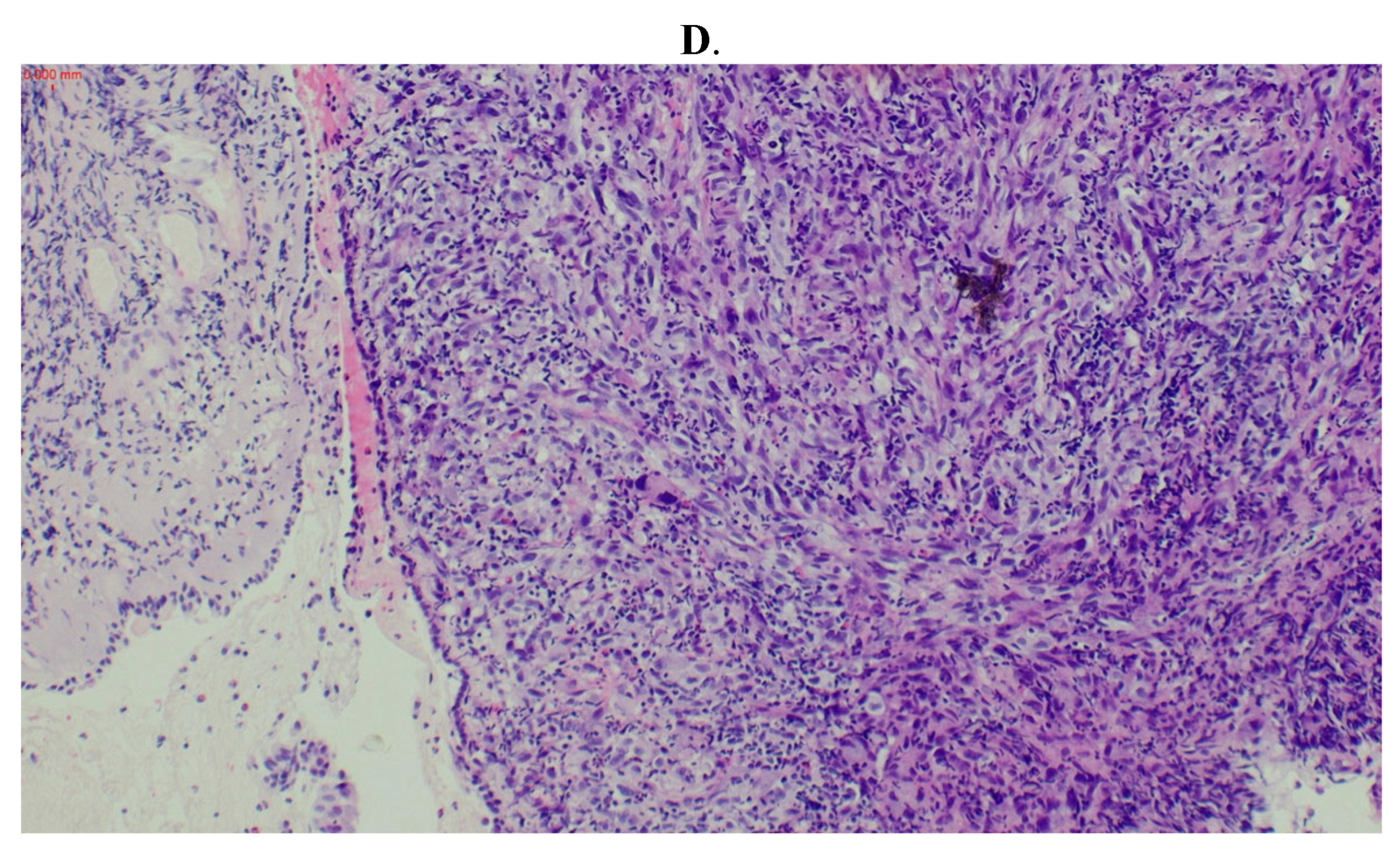
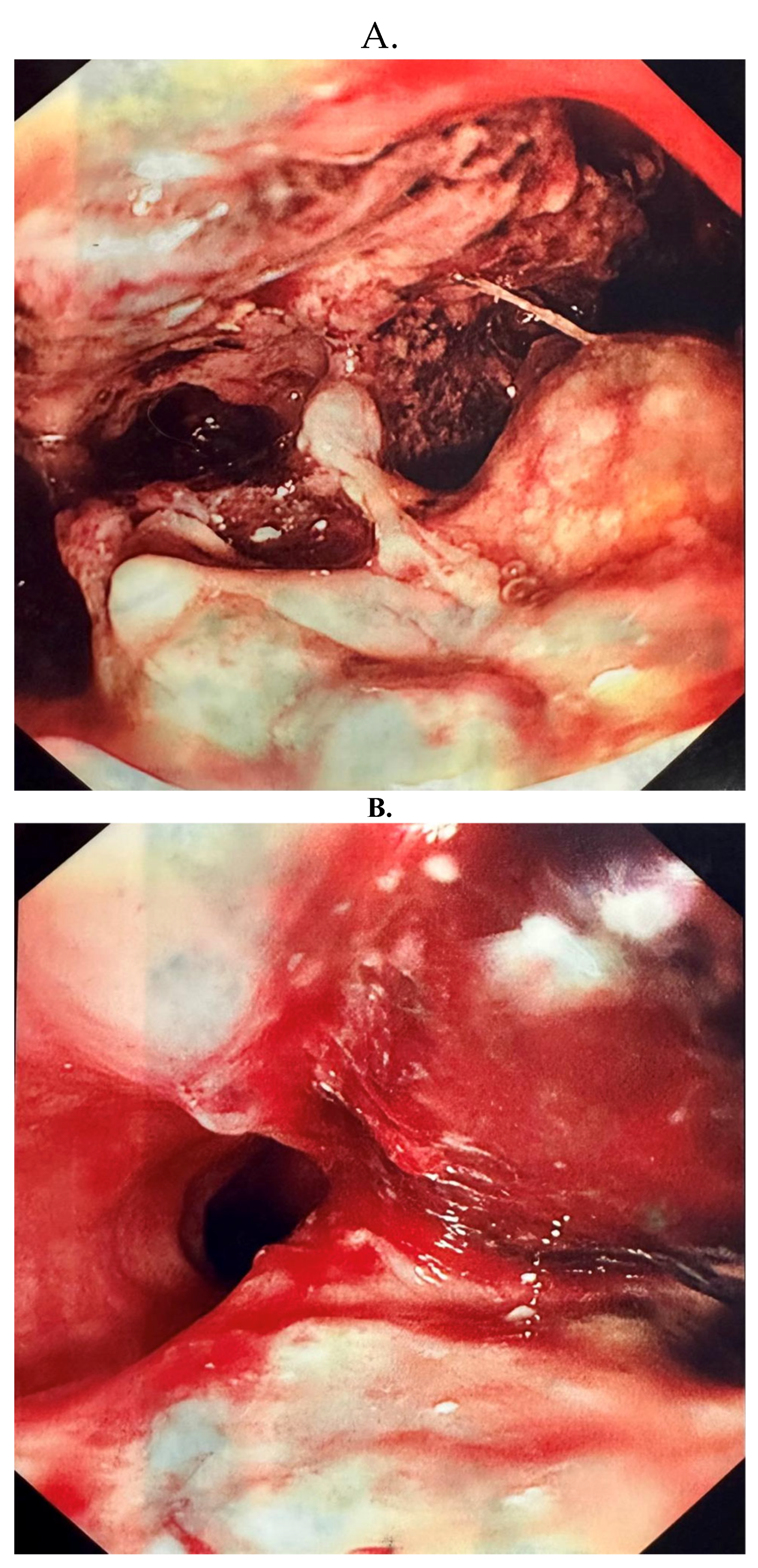
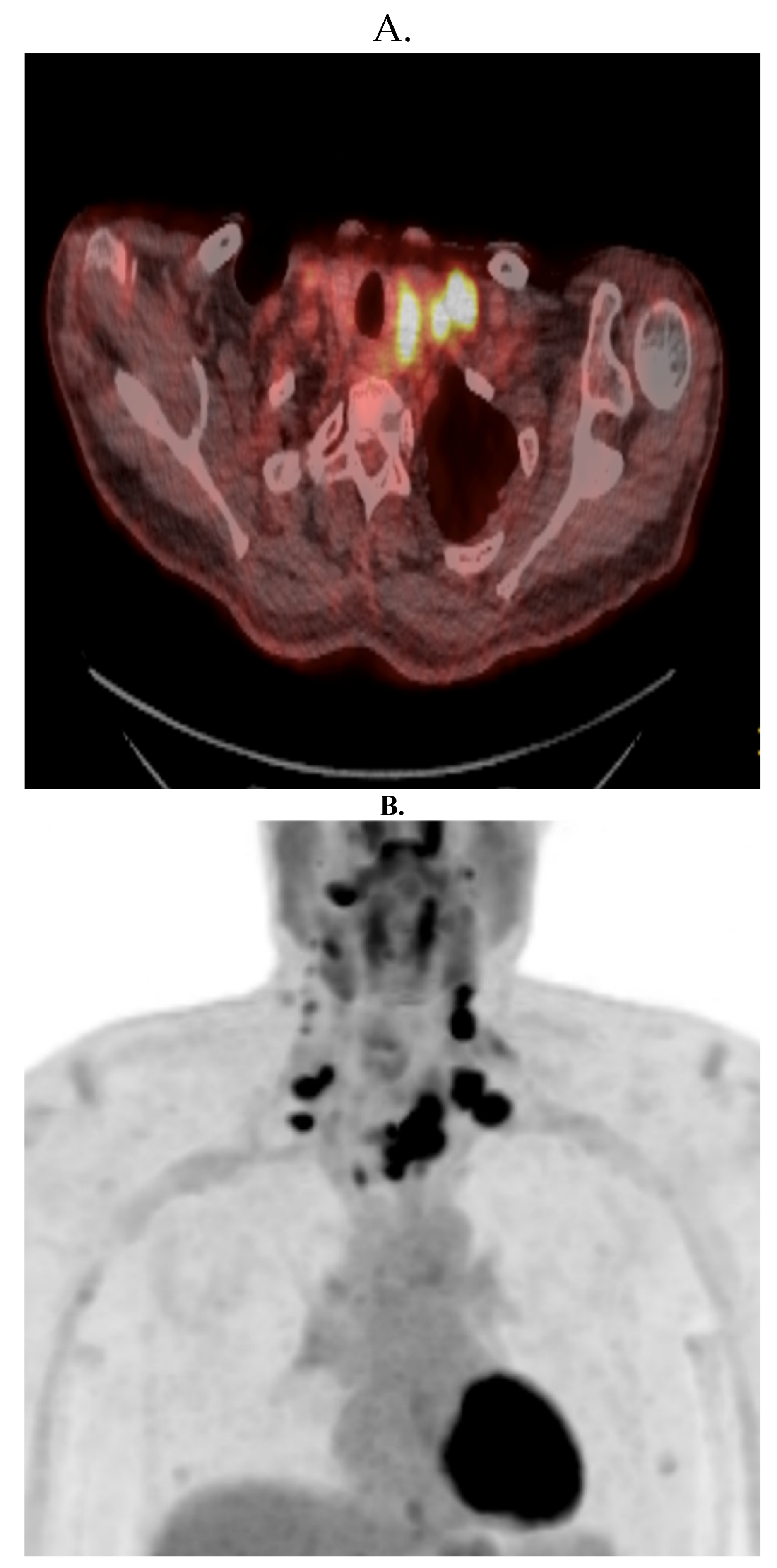
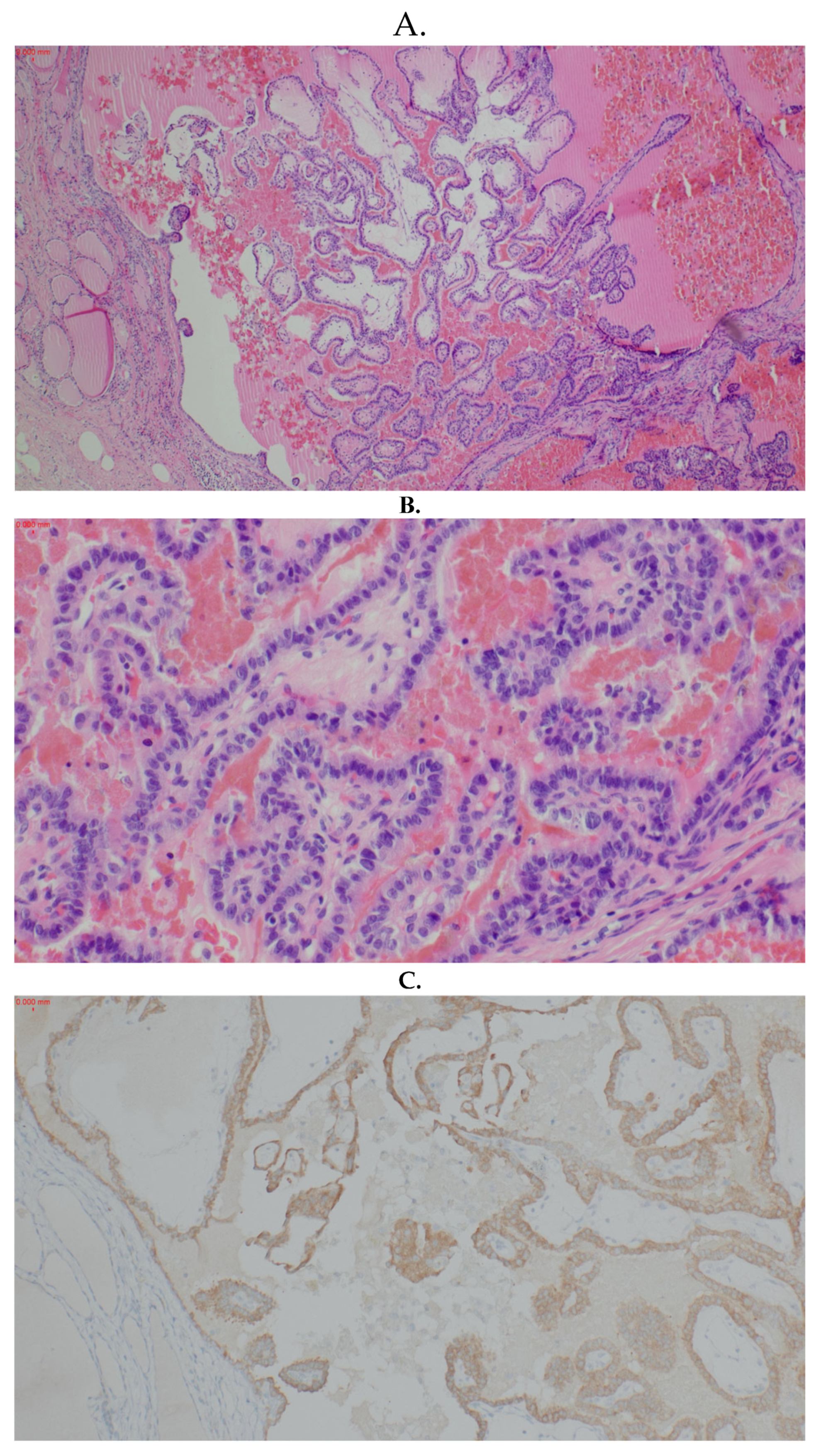
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wächter, S.; Vorländer, C.; Schabram, J.; et al. Anaplastic thyroid carcinoma: changing trends of treatment strategies and associated overall survival. Eur Arch Oto-Rhino-Laryngol 2020, 277, 1507–14. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Copland, J.A. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol 2010, 22, 486–97. [Google Scholar] [CrossRef] [PubMed]
- Stenman, A.; Juhlin, C.C. Novel Insights in the Genomics of Anaplastic Thyroid Carcinoma: A Role for Cyclin-Dependent Kinase Inhibition? Cancers (Basel) 2023, 15, 4621. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr Rev 2019, 40, 1573–604. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Weng, J.; Bauer, J.; et al. The Prevalence and Prognostic Value of BRAF Mutation in Thyroid Cancer. Ann Surg 2007, 246, 466–71. [Google Scholar] [CrossRef]
- Sidaway, P. BRAF plus MEK inhibition effective in papillary craniopharyngioma. Nat Rev Clin Oncol 2023, 20, 661. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; et al. Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Eng J Med 2015, 373, 726–36. