Submitted:
06 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Aims
3. Materials and Methods
3.1. Study Design
3.2. Patient Selection and Randomization
3.3. Breast and Tumor Characterization
3.4. Tumor Marking Procedures
3.5. Lymph Node Marking
3.6. Procedural Assessment
3.7. Post-Marking Procedures
3.8. Histopathological and Imaging Analysis
3.9. Statistical Analysis
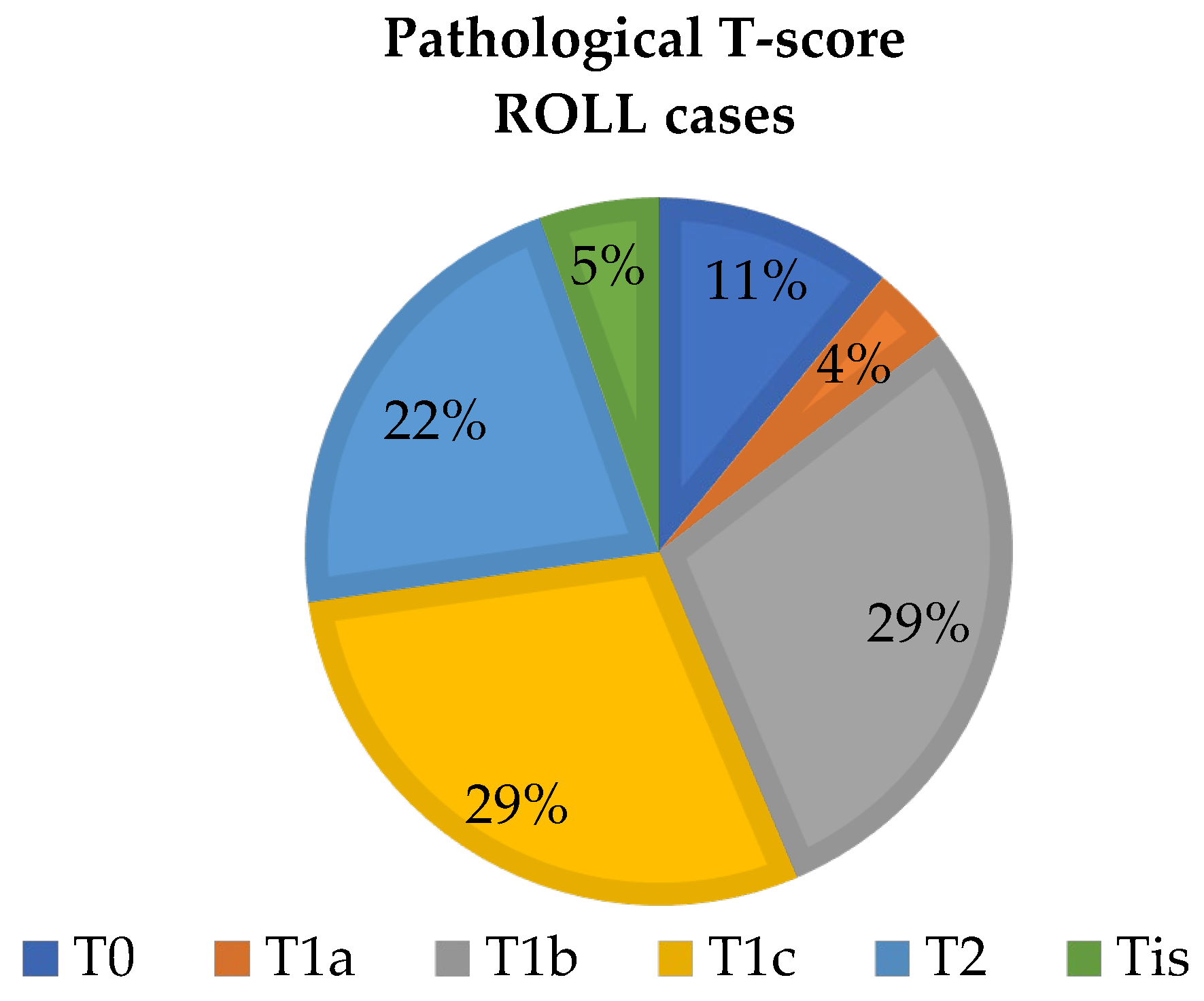
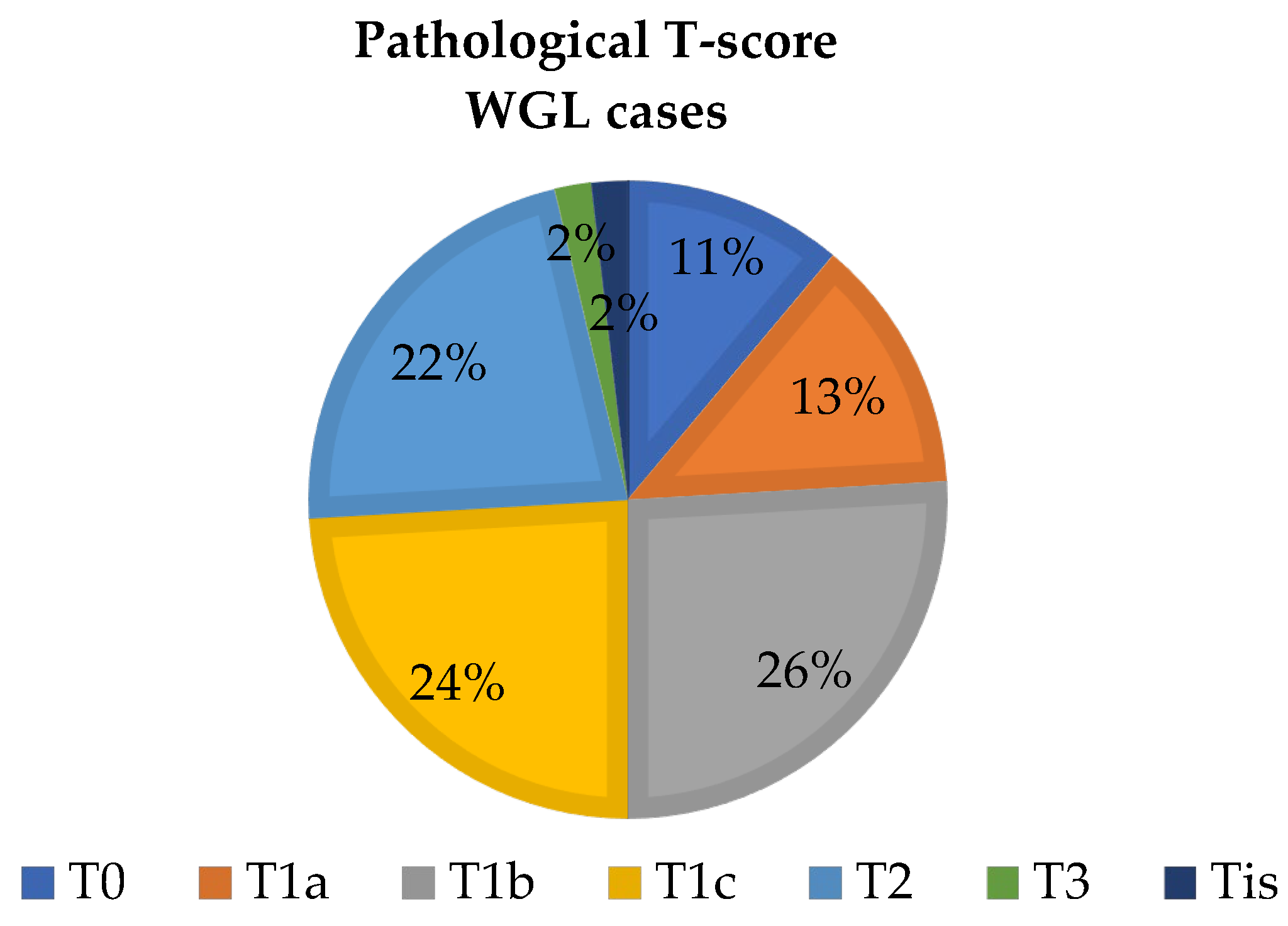
4. Results
4.1. Sentinel Lymph Node Marking Results
5. Discussion
5.1. Suggested Protocol for Non-Palpable Breast Tumor Localization
5.1.1. Patient Assessment
- Evaluate breast size, density, and tumor location (with special attention to inner quadrant tumors).
