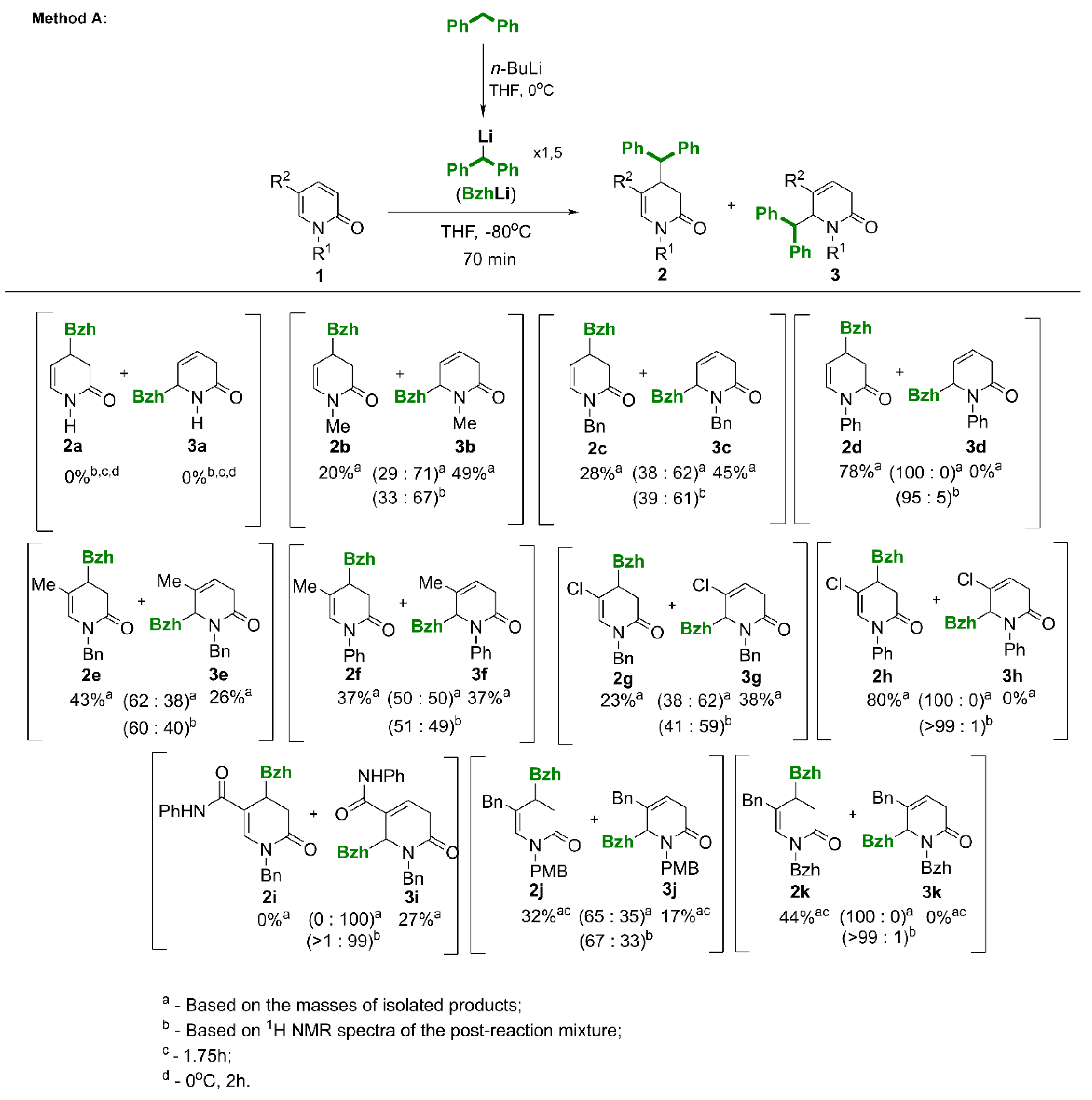
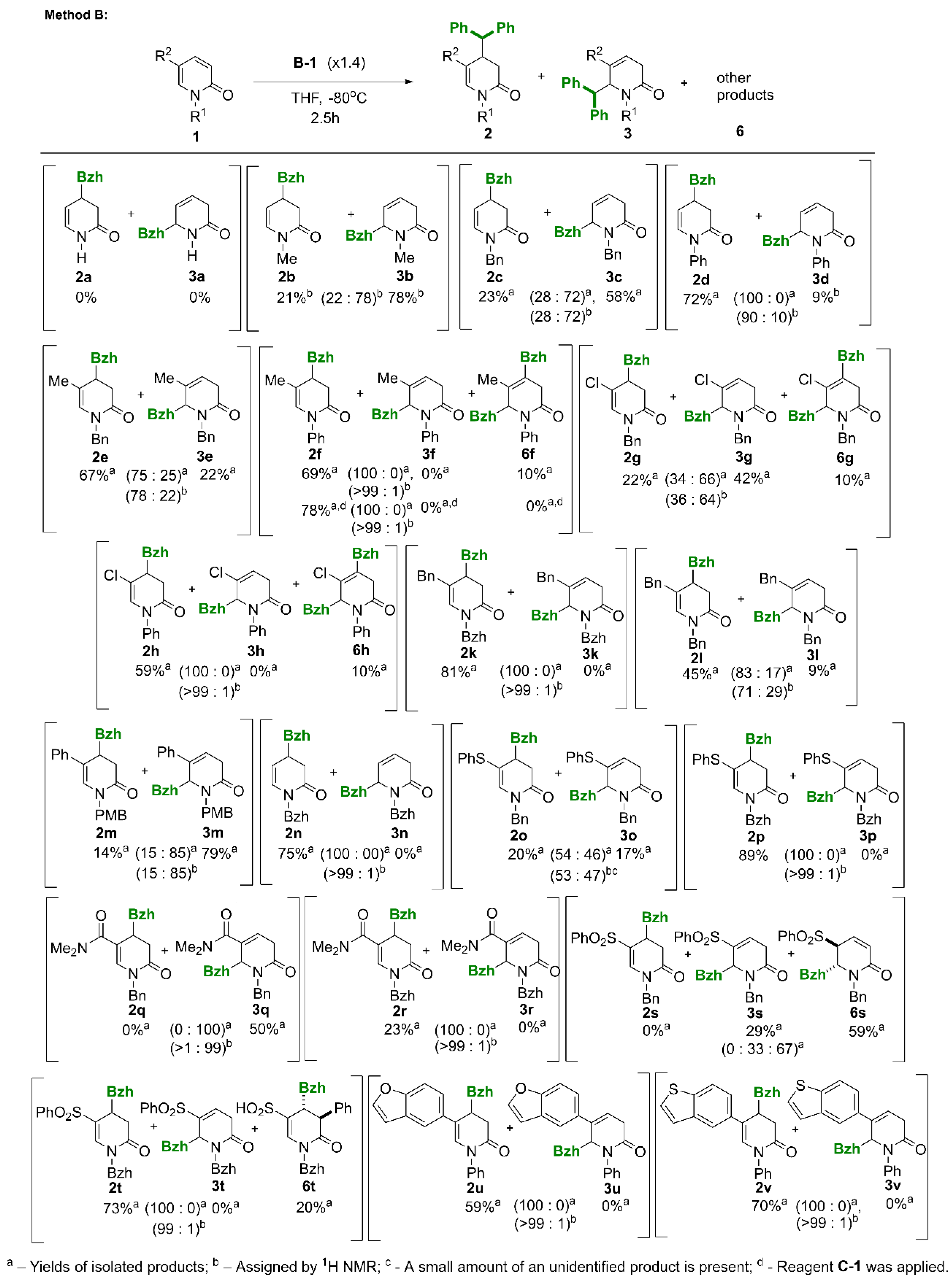
3.2. Synthesis of 4-Benzhydryl-3,4-dihydropyridin-2(1H)-ones (2) / 6-Benzhydryl-3,6-dihydropyridin-2(1H)-ones (3) and by-Products 6
Method A (addition of BzhLi to 2-pyridones).
A 25 mL Schlenk flask was charged with 16 mL of anhydrous THF, placed in an ice bath at 0°C, 0.815g of diphenylmethane (1.5 equiv, 4.858 mmol) was added, and subsequently 2.04 mL of n-BuLi (2.5 M in hexanes, 1.575 equiv, 5.102 mmol) was carefully added dropwise with a syringe and stirred for 25 min at 0°C. The orange-red solution was then transferred with a syringe to a second 50 mL flask placed in a -80°C bath in which 2-pyridone (3.239 mmol) had been previously dissolved in 16 mL of anhydrous tetrahydrofuran. The reaction was carried out for 70 min at -80°C, after which saturated ammonium chloride solution (ca 5 mL) was added. The solution was warmed to rt, extracted with ethyl acetate (3 x 80 mL), and the organic layer was dried over anhydrous magnesium sulfate, concentrated, and purified by column chromatography using silica gel as the stationary phase.
Method B (addition of magnesiate B-1 to 2-pyridones).
A 50 mL Schlenk flask was charged with 10.7 mL of anhydrous THF under argon, placed in an ice bath, and then 0.8g of diphenylmethane (2.8 equiv, 4.756 mmol) and 2 mL of n-BuLi (2.94 equiv, 4.993 mmol, 2.5M solution in hexanes) were added dropwise with a syringe and stirred for 25 min maintaining the temperature at 0°C. Then 0.8 mL of MeMgCl (1.4 equiv, 3.0 M solution in THF) was added and stirred at 0°C for a further 25 min (a slight color change to carmine red was observed). The solution was then transferred with a syringe to a 100 mL Schlenk flask placed in a -80°C bath, in which 1.698 mmol of 2-pyridone was dissolved in 36 mL of anhydrous tetrahydrofuran. The reactions were carried out for 2.5-3 h. Further procedure as in method A.
(Note: In the syntheses of the compounds listed below, proportional amounts of reagents and solvents were used in relation to the amount of starting 2-pyridone.)
(4RS)-4-Benzhydry-1-methyl-3,4-dihydropyridin-2(1H)-one (2b).
Yield 20% (method A, 0.127g, from 0.25g of 1b); yield 21% (method B. Yield determined by internal standard method using 1H NMR). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 3:1) gave a beige solid, m.p. 103−105 °C. 1H NMR (400 MHz, CDCl3): δ 2.28 (dd, J = 16.2, 10.0 Hz, 1H, CHH-3), 2.46 (dd, J = 16.2, 6.4 Hz, 1H, CHH-3), 3.03 (s, 3H, NCH3), 3.37 (ddddd, J = 11.0, 10.0, 6.4, 3.5, 2.0 Hz, 1H, CH-4), 3.72 (d, J = 11.2 Hz, 1H, 4-CH), 4.93 (ddd, J = 7.8, 3.5, 0.8 Hz, 1H, =CH-5), 5.95 (dd, J = 7.8, 1.8 Hz, 1H, =CH-6), 7.11 – 7.34 (m, 10H, 2 x C6H5). 13C{H} NMR (100.6 MHz, CDCl3): δ 33.47 (NCH3), 36.07 (CH-4), 36.42 (CH2-3), 56.10 (4-CH), 109.29 (=CH-5), 126.59, 126.70, 127.85 (2C), 128.21 (2C), 128.65 (2C), 128.79 (2C), ArH, 130.50 (=CH-6), 142.35, 142.52 (Ar), 169.16 (C=O). GC−MS (EI, 70 eV) m/z: 277 (<1) [M+•], 167 (41), 165 (30), 152 (13), 110 (100). HRMS (ESI-TOF): m/z Calcd for C19H20NO[M + H]+, 278.1545; Found 278.1539.
(6RS)-6-Benzhydryl-1-methyl-3,6-dihydropyridin-2(1H)-one (3b).
Yield 49% (method A, 0.31g from 0.25g of 1b); yield 78% (method B. Yield determined by internal standard method using 1H NMR). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 3:1) gave a colorless oil. 1H NMR (400 MHz, CDCl3) δ 2.08 (dq, J = 22.0, 3.4 Hz, 1H, CHH-3), 2.68 (ddd, J = 22.0, 4.9, 2.2 Hz, 1H, CHH-3), 2.93 (s, 3H, NCH3), 4.41 (d, J = 5.5 Hz, 1H, 6-CH), 4.63 – 4.72 (m, 1H, CH-6), 5.73 (dddd, J = 10.1, 5.0, 2.2, 0.7 Hz, 1H, =CH-5), 5.89 (ddd, J = 10.1, 4.6, 3.1 Hz, 1H, =CH-6), 7.19 – 7.35 (m, 10H, 2 x Ph). 13C{H} NMR (100.6 MHz, CDCl3): δ 32.17 (CH2-3), 33.98, NCH3, 54.49 (6-CH), 64.68 (CH-6), 124.13 (=CH-4), 125.35 (=CH-5), 126.87, 127.21, 128.23, 128.53, 128.60, 129.74, 138.82, 140.22 (2 x Ph), 168.82 (C=O). GC−MS (EI, 70 eV) m/z: 277 (<1) [M+•], 202 (100), 167 (17), 165 (16), 110 (100). HRMS (ESI-TOF): m/z Calcd for C19H20NO[M + H]+, 278.1545; Found, 278.1539.
(4RS)-4-Benzhydryl-1-benzyl-3,4-dihydropyridin-2(1H)-one (2c).
Yield 28% (method A, 0.114g from 0.234g of 1c); yield 23% (method B, 0.138g from 0.315 g of 1c). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 3:1) gave a white solid, m.p. = 105-114°C. 1H NMR (400 MHz, CDCl3): δ 2.35 (dd, J = 16.1, 9.7 Hz, 1H, CHH-3), 2.53 (dd, J = 16.1, 6.2 Hz, 1H, CHH-3), 3.26 – 3.45 (m, 1H, CH-4), 3.69 (d, J = 11.3 Hz, 1H, 4-CH), 4.62 – 4.72 (m, 2H, NCH2), 4.93 (dd, J = 7.9, 3.6 Hz, 1H, =CH-5), 5.97 (dd, J = 7.9, 1.7 Hz, 1H, =CH-6), 7.09 – 7.41 (m, 15H, ArH). 13C{H} NMR (101 MHz, CDCl3): δ 36.02 (CH-4), 36.62 (CH2-3), 48.79 (NCH2), 55.99 (4-CH2),109.99 (=CH-5), 126.56, 126.72, 127.57, 127.77, 127.85, 128.15, 128.62, 128.69, 128.81 (ArH), 129.03 (=CH-6), 137.22, 142.27, 142.51 (Ar), 168.84 (C=O). GC−MS (EI, 70 eV) m/z: 353 (>1) [M+•], 186 (100) [M-167(benzhydryl radical)], 165 (15), 91 (97). HRMS (ESI-TOF): m/z Calcd for C25H24NO[M + H]+, 354.1858; Found 354.1852.
