1. Introduction and Background
Glioblastomas (GBMs) are the most common malignant brain tumors, constituting 16% of all primary CNS malignancies. They can emerge de novo or through the malignant transformation of more benign tumors, primarily affecting the brain, with 61% located in the frontal, temporal, parietal, and occipital lobes. Standard treatment involves surgical resection followed by radiotherapy and chemotherapy with temozolomide. The annual incidence is approximately 3.20 cases per 100,000 in the USA, according to the Central Brain Tumor Registry of the USA (CBTRUS 2016). Despite optimal surgical interventions and postoperative adjuvant therapies, GBMs remain almost invariably fatal due to their aggressive and invasive nature. The 5-year survival rate for GBM patients in the USA is about 5.5%, with a median overall survival (OS) of roughly one year. The median OS for patients undergoing gross total resection is reported to be 15.5 months, compared to 11.7 months for those with subtotal resection and 5.9 months for those without resection. Recent research indicates that GBMs may originate from various cell types with neural progenitor-like properties, ranging from neural stem cells to glial cells, each exhibiting different alterations in signaling pathways. This finding challenges the previous belief that all GBMs arise exclusively from glial cells [
1,
2].
Current challenges in GBM treatment include incomplete resection, significant genetic heterogeneity affecting response to treatment, the restrictive blood-brain barrier (BBB), and an immunosuppressive microenvironment. Glioblastoma is also known for its highly invasive and therapy-resistant nature. In the last decade, there has been a gradual increase in clinical trials testing new drugs, particularly those focused on immunotherapy and targeted therapies with limited success to date [
3,
4].
Surgical resection and radiotherapy are standard procedures for glioblastoma treatment, but there are several options for chemotherapy. Temozolomide is considered the most effective, as it significantly improves overall survival (circa 3 months) when used in conjunction with radiotherapy. However, other chemotherapeutic agents are available, although they may not provide the same level of efficacy as temozolomide. These alternatives are valuable, especially in regions where temozolomide is not available. Notable examples include Gliadel, which is a biodegradable implant that releases Carmustine directly at the tumour site, and Lomustine, commonly used in Europe as an alternative to Bevacizumab, which is more frequently used in the US and Canada [
5,
6].
However, the primary challenge, regardless of the efficacy or mechanism of the selected chemotherapeutic agent, is its ability to reach the tumor site. The blood-brain barrier (BBB) poses a significant obstacle. Various therapeutic strategies have been proposed to address this issue, including drug delivery using biological scaffolds (the subject of this review), and nanoparticle-based modulation for example using intravenously delivered ultrasound microbubbles, or optical enhancement to break-down the BBB-associated tight junctions [
7].
Hyaluronan has garnered significant interest among researchers in recent years due to its unique hydrogel-like properties and capacity for drug absorption and release. Its prevalence in the extracellular matrix of the brain positions it as a highly attractive option for creation of novel, modified scaffolds that could be used in drug delivery for enhanced targeting in brain cancer treatment.
2. Hyaluronan—More than a Naturally Occuring Scaffold?
Hyaluronic acid (HA) also known as hyaluronan is a polysaccharide prominently found in the ECM throughout the human body. Its molecular mass ranges between 0.2 and 10 MDa, and its physiological properties are influenced by its polyelectrolyte and polymeric characteristics, as well as its viscous nature. Along with HA, the ECM also contains proteoglycans (versican, neurocan, aggrecan etc), link proteins and other glycosaminoglycans such as chondroitin sulfate, keratan sulfate and heparan sulfate. HA is composed of multiple units of D-glucuronide acid and N-acetyl-D-glucosamine to which different proteins and proteoglycans can attach, resulting in a three-dimensional (3D) network. HA is biocompatible and frequently interacts with various cell receptors, as a ligand for the CD44 glycoprotein and the receptor for HA-mediated cell motility RHAMM to facilitate cell communication and behavior [
8]. Moreover, HA can undergo a variety of relatively simple chemical modifications that enable cross-linking between polymer chains and the formation of highly tunable scaffolds [
9,
10].