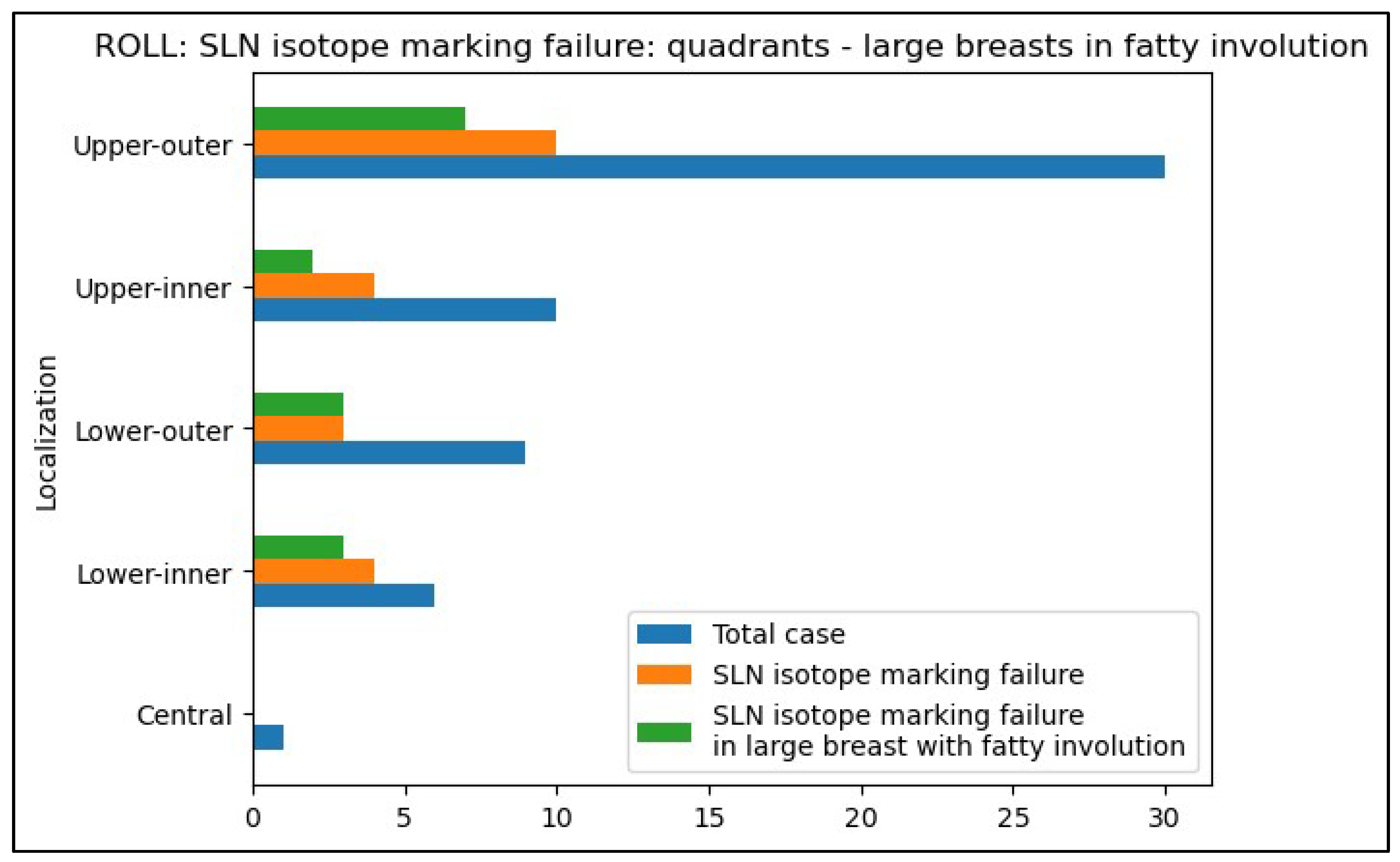
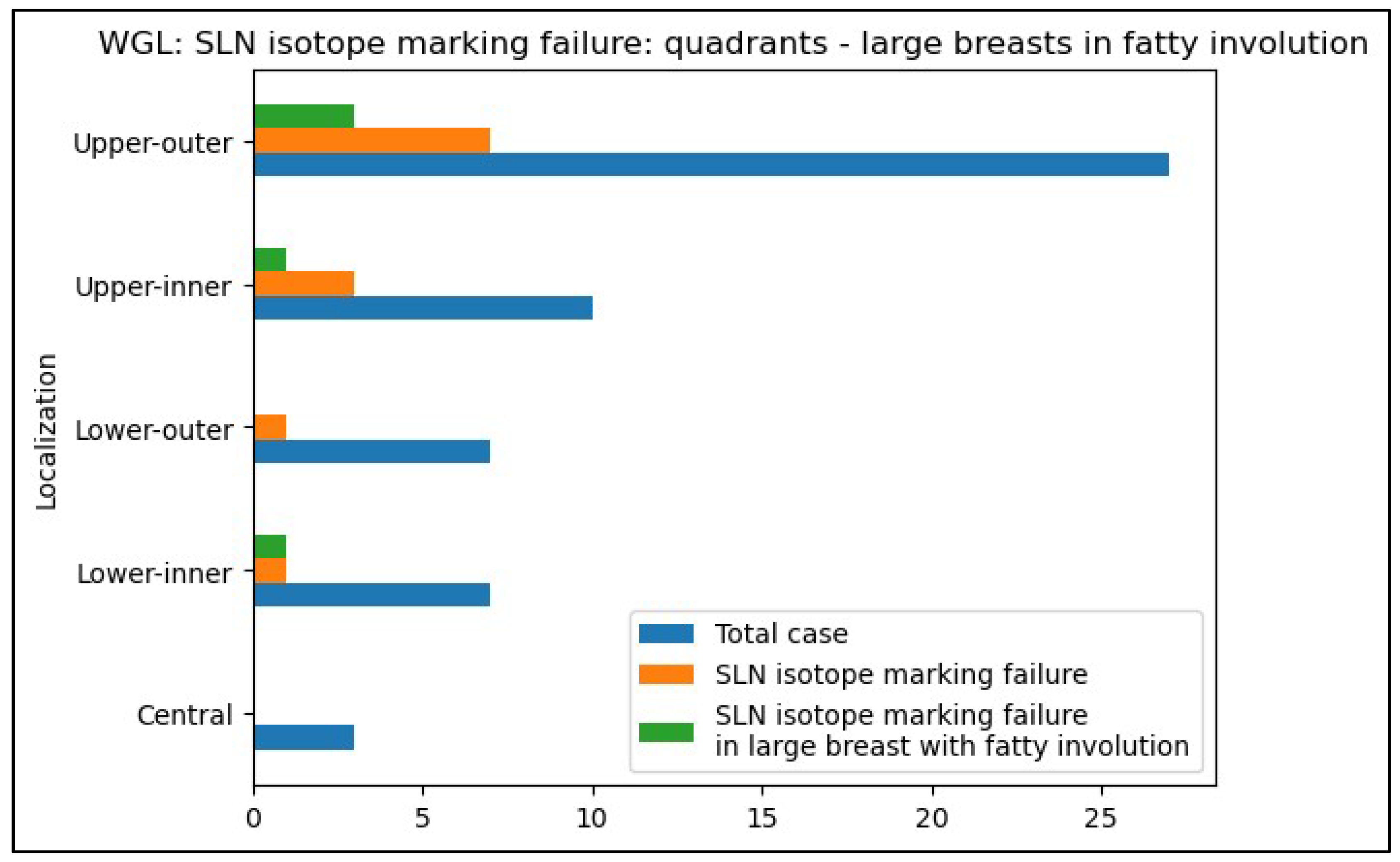
- Prioritize WGL or other non-palpable breast tumor localization method that uses peri-areolar radiocolloid injection for tumors located in the inner quadrants, especially in large, adipose breasts, where ROLL is less likely to achieve adequate SLN marking.
5.1.2. Preferred Technique
- ROLL: Use as the primary localization method for non-palpable tumors, given its advantages in patient comfort, procedural simplicity, and cosmetic outcomes.
- WGL: Prefer for patients with tumors in the inner quadrants, particularly those who have undergone neoadjuvant chemotherapy, as WGL demonstrates superior SLN identification in these cases.
5.1.3. Intraoperative Contingency Plan
- If SLN marking fails using ROLL, intraoperative blue dye injection into the subareolar plexus should be employed.
- Surgeons should be prepared to perform axillary lymph node dissection if SLN identification remains unsuccessful.
5.1.4. Post-Chemotherapy Considerations
- For patients with tumor regression after neoadjuvant chemotherapy, consider avoiding ROLL when SLN identification is critical, as its success rate is lower without peri-areolar isotope injection in such cases compared to WGL. Especially in adipose involution of large breasts.
