Introduction
Ribonucleic acid (RNA) and proteins represent the essential components of biological activity that occur between genes, facilitating a process known as "protein synthesis." This process is executed through RNA processing, with RNA splicing being particularly relevant in this context. The RNA chaperone hypothesis presents the idea that the challenge of correct folding is intrinsic to the history of life on Earth and could have significantly influenced the development of RNA-protein interactions. The proposed hypothesis indicates that RNA encounters two primary challenges in achieving its accurate structural configuration: firstly, there is a tendency for RNA to misfold and develop alternative conformations that may become kinetically trapped; secondly, the inherent instability of the tertiary structure and its predisposition to transition towards the most thermodynamically favourable configuration present further complications. It has been posited that RNA-binding proteins play a crucial role in addressing both of these issues. An illustrative example is the category of non-wide ranging RNA-binding proteins, which typically function as RNA chaperones, rapidly reorganizing RNA molecules by preventing misfolding and rectifying any atypical folding that has taken place. Consequently, RNA performs its biological functions efficiently. Furthermore, RNA achieves proper folding and the synthesis of its tertiary structure via interactions with proteins, thus addressing the thermodynamic challenges and guiding RNA molecules toward their appropriate functional conformation. The emergence of these non-specific RNA-binding proteins might be considered a significant transition period from an RNA-dominated world, in which RNA was the central element of biological functions, to the contemporary RNA-protein world. While some RNA-binding proteins function to solve RNA misfolding issues, other RNA-binding proteins have chaperoning properties on specific RNA targets. RNA-dependent ATPases are part of the third class of protein, also thought to be RNA chaperones, which can facilitate spatial and temporal RNA folding regulation. The term "RNA chaperone" is specifically used for proteins that interact with RNA to allow correct folding; thus, cells recognize these proteins but not the RNA molecules themselves, which actually defines the function of other proteins acting as chaperones. Moreover, protein chaperones besides RNA chaperones refer to those proteins that ensure correct folding of other proteins. Alternatively, one can verbalize the term to refer to RNA chaperones as proteins that ensure accurate folding of RNA by either preventing misfolding or correcting overly twisted RNA structures. This is in contrast to proteins that drive the folding of RNA either by facilitating intermediate steps in the folding pathway or by stabilizing the folded form. Speculations about the RNA chaperones being responsible for the biological activity of RNA in living beings without any direct evidence have been constantly made in the literature. The RNA chaperone hypothesis thus highlights the dissipation of kinetic barriers that hinder proper RNA folding and thus provides a unique framework for addressing the physical mechanisms of RNA-protein interaction in relation to RNA structure. Although this discourse is mainly focused on the challenges that arise in kinetic folding, thermodynamic folding challenges are recognized as essential constituent parts of the overall complexity surrounding RNA folding.
The Two Core Challenges in RNA Folding
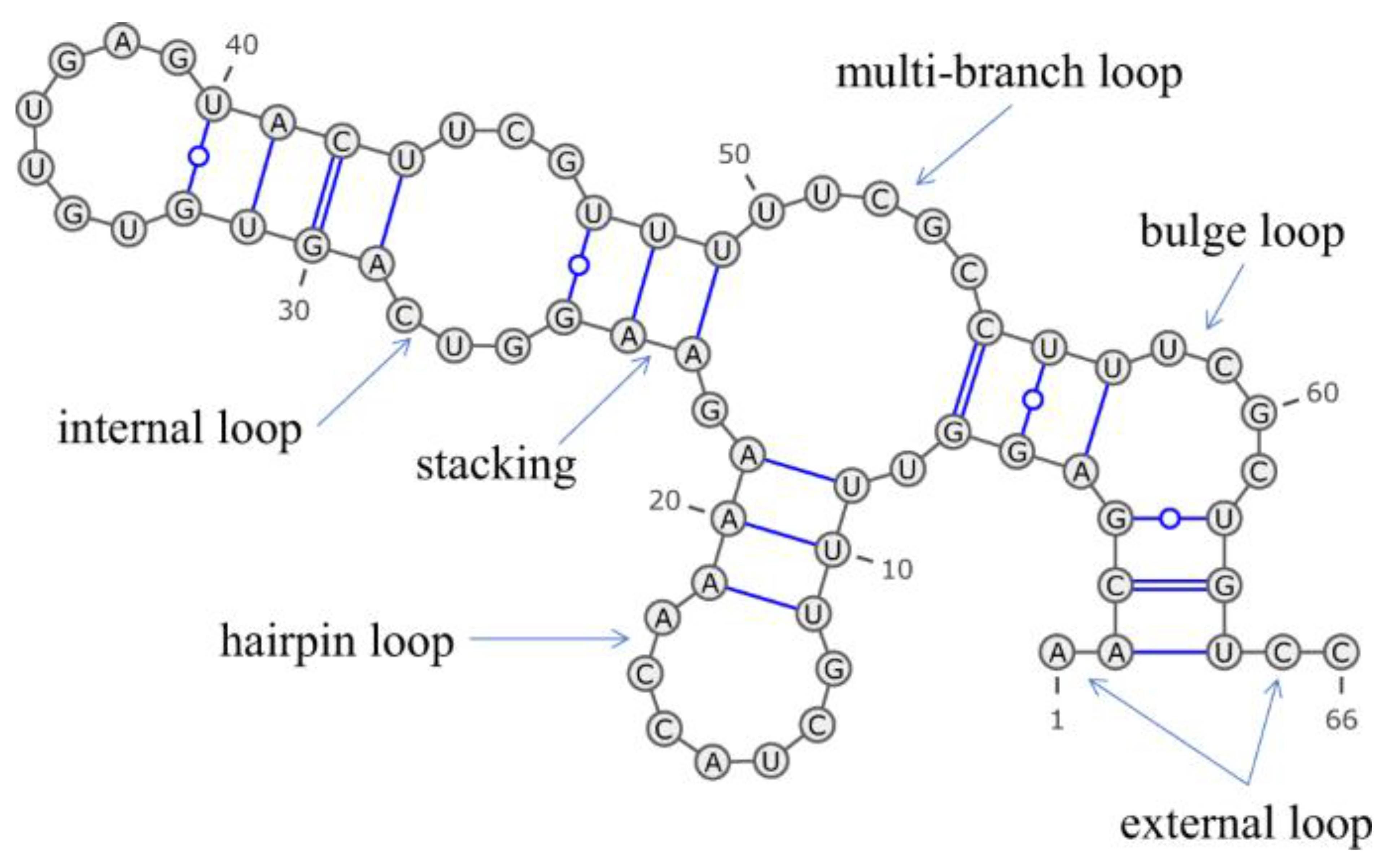
Kinetic traps and thermodynamic instability are two of the major challenges in attaining precise three-dimensional structure for RNA molecules. In kinetic traps, the RNA may take erroneous, nonfunctional conformations much faster than it could change over into the correct configuration. That way, the RNA molecules might be adversely affected from achieving the most stable and biologically active form. On the other hand, there is thermodynamic instability when the final folded form of RNA, very often possessing complex tertiary structures, does not exhibit enough stability in physiological conditions. In such cases, the molecule may switch to less stable or other conformers, which further complicates the folding mechanism. In vitro studies concerning RNA misfolding have shown that not only may inactive or inappropriate conformations of RNA be kinetically trapped but also prevent such molecules from being able to reform into their functional forms, even for very prolonged times. Early results showed that tRNAs come in two general types, but only one of those can be activated by the specific aminoacyl-tRNAsynthetase [
2,
3]. Specifically, the inactive forms of tRNALeu turned out to be stable for several hours, irrespective of the addition or exclusion of magnesium ions. However, these inactive tRNAs can be converted into an active structure by the use of heat in combination with Mg2+ ions [
2]. This might indicate that these inactive tRNAs take a stable, alternative secondary structures (
Figure 1) [
4,
5]. For larger RNA molecules, further evidence for kinetic barriers to the correct folding is provided. For example, full execution of in vitro self-splicing reactions involving group I introns with sequences longer than 200 nucleotides is usually impossible. Presumably, this occurs because misfolded structures become kinetically trapped at various stages along the folding pathway [
6,
7].
Although the findings regarding RNA folding made in vitro have been very illuminating, just how applicable they are to the RNA within living cells is obscure. Probably, at least in some measure, the difficulties arise because of the methods used in an in vitro experiment, such as purifying RNA under denaturing conditions and then refolding. A comparative analysis of RNA's primary, secondary, and tertiary structures in comparison with similar studies of protein structure indicates that kinetic and thermodynamic barriers inherent to the folding of RNA, as observed in studies of tRNA [
8].
Primary Structure
When comparing RNA and proteins at the primary structural level, RNA exhibits a much lower degree of diversity. While proteins are made up of 20 different amino acids, RNA is composed of only four nucleotide bases. These four nucleotides are more similar to each other than the side chains of proteins, as they only vary between purines and pyrimidines. The flat, planar nature of these RNA bases, each equipped with hydrogen bond donors and acceptors, further contributes to the low diversity. By contrast, protein side chains are highly variable in terms of size, charge, and hydrophobicity, which help proteins adopt unique tertiary structures. The limited diversity of RNA's primary structure reduces its "information content," making it more challenging for an RNA sequence to fold into a distinct, stable tertiary structure.
Secondary Structure
RNA secondary structures, primarily duplexes, are highly stable and exhibit different dynamics compared to protein folding. For example, the most stable protein alpha-helices unfold on the sub-microsecond scale [
9]. In contrast, an RNA duplex of 10 base pairs has a dissociation half-time of around 30 minutes, with G/C-rich duplexes taking up to 100 years to dissociate at 30°C [
10]. This immense stability can cause RNA molecules to become stuck in misfolded conformations, unable to transition to their correct forms. The persistence of such incorrect folds could block access to mRNA and even hinder the turnover of RNA after successful folding.The propensity for forming alternative folds appears to be a widespread feature of RNA molecules. Even random sequences of RNA tend to form secondary structures, with predictions suggesting that nearly half of the residues in such sequences are base-paired. This estimate aligns with experimental observations of the helical content in randomly folded RNA molecules [
11,
12].
Tertiary Structure
The issues surrounding RNA secondary structure become even more pronounced when tertiary interactions come into play. Various molecular interactions, such as those involving 2′-hydroxyl groups, phosphate groups, and metal ions, alongside non-standard base pairings, can further stabilize misfolded RNA conformations. Even when an RNA molecule has successfully folded into its correct secondary structure, the challenges are far from over. The limited variability of RNA's primary structure is compounded by the fact that its base-pairing faces are often hidden within duplexed regions, whereas in proteins, the side chains are exposed in alpha-helices and beta-sheets. This means that many secondary structural elements in RNA look alike, making it difficult for RNA to consistently specify a unique tertiary structure.
An example of this challenge can be seen in the Tetrahymena group I ribozyme, where approximately 1 in 1,000 RNA duplexes misdock into incorrect tertiary interactions, and mutations can increase this misdocking rate to nearly 50% [
13]. However, while the formation of correct tertiary structures in RNA can be challenging, it is not impossible. A relatively small thermodynamic advantage—on the order of just 2 kcal/mol—is often enough to ensure that RNA folds correctly in more than 95% of cases [
1].These insights underline the complexities and barriers that RNA molecules face in achieving their proper structures, both kinetically and thermodynamically.
RNA Chaperones: Solutions to RNA Misfolding Problems
The general concept of RNA chaperones stems from the inherent problem that RNA commonly becomes kinetically stuck in inappropriate conformations during folding. Experimental studies established that non-specific RNA-binding proteins reduce this challenge in vitro by enabling the proper structure of RNA to be reached. It has been suggested, therefore, that RNA-binding proteins act like RNA chaperones within living organisms to avoid RNA misfolding and to facilitate the proper folding of RNA configurations. This concept was initially proposed more than twenty years prior, when it was revealed that UP1, a truncated variant of the hnRNP A1 protein, was capable of refolding kinetically trapped 5S rRNA and tRNAs. This finding implies that analogous RNA chaperone functions may be crucial for various biological processes [
14,
15].
