1. Introduction
Waldenstrom’s Macroglobulinemia (WM) is a rare (1-2% of all hematological malignancies), indolent B-cell lymphoma, with the hallmark of clonal lymphoplasmacytic bone marrow (BM) infiltration (lymphoplasmacytic lymphoma-LPL) and monoclonal IgM secretion [
1,
2,
3]. There are three entities in the spectrum of WM; IgM Monoclonal Gammopathy of Undetermined Significance (IgM-MGUS), asymptomatic WM (AWM) and symptomatic WM [
3,
4,
5,
6,
7]. In the heterogenous WM disease spectrum, both IgM-MGUS and AWM are asymptomatic, but AWM is more likely to progress to WM [
4]. IgM-MGUS is defined by the presence of monoclonal IgM <3 g/dL and BM infiltration <10%. WM is defined by the presence of BM infiltration≥10% with IgM of any concentration, along with the presence of symptoms related to the disease, while AWM fulfills the diagnostic criteria of WM but requires no treatment initiation [
3,
4,
5,
6,
7]. Indications for treatment include constitutional symptoms (recurrent fever, night sweats, fatigue, or weight loss), progressive symptomatic lymphadenopathy or splenomegaly, hemoglobin <10g/dL or platelets <100x10*9/L, systemic amyloidosis, signs of hyperviscocity syndrome and autoimmune manifestations [
2,
3,
4].
The availability of prognostic markers is important in order to discriminate on one hand the subgroup of patients at risk to present with an aggressive disease, and on the other hand detect patients who although asymptomatic at diagnosis might need treatment in the near future [
1].
Tumor-associated macrophages (TAMs) are macrophages that constitute a vital part of the tumor microenvironment. TAMs are widely present in various tumors, promoting tumor cell growth, dissemination, and drug resistance [
8].
TAMs can decompose the extracellular matrix by secreting various enzymes {matrix metalloproteinases (MMPs) and cathepsins}, aiding the migration of tumor cells and their extravasation into the circulation [
8,
9]. Additionally, extravasation is enhanced by increasing the vascular permeability through secretion of vascular endothelial growth factor (VEGF) and nitric oxide (NO) by TAMs [
9,
10]. Moreover, they release molecules including MMP-2, MMP-7, MMP-9, MMP-12, and cyclooxygenase-2, that promote angiogenesis [
8,
10]. Thus, TAMs not only increase the ability of tumor cells to migrate, but also enlarge the actual density of blood vessels [
8,
9,
10].
A plethora of growth factors is secreted by TAMs, such as epithelial growth factor (EGF), platelet-derived growth factor (PDGF), and epithelial growth ligands of the factor receptor (EGFR) family and basic fibroblast growth factor (bFGF), all stimulating tumor cell proliferation. Noteworthy, TAMs are an important cell source for EGF secretion in tumor microenvironment and play an exceptionally important role in the tumor growth of breast and lung cancer, where EGFR ligand is abundant [
8].
Moreover, TAMs seem to facilitate the formation of premetastatic niches in distant organs [
9,
10]. TAMs-derived tumor necrosis factor-a (TNF-α), VEGF, and transforming growing factor-β (TGF-β), induce in specific organs the production of S100A8 and serum amyloid A3, both of which can recruit macrophages and tumor cells [
10].
With regards to altered immune regulation in tumor microenvironment, TAMs-derived IL-6 promotes signal transducer and activator of transcription 3 (STAT3) phosphorylation leading to decreased apoptosis in tumors cells [
10,
11]. In addition, they secrete arginase I (ARGI) which metabolizes and depletes L-arginine, an essential amino acid for CD8+ T-cell proliferation. Besides, TAMs recruit natural regulatory T (nTreg) cells, further suppressing the antitumor immune activity of CD8+ T-cells [
8,
9,
10,
12].
Recruited macrophages most probably account for the majority of TAMs [
8,
9], while M-MDSCs (monocyte-related myeloid-derived suppressor cells) are another main circulating precursor of TAMs [
8]. TAMs receive signals from the microenvironment in which they reside via multiple mechanisms and have mainly the characteristics of M2 macrophages [
10]. M2 TAMs express a large number of scavenger receptors, including CD163 [
8,
11,
12]. CD163 is identified as the ‘‘hemoglobin (Hb) scavenger receptor’’ and is responsible for the uptake of Hb released into the plasma. It is expressed almost exclusively on macrophages and monocytes [
13,
14], hence TAMs are commonly identified with immunochemistry by the expression of CD163 [
10]. Soluble variant of CD163 (sCD163), a product of shedding, is present in plasma and other tissue fluids [
13] and has been found to be increased in various solid tumors and hematological malignancies, reflecting increased burden of TAMs overexpressing CD163 [
15].
CCL2 {Monocyte Chemoattractant Protein-1 (MCP-1)} is a member of the C-C chemokine family [
16,
17] and high levels of CCL2 induce polarization of macrophages to the tumor-promoting phenotype of TAMs [
18]. Moreover, CCL4 {Macrophage Inflammatory Protein 1 beta (MIP-1b)} also belongs to the CC chemokine subfamily [
19] and is secreted by tumor cells together with other chemokines such as CCL2, inducing TAMs infiltration and promoting cancer progression [
19,
20].
Both CCL2 and CCL4 can be secreted by activated leukocytes, lymphocytes, endothelial and cancer cells, and are chemoattractant for natural killer cells and monocytes [
16,
17,
19]. High levels of CCL2 promote polarization of tumor microenvironment macrophages to the phenotype of TAMs and enhance the interaction between them and tumor cells. Additionally, CCL2 may promote macrophages for epithelial-to-mesenchymal transition, making the tumor more prone to metastasis [
18].
Recognizing the interplay between sCD163 which acts as a surrogate of CD-163 positive TAMs burden, with CCL2 and CCL4 that enforce TAMs’ tumor infiltration, could reveal new biomarkers. Hence, in our study we aimed to demonstrate the association between sCD163, CCL2 and CCL4, as prognostic markers in WM, helping to detect high-risk WM patients.
