Submitted:
04 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
- Tryptophan metabolism via the kynurenine pathway
- NMDARs and α-7nAChRs, and neuroprotection
- Kynurenic acid and significant abnormalities
- Kynurenic acid metabolism throughout life
- Glutamatergic and acetyl cholinergic activity and dementia
- Kynurenic acid and neuropsychiatric disorders and dementia
- Different types of pathology after HIV-1
- Glia depressing Factor
-
Anti-dementia approaches
- 9.1
- Stochastic Resonance Therapy (SRT)
- 9.2
- Antidementia drugs
- 9.3
- Herbal medicines - prophylaxis - protection
- Future perspectives
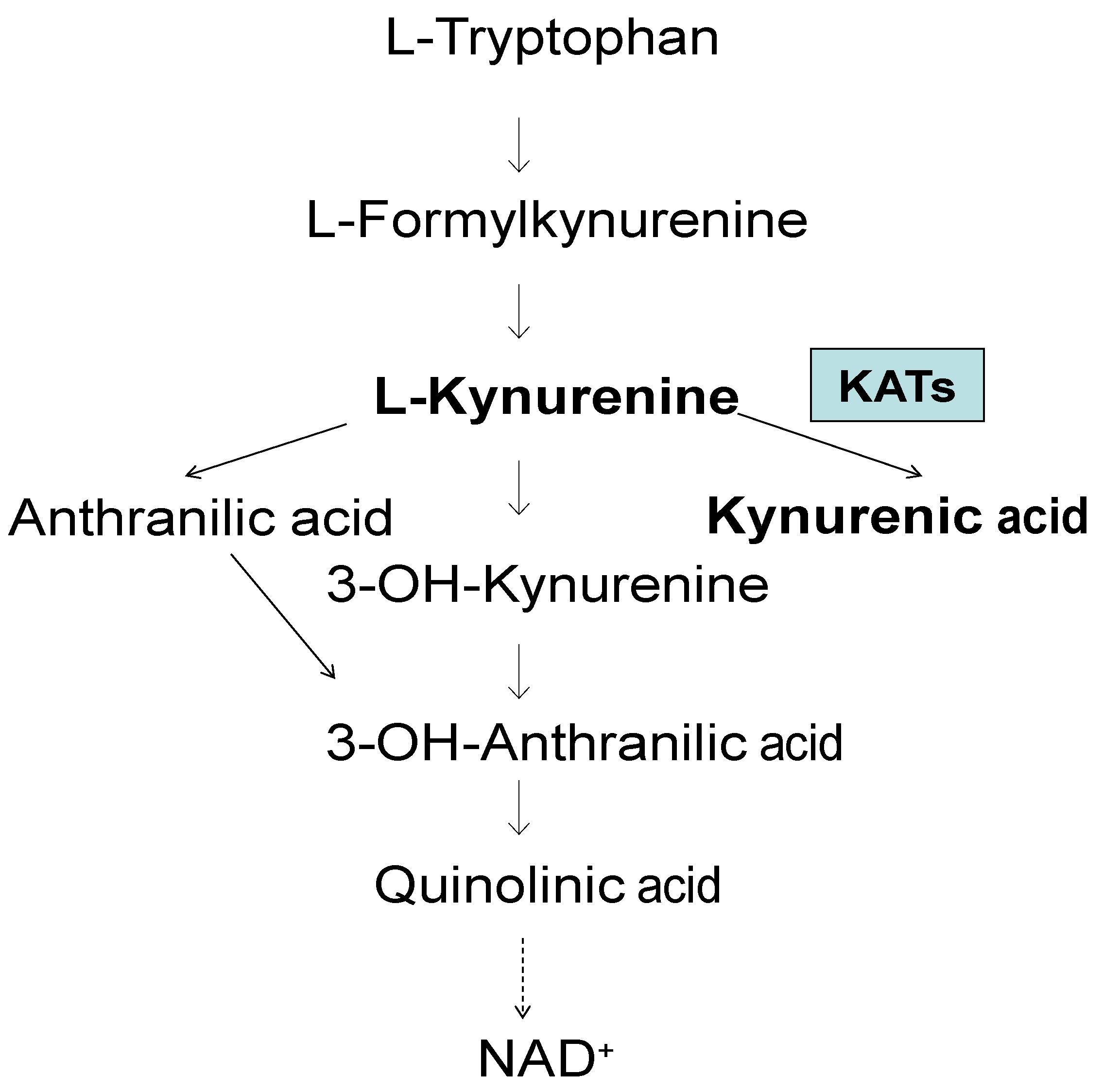
1. Tryptophan Metabolism via the Kynurenine Pathway
2. NMDARs and α-7nAChRs, and Neuroprotection
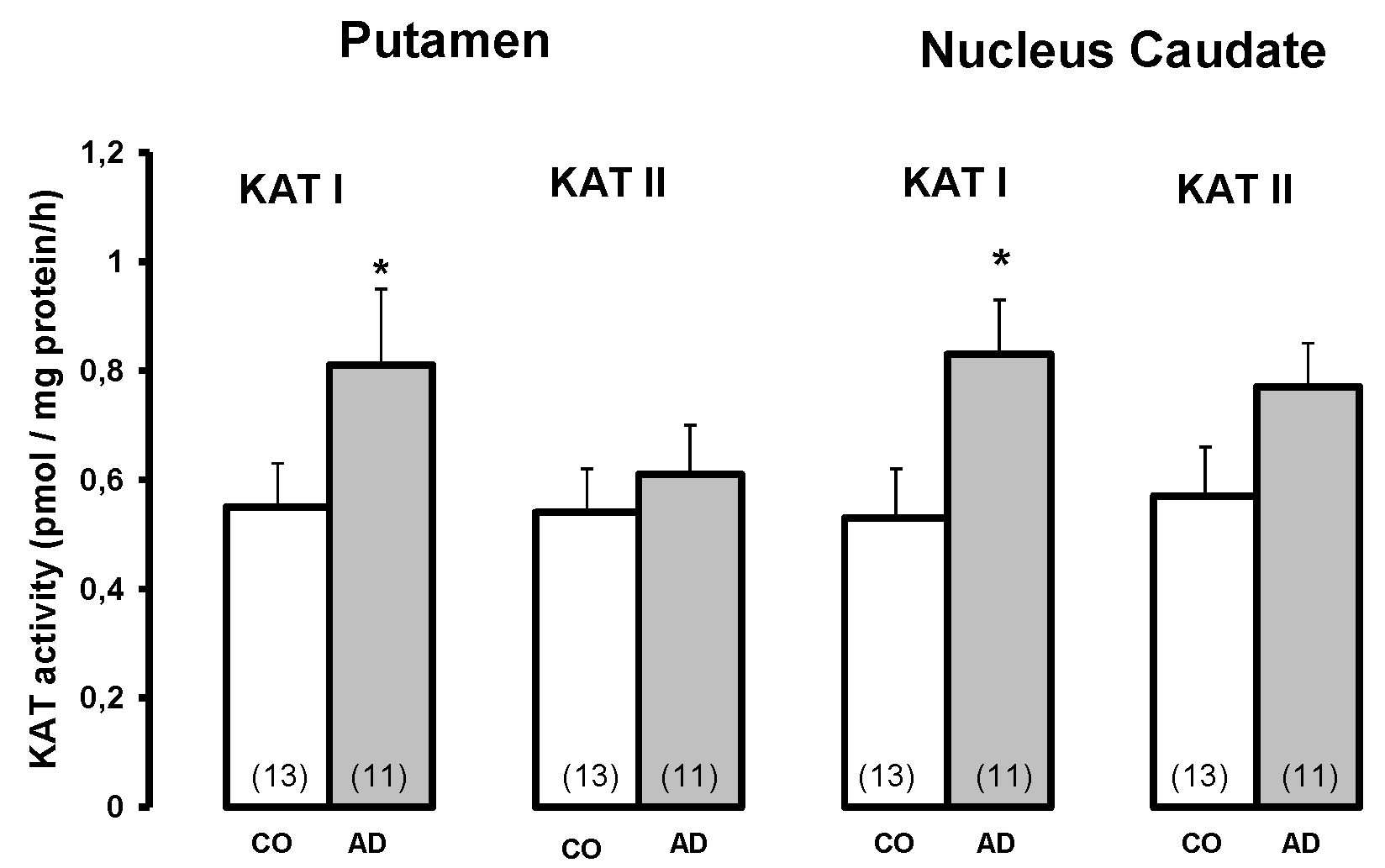
3. Kynurenic Acid and Significant Abnormalities
4. Kynurenic Acid Metabolism throughout Life
5. Glutamatergic and Acetylcholinergic Activity and Dementia
6. Kynurenic Acid and Neuropsychiatric Disorders and Dementia
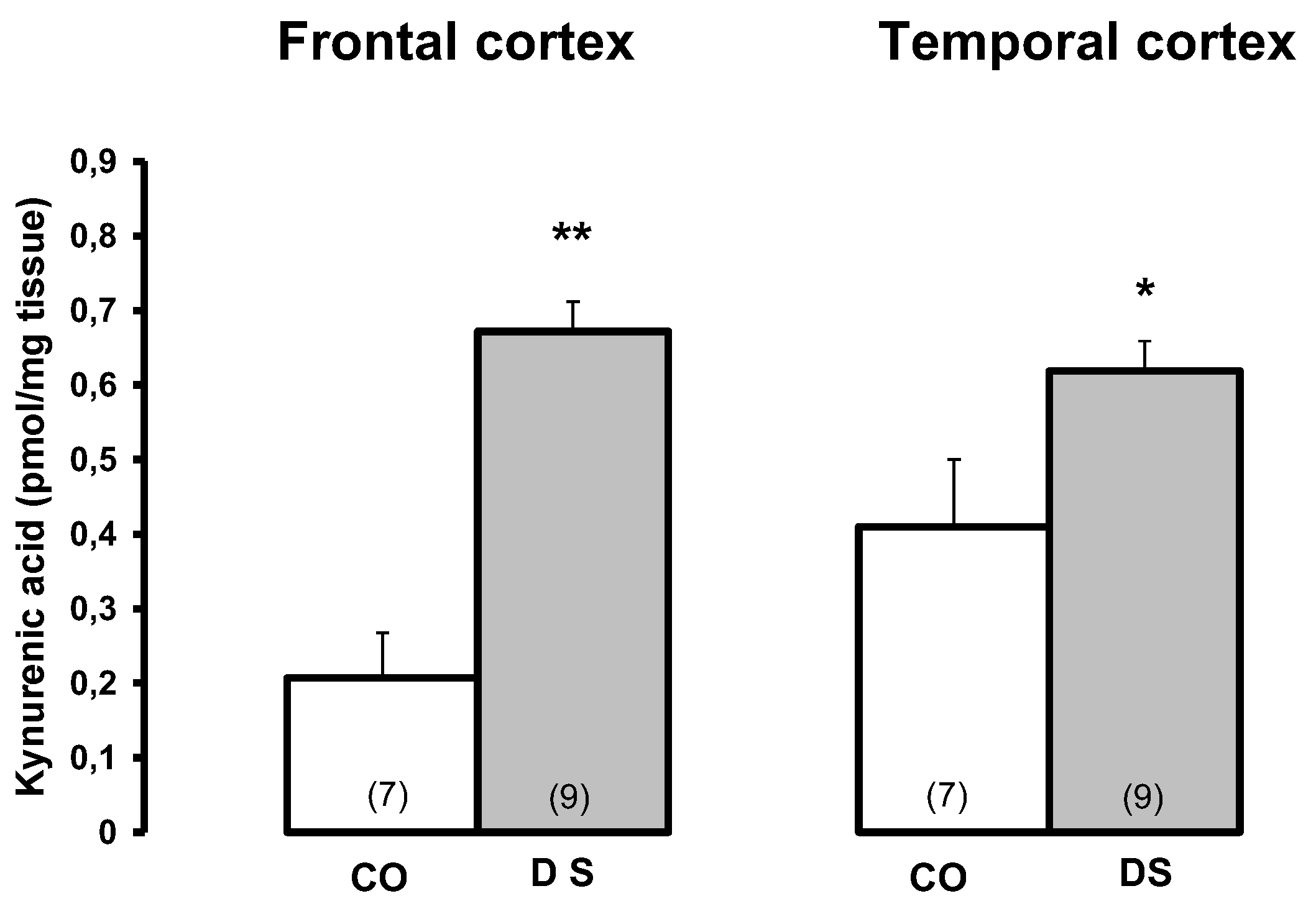
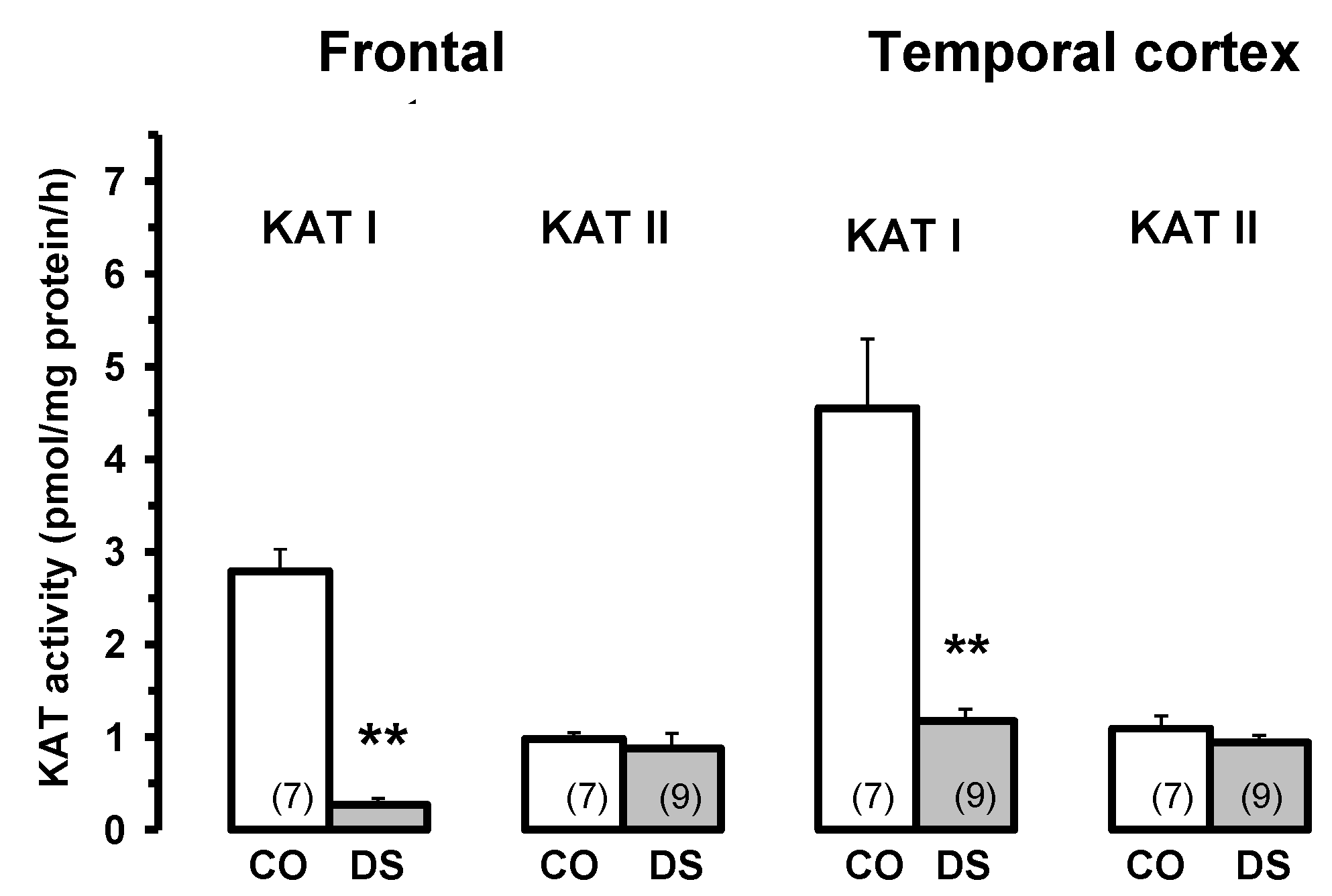
7. Different Types of Pathology after HIV-1
8. Glia Depressing Factor
9. Anti-Dementia Approaches
9.1. Stochastic Resonance Therapy (SRT)
9.2. Antidementia Drugs
9.2.1. Cerebrolysin
9.2.2. D-Cycloserine
9.3. Herbal Medicines - Prophylaxis – Protection
10. Future Perspectives
Authors' contributions
Acknowledgments
Disclosure statement
Abbreviations
References