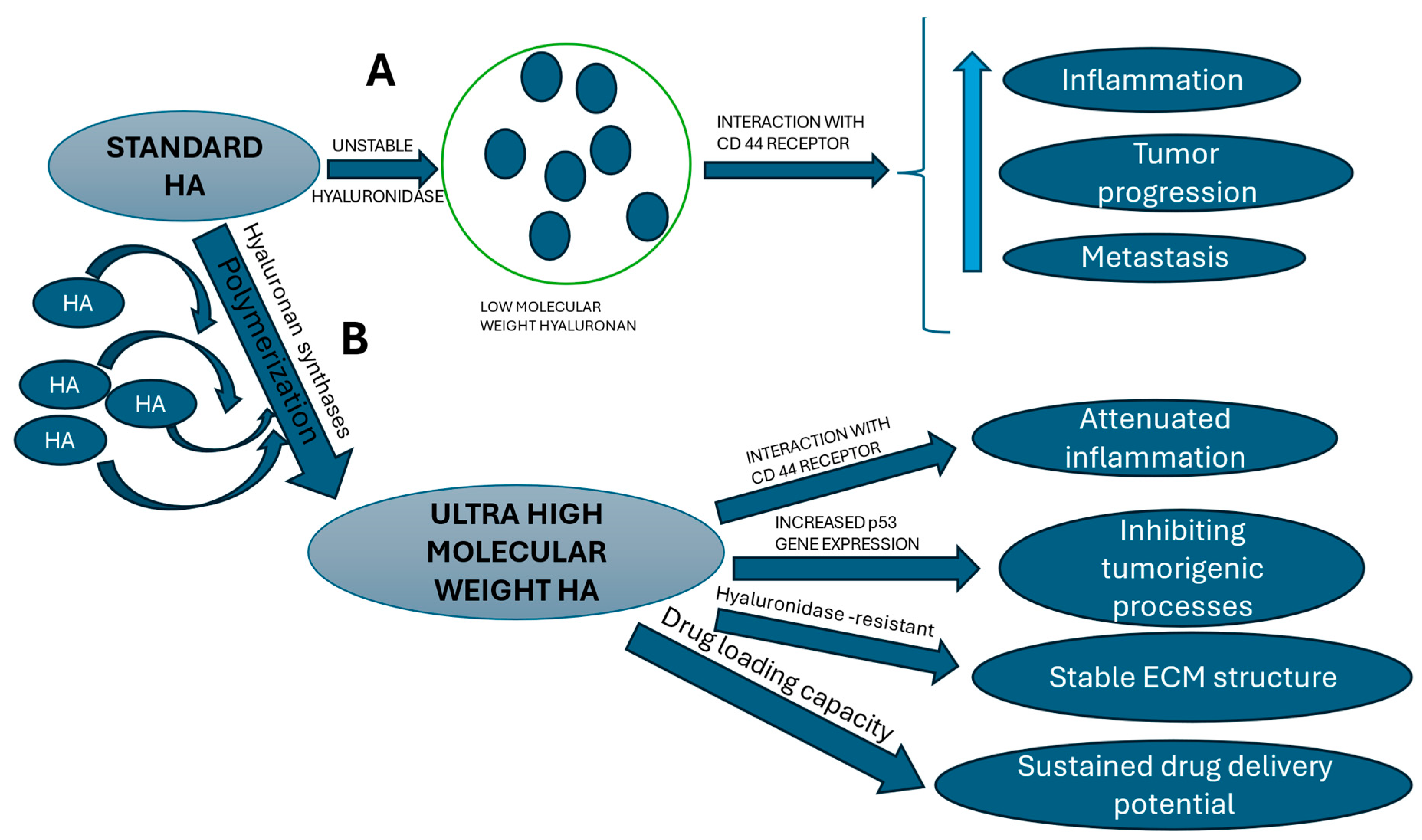
HA is in fact the most concentrated biomolecule within the brain ECM, where its molecular weight dictates its behavior and properties (see
Figure 1). Due to this abundance, HA is critically involved in modulating and maintaining balance within numerous processes, including cell migration, proliferation, differentiation, maturation of neural stem cell progenitors (NSCP) during brain development and repair, and other cellular behaviors [
10,
11].