2. Materials and Methods
4.1. Patient Selection
We retrospectively reviewed 204 WM patients diagnosed in Laikon General Hospital of Athens from 1990 to 2023. As a control group, plasma from 25 healthy volunteers was obtained. The inclusion criteria were a confirmed diagnosis of WM, IgM-MGUS or LPL according to the latest WHO classification, availability of frozen sera samples from diagnosis before any treatment, and consent to participate in the study. Patients who had received any prior treatment including corticosteroids before the collection of the serum or had been diagnosed with other malignancies or autoimmune diseases that could influence serum sCD163 levels, were excluded from the study.
4.2. Cytokine Measurement
Measurements of the cytokines were performed using an enzyme-linked immunosorbent assay (ELISA) (Quantikine, Duo-Set R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. These assays are solid phase sandwich ELISA that utilize natural human CD163, CCL2 and CCL4 (catalog numbers DC1630, DY279 and DY271 respectively), with a < 0.5% cross-reactivity and no significant interference observed with available related molecules. Their sensitivities for CD163, CCL2 and CCL4, are 0.613 ng/mL, 10 pg/mL and 11 pg/mL respectively. The method includes reagent preparation, plate preparation, and sample determination. The microplate was pre-coated with a monoclonal antibody specific for human CD163, CCL2 and CCL4 respectively, and incubated overnight. All measurements were performed in duplicate. Unfortunately, all WM patients displayed levels of serum-soluble receptor CD163, exceeding the highest standard value, and it was necessary to dilute the sample with a diluent and repeat the assay, utilizing a dilution ratio of 1:4. All cytokine levels were expressed in picogram/mL.
4.3. Statistical Analysis
Statistical analysis was performed using the SPSS v.28 software. Kaplan–Meier curves depicted survival, while the log-rank test was used to estimate the differences in outcomes between the subgroups that were studied. Statistical significance was set at p < 0.05. To compare plasma levels of cytokines from controls versus patients, statistical analysis was performed using the Mann–Whitney test, and p values <0.05 were considered significant. We utilized the median values as the cut-off for statistical analysis, since it can dichotomize a continuous variable and guarantees an equal sample size for both groups. Additionally, since there are no known normal ranges for these cytokines, we attempted to find a cut-off that would provide a statistical significance. Spearman’s test was used to analyze correlation between various cytokines.
4.4. Study Approval
This study was approved by the institutional review board of Laikon General Hospital of Athens with protocol number 2782 and written informed consent was obtained from all participants in research for analysis of medical history and sera collection. Patient confidentiality and data anonymization was maintained throughout the study.
3. Results
204 patients were included in the study of whom 82 had WM, 88 had AWM, 14 had IgM-MGUS and 20 had LPL. 56% were males and 44% were females, with a mean age of 66.5 years (range, 33-92 years). As AWM patients were defined those fulfilling the diagnostic criteria for WM but not requiring treatment at diagnosis; the term also includes patients receiving treatment 6 months or more after the initial diagnosis. Time to Treatment (TTT) was defined as the interval between the initial diagnosis and treatment initiation.
The patients’ characteristics and their laboratory tests at diagnosis are shown in
Table 1. At diagnosis the median levels of platelets, hemoglobin, white blood cells, lymphocytes, monocytes, IgM, LDH, b2-microglobulin and ESR were 215 K/μL, 11.3 gr/dL, 7.14 K/μL, 6.7 K/μL, 2 K/μL, 0.49 K/μL, 2088 mg/dL, 290 IU/L, 3.29 mg/dL and 86.5mm/hr respectively.
In our study median values of the cytokines’ measurements were chosen as cut-offs for statistical analysis. sCD163 was measured in 75 patients and the median value was 28163 pg/ml (range: 16696 to 97286 pg/mL) in WM, and 27368 pg/mL (range: 25410 to 51319 pg/ml) in patients with LPL; it was statistically significantly higher than in patients with IgM-MGUS (median: 26821 pg/mL (range: 14281 to 97280 pg/ml) and healthy individuals (HI) (median: 26826 pg/ml; range: 11831-97286 pg/mL)(p < 0.001). Serum CCL2 was measured in 64 patients and the median value was 347.5 pg/ml (range: 291 to 1829 pg/mL) in HI and 497.45 pg/ml (range: 6.64 to 1713.11 pg/mL) in patients; serum CCL4 was measured in 65 patients the median value was 202 pg/ml (range: 185.53 to 578.61 pg/mL) in HI and 278.61 pg/mL (range: 0-2462 pg/mL) in patients (
Table 2).