- Brown, R.R. Biochemistry and pathology of tryptophan metabolism and its regulation by amino acids, vitamin B6 and steroid hormone. Am J Clin Nutr. 1971, 24, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Eastmann, C.L.; Guilarte, T.R. Cytotoxicity of 3-hydroxykynurenine in aneuronal hybrid cell line. Brain Res. 1989, 495, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolonic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993, 45, 309–379. [Google Scholar]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991, 56, 2007–2015. [Google Scholar] [CrossRef]
- Speciale, C.; Hares, K.; Schwarcz, R.; Brookes, N. High-affinity uptake of L-kynurenine by a Na+-independent transport of neutral amino acids in astrocytes. J Neurosci. 1989, 9, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Turski, W.A.; Gramsbergen, J.B.P.; Traitler, H.; Schwarcz, R. Rat brain slices produce and liberate kynurenic acid upon expose to L-kynurenine. J Neurochem. 1989, 52, 1629–1636. [Google Scholar] [CrossRef]
- Gal, E.M.; Sherman, A.D. L-Kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980, 5, 223–239. [Google Scholar] [CrossRef]
- Baran, H.; Schwarcz, R. Presence of 3-hydroxyanthranilic acid in rat tissue and evidence for its production from anthranilic acid in the brain. J Neurochem. 1990, 55, 738–744. [Google Scholar] [CrossRef]
- Moroni, F.; Russi, P.; Lombardi, G.; Beni, M.; Carla, V. Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988, 51, 177–180. [Google Scholar] [CrossRef]
- Moroni, F.; Russi, P.; Carla, V.; De Luca, G.; Politi, V. The regulation of brain kynurenic acid content: Focus on indole-3-pyruvic acid. In: Schwarcz, R.; Young, S.N.; Brown, R.R. (eds). Kynurenine and serotonin pathway progress in tryptophan research. Adv Exp Med Biol. Plenum Press, New York, London. 1991, 294, 299–308. [Google Scholar] [PubMed]