(6RS)-6-Benzhydryl-1-benzyl-3,6-dihydropyridyn-2(1H)-one (3c).
Yield 45% (method A, 0.2g); yield 58% (0.346g, method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 3:1) gave a white solid, m.p. = 124-125°C. 1H NMR (400 MHz, CDCl3): δ 2.23 (dq, J = 21.2, 2.8 Hz, 1H, CHH-3), 2.82 (ddd, J = 21.1, 5.1, 1.8 Hz, 1H,CHH-3), 3.53 (d, J = 15.3 Hz, 1H, NCHH), 4.41 (d, J = 6.1 Hz, 1H, 6-CH), 4.52-4.63 (m, 1H, CH-6), 5.54 (d, J = 15.3 Hz, 1H,NCHH), 5.77 (ddd, J = 10.0, 5.1, 2.0 Hz, 1H, =CH-4), 5.85 (ddd, J = 10.0, 4.8, 3.1 Hz, 1H, =CH-5), 7.09-7.18 (m, 2H, ArH), 7.19 – 7.37 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3): δ 32.63 (CH2-2), 47.12 (NCH2), 54.54 (6-CH), 60.47 (CH-6), 125.13 (=CH-5), 125.29 (=CH-4), 126.93, 127.19, 127.41, 127.81, 128.30, 128.61, 128.69. 129.70 (ArH), 136.89, 139.10, 140.27 (Ar), 169.25 (C=O). GC−MS (EI, 70 eV) m/z: 353 (<1) [M+•], 186 (76) [M+•-167(benzhydryl radical)], 165 (20), 91 (100). HRMS (ESI-TOF): m/z Calcd for C25H24NO[M + H]+, 354.1858; Found 354.1852.
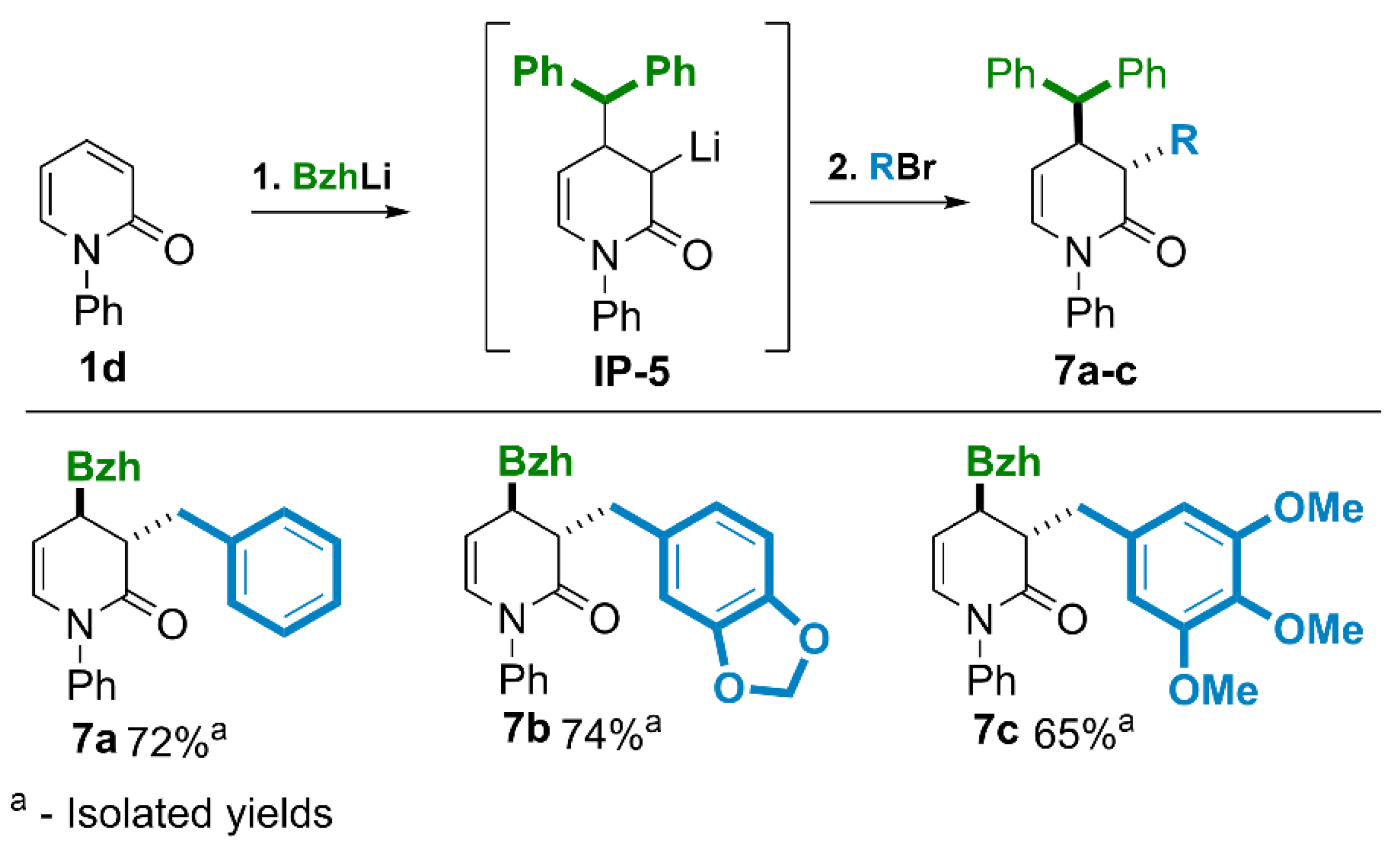
(4RS)-4-Benzhydryl-1-phenyl-3,4-dihydropyridin-2(1H)-one (2d).
Yield 78% (3.088g, method A, from 2 g of 1d); yield 72% (0.339g, method B, from 0.29 g of 1d). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave a pink solid, m.p. = 100-106°C. 1H NMR (400 MHz, CDCl3): δ 2.49 (dd, J = 15.9, 9.9 Hz, 1H, CHH-3), 2.65 (ddd, J = 15.9, 6.1, 1.0 Hz, 1H, CHH-3), 3.41 – 3.59 (m, 1H, CH-4), 3.81 (d, J = 11.1 Hz, 1H, 4-CH), 5.09 (ddd, J = 7.9, 3.6, 1.0 Hz, 1H, =CH-5), 6.23 (dd, J = 7.9, 1.8 Hz, 1H, =CH-6), 7.03 – 7.45 (m, 15H, ArH). 13C{H} NMR (101 MHz, CDCl3): δ 36.00 (CH-4), 37.49 (CH2-3), 56.21 (4-CH), 110.14 (=CH-5), 125.91 (2C), 126.67, 126.77, 127.01, 127.89 (2C), 128.24 (2C), 128.72 (2C), 128.83 (2C), 129.06 (2C), 130.51 (=CH-6), (ArH) 140.29, 142.20, 142.43 (Ar), 168.54 (C=O). GC−MS (EI, 70 eV) m/z: 339 (<1), [M+•], 172 (100) [M+•-167(benzhydryl radical)], 144 (8), 77 (10). HRMS (ESI-TOF): m/z Calcd for C24H22NO[M + H]+, 340.1701; Found 340.1696.
(4RS)-4-Benzhydryl-1-benzyl-5-methyl-3,4-dihydropyridin-2(1H)-one (2e).
Yield 43% (0.198g, method A, from z 0.25g of 1e); yield 67% (0.246g, method B, from 0.2g of 1e); The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave a white solid, m.p. = 132-134°C. 1H NMR (400 MHz, CDCl3): δ 1.04 (d, J = 1.5 Hz, 3H, 5-CH3), 2.44 (dd, J = 16.0, 1.8 Hz, 1H, CHH-3), 2.61 (dd, J = 16.0, 6.3 Hz, 1H, CHH-3), 2.88 (ddd, J = 11.2, 6.3, 1.8 Hz, 1H, CH-4), 3.69 (d, J = 11.2 Hz, 1H, 4-CH), 4.33 (d, J = 14.6 Hz, 1H, NCHH), 5.00 (d, J = 14.6 Hz, 1H, NCHH), 5.79 (d, J = 1.5 Hz, 1H, =CH-6), 6.76 – 7.01 (m, 2H, ArH), 7.04 – 7.49 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 19.88 (5-CH3), 35.81 (CH2-3), 41.36 (CH-4), 48.65 (NCH2), 53.13 (4-CH), 120.52 (=C-5), 124.57 (=CH-6), 126.35, 126.69, 127.71, 128.11 (2C), 128.22 (2C), 128.29 (2C), 128.37 (2C), 128.71 (2C), 128.75 (2C), ArH), 137.74, 141.61, 143.05 (Ar), 167.83 (C=O). GC−MS (EI, 70 eV) m/z: 367 (<1), [M+•], 200 (68) [M+•-167(benzhydryl radical)], 165 (14), 158 (12), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H25NO 368.2014; Found 368.2009.
(6RS)-6-Benzhydryl-1-benzyl-5-methyl-3,6-dihydropyridin-2(1H)-one (3e).
Yield 26% (0.12g, method A, from z 0.25g of 1e); yield 22% (0.08g, method B, from 0.2g of 1e); The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave yellow oil. 1H NMR (400 MHz, CDCl3): δ 1.47 (p, J = 1.2 Hz, 3H, 5-CH3), 2.04 (dq, J = 20.7, 2.6 Hz, 1H, CHH-3), 2.70 (ddt, J = 20.7, 5.9, 1.0 Hz, 1H, CHH-3), 3.30 (d, J = 15.4 Hz, 1H, NCHH), 4.22 – 4.44 (m, 2H, CH-6, 6-CH), 5.33 – 5.53 (m, 2H, NCHH, =CH-4), 6.97 – 7.04 (m, 2H, ArH), 7.21 – 7.38 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 21.45 (5-CH3), 32.86 (CH2-3), 47.80 (NCH2), 54.46 (6-CH), 65.66 (CH-6), 120.89 (=CH-4), 127.11, 127.26 (2C), 127.54 (2C), 128.42 (2C), 128.45 (2C), 128.60 (2C), 129.09 (2C), 129.75 (2C), (ArH), 134.23 (=C-5), 137.07, 138.51, 139.57 (Ar), 170.27 (C=O). GC-MS: m/z = 367 (<1), [M+•], 200 (69) [M+•-167(benzhydryl radical)], 165 (18), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H25NO 368.2014; Found 368.2009.
(4RS)-4-Benzhydryl-5-methyl-1-phenyl-3,4-dihydropyridin-2(1H)-one (2f).
Yield 37% (0.42g, method A, from z 0.6g of 1f); yield 69% (method B, from 0.3146g of 1e), yield 78% (magnesiate C-1 was used). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave a white solid, m.p. = 154-155°C. 1H NMR (400 MHz, CDCl3): δ 1.23 (d, J = 1.3 Hz, 3 H, 5-CH3), 2.59 (dd, J = 15.9, 1.6Hz, 1 H, CHH-3), 2.81 (dd, J = 15.9, 6.5 Hz, 1 H, CHH-3), 3.04 (ddd, 1 H, J = 10.4, 6.5, 1.6 Hz, CH-4), 4.01 (d, J = 10.4 Hz, 1 H, 4-CH), 6.06 (q, J = 1.3 Hz, 1 H, =CH-6), 7.16 – 7.22 (m, 2 H, ArH), 7.24 – 7.33 (m, 11 H, ArH), 7.38 – 7.43 (m, 2 H, ArH). 13C{H} NMR (100 MHz, CDCl3): δ 19.84 (5-CH3), 36.59 (CH2-3), 41.27 (CH-4), 53.55 (4-CH),120.31 (C-5), 125.65 (ArH), 126.34 (=CH-6), 126.58, 126.69, 126.80, 128.35, 128.42, 128.47, 128.71, 128.96 (ArH), 140.35, 141.38, 142.98 (Ar), 167.50 (C=O). GC-MS (EI, 70eV) m/z: 353 (<1), [M+•], 186 (100) [M+•-167(benzhydryl radical)], 158 (12), 143 (15), 77 (11). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H23NO 354.1858; Found 354.1852.
(6RS)-6-Benzhydryl-5-methyl-1-phenyl-3,6-dihydropyridin-2(1H)-one (3f).
Yield 37% (0.42g, method A, from z 0.6g of 1f); yield 0% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave yellow oil.1H NMR (400 MHz, CDCl3) δ 1.44 (dt, J = 2.6, 1.1 Hz, 3H, CH3), 2.38 (dq, J = 20.4, 2.6 Hz, 1H, CHH-3), 2.76 (ddt, J = 20.4, 5.8, 1.1 Hz, 1H, CHH-3), 4.35 (d, J = 5.9 Hz, 1H, 6-CH), 5.03 (dd, J = 5.9, 2.6 Hz, 1H, CH-6), 5.58 (dt, J = 5.8, 1.7 Hz, 1H, =CH-4), 7.10 – 7.37 (m, 15H, 3 x C6H5). 13C{H} NMR (101 MHz, CDCl3) δ 22.14 (CH3), 33.51 (CH2-3), 54.66 (6-CH), 71.33 (CH-6), 121.67 (=CH-4), 126.65 , 126.67, 127.32, 127.50 (2C), 128.26 (4C), 128.34 (2C), 128.95 (2C), 130.10 (2C), (ArH), 134.29 (=C-5), 137.92, 139.91, 141.79 (Ar), 168.96 (C=O). GC-MS (EI, 70eV) m/z: 353 (<1) [M+•], 186 (100) [M+•-167(benzhydryl radical)], 158 (13), 143 (14). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H23NO 354.1858; Found 354.1852.
