1. Introduction
In 2020, female breast cancer (BC) was the most commonly diagnosed cancer worldwide at 11.7% [
1] and should be considered a global health concern, especially due to the rising incidence rates anticipated in regions of the world that are currently undergoing economic transformation. [
1,
2] With BC therapy continuously being optimized and, hence, survival improving over the last few decades [
3], reducing comorbidities and enhancing quality of life have become increasingly relevant aspects of therapy. [
4] Both the cancer diagnosis itself and the treatment, with its potential side effects, are often intensely distressing and pose a risk to mental well-being. [
5,
6,
7,
8] However, prevalence rates of mental disorders in cancer patients vary widely in the literature [
9,
10,
11,
12]: For diseases within the depressive spectrum alone, figures between 5% and 60% were measured in past analyses. [
13] In principle, it must therefore be assumed that mental illness constitutes a relevant comorbidity in cancer patients in general, and especially in BC patients. Such psychological distress is also associated with negative effects on health outcome and may impact the course of the cancer disease and compliance. [
14] Results of a meta-analysis demonstrated that depression functions as a negative predictive factor for adherence to endocrine treatment. [
15] Another review from 2020 suggests that depression and anxiety disorders in BC patients may even be associated with higher mortality. [
16] Thus, the influence of the psyche on the treatment and prognosis of BC has apparently not been fully clarified yet. While studies on depression and anxiety disorders in cancer patients are increasing, there is still a lack of valid data regarding the occurrence of other mental illnesses in cancer patients.
The primary objective of this study was to analyze the incidence of different mental disorders in BC patients compared to the general population and, as a secondary aim, to identify potential risk factors for mental disorders in BC patients.
2. Materials and Methods
The anonymized dataset provided by the AOK Baden-Wuerttemberg consisted of 97,121 patients (95,499 women and 1622 men) who received a BC diagnosis (ICD10 code “C50”) and 94,849 age-matched control patients (also 93,253 women and 1596 men) without a BC diagnosis between January 2010 and December 2020 (observation period). [
17] For analysis in our BC group (BCG), we initially had to exclude 69,252 patients, either due to unreliable C50 diagnosis (no inpatient treatment for invasive BC between 1 July 2010 and 31 December 2019, the analysis period), C50 diagnosis outside the time window of the analysis period, encoding of secondary neoplasia (before or after the first diagnosis of invasive BC, except nonmelanoma skin cancer, ICD code C44), occurrence of distant metastases at least 6 months prior to the first encoding of C50, a too-short period of insurance coverage (overall insurance duration less than 40% of the observation period), death before diagnosis, or male gender. From our control group (CG) we also had to exclude 39,071 patients owing to missing data concerning the insurance period or a too-short period of insurance coverage, development of a neoplastic disease (except for nonmelanoma skin cancer, ICD code 44), male gender, and due to matching. As previously described, matching was performed to pair each BC patient with two unique patients in the control group (1:2 ratio). Matching was implemented using the R package optmatch. Age at first diagnosis was selected as the main matching criterion. As the second matching criterion, a “no exclusion before diagnosis” constraint was applied. For a more detailed description of our matching process we refer to our prior publication. [
17] As also described in our previous publication, we differentiated three distinct breast cancer stages: 1. Stage A: early BC without pathological axillary lymph node involvement (encoding of C50 without encoding of C77.3); 2. Stage B: early BC with pathological axillary lymph node involvement (encoding of C77.3 within 6 months of BC diagnosis date); and 3. Stage C: primary distant metastatic BC [encoding for distant metastatic disease by C77–C79, except C77.3 (axillary lymph node involvement) and C77.9 (lymph node involvement, not otherwise specified) within 6 months of BC diagnosis date]. [
17] The BC histologic subtypes were reconstructed from the medication the patient received according to the Anatomic Therapeutic Chemical codes (ATC) recorded. [
18] If our BC patients received the corresponding medication at least once in the observation period after the first diagnosis of C50, they were defined as either hormone receptor (HR)- or HER2-positive, otherwise they were assumed to be HR- or Her2-negative.
For this analysis regarding mental disorders in BC patients, we excluded an additional 16,316 BC patients in whom a mental disease was diagnosed 12 months (4 quarters) before their BC diagnosis and 23,794 control patients receiving a mental illness diagnosis 12 months prior to BC diagnosis of the matched BC patient. For our analysis the following ICD codes were used to categorize mental diseases: F32-34 and F38 were summarized as affective disorders, F40 and F41 for anxiety disorders, F42 for obsessive compulsive disorders, F45 for somatic symptom disorders and hypochondriac disorders, F44 for dissociative disorders, F30 and F31 for mania and bipolar disorders, F43 for adjustment disorders, and F48 for other neurotic disorders. In some of our analyses F40-42, F44-45, and F48 were regarded together as neurotic disorders. For patient characteristics, the following ICD codes/EBM codes were used: diabetes mellitus ICD E10-E13, obesity ICD E66, and hereditary BC/familial BC predisposition ICD Z40+Z80, EBM 11440/11518/11601/11230/11233.
Statistics
Data processing and statistical analysis were performed using R version 4.3.3 and RStudio (version 2024.04.0+735, Posit PBC, Boston, Massachusetts, USA) with the tidyverse packages dplyr 1.1.4, readr 2.1.5, forcats 1.0.0, stringr 1.5.1, ggplot2 3.5.1, tibble 3.2.1, lubridate 1.9.3, tidyr 1.3.1, and purrr 1.0.2. For generating tables, we used the packages janitor 2.2.0, gt 0.11.0, and gtsummary 2.0.0. Kaplan-Meier curves were generated using the packages survival 3.7-0 and ggsurvfit 1.1.0 to estimate time to disease together with the associated 95% confidence intervals (CI). We used chi-squared tests to compare the BC group and the control group. Given the large group sizes, p-values were often extremely small; thus, we considered effect sizes to gauge significance. In detail, we used Cohen’s D and W measures to quantify effect size calculated with the package effect size 0.8.9. The incidence of mental diseases was registered using the ICD codes. To analyze factors affecting the occurrence of a mental disorder after BC diagnosis we applied a logistic regression model. We considered factors significant when they had a p-value <0.001.
3. Results
3.1. Patient Characteristics
After applying the aforementioned inclusion and exclusion criteria, a total of 43,497 patients (11,553 BC patients and 31,944 control patients) were included in the analysis. Mean age of our BC patients was 66.1 years versus 65.0 years in the control group (CG).