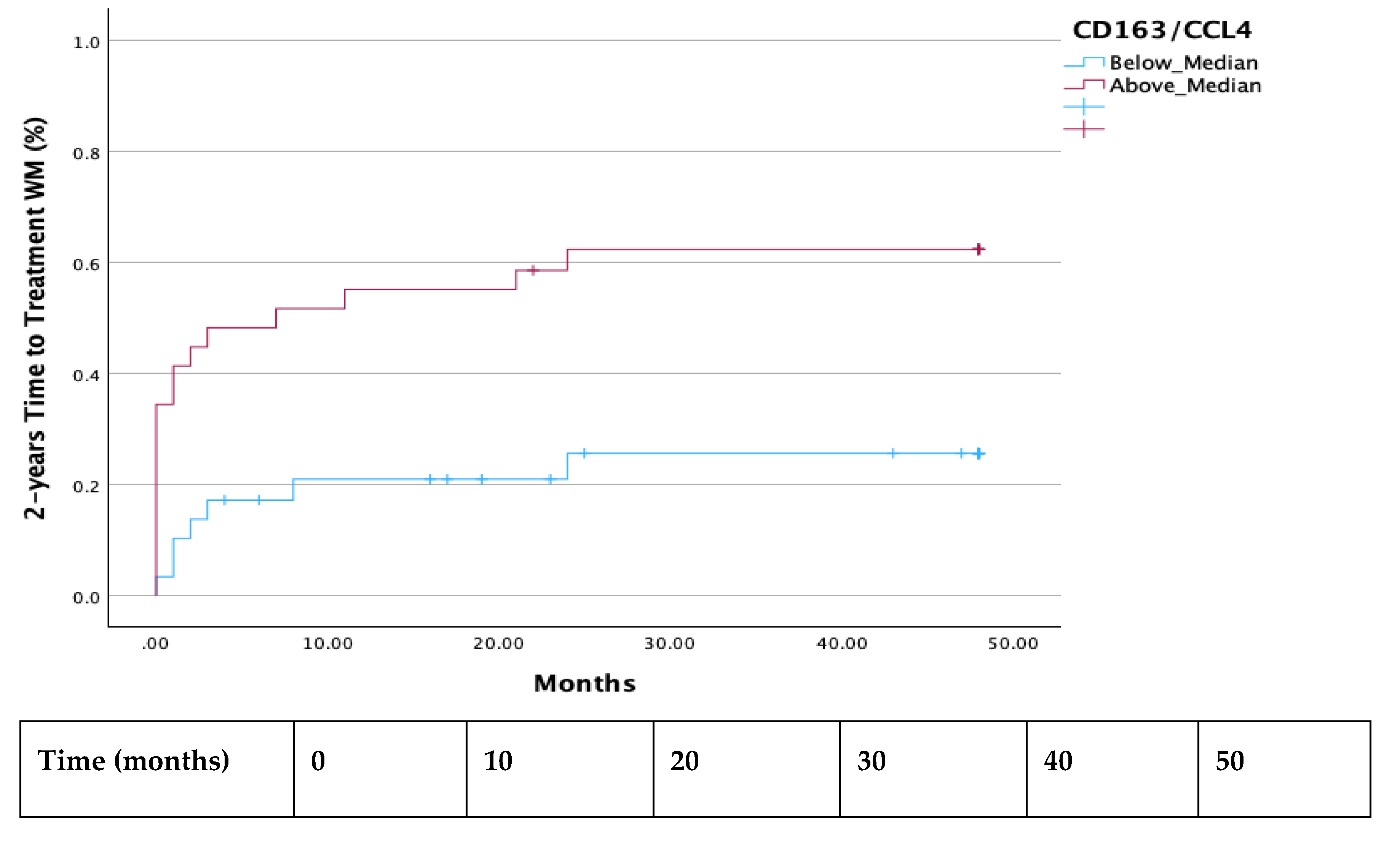
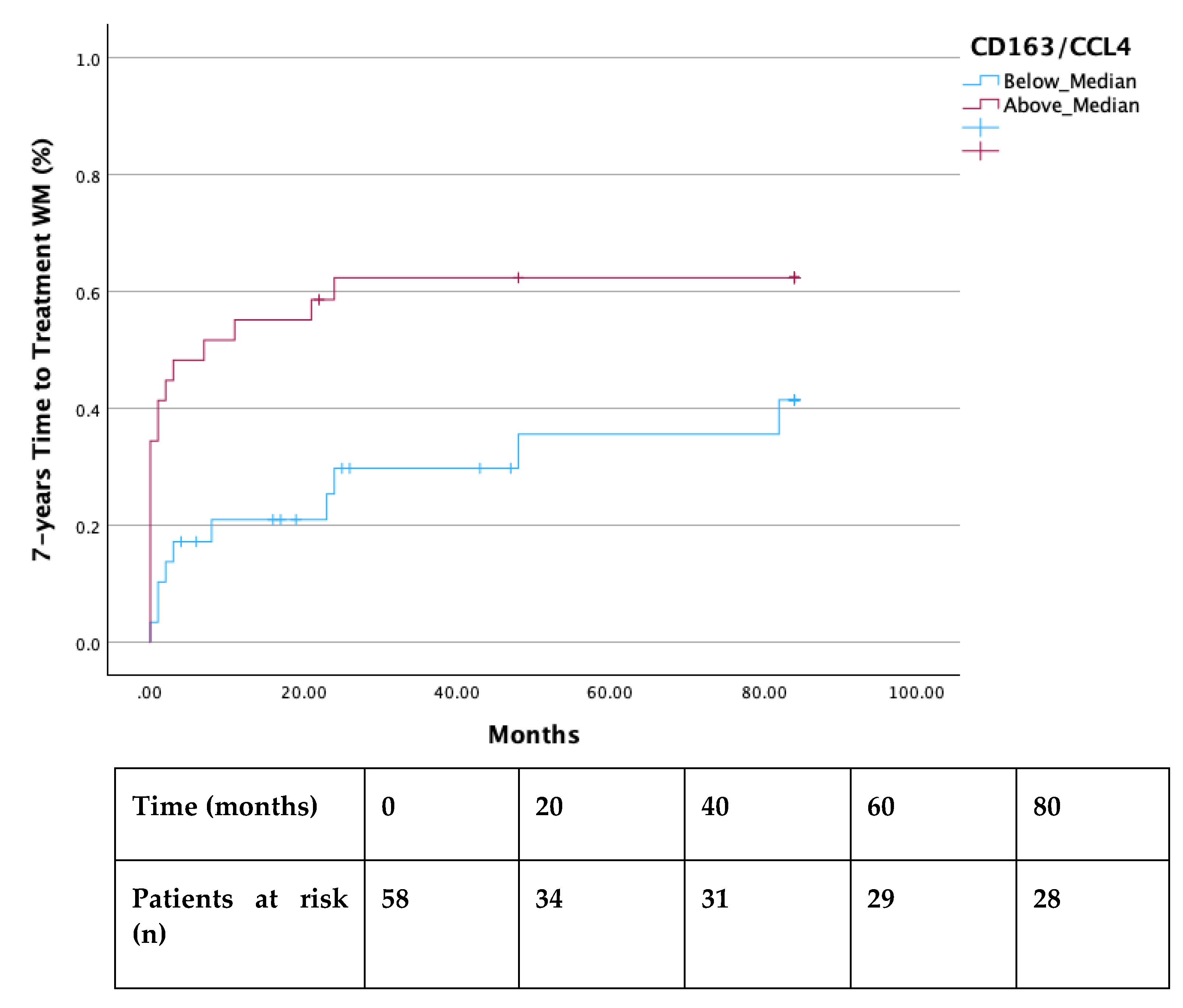
Both the 2-year and 7-year TTT were shorter in all patients with a ratio of CD163/CCL4 above median (p =0.003 and p=0.024 respectively) (