The potential of HA for drug delivery is well-established and has been extensively explored. HA is highly reported as a matrix suitable for controlled drug release and has already been widely used in various biomedical applications [
12]. It can be cross-linked or conjugated with a range of bio-macromolecules, allowing it to encapsulate different drugs, even at the nanoscale. In a study by Xu et al., HA-based hydrogel particles containing varying amounts of heparin (HP) were synthesized using an inverse emulsion crosslinking method. The resulting HA/HP hydrogel was then loaded with bone morphogenetic protein-2 (BMP-2). To assess the in vitro efficiency of BMP-2 release, these HA/HP hybrid particles loaded with BMP-2 were added directly to micromass cultures of murine multi-potential C3H10T1/2 cells. The chondrogenic potential was determined by analyzing key chondrogenic markers, including collagen type II, aggrecan, and Sox 9, which were all significantly upregulated after incubation with the BMP-2-loaded hydrogel particles. The hydrogel particles proved effective in both loading and sustaining the release of BMP-2. The results also showed that BMP-2 release could be fine-tuned by adjusting the heparin content within the particles [
13].
Hydrogels containing PLGA microspheres loaded with vancomycin have also been used for drug delivery, showing effectiveness in providing sustained drug release in non-healing infected wounds. Huang et al., created infected wounds in rats by inducing circular skin defects of 1 cm in diameter on their backs and injecting 0.1 mL of bacterial suspension into the wound areas. The rats were divided into different treatment groups: gauze,
carboxymethyl chitosan (CC) and aldehyde hyaluronic acid (CC/AHA) hydrogels, CC/AHA hydrogels with vancomycin, CC/AHA hydrogels with PLGA microspheres, and CC/AHA hydrogels with vancomycin and PLGA microspheres. The group treated with CC/AHA hydrogels combined with vancomycin and PLGA microspheres showed the most favorable results in terms of granulation tissue thickness, and reduced levels of inflammatory markers at the wound site (including IL-12, IL-1alpha, IL-10, and TNF-alpha), increased angiogenesis, and overall wound area reduction [
14]
.
CNS regeneration is challenging in part due to its limited response to treatment, and, to date, no effective therapies have been identified to rectify CNS injuries which most often
cause impairment of neurological functions in corresponding sites of injury - be it traumatic or due to a tumor formation with mass effect [
15]
. HA hydrogels have shown success in treating spinal cord injuries (SCI) in experimental animals. Mothe et al. utilized a hydrogel blend of HA and methyl cellulose (HAMC) injected with adult brain-derived neural stem/progenitor cells (NSPCs) in a subacute rat SCI model. The HAMC was covalently modified with recombinant rat platelet-derived growth factor-A (rPDGF-A) to promote oligodendrocytic differentiation. This approach resulted in reduced cavitation, improved graft survival, increased oligodendrocytic differentiation, and preservation of perilesional host oligodendrocytes, indicating that HAMC-rPDGF-A could be a promising vehicle for cell delivery in such spinal cord injuries [
16]
. Similarly, Moshayedi et al. used a hyaluronan hydrogel to deliver and attempt to enhance the survival of encapsulated human neural progenitor cells (iPS-NPCs) transplanted into a mouse cortical model of photothrombotic stroke. The optimized HA hydrogel significantly promoted the survival of iPS-NPCs and influenced cell fate, promoting glial, neuronal, or progenitor states. The fate of the stem cells within the hydrogel was tracked in vivo with MRI and outcomes indicated that the system was effective in selectively controlling stem cell survival and differentiation and could be used as a therapeutic adjunct in stem cell therapy [
17].