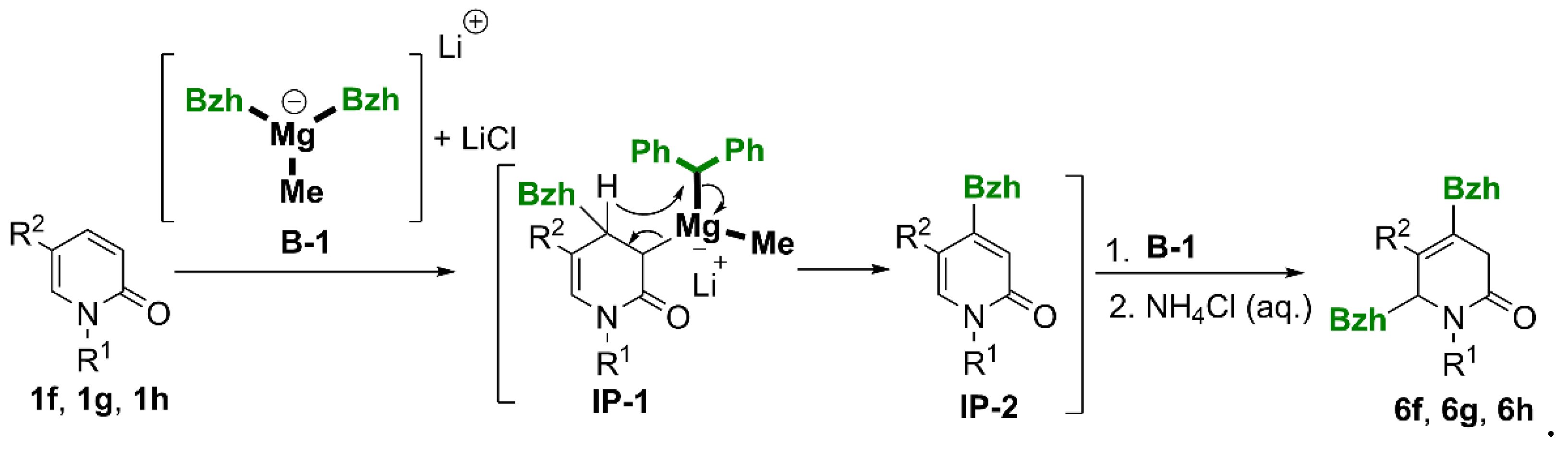
(6RS)-4,6-Dibenzhydryl-5-methyl-1-phenyl-3,6-dihydropyridin-2(1H)-one (6f).
Yield 0% (method A); yield 10% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave a white solid, m.p.= 128 – 129°C. 1H NMR (400 MHz, CDCl3) δ 1.47 (d, J = 2.4 Hz, 3H, CH3), 2.42 (dt, J = 19.9, 2.4 Hz, 1H, CHH-3), 2.92 (d, J = 19.9 Hz, 1H, CHH-3), 4.26 (d, J = 7.5 Hz, 1H, 6-CH), 5.08 (dd, J = 7.5, 1.7 Hz, 1H, CH-6), 5.27 (s, 1H, 4-CH), 6.95 – 7.00 (m, 2H, C6H5), 7.03 – 7.44 (m, 23H, 5 x C6H5). 13C{H} NMR (101 MHz, CDCl3) δ 18.52 (CH3), 36.07 (CH2-3), 51.76 (4-CH), 55.57 (6-CH), 72.38 (CH-6), 126.41, 126.53, 126.72, 126.82, 127.09, 127.23 (2C), 128.26 (2C), 128.28 (2C), 128.36 (2C), 128.53 (2C), 128.58 (2C), 128.60 (2C), 128.76 (2C), 129.51 (2C), 129.68 (2C), (ArH), 130.35 (C-5), 131.72, 138.81, 139.78, 140.70, 141.53, 141.87 (Ar, C-4), 169.40 (C=O). GC-MS (EI, 70eV) m/z = 519 (>1), [M+•], 351 (100) [M+•-1 -167 (benzhydryl radical)], 350 (68), 246 (45), 208 (32), 165 (23), 77(51). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H34NO: 520.2640; Found 520.2635.
(4RS)-4-Benzhydryl-1-benzyl-5-chloro-3,4-dihydropyridin-2(1H)-one (2g).
Yield 23% (0.08g, method A, from 0.2g of 1g); yield 22% (0.15g, method B, from 0.373g of 1g). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave a white solid, m.p. = 127-131°C. 1H NMR (400 MHz, CDCl3) δ 2.66 (dd, J = 16.5, 1.9 Hz, 1H, CHH-3), 2.88 (dd, J = 16.5, 7.5 Hz, 1H, CHH-3), 3.32 (td, J = 8.8, 7.5, 1.9 Hz, 1H, CH-4), 4.00 (d, J = 8.8 Hz, 1H, 4-CH), 4.40 (d, J = 14.7 Hz, 1H, NCHH), 4.57 (d, J = 14.7 Hz, 1H, NCHH), 6.14 (s, 1H, =CH-6), 6.96 – 7.10 (m, 2H, ArH), 7.12 – 7.45 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 35.32 (CH2-3), 43.28 (CH-4), 48.81 (NCH2), 52.31 (4-CH), 116.89 (=C-5), 126.62, 126.91, 127.09, 127.96, 128.19 (2C), 128.22 (2C), 128.49 (2C), 128.61 (2C), 128.72 (2C), 128.85 (2C), (ArH), 136.60, 140.41, 141.35 (Ar), 166.59 (C=O). GC-MS (EI 70eV) m/z: 387 (<1) [M+•], 222 (12), 220 (35) [M+•-167(benzhydryl radical)], 167 (49), 165 (23), 152 (12), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H22ClNO 388.1468; Found 388.1463.
(6RS)-6-Benzhydryl-1-benzyl-5-chloro-3,6-dihydropyridin-2(1H)-one (3g).
Yield 38% (0.133g, method A); yield 42% (0.274g, method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave a semi-solid. 1H NMR (400 MHz, CDCl3) δ 1.61 (dt, J = 21.1, 2.5 Hz, 1H, CHH-3), 2.63 (ddd, J = 21.1, 5.9, 1.0 Hz, 1H, CHH-3), 3.35 (d, J = 15.3 Hz, 1H, NCHH), 4.62 – 4.68 (m, 2H, CH-6, 6-CH), 5.52 (d, J = 15.3 Hz, 1H, NCHH), 5.68 (dd, J = 5.9, 2.5 Hz, 1H, =CH-4), 6.90 (dd, J = 7.3, 2.2 Hz, 2H, ArH), 7.21 – 7.41 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 32.80 (CH2-3), 47.71 (NCH2), 52.98 (6-CH), 66.58 (CH-6), 122.94 (=CH-4), 127.06, 127.51, 127.66 (2C), 127.79 (ArH), 127.91 (=C-5), 128.28 (2C), 128.37 (2C), 128.62 (2C), 128.70 (2C), 131.03 (2C), (ArH), 135.75, 136.20, 139.54 (Ar), 168.25 (C=O). GC-MS (EI 70eV) m/z: 387 (<1) [M+•], 222 (12), 220 (36) [M+•-167(benzhydryl radical)],167 (47), 165 (21), 152 (11), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H22ClNO 388.1468; Found 388.1463.
(6RS)-4,6-Dibenzhydryl-1-benzyl-5-chloro-3,6-dihydropyridin-2(1H)-one (6g).
Yield 0% (method A); yield 10% (0.095g, method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 5:1) gave a white solid, m.p. = 171-174°C. 1H NMR (400 MHz, CDCl3) δ 1.42 (dd, J = 20.5, 2.8 Hz, 1H, CHH-3), 2.66 (d, J = 20.5 Hz, 1H, CHH-3), 3.30 (d, J = 15.3 Hz, 1H, NCHH), 4.66 (d, J = 2.8 Hz, 1H, 6-CH), 4.73 (t, J = 2.8 Hz, 1H, CH-6), 5.48 (d, J = 15.3 Hz, 1H, NCHH), 5.51 (s, 1H, 4-CH), 6.84 – 6.95 (m, 4H, ArH), 7.01 (d, J = 7.5 Hz, 2H, ArH), 7.16 – 7.38 (m, 19H). 13C{H} NMR (101 MHz, CDCl3) δ 35.05 (CH2-5), 47.77 (NCH2), 52.22 (4-CH), 53.38 (6-CH), 67.14 (CH-6), 124.84 (=C-5), 126.82, 126.94, 127.02, 127.48 (2C), 127.55, 127.66, 128.18 (2C), 128.26 (2C), 128.42 (2C), 128.45 (2C), 128.63 (2C), 128.70 (4C), 129.43 (2C), 130.79 (2C), (ArH), 133.42, 136.03, 136.31, 139.67 (2C), 139.80 (Ar), 169.12 (C=O). GC-MS (EI, 70eV) m/z: 553 (<1), [M+•], 387 (14) [M+•-167 (benzhydryl radical) 385 (30), 384 (18), 165 (11), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H32ClNO 554.2251; Found 554.2245.
(4RS)-4-Benzhydryl-5-chloro-1-phenyl-3,4-dihydropyridin-2(1H)-one (2h).
Yield 80% (0.269g, method A, from 0.185g of 1h); yield 59% (0.377g, method B, from 0.349g of 1h). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 3:1) gave a white solid, m.p. = 117-119°C. 1H NMR (400 MHz, CDCl3) δ 2.88 (dd, J = 16.6, 1.6 Hz, 1 H, CHH-3), 3.10 (dd, J = 16.6, 8.2 Hz, 1 H, CHH-3), 3.52 (ddd, J = 8.2, 6.9, 1.6 Hz, 1 H, CH-4), 4.36 (d, J = 6.9 Hz, 1H), 6.38 (s, 1 H =CH-6), 6.90 – 7.01 (m, 2 H, C6H5), 7.20 – 7.37 (m, 13 H, ArH). 13C({H} NMR (101 MHz, CDCl3) δ 35.47 (CH2-3), 43.15 (CH-4), 51.88 (4-CH), 116.55 (=C-5), 125.82 (2C), 126.72, 127.26, 127.34, 128.43 (2C), 128.50 (2C), 128.54 (2C), (ArH), 128.82 (=CH-6), 129.02 (2C), 129.32 (2C), 139.43, 140.15, 141, 27 (Ar), 165.99 (C=O). GC-MS (EI 70eV) m/z: 373 (1) [M+•], 208 (46), 207 (26), 206 (100), 168 (15), 167 (83), 165 (31), 152 (18), 77 (13). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20ClNO 374.1312; Found 374.1306.
(6RS)-4,6-dibenzhydryl-5-chloro-1-phenyl-3,6-dihydropyridin-2(1H)-one (3h).
Yield 0% (method A); yield 10% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave yellow oil. 1H NMR (400 MHz, CDCl3) δ 1.97 (dd, J = 20.2, 2.5 Hz, 1H, CHH-3), 2.86 (d, J = 20.2 Hz, 1H, CHH-3), 4.52 (d, J = 4.5 Hz, 1H, 6-CH), 5.46 (dd, J = 4.5, 2.5 Hz, 1H, CH-6), 5.60 (s, 1H, 4-CH), 6.95 – 7.37 (m, 25H). 13C{H} NMR (101 MHz, CDCl3) δ 35.80 (CH2-3), 52.33 (4-CH), 54.24 (6-CH), 71.51 (CH-6), 124.86 (=C-5), 126.82, 127.00, 127.05 (2C), 127.14, 127.40 (2C), 128.16 (2C), 128.19 (2C), 128.31 (2C), 128.61 (2C), 128.67 (2C), 129.04 (2C), 129.21 (2C), 129.46 (2C), 129.66 (2C), (ArH), 133.88, 137.39, 138.23, 139.62, 139.70, 140.89 (Ar), 167.89 (C=O). GC-MS (EI 70eV) m/z: 539 (<1), [M+•], 374 (10), 373 (52), 372 (42), 371 (100), 370 (55), 336 (27), 308 (17), 306 (32), 307 (11), 266 (20), 130(20), 202 (25), 167 (15), 165 (45), 152 (22), 141 (10), 104 (23), 77 (64), 51 (17). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C37H30ClNO 540.2094; Found 540.2089.
(6RS)-2-Benzhydryl-1-benzyl-6-oxo-N-phenyl-1,2,5,6-tetrahydropyridine-3-carbox-amide (3i).
Yield 27% (0.0633g method A, using 0.154g of 1i). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 1:1) gave a white solid, m.p. = 206 – 210°C. 1H NMR (400 MHz, CDCl3) δ 2.30 (br s, 3H, NCH3), 2.67 (br s, 3H, NCH3), 2.86 (d, J = 15.4 Hz, 1H, NCHH), 2.96 (ddd, J = 20.7, 2.2, 1.6 Hz, 1H, CHH-3), 3.10 (dd, J = 20.7, 6.2 Hz, 1H, CHH-3), 4.23 (d, J = 9.0 Hz, 1H, 6-CH), 5.08 (d, J = 15.4 Hz, 1H, NCHH), 5.17 (dd, J = 9.0, 1.6 Hz, 1H, CH-6), 5.92 (dd, J = 6.2, 2.2 Hz, 1H, =CH-4), 7.01 – 7.10 (m, 2H, ArH), 7.17 – 7.38 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.03 (CH3-2), 34.90 (br, NCH3), 38.08 (br, NCH3), 49.33 (NCH2), 57.29 (6-CH), 62.57 (CH-6), 126.30 (=CH-4), 127.35, 127.38, 127.46, 127.75 (2C), 128.48 (2C), 128.53 (2C), 128.77 (2C), 128.81 (2C), 129.12 (2C), 135.93, 136.63, 139.59, 139.79 (Ar), 168.63 (C=O), 168.69 (C=O). GC-MS (EI 70eV) m/z: 472 (<1), [M+•], 305 (43) [M+•- benzhydryl radical (167)], 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H28N2O2 473.2229; Found 473.2224.
(4RS)-4-Benzhydryl-5-benzyl-1-(4-methoxybenzyl)-3,4-dihydropyridin-2(1H)-one (2j).
Yield 32% (0.159g method A, using 0.32g of 1i). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 6:1) gave a white solid, m.p. = 70 – 72°C. 1H NMR (400 MHz, CDCl3) δ 2.11 (dd, J = 15.5, 1.7 Hz, 1H, 5-CHH), 2.36 (dd, J = 15.9, 2.1 Hz, 1H, CHH-3), 2.43 (dd, J = 15.9, 5.7 Hz, 1H, CHH-3), 2.71 (d, J = 15.5 Hz, 1H, 5-CHH), 2.80 (ddd, J = 11.7, 5.7, 2.1 Hz, 1H, CH-4), 3.65 (d, J = 11.7 Hz, 1H, 4-CH), 4.15 (d, J = 14.4 Hz, 1H, NCHH), 3.85 (s, 3H, OCH3), 5.11 (d, J = 14.4 Hz, 1H, NCHH), 5.80 (d, J = 1.7 Hz, 1H, =CH-6), 6.85 – 7.03 (m, 6H, ArH), 7.12 – 7.30 (m, 11H, ArH), 7.30 – 7.40 (m, 2H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 36.34 (CH2-3), 38.52 (CH-4), 39.69 (5-CH2), 48.26 (NCH2), 53.20 (4-CH), 55.40 (OCH3), 114.11 (2C, ArH), 124.54 (=C-5), 126.16 (=CH-6), 126.32, 126.62, 126.72, 128.14 (2C), 128.22 (2C), 128.39 (2C), 128.53 (2C), 128.76 (2C), 128.82 (2C), 129.74 (2C), (ArH), 129.93, 139.29, 141.48, 143.11, 159.32 (Ar), 167.83 (C=O). GC-MS (EI 70eV) m/z: 473 (<1) [M+•], 306 [M+•-167(benzhydryl radical)], 207 (12), 121 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C33H31NO2 474.2433; Found 474.2428.
(6RS)-6-Benzhydryl-5-benzyl-1-(4-methoxybenzyl)-3,6-dihydropyridin-2(1H)-one (3j).
