1. Introduction
Hereditary angioedema (HAE) is a rare (1.5:100,000), life-threatening, autosomal dominant disorder characterized by unpredictable and recurrent, episodic swelling most commonly involving the skin, gastrointestinal tract and/or upper airways [
1,
2]. In HAE due to C1-inhibitor (C1-INH) deficiency, two main forms are recognized: HAE type 1, which is characterized by quantitatively low levels of C1-INH, and make up 85% of all cases, and HAE type 2, which involves a normal C1-INH antigenic level but impaired functional activity, and make up approximately 15% of cases [
3]. C1INH regulates various interrelated proteolytic cascades, including the contact, fibrinolytic, coagulation and complement systems. C1INH inhibits proteases across various systems, including the contact (kallikrein), fibrinolytic (plasmin, fibrinogen, fibrin), coagulation (Factors XIIa, XIa), and the complement cascades-specifically the classical (C1r, C1s) and lectin pathways (MASP-1, MASP-2) [
4].
Bradykinin, the primary mediator of HAE, is potent vasodilator generated in the contact system. It is released from its precursor, high molecular weight kininogen, following the activation of prekallikrein into kallikrein by Factor XII (FXIIa). C1 inhibitor serves as the main regulator primary of contact factors, inhibiting 93% of FXIIa and 52% of prekallikrein in plasma [
5].
Clinical management of HAE includes LTP to reduce angioedema episodes, on-demand treatment (ODT) for reduce the duration of acute episodes, and short-term prophylaxis (STP) for situations that may trigger an outbreak. Lanadelumab, a fully humanized monoclonal antibody that specifically inhibits plasma kallikrein and reduces bradykinin production, was approved for long-term prophylaxis (LTP) of HAE in Spain in April 2021. The recommended dosage for profilaxis is 300 mg administered subcutaneously (SC) every 2 weeks (q2W), while an alternative dosing interval of 300 mg SC q4W may be effective for patients who have been well controlled (attack-free) for more than 6 months [
6].
The primary aim of this study was to investigate potential cost reductions for payers and the healthcare system associated with LTP of HAE using lanadelumab. To achieve this, we evaluated the impact of lanadelumab treatment on hospital consultations including medical, nursing, pharmacy, and emergency visits, as well as consultations via WhatsApp and phone. Secondary objectives included analyzing angioedema episode frequency, the need for ODT with icatibant, and STP before high-risk medical procedures. Additionally, the study compared HAE-QoL quality of life questionnaire results and monitored any adverse reactions related to lanadelumab use.
2. Materials and Methods
2.1. Subjects and Study Variables
As of 2023-2024, the population of Tenerife, Spain exceeds 950,000 residents [
7]. Approximately half of these individuals (498,208 healthcare users) are served by our institution, which covers the northern health district. This study was a single-center, observational, retrospective analysis that included only participants meeting specific inclusion criteria: patients with a bradykinin-mediated HAE type 1 diagnosis confirmed by a trained allergist who were receiving treatment with lanadelumab 300 mg SC q2W. Pregnant and breastfeeding women were excluded from the study. Approval was obtained from the local Ethical Committee of our Institution (code number CHU-2024-94), and informed consent was provided by all participants, with parents or guardians signing for those under 18 years of age.
Data were retrospectively gathered from patients' clinical records between March 2021 and June 2024 to assess their clinical progression under lanadelumab therapy compared to their previous clinical status. The analysis focused on the following metrics: clinical outcomes (frequency and detailed descriptions of angioedema attacks, the need for on-demand treatment with icatibant, and short-term prophylaxis before high-risk medical procedures), healthcare resource utilization (number of in-person hospital visits, including medical, nursing, pharmacy, and emergency room consultations, as well as remote consultations via WhatsApp and/or phone), QoL using validated tools (HAE-QoL questionnaire), and monitoring of any adverse reactions potentially related to lanadelumab use. The study flow is illustrated in
Figure 1.
2.2. Statistical Analysis
The median and interquartile range (IQR) were utilized to assess age and post-lanadelumab follow-up periods. ANOVA was employed for comparision involving more than two groups, with a p-value value of less than 0.05 considered statistically significant. All statistical analysis were conducting using GraphPad Prism version 10.0.0 for Windows, GraphPad Software, La Jolla, CA, USA.
3. Results
3.1. Characteristics of Investigated Patients
Currently, our Institution is actively monitoring 21 patients with distinct types of bradykinin-mediated angioedema, excluding those cases caused by angiotensin-converting enzyme (ACE) inhibitors. Among these patients:
HAE Type 1: 10 patients (47.6%) with a fully confirmed diagnosis
HAE Type 2: 8 patients (38%)
HAE-FXII: 2 patients (9.5%), with genetic testing confirming mutations in Factor XII
Acquired Angioedema (AEA): 1 patient with C1q deficiency
Of the 21 patients:
14 (66.6%) did not require LTP
2 males (9.5%) were on LTP with danazol
4 females (19%) were receiving LTP with lanadelumab
The remaining patient with AEA was on LTP with amchafibrin
Three infants were undergoing evaluation for angioedema but were not included in this study. All patients had icatibant available as ODT. Among those patients receiving LTP (19%) with lanadelumab, 3 switched from plasma-derived C1-esterase inhibitor (PdC1-INH) treatment, which was administered intravenously twice a week, due to lack of efficacy. The fourth patient switched after failure of berotralstat, an oral daily tablet, in conjunction with danazol (200 mg orally every 48 hours). All doses of PdC1-INH were given in the hospital, as the patients refused self-administration.