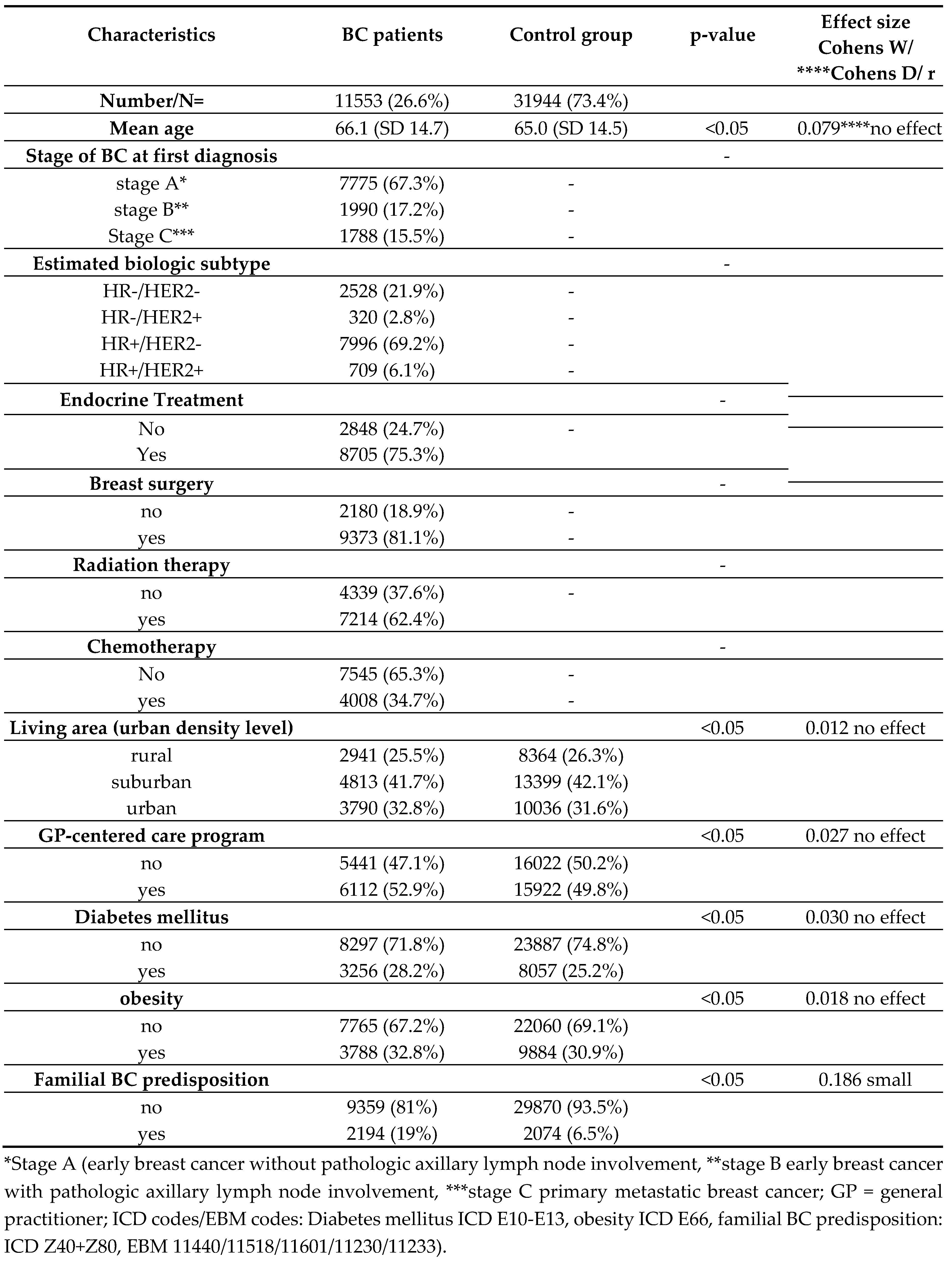
Table 1 displays the baseline patient characteristics that were reconstructed using claims data as described in the Materials and Methods section as well as in our previous publication [
17].
Regarding general characteristics in both groups the majority of patients lived in the suburbs (BCG 41.7%; 4813/11553 versus CG 42.1%; 13399/31944) and about half of each group participated in GP-centered care programs (BCG 52.9%; 6112/11553 versus CG 49.8%; 15922/31944). About one fourth of each group’s patients presented with diabetes mellitus (BCG 28.2%; 3256/11553 versus CG 25.2%, 8057/31944) and one third presented with obesity (BCG 32.8%; 3788/11553 versus CG 30.9%; 9884/31944). At 19% (2194/11553), significantly more patients in the BCG had a familial BC predisposition as compared to 6.1% (2074/31944) of the controls (p<0.05; Cohens D 0.186 small effect). Regarding the BC patients in this analysis, the most common tumor subtype was HR+/HER2− (7996/11553; 69.2%), followed by HR−/HER2− (2528/11553; 21.9%), HR+/HER2+ (709/11553; 6.1%), and HR−/HER2+ (320/11553; 2.8%). Furthermore, the majority of our BC patients (7775/11553; 67.3%) were assigned to stage A, 17.2% to stage B (1990/11553), and 15.5% to stage C (1788/11553). Furthermore, 75.3% (8705/11553) of BC patients received endocrine treatment, 81.1% (9373/11553) breast surgery, 62.4% (7214/11553) radiation therapy, and 34.7% (4008/11553) were treated with chemotherapy.
3.2. Mental Illnesses in BC Patients
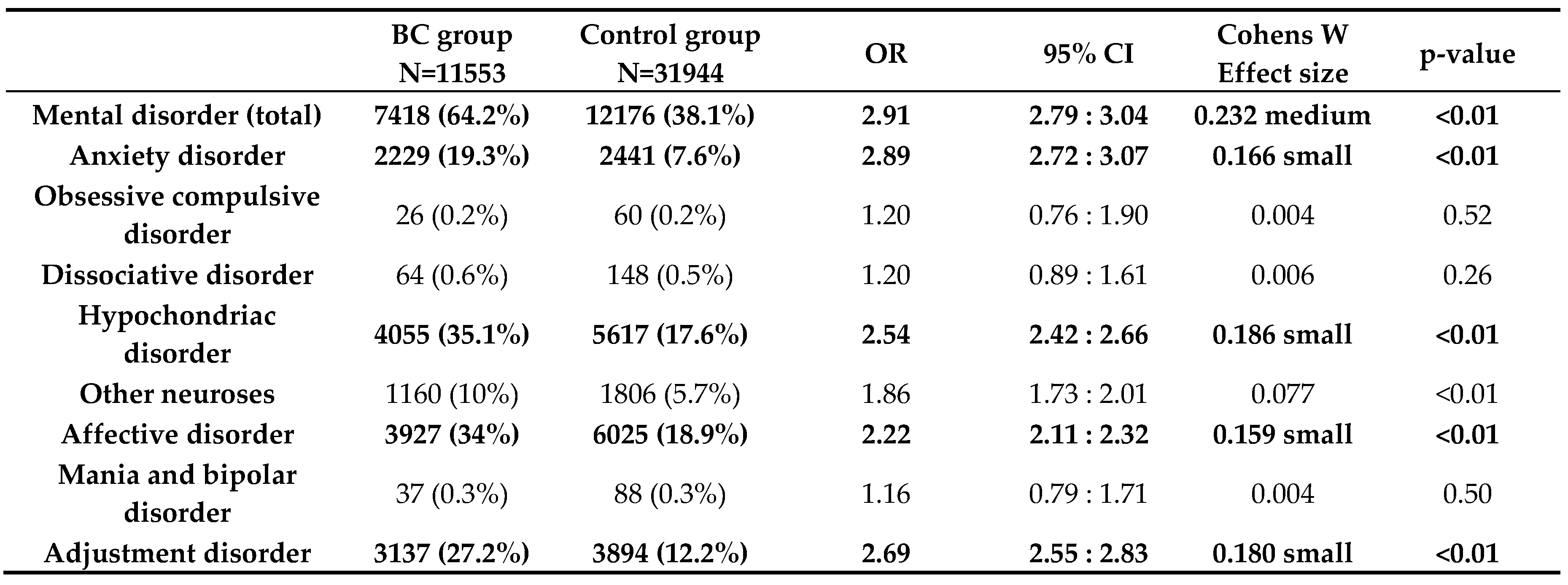
Concerning our primary objective, we found that at 64.2% (7418/11553) our BC patients developed a mental disorder significantly more often than our control patients (38.1% (12176/31944); p<0.01; OR 2.91). Regarding each psychological illness separately, we also found significantly higher rates among BC patients for anxiety (BCG 19.3%; 2229/11553 versus CG 7.6%; 2441/31944, p<0.01 OR 2.889), somatic symptom disorders and hypochondriac disorders (BCG 35.1%; 4055/11553 versus CG 17.6%; 5617/31944, p<0.01, OR 2.54), affective disorders (BCG 34%; 3927/11553 versus CG 18.9%;6025/31944, p<0.01, OR 2.22), and also for adjustment disorders (BCG 27.2%; 3137/11553 versus CG 12.2%;3894/31944, p<0.01, OR 2.69); see
Table 2.