3. CD44 and Hyaluronan: Exploring Their Link in the Glioblastoma Microenvironment
The majority of interactions between glioblastoma cells and hyaluronan (HA) within the brain extracellular matrix (ECM) are mediated through the CD44 receptor, a prominent vascular and tumour cell modulator that could represent a viable therapeutic target. CD44 is recognized as a marker of mesenchymal glioma stem cells (GSCs) and the mesenchymal subtype of GBM, associated with poor prognosis and radiation resistance in human GBM [
18].
In vivo studies by Xu et al. reported elevated CD44 expression in U87MG and U251 glioma cells, which were used in subcutaneous and intracranial tumor growth studies in immunocompromised mice. CD44 knockdown via lentiviral shRNA reduced both tumor volume and proliferation rates in subcutaneous tumors, while significantly inhibiting intracranial tumor growth and extending survival in the intracranial models. When combined with standard GBM drugs (Temozolomide and Carmustine), CD44 depletion resulted in a synergistic inhibition of intracranial tumor progression, further prolonging the median survival of mice [
19].
This chemosensitizing effect of CD44 depletion was linked to oxidative stress from reactive oxygen species (H
2O
2) and cytotoxic stress from Temozolomide, leading to activation of mammalian sterile 20-like kinase-1/2 (MST1/2) and Hippo pathway kinases Lats1/2 (tumour promoters), as well as inactivation of yes-associated protein (YAP), reduced merlin phosphorylation, increased cleaved caspase-3, and decreased cell viability [
19].
To examine how HA concentrations influenced GBM invasion, patient-derived GBM cells were cultured in a 3D matrix with controlled HA levels. HA concentration was found to regulate cell invasion in a biphasic, patient-specific manner, potentially through phosphorylated ezrin linking HA-bound CD44 to the actin cytoskeleton. Thereby, targeting HA-CD44-ezrin interactions may offer a promising approach to preventing tumor cell invasion in the brain [
20].
Lubanska et al (2022), synthesized spherical diketopyrrolopyrrole-based Conjugated Polymer Nanoparticles (CPNs) containing fluorescein-conjugated Hyaluronic Acid (HA), (as a ligand for the CD44 receptor present on stem cell-like tumour initiating cells) in a patient-derived Zebra fish xenograft model of glioblastoma. They showed effective blood–brain barrier permeability of this system and a concentration and cell cycle phase-dependent selective uptake of HA-CPNs in CD44 positive GBM-patient derived cultures, with a concomitant decrease in cell stemness and capacity for invasion indicating potential use of this system as a therapeutic [
21].
Ooki T et al. demonstrated that specifically, the high-molecular-weight hyaluronan (HMW-HA), [a very large glycosaminoglycan], plays a critical role in stimulating tumor-suppressive Hippo signaling pathways in breast epithelial cells. This tumor-suppressive effect was mediated through the clustering of the CD44 extracellular domain, a cell surface glycoprotein known to interact with hyaluronan. The clustering of CD44 led to the recruitment of the polarity-regulating kinase PAR1b via the CD44 intracellular domain and subsequent disruption of the inhibitory interaction between PAR1b and the MST (mammalian Ste20-like kinase) complex, which is a core component of the Hippo pathway. By releasing MST from this inhibitory complex, HMW-HA promotes the activation of Hippo signaling, leading to the suppression of tumor growth and proliferation. Conversely, low molecular weight hyaluronan (LMW-HA), which is a smaller fragment of the same molecule, exerted an opposite, pro-tumorigenic effect. It competes with HMW-HA for binding to the CD44 receptor, thereby preventing the beneficial clustering of CD44 and enabling tumor progression by inhibiting Hippo signaling [
22]. This dual role of hyaluronan in tumorigenesis highlights the complex and context-dependent nature of its interactions with cellular signaling pathways in cancer development.