6. Conclusions
Informed Consent Statement:
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A. Statistical Results
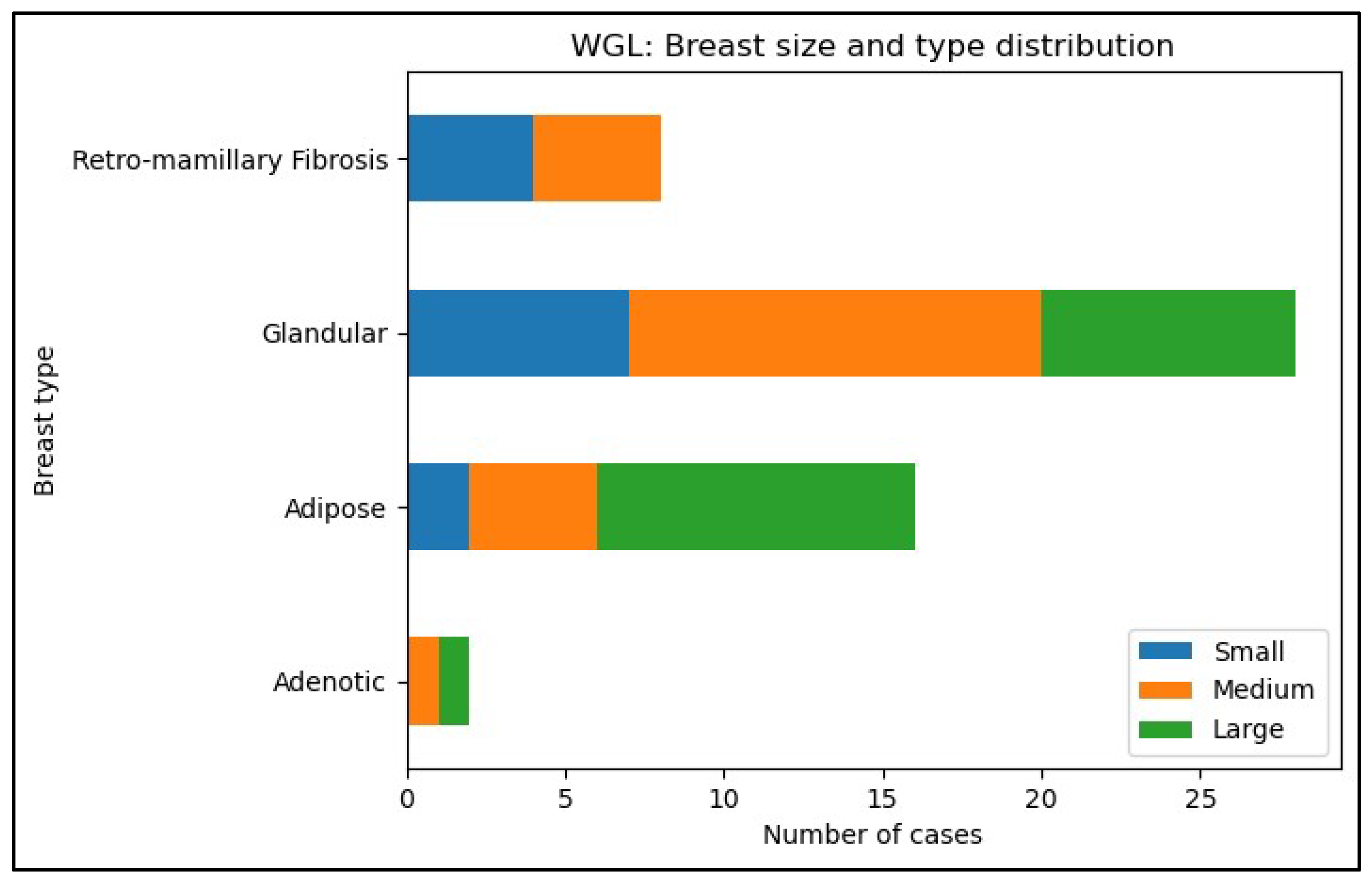
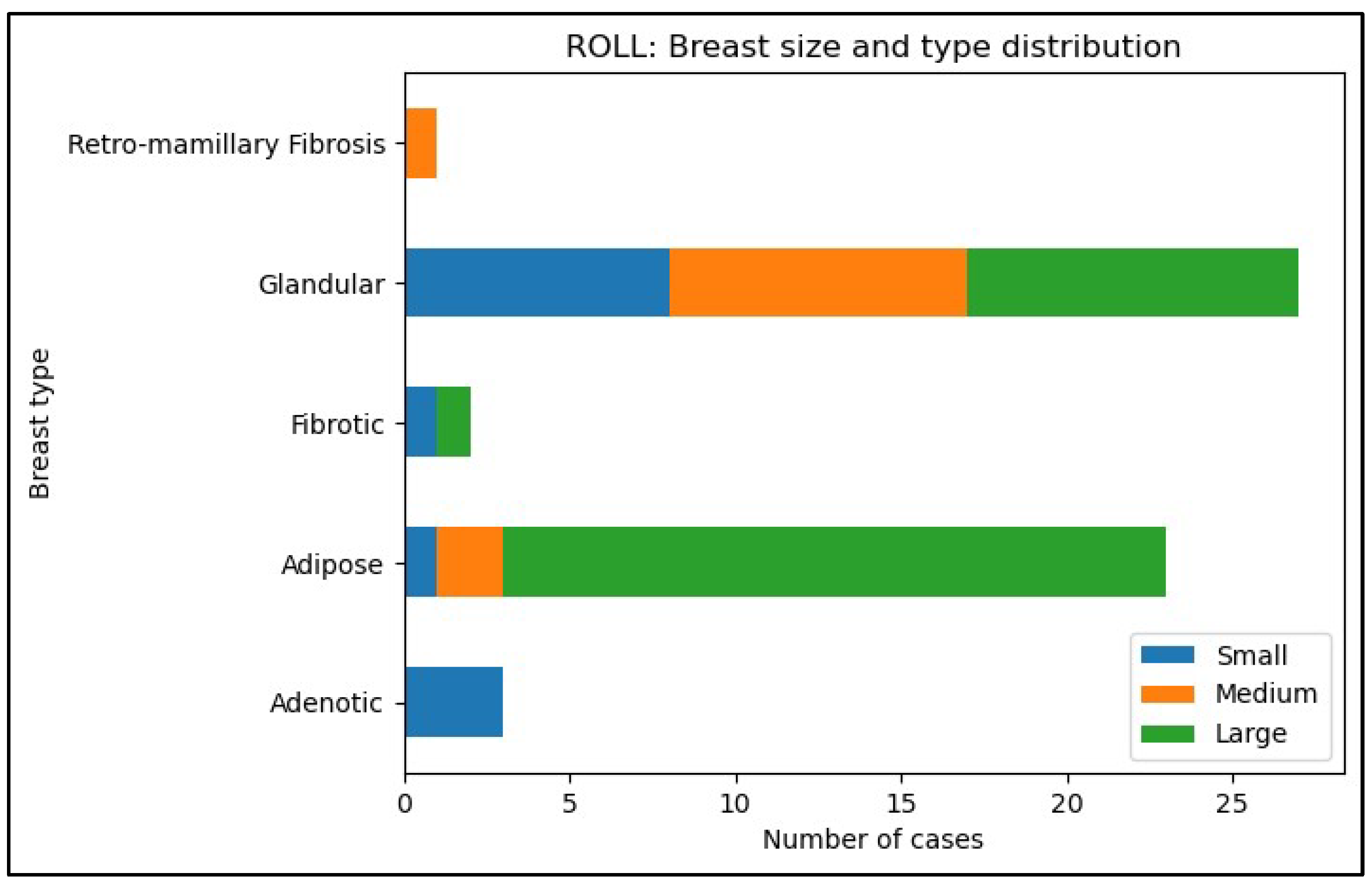
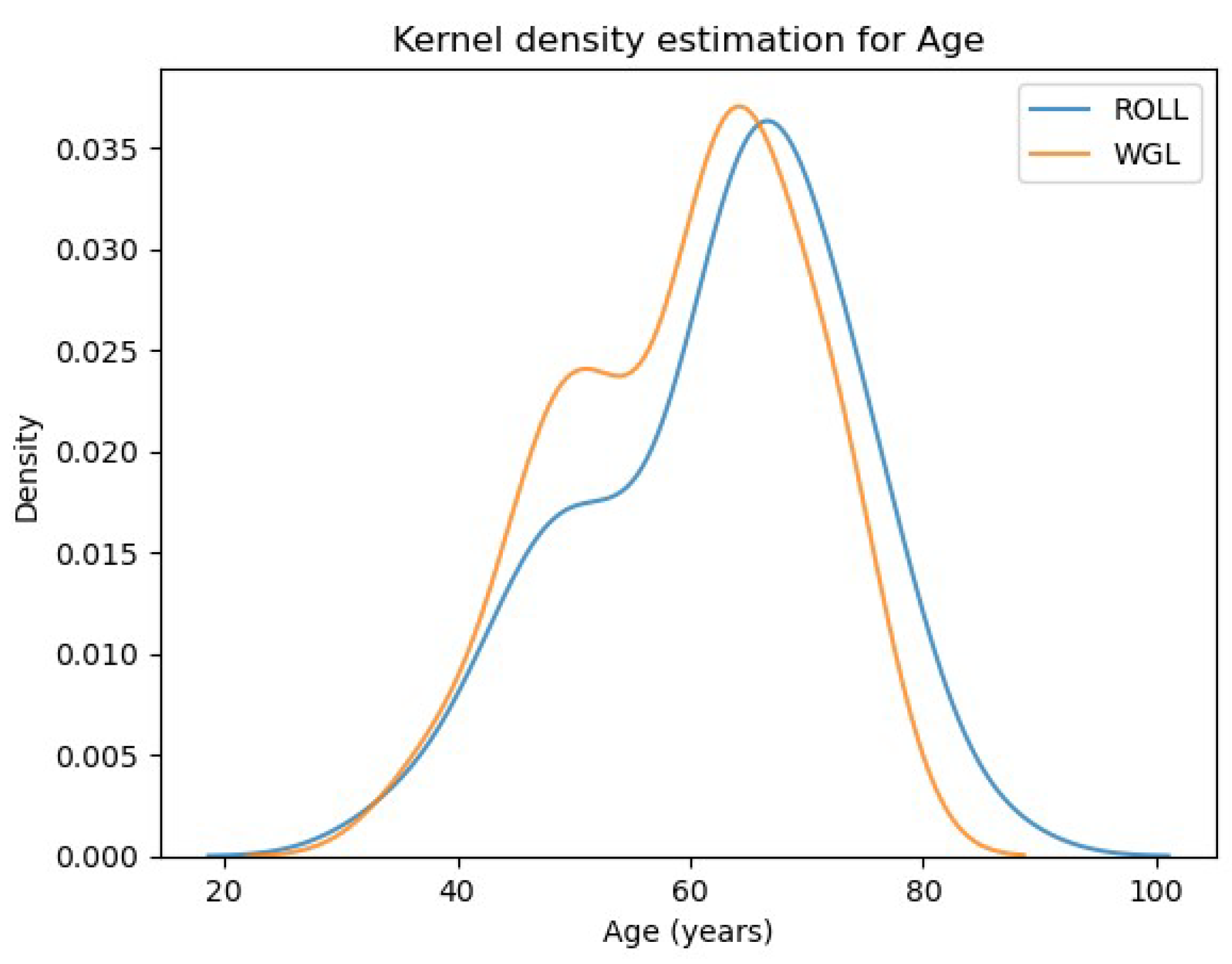
- 1.