Yield 17% (0.084g method A). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 6:1) gave brown oil. 1H NMR (400 MHz, CDCl3) δ 2.00 (d, J = 20.7 Hz, 1H, CHH-3), 2.71 (dd, J = 20.7, 5.9 Hz, 1H, CHH-3), 2.87 (ddd, J = 15.3, 2.4, 1.5 Hz, 1H, 5-CHH), 3.14 (d, J = 15.3 Hz, 1H, 5-CHH), 3.18 (d, J = 14.9 Hz, 1H, NCHH), 3.78 (s, 3H, OCH3), 4.34 (d, J = 5.1 Hz, 1H, 6-CH), 4.38 (dd, J = 5.1, 1.5 Hz, 1H, CH-6), 5.28 (d, J = 14.9 Hz, 1H, NCHH), 5.44 (dd, J = 5.8, 1.5 Hz, 1H, =CH-3), 6.68 (d, J = 8.4 Hz, 2H, ArH), 6.74 – 6.84 (m, 4H, ArH), 7.08 – 7.16 (m, 3H, ArH), 7.26 – 7.38 (m, 10H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.03 (CH2-3), 40.97 (5-CH2), 47.37 (NCH2), 54.63 (6-CH), 55.19 (OCH3), 63.21, 113.81 (2C), 122.64 (=CH-4), 126.30, 127.18, 127.29, 128.35 (2C), 128.46 (2C), 128.48 (2C), 128.73 (2C), (ArH), 128.77 (=C-5), 129.01 (2C), 129.26 (2C), 129.82 (2C), (ArH), 137.75, 138.01, 138.41, 139.42, 158.68 (Ar), 170.14 (C=O). GC-MS (EI 70eV) m/z: 473 (<1) [M+•], 306 (8) [M+•-167 (benzhydryl radical)], 281 (37), 253 (15), 208 (12), 207 (100), 191 (11), 133 (12), 121 (62), 73 (26). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C33H31NO2 474.2433; Found 474.2428.
(4RS-)-1,4-Dibenzhydryl-5-benzyl-3,4-dihydropyridin-2(1H)-one (2k).
Yield 44% (0.065g method A, using 0.1g of 1k). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 8:1) gave solid, m.p. = 153–155°C. 1H NMR (400 MHz, CDCl3) δ 2.07 (dd, J = 15.5, 1.7 Hz, 1H, 5-CHH), 2.42 (dd, J = 15.9, 1.9 Hz, 1H, CHH-3), 2.54 (dd, J = 15.9, 5.9 Hz, 1H, CHH-3), 2.67 (d, J = 15.5 Hz, 1H, 5-CHH), 2.81 (ddd, J = 11.8, 5.9, 1.9 Hz, 1H, CH-4), 3.63 (d, J = 11.8 Hz, 1H, 4-CH), 5.85 (d, J = 1.7 Hz, 1H, =CH-6), 6.79 – 6.87 (m, 2H, ArH), 6.90 – 6.96 (m, 2H, ArH), 7.12 – 7.37 (m, 17H, ArH, NCH), 7.39 – 7.56 (m, 5H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 36.60 (CH3-2), 38.03 (CH-4), 39.92 (5-CH2), 53.22 (4-CH), 58.58 (NCH), 123.94 (=CH-6), 124.35 (=C-5), 126.28, 126.63, 126.73, 127.38, 127.96, 128.12 (4C), 128.22 (2C), 128.36 (2C), 128.49 (2C), 128.53 (2C), 128.72 (2C), 128.76 (2C), 128.80 (2C), 129.24 (2C), (ArH), 138.41, 139.28, 140.06, 141.48, 143.13 (Ar), 168.00 (C=O). GC-MS (EI 70eV) m/z: 519 (<1) [M+•], 352 (18) [M+•-167 (benzhydryl radical], 207 (21), 167 (100), 165 (20). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H34NO 520.2640; Found 520.2635.
(6RS)-4-benzhydryl-1,5-dibenzyl-3,4-dihydropyridin-2(1H)-one (2l).
Yield 45% (0.1383g method B, using 0.1g of 1l). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 8:1) gave white solid, m.p. = 184–187°C. 1H NMR (400 MHz, CDCl3) δ 2.11 (dd, J = 15.5, 1.7 Hz, 1H, 5-CHH), 2.38 (dd, J = 15.9, 2.1 Hz, 1H, CHH-3), 2.45 (dd, J = 15.9, 5.7 Hz, 1H, CHH-3), 2.71 (d, J = 15.5 Hz, 1H, 5-CHH), 2.81 (ddd, J = 11.6, 5.7, 2.1 Hz, 1H, CH-4), 3.66 (d, J = 11.6 Hz, 1H, 4-CH), 4.19 (d, J = 14.5 Hz, 1H, NCHH), 5.20 (d, J = 14.4 Hz, 1H, NCHH), 5.81 (d, J = 1.7 Hz, 1H, =CH-6), 6.87 – 6.97 (m, 4H, ArH), 7.13 – 7.29 (m, 11H, ArH), 7.36 – 7.49 (m, 5H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 36.29 (CH2-3), 38.52 (CH-4), 39.67 (5-CH2), 48.88 (NCH2), 53.22 (4-CH), 124.58 (=C-5), 126.22 (=CH-6), 126.33, 126.62, 126.73, 127.82, 128.13 (2C), 128.23 (2C), 128.41 (4C), 128.50 (2C), 128.78 (4C), 128.82 (2C), (ArH), 137.76, 139.27, 141.47, 143.10 (Ar), 167.89 (C=O). GC-MS (EI 70eV) m/z: 443 (<1), [M+•], 277 (17) [M+•-167 (benzhydryl radical)], 276 (77), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H29ClNO 444.2327; Found 444.2322.
(6RS)-6-Benzhydryl-1,5-dibenzyl-3,6-dihydropyridin-2(1H)-one (3l).
Yield 9% (0.029g method B, using 0.1g of 1l). White solid, m.p. = 148–150°C. 1H NMR (400 MHz, CDCl3) δ 2.04 (d, J = 20.7 Hz, 1H, CHH-3), 2.74 (dd, J = 20.7, 5.8 Hz, 1H, CHH-3), 2.89 (dt, J = 16.2, 3.0, 2.0 Hz, 1H, 5-CHH), 3.18 (d, J = 16.2 Hz, 1H, 5-CHH), 3.23 (d, J = 15.7 Hz, 1H, NCHH), 4.35 (d, J = 5.1 Hz, 1H, 6-CH), 4.40 (dd, J = 5.1, 2.0 Hz, 1H, CH-6), 5.32 (d, J = 15.3 Hz, 1H, NCHH), 5.45 (d, J = 5.8 Hz, 1H, =CH-4), 6.78 – 6.89 (m, 4H, ArH), 7.11 – 7.20 (m, 6H, ArH), 7.24 – 7.37 (m, 10H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.03 (CH2-3), 41.00 (5-CH2), 48.10 (NCH2), 54.71 (6-CH), 63.73 (CH-6), 122.64 (=CH-4), 126.42, 127.12, 127.21, 127.34, 127.60 (2C), 128.42 (4C), 128.49 (2C), 128.51 (2C), 128.79 (2C), 129.22 (2C), 129.85 (2C), (ArH), 136.78, 137.83, 137.97, 138.40, 139.45 (Ar, =C-5), 170.19 (C=O). GC-MS (EI 70 eV) m/z: 443 (<1) [M+•], 276 (100) [M+•-167(benzhydryl radical)], 91 (86). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H29NO 444.2327; Found 444.2322.
(4RS)-4-Benzhydryl-1-(4-methoxybenzyl)-5-phenyl-3,4-dihydropyridin-2(1H)-one (2m).
Yield 14% (0.426g method B, using 1.94g of 1m). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 8:1) gave a white solid, m.p. = 122–124°C. 1H NMR (400 MHz, CDCl3) δ 2.70 (dd, J = 16.1, 1.7 Hz, 1H, CHH-3), 2.81 (dd, J = 16.1, 6.4 Hz, 1H, CHH-3), 3.74 (ddd, J = 9.6, 6.4, 1.7 Hz, 1H, CH-4), 3.81 (d, J = 9.6 Hz, 1H, 4-CH), 3.83 (s, 3H, OCH3), 4.43 (d, J = 14.5 Hz, 1H, NCHH), 4.80 (d, J = 14.5 Hz, 1H, NCHH), 6.23 (s, 1H, =CH-6), 6.78 – 6.83 (m, 2H, ArH), 6.88 (ddd, J = 8.0, 4.3, 1.8 Hz, 5H, ArH), 6.91 – 6.96 (m, 2H, ArH), 7.02 – 7.11 (m, 3H, ArH), 7.16 – 7.34 (m, 7H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 35.70 (CH2-3), 38.89 (CH-4), 48.42 (NCH2), 53.18 (4-CH), 55.37 (OCH3), 114.19 (2C, ArH), 123.90 (=C-5), 125.85 (2C), 126.06, 126.21 (ArH), 126.31 (=CH-6), 126.82, 127.64 (2C), 128.08 (2C), 128.46 (2C), 128.58 (4C), (ArH), 129.51 (Ar), 129.63 (2C, ArH), 138.81, 141.35, 141.89, 159.32 (Ar), 167.96 (C=O). GC-MS (EI 70eV) m/z: 459 (<1) [M+•], 292 (15) [M+•-167 (benzhydryl radical)], 207 (16), 167 (10), 121 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H29NO2 460.2277; Found 360.2271.
(6RS)-6-Benzhydryl-1-(4-methoxybenzyl)-5-phenyl-3,6-dihydropyridin-2(1H)-one (3m).
Yield 79% (2.41g method B, using 1.94g of 1m).). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 4:1) gave yellow oil. 1H NMR (400 MHz, CDCl3) δ 2.17 (dt, J = 20.8, 2.4 Hz, 1H, CHH-3), 2.92 (dd, J = 20.8, 6.0 Hz, 1H, CHH-3), 3.11 (d, J = 15.1 Hz, 1H, NCHH), 3.82 (s, 3H, OCH3), 4.15 (d, J = 4.8 Hz, 1H, 6-CH), 5.11 (dd, J = 4.8, 2.4 Hz, 1H, CH-6), 5.52 (d, J = 15.1 Hz, 1H, NCHH), 5.76 (dd, J = 6.0, 2.4 Hz, 1H, =CH-4), 6.83 – 6.96 (m, 6H, ArH), 7.11 – 7.40 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.37 (CH2-3), 47.46 (NCH2), 54.98 (6-CH), 55.33 (OCH3), 63.89 (CH-6), 114.05 (2C), 122.28, 126.34 (2C), 126.65, 127.49, 127.54, 128.20 (2C), 128.24 (2C), 128.40 (2C), 128.64 (2C), 128.87 (=C-5), 129.06 (2C), 130.50 (2C), (ArH), 137.75, 139.21, 139.63, 140.24, 158.97 (Ar), 170.08 (C=O). (EI 70eV) m/z: 459 (<1) [M+•], 458 (3), 292 (15) [M+•-167 (benzhydryl radical)], 291 (56), 121 (100), 116 (20), 89 (53), 73 (17), 51 (38). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H21NS 460.2277; Found 460.2271.
(4RS)-1,4-Dibenzhydryl-3,4-dihydropyridin-2(1H)-one (2n).
Yield 75% (0.436g method B, using 0.35g of 1n). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 7:1) gave transparent oil. 1H NMR (400 MHz, CDCl3) δ 2.40 (dd, J = 16.1, 9.2 Hz, 1H, CHH-3), 2.60 (dd, J = 16.1, 6.1 Hz, 1H, CHH-3), 3.25 – 3.42 (m, 1H, CH-4), 3.68 (d, J = 11.5 Hz, 1H, 4-CH), 4.92 (dd, J = 8.1, 3.8 Hz, 1H, =CH-5), 5.95 (dd, J = 8.1, 1.5 Hz, 1H, =CH-6), 6.52 – 7.53 (m, 21H, 4 x C6H5, NCH). 13C{H} NMR (101 MHz, CDCl3) δ 35.74 (CH-4), 36.85 (CH2-3), 55.91 (4-CH), 58.97 (NCH), 109.64 (=CH-5), 126.55, 126.73 (ArH), 126.92 (=CH-6), 127.59, 127.66, 127.88 (2C), 128.13 (2C), 128.56 (4C), 128.60 (4C), 128.63 (2C), 128.81 (2C), (ArH), 139.18, 139.40, 142.23, 142.62 (Ar), 168.80 (C=O). GC-MS (EI 70eV) m/z: 429 (<1) [M+•], 262 (21) [M+•-167 (benzhydryl radical)], 167 (100), 165 (24), 152 (13). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H27NO 430.2171; Found 430.2165.
(4RS)-4-Benzhydryl-1-benzyl-5-(phenylthio)-3,4-dihydropyridin-2(1H)-one (2o).
Yield 20% (0.05g method B, using 0.155g of 1o). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 6:1) gave a white solid, m.p. 168-170°C. 1H NMR (400 MHz, CDCl3) δ 2.68 (dd, J = 16.4, 2.7 Hz, 1H, CHH-3), 2.74 (dd, J = 16.4, 6.2 Hz, 1H, CHH-3), 3.17 (ddd, J = 8.9, 6.3, 2.6 Hz, 1H, CH-4), 3.99 (d, J = 8.9 Hz, 1H, 4-CH), 4.38 (d, J = 14.6 Hz, 1H, NCHH), 4.69 (d, J = 14.7 Hz, 1H, NCHH), 6.46 (s, 1H, =CH-6), 6.97 (dd, J = 7.6, 1.9 Hz, 2H, ArH), 7.11 – 7.44 (m, 18H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 36.02 (CH2-3), 40.44 (CH-4), 48.94 (NCH2), 52.71 (4-CH), 115.03 (=C-5), 126.39, 126.48, 126.92, 127.95, 128.08 (2C), 128.27 (2C), 128.50 (2C), 128.55 (2C), 128.56 (2C), 128.75 (2C), 128.86 (2C), 129.11 (2C), 135.29 (=CH-6), 136.22, 136.81, 140.84, 141.86 (Ar), 167.49 (C=O). GC-MS (EI 70eV) m/z: 461 (3) [M+•], 294 (97) [M+•-167 (benzhydryl radical)], 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H27NOS 462.1892; Found 462.1886.
(6RS)-6-Benzhydryl-1-benzyl-5-(phenylthio)-3,6-dihydropyridin-2(1H)-one (3o).