Figure 1 and
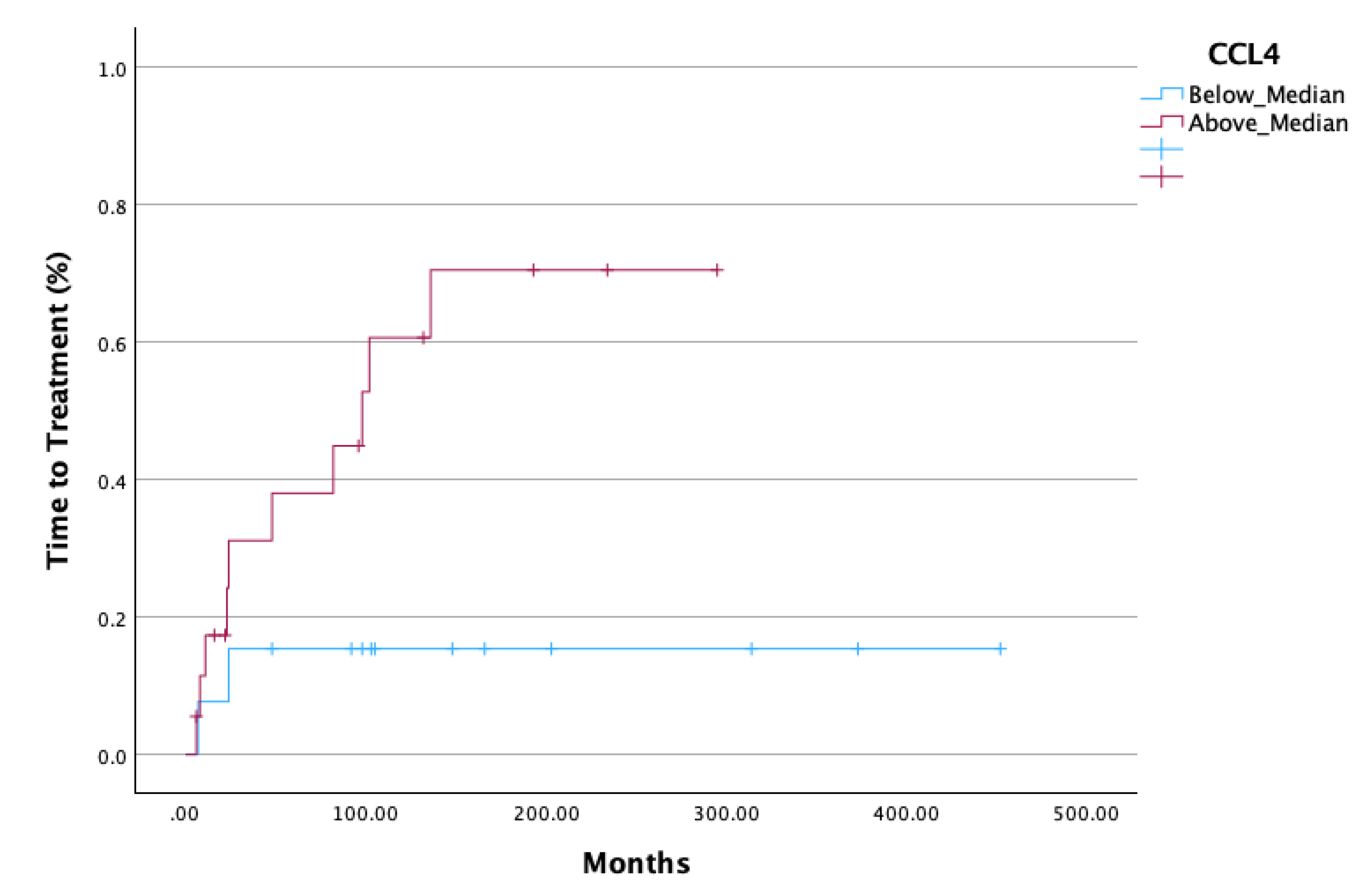
Figure 2). Additionally, significantly decreased TTT was observed in all AWM patients with values of CCL4 above the median (p=0.018) (
Figure 3).