- Ages of patients in the two examined groups
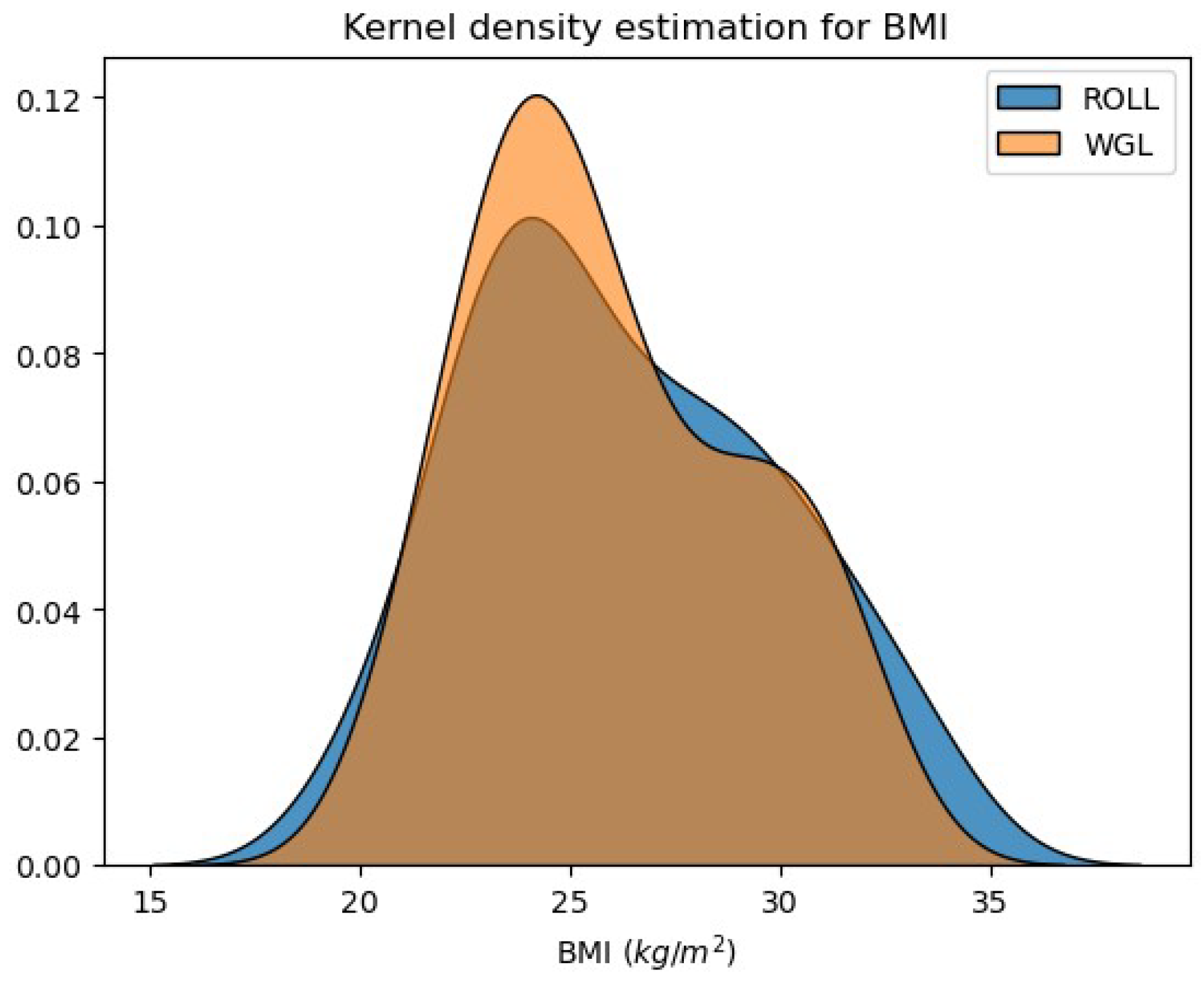
- 2.
- BMI of patients in the two examined groups
- 3.
- Pre-operative difficulty of non-palpable breast lesion marking with each procedure - Radiologists’ perspective
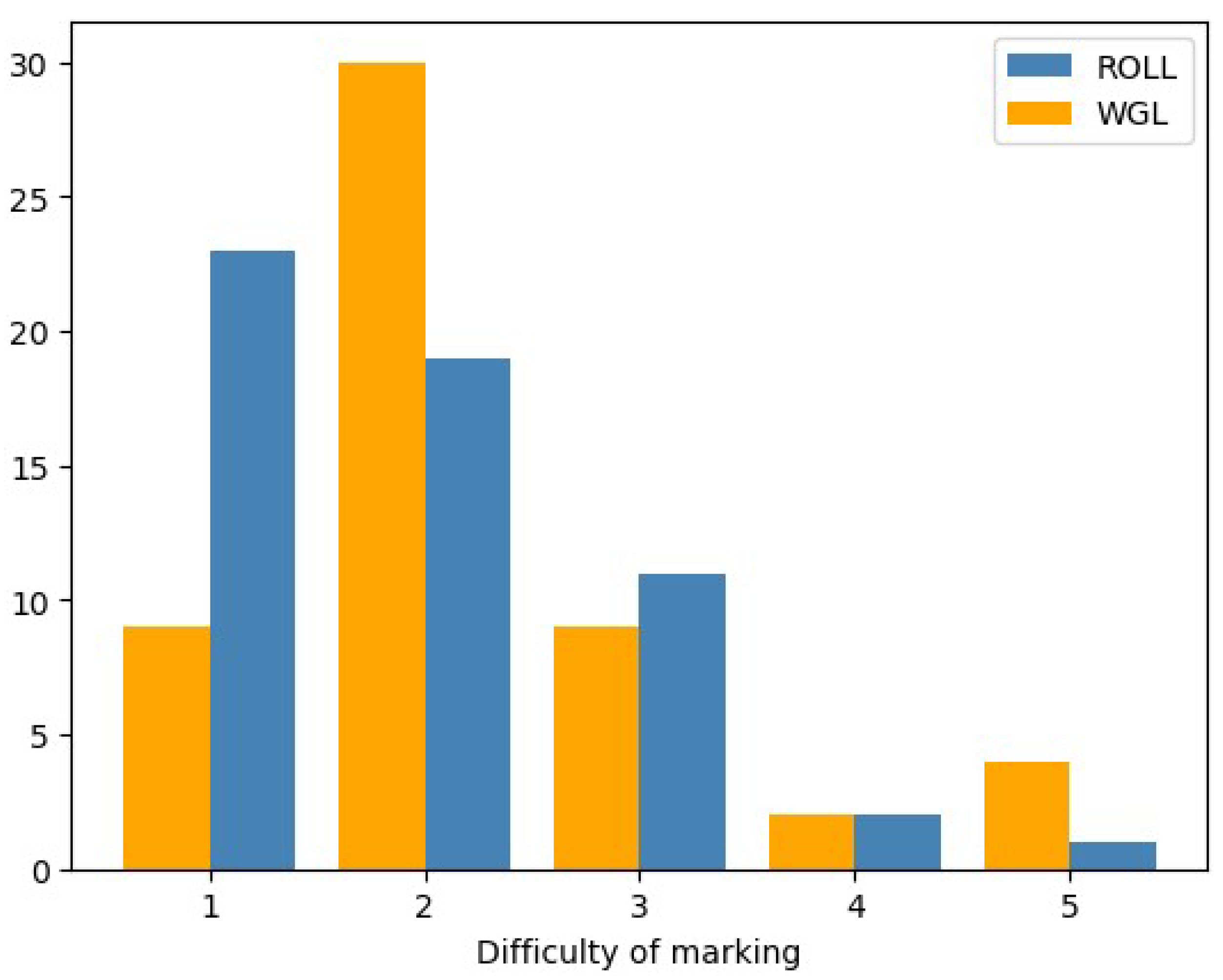
- 4.