This hypothesis follows on the premise from previous DNA annealing experiments that show lengthy single strands of RNA or DNA reassociate at an enormously slower rate than oligonucleotides of shorter lengths [
16]. The reason for this slow rate was attributed to intramolecular structure formation in longer nucleic acid chains that interfere with the joining of complementary strands. Single-strand nucleic acid-binding proteins, such as T4 gene 32 protein and
Escherichia coli SSB, supported the annealing of polynucleotides through interruption of these intramolecular configurations, thus promoting intermolecular base-pairing [
16]. The RNA chaperone model extended this analogy further between RNA and protein folding. Just like protein chaperones, which prevent protein misfolding, it was suggested that RNA chaperones help in the proper folding of RNA by reordering misfolded intermediates [
17]. Recent experimental results have provided strong evidence for the RNA chaperone function of RNA-binding proteins.
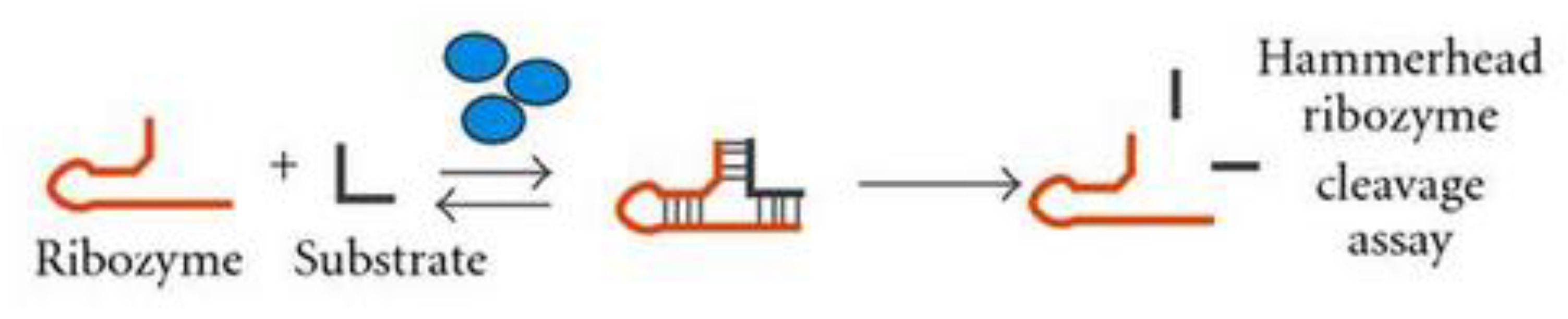
The turnover rate and substrate specificity linked with the hammerhead RNA-based enzymes (ribozymes) reaction is hampered by slow physical processes, making it a significant model in probing the kinetics of RNA folding (
Figure 2). Here, the disassociation of intermolecular duplexes is critical for high turnover as well as distinguishing correct from incorrect RNA substrates [
18]. These challenges mimic the process of misfolded RNA secondary structures unfolding at any time in the folding process. It has been shown that proteins such as HIV-1 NC and hnRNP A1 enhance these kinetic phases, thereby enhancing the efficacy of ribozymes. The HIV-1 NC protein can actually correct a misfolded complex that is stuck in an alternative structure [
19,
20]. Furthermore, whereas the self-splicing mechanism of group I introns is highly inefficient and takes place very slowly in vitro, this reaction proceeds rapidly and efficiently in vivo [
1].
The detected inconsistency suggests that proteins may aid splicing in biological organisms by associating with introns and stabilizing their catalytically competent conformations [
21]. For instance, S12 ribosomal protein from E. coli encourages correct folding of group I introns through unspecific interactions indicating a special mechanism for the support of RNA folding and splicing under biological conditions [
1]. The function of S12 in the process of intron splicing has further strengthened the comparison between RNA chaperones and their protein counterparts. Numerous attributes of the S12 protein lend credence to this perspective.
Initially, the protein shows no distinct preference for group I introns over exons or other RNA sequences, indicating that it does not somehow stabilize the active conformation of the intron through specific interactions. Notably, S12 can stimulate the hammerhead ribozyme reaction similarly to nonspecific activity observed with proteins HIV-1 NC and hnRNP A1 (
Figure 3) [
1]. Thirdly, S12 is able to assist the splicing of unreactive precursor RNAs which are kinetically trapped, thus implying its ability to deal with misfolded RNA intermediates. Lastly, similarly to protein chaperones, S12 is only required during the formation of the RNA fold and is not part of the mature active RNA species. The removal of the protein by proteolysis before the reaction, however, does not make it lose its stimulatory effect to group I intron self-splicing, hence confirming its role as a true chaperone that functions only during folding.
Binding Mechanism of RNA chaperone
A potential RNA-binding protein could also in principle act as an RNA chaperone by "pre-association binding," which might act to overcome the kinetic barriers associated with RNA folding. Although an RNA-binding protein could in principle sequester the RNA into the correct conformation to overcome the thermodynamic folding problem, that conformation may still be transitorily inaccessible because of the kinetically-based problem. The two bottlenecks encountered are: (i) kinetic entrapment of RNA in misfolded conformations (k
misfold); and (ii) the insufficient thermodynamic stability of correctly folded RNA that prevents their survival for enough time to effectively interact with the corresponding protein (k
trap versus k
unfold). The said tribulation indicates that the ribosomal protein S12 acting as a chaperone for the folding of group I introns may be helpful in dealing with such types of kinetic-folding problems [
1]. The pre-association mechanism for the protein allows it to make an initial binding with the unfolded RNA through a nonspecific interaction or through partial specific contacts to avoid misfolding. Many RNA-binding proteins have significant levels of nonspecific binding that may be involved in this chaperone-like function. Actually, after the initial binding, the RNA undergoes rearrangements either within the complex itself or through cycles of its dissociation followed by reassociation until the proper structure is achieved and stabilized through more specific interactions of proteins with RNAs. Such proteins represent Specific RNA-binding proteins that, besides, possess functionality as RNA chaperones [
1].
Such a function would resemble protein chaperones; for example, they promote proper folding of proteins, for the most part by preventing improper folding [
22]. Hence, RNA chaperones would seemingly facilitate folding of RNA for the most part by excluding improper conformations. In some depth, subsequent comparisons and contrasts it draws between RNA chaperones and protein chaperones provide insightful observations. Protein chaperones form one distinct category that assist in the folding of proteins; in stark contrast, the very promiscuous character of RNA-binding proteins interactions suggests that the vast majority of such proteins must act as RNA chaperones in vitro [
1]. The hnRNPs that package pre-mRNA during transcription of it naturally qualify as prima facie candidates for the roles of cellular RNA chaperoning [
23].
A clear difference in the binding properties of RNA versus protein chaperones can be seen. While protein chaperones are to be involved in the folding process but then leave the folded product, it would seem an RNA chaperone cannot be released from folded RNA because it exhibits strong nonspecific binding ability. The recognition elements at the foundation of these two classes of chaperone may explain this. Protein chaperones recognize misfolded proteins through the exposure of hydrophobic regions that are sequestered in the native fold, leading to the release of the chaperone [
1]. In contrast, the anionic phosphodiester backbone of RNA, as well as the exposed bases in both the misfolded and unfolded conformations, leads to electrostatic-based nonspecific binding. Such nonspecific binding may be critical for the ability of RNA chaperons to correct misfolded RNA structures that otherwise would be stable.
Mechanistic studies on protein chaperones suggest that these proteins prevent protein misfolding through inhibition by sequestering unfolded proteins and preventing their aggregation [
24]. RNA chaperones could work similarly to protein chaperones, essentially by having a high-affinity interaction with specific regions of an RNA molecule, thereby preventing or slowing the incorrect intramolecular interactions. The advantage of RNA chaperones includes rescuing of misfolded RNA structures that most of the classical protein chaperones studied can only unfold, but cannot disaggregate the protein highly efficiently. At the same time, due to the very high potential of nonspecific binding, RNA chaperones are actively able to contribute to rescuing those misplaced RNA structures. Only the discovery of the Hsp104 protein, which can resolubilize protein aggregates, provides an exception, implying that some protein chaperones may disassemble aggregates, while the mechanism certainly has to be sorted out [
25].
Apart from the above-mentioned chaperones, other proteins, such as prolylisomerases and protein disulfideisomerases, have consequential roles in helping in the correct folding of proteins [
26]. There are also certain RNA-binding proteins with RNA chaperone activity that prevent or even reverse the misfolding of cognate and non-cognate RNAs. These proteins themselves could also be "chaperones" in folding, sequestering correctly folded domains or subdomains to help guide the RNA toward its final, functional conformation. Protein-protein contacts may also promote folding by positioning two RNA molecules (or different parts of one RNA) in proximity so that duplex formation or other crucial interactions are more likely [
27]. These proteins probably shouldn't be called chaperones; they are better considered as "matchmakers," promoting self-assembly, perhaps in very complex, RNA-processing reactions like spliceosome assembly [
1]. HnRNP A1 has been propounded to also act as an RNA chaperone and as a matchmaker. Another difficult task is to separate these two mechanisms mechanistically, since both lead to an increase in RNA/RNA assembly rates [
1].
Moreover, some RNAs themselves could be the RNA chaperones and fold other RNA molecules into their native structures. For example, "facilitator" RNAs bind in a base-pairing interaction with a ribozyme close to the substrate, locking out the ribozyme from misfolding and thereby making the substrate more accessible to base-pairing [
28]. This is analogous to the action of single strand binding proteins, which stabilize duplexes. Some of these RNA molecules can be modified intramolecularly in a fashion that either forms or repairs folding problems, much like a prosequence removes kinetic barriers to the folding of some bacterial proteases [
29,
30].
The analogy between the folding machinery of RNA and proteins can also be extended to RNA-dependent ATPases (Rd-ATPases), a class of protein which hydrolyzes ATP to catalyze structural changes [
1]. While most protein chaperones are strictly ATP-requiring enzymes, RNA chaperones often function independently of this nucleotide [
31]. Still, Rd-ATPases- analogous to helicases-rely on ATP to elastically unwind duplexes and other conformers of RNA in sequence [
32]. The ATP-driven RNA folding and unfolding activity facilitates incorporation into wider biological mechanisms. Thus, it may be conceived that during some stage of the assembly process of the spliceosome, an Rd-ATPase can dissociate the U4-U6 snRNP complex at a time such that splicing is hindered at that moment but goes on later to efficiently form a catalytically competent spliceosome [
33]. Alternatively, the primary role of U4 snRNA could be as an RNA chaperone, preventing U6 misfolding. Rd-ATPases could also serve as a means of distinguishing among several splice sites, or alternatively as a proofreading process so that the splicing was correct by limiting the time each assembly and catalytic step required [
34]. Thirdly, Rd-ATPases are implicated in ribosome assembly and translation initiation, another broad functionality favouring their contribution to RNA metabolism.
An Evolutionary Outlook of RNA Chaperones
From an evolutionary standpoint, the concepts discussed earlier can be framed within a broader, yet speculative, hypothesis that addresses the shift from an RNA-only world to one where both RNA and proteins coexisted and interacted. A key step in this transition likely involved the emergence of primitive peptides with nonspecific RNA-binding abilities, which displayed chaperone-like functions. These early peptides may have provided a significant evolutionary advantage by preventing RNA molecules from becoming trapped in nonfunctional, kinetically unfavorable conformations. These peptides could have facilitated the transition of RNA from a post-replicative double-stranded form into its proper, functional single-stranded configuration. This process would have enabled RNA molecules to explore a wider array of structural possibilities, contributing to functional diversity in an RNA-dominated world. The likelihood of these nonspecific RNA-binding peptides emerging is considered higher than that of highly specific RNA-binding proteins. This is because the structural and functional requirements for nonspecific binding are much less stringent, and a nonspecific peptide would have had many potential RNA targets to interact with. Thus, nonspecific binding represents a more accessible solution to the problem of early RNA folding and stabilization.