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; et al. Dabrafenib plus trametinib in BRAF V600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med 2023, 1103–1112. [Google Scholar] [CrossRef]
- Agarwal, R.; Wang, J.; Wilson, K.; et al. Response to Targeted Therapy in BRAF Mutant Anaplastic Thyroid Cancer. J Natl Compr Canc Netw 2016, 14, 1203–1207. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; et al. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; et al. Evaluation of Overall Survival in Patients with Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol 2020, 6, 1397. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–6. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Kiyota, N.; Imai, H.; Takahashi, S.; Nishiyama, A.; Tamura, S.; Shimizu, Y.; Kadowaki, S.; Ito, K.I.; Toyoshima, M.; Hirashima, Y.; Ueno, S.; Sugitani, I. A Phase 2 Study of Encorafenib in Combination with Binimetinib in Patients with Metastatic BRAF-Mutated Thyroid Cancer in Japan. Thyroid. 2024, 34, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Lau, S.C.; Velcheti, V. To crush or not to crush: Administering dabrafenib and trametinib through a nasogastric tube in a critically ill patient with non small cell lung cancer – a case report and review of literature of targeted therapies given through enteral feeding tubes. In Clinical Lung Cancer; 2023. [Google Scholar] [CrossRef]
- Wu, S.S.; Lamarre, E.D.; Yalamanchali, A.; et al. Association of Treatment Strategies and Tumor Characteristics With Overall Survival Among Patients With Anaplastic Thyroid Cancer: A Single-Institution 21-Year Experience. JAMA Otolaryngol Head Neck Surg 2023, 149, 300–309. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.R.; Dadu, R.; et al. Surgery After BRAF-Directed Therapy Is Associated with Improved Survival in BRAF V600E Mutant Anaplastic Thyroid Cancer: A Single-Center Retrospective Cohort Study. Thyroid 2023, 33, 484–491. [Google Scholar] [CrossRef]
- Yang, K.M.; Jeong, M.J.; Yoon, K.H.; et al. Oncologic outcome of colon cancer with perforation and obstruction. BMC Gastroenterol 2022, 22, 247. [Google Scholar] [CrossRef]
- Vaidya, R.; Habermann, T.M.; Donohue, J.H.; et al. Bowel perforation in intestinal lymphoma: Incidence and clinical features. Ann Oncol 2013, 24, 2439–43. [Google Scholar] [CrossRef]
- Obata, K.; Sugitani, I.; Ebina, A.; et al. Common carotid artery rupture during treatment with Lenvatinib for anaplastic thyroid cancer. Int Cancer Conf J 2016, 5, 197–201. [Google Scholar] [CrossRef]