A total of 4 Caucasian female patients, all diagnosed with HAE type 1, were finally enrolled in the study. Their median age was 50.5 years (interquartile range [IQR] 13.5), with ages ranging from 39 to 78 years. These 4 patients began long-term prophylaxis (LTP) with lanadelumab between March 2021 and February 2024, and the follow-up period varied from 5 to 40 months, with a median duration of 23 months (IQR 16.25), resulting in a cumulative follow-up time of 91 months across all patients. All patients commenced lanadelumab treatment with a dosage of 300 mg administered subcutaneously every two weeks (SC q2W). After the first six months, the dosing interval was adjusted to 300 mg SC q4W for 2 patients (50%). One patient initially required an adjustment to receive lanadelumab 300 mg SC every ten days due to episodes occurring shortly before the two-week interval; however, this patient eventually returned to the standard two-week interval. Throughout the study, all patients continued to have icatibant available for ODT. Demographic and clinical characteristics are detailed in
Table 1.
3.2. Healthcare Resource Utilization
3.2.1. Allergy Department (Allergist and Nursing) Consultations
In the year prior to switching to lanadelumab for LTP, the 4 patients in this investigation collectively made 343 visits to the allergy department, consisting of 119 medical consultations and 224 nursing consultations. This equated to an average of 7.1 visits per month per patient, with 2.4 medical visits and 4.6 nursing visits each month. During the cumulative 91 months of follow-up after starting LTP with lanadelumab, these patients made a total of 135 visits to the allergy department—41 medical consultations and 94 nursing consultations—averaging 1.4 visits per month per patient (0.4 medical visits and 1 nursing visit). Additionally, two patients transitioned to telephone medical consultations, with 4% of all medical consultations after starting lanadelumab conducted by phone.