As evolution progressed, the challenges associated with RNA folding and function could have been transformed into new biological opportunities. The cooperative interaction between RNA and proteins likely became more sophisticated, with nonspecific RNA-binding proteins evolving to exhibit chaperone activity. Over time, these proteins may have developed more specific binding preferences and acquired ATP-dependent mechanisms, which allowed for greater regulatory control and functional specialization. This transition from generalist chaperones to more specialized protein-RNA interactions likely opened new avenues for the regulation of RNA structure and function within the cell.
One example of this evolutionary progression is seen in the heterogeneous nuclear ribonucleoprotein (hnRNP) A1. This protein not only displays RNA chaperone activity but also plays a role in the regulation of splice site selection during pre-mRNA processing [
35,
36]. Such dual functionality illustrates how proteins that originally evolved as general RNA chaperones could later acquire more specific roles in RNA metabolism and regulation. Similarly, the nucleocapsid (NC) protein from the human immunodeficiency virus (HIV) serves as an RNA chaperone and also binds viral RNA with high specificity during the viral packaging process [
1]. This suggests that the functional transition from nonspecific binding to targeted interactions with RNA was a critical evolutionary step in the development of complex RNA-protein regulatory systems.
RNA Chaperone Mechanisms, Regulation & Thermodynamics
The unfolding of RNA by molecular chaperones is governed by the principle that these proteins must exhibit a preferential binding affinity for single-stranded RNA as compared to double-stranded regions [
1,
37]. This concept finds early experimental support in studies on hnRNP A1 and StpA, which demonstrated their ability to selectively interact with RNA molecules containing unstructured or partially unfolded regions [
38,
39]. According to basic thermodynamic principles, the stronger a chaperone binds to the unfolded RNA, the more effective it will be at promoting RNA unfolding. However, excessively strong binding interactions between a chaperone and RNA may result in the failure of the RNA to be released, thereby restricting the chaperone’s capacity to act on a wide variety of RNA structures [
40]. In practical scenarios, RNA chaperones can unfold both correctly folded and misfolded RNAs with little to no discrimination between them, implying that thermodynamic models alone are insufficient to fully capture the intricacies of chaperone function [
37]. Most chaperones, rather than relying solely on thermodynamic stability, exploit differences in the dynamic behaviour of folded and unfolded RNA to facilitate efficient RNA folding pathways in the short term.
A key feature of RNA chaperones is their transient interaction with substrates, as this is essential for enabling regulatory RNA molecules to form functional structures. The classic iterative annealing model of chaperone action provides a conceptual framework in which chaperones engage in cycles of binding and partial unfolding of their substrates, followed by their release, allowing the RNA or protein to fold anew [
41]. This cycle is repeated multiple times, thereby increasing the likelihood of correct folding and ultimately leading to a greater proportion of properly folded RNA or proteins. During each cycle, the RNA has the potential to partition into folding pathways that either lead to the formation of native, functional structures or misfolded, non-functional structures [
42]. Should the pool of native RNA become depleted due to subsequent biochemical reactions or the introduction of additional interacting proteins, the iterative chaperone action ensures an increased throughput of RNA folding and assembly processes, regardless of whether the chaperone is capable of distinguishing between native and non-native RNA structures.
The iterative annealing mechanism has been experimentally validated by studies on the GroEL protein chaperone and the CYT-19 DEAD-box RNA chaperone, both of which use ATP hydrolysis to drive multiple rounds of substrate unfolding [
41,
43]. Recent theoretical analyses of the reaction kinetics involved in GroEL and CYT-19 ATPases suggest that active protein and RNA chaperones operate under nonequilibrium conditions, enhancing the short-term accumulation of native substrate, despite the fact that some portion of the native protein or RNA may also be unfolded during these cycles [
44]. This model proposes that repeated unfolding events expedite the process by which substrates attain their native structure, although the overall efficiency of the chaperone system hinges on its ability to differentiate between correctly folded and misfolded structures.
Passive RNA chaperones similarly undergo cycles of binding and dissociation with their substrates, transiently unfolding and refolding RNA molecules in the process [
37]. This cyclical activity plays a crucial role in facilitating annealing events between trans-acting regulatory RNAs and their respective target sequences. In this context, the chaperone must simultaneously bind both the regulatory RNA and its target, forming a ternary complex that permits base pairing between the two RNA molecules [
45]. Once this base pairing occurs, the chaperone either releases the newly formed RNA duplex or becomes recycled as the RNA complex undergoes turnover [
46].
For both intra- and intermolecular RNA folding events, the rapid on-and-off binding cycles of chaperones are critical for allowing RNA molecules to explore the conformational space necessary to identify their most thermodynamically stable structure [
47]. This principle has been reinforced by studies on various RNA-binding proteins, including variants of HIV NCp7 and E. coli StpA [
48,
49]. The Hfq protein, known to promote annealing between small regulatory RNAs (sRNAs) and their mRNA targets in vitro, is also involved in similar cycling behaviour, dissociating from the sRNA-mRNA duplex after formation [
50,
51]. Efficient recognition and binding of the correct target by the Hfq-sRNA complex is achieved through rapid searching among potential RNA targets, with base pairing occurring only when a complementary site is identified [
52].
Understanding of RNA Chaperone-Mediated Unfolding and Refolding
Recent biophysical investigations have provided a detailed understanding of how passive RNA chaperones, such as CspA and HIV NCp7, function to disrupt RNA secondary structures and facilitate proper folding. CspA, which is the predominant cold shock protein in E. coli, becomes highly upregulated following a temperature drop to 10°C, where it participates in various cellular functions. One of its critical roles, along with its homolog CspE, is to mitigate the stabilization of mRNA secondary structures that might otherwise cause premature transcription termination or hinder translation under cold stress conditions [
53]. The structural basis for the RNA chaperone function of CspA lies in its cold shock domain, a β-barrel fold that has been found to bear significant similarity to eukaryotic Y-box proteins. The RNA-binding surface of the cold shock domain features a set of aromatic side chains that play anessential role in the protein's ability to destabilize RNA structures [
54]. A study by Phadtare et al., 2004 have demonstrated that these aromatic residues are essential for CspA’s RNA chaperone activity [
55].
Using time-resolved NMR spectroscopy, Rennella et al., 2017 observed that multiple CspA molecules can promote the dimerization of two complementary RNA hairpins, a process that involves the gradual unfolding of the hairpins [
56]. Notably, they discovered that the slowest unfolding step occurred at a rate that matched the rate of RNA dimerization, implying that the individual hairpins must completely unzip before they can pair with one another. Intriguingly, CspA accelerates the rate of hairpin unfolding, primarily by destabilizing the base pairs located at the hairpin loop and helix junction. The disruption of these base pairs is mediated through stacking interactions with the exposed aromatic side chains of CspA, which are coupled with hydrogen bonding interactions involving the surrounding basic residues. This aromatic–basic residue interplay offers an energetically favourable yet flexible binding interface for single-stranded RNA. Importantly, these aromatic side chains are highly dynamic when not bound to RNA but become structurally stabilized upon interaction with RNA substrates, thereby reducing the motion of RNA base pairs as chaperone binding induces conformational changes [
56].These findings support the broader hypothesis that many RNA chaperones, including CspA, contain disordered regions that contribute to their ability to manipulate RNA structure. It is thought that these disordered regions transfer entropy from the chaperone to the RNA substrate, thereby facilitating RNA unfolding and refolding [
57].
The RNA chaperone activities of viral nucleocapsid proteins, particularly those of HIV NCp7, have been extensively studied, revealing their role in both RNA destabilization and strand annealing processes [
58]. HIV NCp7 is involved in multiple stages of the retroviral life cycle, including the dimerization and packaging of the genomic RNA, priming of tRNA, and transactivation events [
59]. Processed from the larger gag polyprotein precursor, NCp7 contains two zinc finger domains, each of which plays a key role in binding and destabilizing RNA structures [
60]. Each zinc finger domain features a hydrophobic pocket that specifically interacts with unpaired guanosinenucleobases, while the positively charged and disordered N-terminal region of NCp7 has been associated with its propensity to aggregate RNA molecules [
61].
In a manner similar to CspA, multiple NCp7 molecules can bind to a single RNA substrate, destabilizing the structure in a cooperative fashion [
62]. Recent studies using force-stretching techniques have provided significant insight into how NCp7 destabilizes the TAR RNA hairpin during the reverse transcription of the HIV genome. In these experiments, NCp7 was shown to increase the probability of TAR unfolding by approximately 10,000-fold by shifting the position of the transition state for RNA unfolding. This shift reduces the number of base pairs that need to be disrupted simultaneously before the TAR hairpin completely unzips [
63]. Consistent with earlier observations, the mechanism underlying this effect is primarily mediated by NCp7’s ability to preferentially bind to guanosines near RNA defects such as G-U wobble pairs, bulges, and loops, thereby disrupting base pairing in these regions [
64]. At moderate protein-to-RNA ratios (approximately one protein molecule per 7 to 15 nucleotides), NCp7 is able to progressively destabilize large RNA structures, a condition where its chaperone activity is most prominent [
65]. Furthermore, NCp7's weak interactions with most binding sites allow for frequent dissociation, providing the RNA with opportunities to form more stable interactions in the presence of the chaperone [
48].
The interaction between RNA chaperones and their substrates typically involves multiple weak, but collectively significant, interactions that modulate RNA structure. This distributive binding mechanism allows the chaperone to rearrange its contacts with RNA without completely dissociating, thus maintaining a persistent yet flexible interaction with the RNA substrate. The ability to establish multiple contacts with RNA also enables chaperones to recognize a diverse range of RNA structures or selectively target specific classes of RNA molecules.RNA chaperones with strand annealing capabilities utilize multiple binding surfaces to bring together two RNA strands, facilitating their proper interaction and folding. Many RNA-binding proteins employ multiple domains or multiple copies of the same protein to enhance RNA refolding and improve substrate selectivity, even though the three-dimensional structures of these proteins can vary significantly across different families [
37,
39].
Small RNA chaperones, such as CspA, NCp7, and StpA, often bind to their RNA targets with multiple copies, explaining their efficacy at higher protein-to-RNA ratios [
37,
39]. These proteins typically contain several RNA-binding domains that function cooperatively to unwind or anneal RNA molecules [
37]. For instance, HIV NCp7's chaperone activity requires the concerted action of both zinc finger domains and its N-terminal basic region [
66,
67]. Ribosomal protein S1, another well-studied RNA chaperone, utilizes its six oligonucleotide-binding (OB) domains to unwind RNA helices, with at least three domains required for structured mRNAs to be accommodated by the 30S ribosome [
68]. Experiments using optical tweezers have shown that each molecule of S1 unwinds RNA helices in a stepwise manner, reducing the energy barrier needed to unfold large RNA secondary structures by tackling them in smaller increments [
69].
Eukaryotic RNA chaperones often consist of multiple RNA-binding domains connected by flexible linkers, providing versatility in RNA substrate recognition and enabling the protein to adapt to conformational changes in the RNA. This "pearl necklace" arrangement allows for greater specificity in recognizing RNA sequence motifs and accommodates structural rearrangements in the RNA substrate. For example, the yeast protein Prp24 facilitates the annealing of U6 and U4 snRNAs, a critical step in the recycling of spliceosomal complexes [
70]. Prp24 contains four RNA recognition motif (RRM) domains that wrap around the U6 snRNA, making extensive contacts with both double-stranded and single-stranded regions of the RNA [
71]. These domains likely undergo reorientation to allow for the remodelling of U6 base pairs, an essential aspect of spliceosome function [
72]. Similarly, the yeast La protein, which binds the 3′ ends of pol III transcripts, plays a crucial role in chaperoning misfolded pre-tRNAs and other small nuclear RNAs [
73,
74]. When La binds to the RNA, a flexible linker between its La motif and RRM domain becomes ordered, resulting in a significant increase in the accuracy of substrate selection due to improved orientation of the two domains.