- Staub, Y.; Nishiyama, A.; Suga, Y.; et al. Clinical Characteristics Associated with Lenvatinib-induced Fistula and Tumor-related Bleeding in Patients with Thyroid Cancer. Anticancer Res 2019, 39, 3871–8. [Google Scholar] [CrossRef]
- Wreesmann, V.B.; et al. Genome-wide appraisal of thyroid cancer progression. The Am J Pathol 2002, 161, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, E.A.; Rosai, J.; Carcangiu, M.; et al. Tumors of the thyroid gland. J Pathol 1992, 171, 247–248. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Kimura, E.T.; Gandhi, M.; et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003, 88, 5399–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; et al. Clinical responses to Vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAFv600emutation. Thyroid 2013, 23, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; et al. ‘Vemurafenib in patients with BRAF v600e-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial’. Lancet Oncol 2016, 17, 1272–1282. [Google Scholar] [CrossRef]
- Rubino, S.; Oliver, D.E.; Tran, N.D.; et al. Improving Brain Metastases Outcomes Through Therapeutic Synergy Between Stereotactic Radiosurgery and Targeted Cancer Therapies. Front Oncol 2022, 12, 854402. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016, 126, 1052–66. [Google Scholar] [CrossRef]
- Haddad, R.I.; Bischoff, L.; Ball, D.; et al. NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma Version 2.2022. Available at: NCCN.org. Accessed December 1, 2023.
- Drilon, A.; Laetsch, T.W.; Kummar, S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N Eng J Med 2018, 378, 731–9. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 2020, 21, 271–82. [Google Scholar] [CrossRef]
- Hong, D.S.; Bauer, T.M.; Lee, J.J.; et al. Larotrectinib in adult patients with solid tumours: a multi-centre, open-label, phase I dose-escalation study. Ann Oncol. 2019, 30, 325–31. [Google Scholar] [CrossRef]
- Capdevila, J.; Wirth, L.J.; Ernst, T.; et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol 2020, 38, 2620–7. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020, 21, 1353–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAF V600E -Mutated Anaplastic Thyroid Carcinoma. Thyroid 2019, 29, 1036–43. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Eizaguirre, G.; Rodríguez, I.; De la Vieja, A.; et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res 2009, 69, 8317–8325. [Google Scholar] [CrossRef]
- Rothenberg, S.M.; et al. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with Dabrafenib. Clin Cancer Res 2015, 21, 1028–1035. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