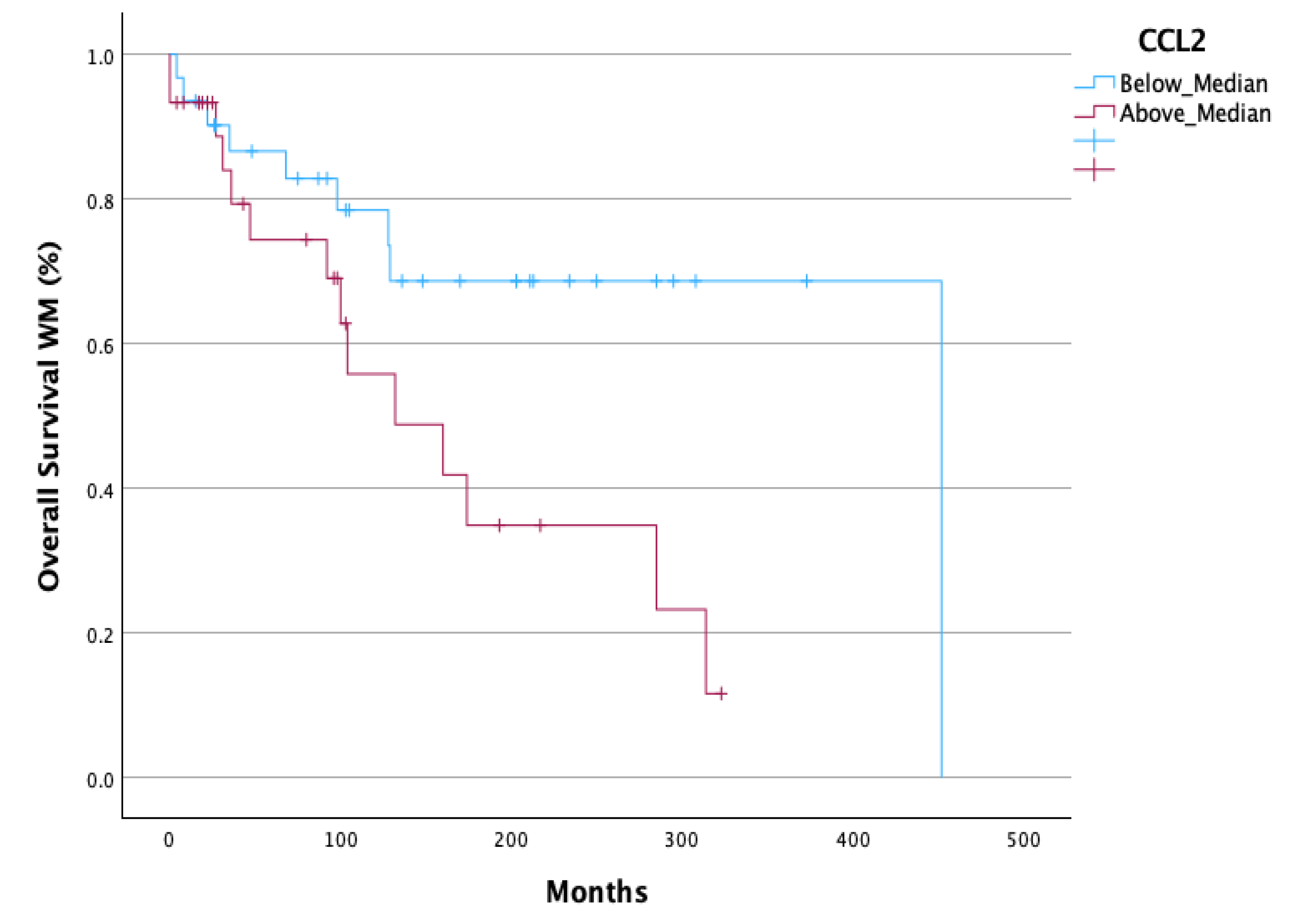
A statistically significant decreased Overall Survival (OS) (p=0.033) (
Figure 4) was observed in all WM patients with CCL2 values above median. In AWM patients, CCL2 above median had a strong tendency for decreased OS (p=0.08) (Supplementary material).
It is worth noticing that the absolute number of monocytes count, and the ratio of monocytes-to-lymphocytes were not associated with OS or TTT.
Correlation bivariate analysis amongst sCD163, CCL2, CCL4 demonstrated a positive correlation by Spearman between CCL2 and CCL4 (Spearman correlation coefficient: 0.433, p <0.001) and CCL2 with the ratio sCD163/CCL4 (Spearman correlation coefficient: 0.461, p <0.001). Similarly, correlation analysis between sCD163, CCL2 and CCL4, and other cytokines known to have a prognostic impact in WM pathogenesis showed the following positive correlations; sCD163 with TGFb (0.343, p=0.037), sCD163 with serum Syndecan (CD138) (0.326, p=0.034), CCL4 with VEGF (0.394, p=0.023), and CD163/CCL4 with VEGF (0.453, p-=0.011). Moreover, we examined the correlation of sCD163, CCL2 and CCL4 with clinical factors of WM and the following correlations were revealed; bone marrow infiltration was correlated with both sCD163 (0.249, p=0.04) and CCL2 (0.272, p=0.043), and occurrence of splenomegaly was associated with CCL4 (0.311, p=0.023).
4. Discussion
In our study two hundred and four patients were included of whom 82 were diagnosed with WM, 88 with AWM, 14 with IgM-MGUS and 20 with LPL.
WM mortality is mainly attributed to disease-related symptoms and complications, thus IgM-MGUS and AWM have an OS similar, or slightly decreased, compared to that of the general population, and can remain stable for years [
6]. Conversely, symptomatic WM has a median survival ranging from 5 to greater than 10 years [
2,
6,
21,
22]. OS was not improved drastically after the introduction of the rituximab-based regimen in WM, an observation mainly attributed to the low rate of patients achieving Complete Response (CR) [
23,
24]. Therefore, over the past few years many studies have focused on associating patients’ characteristics and biomarkers with their clinical outcome, attempting to detect patients who present with adverse characteristics [
1,
21,
25]. However, the rarity and the heterogeneity of WM, along with the long follow-up needed to extract safe conclusions, make the development of new biomarkers challenging [
6,
21].
TAMs and their contribution in the tumor microenvironment have been studied for years. They secrete numerous cytokines that stimulate tumor cell proliferation and survival, with the enhancement of invasion/metastasis and the creation of premetastatic niches being the most thoroughly described mechanism by which TAMs promote solid tumor progression [
8,
10].