Role of Ring-shaped Proteins and RNA Chaperones in RNA Structure Regulation
Related to RNA chaperones, ring-shaped proteins exhibit a unique ability to interact with multiple RNA segments simultaneously, enabling structural manipulation of RNA. Unlike multi-domain RNA-binding proteins, which rely on flexible interactions, ring-shaped proteins provide a more rigid, three-dimensional framework. This configuration enables RNA molecules to wrap around or pass through the ring, causing distortions or aligning distinct RNA strands. A prime example is the
B. subtilis trp RNA-binding attenuator protein (TRAP), which wraps up to 33 nucleotides of RNA around its large, wheel-shaped 11-subunit structure [
75]. A smaller yet significant example is the Ro antigen, which employs a ring of HEAT repeats to bind and stabilize eukaryotic Y box small RNAs [
76]. Among bacterial small RNA (sRNA) chaperones, Hfq (Host Factor for phage Qbeta replication) is one of the most extensively studied examples [
77]. Hfq belongs to the Sm/Lsm protein family and forms a six-subunit ring structure that binds to sRNAs, protects them from degradation, and facilitates their annealing with target mRNAs [
78,
79]. In addition to post-transcriptional regulation by sRNAs, Hfq also exerts direct control over its own mRNA translation as well as that of other mRNAs, further illustrating its broad regulatory roles [
79,
80].
Hfq's versatility in RNA recognition stems from the combination of multiple RNA-binding surfaces and disordered domains that allow it to bind a wide range of RNA substrates while selectively avoiding others. The proximal face of Hfq is highly conserved across Sm/Lsm proteins and is critical for interactions with uridine-rich sequences located at the 3′ ends of sRNAs [
81]. These interactions are essential for the stabilization of sRNAs within the cellular environment [
82]. On the opposite side, the distal face of Hfq binds single-stranded AAN triplet motifs found in the mRNA targets of sRNA regulation, as well as a specific class of sRNAs [
82]. The AAN triplet is particularly important in the up-regulation of rpoS translation in E. coli, as well as in the down-regulation of various mRNA targets by the Spot 42 sRNA [
83,
84]. Consequently, the Hfq ring can facilitate the pairing of sRNAs with their corresponding mRNA targets, enhancing their regulatory interactions [
85].
Hfq possesses basic residues, predominantly arginines, along the lateral edge of its hexameric ring. These residues interact with complementary RNA regions, creating multi-lateral interactions that are vital for Hfq-dependent sRNA regulation. This has been demonstrated through mutational analyses, in-cell crosslinking, and co-immunoprecipitation studies [
85]. Biophysical study by Zheng et al., 2016 revealed that Hfq accelerates the annealing of complementary RNA strands, a process that depends on the basic patches on the rim of the hexamer [
86]. Like other RNA chaperones, Hfq rapidly binds RNA, facilitating pairing before detaching from the duplex once base pairing is established [
50]. Single-molecule FRET studies further reveal that this stable RNA annealing is preceded by transient binding, with Hfq dissociating from the duplex soon after pairing occurs [
87]. Though Hfq has been shown to bind ATP [
88], ATP hydrolysis is not necessary for its annealing function [
89].
The precise mechanism by which the arginine residues on Hfq’s rim facilitate RNA base pairing remains unclear, but several aspects of the annealing process are known. Firstly, interactions between the rim and UA motifs in the sRNA prime the seed region for pairing with complementary RNA strands, potentially by increasing the flexibility of the bound sRNA [
90,
91,
92]. Secondly, arginine residues directly stabilize the formation of a helix initiation complex, potentially by counteracting the electrostatic repulsion between the RNA strands or forming hydrogen bonds [
93]. Finally, through simultaneous interactions with RNA via its proximal, distal, and lateral rim surfaces, Hfq can refold RNA into configurations more conducive to base pairing. For instance, the distal face of Hfq recognizes an AAN motif in the 5′ UTR of the rpoS mRNA, while the rim engages a U-rich loop downstream of the sRNA target site [
94]. SHAPE modification and SAXS experiments have shown that this multi-site recognition induces a distorted conformation in the rpoS 5′ UTR, enabling it to pair with regulatory sRNAs [
95]. Similar recognition sites have been identified in other mRNA targets of Hfq and sRNA regulation, suggesting that mRNA distortion may be a common strategy used to enhance sRNA regulatory efficiency [
96].
Bacterial small RNAs must efficiently locate their target mRNAs in a highly complex cellular environment. The kinetics of target search relies on co-localization, selective searching among potential targets, and accurate recognition of the complementary sequence. In contrast to systems like CRISPR and eukaryotic microRNAs, which employ Cas and RISC proteins for rapid target scanning and increased fidelity, bacterial sRNAs rely on Hfqhexamers [
97]. Overexpression studies have demonstrated that sRNAs compete for a limited pool of Hfqhexamers in the cell, rapidly cycling on and off the protein [
98].
Although Hfq-bound sRNAs and mRNAs containing U-rich or AAN motifs have dissociation constants of 1–30 nM, it remains unclear how RNA molecules exchange on Hfq [
99]. Fender and Wagner proposed that sRNA dissociation kinetics depend on the availability of free RNA, with sRNAs actively displacing one another from the proximal face of the Hfqhexamer [
100]. These dynamic interactions are critical for matching sRNAs with their complementary mRNA targets within minutes of induction and for quickly adapting regulatory circuits to changing growth conditions [
101].
Chaperone-Assisted Nucleic Acid Conformational Transitions Mechanisms
Intrinsically disordered peptide (IDP) regions are commonly found in RNA chaperones and play essential roles in chaperone activity [
57]. The presence of IDPs in RNA chaperones supports the "entropy transfer" hypothesis, which suggests that chaperone folding is coupled with increased disorder in the RNA substrate [
57]. Depending on their charge, these disordered regions can influence RNA binding and remodelling. For instance, basic N-terminal polypeptides often enhance RNA affinity and remodelling capabilities, as seen with the basic N-terminal domain of HIV NCp7, which significantly accelerates TAR hairpin annealing with complementary DNA during minus-strand transfer [
102]. Other examples include the Mss116p and CYT-19 DEAD-box helicases, which possess unstructured, arginine-rich C-terminal extensions that facilitate RNA substrate binding and may even loosen RNA structures [
103,
104]. Disordered regions also allow chaperones to accommodate a variety of RNA structures, as proposed for the La protein [
105].
Conversely, acidic peptides found in many DNA and RNA-binding proteins can mimic nucleic acids and inhibit binding to the protein core [
106]. For Hfq, recent studies have highlighted the importance of acidic peptides in RNA chaperone function [
107]. The C-terminal tail (CTD) of E. coli Hfq, consisting of approximately 30 residues, is disordered in solution and extends from the hexameric ring formed by the stable Sm domain [
108]. Acidic residues at the CTD tip interact with the arginine patches on the rim of the hexamer, displacing double-stranded RNA and preventing the binding of non-specific RNA and DNA [
107]. This displacement has significant consequences for Hfq’s role in sRNA regulation. Firstly, the CTD prevents the dissociation of sRNA-mRNA strands, thus maintaining RNA pairing while recycling Hfq for further RNA interactions. Secondly, the CTD creates a hierarchy among sRNAs, allowing those with AAN motifs to outcompete other sRNAs for access to Hfq in the cell [
107]. The variability in CTD length and acidic residue content among bacterial species may serve as a regulatory mechanism to fine-tune Hfq turnover and RNA regulation across different organisms [
109].
Holmstrom et al. (2019) describe that hairpin structures within nucleic acids, both RNA and DNA, represent a ubiquitous motif that plays a fundamental role in various biological processes [
110]. Given their prevalence in folded nucleic acids, the mechanisms by which RNA and DNA chaperones assist in conformational transitions are likely to be widely applicable to a broad array of nucleic acid conformations. The hypothesis presented by Holmstrom et al. (2019) posits that this functional mechanism extends beyond the context of the hairpin and has the potential to influence other chaperone-assisted structural transitions in nucleic acids. One notable example worth investigating further, as proposed by Holmstrom et al., is the process of genome dimerization in the hepatitis C virus (HCV). The initiation of dimerization is believed to involve intermolecular base pairing between unpaired nucleotides within the stem-loop of the dimer linkage sequence (DLS). This interaction is also commonly referred to as RNA kissing loops, which could be facilitated by viral nucleocapsid proteins (NCDs), aiding in genome dimerization through charge screening mechanisms similar to those observed in hairpin formation [
111].
Holmstrom et al. (2019) further elaborate on how NCDs, when bound to the DLS, serve as macromolecular counter-ions that neutralize the charge associated with the nucleic acid complex, thereby decreasing the electrostatic repulsion between the RNA loops in the DLS. This mechanism, which is crucial for the formation of RNA kissing loops, may effectively accelerate the formation of these loops and favour genome dimerization by shifting the equilibrium towards a dimeric state. Thus, the presence of the NCD not only modulates the charge but also enhances the structural stability of the genome, facilitating viral replication.
Intrinsically disordered RNA chaperones have garnered increasing attention for their remarkable ability to bind to nucleic acids with high affinity. These chaperones provide an efficient and controllable means of influencing nucleic acid conformation and dynamics within a cellular environment. Unlike cellular factors such as ion concentrations, which are restricted to narrow physiological ranges, these chaperones exhibit a degree of flexibility in regulating nucleic acid structures that is unparalleled. Such interactions are likely responsible for the observed differences in RNA structure and dynamics when comparing in vitro studies to those carried out in cellular extracts [
112]. Proteins like NCp7 and Tat from HIV-1, along with the core protein from the West Nile virus, exemplify how viral proteins with intrinsically disordered regions contribute to nucleic acid regulation through mechanisms such as charge screening [
113]. By modulating nucleic acid affinity for various proteins, these positively charged regions may imbue proteins like transcription factors, nucleocapsid proteins, and histone-like proteins with chaperone-like activities [
114].
Although Holmstrom et al. (2019) highlight charge screening as a central mechanism employed by RNA chaperones, they acknowledge the existence of a variety of complementary pathways [
110]. For instance, the Moloney murine leukemia virus nucleocapsid protein displays a structural specificity by binding selectively to guanosine residues, preventing the formation of non-native contacts that could hinder proper folding [
115]. In contrast, bacterial RNA chaperones like Hfq rely on the flexibility of intrinsically disordered tails to facilitate the release of RNA substrates [
107,
116]. RNA chaperones with ATP-dependent helicase activity, such as those involved in iterative RNA annealing, provide an additional layer of regulation [
117]. While these mechanisms are diverse, they are not mutually exclusive. Instead, they likely work in concert with macromolecular charge screening to enhance the efficiency of nucleic acid conformational transitions, thus underscoring the complexity of RNA and DNA chaperone systems.
Molecular Evolutions of RNA Chaperones and Protein Folding
The hypothesis known as the "RNA world" suggests that during the early stages of Earth's existence, RNA molecules functioned as the essential building blocks of life [
118,
119]. A living entity fundamentally requires two key elements: a genetic template for storing information and a catalytic system for replicating itself and producing offspring. RNA, composed of four unique nucleotides and capable of adopting various structural conformations, was theorized to fulfil both these roles, making it a prime candidate for early self-replicating systems. The dual capability of RNA to act as both genetic material and a catalyst was seen as a critical feature for sustaining primordial biological processes. However, the existence of an RNA-based organism has not yet been physically proven, and the "RNA-first" hypothesis, while intellectually valuable for understanding the molecular evolution of life, is met with caution. It is important to consider that other types of self-replicating molecules may have preceded RNA in Earth's early history [
120]. Despite this, molecular biology research has successfully recreated artefacts of this ancient era under laboratory conditions, providing a glimpse into RNA's role in early life forms [
121].