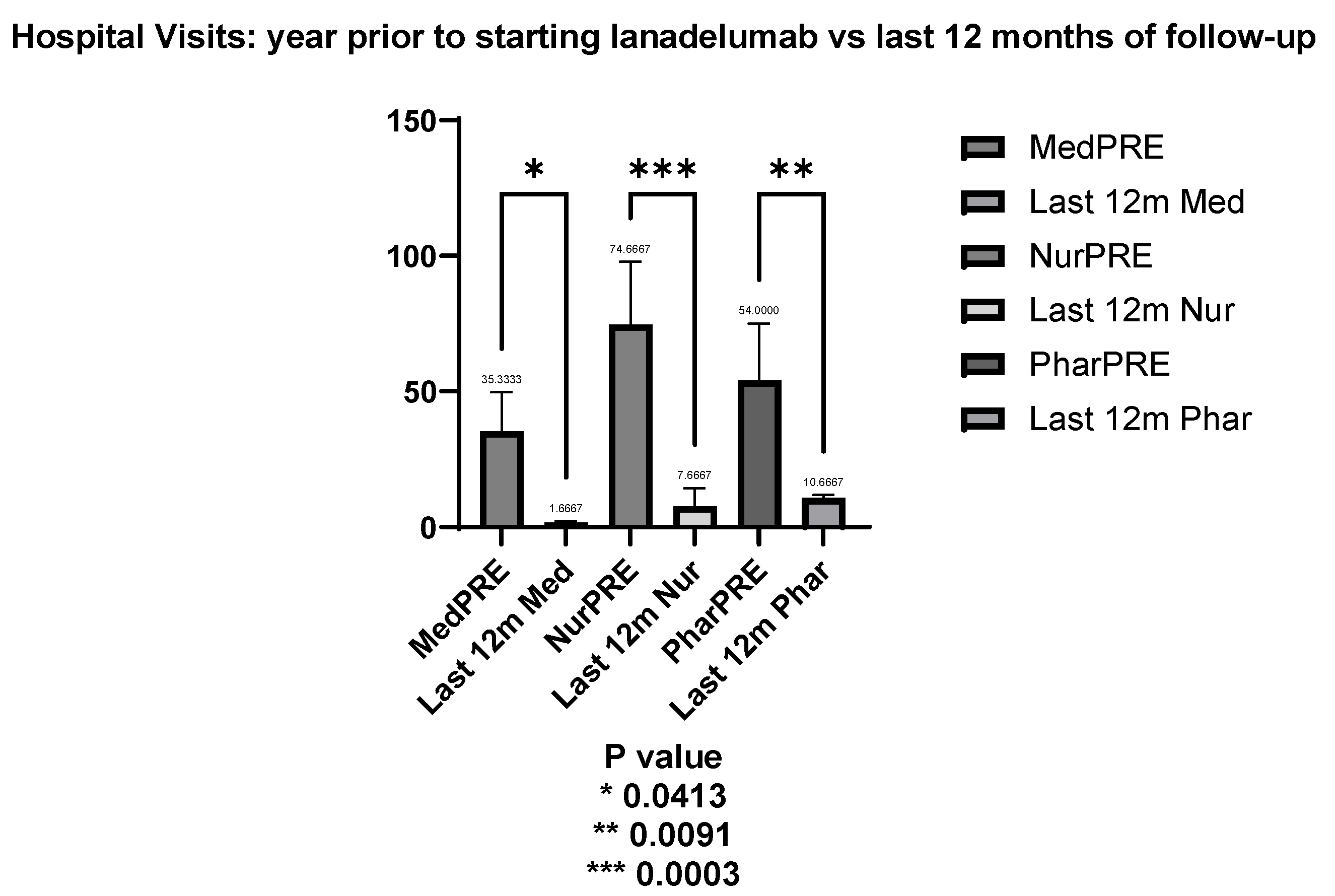
When comparing hospital visits before and during the last 12 months of follow-up for the 3 of 4 patients who have been on lanadelumab for over a year, there was a significant reduction in both medical and nursing consultations. Prior to starting lanadelumab, the average number of medical visits was 35.33 per year. In the last 12 months of follow-up, the mean dropped to 1.66, reflecting a significant decrease of 33.67 visits (t = 2.876, p = 0.041). Similarly, the mean number of nursing visits decreased from 74.67 in the year before treatment to 7.67 in the last year, representing a reduction of 67.00 visits with strong statistical significance (t = 5.723, p = 0.0003) as shown in
Figure 2.
3.2.2. Hospital Pharmacy Visits
In the year before switching to LTP with lanadelumab, all 4 patients made a total of 172 visits to the hospital pharmacy. Over the 91 months of follow-up after starting lanadelumab, this number dropped to 107 visits, reducing the average from 3.5 visits per month per patient to 1.1 visits. For the 3 patients who had been on lanadelumab for more than a year, the mean number of pharmacy visits was 54.00 in the year prior to treatment, compared to 10.67 in the last year of follow-up. This decrease of 43.33 visits is statistically significant (t = 3.701, p = 0.0091) (
Figure 2).
3.2.3. Trends in Hospital Visits after LTP with Lanadelumab
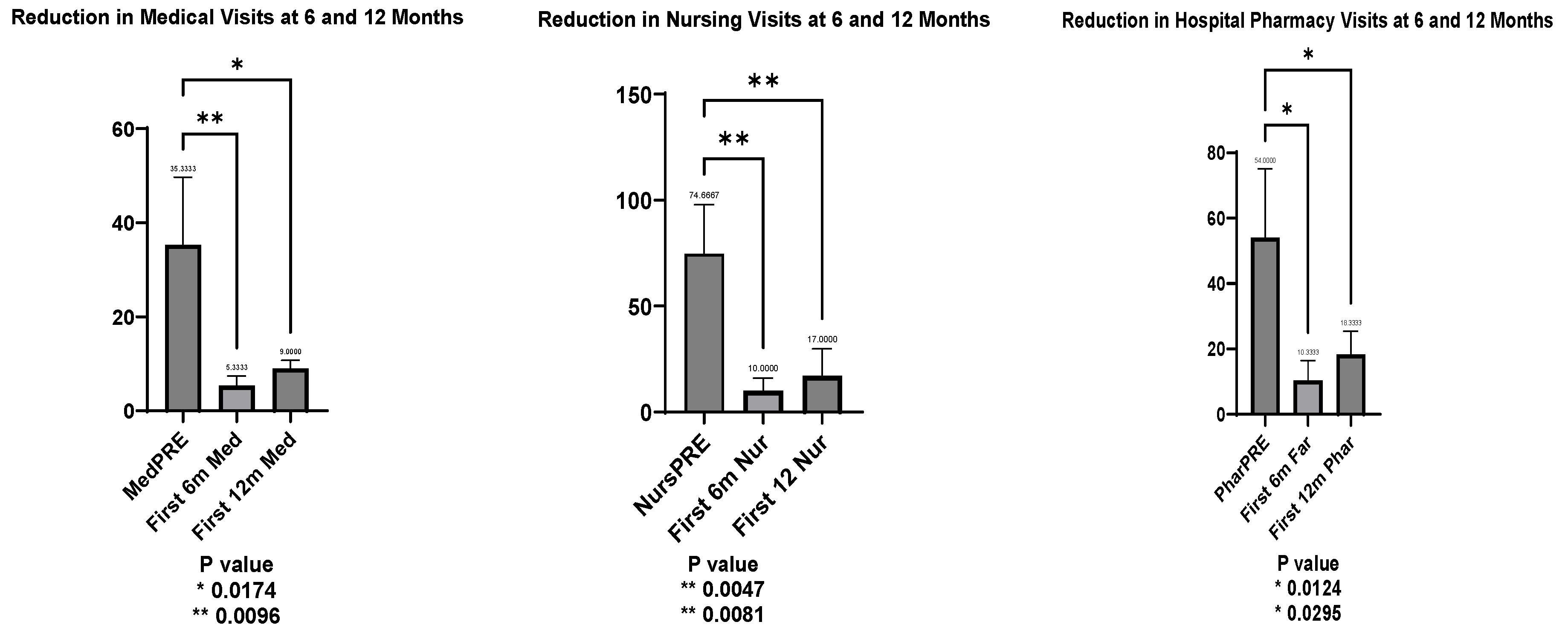
An early response to lanadelumab therapy was observed when comparing hospital visits in the year prior to treatment with visits in the first 6 and 12 months afterward. This analysis, conducted for the 3 patients who had been on lanadelumab for more than six months, showed statistically significant reductions across all areas.