- Politi, V.; Lavaggi, M.V.; DiStazio, G.; Margonelli, A. Indole-3-pyruvic acid as a direct precursor of kynurenic acid. In: Schwarcz, R.; Young, S.N.; Brown, R.R. (eds). Kynurenine and serotonin pathway progress in tryptophan research. Adv Exp Med Biol. 1991, 294, 515–518. [Google Scholar] [PubMed]
- Kido, R. Kynurenate forming enzymes in liver, kidney and brain. In Schwarcz, R.; Young, S.N.; Brown, R.R. (eds). Kynurenine and serotonin pathways: Progress in tryptophan Research. Adv Exp Med Biol, Plenum Publishing Corporation, New York, ISBN 0-306-43929-8. 1989, Vol 294, 201-205. ISBN 0-306-43929-8.
- Baran, H.; Amann, G.; Lubec, B.; Lubec, G. Kynurenic acid and kynurenine aminotransferase in heart. Pediatr Res. 1997, 41, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Okuno, E.; Tsujimoto, M.; Nakamura, M.; Kido, R. Kynurenine-pyruvate aminotransferase in rat kidney and brain. In Schwarcz, R.; Young, S.N.; Brown, R.R. (eds). Kynurenine and serotonin pathways: Progress in tryptophan Research. Adv Exp Med Biol, Plenum Publishing Corporation, New York, ISBN 0-306-43929-8. 1989, Vol 294, 567-572.
- Okuno, E.; Du, F.; Ishikawa, T.; Tsujimoto, M.; Nakamura, M.; Schwarcz, R.; Kido, R. Purification and characterisation of kynurenine-pyruvate aminotransferase from rat kidney and brain. Brain Res. 1990, 534, 37–44. [Google Scholar] [CrossRef]
- Baran, H.; Kepplinger, B.; Draxler, M.; Ferraz-Leite, H. Kynurenic acid metabolism in rat, piglet and human tissues. 7th Congress of the European Society for Clinical Neuropharmacology Battistin, L. (ed) Medimond Srl, International Proceedings. 2004, 227-231.