A conventional view within the RNA world theory suggests that catalysis progressed in stages, from ribozymes to RNP enzymes, and eventually to protein-based enzymes. While protein enzymes predominantly perform catalytic functions in modern cells, ribozymes still play crucial roles, even though they are significantly outnumbered. Among the four core processes involved in the transmission of genetic information, two (DNA replication and transcription) are catalyzed by proteins alone, whereas mRNA splicing and protein synthesis rely on ribozymes for their catalytic actions. RNA molecules participate in a variety of essential biological processes, such as RNA processing, replication of RNA viruses, and the peptide-bond formation within ribosomes, which is critical for protein synthesis across all living organisms [
118]. The continued involvement of RNA-based catalysts in these key cellular functions indicates that the RNA world may not have completely vanished but instead persists alongside proteins in what could be considered an RNA-protein world [
122]. In modern cells, RNA often functions in partnership with proteins, further cementing its significance in contemporary biological systems [
123].
The complex relationship between RNA and protein raises the possibility of mutual influence, especially in the context of energy or information transfer between the two [
118]. For instance, when RNA interacts with proteins, it might influence how the protein folds into its functional three-dimensional structure. This RNA-mediated effect could be particularly critical for proteins that interact with multiple RNA molecules, potentially affecting their stability and proper folding. Such RNA chaperone-like activity may have been particularly important for early proteins, which lacked the sophisticated molecular chaperones found in modern cells. In the early RNA world, primitive peptides and proteins were likely small and able to fold independently of chaperones. However, another possibility is that ribozymes, in addition to catalyzing peptide-bond formation, also acted as molecular chaperones, aiding in the correct folding of the proteins they helped synthesize. This idea suggests that ribozymes may have possessed an inherent ability to assist protein folding, a "moonlighting" function that has yet to be fully explored or understood. One prominent example of an RNA-based chaperone is Ribonuclease P (RNase P), an ancient ribozyme responsible for the maturation of tRNA molecules through endonucleolytic cleavage of tRNA precursors [
124]. The presence of RNase P in all domains of life strongly supports its evolutionary significance, suggesting that it was present in the last universal common ancestor and thus serves as a remnant of the prebiotic RNA world [
120]. RNase P has been found to function universally, with only a few exceptions, such as certain endosymbiont organelles where a protein-based version of RNaseP is used [
125]. In the case of E. coli, RNase P is a ribonucleoprotein (RNP) complex consisting of an RNA subunit (M1 RNA) that catalyzes the reaction and a protein subunit (C5 protein) that enhances both catalytic efficiency and specificity for tRNA substrates [
124].
Interestingly, recent research has uncovered an additional role for M1 RNA, which, beyond its catalytic activity, assists in the proper folding of its associated C5 protein both in vivo and in vitro [
118]. It is hypothesized that during protein synthesis, the nascent C5 polypeptide may interact with M1 RNA, which facilitates its folding into the correct conformation as part of the ribonucleoprotein complex [
125]. M1 RNA also appears to serve a quality control function, promoting the degradation of aberrant C5 proteins that arise from mutations, thus ensuring their efficient removal from the cytoplasm. This chaperone-like activity of M1 RNA parallels the role of protein-based molecular chaperones, which assist in protein folding and maintain proteostasis in modern cells [
126].
While the enzymatic activity of RNase P resides within its RNA subunit, the protein component is critical for enhancing the overall functionality of the holoenzyme by improving substrate specificity and catalytic efficiency [
127]. Given the central role of tRNAs in translating mRNA codons into amino acids, it is essential that RNase P functions efficiently to ensure the proper folding of tRNAs. Correctly synthesized and folded tRNAs are fundamental for high-fidelity protein synthesis, bridging the gap between the RNA and protein worlds. This efficient synthesis and maturation of tRNAs were likely crucial in early life, facilitating the transition from an RNA-dominated metabolism to one governed by protein-based enzymes. Remarkably, although ribozymes may have gradually been replaced by more efficient protein enzymes, the enzyme responsible for peptide-bond formation during protein synthesis remains a ribozyme. This phenomenon accentuates the ribosome's unique role as the catalyst for nearly all protein synthesis, a process that remains universally conserved across life, except in a few specialized cases such as the synthesis of certain peptide antibiotics [
128].
A wealth of structural and biochemical data has confirmed that the large subunit of the ribosome, specifically its 23S rRNA (in bacteria), contains the peptidyl-transferasecenter (PTC), which is responsible for the ribozyme-catalyzed formation of peptide bonds [
129]. In particular, adenine 2451 within domain V of 23S rRNA, based on E. coli numbering, plays a central role in the peptidyl transfer reaction, a critical step in the elongation of nascent polypeptides [
130].
In addition to its well-known role in protein synthesis, the ribosome also possesses protein folding activity (PFAR), a lesser-known but equally important function. First discovered in bacterial ribosomes about two decades ago, this folding activity has since been identified in organisms ranging from bacteria to eukaryotes, including mitochondria [
131]. Biochemical evidence suggests that PFAR, which resides in the same domain V of rRNA responsible for peptidyl transfer, may serve as an RNA-based chaperone, helping newly synthesized proteins fold into their functional conformations [
132]. Interestingly, the regions of domain V responsible for PFAR and peptide-bond formation are distinct, allowing these two functions to operate independently but synergistically within the ribosome [
132]. The physical dissociation of ribosomal subunits, triggered by unfolded polypeptides, may facilitate the accessibility of the PFAR center, further highlighting its importance in protein quality control and proteostasis [
133]. Anti-prion compounds have been found to inhibit PFAR, reinforcing the connection between this activity and the maintenance of cellular protein homeostasis [
134]. Aberrant protein folding, if left unchecked, can lead to diseases associated with protein misfolding, further underscoring the significance of PFAR in cellular health [
135].
Emerging biochemical data increasingly support the view that the ribosome's role in protein folding should be considered integral to its function, alongside its peptidyl-transferase activity. This dual functionality of the ribosome, as both a catalyst for peptide-bond formation and a chaperone for protein folding, may represent an evolutionary remnant of the RNA world, where RNA-based catalysts performed multiple roles essential for early life [
136]. Before the emergence of protein-based molecular chaperones, it is plausible that ribozymes, such as the ribosome itself, facilitated the folding of nascent proteins, helping to bridge the transition from RNA-based to protein-based cellular machinery. Thus, the ribosome’s moonlighting activities, once critical for early life, continue to be vital in all living organisms, illustrating the deep evolutionary roots of RNA-based catalysis in modern biology.
Chaperone-Mediated Regulation of rRNA Folding
Ribosomal RNAs (rRNAs), like all RNAs, exhibit a tendency to misfold in vitro in the absence of associated assembly factors (AFs) [
137,
138]. The efficiency of rRNA folding in vivo is thought to be facilitated by a complex machinery of proteins, which likely includes AFs functioning as molecular chaperones to guide the proper folding of rRNA. Despite these observations, the precise causes behind RNA misfolding and the specific mechanisms through which chaperones prevent such events have remained largely enigmatic. While the role of alternative secondary structures contributing to RNA misfolding has been documented in model RNAs, the extent to which these alternative structures play a role in rRNAmisfolding is less clear [
139,
140]. Similarly, the contributions of misfolded helical junctions or tertiary structures to RNA misfolding have not been thoroughly characterized, possibly due to the complexity and difficulty of dissecting or manipulating these interactions [
141,
142]. Whether misfolding in large RNAs leads to local perturbations or global structural changes remains unclear.
In addressing these questions, Huang and Karbstein (2021) conducted experiments to examine the formation of specific structural elements in the small ribosomal subunit [
137]. These elements include a three-helix junction (j34-35-38) and a loop, which establish a tertiary contact at the P-site of the subunit. Their findings reveal that the junction is highly prone to misfolding, and this misfolded intermediate becomes kinetically trapped when it forms a tertiary interaction with the helical loop. Importantly, the study demonstrates that AFs act in a sequential manner, delaying the folding of the helical loop until the last stages of subunit assembly. This stepwise process prevents the formation of kinetic traps and enables the correct refolding of the misfolded intermediate. Instead of directly binding to the three-helix junction and guiding its folding, the AFs interact with the junction’s partners, decreasing the stability of the misfolded intermediate and promoting refolding into the native structure. These understandings into the role of AFs highlight their indirect but critical role in chaperoning rRNA folding and provide a mechanistic basis for this process.
It has been seen that specifically, the snoRNA snR35 appears to inhibit the premature folding of both helix 31 and its loop during transcription. After snR35 dissociates, the nucleolar protein Emg1 binds and chaperones l31, followed by the AF Rio2, which acts in conjunction with Tsr1 in the cytoplasm. Each of these factors binds l31, preventing it from adopting its final conformation until the late stages of assembly, just before the formation of 80S-like ribosomes, which are intermediates in the assembly process [
143]. In both wild-type and unchaperoned yeast, the j34-35-38 junction is more likely to misfold than to fold correctly, as indicated by a larger arrow pointing to the misfolded form in. However, in wild-type cells, the misfolded intermediate is capable of unfolding and repopulating the correct pathway, aided by the slow dissociation of Enp1 from the misfolded state [
144]. Once Rio2 dissociates from the subunit, l31 completes its folding and binds the preformed j34-35-38, rendering the overall process irreversible as the 80S-like ribosomes assemble with the aid of eIF5B and GTP.
In the absence of chaperones, the rapid folding of the l31 loop precedes the folding of j34-35-38, leading to the stabilization of both native and misfolded forms through tertiary interactions [
145]. In such cases, misfolded ribosomes may accumulate due to the slow unfolding of the misfolded junction, especially when Enp1 dissociation is faster than junction unfolding. Furthermore, the lack of Ltv1 results in misfolded ribosomes, with mature 18S rRNA levels reduced while pre-18S rRNA does not accumulate significantly, indicating degradation of the misfolded intermediates [
146].
The analysis of these pathways reveals that Rio2, Emg1, and snR35 act by preventing the premature folding of l31, which in turn delays the formation of tertiary interactions between l31 and j34-35-38. This protective role is achieved not by directly interacting with the misfolding-prone RNA elements but by binding and blocking the interaction partners, thereby isolating the folding process of the junctions. Interestingly, previous research has shown that native tertiary interactions can stabilize both native and misfolded intermediates in vitro, further supporting this mechanism [
142].
Importantly, misfolding in large RNA molecules, such as rRNA, tends to have local rather than global effects, as evidenced by structural studies of small ribosomal subunits from Ltv1-deficient yeast and inactive bacterial subunits [
147]. This localized nature of misfoldingaccentuates the modular assembly of complex RNA structures through junctions and tertiary interactions. AFs appear to facilitate local corrections without necessitating global unfolding, which is distinct from the mechanism employed by protein chaperones.
Huang and Karbstein (2021) propose that the folding of three-helix junctions, such as j34-35-38, represents a significant bottleneck in the assembly of ribosomal RNA [
137]. These junctions are prone to misfolding and can become trapped in non-native structures, particularly when premature tertiary interactions form. The data suggest that this problem extends beyond j34-35-38, as other three-helix junctions in rRNA also face similar folding challenges. The role of AFs, particularly Rio2, Emg1, and snR35, in delaying the folding of l31 and similar elements allows the RNA to refold and adopt its native structure without becoming kinetically trapped in misfolded states.
A comparison with studies of bacterial ribosomes shows that similar misfolding events occur during the assembly of 16S rRNA [
138,148]. Misfolded intermediates, termed RI, must undergo heat-dependent refolding (stage II) before binding to tertiary proteins in stage III. The refolding of j34-35-38 requires the unfolding of l31, which in turn enables proper binding of interacting proteins (uS3, uS10, and uS14) in the final stage of assembly. Moreover, other three-helix junctions, such as j28-29-43 and j22-23-23a, also display misfolding and refolding dynamics during the assembly process [148].