Yield 17% (0.0324g method B, using 0.155g of 1o). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 6:1) gave a brown oil. 1H NMR (400 MHz, CDCl3) δ 1.47 (dt, J = 21.2, 2.2 Hz, 1H, CHH-3), 2.68 (ddd, J = 21.2, 5.8, 1.2 Hz, 1H, CHH-3), 3.12 (d, J = 14.9 Hz, 1H, NCHH), 4.49 – 4.63 (m, 1H, CH-6), 4.84 (d, J = 2.1 Hz, 1H, 6-CH), 5.49 (d, J = 14.9 Hz, 1H, NCHH), 5.98 (dd, J = 5.8, 2.2 Hz, 1H, =CH-4), 6.81 (dd, J = 7.3, 1.7 Hz, 2H, ArH), 6.98 – 7.10 (m, 6H, ArH), 7.12 – 7.41 (m, 12H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.60 (CH2-3), 47.66 (NCH2), 53.17 (6-CH), 64.28 (CH-6), 126.67, 127.06, 127.37, 127.68, 128.01 (2C), 128.06 (2C), 128.29 (2C), 128.41 (2C), 128.56 (2C), 129.24 (2C), 130.20 (3C), (ArH, =CH-4), 130.91 (Ar), 131.32 (2C, ArH), 132.75, 135.99, 136.74 (Ar), 140.46 (=C-5), 169.11 (C=O). GC-MS (EI 70eV) m/z: 461 (3) [M+•], 294 (97) [M+•-167 (benzhydryl radical)], 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H27NOS 462.1892; Found 462.1886.
(4RS)-1,4-Dibenzhydryl-5-(phenylthio)-3,4-dihydropyridin-2(1H)-one (2p).
Yield 89% (0.387g method B, using 0.3g of 1p). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate = 8:1) gave a white solid, m.p. 63–65°C. 1H NMR (400 MHz, CDCl3) δ 2.67 (dd, J = 16.2, 2.0 Hz, 1H, CHH-3), 2.78 (dd, J = 16.2, 6.8 Hz, 1H, CHH-3), 3.16 (ddd, J = 10.0, 6.8, 2.0 Hz, 1H, CH-4), 3.89 (d, J = 10.0 Hz, 1H, 4-CH), 6.44 (s, 1H, =CH-6), 6.87 – 7.02 (m, 2H, ArH), 7.06 – 7.53 (m, 24H, ArH, NCH). 13C{H} NMR (101 MHz, CDCl3) δ 36.52 (CH2-3), 40.32 (CH-4), 53.10 (4-CH), 59.23 (NCH), 115.32 (=C-5), 126.40, 126.51, 126.89, 127.72, 128.02 (3C), 128.18 (2C), 128.50 (2C), 128.58 (2C), 128.70 (6C), 128.81 (2C), 128.91 (2C), 129.07 (2C), 132.86 (=CH-6), 136.13, 138.22, 139.17, 141.02, 141.93 (Ar), 167.62 (C=O). GC-MS (EI 70eV): decomposition. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C37H31NOS 538.2205; Found 538.2199.
(6RS)-2-Benzhydryl-1-benzyl-N,N-dimethyl-6-oxo-1,2,5,6-tetrahydropyridine-3-carboxamide (3q).
Yield 50% (0.085g method B, using 0.103g of 1q). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate) gave a white solid, m.p. 204–208°C. 1H NMR (400 MHz, CDCl3) δ 2.30 (br. s, 3H, NCH3), 2.67 (br. s, 3H, NCH3), 2.86 (d, J = 15.4 Hz, 1H, NCHH), 2.96 (ddd, J = 20.6, 2.1, 1.6 Hz, 1H, CHH-5), 3.10 (dd, J = 20.6, 6.2 Hz, 1H, CHH-5), 4.23 (d, J = 9.1 Hz, 1H, 2-CH), 5.08 (d, J = 15.4 Hz, 1H, NCHH), 5.17 (dd, J = 9.1, 1.6 Hz, 1H, CH-2), 5.92 (dd, J = 6.2, 2.1 Hz, 1H, =CH-4), 7.01 – 7.11 (m, 2H, ArH), 7.18 – 7.37 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.03 (CH2-5), 34.90 br. (NCH3), 38.08 br. (NCH3), 49.33 (NCH2), 57.29 (2-CH), 62.57 (CH-2), 126.30 (=CH-4), 127.35, 127.38, 127.46, 127.75 (2C), 128.48 (2C), 128.53 (2C), 128.77 (2C), 128.81 (2C), 129.12 (2C), 135.93, 136.63, 139.59, 139.79 (Ar, =C-3), 168.63 (C=O), 168.69 (C=O). GC-MS (EI 70eV) m/z: 424 (<1) [M+•], 257 [M+• - 167] (82), 207(44), 167 (32), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C28H28N2O2 425.2229; Found 425.2224.
(4RS)-1,4-Dibenzhydryl-N,N-dimethyl-6-oxo-1,4,5,6-tetrahydropyridine-3-carboxamide (2r).
Yield 23% (method B, using 0.165g of 1r). White solid, m.p. = 212–213°C. 1H NMR (400 MHz, CDCl3) δ 1.75 (br s, 3H, CH3), 2.52 (dd, J = 16.3, 1.7 Hz, 1H, CHH-3), 2.32 – 2.73 (br s, 3H, CH3), 2.84 (dd, J = 16.3, 6.5 Hz, 1H, CHH-3), 3.75 (d, J = 12.3 Hz, 1H, 4-CH), 3.91 (ddd, J = 12.3, 6.5, 1.7 Hz, 1H, CH-4), 6.08 (s, 1H, =CH-6), 7.04 – 7.20 (m, 8H, ArH, NCH), 7.24 – 7.45 (m, 13H, ArH). 13C{H} NMR (101 MHz, CDCl3)* δ 35.79 (CH2-3), 35.94 (CH-4), 54.54 (4-CH), 58.94 (NCH), 117.44 (=C-5), 126.79, 126.92 (2C), 127.83 (2C), 127.94, 128.05 (2C), 128.18 (2C), 128.30, 128.80 (2C), 128.83 (2C), 128.89 (2C), 128.95 (2C), 129.35 (ArH), 137.53, 140.34, 141.85, 142.35 (Ar), 168.42 (C=O), 169.83 (C=O). (*Note: signals for N(CH3)2 groups are invisible). GC-MS (EI 70eV): decomposition. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C34H33N2O2 501.2542; Found 501.2537.
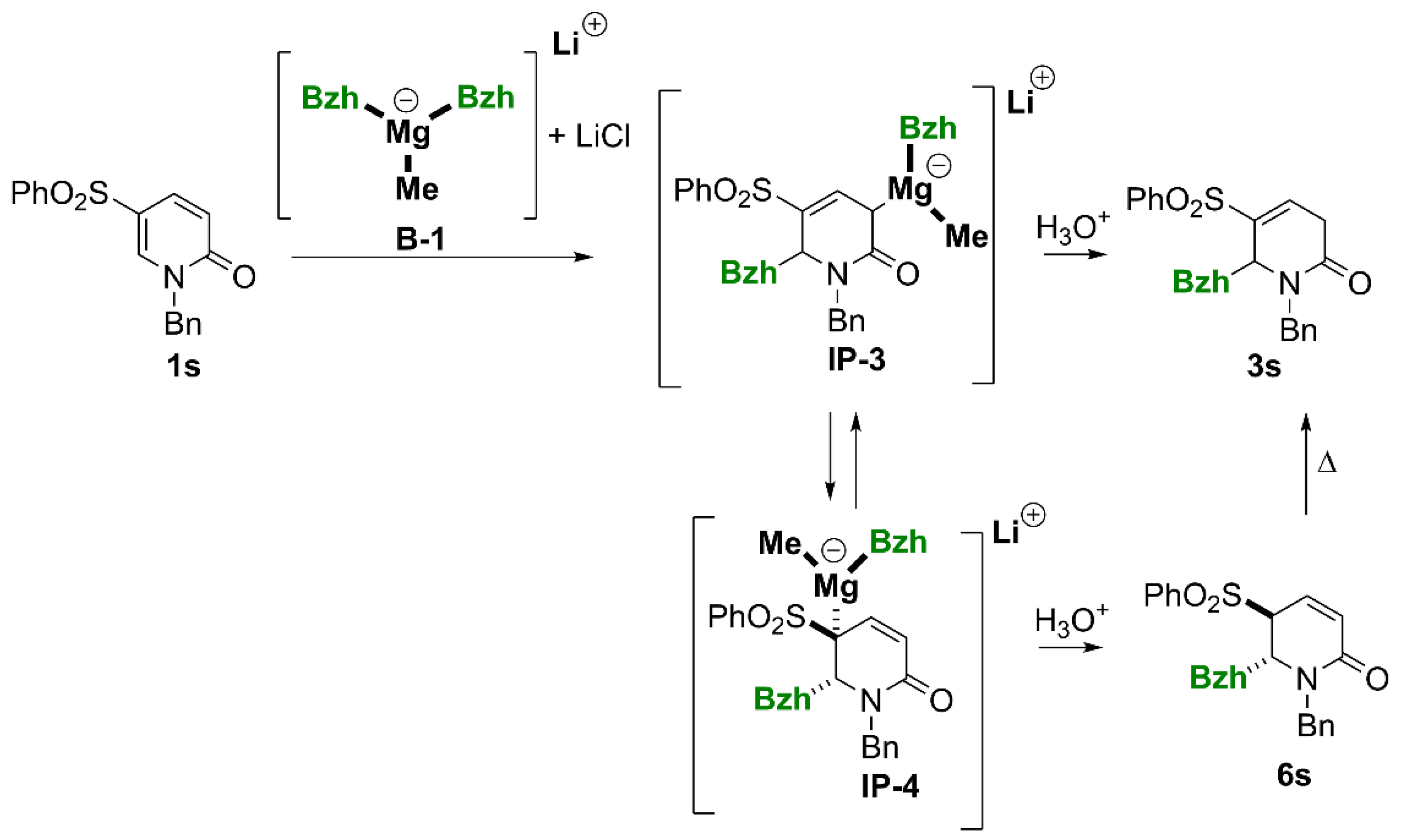
(6RS)-6-Benzhydryl-1-benzyl-5-(phenylsulfonyl)-3,6-dihydropyridin-2(1H)-one (3s).
Yield 29% (0.0654g method B, using 0.149g of 1s). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white solid, m.p. 167–176°C. 1H NMR (400 MHz, CDCl3) δ 1.26 (dt, J = 21.7, 2.0, 1.5 Hz, 1H, CHH-3), 2.67 (dd, J = 21.7, 6.3 Hz, 1H, CHH-3), 3.20 (d, J = 15.0 Hz, 1H, NCHH), 4.88 (dd, J = 1.5, 1.2 Hz, 1H, CH-6 or 6-CH), 4.98 (d, J = 1.2 Hz, 1H, CH-6 or 6-CH), 5.42 (d, J = 15.0 Hz, 1H, NCHH), 6.46 (d, J = 7.6 Hz, 2H, ArH), 6.93 (dd, J = 6.3, 2.0 Hz, 1H, =CH-4), 6.94 – 7.00 (m, 2H, ArH), 7.10 (t, J = 7.3 Hz, 1H, ArH), 7.28 – 7.48 (m, 12H, ArH), 7.55 – 7.61 (m, 1H, ArH), 7.70 – 7.77 (m, 2H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 32.89 (CH2-3), 48.01 (NCH2), 54.49, 61.14 (CH-6, 6-CH), 126.97, 127.27, 127.35 (2C), 127.58 (2C), 127.73 (2C), 128.11, 128.40 (2C), 128.59 (2C), 128.86 (2C), 129.48 (2C), 132.37 (2C), 133.80 (ArH), 134.63, 135.76 (Ar), 138.56 (=CH-4), 138.86, 139.45, 140.07 (Ar, =C-5), 167.86 (C=O). GC-MS (EI 70eV) m/z: 493 (<1) [M+•], 325 (32) [M+•- 167], 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H27NO3S 494.1790; Found 494.1784.
(5SR,6RS)-6-Benzhydryl-1-benzyl-5-(phenylsulfonyl)-5,6-dihydropyridin-2(1H)-one (6s).
Yield 59% (0.1338g method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white solid, m.p. 180–182°C. 1H NMR (400 MHz, CDCl3) δ 2.46 (d, J = 14.8 Hz, 1H, NCHH), 3.82 (d, J = 6.0 Hz, 1H, CH-5), 4.22 (d, J = 10.0 Hz, 1H, 6-CH), 4.65 (dd, J = 10.0, 1.5 Hz, 1H, CH-6), 5.02 (d, J = 14.8 Hz, 1H, NCHH), 6.20 (ddd, J = 9.8, 6.0, 1.5 Hz, 1H, =CH-4), 6.40 (dd, J = 9.8, 0.9 Hz, 1H, =CH-3), 6.86 – 6.93 (m, 2H, ArH), 7.07 – 7.14 (m, 2H, ArH), 7.15 – 7.41 (m, 15H), 7.57 – 7.67 (m, 1H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 48.93 (NCH2), 55.12 (6-CH), 56.97 (CH-6), 62.35 (CH-5), 127.36 (=CH-4), 127.55, 127.67, 127.90, 128.09 (2C), 128.36 (2C), 128.92 (2C), 128.94 (2C), 129.03 (2C), 129.27 (2C), 129.34 (2C), 130.57 (2C), (ArH), 131.53 (=CH-3), 134.06 (ArH), 135.86, 137.00, 139.59, 139.82 (Ar), 161.43 (C=O). GC-MS (EI 70eV) m/z: 493 (<1) [M+•], 325 (48) [M+•- 167], 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H27NO3S 494.1790; Found 494.1784.
(4RS)-1,4-Dibenzhydryl-5-(phenylsulfonyl)-3,4-dihydropyridin-2(1H)-one (2t).
Yield 73% (0.0942g method B, using 0.091g of 1t). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white solid, m.p. 154–156°C. 1H NMR (400 MHz, CDCl3) δ 2.68 (dd, J = 17.2, 9.3 Hz, 1H, CHH-3), 3.02 (dd, J = 17.2, 1.1 Hz, 1H, CHH-3), 3.73 (dud, J = 9.3, 4.3, 1.1 Hz, 1H, CH-4), 4.41 (d, J = 4.3 Hz, 1H, 4-CH), 6.58 – 6.65 (m, 2H, ArH), 6.67 (s, 1H,NCH), 6.88 – 6.98 (m, 2H, ArH), 7.01 – 7.13 (m, 4H, ArH), 7.13 – 7.39 (m, 13H, ArH, =CH-6), 7.49 (t, J = 7.8 Hz, 2H, ArH), 7.55 – 7.67 (m, 3H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 34.01 (CH2-3), 35.49 (CH-4), 51.59 (4-CH), 60.14 (NCH), 118.45 (=C-5), 126.56, 127.43, 127.45 (2C), 127.64 (2C), 128.10 (2C), 128.15 (2C), 128.34 (2C), 128.46 (2C), 128.81 (2C), 128.90 (2C), 129.00 (2C), 129.31 (2C), 130.22 (2C), 133.05 (ArH) , 137.67, 138.15, 139.15 (Ar), 139.56 (=CH-6), 141.37, 141.54 (Ar), 167.43 (C=O). GC-MS (EI 70eV) m/z: 569 (<1), (M+•), 535 (12) [569 (M+•) - H2S], 207 (11), 168 (17), 167 (100), 165 (33), 152 (19). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C37H31NO3S 570.2103; Found 570.2097.