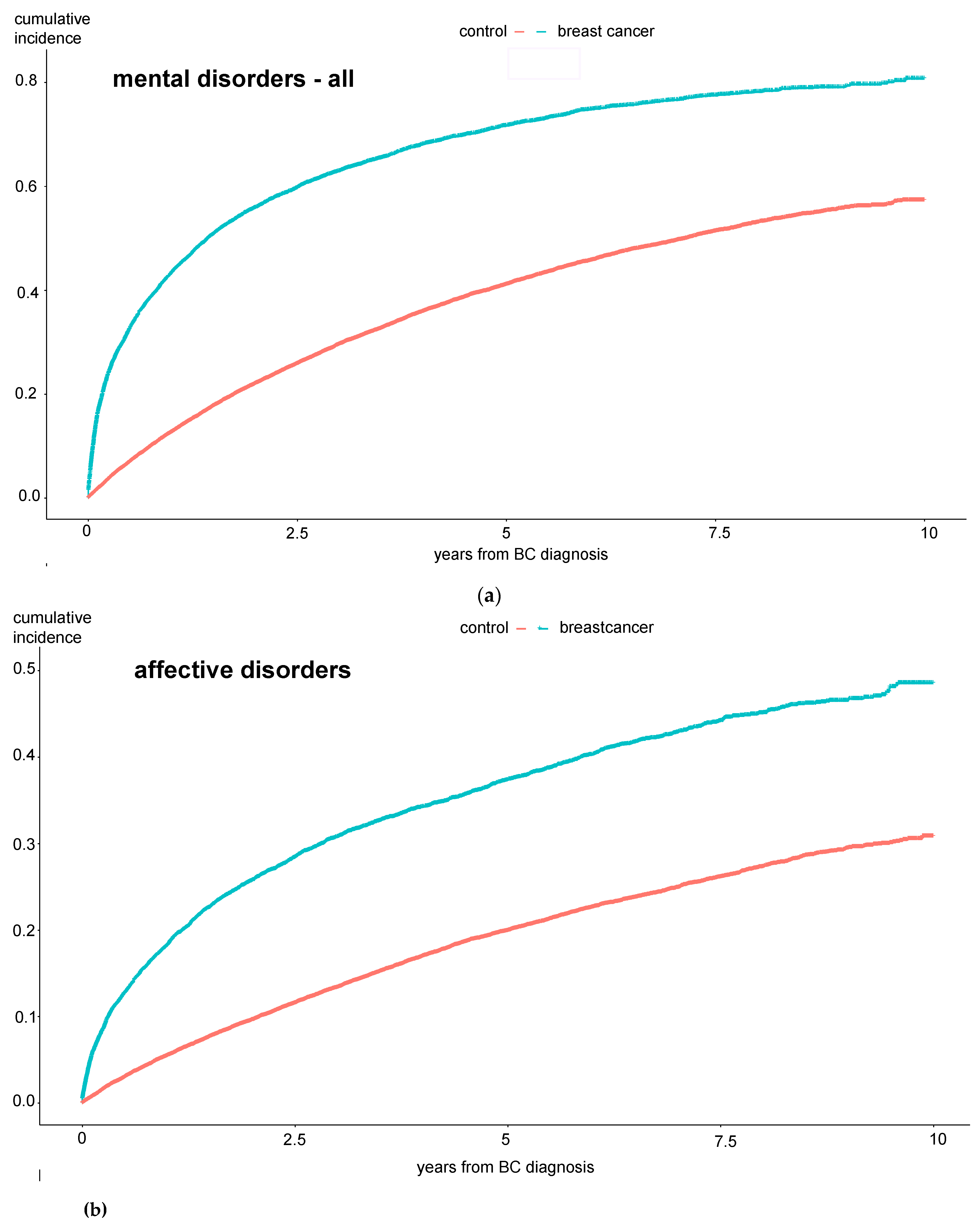
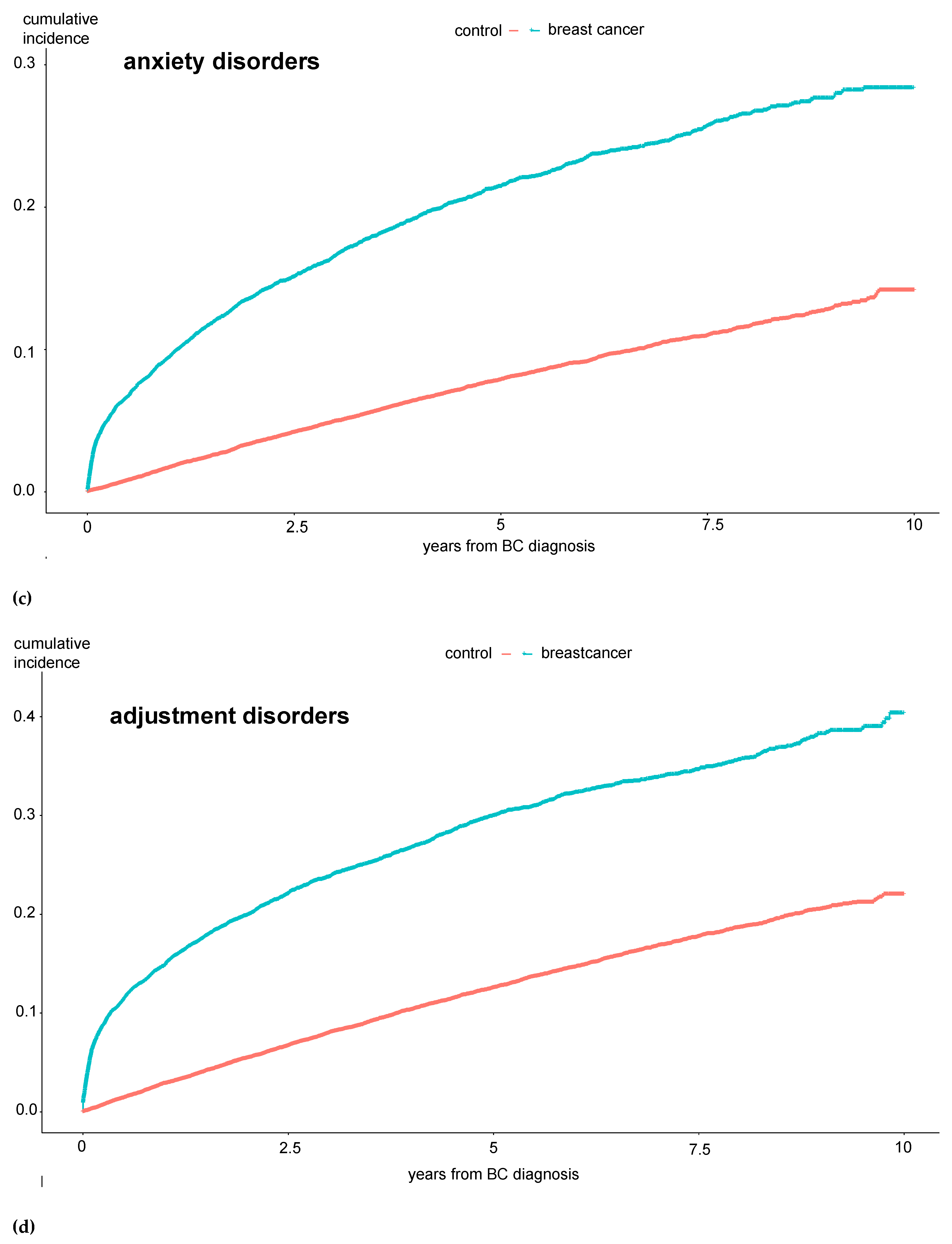
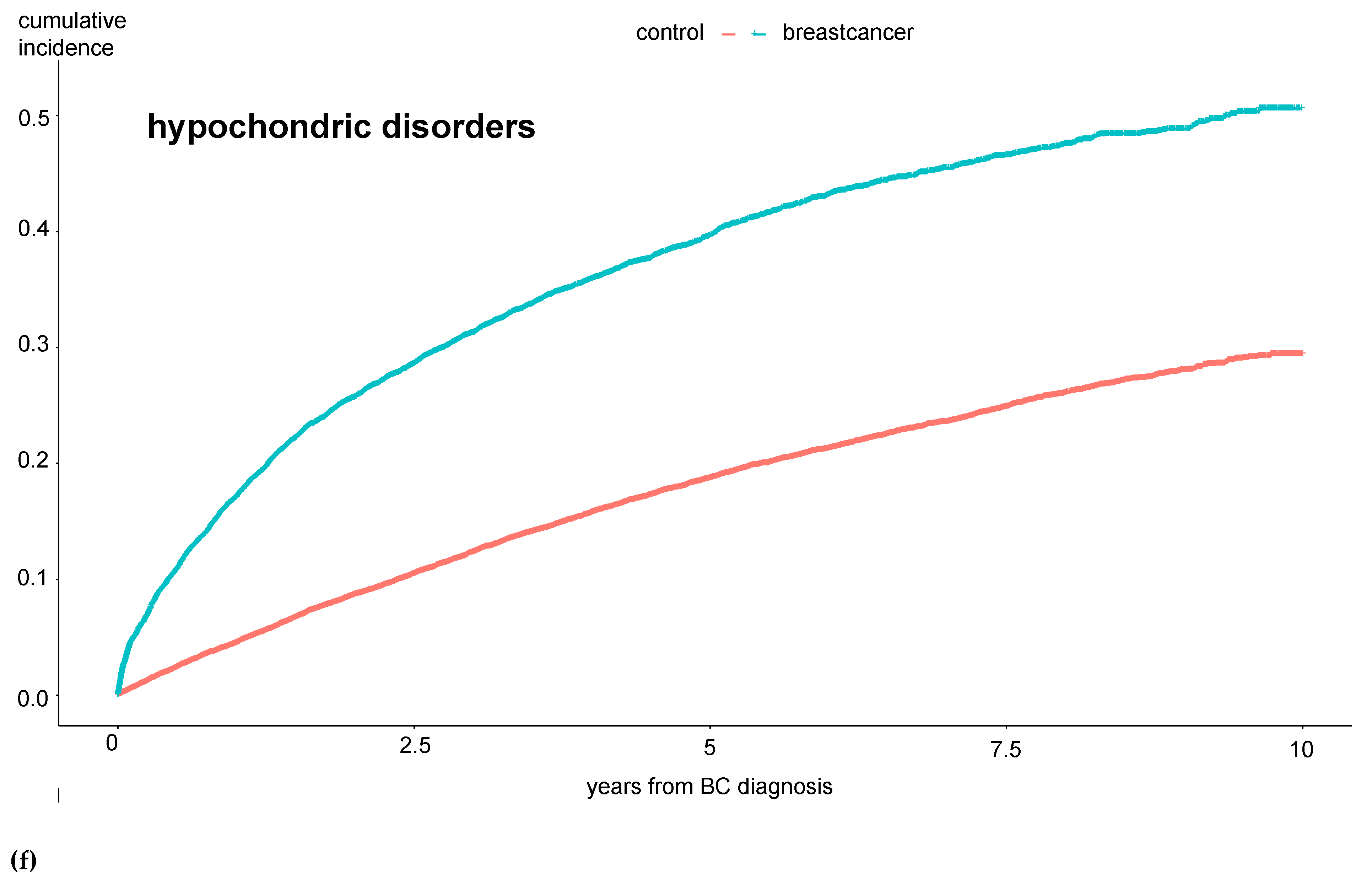
Incidence rates of mania and bipolar disorders, obsessive compulsive disorders, dissociative disorders, and “other neuroses” did not differ between the groups. Furthermore, Kaplan-Maier curves were generated to analyze the cumulative incidences of mental diseases over a 10-year observation period. Here, the same significant differences between BC and control patients were observed: the total number of mental disorders, especially affective disorders, anxiety disorders, hypochondriac disorders, and adjustment disorders, were significantly more common among BC patients (see
Figure 1 a-e).
The results in
Figure 1 show that these patients develop the mental disorder particularly within the first year after the BC diagnosis, i.e., while still undergoing treatment. To further investigate the extent to which the therapy directly influences the occurrence of a psychological comorbidity, we examined the experimental group in more detail.
3.3. Risk Factors for Mental Illnesses
Furthermore, a logistic regression model was applied to assess the occurrence of mental illness considering the following factors: chemotherapy, radiation therapy, mastectomy, endocrine therapy, age at first diagnosis, stage of BC, and family history of BC predisposition. An OR >1.68 or <0.05 with a p-value < 0.001 was considered a significant difference.