- Kronsteiner, C.; Baran, H.; Kepplinger, B. Kynurenic acid levels and kynurenine aminotransferase I, II and III activities in ganglia, heart and liver of snail Helix Pomatia. Cell Physiol Biochem. 2023, 57, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Okuno, E.; Kido, R.; Schwarcz, R. Purification and characterisation of kynurenine aminotransferase I from human brain. J Neurochem. 1994, 62, 730–738. [Google Scholar] [CrossRef]
- Han, Q.; Li, J.; Li, J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur J Biochem. 2004, 271, 4804–4814. [Google Scholar] [CrossRef]
- Okuno, E.; Nakamura, M.; Schwarcz, R. Two kynurenine aminotransferases in human brain. Brain Res. 1991, 542, 307–312. [Google Scholar] [CrossRef]
- Schmidt, W.; Guidetti, P.; Okuno, E.; Schwarcz, R. Characterization of human brain kynurenine aminotransferases using [3H]kynurenine as a substrate. Neuroscience. 1993, 55, 177–184. [Google Scholar] [CrossRef]
- Guidetti, P.; Amori, L.; Sapko, M.T.; Okuno, E.; Schwarcz, R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007, 102, 103–111. [Google Scholar] [CrossRef]
- Yu, P.; Li, Z.; Zhang, L.; Tagle, D.A.; Cai, T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006, 365, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J.Y. Biochemical and Structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol. 2009, 29, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.; Ceresoli-Borroni, G.; Guidetti, P.; Zielke, C.L.; Zielke, H.R.; Schwarcz, R. Kynurenate production by cultured human astrocytes. J Neural Transm. 2003, 110, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Schwarcz, R.; Rizzi, M. Curiosity to kill the KAT (kynurenine aminotransferase): structural insights into brain kynurenic acid synthesis. Curr Opin Struct Biol. 2008, 18, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.C.; Du, F.; McCarthy, K.E.; Okuno, E.; Schwarcz, R. Immunocytochemical localization of kynurenine aminotransferase in the rat striatum: A light and electron microscopic study. J Comp Neurol. 1992, 326, 82–90. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonizes responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacology. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An Expanding range of targets for kynurenine metabolism of tryptophan. Trends in Paharmacological Science. 2013, 34, 34,136–143. [Google Scholar]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The Mechanism of the Neuroprotective Effect of Kynurenic Acid in the Experimental Model of Neonatal Hypoxia–Ischemia: The Link to Oxidative Stress. Antioxidants. 2021, 10, 1775. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Alkondon, M.; Pereira, E.F.R.; Castro, N.G.; Schrattenholz, A.; Barbosa, C.T.F.; Bonfante-Carbarcas, R.; Aracava, Y.; Eisenberg, H.M.; Maelike, A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997, 280, 1117–1136. [Google Scholar] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic receptors: from structure to function. Physiol Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Bergeron, R.; Meyer, T.M.; Coyle, J.T.; Greene, R.W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci USA. 1998, 22, 15730–15734. [Google Scholar] [CrossRef] [PubMed]
- D´Angelo, E.; Rossi, P.; Garthwaite, J. Dual component NMDA receptor currents at a single central synapse. Nature. 1990, 346, 467–470. [Google Scholar] [CrossRef]
- Koukouli, F.; Maskos, U. The multiple roles of the α7 nicotinic acetylcholine receptor in modulating glutamatergic systems in the normal and diseased nervous system. Biochem Pharmacol. 2015, 4, 378–387. [Google Scholar] [CrossRef]
- Rassoulpour, A.; Wu, H.Q.; Albuquerque, E.X.; Schwarcz, R. Prolonged nicotine administration results in biphasic, brain-specific changes in kynurenate levels in the rat. Neuropsychopharmacol. 2005, 30, 697–704. [Google Scholar] [CrossRef]
- Pearce, I.A.; Cambray-Deakin, M.A.; Burgoyne, R.D. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 1987, 223, 143–147. [Google Scholar] [CrossRef]
- Pugh, P.C.; Berg, D.K. Neuronal acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994, 14, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Damaj, M.E.; Glassco, W.; Dukat, M.; Martin, B.R. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 1999, 291, 1284–1291. [Google Scholar]
- Leeson, P.D.; Iversen, L.L. The glycine site on the NMDA receptor: structure-activity relationships and therapeutic potential. J Med Chem. 1994, 37, 4053–4067. [Google Scholar] [CrossRef]
- Sun, A.; Cheng, J. Novel targets for therapeutic intervention against ischemic brain injury. Clin Neuropharmacol. 1999, 22, 164–171. [Google Scholar] [PubMed]
- Du, F.; Eid, T.; Schwarcz, R. Neuronal damage after the injection of aminooxy acetic acid into the entorhinal cortex: a silver impregnation. Neuroscience. 1988, 82, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Whetsell, W.O., Jr; Abou-Khalil, B.; Lothman, E.W.; Schwarcz, R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- McMaster, O.G.; Baran, H.; Wu, H.Q.; Du, F.; French, E.; Schwarcz, R. Gamma-Acetylenic GABA produces axon-sparing neurodegeneration after focal injection into the rat hippocampus. Exp Neurol. 1993, 124, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Baran, H.; Gramer, M.; Hönack, D.; Löscher, W. Systemic administration of kainate induces marked increase of endogenous kynurenic acid in various brain regions and plasma of rats. Eur J Pharmacol. 1995, 286, 167–175. [Google Scholar] [CrossRef]