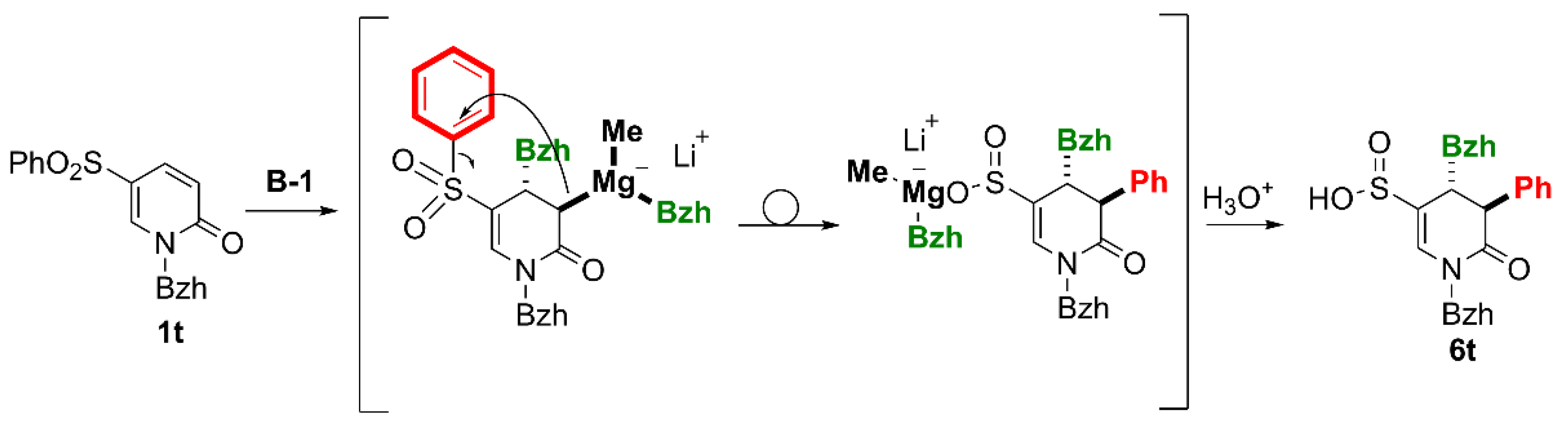
(4RS,5SR)-1,4-Dibenzhydryl-6-oxo-5-phenyl-1,4,5,6-tetrahydropyridine-3-sulfinic acid (6t).
Yield 20% (0.026g method B, using 0.091g of 1t). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white solid, m.p. 208–210°C. 1H NMR (400 MHz, CDCl3) δ 2.41 – 2.44 (m, 1H, OH), 3.58 (dd, J = 4.2, 1.3 Hz, 1H, CH-4), 4.59 (dd, J = 3.7, 1.3 Hz, 1H, 4-CH), 4.66 (d, J = 4.1 Hz, 1H, CH-5), 6.54 (s, 1H, NCH), 6.69 – 6.76 (m, 2H, ArH), 6.92 – 7.00 (m, 2H, ArH), 7.00 – 7.06 (m, 2H, ArH), 7.10 – 7.40 (m, 15H, ArH, =CH-2), 7.46 – 7.54 (m, 2H, ArH), 7.58 – 7.64 (m, 1H, ArH), 7.69 – 7.75 (m, 2H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 43.55 (CH-4), 49.45 (CH-5), 60.31 (NCH), 69.60 (4-CH), 117.01 (=C-3), 126.62, 127.40 (2C), 127.48, 127.57 (2C), 127.99 (2C), 128.02, 128.34 (2C), 128.40, 128.51 (2C), 128.85 (2C), 128.92 (2C), 129.18 (2C), 129.28 (2C), 129.89 (2C), 133.10 (ArH), 137.18 (Ar), 137.59 (=CH-2), 137.90, 139.27, 140.46, 141.17 (Ar), 167.32 (C=O). GC-MS (EI 70eV): decomposition. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C37H31NO3S 570.2103; Found 570.2097.
(4RS)-4-Benzhydryl-5-(benzofuran-5-yl)-1-phenyl-3,4-dihydropyridin-2(1H)-one (2u).
Yield 59% (0.15g method B, using 0.160g of 1u). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 4 : 1) gave a white solid, m.p. 148–149°C. 1H NMR (400 MHz, CDCl3) δ 2.96 (dd, J = 16.3, 1.6 Hz, 1H, CHH-3), 3.09 (dd, J = 16.3, 7.3 Hz, 1H, CHH-3), 3.99 (ddd, J = 7.9, 7.3, 1.6 Hz, 1H, CH-4), 4.15 (d, J = 7.9 Hz, 1H, 4-CH), 6.48 (s, 1H, =CH-6), 6.67 (dd, J = 2.2, 1.0 Hz, 1H, ArH), 6.94 – 7.09 (m, 4H, ArH), 7.11 – 7.19 (m, 4H, ArH), 7.19 – 7.35 (m, 8H, ArH), 7.37 – 7.45 (m, 2H, ArH), 7.59 (d, J = 2.2 Hz, 1H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 35.93 (CH2-3), 39.36 (CH-4), 53.15 (4-CH), 106.54, 111.17, 118.66, 122.88 (ArH), 123.29 (=C-5), 126.08 (2C), 126.26, 126.98, 127.12 (ArH), 127.57 (Ar), 127.96 (3C, ArH, =CH-6), 128.47 (2C), 128.51 (2C), 129.08 (2C), 129.13 (2C), (ArH), 133.53, 140.36, 141.10, 142.07 (Ar), 145.46 (ArH), 153.96 (Ar), 167.55 (C=O). GC-MS (EI 70eV) m/z: 455 (<1) [M+•], 287 (36) [M+•- 168], 167 (100), 77 (28). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H25NO2 456.1964; Found 456.1958.
(4RS)-4-Benzhydryl-5-(benzo[b]thiophen-5-yl)-1-phenyl-3,4-dihydropyridin-2(1H)-one (2v).
Yield 70% (0.13g method B, using 0.119g of 1v). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 4 : 1) gave a solid, m.p. 90–92°C. 1H NMR (400 MHz, CDCl3) δ 2.86 (dd, J = 16.0, 1.7 Hz, 1H, CHH-3), 3.16 (dd, J = 16.1, 6.8 Hz, 1H, CHH-3), 3.96 (ddd, J = 9.7, 6.8, 1.7 Hz, 1H, CH-4), 4.12 (d, J = 9.7 Hz, 1H, 4-CH), 6.46 (s, 1H, =CH-6), 6.76 – 6.88 (m, 4H, ArH), 6.98 (dd, J = 6.5, 3.1 Hz, 2H, ArH), 7.10 (t, J = 7.7 Hz, 1H, ArH), 7.16 – 7.34 (m, 9H, ArH), 7.37 (d, J = 5.6 Hz, 1H, ArH), 7.45 (t, J = 7.8 Hz, 2H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 36.44 (CH2-3), 40.63 (CH-4), 54.41 (4-CH), 121.02, 122.64 (ArH), 123.33 (=C-5), 123.70, 123.97, 125.98 (3C), 126.15, 126.92, 127.21, 127.79 (2C), 128.12 (2C), 128.48 (2C), 128.70 (2C), 129.21 (2C), (ArH), 129.85 (=CH-6), 135.10, 137.27, 140.06, 140.19, 141.60, 141.78 (Ar), 167.75 (C=O). GC-MS m/z: 470 (<1), (M+•) (304 (6), (M+•-167), 303 (49), 275 (12), 171 (26), 168 (39), 167 (100), 165 (33), 152 (13), 77 (17). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H26NOS 472.1735; Found 472.1729.
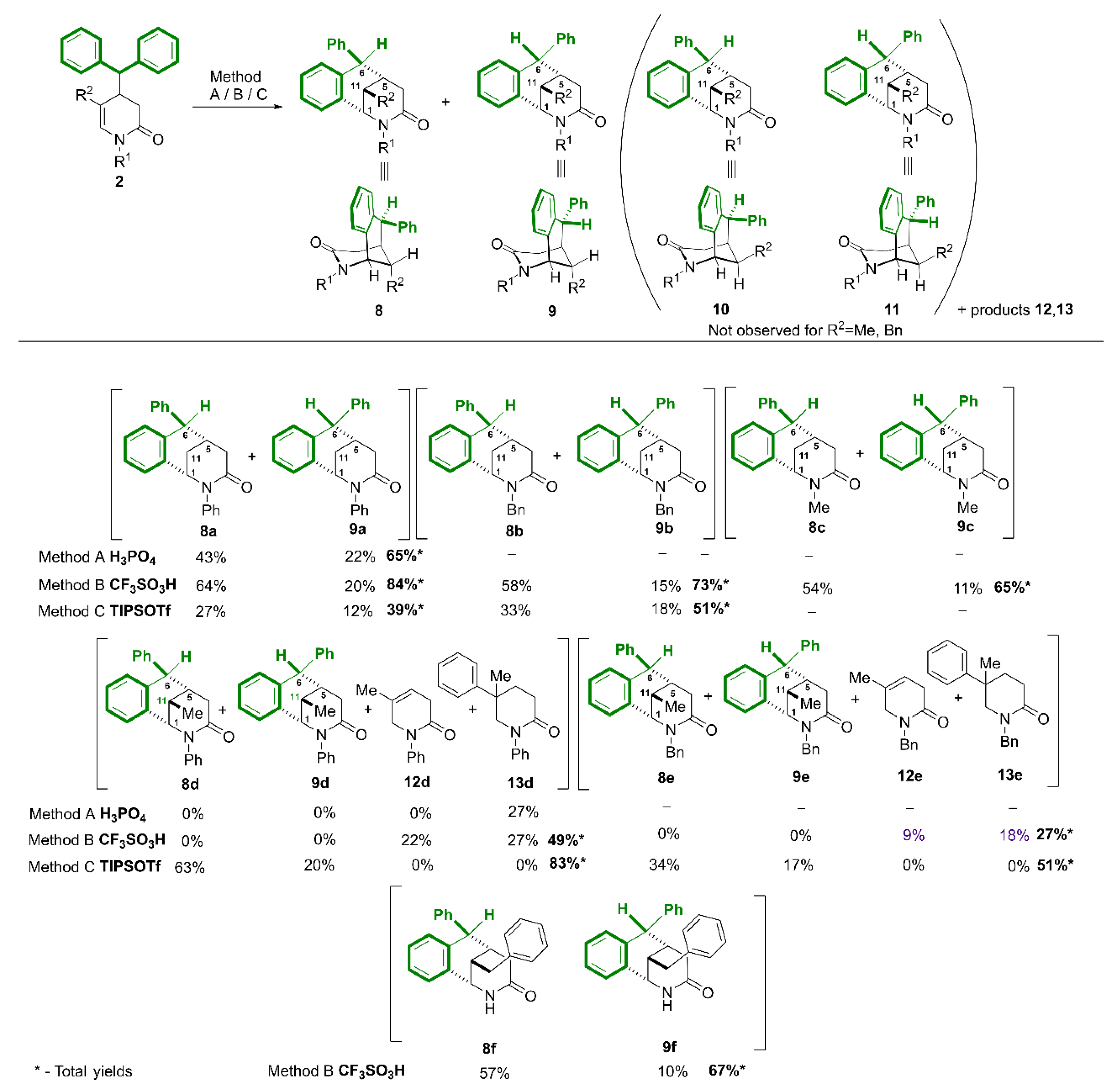
3.4. Synthesis of Compounds 8, 9
Method A: In a round-bottomed flask fitted with a reflux condenser and an argon balloon, 3,4-dihydropyridin-2-one (0.295 mmol) and 6 mL (18.24 mmol) of 85% phosphoric(V) acid were placed, heated to 120°C and stirred for 2-3 h. After this time, the reaction mixture was cooled, the flask was placed in an ice bath and saturated sodium bicarbonate solution was carefully added, extracted with ethyl acetate (3 x 20 mL), the organic layer was dried over anhydrous magnesium sulfate, filtered and the solvent was distilled off under reduced pressure. The crude product was purified by column chromatography (SiO2) using a mixture of hexane and ethyl acetate as eluent.
Method B: The procedure was similar to method A, except that 0.295 mmol of 3,4-dihydropyridin-2-one was dissolved in 4 mL of anhydrous acetonitrile. Then, 0.21 mL of triflic acid (7.5-fold excess, 2.21 mmol) was slowly added dropwise and the reaction was carried out for 24 h at room temperature. After this time, while cooling the flask, a saturated solution of NaHCO3 was slowly added. The further procedure is the same as in method A.
Method C: The procedure was similar to method A, except that 0.587 mmol of 3,4-dihydropyridin-2-one was dissolved in 8 mL of anhydrous acetonitrile in the flask and 2.5-fold excess of TIPSOTf (1.469 mmol) was added dropwise from a syringe then the mixture was refluxed for 24 h (monitoring by 1H NMR). Then the reaction mixture was cooled to room temperature and 10 mL of saturated NaHCO3 solution was added and the aqueous layer was extracted with ethyl acetate (3 x 40 mL). The further procedure is the same as in method A.
(1SR,5SR,6RS)-2,6-Diphenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (8a).
Yield 43% (method A), 64% (method B) 27% (method C). White solid, m.p. 183 – 185°C. 1H NMR (400 MHz, CDCl3) δ 2.28 (dt, J = 13.3, 3.2 Hz, 1H, CHHax-11), 2.39 (dtd, J = 13.3, 3.2, 1.4 Hz, 1H, CHHeq-11), 2.60 – 2.67 (m, 1H, CH-5), 2.74 (dt, J = 18.8, 1.4 Hz, 1H, CHHeq-4), 3.11 (dd, J = 18.8, 8.3 Hz, 1H, CHHax-4), 4.26 (s, 1H, CHα-6), 4.74 (td, J = 3.2, 1.4 Hz, 1H, CH-1), 6.59 (dd, J = 7.7, 1.2 Hz, 1H, ArH), 6.90 – 6.96 (m, 2H, ArH), 7.01 – 7.06 (m, 4H, ArH), 7.16 – 7.33 (m, 5H, ArH), 7.37 – 7.44 (m, 2H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 25.57 (CH2-11), 35.06 (CH-5), 39.98 (CH2-4), 52.08 (CH-6), 59.24 (CH-1), 126.33, 126.41, 127.27, 128.02 (2C), 128.39 (2C), 128.43, 128.53 (2C), 128.58, 129.26 (2C), 132.05 (ArH), 134.87, 136.55, 142.09, 146.61 (Ar), 169.51 (C=O). GC-MS (EI 70eV) m/z: 339 (100) [M+•], 204 (44), 178 (28), 141 (35), 135 (83). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H22NO 340.1701; Found 340.1696.