In parallel, other studies have explored the therapeutic potential of HA-micelles (HA-M) as a drug delivery system, specifically encapsulating a 1:1 molar ratio of lauroyl-gemcitabine (Gem-C12) and honokiol (HNK). HA-M takes advantage of the CD44 receptor, which is often overexpressed in various tumor cells, to facilitate receptor-mediated endocytosis. This targeted delivery enhances the micelles' penetration into dense tumor spheroids, which are typically resistant to conventional therapies, and increases the cytotoxic effects specifically against glioma cells. In in vivo studies, drug-loaded HA-M demonstrated significant therapeutic efficacy, leading to improved survival rates in mice bearing orthotopic xenograft glioblastomas, a model that closely mimics the human condition. These findings underscore the potential of HA-M as a promising strategy for enhancing drug delivery and efficacy in treating aggressive cancers like glioblastoma, where current treatment options are limited [
23].
4. The Potential of Ultra High Molecular Weight [Naked Mole Rat] Hyaluronan in Targeted Cancer Therapy
The naked mole rat is a remarkable species, notable for its adaptation to low oxygen levels and exceptional cancer resistance. Its subterranean lifestyle has led to elevated levels of high molecular weight hyaluronan in their tissues. This adaptation confers numerous benefits, including preserved skin elasticity, youthful appearance, accelerated wound healing, protection against oxidative stress, and resistance to cancer and arthritis [
24].
The HMW- HA present in the ECM of the NMR brain appears to form three-dimensional folded structures that resemble the macroscopic configuration of the gyri and sulci observed in the human brain [
25]. The NMR fibroblasts secrete very high molecular weight hyaluronan (6-12 MDa), almost five times larger than mouse HA (0,5-3 MDa) [
26] and human (0,5-2 MDa) [
27]. The three main synthases involved in the production of HA are HAS1, HAS2 and HAS3, all having distinct functionality and regulatory mechanisms [
28].
This characteristic of the naked mole-rat brain's HA may provide a robust basis for the resistance these mammals exhibit against various forms of cancer, potentially including glioblastoma and other central nervous system tumors. One of the processes involved in the resistance to cancer developed by the NMR is contact inhibition, a key anticancer mechanism that halts the cell cycle upon contact between cells. In humans, this process is mainly regulated by the p27 cyclin-dependent kinase inhibitor. NMRs exhibit heightened sensitivity to this process, known as early contact inhibition (ECI), which prevents cell proliferation even with minimal contact. The ECI is thought to be modulated by HA, as it represents a critical component of the ECM [
26]. In NMRs, the cyclin-dependent kinase inhibitor acts as a secondary defense, only engaging if ECI fails, offering extra protection against excessive cell division [
29].
Tian X et al [
26] demonstrated that the naked mole-rat has extremely HMW-HA compared to mice, concomitant with increased binding to the CD44 receptor, and lower hyaluronidase activity. All these characteristics play a role in mediating the cancer resistance of the NMR. In addition, another study [
30] compared the HMW levels of the naked mole-rat, mouse and guinea pig, using Alcian blue staining (non-specific for hyaluronan). The results showed that the NMR had the higher levels of HA, the strongest signals being in epidermis, renal glomeruli and lymph nodes, and that HMW HA composed the majority compared to LMW HA, thus having a positive anti-aging and anti-cancer effect.