The analysis of the various treatments revealed that endocrine therapy, with an OR of 1.69 (p<0.0001, small effect size), was significantly associated with an increased occurrence of mental illness. For the other therapies examined, only a trend toward more mental illness was observed (chemotherapy OR 1.33, no effect; radiation therapy OR 1.53; and mastectomy OR 1.31, no effect). Furthermore, primarily metastasized patients (stage C) had a significantly lower risk to be diagnosed with mental illness compared to those with early breast cancer without lymph node involvement (stage A) (OR 0.55, p<0.0001, small effect). In this model no significant difference was found for stage B compared to stage A (OR 0.87, p=0.022, no effect).
Regarding age at first diagnosis, we observed a trend toward more mental illness in younger patients, but, again, the effect size was not significant (OR 0.63, p<0.001, no effect). For patients with a family history of BC predisposition, there was also only a trend toward more mental illness (OR 1.24, p<0.001, no effect).
Table 3.
Log. regression model considering the following factors: chemotherapy, radiation therapy, endocrine therapy, mastectomy, age at first diagnosis, stage of breast cancer, and familial breast cancer predisposition.
Table 3.
Log. regression model considering the following factors: chemotherapy, radiation therapy, endocrine therapy, mastectomy, age at first diagnosis, stage of breast cancer, and familial breast cancer predisposition.
| Factors |
Log(OR) |
SE |
OR |
95%CI |
p-value |
| Chemotherapy |
0.29** |
0.051 |
1.33 |
1.21, 1.47 |
<0.001 |
| Radiation therapy |
0.42** |
0.048 |
1.53 |
1.39, 1.68 |
<0.001 |
| Endocrine therapy |
0.52** |
0.049 |
1.69 |
1.53, 1.86 |
<0.001 |
| Mastectomy |
0.27** |
0.050 |
1.31 |
1.19, 1.45 |
<0.001 |
| Age at first diagnosis |
-0.47** |
0.025 |
0.63 |
0.60, 0.66 |
<0.001 |
| Stage of BC |
|
|
|
|
|
| 1 versus 2 |
-0.14* |
0.060 |
0.87 |
0.78, 0.98 |
0.022 |
| 1 versus 3 |
-0.60** |
0.058 |
0.55 |
0.49, 0.61 |
<0.001 |
| Familial BC predisposition |
0.22** |
0.059 |
1.24 |
1.11, 1.40 |
<0.001 |
Since the initial model did not account for the fact that patients could have undergone breast- conserving surgery alone as well as reconstructive breast surgery after mastectomy, this was examined in more detail in a subsequent analysis. Here, we conducted a further analysis and distinguished the surgical groups more precisely.
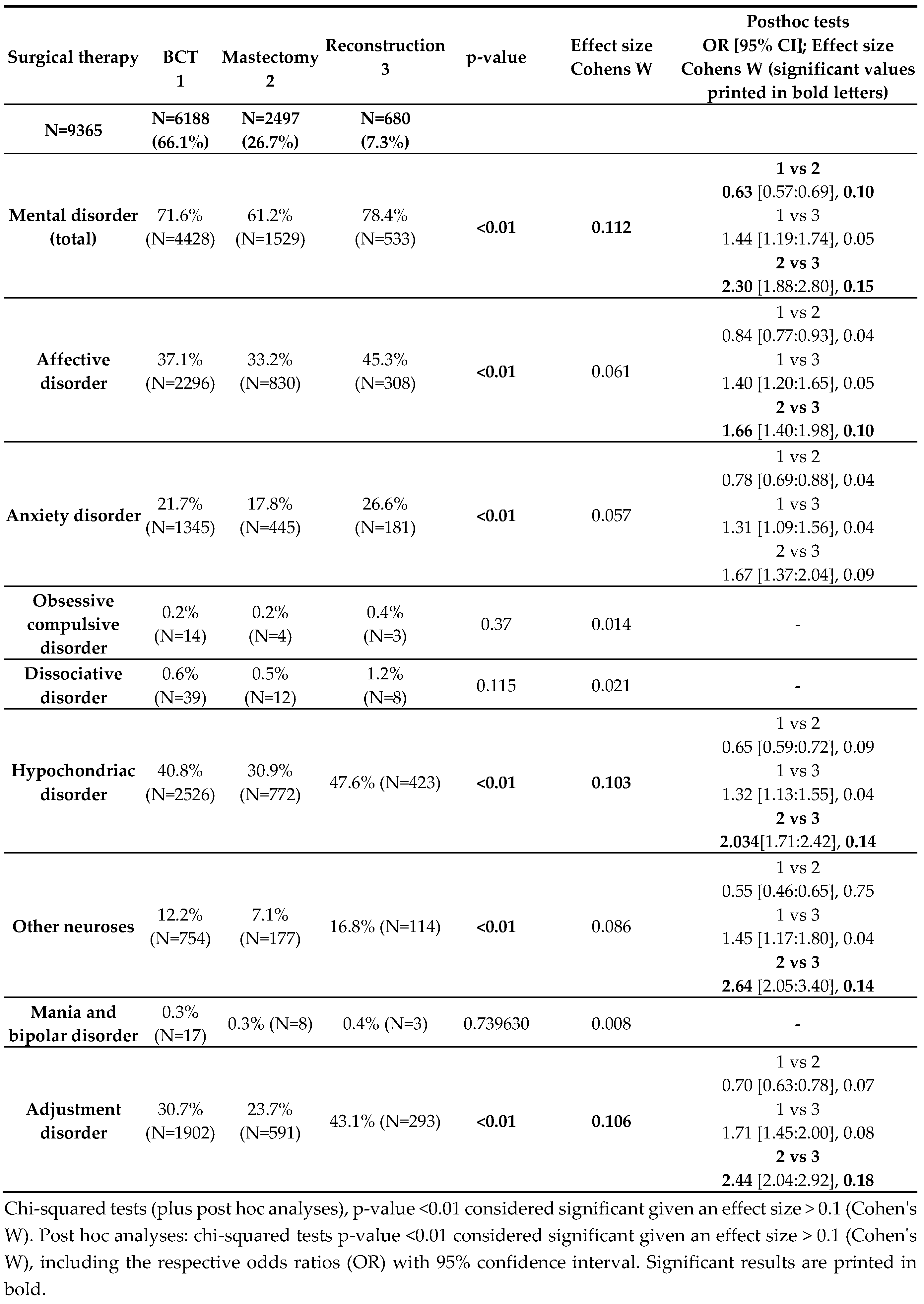
BC patients were now divided into three groups based on their surgical therapy. The first group, "BCT", included patients who received breast-conserving therapy with or without reconstructive surgery – essentially, all patients who never completely lost their breast. The second group, "Mastectomy," included patients in whom mastectomy without reconstruction was performed (including primary mastectomy patients and patients with initial BCT followed by secondary mastectomy). The third group, "Reconstruction," included all patients with primary or secondary mastectomy followed by breast reconstruction. Patients who, according to the coding, only received "reconstructive surgery" were excluded from our analysis, as they could not be clearly assigned to any of these three groups and this does not represent a standard surgical therapy for an oncological breast procedure.