(1SR,5SR,6SR)-2,6-Diphenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (9a).
Yield 22% (method A), 20% (method B) 12% (method C). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white solid, m.p. 218 – 220°C. 1H NMR (400 MHz, CDCl3) δ 2.35 – 2.53 (m, 3H, CH2-4, CHH-11), 2.68 – 2.81 (m, 2H, CHH-11, CH-5), 4.63 (d, J = 6.1 Hz, 1H, CHb-6), 4.70 – 4.76 (m, 1H, CH-1), 6.49 (dd, J = 7.6, 1.4 Hz, 1H, ArH), 6.93 – 7.22 (m, 6H, ArH), 7.25 – 7.43 (m, 7H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 31.68 (CH2-11), 33.33 (CH-5), 33.83 (CH2-4), 50.67 (CH-6), 59.53 (CH-1), 125.85, 126.84, 127.22, 128.22 (4C), 128.47, 128.72 (2C), 129.23 (2C), 129.83 (br), 130.63 (ArH), 136.23, 137.08, 142.08, 143.16 (Ar), 169.48 (C=O). GC-MS (EI 70eV) m/z: 339 (100) [M+•], 204 (45), 178 (27), 135 (93). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H22NO 340.1701 Found 340.1696.
(1SR,5SR,6RS)-2-Benzyl-6-phenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (8b).
Yield 58% (method B), 33% (method C). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 3 : 1) gave a white solid, m.p. 149 – 151°C. 1H NMR (400 MHz, CDCl3) δ 1.90 (dt, J = 13.3, 3.4 Hz, 1H, CHHax-11), 2.19 (dq, J = 13.0, 2.6 Hz, 1H, CHHeq-11), 2.46 – 2.59 (m, 1H, CH-5), 2.68 (d, J = 18.6 Hz, 1H, CHHeq-4), 3.06 (dd, J = 18.6, 8.0 Hz, 1H, CHHax-4), 3.81 (d, J = 15.4 Hz, 1H, NCHH), 4.18 (s, 1H, CHα-6), 4.23 (td, J = 3.2, 1.3 Hz, 1H, CH-1), 5.56 (d, J = 15.4 Hz, 1H, NCHH), 6.83 – 6.92 (m, 2H, ArH), 6.96 – 7.04 (m, 1H, ArH), 7.14 – 7.27 (m, 6H, ArH), 7.30 – 7.43 (m, 5H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 25.60 (CH2-11), 35.09 (CH-5), 40.00 (CH2-4), 46.99 (NCH2), 52.00 (CH-6), 52.49 (CH-1), 126.36, 126.51, 127.46, 127.82, 128.15 (2C), 128.39 (4C), 128.50, 128.81 (2C), 132.22 (ArH), 135.82, 136.92, 137.19, 147.00 (Ar), 169.49 (C=O). GC-MS (EI 70eV) m/z: 353 (78) [M+•], 206 (100), 148 (75), 106 (61), 91 (85). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H24NO 354.1858 Found 354.1852.
(1SR,5SR,6SR)-2-Benzyl-6-phenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (9b).
Yield 15% (method B), 18% (method C). The crude product purified by column chromatography (SiO2, n-hexane/ethyl acetate, 3 : 1) gave a white solid, m.p. 150 – 152°C. 1H NMR (400 MHz, CDCl3) δ 2.26 – 2.38 (m, 3H, CHHeq-4, CH2-11), 2.44 (dd, J = 19.0, 7.3 Hz, 1H, CHHax-4), 2.59 – 2.76 (m, 1H, CH-5), 3.83 (d, J = 15.3 Hz, 1H, NCHH), 4.20 (td, J = 3.1, 1.3 Hz, 1H, CH-1), 4.57 (d, J = 6.3 Hz, 1H, CHβ-6), 5.62 (d, J = 15.3 Hz, 1H, NCHH), 6.95 – 7.46 (m, 14H). 13C{H} NMR (101 MHz, CDCl3) δ 31.49 (CH2-11), 33.22 (CH-5), 33.51 (CH2-4), 46.77 (NCH2), 50.76 (CH-6), 52.80 (CH-1), 126.21, 126.84, 127.48, 128.01, 128.11 (2C), 128.28, 128.70 (2C), 128.84 (2C), 129.84 br (2C), 130.84 (ArH), 136.89, 137.22, 137.28, 143.14 (Ar), 169.86 (C=O). GC-MS (EI 70eV) m/z: 353 (99) [M+•·], 204 (57), 148 (47), 128 (44), 106 (78), 91 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H24NO 354.1858; Found 354.1852.
(1SR,5SR,6RS)-2-Methyl-6-phenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (8c).
Yield 54% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1 then 1 : 1) gave a white solid, m.p. 164 – 166°C. 1H NMR (400 MHz, CDCl3) δ 2.06 (dt, J = 13.1, 3.3 Hz, 1H, CHHax-11), 2.26 (dq, J = 13.1, 2.7 Hz, 1H, CHHeq-11), 2.48 – 2.55 (m, 1H, CH-5), 2.56 (d, J = 18.5 Hz, 1H, CHHeq-4), 2.92 (dd, J = 18.5, 7.9 Hz, 1H, CHHax-4), 3.00 (s, 3H, NCH3), 4.14 (s, 1H, CHα-6), 4.25 (td, J = 3.3, 1.3 Hz, 1H, CH-1), 6.87 – 6.94 (m, 2H, ArH), 6.96 – 7.02 (m, 1H, ArH), 7.17 – 7.30 (m, 6H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 25.33 (CH2-11), 33.66 (NCH3), 35.37 (CH-5), 39.83 (CH2-4), 51.92 (CH-6), 57.27 (CH-1), 126.36, 126.51, 127.69, 128.41 (4C), 128.49, 132.25 (ArH), 135.48, 136.83, 147.05 (Ar), 169.61 (C=O). GC-MS (EI 70eV) m/z: 277 (23) [M+•], 204 (48), 73 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H20NO 278.1545; Found 278.1539.
(1SR,5SR,6SR)-2-Methyl-6-phenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (9c).
Yield 11% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1 then 1 : 1) gave a white solid, m.p. 192 – 193°C. 1H NMR (400 MHz, CDCl3) δ 2.19 (dt, J = 18.5, 1.7 Hz, 1H, CHHeq-11), 2.30 (dd, J = 18.5, 7.3 Hz, 1H, CHHax-11), 2.36 (dq, J = 13.0, 2.8 Hz, 1H, CHHeq-4), 2.50 (dt, J = 13.0, 3.5 Hz, 1H, CHHax-4), 2.61 – 2.70 (m, 1H, CH-5), 3.03 (s, 3H, NCH3), 4.23 (td, J = 3.2, 1.3 Hz, 1H, CH-1), 4.57 (d, J = 6.4 Hz, 1H, CHβ-6), 7.02 – 7.28 (m, 7H, ArH), 7.32 (t, J = 7.4 Hz, 2H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 31.26 (CH2-11), 33.46 (2C), (CH3, CH2-4), 33.50 (CH-5), 50.72 (CH-6), 57.55 (CH-1), 126.15, 126.77, 127.81, 128.22, 128.63 (2C), 129.80 br, (2C), 130.82 (ArH), 136.61, 137.25, 143.16 (Ar), 169.92 (C=O). GC-MS (EI 70eV) m/z: 277 (23) [M+•], 204 (48), 73 (100). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H20NO 278.1545; Found 278.1539.
(1SR,5SR,6RS,11SR)-11-Methyl-2,6-diphenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (8d).
Yield 63% (method C). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 3 : 1) gave a white solid, m.p. 170 – 172°C. 1H NMR (400 MHz, CDCl3) δ 1.33 (d, J = 6.9 Hz, 3H, 11-CH3), 2.37 (ddd, J = 8.6, 2.7, 1.7 Hz, 1H, CH-5), 2.53 (qt, J = 6.9, 2.7 Hz, 1H, CH-11), 2.60 (d, J = 19.1 Hz, 1H, CHHeq-4), 3.08 (dd, J = 19.1, 8.6 Hz, 1H, CHHax-4), 4.32 (s, 1H, CHa-6), 4.46 (dd, J = 2.7, 1.7 Hz, 1H, CH-1), 6.63 (dd, J = 7.6, 1.4 Hz, 1H, ArH), 6.88 – 6.95 (m, 2H, ArH), 7.02 – 7.13 (m, 4H, ArH), 7.17 – 7.35 (m, 5H), 7.36 – 7.47 (m, 2H). 13C{H} NMR (101 MHz, CDCl3) δ 17.06 (11-CH3), 27.28 (CH-11), 36.65 (CH2-4), 41.02 (CH-5), 53.92 (CHa-6), 64.89 (CH-1), 126.40 (2C), 127.13, 128.06 (2C), 128.31 (2C), 128.42, 128.56, 128.70 (2C), 129.25 (2C), 132.09 (ArH), 134.31, 137.85, 142.31, 146.44 (Ar), 169.07 (C=O). GC-MS (EI 70eV) m/z: 353 (86) [M+•], 338 (39) [M+•- Me], 218 (100), 203 (46), 178 (33), 135 (77), 92 (35), 77 (23). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H24NO 354.1858; Found 354.1852.
(1SR,5SR,6SR,11SR)-11-Methyl-2,6-diphenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (9d).
Yield 20% (method C). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 3 : 1) gave a white solid, m.p. 220 – 222°C. 1H NMR (400 MHz, CDCl3) δ 1.52 (d, J = 6.9 Hz, 3H, 11-CH3), 2.35 – 2.41 (m, 2H, CH2-4), 2.43 – 2.51 (m, 1H, CH-5), 2.60 (qt, J = 6.9, 2.4 Hz, 1H, CH-11), 4.45 (t, J = 2.2 Hz, 1H, CH-1), 4.62 (d, J = 5.6 Hz, 1H, CHb-6), 6.55 (dd, J = 7.6, 1.4 Hz, 1H, ArH), 7.00 – 7.23 (m, 6H), 7.24 – 7.46 (m, 7H). 13C{H} NMR (101 MHz, CDCl3) δ 17.37 (11-Me), 29.45 (CH2-4), 34.45 (CH-11), 39.44 (CH-5), 52.47 (CH-6), 65.26 (CH-1), 126.00, 126.85, 127.15, 128.17, 128.21 (3C), 128.52, 128.66 (2C), 129.25 (2C), 130.02, 130.61 (ArH), 135.62, 138.12, 142.32, 142.86 (Ar), 169.21 (C=O). GC-MS (EI 70eV) m/z: 353 (100) [M+•], 218 (38), 178 (23), 133 (32), 91 (24); HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H24NO 354.1858; Found 354.1852.
(1SR,5SR,6RS,11SR)-2-Benzyl-11-methyl-6-phenyl-1,4,5,6-tetrahydro-1,5-methano-benzo[c]azocin-3(2H)-one (8e).
Yield 34% (method C). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 3 : 1) gave a colorless oil. 1H NMR (400 MHz, CDCl3) δ 0.85 (d, J = 6.9 Hz, 3H, 11-CH3), 2.22 (dd, J = 8.2, 2.3 Hz, 1H, CH-5), 2.27 – 2.35 (m, 1H, CH-11), 2.54 (d, J = 18.9 Hz, 1H, CHHeq-4), 3.03 (dd, J = 18.9, 8.2 Hz, 1H, CHHax-4), 3.75 (d, J = 14.8 Hz, 1H, NCHH), 3.96 (t, J = 2.3 Hz, 1H, CH-1), 4.24 (s, 1H, CHα-6), 5.58 (d, J = 14.9 Hz, 1H, NCHH), 6.80 – 6.90 (m, 2H, ArH), 6.98 – 7.04 (m, 1H, ArH), 7.11 – 7.26 (m, 6H, ArH), 7.28 – 7.41 (m, 5H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 16.42 (11-CH3), 27.24 (CH-11), 36.47 (CH2-4), 40.84 (CH-5), 46.98 (NCH2), 53.84 (CH-6), 57.61 (CH-1), 126.30, 126.46, 127.58, 127.76, 128.28 (2C), 128.43, 128.52 (2C), 128.61 (2C), 129.16 (2C), 132.28, (ArH), 135.26, 136.91, 138.19, 146.91 (Ar), 169.12 (C=O). GC-MS m/z: 367 (82) [M+•], 220 (100), 205 (78), 146 (23), 25 (78), 146 (23), 106 (25), 91 (55). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H26NO 368.2014 Found 368.2009.
(1SR,5SR,6SR,11SR)-2-Benzyl-11-methyl-6-phenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (9e).
Yield 17% (method C). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 3 : 1) gave a white solid, m.p. = 220 - 221°C. 1H NMR (400 MHz, CDCl3) δ 1.07 (d, J = 6.8 Hz, 3H, 11-CH3), 2.25 (d, J = 17.6 Hz, 1H, CHH-4), 2.31 – 2.47 (m, 3H, CHH-4, CH-5, CH-11), 3.77 (d, J = 14.8 Hz, 1H, NCHH), 3.93 (t, J = 2.1 Hz, 1H, CH-1), 4.56 (d, J = 5.6 Hz, 1H, CH-6b), 5.65 (d, J = 14.9 Hz, 1H, NCHH), 7.05 – 7.45 (m, 14H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 16.86 (11-CH3), 29.25 (CH2-4), 34.21 (CH-11), 39.16 (CH-5), 46.72 (NCH2), 52.51 (CH-6), 57.88 (CH-1), 126.18, 126.80, 127.57, 127.98, 128.15, 128.65 (4C), 129.05 (2C), 130.01 (2C, br), 130.81 (ArH), 136.40, 137.05, 138.41, 142.95 (Ar), 169.41 (C=O). 1H NMR (400 MHz, Toluene-d8) δ 0.74 (d, J = 6.9 Hz, 3H, 11-CH3), 1.74 – 1.83 (m, 1H, CH-11), 1.83 – 1.90 (m, 1H, CH-5), 2.18 (dd, J = 19.0, 7.3 Hz, 1H, CHHax-4), 2.28 (d, J = 19.0 Hz, 1H, CHHeq-4), 3.58 (d, J = 14.8 Hz, 1H, NCHH), 3.65 (t, J = 2.2 Hz, 1H, CH-1), 4.14 (d, J = 6.2 Hz, 1H, CH-6b), 5.83 (d, J = 14.8 Hz, 1H, NCHH), 6.87 – 7.32 (m, 14H, ArH). GC-MS (EI 70eV): m/z= 367 (100) [M+•], 220 (37), 205 (34), 179 (19), 143 (21), 106 (43), 91 (71). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H26NO 368.2014 Found 368.2009.