- Time duration of marking procedures
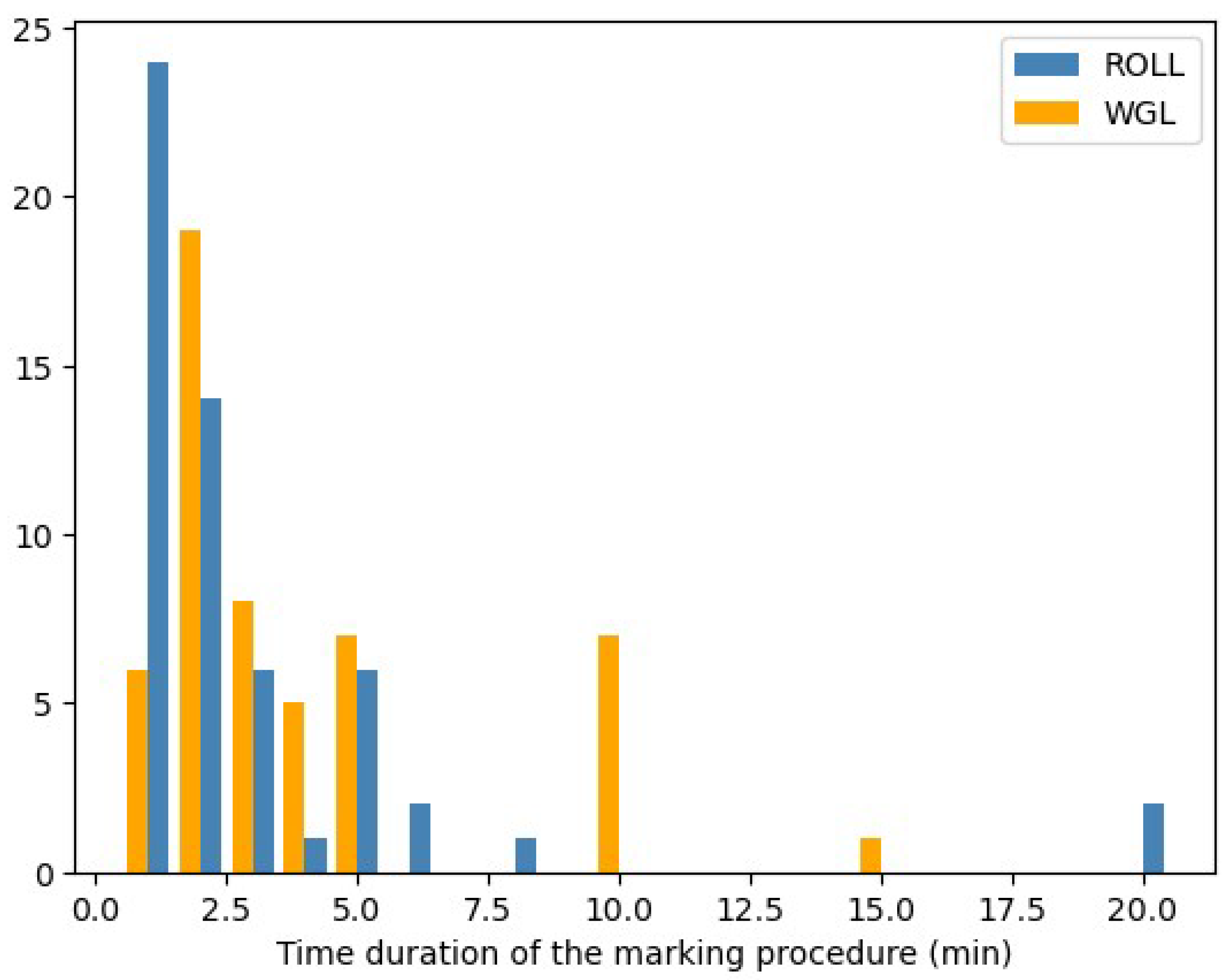
- 5.
- The pain of tumor marking with each technique – Patients’ perspective
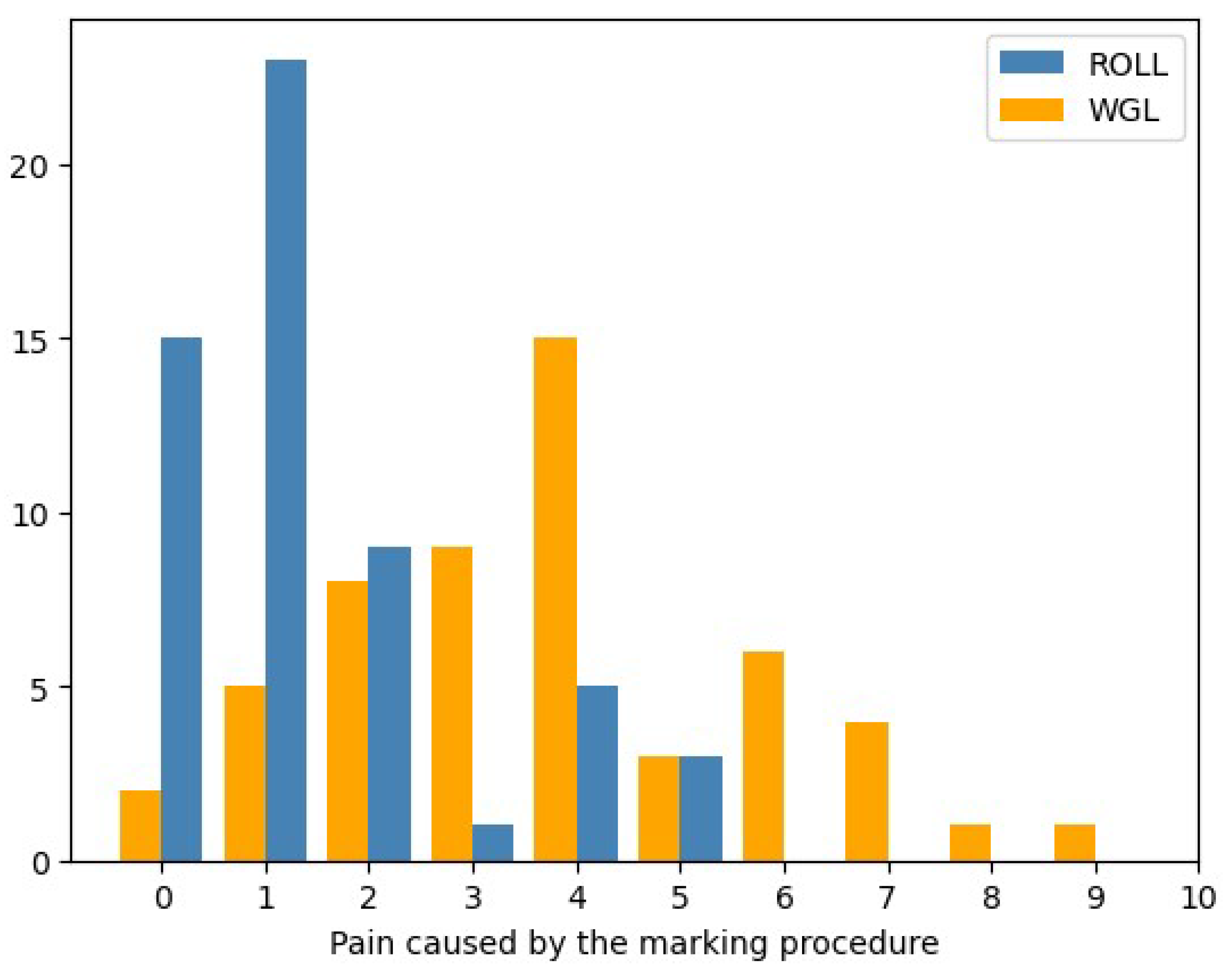
- 6.
- Operative difficulty of lesion localization – Surgeons’ perspective
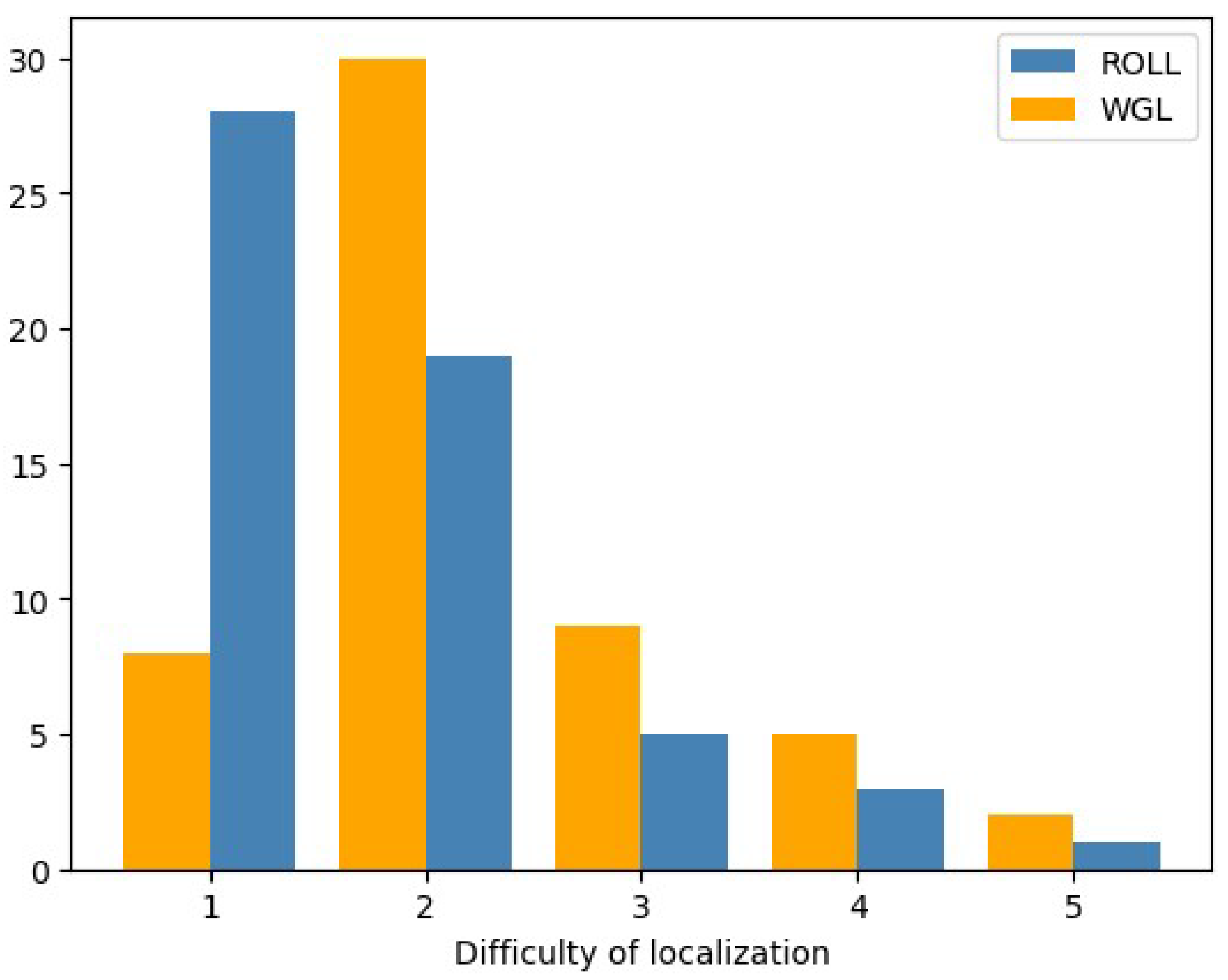
- 7.