- Baran, H.; Kepplinger, B.; Herrera-Marschitz, M.; Stolze, K.; Lubec, G.; Nohl, H. Increased kynurenic acid in the brain after neonatal asphyxia. Life Sci. 2001, 69, 1249–1256. [Google Scholar] [CrossRef]
- Richter, A.; Löscher, W.; Baran, H.; Gramer, M. Increased levels of kynurenic acid in brains of genetically dystonic hamsters. Dev Brain Res. 1996, 92, 111–116. [Google Scholar] [CrossRef]
- Baran, H.; Du, F.; Schwarcz, R. Transient increase in striatal kynurenate synthesis following systemic kainate administration in rat. Soc for Neurosci Abs 1991, 17, 356.10. [Google Scholar]
- Baran, H.; Draxler, M.; Kronsteiner, C.; Kepplinger, B. Increase of kynurenic acid after Encephalomyocarditis virus infection and ist significances. Neurosignals. 2021, 29, 24–34. [Google Scholar]
- Baran, H.; Hainfellner, J.A.; Kepplinger, B.; Mazal, P.R.; Schmid, H.; Budka, H. Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm. 2000, 107, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Kerr-Pontes, L.R.; Oliveira, F.A.; Freire, C.A. Tuberculosis associated with AIDS: the situation in a Northeastern region of Brazil. Rev Saude Publica 1997, 31, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gordin, F.M.; Roediger, M.P.; Girard, P.M.; Lundgren, JD.; Miro, J.M.; Palfreeman, A.; Rodriguez-Barradas, M.C.; Wolff, M.J.; Easterbrook, P.J.; Clezy, K.; Slater, L.N. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med. 2008, 178, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.Y.; Wang, X.T.; Zhang, L.N. Chinese Critical Care Ultrasound Study Group (CCUSG): Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med Letter. 2020, 12, 1–2. [Google Scholar]
- Peters, C.E.; Carette, J.A. Return of the neurotropic enteroviruses: Co-opting cellular pathways for infection. Viruses. 2021, 13, 1–23. [Google Scholar] [CrossRef]
- Lucas, S.B.; Hounnou, A.; Peacock, C.; Beaumel, A.; Djomand, G.; N'Gbichi, J.M.; Yeboue, K.; Hondé, M.; Diomande, M.; C Giordano, C.; et al. The mortality and pathology of HIV infection in a West African city. AIDS. 1993, 7, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Lubec, B.; Marx, M.; Herrera-Marschitz, M.; Labudowa, O.; Hoeger, H.; Gille, L.; Nohl, H.; Mosgoeller, W.; Lubec, G. Decrease of heart protein kinase C and cyclin-dependent kinase precedes death in perinatal asphyxia of the rat. The FASEB Journal. 1997, 11, 482–492. [Google Scholar] [CrossRef]
- Urbańska, E.M.; Luchowski, P.; Luchowska, E.; Pniewski, J.; Woźniak, R.; Chodakowska-Zebrowska, M.; Lazarewicz, J. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol Rep. 2006, 58, 507–511. [Google Scholar]
- Vécsei, L.; Beal, M.F. Intracerebroventricular injection of kynurenic acid, but not kynurenine induces ataxia and stereotyped behaviour in rats. Brain Res Bull. 1990, 25, 623–627. [Google Scholar] [CrossRef]
- Vécsei, L.; Beal, M.F. Comparative behavioral and pharmacological studies with centrally administered kynurenine and kynurenic acid in rats. Eur J Pharmacol. 1991, 196, 239–246. [Google Scholar] [CrossRef]
- Baran, H.; Draxler, M.; Kalbacher, H.; Nowotny, N.; Schmidt, P.; Kepplinger, B.; Url, A.; Schuh, M.; Hofecker, G. Kynurenine aminotransferase I and II (KAT I and KAT II) in piglet’s brain after Encephalomyocarditis (EMCV) infection. Soc Neurosci Abstr. 200, 33, 215.8. [Google Scholar]
- Baran, H.; Draxler, M.; Kepplinger, B.; Kahlbacher, H.; Nowotny, N.; Schmidt, P.; Url, A.; Schuh, M.; Hofecker, G. Kynurenine metabolism in piglet brain due to EMCV infection. Pharmacology. 2003, 69, 212–217. [Google Scholar]
- Baran, H.; Staniek, K.; Kepplinger, B.; Stur, I.; Draxler, M.; Nohl, H. Kynurenines and the respiratory parameters in rat heart mitochondria. Life Sci. 2003, 72, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Staniek, K.; Bertignol-Spörr, M.; Attam, M.; Kronsteiner, C.; Kepplinger, B. Effects of various kynurenine metabolites on respiratory parameters of rat brain, liver and heart mitochondria. Int J Tryptophan Res. 2016, 9, 17–29. [Google Scholar] [CrossRef]
- Baran, H.; Staniek, K.; Bertignol, Attam, M.; Kepplinger, B. Influence of tryptophan metabolites on the respiratory parameters of rat brain mitochondria during aging. Soc for Neurosci Abs 2010, 406.9. [Google Scholar]
- Beal, M.F.; Swartz, K.J.; Isacson, O. Developmental changes in brain kynurenic acid concentrations. Dev Brain Res. 1992, 68, 136–139. [Google Scholar] [CrossRef]
- Baran, H.; Schwarcz, R. Regional differences in the ontogenetic pattern of kynurenine aminotransferase in the rat brain. Dev Brain Res. 1993, 74, 283–286. [Google Scholar] [CrossRef]
- Gramsbergen, J.B.P.; Schmidt, W.; Turski, W.A.; Schwarcz, R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992, 588, 1–5. [Google Scholar] [CrossRef]
- Wada, H.; Ito, H.; Orimo, H.; Sato, A. Kynurenine specifically increases in the cerebrospinal fluid of the aged rats. Biogenic Amines. 1994, 19, 221–225. [Google Scholar]