Future Perspective
Proteins that interact with and assist non-coding RNAs are widespread across biological systems and play crucial roles in regulating gene expression, responding to cellular stress, facilitating viral replication, and maintaining RNA metabolism. These RNA-binding proteins are instrumental in reshaping RNA structures and enabling antisense interactions, which are essential for regulatory RNAs to function effectively. A key line of research can be to address the limitations of computational models used for RNA secondary structure prediction. While current models exhibit excellent predictive capabilities, a detailed evaluation of their ability to identify biologically significant features—such as pseudoknots, which are often challenging to predict—remains essential. The absence of such features could significantly impact the biological functionality of RNA structures.
The long-term objective of these future investigations is to engineer RNA sequences with specific functional characteristics. The findings from this study indicate that the proposed predictive models could serve as foundational tools in this design process. However, it is yet to be determined how effectively these models can adapt to novel RNA sequences introduced during RNA design. Extending these models to allow for multiple secondary structure predictions for a single sequence might increase the likelihood of identifying structures that meet the desired functional criteria.
References
- Herschlag D. (1995).RNA chaperones and the RNA folding problem. The Journal of biological chemistry, 270(36), 20871–20874. [CrossRef]
- Lindahl, T., Adams, A., & Fresco, J. R. (1966). Renaturation of transfer ribonucleic acids through site binding of magnesium. Proceedings of the National Academy of Sciences of the United States of America, 55(4), 941–948. [CrossRef]
- Adams, A., Lindahl, T., & Fresco, J. R. (1967). Conformational differences between the biologically active and inactive forms of a transfer ribonucleic acid. Proceedings of the National Academy of Sciences of the United States of America, 57(6), 1684–1691. [CrossRef]
- Cole, P. E., Yang, S. K., & Crothers, D. M. (1972). Conformational changes of transfer ribonucleic acid. Equilibrium phase diagrams. Biochemistry, 11(23), 4358–4368. [CrossRef]
- Uhlenbeck, O. C., Chirikjian, J. G., & Fresco, J. R. (1974). Oligonucleotide binding to the native and denatured conformers of yeast transfer RNA-3 Lea. Journal of molecular biology, 89(3), 495–504. [CrossRef]
- Dávila-Aponte, J. A. , Huss, V. A., Sogin, M. L., &Cech, T. R. (1991). A self-splicing group I intron in the nuclear pre-rRNA of the green alga, Ankistrodesmusstipitatus. Nucleic acids research, 19(16), 4429-4436.
- Jaeger, L. , Westhof, E., & Michel, F. (1991). Function of P11, a tertiary base pairing in self-splicing introns of subgroup IA. Journal of molecular biology, 221(4), 1153-1164.
- Sigler, P. B. (1975). An analysis of the structure of tRNA.Annual review of biophysics and bioengineering, 4(1), 477-527.
- Gruenewald, B. , Nicola, C. U., Lustig, A., Schwarz, G., &Klump, H. (1979). Kinetics of the helix—coil transition of a polypeptide with non-ionic side groups, derived from ultrasonic relaxation measurements. Biophysical chemistry, 9(2), 137-147.
- Turner, D. H. (1989). Thermodynamics and kinetics of base-pairing and of DNA and RNA self-assembly and helix coil transition.Nucleic acids.
- Doty, P., Boedtker, H., Fresco, J. R., Haselkorn, R., &Litt, M. (1959). Secondary structure in ribonucleic acids. Proceedings of the National Academy of Sciences, 45(4), 482-499.
- Gralla, J. , &Delisi, C. (1974). Biological sciences: mRNA is expected to form stable secondary structures. Nature, 248(5446), 330-332.
- Herschlag, D. (1992). Evidence for processivity and two-step binding of the RNA substrate from studies of J1/2 mutants of the Tetrahymena ribozyme. Biochemistry, 31(5), 1386-1399.
- Karpel, R. L. , Swistel, D. G., Miller, N. S., Geroch, M. E., Lu, C., & Fresco, J. R. (1975, July). Acceleration of RNA renaturation by nucleic acid unwinding proteins.In Brookhaven symposia in biology (No. 26, pp. 165-174).
- Karpel, R. L. , Miller, N. S., & Fresco, J. R. (1982). Mechanistic studies of ribonucleic acid renaturation by a helix-destabilizing protein. Biochemistry, 21(9), 2102-2108.
- Alberts, B. M. , & Frey, L. (1970). T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature, 227(5265), 1313-1318.
- Rothman, J. E. , & Kornberg, R. D. (1986). Cell biology: An unfolding story of protein translocation. Nature, 322(6076), 209-210.
- Hertel, K. J., Herschlag, D., &Uhlenbeck, O. C. (1994). A kinetic and thermodynamic framework for the hammerhead ribozyme reaction. Biochemistry, 33(11), 3374-3385.
- Müller, G. , Strack, B., Dannull, J., Sproat, B. S., Surovoy, A., Jung, G., &Moelling, K. (1994). Amino acid requirements of the nucleocapsid protein of HIV-1 for increasing catalytic activity of a Ki-ras ribozyme in vitro.Journal of molecular biology, 242(4), 422-429.
- Bertrand, E. L. , & Rossi, J. J. (1994). Facilitation of hammerhead ribozyme catalysis by the nucleocapsid protein of HIV-1 and the heterogeneous nuclear ribonucleoprotein A1. The EMBO Journal, 13(12), 2904-2912.
- Mohr, G. , Zhang, A., Gianelos, J. A., Belfort, M., &Lambowitz, A. M. (1992). The neurospora CYT-18 protein suppresses defects in the phage T4 td intron by stabilizing the catalytically active structure of the intron core. Cell, 69(3), 483-494.
- Todd, M. J. , Viitanen, P. V., &Lorimer, G. H. (1994). Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science, 265(5172), 659-666.
- VAN DIEIJEN, G. , van Knippenberg, P. H., & van Duin, J. (1976).The specific role of ribosomal protein S1 in the recognition of native phage RNA. European Journal of Biochemistry, 64(2), 511-518.
- Saibil, H. (2013). Chaperone machines for protein folding, unfolding and disaggregation. Nature reviews. Molecular cell biology, 14(10), 630–642. [CrossRef]
- Chernoff, Y. O. , Lindquist, S. L., Ono, B. I., Inge-Vechtomov, S. G., &Liebman, S. W. (1995). Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science, 268(5212), 880-884.
- Gething, M. J., &Sambrook, J. (1992). Protein folding in the cell. Nature, 355(6355), 33-45.
- Pontius, B. W. (1993). Close encounters: why unstructured, polymeric domains can increase rates of specific macromolecular association. Trends in biochemical sciences, 18(5), 181-186.
- Goodchild, J. (1992). Enhancement of ribozyme catalytic activity by a contiguous oligodeoxynucleotide (facilitator) and by 2′-O-methylation.Nucleic acids research, 20(17), 4607-4612.
- Woodson, S. A., &Emerick, V. L. (1993). An alternative helix in the 26S rRNA promotes excision and integration of the Tetrahymena intervening sequence. Molecular and cellular biology, 13(2), 1137-1145.
- Baker, D., &Agard, D. A. (1994). Kinetics versus thermodynamics in protein folding. Biochemistry, 33(24), 7505-7509.
- Ellis, R. J. , & Van der Vies, S. M. (1991). Molecular chaperones. Annual review of biochemistry, 60(1), 321-347.
- Lohman, T. M. (1993). Helicase-catalyzed DNA unwinding.Journal of Biological Chemistry, 268(4), 2269-2272.
- Guthrie, C., & Patterson, B. (1988). SpliceosomalsnRNAs.Annual review of genetics, 22(1), 387-419.
- Burgess, S. M. , & Guthrie, C. (1993). Beat the clock: paradigms for NTPases in the maintenance of biological fidelity. Trends in biochemical sciences, 18(10), 381-384.
- Bertrand, E. L. , & Rossi, J. J. (1994). Facilitation of hammerhead ribozyme catalysis by the nucleocapsid protein of HIV-1 and the heterogeneous nuclear ribonucleoprotein A1. The EMBO Journal, 13(12), 2904-2912.
- Cáceres, J. F., Stamm, S., Helfman, D. M., &Krainer, A. R. (1994). Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science, 265(5179), 1706-1709.
- Woodson, S. A. , Panja, S., & Santiago-Frangos, A. (2018). Proteins That Chaperone RNA Regulation. Microbiology spectrum, 2018, 6(4),. [CrossRef]
- Herschlag, D., Khosla, M., Tsuchihashi, Z., &Karpel, R. L. (1994). An RNA chaperone activity of non-specific RNA binding proteins in hammerhead ribozyme catalysis. The EMBO journal, 13(12), 2913–2924. [CrossRef]
- Mayer, O., Rajkowitsch, L., Lorenz, C., Konrat, R., & Schroeder, R. (2007). RNA chaperone activity and RNA-binding properties of the E. coli protein StpA. Nucleic acids research, 35(4), 1257–1269. [CrossRef]
- Woodson S. A. (2010). Taming free energy landscapes with RNA chaperones. RNA biology, 7(6), 677–686. [CrossRef]
- Todd, M. J., Lorimer, G. H., &Thirumalai, D. (1996). Chaperonin-facilitated protein folding: optimization of rate and yield by an iterative annealing mechanism. Proceedings of the National Academy of Sciences of the United States of America, 93(9), 4030–4035. [CrossRef]
- Thirumalai, D., & Woodson, S. A. (1996). Kinetics of folding of proteins and RNA.Accounts of chemical research, 29(9), 433-439.