Zhao Y et al. conducted a comprehensive investigation into the effects of extremely high molecular weight hyaluronan (EHMW-HA) derived from the NMR on human breast cancer cells. By utilizing genetically modified cancer cell lines, 4T1/BT549-nmrHas2, they demonstrated that these cells were capable of secreting EHMW-HA, with molecular weights reaching up to 6 MDa, similar to what is observed in NMR. The overexpression of the nmrHas2 gene led to an increased accumulation of EHMW-HA in both two-dimensional (2D) and three-dimensional (3D) in vitro models, as well as in living tissue models. In their in vitro studies, the overexpression of nmrHas2 in 4T1/BT549-nmrHas2 cancer cells resulted in a significant increase in EHMW-HA production, which subsequently induced enhanced apoptosis and inhibited cancer cell proliferation in 2D and 3D cultures.Furthermore, in vivo experiments focusing on nmrHas2-4T1 cells demonstrated that EHMW-HA effectively suppressed tumor formation in nude mice, with overexpression of nmrHas2 resulting in an approximately five-fold decrease in the mean weight of tumors collected three weeks after inoculation. Mechanistically, EHMW-HA was found to induce higher expression levels of the tumor suppressor protein p53, which in turn promoted the expression of pro-apoptotic proteins, such as p21 and Bax, thereby facilitating apoptosis in breast cancer cells. These findings suggest that EHMW-HA could have potential therapeutic applications in targeting breast cancer by promoting tumor cell apoptosis and inhibiting tumor growth [
31].
Similarly, Zhang Z et al. demonstrated cancer resistance and extended lifespan conferred by HMW-HA using C57BL/6 transgenic mice overexpressing the naked mole-rat hyaluronic acid synthase 2 gene (nmrHas2). These genetically modified mice exhibited increased HA levels in various tissues, reduced incidence of spontaneous (lymphoma) and induced (skin papillomas) cancer, extended lifespan, and improved overall health. The most notable changes were attenuated systemic inflammation across multiple tissues, and transcriptomic/epigenetic alterations associated with maintenance of a lower biological age, suggesting that the longevity mechanisms evolved in the naked mole-rat can be transferred to other species [
32].
The importance of MWt of HA to the overall physiological performance and health is in many ways astonishing, and this was reviewed eloquently by Michalczyk et al (2023), and hence optimal modulation of the homeostatic balance of HA within an organism may provide a basis for ensuring stability within the ECM that enables normal cellular function with a minimal stress [
33]. Further studies are warranted in order to fully characterize this phenomenon and understand how to utilize NMR-HA in cancer and more specifically brain cancer and glioblastoma therapies (
Figure 1).
5. Conclusions
Ultra-high molecular weight HA may have enhanced stability and offer greater protection against chronic inflammatory conditions, providing a moderating scaffold that could also be suitable for extended drug delivery for example at sites of injury or for sustained chemotherapeutic treatment. The mechanisms by which UHMW-HA differs from regular HA are primarily regulated by its interaction with the CD44 receptor, triggering distinct biological responses, which are also related to the ECI phenomenon observed in the naked mole-rat. Considering that in most mammalian species the average molecular weight of HA tends to decrease with age and yet the total amount remains consistent a more detailed characterization of the naked mole-rat HA and its unique properties is required.
6. Future Perspectives
Further studies are needed in order to understand for example how the mole rat is able to synthesize and maintain such a high concentration throughout life, and what is required of the local micro-environment within the extracellular matrix in order to retain such an anti-ageing protective 3D milieu and how to optimise such a system for sustained, effective and targeted drug delivery in human cancer and other diseases.