Medical visits decreased significantly, with a mean reduction to 30.00 visits at 6 months (t = 4.354, p = 0.0096) and 26.33 visits at 12 months (t = 3.822, p = 0.017).
Nursing visits followed a similar pattern, with a mean reduction of 64.67 visits at 6 months (t = 5.052, p = 0.0047) and 57.67 visits at 12 months (t = 4.5054, p = 0.0081).
Hospital pharmacy visits also declined, with a mean reduction of 43.67 visits at 6 months (t = 4.017, p = 0.0124) and 35.67 visits at 12 months (t = 3.281, p = 0.0295).
These results confirmed significant improvements across all measured areas within the first year of lanadelumab treatment (
Figure 3).
3.2.4. Emergency Department Visits
Two of the patients utilized private insurance for their emergency visits exclusively, so data for these visits were not available in the hospital's EMR. For the remaining 2 patients, the average number of emergency visits in the year before switching to lanadelumab was 7 episodes, or 0.30 visits per patient per month. After initiating lanadelumab, none of the patients required emergency department visits. However, due to the limited data, statistical significance could not be demonstrated.
3.2.5. WhatsApp consultations
Most of the patients included in the study (75%) utilized the WhatsApp communication system. In the year prior to switching to lanadelumab, the mean number of WhatsApp messages for these patients was 6.6, averaging 0.55 messages per month per patient. After starting lanadelumab, this average decreased to 0.45 messages per month per patient. However, this trend did not reach statistical significance.
3.3. Number of Angioedema Episodes and use of ODT with Icatibant
As noted, all 4 patients had immediate access to icatibant for ODT. In the year prior to switching to lanadelumab, the average use of icatibant was 1.3 doses per patient per month, totaling 67 icatibant units. After transitioning to lanadelumab, this decreased to 0.2 doses per patient per month, totaling 26 units, representing a statistically significant (p<0.05) reduction of 61% in the use of ODT icatibant (
Table 2).
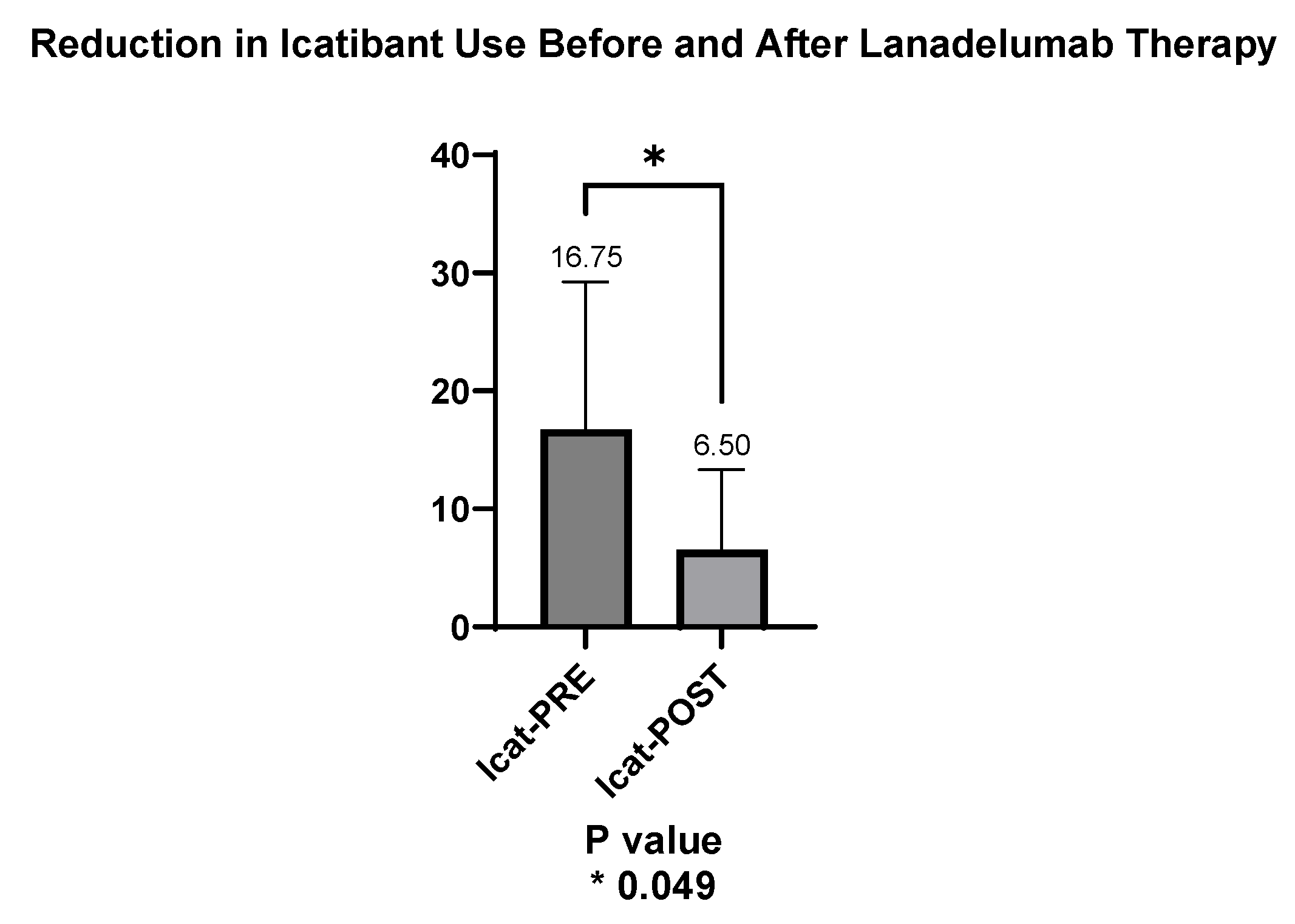