- Bhaskaran, H., & Russell, R. (2007). Kinetic redistribution of native and misfolded RNAs by a DEAD-box chaperone. Nature, 449(7165), 1014–1018. [CrossRef]
- Chakrabarti, S., Hyeon, C., Ye, X., Lorimer, G. H., &Thirumalai, D. (2017). Molecular chaperones maximize the native state yield on biological times by driving substrates out of equilibrium. Proceedings of the National Academy of Sciences of the United States of America, 114(51), E10919–E10927. [CrossRef]
- Storz, G., Opdyke, J. A., & Zhang, A. (2004). Controlling mRNA stability and translation with small, noncoding RNAs. Current opinion in microbiology, 7(2), 140–144. [CrossRef]
- Wagner E. G. (2013). Cycling of RNAs on Hfq. RNA biology, 10(4), 619–626. [CrossRef]
- Doetsch, M., Schroeder, R., &Fürtig, B. (2011). Transient RNA-protein interactions in RNA folding. The FEBS journal, 278(10), 1634–1642. [CrossRef]
- Cruceanu, M., Gorelick, R. J., Musier-Forsyth, K., Rouzina, I., & Williams, M. C. (2006). Rapid kinetics of protein-nucleic acid interaction is a major component of HIV-1 nucleocapsid protein's nucleic acid chaperone function. Journal of molecular biology, 363(5), 867–877. [CrossRef]
- Cusick, M. E., & Belfort, M. (1998). Domain structure and RNA annealing activity of the Escherichia coli regulatory protein StpA. Molecular microbiology, 28(4), 847–857. [CrossRef]
- Hopkins, J. F., Panja, S., & Woodson, S. A. (2011). Rapid binding and release of Hfq from ternary complexes during RNA annealing. Nucleic acids research, 39(12), 5193–5202. [CrossRef]
- Rajkowitsch, L., & Schroeder, R. (2007). Dissecting RNA chaperone activity. RNA (New York, N.Y.), 13(12), 2053–2060. [CrossRef]
- Adamson, D. N., & Lim, H. N. (2011). Essential requirements for robust signaling in Hfq dependent small RNA networks. PLoS computational biology, 7(8), e1002138. [CrossRef]
- Phadtare, S., Tadigotla, V., Shin, W. H., Sengupta, A., &Severinov, K. (2006). Analysis of Escherichia coli global gene expression profiles in response to overexpression and deletion of CspC and CspE. Journal of bacteriology, 188(7), 2521–2527. [CrossRef]
- Schindelin, H., Jiang, W., Inouye, M., & Heinemann, U. (1994). Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 91(11), 5119–5123. [CrossRef]
- Phadtare, S., Inouye, M., &Severinov, K. (2004). The mechanism of nucleic acid melting by a CspA family protein. Journal of molecular biology, 337(1), 147–155. [CrossRef]
- Rennella, E., Sára, T., Juen, M., Wunderlich, C., Imbert, L., Solyom, Z., Favier, A., Ayala, I., Weinhäupl, K., Schanda, P., Konrat, R., Kreutz, C., &Brutscher, B. (2017). RNA binding and chaperone activity of the E. coli cold-shock protein CspA. Nucleic acids research, 45(7), 4255–4268. [CrossRef]
- Tompa, P., & Kovacs, D. (2010). Intrinsically disordered chaperones in plants and animals. Biochemistry and cell biology = Biochimieetbiologiecellulaire, 88(2), 167–174. [CrossRef]
- Rein, A., Henderson, L. E., & Levin, J. G. (1998). Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends in biochemical sciences, 23(8), 297–301. [CrossRef]
- Darlix, J. L., de Rocquigny, H., &Mély, Y. (2016). The multiple roles of the nucleocapsid in retroviral RNA conversion into proviral DNA by reverse transcriptase. Biochemical Society transactions, 44(5), 1427–1440. [CrossRef]
- Williams, M. C., Rouzina, I., Wenner, J. R., Gorelick, R. J., Musier-Forsyth, K., & Bloomfield, V. A. (2001). Mechanism for nucleic acid chaperone activity of HIV-1 nucleocapsid protein revealed by single molecule stretching. Proceedings of the National Academy of Sciences of the United States of America, 98(11), 6121–6126. [CrossRef]
- Le Cam, E., Coulaud, D., Delain, E., Petitjean, P., Roques, B. P., Gérard, D., Stoylova, E., Vuilleumier, C., Stoylov, S. P., &Mély, Y. (1998). Properties and growth mechanism of the ordered aggregation of a model RNA by the HIV-1 nucleocapsid protein: an electron microscopy investigation. Biopolymers, 45(3), 217–229. [CrossRef]
- Heilman-Miller, S. L., Wu, T., & Levin, J. G. (2004). Alteration of nucleic acid structure and stability modulates the efficiency of minus-strand transfer mediated by the HIV-1 nucleocapsid protein. The Journal of biological chemistry, 279(42), 44154–44165. [CrossRef]
- McCauley, M. J., Rouzina, I., Manthei, K. A., Gorelick, R. J., Musier-Forsyth, K., & Williams, M. C. (2015). Targeted binding of nucleocapsid protein transforms the folding landscape of HIV-1 TAR RNA. Proceedings of the National Academy of Sciences of the United States of America, 112(44), 13555–13560. [CrossRef]
- Grohman, J. K., Gorelick, R. J., Lickwar, C. R., Lieb, J. D., Bower, B. D., Znosko, B. M., & Weeks, K. M. (2013). A guanosine-centric mechanism for RNA chaperone function. Science (New York, N.Y.), 340(6129), 190–195. [CrossRef]
- Darlix, J. L., Godet, J., Ivanyi-Nagy, R., Fossé, P., Mauffret, O., &Mély, Y. (2011). Flexible nature and specific functions of the HIV-1 nucleocapsid protein. Journal of molecular biology, 410(4), 565–581. [CrossRef]
- Wu, H. Wu, H., Mitra, M., Naufer, M. N., McCauley, M. J., Gorelick, R. J., Rouzina, I., Musier-Forsyth, K., & Williams, M. C. (2014). Differential contribution of basic residues to HIV-1 nucleocapsid protein's nucleic acid chaperone function and retroviral replication. Nucleic acids research, 42(4), 2525–2537. [CrossRef]
- Godet, J., Ramalanjaona, N., Sharma, K. K., Richert, L., de Rocquigny, H., Darlix, J. L., Duportail, G., &Mély, Y. (2011). Specific implications of the HIV-1 nucleocapsid zinc fingers in the annealing of the primer binding site complementary sequences during the obligatory plus strand transfer. Nucleic acids research, 39(15), 6633–6645. [CrossRef]
- Duval, M., Korepanov, A., Fuchsbauer, O., Fechter, P., Haller, A., Fabbretti, A., Choulier, L., Micura, R., Klaholz, B. P., Romby, P., Springer, M., &Marzi, S. (2013). Escherichia coli ribosomal protein S1 unfolds structured mRNAs onto the ribosome for active translation initiation. PLoS biology, 11(12), e1001731. [CrossRef]
- Qu, X., Lancaster, L., Noller, H. F., Bustamante, C., &Tinoco, I., Jr (2012). Ribosomal protein S1 unwinds double-stranded RNA in multiple steps. Proceedings of the National Academy of Sciences of the United States of America, 109(36), 14458–14463. [CrossRef]
- Raghunathan, P. L., & Guthrie, C. (1998). A spliceosomal recycling factor that reanneals U4 and U6 small nuclear ribonucleoprotein particles. Science (New York, N.Y.), 279(5352), 857–860. [CrossRef]
- Montemayor, E. J., Curran, E. C., Liao, H. H., Andrews, K. L., Treba, C. N., Butcher, S. E., & Brow, D. A. (2014). Core structure of the U6 small nuclear ribonucleoprotein at 1.7-Å resolution. Nature structural & molecular biology, 21(6), 544–551. [CrossRef]
- Didychuk, A. L., Montemayor, E. J., Brow, D. A., & Butcher, S. E. (2016). Structural requirements for protein-catalyzed annealing of U4 and U6 RNAs during di-snRNP assembly. Nucleic acids research, 44(3), 1398–1410. [CrossRef]
- Belair, C., Sim, S., &Wolin, S. L. (2018). Noncoding RNA Surveillance: The Ends Justify the Means. Chemical reviews, 118(8), 4422–4447. [CrossRef]
- Blewett, N. H., &Maraia, R. J. (2018). La involvement in tRNA and other RNA processing events including differences among yeast and other eukaryotes. Biochimicaetbiophysicaacta. Gene regulatory mechanisms, 1861(4), 361–372. [CrossRef]
- Babitzke P. (2004). Regulation of transcription attenuation and translation initiation by allosteric control of an RNA-binding protein: the Bacillus subtilis TRAP protein. Current opinion in microbiology, 7(2), 132–139. [CrossRef]
- Sim, S., &Wolin, S. L. (2011). Emerging roles for the Ro 60-kDa autoantigen in noncoding RNA metabolism. Wiley interdisciplinary reviews. RNA, 2(5), 686–699. [CrossRef]
- Waters, L. S., &Storz, G. (2009). Regulatory RNAs in bacteria. Cell, 136(4), 615–628. [CrossRef]
- Updegrove, T. B., Zhang, A., &Storz, G. (2016). Hfq: the flexible RNA matchmaker. Current opinion in microbiology, 30, 133–138. [CrossRef]
- Kavita, K., de Mets, F., &Gottesman, S. (2018). New aspects of RNA-based regulation by Hfq and its partner sRNAs. Current opinion in microbiology, 42, 53–61. [CrossRef]
- Ellis, M. J., Trussler, R. S., &Haniford, D. B. (2015). Hfq binds directly to the ribosome-binding site of IS10 transposase mRNA to inhibit translation. Molecular microbiology, 96(3), 633–650. [CrossRef]
- Sauer, E., &Weichenrieder, O. (2011). Structural basis for RNA 3'-end recognition by Hfq. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13065–13070. [CrossRef]
- Zhang, A., Schu, D. J., Tjaden, B. C., Storz, G., &Gottesman, S. (2013). Mutations in interaction surfaces differentially impact E. coli Hfq association with small RNAs and their mRNA targets. Journal of molecular biology, 425(19), 3678–3697. [CrossRef]
- Soper, T. J., & Woodson, S. A. (2008). The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrAsRNA. RNA (New York, N.Y.), 14(9), 1907–1917. [CrossRef]
- Beisel, C. L., Updegrove, T. B., Janson, B. J., &Storz, G. (2012). Multiple factors dictate target selection by Hfq-binding small RNAs. The EMBO journal, 31(8), 1961–1974. [CrossRef]
- Panja, S., Schu, D. J., & Woodson, S. A. (2013). Conserved arginines on the rim of Hfqcatalyze base pair formation and exchange. Nucleic acids research, 41(15), 7536–7546. [CrossRef]
- Zheng, A., Panja, S., & Woodson, S. A. (2016). Arginine Patch Predicts the RNA Annealing Activity of Hfq from Gram-Negative and Gram-Positive Bacteria. Journal of molecular biology, 428(11), 2259–2264. [CrossRef]
- Hwang, W., Arluison, V., &Hohng, S. (2011). Dynamic competition of DsrA and rpoS fragments for the proximal binding site of Hfq as a means for efficient annealing. Nucleic acids research, 39(12), 5131–5139. [CrossRef]
- Wang, W. Wang, W., Wang, L., Zou, Y., Zhang, J., Gong, Q., Wu, J., & Shi, Y. (2011). Cooperation of Escherichia coli Hfqhexamers in DsrA binding. Genes & development, 25(19), 2106–2117. [CrossRef]
- Hopkins, J. F. Hopkins, J. F., Panja, S., McNeil, S. A., & Woodson, S. A. (2009). Effect of salt and RNA structure on annealing and strand displacement by Hfq. Nucleic acids research, 37(18), 6205–6213. [CrossRef]
- Ishikawa, H., Otaka, H., Maki, K., Morita, T., &Aiba, H. (2012). The functional Hfq-binding module of bacterial sRNAs consists of a double or single hairpin preceded by a U-rich sequence and followed by a 3' poly(U) tail. RNA (New York, N.Y.), 18(5), 1062–1074. [CrossRef]
- Sauer, E. Sauer, E., Schmidt, S., &Weichenrieder, O. (2012). Small RNA binding to the lateral surface of Hfqhexamers and structural rearrangements upon mRNA target recognition. Proceedings of the National Academy of Sciences of the United States of America, 109(24), 9396–9401. [CrossRef]
- Ribeiro, E.deA., Jr, Beich-Frandsen, M., Konarev, P. V., Shang, W., Vecerek, B., Kontaxis, G., Hämmerle, H., Peterlik, H., Svergun, D. I., Bläsi, U., &Djinović-Carugo, K. (2012). Structural flexibility of RNA as molecular basis for Hfq chaperone function. Nucleic acids research, 40(16), 8072–8084. [CrossRef]
- Panja, S., Paul, R., Greenberg, M. M., & Woodson, S. A. (2015). Light-Triggered RNA Annealing by an RNA Chaperone. AngewandteChemie (International ed. in English), 54(25), 7281–7284. [CrossRef]