Thus, this analysis was conducted on a cohort of 9365 BC patients.
This analysis showed that patients in the mastectomy group did not have higher rates of mental disorders (see
Table 4). On the contrary, a significantly lower proportion of patients in the mastectomy group (61.2%) suffered from a mental disorder compared to 71.6% in the BCT group and 78.4% in the reconstruction group. Furthermore, 47.6% of patients in the reconstruction group showed significantly more hypochondriac disorders than patients in whom mastectomy was performed (30.9%). A significantly different result was also found for the development of adjustment disorders: they were also most common in the reconstruction group (43.1%) and least common in the mastectomy group (23.7%). Overall, the reconstruction group also showed more "other neuroses" and "affective disorders" than the mastectomy group in the post hoc analyses. No differences were found between the BCT and reconstruction groups in terms of the occurrence of a mental disorder. Regarding mania, dissociative disorder, and obsessive-compulsive disorder, no significant differences were found between the three groups based on surgical therapy. In conclusion, our initial hypothesis that women who undergo mastectomy without subsequent reconstruction suffer more and therefore potentially develop more psychological comorbidities was not confirmed.
4. Discussion
With 64% of patients affected in our BC cohort, we observed significantly more mental disorders during the observational period compared to 38.1% in the healthy control patients; in particular, anxiety disorders, adjustment disorders, hypochondriac disorders, and affective disorders were more common. Thus, overall, we can confirm our previously stated main hypothesis that BC patients suffer from mental disorders more frequently. Considering the existing literature, the reported prevalence of depression and anxiety disorders among BC patients varies around approximately 30%. In a 2018 study by Tsaras et al., symptoms of depression or anxiety disorder were diagnosed in nearly 38% and 32%, respectively, of the included patients. [
9] Similar results were seen in a study from Germany, showing a 5-year incidence of depression and/or anxiety disorder of 35.1% of BC patients treated in gynecological private practices. [
10] Moreover, the analysis of our control group indicates that 38.1% of the women were affected by a mental illness, a figure consistent with representative data for Germany. A national survey from 2014 demonstrated that approximately one-third of adult women in Germany suffer from a mental disorder. [
19] Thus, our results in this analysis both regarding the BC group and the control group are in line with the literature. In another analysis by Burgess et al., nearly 50% of BC patients in their first year after diagnosis suffered from depression and/or anxiety disorder. [
20] As stated above, in our analysis, too, BC patients developed a mental disorder particularly within the first year after the BC diagnosis. Such an effect was similarly observed in another claims data analysis from Korea. [
21] One possible explanation could be that the life-threatening diagnosis of BC represents a potential trauma with a corresponding risk of an acute or post-traumatic stress reaction and an adjustment disorder. [
22] Already in 2002, Amir et al. reported that diagnosis of post-traumatic stress disorder is common among long-term BC survivors. [
23] As BC patients who are diagnosed with a cancer-related acute stress disease are at heightened risk of a second mental disorder diagnosis [
22], it is essential to provide these affected women with medical support at an early stage to prevent potential long-term mental health consequences.
Regarding the potential influencing factors, our most interesting finding was that patients who received endocrine treatment suffered from mental distress significantly more often (OR 1.69). The most commonly prescribed anti-endocrine therapies are the selective estrogen-receptor modulator (SERM) tamoxifen in premenopausal women and aromatase-inhibitors (AI) such as the third-generation agents anastrozole, letrozole, or exemestane in postmenopausal women: both therapies affect estrogen levels and the estrogen pathway in women, though in different ways. [
24] Estrogen has a significant impact on mood and mental state, e.g., low estrogen levels in women are linked to premenstrual syndrome as well as postpartum and postmenopausal depression. [
25] Thus, based on our finding that endocrine treatment is associated with higher rates of mental disorders, we support the hypothesis that anti-endocrine treatment leads to psychological side effects in women. Despite the common assumption that chemotherapy and radiation therapy represent a significant burden for BC patients, like other studies before, our analysis did not find an association with mental disorders for these treatment-associated factors.[
26] Furthermore, being in stage C (primary distant metastatic BC) was associated with significantly lower numbers of mental disorders in our study. Assessing the clinical stage of disease in BC patients is a crucial step in determining both treatment strategy and prognosis. [
27] As a higher stage usually comes with a poorer prognosis and, with respect to the current literature [
28], we initially assumed that higher disease stages would be associated with more psychological comorbidities. Possible explanations for our contradictory result could be that patients in advanced stages of cancer may prioritize physical survival and immediate medical concerns over mental health issues; thus, the focus on survival could diminish the impact or at least the reporting of psychological distress. Furthermore, patients with more advanced disease might receive more comprehensive palliative care, including psychological support, which could help mitigate feelings of anxiety and depression. The awareness and acknowledgment of their prognosis might also lead to increased support from friends and family, contributing to better psychological outcomes.
Regarding surgical treatment, we found a significant correlation between reconstructive breast surgery and the occurrence of mental disorders, in general, and of both adjustment disorders and hypochondriac disorders, in particular. Patients treated by mastectomy, on the contrary, showed the lowest rates of mental illness (61.2%); these patients were significantly less affected than those in the BCT group (71.6%) and in the breast reconstruction group (78.4%). Regarding the current literature, there are mixed reports on the impact of mastectomy on BC patients. [
29,
30,
31,
32] In a current meta-analysis by Mishra and colleagues, the authors stated that mastectomy has a strong negative impact on women’s appearance and psychology. [
33] However, when examining the literature in more detail, it becomes clear that some studies report contradictory results. For example, a study from Italy comparing mastectomy patients with and without breast reconstruction to healthy women found no difference between mastectomy patients with versus without reconstruction in terms of the occurrence of anxiety disorders, quality of life, and social adaptation. [
34] Additionally, many women regret undergoing breast reconstruction over time. A study from Australia on post-decision regret after breast reconstruction found that 27.6% of women experienced mild regret, while 19.5% experienced moderate to severe regret regarding their decision for breast reconstruction. In this study, decision regret was associated with depression, anxiety, low satisfaction with preparatory information, and stress. [
35] Another prospective longitudinal survey study, which compared the psychological outcomes of mastectomy patients with those who underwent delayed breast reconstruction, showed that women with delayed breast reconstruction had significantly higher levels of total distress, obsessiveness, and cancer-related distress than those with mastectomy alone. Furthermore, in that study, no differences in quality of life between the two groups could be seen at any time point. [
36] Nissen et al. also conducted a prospective study regarding quality of life, which revealed that women with mastectomy and following reconstruction showed greater mood disturbances and poorer well-being after baseline and still 18 months after surgery. [
32] These factors highlight the complexity of psychological responses to different types of BC surgery, necessitating tailored mental health interventions across the disease spectrum. In most BC centers in Germany, women diagnosed with BC are already advised on the pros and cons of mastectomy versus breast-conserving therapy in the context of participatory decision-making. If there is no indication for mastectomy, BCT with subsequent radiation is usually offered as the standard option and mastectomy alone is considered an “equivalent alternative” with or without subsequent reconstruction. Some have argued that psychological stress potentially develops after breast amputation, and the German S3 guideline even speaks of a potential disadvantage if immediate reconstruction cannot follow a mastectomy. [
37] However, from our analysis on over 9000 BC patients and also our current literature research, this argument cannot be supported. As in the comparison of mastectomy, BCT, and breast reconstruction in the present study, a mastectomy was not associated with increased psychological distress, while reconstructive and breast-conserving therapy were, counseling should be more open to a woman’s desire for mastectomy without reconstruction. Furthermore, patients undergoing reconstructive surgery should receive more intensive follow-up and possibly psychological support.