As more light has been shed on the relationship between TAMs and neoplasms, their level of infiltration has begun to be used as potential biomarker for diagnosis and prognosis. In recent years, several studies have demonstrated the roles of TAMs and sCD163 in the pathogenesis of hematopoietic malignancies including Multiple myeloma (MM) [
15,
26,
27], Hodgkin lymphoma (HL) [
28,
29], Chronic Lymphocytic leukemia (CLL) [
30], and Diffuse B-cell large lymphoma (DLBCL) [
31,
32]. In all the aforementioned studies, increased sCD163 evaluated with ELISA and CD163+ TAMs tumor infiltration evaluated with immunohistochemistry, were indicative of unfavorable prognosis, poorer outcome, rapid progression, and increased likelihood of treatment resistance or recurrence.
The vast majority of patients in our study with the diagnosis of WM/AWM exhibited increased levels of serum sCD163, irrespective of their clinical and laboratory findings. Consequently, elevated levels of soluble receptor CD163 appears to be a common feature among patients with WM implicating that TAMs play a crucial role in disease pathogenesis.
Notably, IgM-MGUS patients and HI had similar values and median value of sCD163. Since IgM-MGUS is a naive condition with a very low annual incidence of progression to WM (6), we assume that the infiltration by TAMs should be minimal, hence the sCD163 levels are not elevated. This observation further supports the hypothesis of TAMs being involved in WM disease progression.
TAMs receive signals for their polarization from the microenvironment in which they reside and are not observed in the steady state but rather in pathologic conditions, such as neoplasms [
10,
11]. CCL2 and CCL4 are cytokines that belong to the C-C chemokine family characterized by adjacent cysteine residue [
16,
17,
19]. Tumor-cell derived CCL4 and CCL2 can enhance TAM infiltration leading to cancer progression. The expression levels of both, CCL4 and CCL2, were elevated in colon-cancer and lung adenocarcinoma tissues, and were associated with shorter OS [
19,
20,
33]. It is noteworthy that Inhibition of CCL2 and CCL4 expression has been shown to suppress macrophage infiltration in solid tumors [
19,
33].
To our knowledge there is scarce data in the hematological neoplasms and no other in WM, evaluating CCL2 and CCL4 as chemoattractant cytokines for TAM recruitment, along with CD163. It is vital to recognize not only the sCD163 as a possible biomarker in WM but also other cytokines known to increase BM infiltration of TAMs, such as CCL2 and CCL4, as they could be used as potential therapeutic targets [
19].
In our study we showed that serum CCL4 and its ratio with CD163 (CD163/CCL4) were able to predict TTT in WM and AWM, as patients with values above the median had significantly shortened TTT. Moreover, AWM with CCL4 values above the median demonstrated a more rapid evolution to symptomatic WM as was depicted by their shortened TTT. Further investigation is needed to verify our finding, however it would imply that serum sCD163 levels, CCL4 levels, and their ratio, can eventually discriminate patient with AWM who will evolve to symptomatic WM, guiding decisions regarding the intervals of their follow-up. Additionally, AWM and WM patients with elevated serum CCL2 seem to have a diminished OS, revealing a probable new OS-biomarker in a long-standing disease.
Tumor microenvironment has been well studied in WM and various cytokines have been deemed to orchestrate the pathogenetic mechanisms, such as IL-1, sIL-6, Syndecan (CD138) and TGF-b [
34,
35,
36,
37].Our analysis revealed a strongly positive correlation not only amongst CCL2, CCL4 and CD163, but also with the other cytokines involved in WM pathogenesis, showing that TAMs and the related cytokines should have a crucial role in the disease course.
Biomarkers display an important tool in medicine, facilitating diagnosis and prognosis, and are useful in numerous ways, including measuring disease progression, detecting high-risk patients for treatment failure, and establishing susceptibility to recurrence. WM is a rare and indolent disease, hence long-term follow up is needed to exclude safe results regarding new biomarkers. We achieved to demonstrate in a large group of WM patients with a very long follow-up that sCD163, CCL2 and CCL4, readily measured with ELISA, may be used as biomarkers to predict TTT and OS, reinforcing the hypothesis that TAMs play an important role in disease pathogenesis.
5. Conclusions