- Updegrove, T. B., &Wartell, R. M. (2011). The influence of Escherichia coli Hfq mutations on RNA binding and sRNA•mRNA duplex formation in rpoSriboregulation. Biochimicaetbiophysicaacta, 1809(10), 532–540. [CrossRef]
- Peng, Y., Curtis, J. E., Fang, X., & Woodson, S. A. (2014). Structural model of an mRNA in complex with the bacterial chaperone Hfq. Proceedings of the National Academy of Sciences of the United States of America, 111(48), 17134–17139. [CrossRef]
- Desnoyers, G., &Massé, E. (2012). Noncanonical repression of translation initiation through small RNA recruitment of the RNA chaperone Hfq. Genes & development, 26(7), 726–739. [CrossRef]
- Gorski, S. A., Vogel, J., &Doudna, J. A. (2017). RNA-based recognition and targeting: sowing the seeds of specificity. Nature reviews. Molecular cell biology, 18(4), 215–228. [CrossRef]
- Moon, K., &Gottesman, S. (2011). Competition among Hfq-binding small RNAs in Escherichia coli. Molecular microbiology, 82(6), 1545–1562. [CrossRef]
- Olejniczak M. (2011). Despite similar binding to the Hfq protein regulatory RNAs widely differ in their competition performance. Biochemistry, 50(21), 4427–4440. [CrossRef]
- Fender, A., Elf, J., Hampel, K., Zimmermann, B., & Wagner, E. G. (2010). RNAs actively cycle on the Sm-like protein Hfq. Genes & development, 24(23), 2621–2626. [CrossRef]
- Hussein, R. , & Lim, H. N. (2011). Disruption of small RNA signaling caused by competition for Hfq. Proceedings of the National Academy of Sciences of the United States of America, 108(3), 1110–1115. [CrossRef]
- Vo, M. N. , Barany, G., Rouzina, I., &Musier-Forsyth, K. (2009). HIV-1 nucleocapsid protein switches the pathway of transactivation response element RNA/DNA annealing from loop-loop "kissing" to "zipper". Journal of molecular biology, 386(3), 789–801. [CrossRef]
- Busa, V. F. , Rector, M. J., & Russell, R. (2017). The DEAD-Box Protein CYT-19 Uses Arginine Residues in Its C-Tail To Tether RNA Substrates. Biochemistry, 56(28), 3571–3578. [CrossRef]
- Mohr, G. , Del Campo, M., Mohr, S., Yang, Q., Jia, H., Jankowsky, E., &Lambowitz, A. M. (2008). Function of the C-terminal domain of the DEAD-box protein Mss116p analyzed in vivo and in vitro. Journal of molecular biology, 375(5), 1344–1364. [CrossRef]
- Russell, R. , Jarmoskaite, I., &Lambowitz, A. M. (2013).Toward a molecular understanding of RNA remodeling by DEAD-box proteins. RNA biology, 10(1), 44–55. [CrossRef]
- Wang, C. , Uversky, V. N., & Kurgan, L. (2016). Disordered nucleiome: Abundance of intrinsic disorder in the DNA- and RNA-binding proteins in 1121 species from Eukaryota, Bacteria and Archaea. Proteomics, 16(10), 1486–1498. [CrossRef]
- Santiago-Frangos, A. , Kavita, K., Schu, D. J., Gottesman, S., & Woodson, S. A. (2016). C-terminal domain of the RNA chaperone Hfq drives sRNA competition and release of target RNA. Proceedings of the National Academy of Sciences of the United States of America, 113(41), E6089–E6096. [CrossRef]
- Vincent, H. A. , Henderson, C. A., Stone, C. M., Cary, P. D., Gowers, D. M., Sobott, F., Taylor, J. E. N., & Callaghan, A. J. (2012).The low-resolution solution structure of Vibrio choleraeHfq in complex with Qrr1 sRNA. Nucleic acids research, 40(17), 8698–8710. [CrossRef]
- Santiago-Frangos, A. , & Woodson, S. A. (2018).Hfq chaperone brings speed dating to bacterial sRNA. Wiley interdisciplinary reviews.RNA, 9(4), e1475. [CrossRef]
- Holmstrom, E.D. , Liu, Z., Nettels, D. et al.(2019) Disordered RNA chaperones can enhance nucleic acid folding via local charge screening. Nat Commun 10, 2453. [CrossRef]
- Shetty, S., Kim, S., Shimakami, T., Lemon, S. M., &Mihailescu, M. R. (2010). Hepatitis C virus genomic RNA dimerization is mediated via a kissing complex intermediate. RNA (New York, N.Y.), 16(5), 913–925. [CrossRef]
- Daher, M., Widom, J. R., Tay, W., & Walter, N. G. (2018). Soft Interactions with Model Crowders and Non-canonical Interactions with Cellular Proteins Stabilize RNA Folding. Journal of molecular biology, 430(4), 509–523. [CrossRef]
- Godet, J., Boudier, C., Humbert, N., Ivanyi-Nagy, R., Darlix, J. L., &Mély, Y. (2012). Comparative nucleic acid chaperone properties of the nucleocapsid protein NCp7 and Tat protein of HIV-1. Virus research, 169(2), 349–360. [CrossRef]
- Vuzman, D., & Levy, Y. (2012).Intrinsically disordered regions as affinity tuners in protein-DNA interactions. Molecular bioSystems, 8(1), 47–57. [CrossRef]
- Grohman, J. K., Gorelick, R. J., Lickwar, C. R., Lieb, J. D., Bower, B. D., Znosko, B. M., & Weeks, K. M. (2013). A guanosine-centric mechanism for RNA chaperone function. Science (New York, N.Y.), 340(6129), 190–195. [CrossRef]
- Hyeon, C., &Thirumalai, D. (2013). Generalized iterative annealing model for the action of RNA chaperones. The Journal of chemical physics, 139(12), 121924. [CrossRef]
- Son, A., Horowitz, S., & Seong, B. L. (2021). Chaperna: linking the ancient RNA and protein worlds. RNA biology, 18(1), 16–23. [CrossRef]
- Lazcano, A., & Miller, S. L. (1996). The origin and early evolution of life: prebiotic chemistry, the pre-RNA world, and time. Cell, 85(6), 793–798. [CrossRef]
- Robertson, M. P., & Joyce, G. F. (2012).The origins of the RNA world. Cold Spring Harbor perspectives in biology, 4(5), a003608. [CrossRef]
- Joyce G. F. (2002).The antiquity of RNA-based evolution. Nature, 418(6894), 214–221. [CrossRef]
- Altman S. (2013).The RNA-Protein World. RNA (New York, N.Y.), 19(5), 589–590. [CrossRef]
- Cech T. R. (2012).The RNA worlds in context. Cold Spring Harbor perspectives in biology, 4(7), a006742. [CrossRef]
- Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N., & Altman, S. (1983). The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell, 35(3 Pt 2), 849–857. [CrossRef]
- Gobert, A., Gutmann, B., Taschner, A., Gössringer, M., Holzmann, J., Hartmann, R. K., Rossmanith, W., &Giegé, P. (2010). A single Arabidopsis organellar protein has RNase P activity. Nature structural & molecular biology, 17(6), 740–744. [CrossRef]
- Kim, Y. E., Hipp, M. S., Bracher, A., Hayer-Hartl, M., &Hartl, F. U. (2013). Molecular chaperone functions in protein folding and proteostasis. Annual review of biochemistry, 82, 323–355. [CrossRef]
- Hsieh, J., &Fierke, C. A. (2009). Conformational change in the Bacillus subtilisRNase P holoenzyme--pre-tRNA complex enhances substrate affinity and limits cleavage rate. RNA (New York, N.Y.), 15(8), 1565–1577. [CrossRef]
- Walsh C. T. (2004).Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science (New York, N.Y.), 303(5665), 1805–1810. [CrossRef]
- Noller H. F. (2012).Evolution of protein synthesis from an RNA world. Cold Spring Harbor perspectives in biology, 4(4), a003681. [CrossRef]
- Ban, N. Ban, N., Nissen, P., Hansen, J., Moore, P. B., &Steitz, T. A. (2000). The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science (New York, N.Y.), 289(5481), 905–920. [CrossRef]
- Das, D., Das, A., Samanta, D., Ghosh, J., Dasgupta, S., Bhattacharya, A., Basu, A., Sanyal, S., & Das Gupta, C. (2008).Role of the ribosome in protein folding. Biotechnology journal, 3(8), 999–1009. [CrossRef]
- Samanta, D., Mukhopadhyay, D., Chowdhury, S., Ghosh, J., Pal, S., Basu, A., Bhattacharya, A., Das, A., Das, D., &DasGupta, C. (2008). Protein folding by domain V of Escherichia coli 23S rRNA: specificity of RNA-protein interactions. Journal of bacteriology, 190(9), 3344–3352. [CrossRef]
- Basu, A., Samanta, D., Das, D., Chowdhury, S., Bhattacharya, A., Ghosh, J., Das, A., &Dasgupta, C. (2008). In vitro protein folding by E. coli ribosome: unfolded protein splitting 70S to interact with 50S subunit. Biochemical and biophysical research communications, 366(2), 598–603. [CrossRef]
- Tribouillard-Tanvier, D., Dos Reis, S., Gug, F., Voisset, C., Béringue, V., Sabate, R., Kikovska, E., Talarek, N., Bach, S., Huang, C., Desban, N., Saupe, S. J., Supattapone, S., Thuret, J. Y., Chédin, S., Vilette, D., Galons, H., Sanyal, S., &Blondel, M. (2008). Protein folding activity of ribosomal RNA is a selective target of two unrelated antiprion drugs. PloS one, 3(5), e2174. [CrossRef]
- Voisset, C., Blondel, M., Jones, G. W., Friocourt, G., Stahl, G., Chédin, S., Béringue, V., &Gillet, R. (2017). The double life of the ribosome: When its protein folding activity supports prion propagation. Prion, 11(2), 89–97. [CrossRef]
- Voisset, C., Blondel, M., Jones, G. W., Friocourt, G., Stahl, G., Chédin, S., Béringue, V., &Gillet, R. (2017). The double life of the ribosome: When its protein folding activity supports prion propagation. Prion, 11(2), 89–97. [CrossRef]
- Huang, H., & Karbstein, K. (2021). Assembly factors chaperone ribosomal RNA folding by isolating helical junctions that are prone to misfolding. Proceedings of the National Academy of Sciences of the United States of America, 118(25), e2101164118. [CrossRef]
- Adilakshmi, T., Bellur, D. L., & Woodson, S. A. (2008). Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature, 455(7217), 1268–1272. [CrossRef]
- Duss, O., Stepanyuk, G. A., Puglisi, J. D., & Williamson, J. R. (2019).Transient Protein-RNA Interactions Guide Nascent Ribosomal RNA Folding. Cell, 179(6), 1357–1369.e16. [CrossRef]
- Rodgers, M. L., & Woodson, S. A. (2019). Transcription Increases the Cooperativity of Ribonucleoprotein Assembly. Cell, 179(6), 1370–1381.e12. [CrossRef]
- Gracia, B., Al-Hashimi, H. M., Bisaria, N., Das, R., Herschlag, D., & Russell, R. (2018).Hidden Structural Modules in a Cooperative RNA Folding Transition. Cell reports, 22(12), 3240–3250. [CrossRef]
- Russell, R., Tijerina, P., Chadee, A. B., &Bhaskaran, H. (2007). Deletion of the P5abc peripheral element accelerates early and late folding steps of the Tetrahymena group I ribozyme. Biochemistry, 46(17), 4951–4961. [CrossRef]
- Huang, H., Ghalei, H., &Karbstein, K. (2020). Quality control of 40S ribosome head assembly ensures scanning competence. The Journal of cell biology, 219(11), e202004161. [CrossRef]
- Rai, J., Parker, M. D., Huang, H., Choy, S., Ghalei, H., Johnson, M. C., Karbstein, K., &Stroupe, M. E. (2021). An open interface in the pre-80S ribosome coordinated by ribosome assembly factors Tsr1 and Dim1 enables temporal regulation of Fap7. RNA (New York, N.Y.), 27(2), 221–233. [CrossRef]
- Liphardt, J., Onoa, B., Smith, S. B., Tinoco, I., Jr, & Bustamante, C. (2001).Reversible unfolding of single RNA molecules by mechanical force. Science (New York, N.Y.), 292(5517), 733–737. [CrossRef]
- Ghalei, H., Schaub, F. X., Doherty, J. R., Noguchi, Y., Roush, W. R., Cleveland, J. L., Stroupe, M. E., &Karbstein, K. (2015). Hrr25/CK1δ-directed release of Ltv1 from pre-40S ribosomes is necessary for ribosome assembly and cell growth. The Journal of cell biology, 208(6), 745–759. [CrossRef]
- Jahagirdar, D., Jha, V., Basu, K., Gomez-Blanco, J., Vargas, J., & Ortega, J. (2020). Alternative conformations and motions adopted by 30S ribosomal subunits visualized by cryo-electron microscopy. RNA (New York, N.Y.), 26(12), 2017–2030. [CrossRef]
- Holmes, K. L., & Culver, G. M. (2005).Analysis of conformational changes in 16 S rRNA during the course of 30 S subunit assembly. Journal of molecular biology, 354(2), 340–357. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).