- Time (duration) of operation (min):
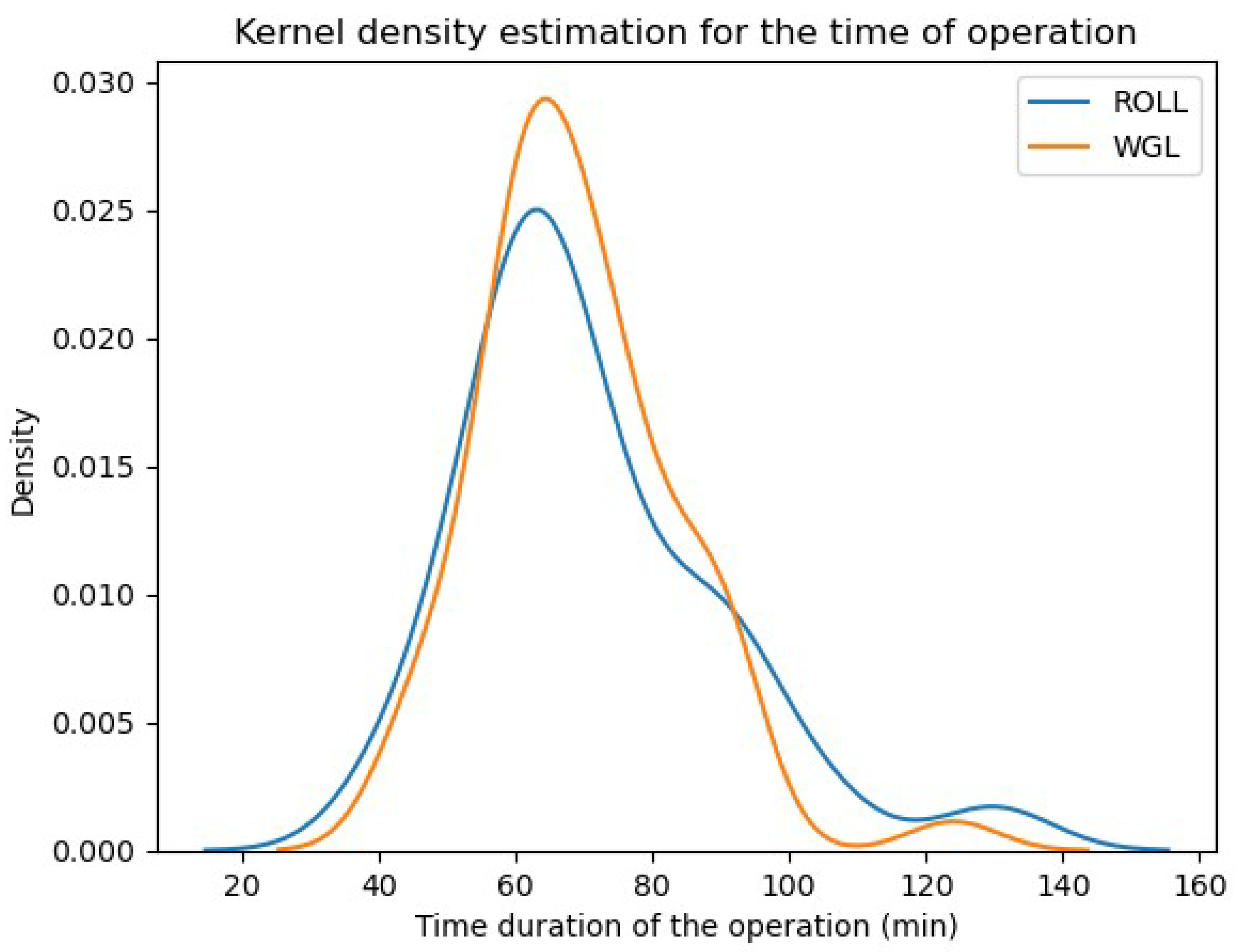
- 8.
- Weight of the specimen:
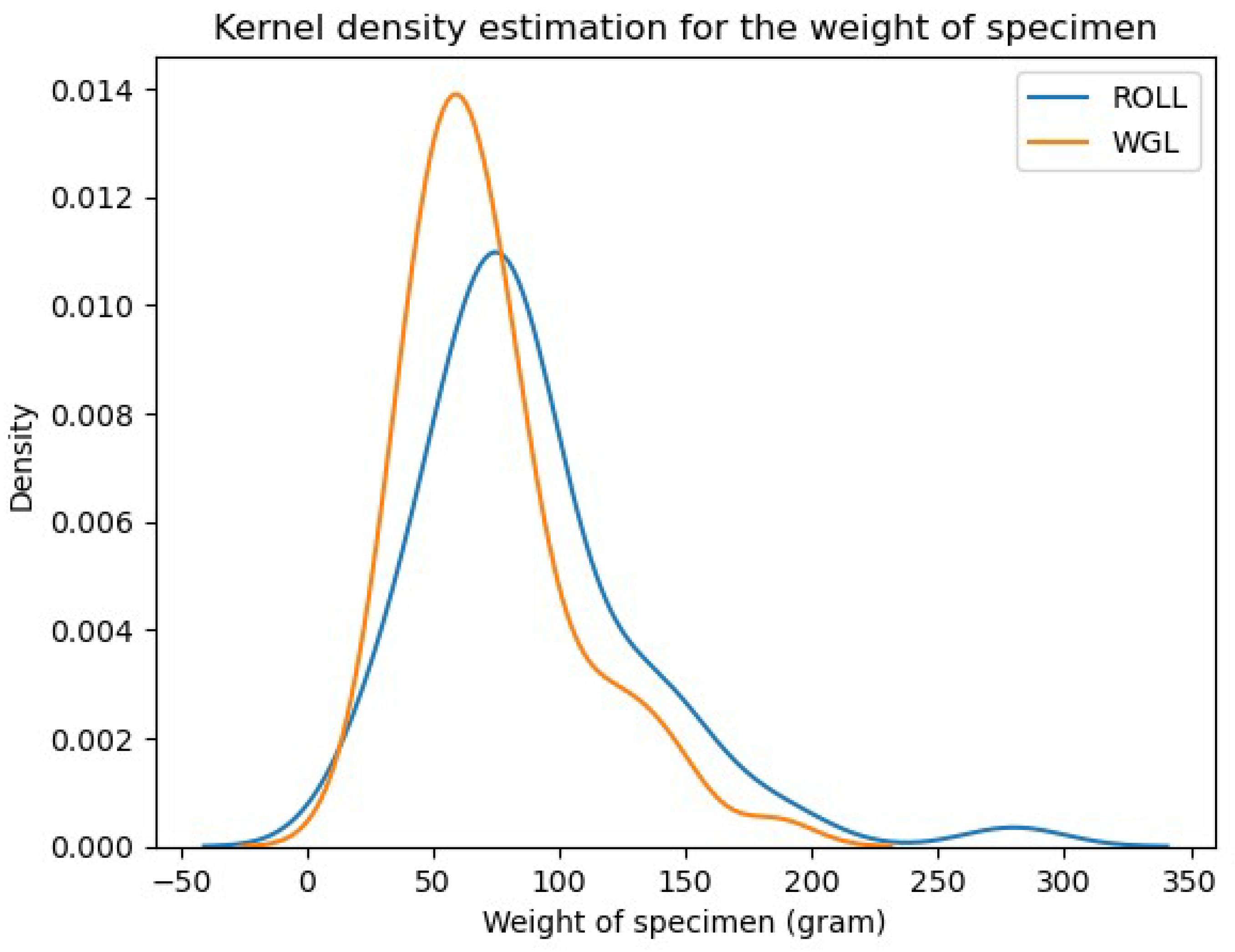
Appendix B
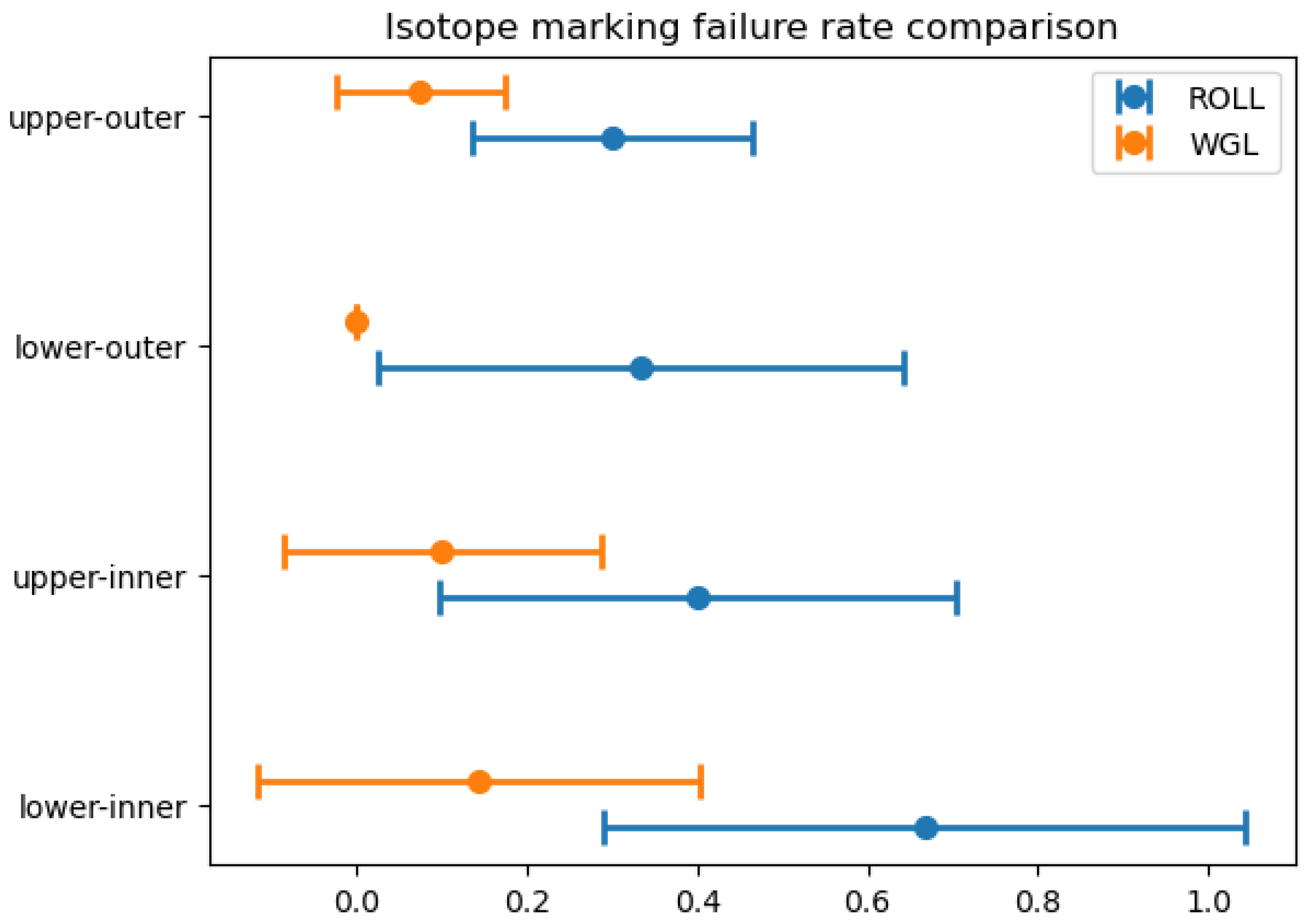
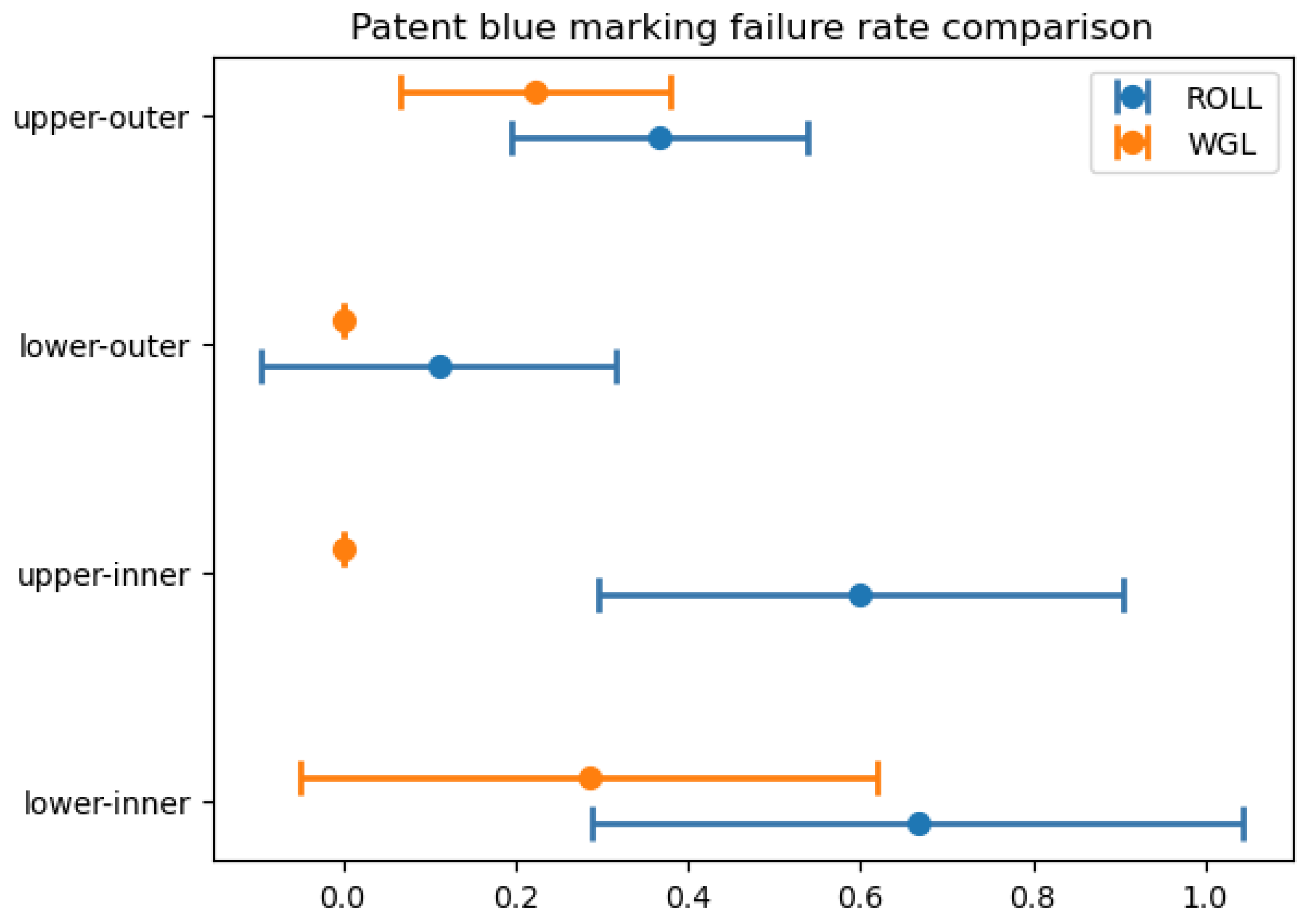
| Localization | Isotope marking failure | Patent blue marking failure |
| Lower-inner q. | WGL better (0.0265) | no difference (0.0847) |
| Upper-inner q. | no difference (0.0607) | WGL better (0.0017) |
| Central | - | - |
| Lower-outer q. | WGL better (0.0451) | no difference (0.1812) |
| Upper-outer q. | WGL better (0.0155) | no difference (0.1169) |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Plevritis, S.K.; Munoz, D.; Kurian, A.W.; Stout, N.K.; Alagoz, O.; Near, A.M.; Lee, S.J.; Broek, J.J.v.D.; Huang, X.; Schechter, C.B.; et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000-2012. JAMA 2018, 319, 154–164. [Google Scholar] [CrossRef]
- Massat, N.J.; Dibden, A.; Parmar, D.; Cuzick, J.; Sasieni, P.D.; Duffy, S.W. Impact of Screening on Breast Cancer Mortality: The UK Program 20 Years On. Cancer Epidemiology Biomarkers Prev. 2016, 25, 455–462. [Google Scholar] [CrossRef]
- Beriwal, S.; Schwartz, G.F.; Komarnicky, L.; Garcia-Young, J.A. Breast-Conserving Therapy after Neoadjuvant Chemotherapy: Long-term Results. Breast J. 2006, 12, 159–164. [Google Scholar] [CrossRef]
- Kopans, D.B.; DeLuca, S. A modified needle-hookwire technique to simplify preoperative localization of occult breast lesions. Radiology 1980, 134, 781. [Google Scholar] [CrossRef]
- Elzohery, Y.H.; Gomaa, M.M.; Mohamed, G.; Fadlalla, W.M.; Taha, S.N.; Ibraheem, M.H. Comparison of wire-guided localization (WGL) and radio-guided occult lesion localization (ROLL) in localization of non-palpable breast lesions. World J. Surg. Oncol. 2023, 21, 1–10. [Google Scholar] [CrossRef]
- the ROLL study group. Postma, E.L.; Verkooijen, H.M.; van Esser, S.; Hobbelink, M.G.; van der Schelling, G.P.; Koelemij, R.; Witkamp, A.J.; Contant, C.; van Diest, P.J.; et al. Efficacy of ‘radioguided occult lesion localisation’ (ROLL) versus ‘wire-guided localisation’ (WGL) in breast conserving surgery for non-palpable breast cancer: a randomised controlled multicentre trial. Breast Cancer Res. Treat. 2012, 136, 469–478. [Google Scholar] [CrossRef]
- A, L.; S, Z.; V, G.; G, P. Correspondence. Eur. J. Cancer 1998, 34, 204–205. [Google Scholar] [CrossRef]
- Feggi, L.; Basaglia, E.; Corcione, S.; Querzoli, P.; Soliani, G.; Ascanelli, S.; Prandini, N.; Bergossi, L.; Carcoforo, P. An original approach in the diagnosis of early breast cancer: use of the same radiopharmaceutical for both non-palpable lesions and sentinel node localisation. Eur. J. Nucl. Med. 2001, 28, 1589–1596. [Google Scholar] [CrossRef]
- Takács, T.; Paszt, A.; Simonka, Z.; Ábrahám, S.; Borda, B.; Ottlakán, A.; Ormándi, K.; Lázár, M.; Vörös, A.; Kahán, Z.; et al. Radioguided Occult Lesion Localisation Versus Wire-Guided Lumpectomy in the Treatment of Non-Palpable Breast Lesions. Pathol. Oncol. Res. 2013, 19, 267–273. [Google Scholar] [CrossRef]