In our study, we achieved to demonstrate that sCD163, along with CCL2 and CCL4, could be utilized as novel biomarkers in WM, predicting TTT, especially in AWM, and OS. As the levels of sCD163 reflect the burden of TAMs in the tumor microenvironment along with CCL2 and CCL4 that are well studied chemoattractant cytokines for TAMs’ infiltration, we hypothesize that TAMs play an important role in WM pathogenesis. The roles of CD163, CCL2 and CCL4 in WM pathogenesis seem to be interwoven and mutually affecting each other, as their values and their ratios seem to be prognostically important. Hence, further study is needed to confirm our findings and fully clarify their interactions on gene and molecular level.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Design study, M.-C.K. and N.K.; Methodology, A.G., M.P-G. A.K., A.I.G., T.M.T., A.A.; validation, A.G. and M.-C.K.; formal analysis, A.G. and M.-C.K.; resources, data curation, A.G., A.K., A.I.G., A.G., M.P-G., A.A. and V.B.; writing—review and editing, A.G.; supervision, M.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Laikon General Hospital of Athens (protocol code 2782).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dimopoulos, M. A.; Kastritis, E. How I Treat Waldenström Macroglobulinemia. Blood 2019, 134(23), 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Johnson, A. C.; Okolo, O. N.; Arnold, S. J.; McBride, A.; Zhang, L.; Baz, R. C.; Anwer, F. Waldenström Macroglobulinemia: Review of Pathogenesis and Management. Clin Lymphoma Myeloma Leuk 2017, 17(5), 252–262. [Google Scholar] [CrossRef] [PubMed]
- Cingam, S.; Sidana, S. Differential Diagnosis of Waldenström’s Macroglobulinemia and Early Management: Perspectives from Clinical Practice. Blood Lymphat Cancer 2022, Volume 12 (July), 107–117. [Google Scholar] [CrossRef]
- Kyle, R. A.; Benson, J. T.; Larson, D. R.; Therneau, T. M.; Dispenzieri, A.; Kumar, S.; Melton, L. J.; Rajkumar, S. V. Progression in Smoldering Waldenström Macroglobulinemia: Long-Term Results. Blood 2012, 119(19), 4462–4466. [Google Scholar] [CrossRef] [PubMed]
- Ocio, E. M.; Del Carpio, D.; Caballero, Á.; Alonso, J.; Paiva, B.; Pesoa, R.; Villaescusa, T.; López-Anglada, L.; Vidriales, B.; García-Sanz, R. Differential Diagnosis of IgM MGUS and WM According to B-Lymphoid Infiltration by Morphology and Flow Cytometry. Clin Lymphoma Myeloma Leuk 2011, 11(1), 93–95. [Google Scholar] [CrossRef]
- Kyle, R. A.; Treon, S. P.; Alexanian, R.; Barlogie, B.; Björkholm, M.; Dhodapkar, M.; Lister, T. A.; Merlini, G.; Morel, P.; Stone, M.; Branagan, A. R.; Leblond, V. Prognostic Markers and Criteria to Initiate Therapy in Waldenstrom’s Macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003, 30(2), 116–120. [Google Scholar] [CrossRef]
- Robert, A. Kyle, Joanne Benson, Dirk Larson, Terry Therneau, Angela Dispenzieri, L. Joseph Melton III, S. V. R. IgM Monoclonal Gammopathy of Undetermined Significance and Smoldering Waldenström’s Macroglobulinemia. Clin Lymphoma Myeloma Leuk 2009, 9 (1), 17–18. [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol 2020, 11 (December). [CrossRef]
- Noy, R.; Pollard, J. W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41(1), 49–61. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-Associated Macrophages: From Basic Research to Clinical Application. J Hematol Oncol 2017, 10(1), 58. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol 2020, 10 (February), 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. The Origin and Function of Tumor-Associated Macrophages. Cell Mol Immunol 2015, 12(1), 1–4. [Google Scholar] [CrossRef]
- Møller, H. J. Soluble CD163. Scand J Clin Lab Invest 2012, 72(1), 1–13. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Moestrup, S. K. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid Redox Signal 2013, 18(17), 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M. N.; Abildgaard, N.; Maniecki, M. B.; Møller, H. J.; Andersen, N. F. Monocyte/Macrophage-Derived Soluble CD163: A Novel Biomarker in Multiple Myeloma. Eur J Haematol 2014, 93(1), 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jian Zhang1, Lalit Patel1, and K. J. P. CC Chemokine Ligand 2 (CCL2) Promotes Prostate Cancer Tumorigenesis and Metastasis. Cytokine Growth Factor Rev. 2010, 21 (1), 41–48. [CrossRef]
- Zhang, L.; Yu, M.; Deng, J.; Lv, X.; Liu, J.; Xiao, Y.; Yang, W.; Zhang, Y.; Li, C. Chemokine Signaling Pathway Involved in CCL2 Expression in Patients with Rheumatoid Arthritis. Yonsei Med J 2015, 56(4), 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lin, J.; Xu, A.; Lou, J.; Qian, C.; Li, X.; Wang, Y.; Yu, W.; Tao, H. CCL2: An Important Mediator Between Tumor Cells and Host Cells in Tumor Microenvironment. Front Oncol 2021, 11 (July), 1–14. [Google Scholar] [CrossRef]
- Crusio, W. E.; Lambris, J. D.; Radeke, H. H. Tumor Microenvironment The Role of Chemokines - Part A; 2020.
- De la Fuente López, M.; Landskron, G.; Parada, D.; Dubois-Camacho, K.; Simian, D.; Martinez, M.; Romero, D.; Roa, J. C.; Chahuán, I.; Gutiérrez, R.; Lopez-K, F.; Alvarez, K.; Kronberg, U.; López, S.; Sanguinetti, A.; Moreno, N.; Abedrapo, M.; González, M. J.; Quera, R.; Hermoso-R, M. A. The Relationship between Chemokines CCL2, CCL3, and CCL4 with the Tumor Microenvironment and Tumor-Associated Macrophage Markers in Colorectal Cancer. Tumor Biology 2018, 40(11), 1–12. [Google Scholar] [CrossRef]
- Dhodapkar, M. V.; Hoering, A.; Gertz, M. A.; Rivkin, S.; Szymonifka, J.; Crowley, J.; Barlogie, B. Long-Term Survival in Waldenstrom Macroglobulinemia: 10-Year Follow-up of Southwest Oncology Group Directed Intergroup Trial S9003. Blood 2009, 113(4), 793–796. [Google Scholar] [CrossRef]