As we already discussed in our prior publication [
17], retrospective claims data analyses are valuable tools in healthcare research, reflecting actual clinical practice in a broad population and offering insights into real-world treatment patterns and outcomes. The most important advantages of retrospective claims data analyses are the large sample sizes, like the one in our present investigation, making it a robust dataset for statistical analyses. However, claims data are primarily collected for billing purposes, and not research. Thus, the data provided on tumor stage, patient characteristics, or social determinants of health are not exact. Therefore, the issue of incomplete data poses a limitation on data analysis. In this analysis, for example, we had to exclude 50,098 patients due to an unreliable C50 diagnosis from the original dataset. Another challenge of claims data analysis in general and also of our study is establishing correct causal relationships. To ensure that the mental illness is indeed a consequence of BC diagnosis or treatment, we selected a cohort in which no psychological comorbidities had been diagnosed within the 12 months preceding the initial BC diagnosis. This approach allowed us to establish a clear temporal sequence of diagnostic events, albeit at the expense of cohort size, as we were required to exclude an additional 16,316 BC patients.
5. Conclusions
Our analyses indicate that patients with BC are at a significantly increased risk of developing a psychological disorder. In particular, adjuvant patients, those receiving endocrine treatment, and those treated with reconstructive breast surgery seem to be at higher risk. Thus, it is particularly important to educate BC patients also about possible psychological side effects before starting the treatment. Currently, in Germany, there is no widespread psycho-oncological co-management for BC patients—especially for those who do not receive chemotherapy, as they are often not referred for psycho-oncological care. Ideally, screening for past or existing psychological distress or disorders should be implemented within the primary care framework at BC centers.
Furthermore, reconstructive surgery should be openly discussed with the patients as it is associated with more – instead of less – psychological distress. Our results challenge the current practice to generally recommend reconstructive surgery following mastectomy.
Author Contributions
Conceptualization: A.v.A., R.G., T.M.H.D, K.H., S.H.-K., S.W.; Methodology: A.v.A., R.G., D.D., A.S.S. and T.M.H.D.; Software: R.G. and T.M.H.D.; Validation: R.G., A.v.A., S.H.-K., A.C., A.I., A.B., A.S.S., K.H., M.W., D.W., S.Y.B., S.W. and A.D.H.; Formal analysis: A.v.A., S.W., R.G., T.M.H.D.; Resources: S.H.-K., A.I., A.C., A.B., M.W., D.W. and S.Y.B.; Data curation: A.v.A., D.D., R.G. and T.M.H.D.; Writing—original draft: A.v.A.; Writing—review and editing: D.D., A.S.S., K.H., M.H., S.H.-K.,A.I., A.C., A.B., M.W., D.W., S.Y.B., A.D.H. and S.W.; Visualization: A.v.A., R.G., T.M.H.D.; Supervision: S.H.-K., A.I., A.C., A.B., M.W., D.W., S.Y.B., A.D.H and S.W..; Project administration: M.H., S.H.-K., A.C., A.I., A.B., M.W., D.W., S.Y.B., A.D.H and S.W.; Funding acquisition: S.H.-K., A.I., A.C., A.B., M.W., D.W., S.Y.B., S.W. and A.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AOK Baden-Wuerttemberg.
Institutional Review Board Statement
The study was conducted in accordance with the Decla- ration of Helsinki and approved by the Ethics Committee of Tuebingen University (protocol code 380/2020BO, 2020).
Informed Consent Statement
Patient consent was waived due to the anonymization of patient data by AOK Baden-Wuerttemberg.
Data Availability Statement
Data were provided by AOK Baden-Wuerttemberg. Due to privacy reasons and data security regulations, data are only available with the consent of AOK Baden- Wuerttemberg.
Conflicts of Interest
All authors declare no conflicts of interest. This work was funded by AOK Baden-Wuerttemberg. This study was designed in close cooperation with AOK Baden-Wuerttemberg. However, the funders had no role in data analysis, interpretation of data, writing of the manuscript, or decision to publish the results.
References
- Sung, H., J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray. "Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries." CA Cancer J Clin 71 (2021): 209-49. [CrossRef]
- Wilkinson, L. and T. Gathani. "Understanding breast cancer as a global health concern." Br J Radiol 95 (2022): 20211033. [CrossRef]
- Wojtyla, C., P. Bertuccio, A. Wojtyla and C. La Vecchia. "European trends in breast cancer mortality, 1980-2017 and predictions to 2025." Eur J Cancer 152 (2021): 4-17. [CrossRef]
- Fann, J. R. Fann, J. R., A. M. Thomas-Rich, W. J. Katon, D. Cowley, M. Pepping, B. A. McGregor and J. Gralow. "Major depression after breast cancer: A review of epidemiology and treatment." Gen Hosp Psychiatry 30 (2008): 112-26. [CrossRef]
- Payne, D. K., M. D. Sullivan and M. J. Massie. "Women's psychological reactions to breast cancer." Semin Oncol 23 (1996): 89-97.