- Hawkins, S.; Brown, I.; King, P.; El-Gammal, M.; Stepp, K.; Widdison, S.; Barta, M.; Jackson, N.; English, R.; Ahmad, S.; et al. Time to go wireless? A 15-year single institution experience of radioisotope occult lesion localisation (ROLL) for impalpable breast lesions. Eur. J. Surg. Oncol. (EJSO) 2016, 43, 62–67. [Google Scholar] [CrossRef]
- Lovrics, P.J.; Cornacchi, S.D.; Vora, R.; Goldsmith, C.H.; Kahnamoui, K. Systematic review of radioguided surgery for non-palpable breast cancer. Eur. J. Surg. Oncol. (EJSO) 2011, 37, 388–397. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Kühn, T.; Masannat, Y.; Rubio, I.; de Boniface, J.; Ditsch, N.; Cakmak, G.K.; Karakatsanis, A.; Dave, R.; Hahn, M.; et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers 2023, 15, 1173. [Google Scholar] [CrossRef]
- Kane, R.L.; Bershadsky, B.; Rockwood, T.; Saleh, K.; Islam, N.C. Visual Analog Scale pain reporting was standardized. J. Clin. Epidemiology 2005, 58, 618–623. [Google Scholar] [CrossRef]
- Drozgyik, A.; Szabó, T.; Kovács, G.; Kollár, D.; Molnár, T.F. A New Approach to Breast Specimen Orientation: Avoiding Pitfalls with the Specimen Plate Concept. Curr. Oncol. 2024, 31, 4589–4598. [Google Scholar] [CrossRef]
- Schrenk, P.; Hochreiner, G.; Fridrik, M.; Wayand, W. Sentinel Node Biopsy Performed Before Preoperative Chemotherapy for Axillary Lymph Node Staging in Breast Cancer. Breast J. 2003, 9, 282–287. [Google Scholar] [CrossRef]
- McGhan, L.J.; McKeever, S.C.; Pockaj, B.A.; Wasif, N.; Giurescu, M.E.; Walton, H.A.; Gray, R.J. Radioactive Seed Localization for Nonpalpable Breast Lesions: Review of 1,000 Consecutive Procedures at a Single Institution. Ann. Surg. Oncol. 2011, 18, 3096–3101. [Google Scholar] [CrossRef]
- Ahmed, M.; Douek, M. ROLL versus RSL: toss of a coin? Breast Cancer Res. Treat. 2013, 140, 213–217. [Google Scholar] [CrossRef]
- Lázár G, Kelemen P, Kósa C, et al. IV. Emlőrák Konszenzus Konferencia – Az Emlőrák Korszerű Sebészi Kezelése [Modern Surgical Treatment of Breast Cancer. 4th Breast Cancer Consensus Conference]. Magy Onkol. 2020;64(4):329-346.
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Hellingman, D.; Wan, O.Y.; Veen, B.J.d.W.-V.d.; van der Ploeg, I.M.; Elkhuizen, P.H.; Rutgers, E.J.; Stokkel, M.P.; Hellingman, D.; Wan, O.Y.; Veen, B.J.d.W.-V.d.; et al. Predictive risk factors for sentinel lymph node nonvisualization on planar lymphoscintigraphy using an intratumoral injection in patients with primary breast cancer. Nucl. Med. Commun. 2019, 40, 317–324. [Google Scholar] [CrossRef]
- Borgstein, P.J.; Meijer, S.; Pijpers, R.J.; van Diest, P.J. Functional Lymphatic Anatomy for Sentinel Node Biopsy in Breast Cancer. Ann. Surg. 2000, 232, 81–89. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Rubio, I.T.; Henry, R.; Cowan, C.; Colvert, M.; Korourian, S. Subareolar Versus Peritumoral Injection for Location of the Sentinel Lymph Node. Ann. Surg. 1999, 229, 860–860. [Google Scholar] [CrossRef]