- Walker, D.W.; Curtis, B.; Lacey, B.; Nitsos, I. Kynurenic acid in brain and cerebrospinal fluid of fetal, newborn, and adult sheep and effects of placental embolization. Pediatr Res. 1999, 45, 820–826. [Google Scholar] [CrossRef]
- Kepplinger, B.; Baran, H.; Kainz, A.; Ferraz-Leite, H.; Newcombe, J.; Kalina, P. Age-related increase of kynurenic acid in human cerebrospinal fluid: Positive correlation with IgG and beta2-microglobulin changes. Neurosignals. 2005, 14, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Kepplinger, B.; Baran, H.; Kronsteiner, C.; Reuss, J. Increased levels of kynurenic acid in the cerebrospinal fluid in patients with hydrocephalus. Neurosignals. 2019, 27, 1–11. [Google Scholar]
- Greenamyre, J.T.; Maragos, W.F.; Albin, R.L.; Penney, J.B.; Young, A.B. Glutamate transmission and toxicity in Alzheimer’s disease. Review Prog Neuropsychopharmacol Biol Psychiatry. 1988, 12, 421–430. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Annals of Neurology. 1981, 10, 122–126. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Ikonomovic, M.D.; Styren, S.D.; Beckett, L.; Wisniewski, S.; Bennett, D.A.; Cochran, E.J.; Kodower, J.H.; Mufson, E.J. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002, 51, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Frölich, L.; Dirr, A.; Götz, M.E.; Gsell, W.; Reichmann, H.; Riederer, P.; Maurer, K. Acetylcholine in human CSF: methodological considerations and levels in dementia of Alzheimer type. J Neural Transm. 1988, 105, 961–973. [Google Scholar] [CrossRef]
- Jia, J.P.; Jia, J.M.; Zhou, W.D.; Xu, M.; Chu, C.B.; Yan, C.; Sun, Y.X. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Chinese Med J. 2004, 117, 1161–1164. [Google Scholar]
- Ikeda, Y.; Okuyama, S.; Fujiki, Y.; Tomoda, K.; Ohshiro, K.; Itoh, T.; Yamauchi, T. Changes of acetylcholine and choline concentrations in cerebrospinal fluids of normal subjects and patients with dementia of Alzheimer-type. J Neural Transm Suppl. 1990, 30, 25–32. [Google Scholar]
- Enquidawork, E.; Gulesserian, T.; Balic, N.; Cairns, N.; Lubec, G. Changes in nicotinic acetylcholine receptor subunits expression in brain of patients with Down syndrome and Alzheimer’s disease. J Neural Transm Suppl. 2001, 61, 211–222. [Google Scholar]
- Brugge, K.L.; Nichols, S.L.; Salmon, D.P.; Hill, L.R.; Delis, D.C.; Aaron, L.; Trauner, D.A. Cognitive impairment in adults with Down's syndrome: similarities to early cognitive changes in Alzheimer's disease. Neurology. 1994, 44, 232–238. [Google Scholar] [CrossRef]
- Baran, H.; Jellinger, K. Does increased kynurenic acid levels in the brain contribute to the impairment of memory in Alzheimer’s disease? Second International Oxidative Stress and Brain Damage Symposium, Abs., Chicago, Illinois, USA. 1997, Lecture.
- Baran, H.; Jellinger, K. Kynurenine metabolism in Alzheimer disease brain. Eur. J. of Neuroscience. 1998, 10 Suppl. 10, 32.09. [Google Scholar]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer’s disease. J Neural Transm. 1999, 106, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Cairns, N.; Lubec, B.; Lubec, G. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with DOWN syndrome. Life Sci. 1996, 58, 1891–1899. [Google Scholar] [CrossRef]
- Kepplinger, B.; Baran, H.; Kainz, A.; Newcombe, J.; Nohl, H. Altered kynurenic acid levels in CSF and serum of patients with multiple sclerosis. Multiple Sclerosis. 2001, 7 Supl. 1, S118, P400. [Google Scholar]
- Heyes, M.P.; Brew, B.J.; Saito, K.; Quearry, B.J.; Price, R.W.; Lee, K.; Bhalla, R.B.; Der, M.; Markey, S.P. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992, 40, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Connick, J.H.; Carla, V.; Moroni, F.; Stone, T.W. Increase in kynurenic acid in Huntington's disease motor cortex. J Neurochem. 1989, 52, 958–987. [Google Scholar] [CrossRef]
- Erhardt, S.; Blennow, K.; Nordin, C.; Skogh, E.; Lindström, L.H.; Engberg, G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001, 313, 96–98. [Google Scholar] [CrossRef]
- Schwarcz, R.; Rassoulpour, A.; Wu, H.Q.; Medoff, D.; Tamminga, C.A.; Robert, R.C. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001, 50, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Matson, W.R.; Beal, M.F.; Myers, R.H.; Bird, E.D.; Milbury, P.; Saso, S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992, 42, 1702–1706. [Google Scholar] [CrossRef]
- Hornykiewicz, O. Topography and behaviour of noradrenaline and dopamine(3-hydroxytryptamine) in the substantia nigra of normal and Parkinsonian patients (In German). Wien Klin Wochenschr. 1963, 75, 309–312. [Google Scholar]
- Hornykiewicz, O.; Kish, S.J. Neurochemical basis of dementia in Parkinson’s disease. Can J Neurol Sci. 1984, 11 (1 Suppl), 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hlinak, Z.; Krejci, I. Kynurenic acid and 5,7-dichlorokynurenic acids improve social and object recognition in male rats. Psychopharmacol. 1995, 120, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.J.; Stewart, M.G. 7-Chlorokynurenate, an antagonist of the glycine binding site on the NMDA receptor, inhibits memory formation in day-old chicks (Gallus domesticus). Behav Neural Biol. 1993, 60, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of kynurenic acid produce working memory deficits. Schizophr Bull. 2007, 33, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Rassoulpour, A.; Wu, H.Q.; Ferré, S.; Schwarcz, R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005, 93, 762–765. [Google Scholar] [CrossRef]