- Cho, J. H.; Shim, J. H.; Yoon, S. E.; Kim, H. J.; Kim, S. H.; Ko, Y. H.; Lee, S. T.; Kim, K.; Kim, W. S.; Kim, S. J. Real-World Data on the Survival Outcome of Patients with Newly Diagnosed Waldenström Macroglobulinemia. Korean Journal of Internal Medicine 2021, 36(3), 668–678. [Google Scholar] [CrossRef]
- Dimopoulos, M. A.; Kastritis, E.; Delimpassi, S.; Zomas, A.; Kyrtsonis, M. C.; Zervas, K. The International Prognostic Scoring System for Waldenström’s Macroglobulinemia Is Applicable in Patients Treated with Rituximab-Based Regimens. Haematologica 2008, 93(9), 1420–1422. [Google Scholar] [CrossRef]
- Kastritis, E.; Kyrtsonis, M. C.; Hatjiharissi, E.; Symeonidis, A.; Michalis, E.; Repoussis, P.; Tsatalas, K.; Michael, M.; Sioni, A.; Kartasis, Z.; Stefanoudaki, E.; Voulgarelis, M.; Delimpasi, S.; Gavriatopoulou, M.; Koulieris, E.; Gika, D.; Vervesou, E.; Konstantopoulos, K.; Kokkini, G.; Zomas, A.; Roussou, P.; Anagnostopoulos, N.; Economopoulos, T.; Terpos, E.; Zervas, K.; Dimopoulos, M. A. No Significant Improvement in the Outcome of Patients with Waldenström’s Macroglobulinemia Treated over the Last 25 Years. Am J Hematol 2011, 86(6), 479–483. [Google Scholar] [CrossRef]
- Kyrtsonis, M. C.; Vassilakopoulos, T.; Angelopoulou, M.; Siakantaris, M.; Kontopidou, F.; Dimopoulou, M.; Boussiotis, V.; Gribabis, D.; Konstantopoulos, K.; Vaiopoulos, G.; Fessas, P.; Kittas, C.; Pangalis, G. Waldenström Macroglobulinemia: Clinical Course and Prognostic Factors in 60 Patients. Experience from a Single Hematology Unit. Ann Hematol 2001, 80(12), 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kvorning SL, Nielsen MC, Andersen NF, Hokland M, Andersen MN, Møller HJ. Circulating extracellular vesicle-associated CD163 and CD206 in multiple myeloma. Eur J Haematol. 2020;104(5):409-419. [CrossRef]
- Wang, H.; Hu, W. M.; Xia, Z. J.; Liang, Y.; Lu, Y.; Lin, S. X.; Tang, H. High Numbers of CD163+ Tumor-Associated Macrophages Correlate with Poor Prognosis in Multiple Myeloma Patients Receiving Bortezomib-Based Regimens. J Cancer 2019, 10(14), 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Steidl, C.; Lee, T.; Shah, S. P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S. J.; Iqbal, J.; Weisenburger, D. D.; Bast, M. A.; Rosenwald, A.; Muller-Hermelink, H.-K.; Rimsza, L. M.; Campo, E.; Delabie, J.; Braziel, R. M.; Cook, J. R.; Tubbs, R. R.; Jaffe, E. S.; Lenz, G.; Connors, J. M.; Staudt, L. M.; Chan, W. C.; Gascoyne, R. D. Tumor-Associated Macrophages and Survival in Classic Hodgkin’s Lymphoma. New England Journal of Medicine 2010, 362(10), 875–885. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Vari, F.; Keane, C.; Crooks, P.; Nourse, J. P.; Seymour, L. A.; Gottlieb, D.; Ritchie, D.; Gill, D.; Gandhi, M. K. Serum CD163 and TARC as Disease Response Biomarkers in Classical Hodgkin Lymphoma. Clinical Cancer Research 2013, 19(3), 731–742. [Google Scholar] [CrossRef]
- Nederby, L.; Roug, A. S.; Knudsen, S. S.; Skovbo, A.; Kjeldsen, E.; Moller, H. J.; Hokland, M. Soluble CD163 as a Prognostic Biomarker in B-Cell Chronic Lymphocytic Leukemia. Leuk Lymphoma 2015, 56(11), 3219–3221. [Google Scholar] [CrossRef]
- Vajavaara, H.; Ekeblad, F.; Holte, H.; Jørgensen, J.; Leivonen, S. K.; Berglund, M.; Kamper, P.; Møller, H. J.; d’Amore, F.; Molin, D.; Enblad, G.; Ludvigsen, M.; Glimelius, I.; Leppä, S. Prognostic Impact of Soluble Cd163 in Patients with Diffuse Large B-Cell Lymphoma. Haematologica 2021, 106(9), 2502–2506. [Google Scholar] [CrossRef]
- Koudouna, A.; Gkioka, A. I.; Gkiokas, A.; Tryfou, T. M.; Papadatou, M.; Alexandropoulos, A.; Bartzi, V.; Kafasi, N.; Kyrtsonis, M. C. Serum-Soluble CD163 Levels as a Prognostic Biomarker in Patients with Diffuse Large B-Cell Lymphoma Treated with Chemoimmunotherapy. Int J Mol Sci 2024, 25 (5). [CrossRef]
- Li, L.; Liu, Y. D.; Zhan, Y. T.; Zhu, Y. H.; Li, Y.; Xie, D.; Guan, X. Y. High Levels of CCL2 or CCL4 in the Tumor Microenvironment Predict Unfavorable Survival in Lung Adenocarcinoma. Thorac Cancer 2018, 9(7), 775–784. [Google Scholar] [CrossRef]
- Kyrtsonis, M. C.; Levidou, G.; Korkolopoulou, P.; Koulieris, E.; Bartzi, V.; Maltezas, D.; Pangalis, G. A.; Kalpadakis, C.; Dimou, M.; Georgiou, G.; Vassilakopoulos, T. P.; Angelopoulou, M. K.; Salpeas, V.; Tsaftaridis, P.; Patsouris, E.; Panayiotidis, P.; Tzenou, T. K. CD138 Expression Helps Distinguishing Waldenström’s Macroglobulinemia (WM) from Splenic Marginal Zone Lymphoma (SMZL). Clin Lymphoma Myeloma Leuk 2011, 11(1), 99–102. [Google Scholar] [CrossRef]
- Navetta-Modrov, B.; Yao, Q. Macroglobulinemia and Autoinflammatory Disease. Rheumatology and Immunology Research 2021, 2(4), 227–232. [Google Scholar] [CrossRef]
- Han, W.; Matissek, S. J.; Jackson, D. A.; Sklavanitis, B.; Elsawa, S. F. Targeting IL-6 Receptor Reduces IgM Levels and Tumor Growth in Waldenström Macroglobulinemia. Oncotarget 2019, 10(36), 3400–3407. [Google Scholar] [CrossRef]
- Elsawa, S. F.; Novak, A. J.; Ziesmer, S. C.; Almada, L. L.; Hodge, L. S.; Grote, D. M.; Witzig, T. E.; Fernandez-Zapico, M. E.; Ansell, S. M. Comprehensive Analysis of Tumor Microenvironment Cytokines in Waldenstrom Macroglobulinemia Identifies CCL5 as a Novel Modulator of IL-6 Activity. Blood 2011, 118(20), 5540–5549. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).