The mean number of icatibant units used per patient in the year prior to starting lanadelumab was 16.75, whereas in the months following the switch, the mean decreased to 6.50 units. This reduction in icatibant use was statistically significant (p = 0.049), as shown in
Figure 4.
All patients reported significant reductions in the frequency of acute attacks. Prior to switching to lanadelumab, the four patients, who had been on long-term prophylaxis with plasma-derived C1-esterase inhibitor (PdC1-INH) for three individuals and berotralstat plus danazol for the fourth, experienced a mean of 21.25 episodes of angioedema in the year before the transition (standard deviation 19.84), averaging 1.77 episodes per month per patient. In contrast, following the switch to lanadelumab, the mean number of episodes decreased significantly to 3.0 in the year after the switch (standard deviation 2.58), resulting in an average of only 0.12 episodes of angioedema per month per patient (
Figure 5).
There appears to be a trend toward improvement in the use of lanadelumab with longer follow-up times, as HAE attacks are concentrated in the year following the initiation of LTP. Currently, all four patients receiving lanadelumab are attack-free, averaging 17 months without further angioedema episodes, with individual durations ranging from 5 to 33 months. Notably, all angioedema episodes that occurred after starting lanadelumab were abdominal in nature and were managed at home with icatibant, thereby eliminating the need for emergency visits. Furthermore, only one patient experienced a life-threatening episode of laryngeal edema in the year prior to starting lanadelumab, and no severe episodes have occurred since (
Table 1)
3.4. Improved Disease Control as Measured by the HAE-QoL (Hereditary Angioedema Quality of Life) Questionnaire
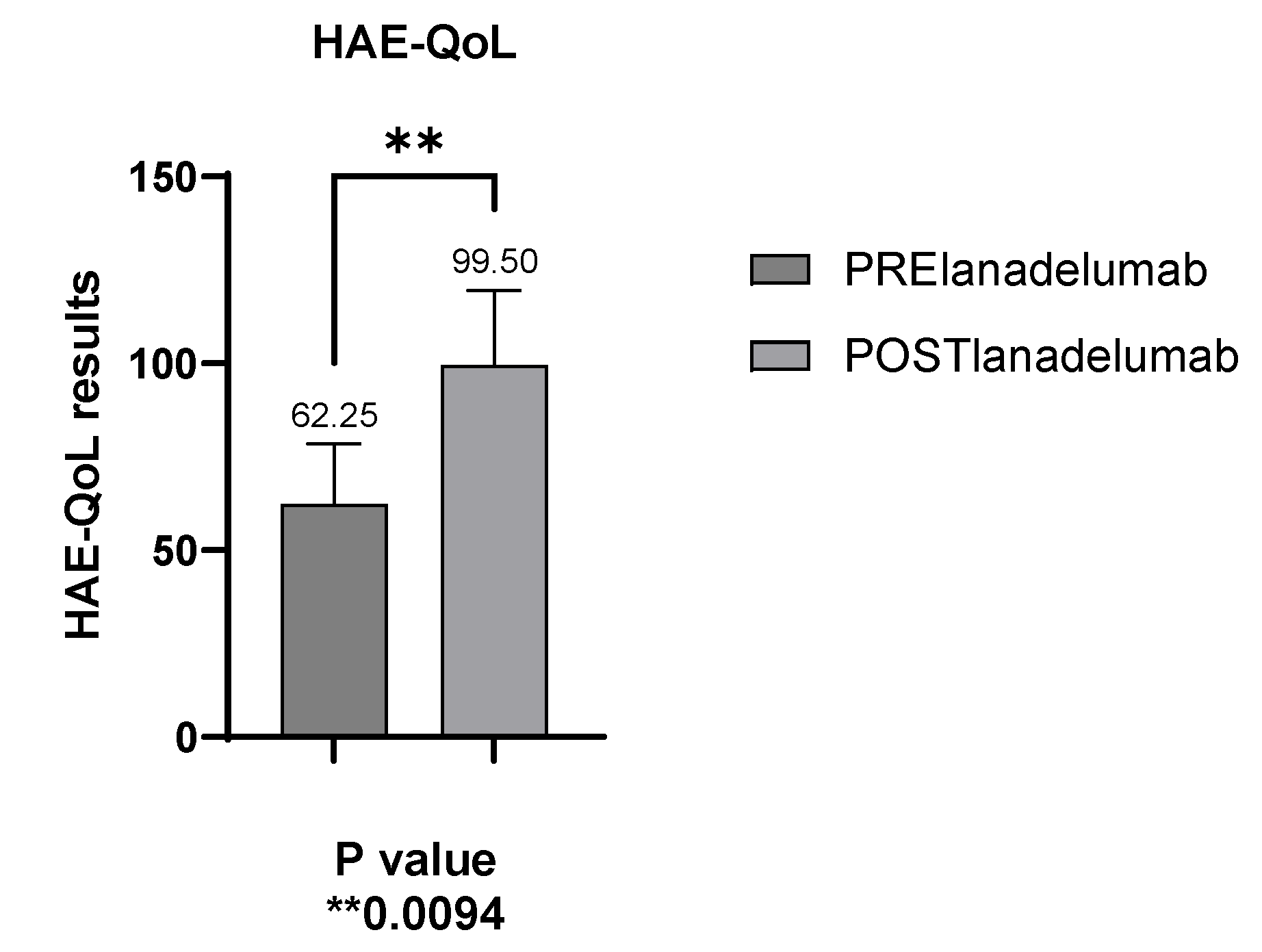
The validated HAE-QoL questionnaire evaluates QoL in hereditary angioedema (HAE) patients, with scores ranging from 25 to 135; higher scores indicate better quality of life. The average score before starting lanadelumab was 62.2 (SD: 16.15), with a range of 44 to 81. After the switch to lanadelumab, the mean score was significantly increased to 99.50 (SD: 20.07), ranging from 73 to 121 (
Figure 6).
3.5. Adverse Reactions Related to the Use of Lanadelumab