References
- Luo C, Song K, Wu S et al. The prognosis of glioblastoma: a large, multifactorial study. Br J Neurosurg. 2021 Oct;35(5):555-561 – 1-2. https://doi.org/10.1080/02688697.2021.1907306. [CrossRef]
- Uyar R. Glioblastoma microenvironment: The stromal interactions. Pathol Res Pract. 2022 Apr;232:153813. https://doi.org/10.1016/j.prp.2022.153813. [CrossRef]
- Tan AC, Ashley DM, López GY et al. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020 Jul;70(4):299-312. https://doi.org/10.3322/caac.21613. [CrossRef]
- Wu W, Klockow JL, Zhang M et al. lioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021 Sep;171:105780. https://doi.org/10.1016/j.phrs.2021.105780. [CrossRef]
- Iuchi T, Inoue A, Hirose Y et al. ong-term effectiveness of Gliadel implant for malignant glioma and prognostic factors for survival: 3-year results of a postmarketing surveillance in Japan. Neurooncol Adv. 2022 Jan 12;4(1):vdab189. https://doi.org/10.1093/noajnl/vdab189. [CrossRef]
- Smolarska A, Pruszynska I, Wasylko W et al. Targeted therapies for glioblastoma treatment. J Physiol Pharmacol. 2023 Jun;74(3). https://doi.org/10.26402/jpp.2023.3.01. [CrossRef]
- Noorani I, de la Rosa J. Breaking barriers for glioblastoma with a path to enhanced drug delivery. Nat Commun. 2023 Sep 22;14(1):5909. https://doi.org/10.1038/s41467-023-41694-9. [CrossRef]
- Pibuel MA, Poodts D, Díaz M, Hajos SE, Lompardía SL. The scrambled story between hyaluronan and glioblastoma. J Biol Chem. 2021 Jan-Jun;296:100549. https://doi.org/10.1016/j.jbc.2021.100549. Epub 2021 Mar 17. PMID: 33744285; PMCID: PMC8050860. [CrossRef] [PubMed]
- Djoudi A, Molina-Peña R, Ferreira N et al. Hyaluronic Acid Scaffolds for Loco-Regional Therapy in Nervous System Related Disorders. Int J Mol Sci. 2022 Oct 12;23(20):12174. https://doi.org/10.3390/ijms23201217. [CrossRef]
- Jensen G, Holloway JL, Stabenfeldt SE. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells. 2020 Sep 17;9(9):2113. https://doi.org/10.3390/cells9092113. [CrossRef]
- Su W, Matsumoto S, Sorg B, Sherman LS. Distinct roles for hyaluronan in neural stem cell niches and perineuronal nets. Matrix Biol. 2019 May;78-79:272-283. https://doi.org/10.1016/j.matbio.2018.01.022. Epub 2018 Jan 31. PMID: 29408010; PMCID: PMC6068007. [CrossRef] [PubMed]
- Agrahari V. The exciting potential of nanotherapy in brain-tumor targeted drug delivery approaches. Neural Regen Res. 2017 Feb;12(2):197-200. https://doi.org/10.4103/1673-5374.200796. [CrossRef]
- Xu X, Jha AK, Duncan RL, Jia X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011 Aug;7(8):3050-9. https://doi.org/10.1016/j.actbio.2011.04.018. [CrossRef]
- Huang J, Ren J, Chen G, Li Z, Liu Y, Wang G, Wu X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater Sci Eng C Mater Biol Appl. 2018 Aug 1;89:213-222. https://doi.org/10.1016/j.msec.2018.04.009. [CrossRef]
- Wang Y, Tan H, Hui X. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. Biomed Res Int. 2018 Apr 1;2018:7848901. https://doi.org/10.1155/2018/7848901. [CrossRef]
- Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013 May;34(15):3775-83. https://doi.org/10.1016/j.biomaterials.2013.02.002. [CrossRef]
- Moshayedi P, Nih LR, Llorente IL et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016 Oct;105:145-155. https://doi.org/10.1016/j.biomaterials.2016.07.028. [CrossRef]