- Al-Azri, M., H. Al-Awisi and M. Al-Moundhri. "Coping with a diagnosis of breast cancer-literature review and implications for developing countries." Breast J 15 (2009): 615-22. [CrossRef]
- Browall, M., K. Ahlberg, P. Karlsson, E. Danielson, L. O. Persson and F. Gaston-Johansson. "Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women." Eur J Oncol Nurs 12 (2008): 180-9. [CrossRef]
- Soqia, J., M. Al-Shafie, L. Y. Agha, M. B. Alameer, D. Alhomsi, R. Saadoun and M. Saifo. "Depression, anxiety and related factors among syrian breast cancer patients: A cross-sectional study." BMC Psychiatry 22 (2022): 796. [CrossRef]
- Tsaras, K., I. V. Papathanasiou, D. Mitsi, A. Veneti, M. Kelesi, S. Zyga and E. C. Fradelos. "Assessment of depression and anxiety in breast cancer patients: Prevalence and associated factors." Asian Pac J Cancer Prev 19 (2018): 1661-69. [CrossRef]
- Jacob, L., L. Bleicher, K. Kostev and M. Kalder. "Prevalence of depression, anxiety and their risk factors in german women with breast cancer in general and gynecological practices." J Cancer Res Clin Oncol 142 (2016): 447-52. [CrossRef]
- Hashemi, S. M., H. Rafiemanesh, T. Aghamohammadi, M. Badakhsh, M. Amirshahi, M. Sari, N. Behnamfar and K. Roudini. "Prevalence of anxiety among breast cancer patients: A systematic review and meta-analysis." Breast Cancer 27 (2020): 166-78. 10.1007/s12282-019-01031-9. [CrossRef]
- Pilevarzadeh, M., M. Amirshahi, R. Afsargharehbagh, H. Rafiemanesh, S. M. Hashemi and A. Balouchi. "Global prevalence of depression among breast cancer patients: A systematic review and meta-analysis." Breast Cancer Res Treat 176 (2019): 519-33. [CrossRef]
- Caruso, R., M. G. Nanni, M. Riba, S. Sabato, A. J. Mitchell, E. Croce and L. Grassi. "Depressive spectrum disorders in cancer: Prevalence, risk factors and screening for depression: A critical review." Acta Oncol 56 (2017): 146-55. [CrossRef]
- Benny, A., M. McLay, R. C. Callaghan, A. Bates and R. Olson. "Population-based comparison of cancer survival outcomes in patients with and without psychiatric disorders." BMC Psychiatry 22 (2022): 543. [CrossRef]
- Mausbach, B. T., R. B. Schwab and S. A. Irwin. "Depression as a predictor of adherence to adjuvant endocrine therapy (aet) in women with breast cancer: A systematic review and meta-analysis." Breast Cancer Res Treat 152 (2015): 239-46. [CrossRef]
- Wang, X., N. Wang, L. Zhong, S. Wang, Y. Zheng, B. Yang, J. Zhang, Y. Lin and Z. Wang. "Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients." Mol Psychiatry 25 (2020): 3186-97. [CrossRef]
- Dannehl, D., A. von Au, T. Engler, L. L. Volmer, R. Gutsfeld, J. F. Englisch, M. Hahn, S. Hawighorst-Knapstein, A. Chaudhuri, A. Bauer, et al. "Implementation and evaluation of a breast cancer disease model using real-world claims data in germany from 2010 to 2020." Cancers (Basel) 16 (2024). [CrossRef]
- Bundesinstitut für Arzneimittel und Medizinprodukte, B. "Anatomic therapeutic chemical (atc) classification 2024." 2024. https://www.bfarm.de/SharedDocs/Downloads/DE/Kodiersysteme/ATC/atc-ddd-amtlich-2024.pdf?__blob=publicationFile. 28 July 2024.
- Jacobi, F., M. Höfler, J. Siegert, S. Mack, A. Gerschler, L. Scholl, M. A. Busch, U. Hapke, U. Maske, I. Seiffert, et al. "Twelve-month prevalence, comorbidity and correlates of mental disorders in germany: The mental health module of the german health interview and examination survey for adults (degs1-mh)." Int J Methods Psychiatr Res 23 (2014): 304-19. [CrossRef]
- Burgess, C., V. Cornelius, S. Love, J. Graham, M. Richards and A. Ramirez. "Depression and anxiety in women with early breast cancer: Five year observational cohort study." Bmj 330 (2005): 702. [CrossRef]
- Heo, J., M. Chun, Y. T. Oh, O. K. Noh and L. Kim. "Psychiatric comorbidities among breast cancer survivors in south korea: A nationwide population-based study." Breast Cancer Res Treat 162 (2017): 151-58. [CrossRef]
- Mehnert, A. and U. Koch. "Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: A prospective study." Psychooncology 16 (2007): 181-8. [CrossRef]
- Amir, M. and A. Ramati. "Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: A preliminary research." J Anxiety Disord 16 (2002): 195-206. [CrossRef]
- Lumachi, F., A. Brunello, M. Maruzzo, U. Basso and S. M. Basso. "Treatment of estrogen receptor-positive breast cancer." Curr Med Chem 20 (2013): 596-604. [CrossRef]
- Fink, G., B. E. Sumner, R. Rosie, O. Grace and J. P. Quinn. "Estrogen control of central neurotransmission: Effect on mood, mental state, and memory." Cell Mol Neurobiol 16 (1996): 325-44. [CrossRef]
- Doege, D., M. S. Y. Thong, L. Koch-Gallenkamp, L. Jansen, H. Bertram, A. Eberle, B. Holleczek, R. Pritzkuleit, A. Waldmann, S. R. Zeissig, et al. "Age-specific prevalence and determinants of depression in long-term breast cancer survivors compared to female population controls." Cancer Med 9 (2020): 8713-21. [CrossRef]
- McDonald, E. S., A. S. Clark, J. Tchou, P. Zhang and G. M. Freedman. "Clinical diagnosis and management of breast cancer." J Nucl Med 57 Suppl 1 (2016): 9s-16s. [CrossRef]
- Brown, L. C., A. R. Murphy, C. S. Lalonde, P. D. Subhedar, A. H. Miller and J. S. Stevens. "Posttraumatic stress disorder and breast cancer: Risk factors and the role of inflammation and endocrine function." Cancer 126 (2020): 3181-91. [CrossRef]
- Al-Ghazal, S. K. and R. W. Blamey. "Subcutaneous mastectomy with implant reconstruction: Cosmetic outcome and patient satisfaction." Eur J Surg Oncol 26 (2000): 137-41. [CrossRef]
- Al-Ghazal, S. K., L. Fallowfield and R. W. Blamey. "Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction." Eur J Cancer 36 (2000): 1938-43. [CrossRef]
- Macadam, S. A., A. L. Ho, E. F. Cook, Jr., P. A. Lennox and A. L. Pusic. "Patient satisfaction and health-related quality of life following breast reconstruction: Patient-reported outcomes among saline and silicone implant recipients." Plast Reconstr Surg 125 (2010): 761-71. [CrossRef]
- Nissen, M. J., K. K. Swenson, L. J. Ritz, J. B. Farrell, M. L. Sladek and R. M. Lally. "Quality of life after breast carcinoma surgery: A comparison of three surgical procedures." Cancer 91 (2001): 1238-46.
- Mishra, A., J. Nair and A. M. Sharan. "Coping in post-mastectomy breast cancer survivors and need for intervention: Systematic review." Breast Cancer (Auckl) 17 (2023): 11782234231209126. [CrossRef]
- Rubino, C., A. Figus, L. Lorettu and G. Sechi. "Post-mastectomy reconstruction: A comparative analysis on psychosocial and psychopathological outcomes." J Plast Reconstr Aesthet Surg 60 (2007): 509-18. [CrossRef]
- Sheehan, J., K. A. Sherman, T. Lam and J. Boyages. "Association of information satisfaction, psychological distress and monitoring coping style with post-decision regret following breast reconstruction." Psychooncology 16 (2007): 342-51. [CrossRef]
- Metcalfe, K. A., T. Zhong, S. A. Narod, M. L. Quan, C. Holloway, S. Hofer, S. Bagher and J. Semple. "A prospective study of mastectomy patients with and without delayed breast reconstruction: Long-term psychosocial functioning in the breast cancer survivorship period." J Surg Oncol 111 (2015): 258-64. [CrossRef]
- Wöckel, A., U. S. Albert, W. Janni, A. Scharl, R. Kreienberg and T. Stüber. "The screening, diagnosis, treatment, and follow-up of breast cancer." Dtsch Arztebl Int 115 (2018): 316-23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).