- Smith, L.F.; Cross, M.J.; Klimberg, V. Subareolar injection is a better technique for sentinel lymph node biopsy. Am. J. Surg. 2000, 180, 434–438. [Google Scholar] [CrossRef]
| ROLL/WGL group | Cancer screening | Neoadjuvant therapy |
| ROLL group | 43 (77%) | 13 (23%) |
| WGL group | 44 (79%) | 10 (18%) |
| Type- and size of breast in the ROLL group (n=56) | Glandular | Adipose | Retro-mamillary Fibrosis | Adenotic | Fibrotic | Total |
| Small | 8(14%) | 1(2%) | 0 | 3(5%) | 1(2%) | 13(23%) |
| Medium | 9(16%) | 2(4%) | 1(2%) | 0 | 0 | 12(21%) |
| Large | 10(18%) | 20(36%) | 0 | 0 | 1(2%) | 31(55%) |
| Type- and size of breast in the WGL group (n=56) | Glandular | Adipose | Retro-mamillary Fibrosis | Adenotic | Fibrotic | Total |
| Small | 7(13%) | 2(4%) | 4(7%) | 0 | 0 | 13(24%) |
| Medium | 13(24%) | 4(7%) | 4(7%) | 1(2%) | 0 | 22(40%) |
| Large | 8(15%) | 10(19%) | 0 | 1(2%) | 0 | 19(35%) |
| Localization* | ROLL(n=56) | WGL (n=54) | ||||
| Laterality | Number of cases | Laterality | Number of cases | |||
| Left | Right | Left | Right | |||
| Lower-inner q. | 3 (5%) | 3 (5%) | 6 (11%) | 4 (7%) | 3 (5%) | 7 (13%) |
| Upper-inner q. | 8 (14%) | 2 (4%) | 10 (18%) | 8 (14%) | 2 (4%) | 10 (18%) |
| Central | 1 (2%) | 0 | 1 (2%) | 1 (2%) | 2 (4%) | 3 (5%) |
| Lower-outer q. | 1 (2%) | 8 (14%) | 9 (16%) | 2 (4%) | 5 (9%) | 7 (13%) |
| Upper-outer q. | 14 (25%) | 16 (29%) | 30 (54%) | 11 (20%) | 16 (29%) | 27 (48%) |
| ROLL (n=56) | WGL (n=54) | |||
| Localization | Isotope marking failure | Patent blue marking failure | Isotope marking failure | Patent blue marking failure |
| Lower-inner q. | 4 (67%) | 4 (67%) | 1 (14%) | 2 (29%) |
| Upper-inner q. | 4 (40%) | 6 (60%) | 1 (10%) | 0 |
| Central | 0 | 0 | 0 | 1 (33%) |
| Lower-outer q. | 3 (33%) | 1 (11%) | 0 | 0 |
| Upper-outer q. | 9 (30%) | 11 (37%) | 2 (7%) | 6 (22%) |
| Localization | Isotope marking failure | Patent blue marking failure |
| Lower-inner quadrant | 0.0265* | 0.0847 |
| Upper-inner quadrant | 0.0607 | 0.0017** |
| Central | - | - |
| Lower-outer quadrant | 0.0451* | 0.1812 |
| Upper-outer quadrant | 0.0155* | 0.1169 |
| Type- and size of breast in the ROLL group (n=56) | Glandular | Adipose | Retro-mamillary Fibrosis | Adenotic | Fibrotic | Total |
| Small | 8/8(100%) | 1/1(1) | 0/0(0) | 3/3(1) | 1/1(1) | 13/13(1) |
| Medium | 9/8(89%) | 2/1(50%) | 1/1(100%) | 0/0 | 0/0(0) | 12/10(83%) |
| Large | 10/6(60%) | 20/5(25%) | 0/0 | 0/0 | 1/1(100%) | 31/12(39%) |
| Type- and size of breast in the WGL group (n=54) | Glandular | Adipose | Retro-mamillary Fibrosis | Adenotic | Fibrotic | Total |
| Small | 7/7(100%) | 2/2(100%) | 4/3(75%) | 0/0 | 0/0 | 13/12(92%) |
| Medium | 13/9(69%) | 4/4(100%) | 4/4(100%) | 1/1(100%) | 0/0 | 22/18(82%) |
| Large | 8/6(75%) | 10/5(50%) | 0/0 | 1/1(100%) | 0/0 | 19/12(63%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