None of the patients (0%) reported adverse reactions to lanadelumab throughout the study period. They unanimously found it easier to administer compared to icatibant, which they described as “more painful”. Furthermore, one patient with a fear of needles depended on her partner to administer both lanadelumab and icatibant.
3.6. Use of Short-Term Prophylaxis after Initiation of Lanadelumab
None of 4 the patients (0%) required STP to prevent potential angioedema attacks during concomitant medical procedures. Collectively, they underwent two cycles of chemotherapy for non-Hodgkin lymphoma, one colonoscopy with polyp removal, and three dental procedures, which included fillings and cleanings.
4. Discussion
Real-world data on lanadelumab treatment are scarce, primarily emphasizing efficacy, safety, and QoL in existing studies [
8,
9,
10,
11,
12,
13,
14]. Our research stands out as the first retrospective analysis of HAE patients receiving lanadelumab in real-world settings, with a specific focus on healthcare utilization. Furthermore, it represents the first Spanish series to present real-world data on this treatment.
The number of healthcare visits for HAE patients varies based on disease burden, personal circumstances, and individual needs. Even patients with similar clinical profiles can demonstrate differing requirements for healthcare utilization and access. In the context of rare diseases, the limited availability of published real-world data creates a knowledge gap for both healthcare providers and managers.
Analyzing the cost-effectiveness of lanadelumab therapy, including its administration frequency and potential reductions in healthcare utilization, is essential for understanding its overall financial impact on both patients and healthcare systems [
15]. Additionally, understanding the balance between drug costs and the economic benefits derived from fewer hospital visits and improved quality of life is essential for making informed treatment decisions. Furthermore, the elevated cost of lanadelumab in Spain [
16] for patients on LTP raises the need to evaluate whether this expense is balanced by a reduction in hospital visits, use of specific rescue medication, fewer angioedema episodes, and ultimately an improvement in their overall QoL.