- Sperk, G.; Lassmann, H.; Baran, H.; Kish, S.J.; Seitelberger, F.; Hornykiewicz, O. Kainic acid induced seizures: Neurochemical and histopathological changes. Neuroscience. 1983, 4, 1301–1315. [Google Scholar] [CrossRef]
- Ting, K.K.; Brew, B.; Guillemin, G. The involvement of astrocytes and kynurenine pathway in Alzheimer’s disease. Neurotox Res. 2007, 12, 247–262. [Google Scholar] [CrossRef]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol of Aging. 1993, 421–429. [Google Scholar] [CrossRef]
- Ajenikoko, M.K.; Ajagbe, A.O.; Onigbinde, O.A.; Okesina, A.A.; Tijani, A.A. Review of Alzheimer’s disease drugs and their relationship with neuron-glia interaction. IBRO Neurosci Rep. 2023, 14, 64–76. [Google Scholar] [CrossRef]
- Monzón-Mayor, M.; Álvarez, M.I.; Arbelo-Galván, J.F.; Romero-Alemán, M.M.; Yanes, C.; Plaza, M.L.; Rodriguez, J.R.; Rodriguez, J.J.; Toledano, A. Long–term evolution of local, proximal and remote astrocyte responses after diverse nucleus basalis lesioning (an experimental Alzheimer model): GFAP immunocytochemical study. Brain Res. 2000, 865, 235–258. [Google Scholar] [CrossRef]
- Du Laping, N.J.; Teter, B.; Nichols, N.R.; Rozovsky, I.; Finch, C.E. Glial fibrillary acidic protein: regulation by hormones, cytokines, and growth factors. Brain Pathol. 1994, 4, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.; Lehmann, L.K.; Kischbaum, J.G.; Borden, E.C.; Lee, C.M.; Brown, R.R. Induction of tryptophan degradation in vitro and in vivo: A gamma-interferon stimulated activity. J Interferon Res. 1986, 6, 389–398. [Google Scholar] [CrossRef]
- Werner, E.R.; Bitterlich, G.; Fuchs, D.; Hausen, A.; Reibneger, G.; Szabo, G.; Dierich, M.; Wachter, H. Human macrophages degrade tryptophan upon induction by interferon gamma. Life Sci. 1987, 42, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Frank, A.; Hernanz, A. Relationship of interleukin-1 beta and beta2-microglobulin with neuropeptides in cerebrospinal fluid of patients with dementia of the Alzheimer type. J Neuroimmunol. 1993, 48, 235–240. [Google Scholar] [CrossRef]
- Brew, B.J.; Bhalla, R.B.; Fleisher, M.; Paul, M.; Khan, A.; Schwartz, M.K.; Price, R.W. Cerebrospinal fluid ß2-microglobulin in patients infected with human immunodeficiency virus. Neurology. 1989, 39, 830–834. [Google Scholar] [CrossRef] [PubMed]
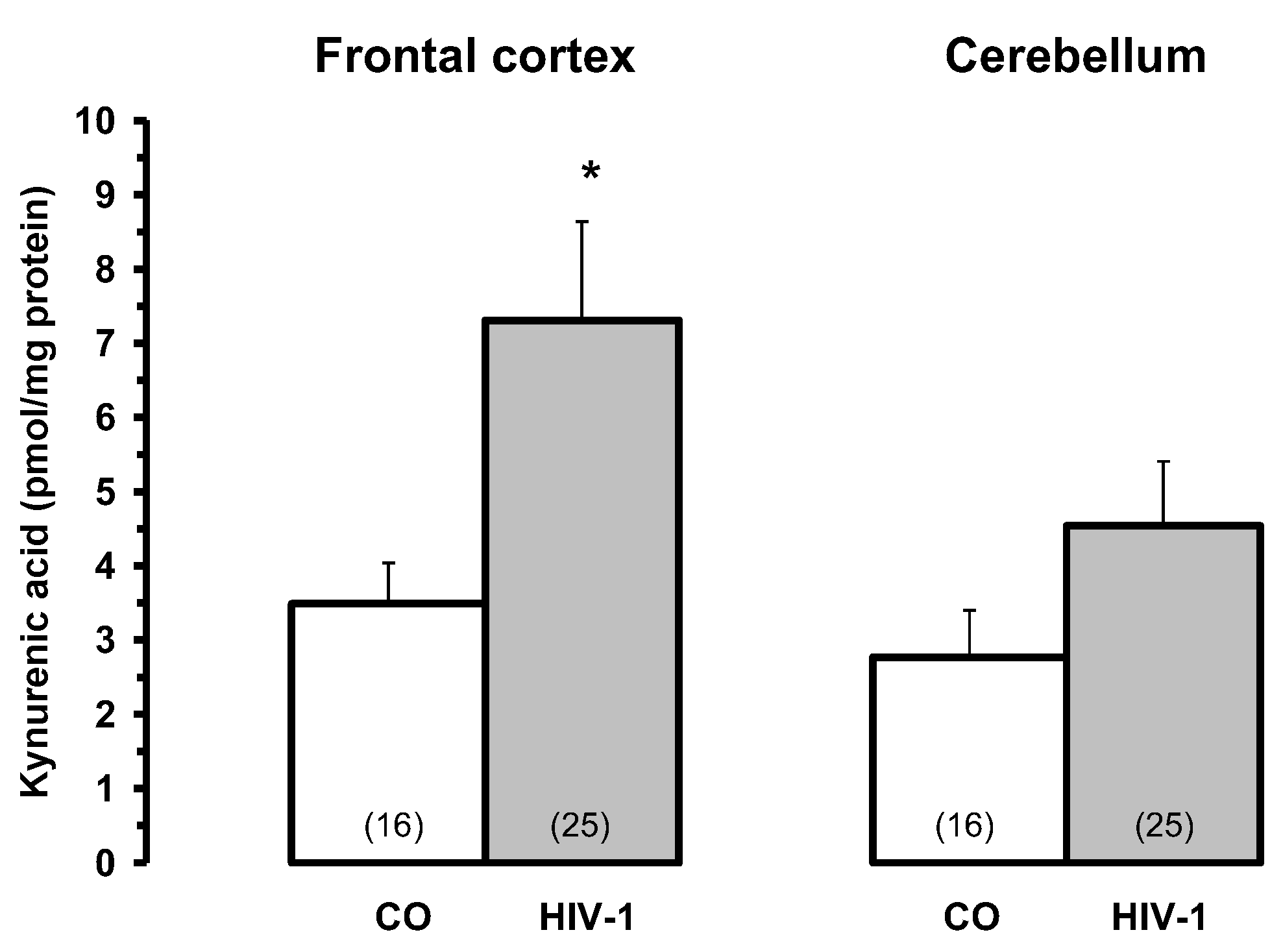
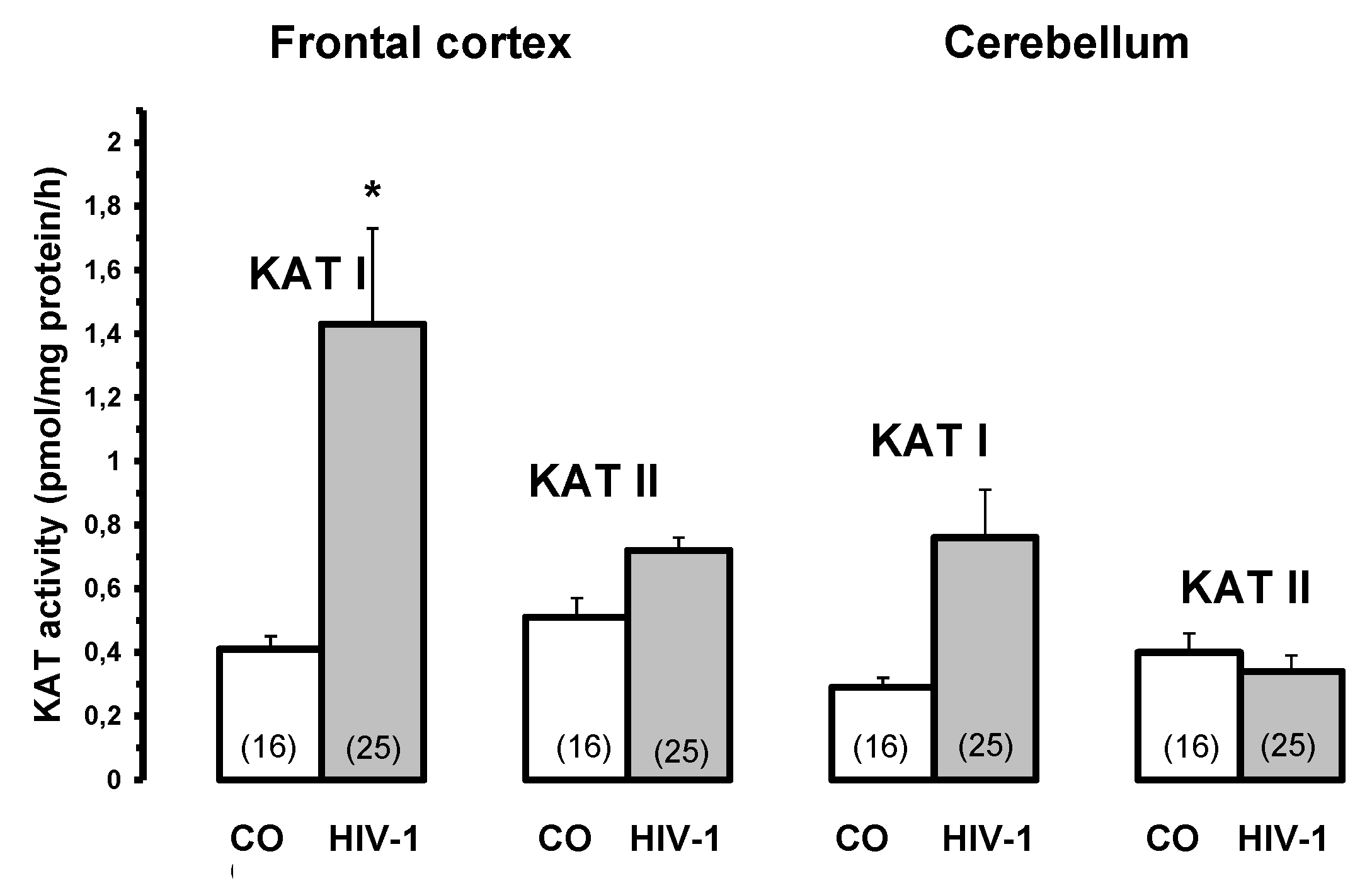
- Baran., H.; Hainfellner, J.A.; Kepplinger, B. Baran. H.; Hainfellner, J.A.; Kepplinger, B. Kynurenic Acid Metabolism in Various Types of Brain Pathology in HIV-1 Infected Patients. Int J Tryptophan Res 2012, 5, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Kepplinger, B.; Draxler, M. Endogenous kynurenine aminotransferases inhibitor is proposed to act as "Glia Depressing Factor" (GDF). Int J Tryptophan Res. 2010, 3, 13–22. [Google Scholar] [CrossRef]
- Vohra, M.; Lemieux, G.A.; Lin, L.; Ashrafi, K. The beneficial effects of dietary restriction on learning are distinct from its effects on longevity and mediated by depletion of a neuroinhibitory metabolite. PLoS Biol 2017, 15, 1–24. [Google Scholar] [CrossRef]
- Lopes, C.; Pereira, E.F.; Wu, H.Q.; Purushottamachar, P.; Njar, V.C.; Schwarcz, R.; Albuquerque, E.X. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha 7 nicotinic receptors. J Pharmacol Exp Ther. 2007, 322, 48–58. [Google Scholar] [CrossRef]
- Rockenstein, E.; Adame, A.; Mante, M.; Larrea, G.; Crews, L.; Windisch, M.; Moessler, H.; Masliah, E. Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer’s disease with the neurotrophic compound Cerebrolysin. J Neural Transm. 2005, 112, 269–282. [Google Scholar] [CrossRef]
- Goetz, C.G. Jean-Martin Charcot and his vibratory chair for Parkinson disease. Neurology. 2009, 73, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.T.; Buhlmann, A.; Turbanski, S.; Schmidtbleicher, D. Proprioceptive and sensorimotor performance in Parkinson's disease. Res Sports Med. 2006, 14, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; Farley, B.G. Exercise and neuroplasticity in persons living with Parkinson's disease. Eur J Phys Rehabil Med. 2009, 45, 215–229. [Google Scholar]
- Turbanski, S.; Haas, C.T.; Schmidtbleicher, D.; Friedrich, A.; Duisberg, P. Effects of random whole-body vibration on postural control in Parkinson's disease. Res Sports Med. 2005, 13, 243–256. [Google Scholar] [CrossRef]
- Hattori, S.; Naoi, M.; Nishino, H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res Bull. 1994, 35, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Rassoulpour, A.; Schwarcz, R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007, 114, 33–41. [Google Scholar] [CrossRef]
- Benzi, R.; Sutera, A.; Vulpiani, A. The mechanism of stochastic resonance. J. Phys. 1981, A14, L453.457. [Google Scholar] [CrossRef]