(1SR,5SR,6RS,11SR)-11-Benzylo-6-fenylo-1,4,5,6-tetrahydro-1,5-metanobenzo[c]azocyn-3(2H)-on (8f).
Yield 57% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white semi-solid. 1H NMR (400 MHz, CDCl3) δ 2.36 (d, J = 8.2 Hz, 1H, CH-5), 2.47 (d, J = 19.1 Hz, 1H, CHH-4), 2.56 – 2.62 (m, 1H, CH-11), 2.66 (dd, J = 13.7, 6.3 Hz, 1H, 11-CHH), 2.88 (dd, J = 13.7, 9.6 Hz, 1H, 11-CHH), 2.95 (dd, J = 19.1, 8.3 Hz, 1H, CHH-4), 4.02 (dt, J = 4.6, 2.1 Hz, 1H, CH-1), 4.24 (s, 1H, CH-6), 6.45 (d, J = 4.6 Hz, 1H, NH), 6.83 – 6.92 (m, 2H, ArH), 6.96 – 7.05 (m, 3H, ArH), 7.05 – 7.10 (m, 1H, ArH), 7.17 – 7.30 (m, 8H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 33.56 (CH-11), 36.07 (CH2-4), 36.51 (11-CH2), 39.18 (CH-5), 52.66 (CH-6), 53.84 (CH-1), 126.42, 126.45, 127.35, 127.99, 128.34 (2C), 128.44, 128.56 (2C), 128.59 (2C), 128.73 (2C), 132.10 (ArH), 134.34, 138.91, 140.14, 146.35 (Ar), 172.13 (C=O). GC-MS (EI 70eV) m/z: 353 (2) [M+•- benzhydryl radical), 294 (100), 217 (31), 91 (37). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H24NO 354.1858; Found 354.1852.
(1SR,5SR,6SR,11SR)-11-Benzylo-6-fenylo-1,4,5,6-tetrahydro-1,5-metanobenzo[c]azocyn-3(2H)-on (9f).
Yield 10% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 2 : 1) gave a white semi-solid. 1H NMR (400 MHz, CDCl3) δ 2.18 (d, J = 19.2 Hz, 1H, CHH), 2.34 (dd, J = 19.2, 7.5 Hz, 1H, CHH-4), 2.45 (dd, J = 9.1, 6.8 Hz, 1H, CH-11), 2.57 – 2.66 (m, 1H, CH-5), 2.92 (dd, J = 13.7, 6.8 Hz, 1H, 11-CHH), 3.02 (dd, J = 13.7, 9.1 Hz, 1H. 11-CHH), 4.08 (dt, J = 4.3, 2.0 Hz, 1H, CH-1), 4.57 (d, J = 6.1 Hz, 1H, CH-6), 6.33 (d, J = 4.3 Hz, 1H, NH), 6.97 – 7.08 (m, 3H, ArH), 7.07 – 7.18 (m, 3H, ArH), 7.22 – 7.30 (m, 5H, ArH), 7.31 – 7.38 (m, 3H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 29.08 (CH2-4), 37.18 (CH-11), 37.28 (11-CH2), 40.88 (CH-5), 52.40 (CH-6), 53.59 (CH-1), 126.63, 126.91 (2C), 127.67, 128.16, 128.66 (2C), 128.78 (2C), 128.98 (2C), 129.99 br (2C), 130.75 (ArH), 135.62, 139.06, 140.42, 142.75 (Ar), 172.35 (C=O). GC-MS (EI 70eV) m/z: 353 (4) [M+•- benzhydryl radical], 294 (100), 217 (20), 91 (42). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H24NO 354.1858; Found 354.1852.
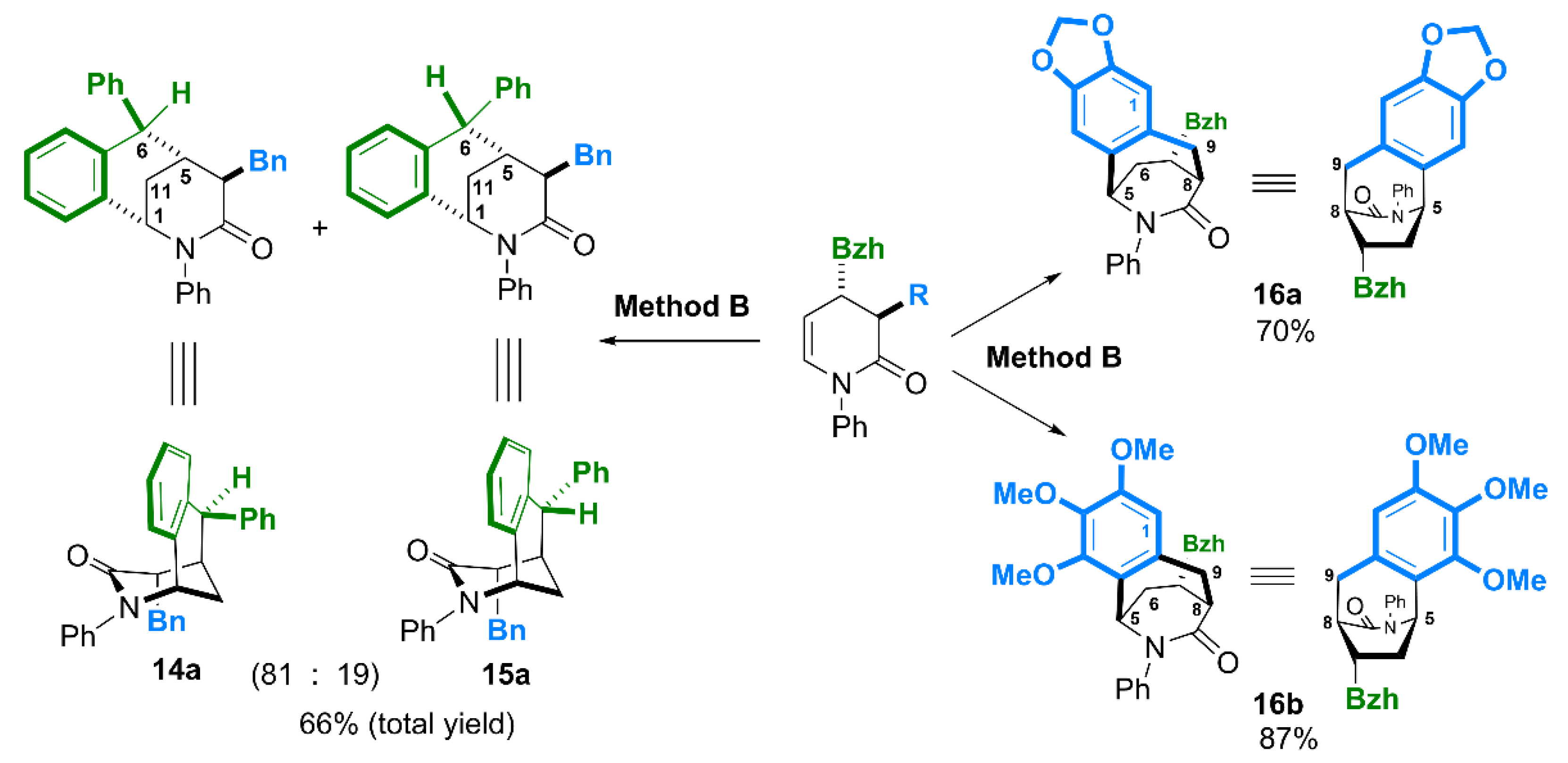
(1SR,4RS,5RS,6RS)-4-Benzyl-2,6-diphenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (14a). Major isomer.
Total yield of 14a and 15a (80 : 20) 66% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 5 : 1) gave a white semi, m.p. = 187–190°C. Spectral data for 14a refined from spectra of mixture 14a : 15a (80 : 20). 1H NMR (400 MHz, CDCl3) δ 2.04 (dt, J = 13.4, 3.6 Hz, 1H, CHH-11), 2.15 (dt, J = 13.4, 3.0 Hz, 1H, CHH-11), 2.36 – 2.43 (m, 1H, CH-5), 2.79 – 2.92 (m, 2H, CH-4, 4-CHH), 3.59 (d, J = 10.9 Hz, 1H, 4-CHH), 3.90 (s, 1H, CH-6), 4.70 (q, J = 2.6 Hz, 1H, CH-1), 6.43 (dd, J = 6.6, 2.9 Hz, 2H, ArH), 6.64 (dd, J = 7.7, 1.4 Hz, 1H, ArH), 6.96 (d, J = 7.5 Hz, 1H, ArH), 7.03 – 7.45 (m, 15H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 23.70 (CH2-11), 38.66 (CH-5), 39.44 (4-CH2), 50.60 (CH-4), 52.58 (CH-6), 59.25 (CH-1), 126.15, 126.48, 126.51, 127.16, 127.92 (2C), 128.11 (2C), 128.49 (2C), 128.53, 128.61 (3C), 129.26 (2C), 129.52 (2C), 132.06 (ArH), 134.56, 136.66, 140.04, 142.57, 146.03 (Ar), 171.74 (C=O). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H28NO 430.2171; Found 430.2166.
(1SR,4RS,5RS,6SR)-4-Benzyl-2,6-diphenyl-1,4,5,6-tetrahydro-1,5-methanobenzo[c]azocin-3(2H)-one (15a). Minor isomer.
Spectral data for 15a refined from spectra of mixture 14a : 15a (80 : 20). 1H NMR (400 MHz, CDCl3) δ 2.26 (dt, J = 13.1, 3.0 Hz, 1H, CHH-11), 2.43 – 2.50 (m, 1H, CH-5), 2.56 (dt, J = 13.1, 3.6 Hz, 1H, CHH-11), 2.72 (dd, J = 10.3, 4.0 Hz, 1H), 2.79 – 2.92 (m, 1H, CH-4,), 3.05 (dd, J = 13.7, 4.0 Hz, 1H), 4.49 (d, J = 6.3 Hz, 1H, CH-6), 4.66 (t, J = 3.3 Hz, 1H, CH-1), 6.45 – 6.50 (m, 1H, ArH), 6.75 – 6.85 (m, 1H, ArH), 6.98 – 7.02 (m, 1H, ArH), 7.03 – 7.45 (m, 16H). 13C{H} NMR (101 MHz, CDCl3) δ 28.65, 35.53, 39.68, 42.55, 50.70, 59.62, 125.82, 126.03, 126.62, 128.17, 128.26 (3C), 128.53 (4C), 128.61 (2C), 128.99 (2C), 129.19 (2C), 129.26, 130.45, 136.00, 137.16, 139.25, 142.24, 142.43, 172.11.
(5
RS,7
RS,8
RS)-7-Benzhydryl-12-phenyl-6,7,8,9-tetrahydro-5
H-5,8-(epiminomethano)cyclohepta [
4,
5]benzo [1,2-
d][
1,
3]dioxol-11-one (
16a).
Yield 70% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 4 : 1) gave a white semi, 151–153°C. 1H NMR (400 MHz, CDCl3) δ 1.95 (ddd, J = 14.0, 8.3, 4.6 Hz, 1H, CHHβ-6), 2.25 (ddd, J = 14.0, 9.1, 2.5 Hz, 1H, CHHα-6), 2.92 (dd, J = 5.1, 2.6 Hz, 1H, CH-8), 2.97 (dd, J = 17.4, 5.1 Hz, 1H, CHHβ -9), 3.12 (ddd, J = 12.0, 9.1, 8.3 Hz, 1H, CH-7), 3.31 (dd, J = 17.4, 2.6 Hz, 1H, CHHα-9), 3.87 (d, J = 12.0 Hz, 1H, 7-CH), 4.43 (dd, J = 4.6, 2.5 Hz, 1H, CHb-5), 5.94 (d, J = 1.1 Hz, 1H, OCHHO), 5.94 (d, J = 1.1 Hz, 1H, OCHHO), 6.51 (d, J = 1.0 Hz, 1H, CH-4), 6.66 (d, J = 1.1 Hz, 1H, CH-1), 7.02 – 7.46 (m, 15H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 37.40 (CH2-9), 39.48 (CH2-6), 39.52 (CH-7), 44.42 (CH-8), 59.14 (7-CH), 64.19 (CH-5), 101.18 (OCH2O), 107.98 (CH-4), 110.86 (CH-1), 124.82 (2C), 126.30, 126.55, 126.71, 127.88 (2C), 128.07 (2C), 128.65 (2C), (ArH), 128.89 (Ar), 128.95 (4C), 131.58, 142.19, 142.94, 143.45, 145.78, 147.57 (Ar), 172.29 (C=O). GC-MS (EI 70eV): decomposition. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H28NO3 474.2069; Found 474.2064.
(5RS,7RS,8RS)-7-Benzhydryl-2,3,4-trimethoxy-11-phenyl-6,7,8,9-tetrahydro-5
H-5,8-(epiminomethano)benzo [
7]annulen-10-one (
16b).
Yield 87% (method B). The crude product purified by column chromatography (SiO2, hexane/ethyl acetate, 4 : 1) gave a white semi, 205–207°C. 1H NMR (400 MHz, CDCl3) δ 2.00 (ddd, J = 14.0, 8.3, 4.8 Hz, 1H, CHHβ-6), 2.16 (ddd, J = 14.0, 9.1, 2.6 Hz, 1H, CHHα-6), 2.92 (dd, J = 4.8, 3.0 Hz, 1H, CH-8), 3.03 (dd, J = 17.9, 4.9 Hz, 1H, CHHβ -9), 3.13 (ddd, J = 12.0, 9.1, 8.3 Hz, 1H, CH-7), 3.36 (ddd, J = 17.9, 3.0, 0.8 Hz, 1H, CHHα-9), 3.61 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.88 (d, J = 12.0 Hz, 1H, 7-CH), 5.29 (dd, J = 4.7, 2.6 Hz, 1H, CH-5), 6.49 (s, 1H, CH-1), 7.09 – 7.46 (m, 15H, ArH). 13C{H} NMR (101 MHz, CDCl3) δ 37.54 (CH2-9), 39.20 (CH2-6), 39.86 (CH-7), 44.51 (CH-8), 54.66 (CH-5), 55.89 (OCH3), 59.18 (7-CH), 60.85 (OCH3), 61.44 (OCH3), 109.41 (CH-1), 124.62 (Ar), 124.82 (2C), 126.17, 126.50, 126.65, 127.89 (2C), 128.07 (2C), 128.63 (2C), 128.86 (2C), 128.91 (2C), (ArH), 131.44, 139.97, 142.11, 143.00, 143.57, 149.78, 152.83 (Ar), 172.72 (C=O). GC-MS (EI 70eV): decomposition. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C34H34NO4 520.2488; Found 520.2482.