Our experience demonstrated a substantial reduction in hospital visits following the initiation of LTP with lanadelumab, including decreases in both medical and nursing visits to the Allergy Department, as well as Hospital Pharmacy visits. While the reduction in Emergency Room visits did not reach statistical significance, there were trends indicating improvement. The four patients in the study made a total of 515 hospital visits (343 to the allergy department and 172 to the hospital pharmacy) during the 48 months prior to starting lanadelumab LTP. In contrast, they accumulated only 242 visits (135 to the allergy department and 107 to the pharmacy) over 91 months of follow-up after beginning treatment. On average, hospital visits per patient dropped from 10.2 visits per month to 2.6 visits per month, marking a significant 75.27% reduction. Additionally, although the decrease in WhatsApp consultations was not statistically significant, the introduction of telephone consultations, which rose to 4%, suggests increased patient autonomy and reduced hospital dependency.
Despite several limitations should be addressed -including the retrospective nature of the data and, the relatively small sample size of 4 difficult-to-treat HAE patients- our study provides valuable insights into real-world management of complex cases. These patients were highly symptomatic and required long-term intravenous prophylaxis prior to switching to lanadelumab. Their close proximity to the hospital may have contributed to a higher frequency of hospital visits, but the significant reductions in healthcare utilization following lanadelumab therapy suggest a meaningful clinical benefit. While the small sample size warrants cautious interpretation, the statistically significant results point to potential improvements in both patient outcomes and healthcare resource utilization. Treatment with lanadelumab has significantly improved the quality of life for HAE patients, consistent with findings from other studies [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17] including both the randomized HELP study and its open-label extension (OLE) [
18,
19]. Moreover, it has shown superior efficacy in preventing attacks compared to other long-term prophylaxis options, including pdC1-INH IV [
20], danazol [
21], and berotralstat [
22]. In addition, the convenience of SC administration, whether monthly or bi-weekly, may further enhance patient autonomy and therapy adherence. Remarkably, in our cohort, the number of HAE attacks dropped by 94.1%, from an average of 1.7 episodes per month per patient before starting lanadelumab to 0.1 episodes during follow-up. Similarly, the need for on-demand treatment (ODT) decreased by 84.6%, from 1.3 doses per patient per month before lanadelumab to 0.2 doses afterward. As of the latest follow-up, the 4 monitored patients (100%) have been attack-free for an average of 17 months, ranging from 5 to 33 months, and have undergone various medical procedures without requiring STP.
5. Conclusions
The present investigation demonstrates that lanadelumab, as LTP, significantly reduces healthcare resource utilization and the frequency of angioedema episodes in selected difficult-to-treat HAE patients. Future studies with larger cohorts and longer follow-up are necessary to confirm these results and evaluate the long-term cost-effectiveness of lanadelumab in managing HAE. Nonetheless, our experience suggests that lanadelumab is a valuable and effective option for difficult-to-treat HAE patients, offering clinical benefits alongside potential reductions in healthcare costs. These findings highlight the potential of lanadelumab in managing complex HAE cases and emphasize the need for further research to validate these trends. Sharing clinical experiences remains essential, particularly in rare diseases like HAE, where real-world data on expensive treatments in public healthcare systems is limited.
Author Contributions
Conceptualization, I.S.-M. and R.G.-P.; methodology, I.S.-M. and R.G.-P.; soft- ware, E.M.-L.; validation and formal analysis, P.P.-G., E.M.-L. and I.S.-M.; investigation, I.S.-M., R.G.-P., P.P.-G., S.G.-G and E.M.-L.; resources, I.S.-M. and R.G.-P.; data curation, E.M.-L., P.P.-G.; writing—original draft preparation, I.S.-M. and R.G.-P.; writing—review and editing, I.S.-M., R.G.-P., P.P.-G., and E.M.-L.; project administration R.G.-P., P.P.-G. and I.S.-M.; funding acquisition R.G.-P., P.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of CEIC Hospital Universitario de Canarias, Tenerife, Spain with the reference number CHU-2024-94 on 27 September 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from Servicio Canario de Salud, however, restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with the permission of Servicio Canario de Salud.
Acknowledgments
The authors would like to express their sincere gratitude to the patients and their families for their participation in this study. We also extend our thanks to our colleagues for their invaluable contributions and support in the completion of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aygören-Pürsün, E., Magerl, M., Maetzel, A., & Maurer, M. Epidemiology of Bradykinin-mediated angioedema: a systematic investigation of epidemiological studies. Orphanet journal of rare diseases. 2018; 13, 1-9.
- Zuraw, B. L., & Christiansen, S. C. HAE pathophysiology and underlying mechanisms. Clinical reviews in allergy & immunology. 2016; 51, 216-229. [CrossRef]
- Rosen FS, Pensky J, Donaldson V, Charache P. Hereditary angioneurotic edema: two genetic variants. Science. 1965; 148(3672): 957-958. [CrossRef]
- Caballero, T. (2021). Treatment of Hereditary Angioedema. Journal of Investigational Allergology & Clinical Immunology. 2021; 31(1), 1-16. [CrossRef]
- De Maat, S., Hofman, Z. L. M., & Maas, C. (2018). Hereditary angioedema: the plasma contact system out of control: reply. Journal of Thrombosis and Haemostasis, 2018, 16(11), 2349-2351. [CrossRef]
- Available online: https://www.aemps.gob.es/informa/informes-de-posicionamiento-terapeutico/informe-de-posicionamiento-terapeutico-de-lanadelumab-takhzyro-en-prevencion-rutinaria-de-las-crisis-recurrentes-de-angioedema-hereditario-aeh-en-pacientes-a-partir-de-los-12-anos-de-edad/. Last accessed, May 2, 2024.