- Levin, J.E.; Miller, J.P. Broadband neural encoding in the cricket cercal sensory system enhanced by stochastic resonance. Nature. 1996, 380, 165–168. [Google Scholar] [CrossRef]
- Collins, J.J.; Imhoff, T.T.; Grigg, P. Noise-enhanced tactile sensation. Nature. 1996, 383, 770. [Google Scholar] [CrossRef]
- Collins, J.J.; Priplata, A.A.; Gravelle, D.C.; Niemi, J.; Harry, J.; Lipsitz, L.A. Noise-enhanced human sensorimotor function. IEEE Eng Med Biol Mag. 2003, 22, 76–83. [Google Scholar] [CrossRef]
- Yousefi, B.; Tadibim, V.; Khoei, A.F.; Montazeri, A. Exercise therapy, quality of life, and activities of daily living in patients with Parkinson disease: a small scale quasi-randomised trial. Trials. 2009, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Wunderer, K.; Schabrun, S.M.; Chipchase, L.S. Effects of whole body vibration on strength and functional mobility in multiple sclerosis. Physiother Theory Pract. 2010, 26, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Andel, R.; Crowe, M.; Pedersen, N.L.; Fratiglioni, L.; Johansson, B.; Gatz, M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008, 63, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Pitkala, K.H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.S.; Kautiainen, H.; Strandberg, T.E. Exercise rehabilitation on home-dwelling patients with Alzheimer's disease-a randomized, controlled trial. Study protocol. Trials. 2010, 11, 92. [Google Scholar] [CrossRef]
- Kluding, P.M.; Tseng, B.Y.; Billinger, S.A. Exercise and executive function in individuals with chronic stroke: a pilot study. J Neurol Phys Ther. 2011, 35, 11–17. [Google Scholar] [CrossRef]
- Kucyi, A.; Alsuwaidan, M.T.; Liauw, S.S.; McIntyre, R.S. Aerobic physical exercise as a possible treatment for neurocognitive dysfunction in bipolar disorder. Postgrad Med. 2010, 122, 122,107–116. [Google Scholar] [CrossRef]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Molteni, R.; Zheng, J.Q.; Ying, Z.; Gómez-Pinilla, F.; Twiss, J.L. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA. 2004, 101, 8473–8478. [Google Scholar] [CrossRef]
- Ying, Z.; Roy, R.R.; Edgerton, V.R.; Gomez-Pinilla, F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005, 19, 283–295. [Google Scholar]
- Kepplinger, B.; Kalina, P.; Zeiner, D.; Eigner, S.; Baran, H. Influence of exercise on kynurenic acid levels in the serum. Amino acids. 2007, 33, LVII. [Google Scholar]
- Kepplinger, B.; Baran, H.; Sedlnitzky-Semler, B.; Badawi, N.R.; Erhart, H. Stochastic Resonance Activity Influences Serum Tryptophan Metabolism in Healthy Human Subjects. Int J Tryptophan Res. 2011, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Kepplinger, B. Porcine tissues influence kynurenic acid formation. Parkinsonism Relat Disord. 2006, Suppl.1, Suppl. [Google Scholar]
- Crook, T.H.; Ferris, S.H.; Alvarez, X.A.; Laredo, M.; Moessler, H. Effect of N-PEP-12 on memory among older adults. Int Clin Psychopharmacol. 2005, 20, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.; Hutter Paier, B.; Windisch, M.; Doppler, E.; Moessler, H.; Wrowski, R. A peptide preparation protects cells in organotypic brain slices against cell death after glutamate intoxication. J Neural Transm. 2006, 113, 103–110. [Google Scholar] [CrossRef]
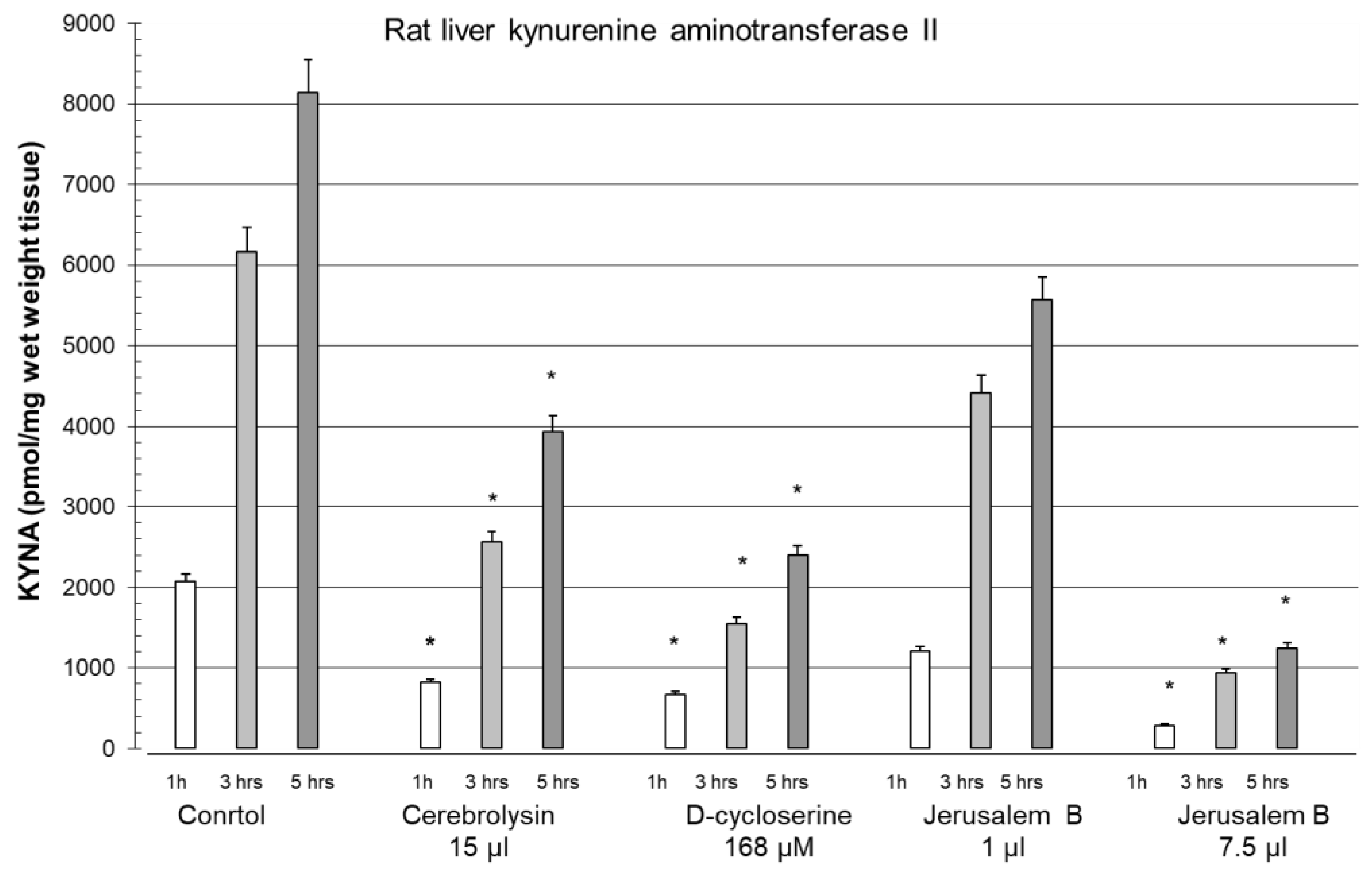
- Baran, H.; Kepplinger, B. Cerebrolysin lowers kynurenic acid formation - An in vitro study. Eur Neuropsychopharmacol. 2009, 19, 161–168. [Google Scholar] [CrossRef]
- Kemp, J.A.; Lesson, P.D. The glycine site of the NMDA receptor – five years on. Trends Pharmacol Sci. 1993, 14, 20–25. [Google Scholar] [CrossRef]
- Goff, D.C. D-Cycloserine: An evolving role in learning and neuroplasicity in Schizophrenia. Schizophrenia Bulletin. 2012, 38, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Löscher, W.; Mevissen, M. The glycine/NMDA receptor partial agonist D-cycloserine blocks kainate-induced seizures in rats. Comparison with MK-801 and diazepam. Brain Res. 1994, 652, 195–200. [Google Scholar] [CrossRef]
- Baran, H.; Gramer, M.; Löscher, W. Alterations in plasma and brain amino acids after administration of the glycine/NMDA receptor partial agonist, D-cycloserine, to mice and rats. Eur J Pharmacol. 1995, 373, 197–201. [Google Scholar] [CrossRef]
- Baran, H.; Kepplinger, B. D-cycloserine lowers kynurenic acid formation – New mechanism of action. Eur Neuropsychopharmacol. 2014, 4, 639–644. [Google Scholar] [CrossRef]
- Goff, D.C. D-cycloserine in Schizophrenia: New Strategies for Improving Clinical Outcomes by Enhancing Plasticity. Curr Neuropharmacol. 2017, 15, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kepplinger, B.; Kronsteiner, C.; Więcek, M.; Baran, H. Hawthorn berry extract lowers kynurenic acid and anthranilic acid formation. 15th ANA Meeting at the IST Austria, 2017 September 24th-26th, Abstract/Lecture.
- Baran, H.; Pietryja, M.J.; Kronsteiner, C.; Kepplinger, B. Jerusalem balsam lowers kynurenic acid formation: an in vitro study. J Tradit Med Clin Natur. 2017, 6, 3. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment o the evidence. J Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