- B. D. Vaillant, K. Bhat, E. P. Sulman, et al. CD44 as a prognostic and predictive marker for GBM. Journal of Clinical Oncology. 2011 May.Volume 29, Number 15. https://doi.org/10.1200/jco.2011.29.15_suppl.2049. [CrossRef]
- Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010 Mar 15;70(6):2455-64. https://doi.org/10.1158/0008-5472.CAN-09-2505. [CrossRef]
- Safarians G, Sohrabi A, Solomon I et al. Glioblastoma Spheroid Invasion through Soft, Brain-Like Matrices Depends on Hyaluronic Acid-CD44 Interactions. Adv Healthc Mater. 2023 Jun;12(14):e2203143. https://doi.org/10.1002/adhm.202203143. [CrossRef]
- Lubanska D, Alrashed S, Mason GT, Nadeem F, Awada A, DiPasquale M, Sorge A, Malik A, Kojic M, Soliman MAR, deCarvalho AC, Shamisa A, Kulkarni S, Marquardt D, Porter LA, Rondeau-Gagné S. Impairing proliferation of glioblastoma multiforme with CD44+ selective conjugated polymer nanoparticles. Sci Rep. 2022 Jul 15;12(1):12078. https://doi.org/10.1038/s41598-022-15244-0. [CrossRef]
- Ooki T, Murata-Kamiya N, Takahashi-Kanemitsu A, Wu W, Hatakeyama M. High-Molecular-Weight Hyaluronan Is a Hippo Pathway Ligand Directing Cell Density-Dependent Growth Inhibition via PAR1b. Dev Cell. 2019 May 20;49(4):590-604.e9. https://doi.org/10.1016/j.devcel.2019.04.018. [CrossRef]
- Liu X, Li W, Chen T et al. Hyaluronic Acid-Modified Micelles Encapsulating Gem-C12 and HNK for Glioblastoma Multiforme Chemotherapy. Mol Pharm. 2018 Mar 5;15(3):1203-1214. https://doi.org/10.1021/acs.molpharmaceut.7b01035. [CrossRef]
- Lagunas-Rangel FA. Naked mole-rat hyaluronan. Biochimie. 2024 May;220:58-66. https://doi.org/10.1016/j.biochi.2023.12.008. [CrossRef]
- Kulaberoglu Y, Bhushan B, Hadi F et al. The material properties of naked mole-rat hyaluronan. Sci Rep. 2019 Apr 29;9(1):6632. https://doi.org/10.1038/s41598-019-43194-7. [CrossRef]
- Tian, X., Azpurua, J., Hine, C. et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349 (2013). https://doi.org/10.1038/nature12234. [CrossRef]
- Holmes MW, Bayliss MT, Muir H. Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochem J. 1988 Mar 1;250(2):435-41. https://doi.org/10.1042/bj2500435. PMID: 3355532; PMCID: PMC1148875. [CrossRef] [PubMed]
- Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002 Oct;54(4):195-9. https://doi.org/10.1080/15216540214929. [CrossRef]
- Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC, Gorbunova V. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19352-7. https://doi.org/10.1073/pnas.0905252106. [CrossRef]
- Del Marmol D, Holtze S, Kichler N, Sahm A, Bihin B, Bourguignon V, Dogné S, Szafranski K, Hildebrandt TB, Flamion B. Abundance and size of hyaluronan in naked mole-rat tissues and plasma. Sci Rep. 2021 Apr 12;11(1):7951. https://doi.org/10.1038/s41598-021-86967-9. PMID: 33846452; PMCID: PMC8041917. [CrossRef] [PubMed]
- Zhao Y, Qiao S, Hou X et al. Bioengineered tumor microenvironments with naked mole rats high-molecular-weight hyaluronan induces apoptosis in breast cancer cells. Oncogene. 2019 May;38(22):4297-4309. https://doi.org/10.1038/s41388-019-0719-4. [CrossRef]
- Zhang Z, Tian X, Lu JY et al. Increased hyaluronan by naked mole-rat Has2 improves healthspan in mice. Nature. 2023 Sep;621(7977):196-205. https://doi.org/10.1038/s41586-023-06463-0. [CrossRef]
- Michalczyk M, Humeniuk E, Adamczuk G, Korga-Plewko A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int J Mol Sci. 2022 Dec 21;24(1):103. https://doi.org/10.3390/ijms24010103. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).