- Available online: https://es.statista.com/estadisticas/474029/poblacion-de-canarias-por-isla/#:~:text=Esta%20estad%C3%ADstica%20muestra%20la%20poblaci%C3%B3n,unas%20869.000%20personas%20en%202024. Last accessed June, 2, 2024.
- Buttgereit, T., Vera Ayala, C., Aykanat, S., Weller, K., Gutsche, A., Maurer, M., & Magerl, M. The real life experience goes on: update after 4 years on the first cohort treated with lanadelumab at our center. Frontiers in Immunology, 2024; 15, 1405317. [CrossRef]
- Martinez-Saguer I, Knop J, Flemming A, Thomann M, Maurer M. Real World treatment patterns of hereditary angioedema with lanadelumab in Germany: A prescription data analysis. J Dtsch Dermatol Ges. 2022; 20:1127–9. [CrossRef]
- Abuzakouk M, Ghorab O, Al-Hameli H, Salvo F, Grandon D, Maurer M. Using an extended treatment regimen of lanadelumab in the prophylaxis of hereditary angioedema: a single-centre experience. World Allergy Organ J. 2022; 15:100664. [CrossRef]
- Dorr AD, Chopra C, Coulter TI, Dempster J, Dziadzio M, El-Shanawany T, et al.. Lanadelumab for the prevention of hereditary angioedema attacks: A real-world UK audit Allergy. 2023; 78:1369–71. [CrossRef]
- Magerl M BL, Martinez Saguer I, Gavini F, Bent-Ennakhil N, Sayegh L, Andresen I. Real-world effectiveness of lanadelumab in european patients with HAE type I/II: results from the retrospective INTEGRATED study. European academy of allergy & Clinical immunology (EAACI) hybrid congress 2023 | 9–11 june 2023 | Hamburg, Germany.
- Hahn J, Trainotti S, Wigand M, Schuler P, Hoffmann T, Greve J. Prospective analysis in patients with HAE under prophylaxis with lanadelumab a real-life experience. J Drugs Dermatol. 2020;19 (10): 978–983. [CrossRef]
- Bernardino, A. G., Ferreira, M. B., Costa, C., Caiado, J., Pedro, E., & Santos, A. S. Experience of lanadelumab administration in hereditary angioedema: A case series of 4 patients in Portugal. Asia Pacific Allergy, 2023;13(2), 91-94. [CrossRef]
- Shah CH, Princic N, Evans KA, Schultz BG. Real-world changes in costs over time among patients in the United States with hereditary angioedema on long-term prophylaxis with lanadelumab. J Med Econ. 2023 Jan-Dec;26(1):871-877.
- Available online: https://www.sanidad.gob.es/areas/farmacia/precios/comisionInteministerial/acuerdosNotasInformativas/docs/ACUERDOS_CIPM_208_de_17_de_DICIEMBRE_web.pdf Last accessed 0ctober, 5, 2024.
- Iaboni, A., Kanani, A., Lacuesta, G., Song, C., Kan, M., & Betschel, S. D. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy, Asthma & Clinical Immunology, 2021;17, 1-7. [CrossRef]
- Banerji A, Riedl M, Bernstein J, Cicardi M, Longhurst H, Zuraw B, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018; 320:2108–21. [CrossRef]
- Johnston D, Banerji A, Nurse C, Lu P, Aygören-Pürsün E, Kiani-Alikhan S, Soteres D, et al. Long-term safety of lanadelumab in hereditary angioedema (HAE): interim results from the HELP OLE Study. Ann Allergy Asthma Immunol. 2019;123:S30. [CrossRef]
- Magerl, M. Magerl, M., Schiffhorst, G., Fanter, L., Müller, G., Hirche, C., Berkemeier, F., & Aygören, E. (2024). Patient-level indirect treatment comparison of lanadelumab versus pdC1-INH iv in hereditary angioedema patients: PATCH study. Allergy, 2024;79(1), 215-224. [CrossRef]
- Zozaya, N., Caballero, T., González-Quevedo, T., Setien, P. G., González, M. Á., Jódar, R., & Hidalgo-Vega, Á. A multicriteria decision analysis (MCDA) applied to three long-term prophylactic treatments for hereditary angioedema in Spain. Global & Regional Health Technology Assessment, 2022;9, 14. [CrossRef]
- Watt, M., Malmenäs, M., Romanus, D., & Haeussler, K. Network meta-analysis for indirect comparison of lanadelumab and berotralstat for the treatment of hereditary angioedema. Journal of Comparative Effectiveness Research, 2023;12(6), e220188. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).