1. Introduction
Formaldehyde is the simplest, smallest and most reactive aldehyde with high toxicity. It is an important metabolic intermediate physiologically produced in all cells [
1]. In food raw materials, formaldehyde is generated through methanol oxidation catalysed by alcohol dehydrogenase, catalases and cytochrome P450 enzymes [
2]. Methanol can be sourced from the demethylation of DNA, RNA, histone [
3] in all living beings and pectin [
4] in plants. Formaldehyde is also produced by methylamine deamination, glycine oxidation, glycine cleavage system in animals, oxidation of trimethylamine in fish and decomposition of lentinic acid in shiitake mushroom [
5]. In processed foods, formaldehyde can be produced using carbohydrates, lipids, ascorbic acid, and amino acids as precursors during food processing, especially from the direct cleavage of α-dicarbonyl compounds or their Strecker degradation [
6]. The multiple formation pathways make it ubiquitously exist in various foods.
Acute formaldehyde exposure causes irritation and injury the skin, eyes and upper respiratory mucosa. Chronic exposure of formaldehyde induces genetic toxicity, neurotoxicity, reproductive toxicity, and respiratory damage [
6]. Continuous long-term low-dose of formaldehyde inhalation impairs the learning and memory functions, and induces patho logical changes in the lung and liver [
7]. Formaldehyde causes nasopharyngeal cancer and leukemia in humans, and has been classified as a Group Ⅰ carcinogen for humans by the International Agency for Research on Cancer (IARC) in 2004 [
1]. Its maximum daily dosage established by the WHO is between 1.5 and 14 mg g
−1 of body weight [
8].
However, the risks of free formaldehyde in foods are seldom considered because of its rapid metabolism to formic acid by dehydrogenase in humans after intake [
9]. EFSA (2014) demonstrated that food-sourced formaldehyde is negligible compared with its high turnover in the body, reaching 878-1310 mg/kg bw per day [
1]. In accordance with the endogenous situation, most of the formed formaldehyde in foods is eliminated rapidly after its formation during processing. Formaldehyde can be removed via evaporation, as well as the reaction with other food components because of its high reactivity [
6]. However, the knowledge of its specific disappearance mechanisms is limited.
In addition to discuss the toxic effects of formaldehyde, this review article focused on the disappearing pathway of formaldehyde from two aspects, the formation of formaldehyde-derived toxins and aromas in foods. We believe that a comprehensive understanding of formaldehyde from positive and negative view may help food scientists take strategies to transform its adverse effects to beneficial effects.
2. Toxic Effects of Formaldehyde
2.1. Acute Toxication
Acute inhalation exposure of formaldehyde (in the form of formalin) affects the eyes, noses, throats, skin, upper and lower respiratory tract [
10]. Formaldehyde corrosion in eyes induces burning sensation, lacrimation, conjunctivitis, corneal clouding, and even loss of vision. It also irritates the upper respiratory system and causes bronchial asthma, pneumonia, pulmonary edema, and bronchospasm [
10]. An acute short-term exposure of formaldehyde causes a rapid decline in vascular function in the upper extremities and an increased oxidative stress [
11]. Acute oral exposure to formaldehyde has been found to cause DNA damage, apoptosis, and central nervous system injury in the rabbits [
12]. High level of formaldehyde caused rapid fixation of blood and acute circulatory disturbance, thus damage almost each organ in a case of human. In a woman who died after accidentally drinking formalin, formaldehyde level in the blood and stomach reached 36.56 mg/kg and 274.48 mg/kg, respectively. This high level of formaldehyde caused rapid fixation of blood and protein coagulation in tissues. The anatomical examination showed that the organs exhibited extreme changes. These changes include the congestion in stomach, liver, kidneys, spleen and pancreas, intestinal and brain oedema, trachea and lung haemorrhage, and cardiomyocytes necrosis [
13].
2.2. Chronic Toxication
The high reactivity of formaldehyde together with its high permeability into cells make it easily modify proteins as well as nucleic acid. Formaldehyde is a well known cross-linking agent that can modify the proteins, including nucleoprotein. It reacts with amino groups in proteins to produce a methylol adducts that is dehydrated to yield a labile Schiff-bases (Mannich base). This intermediate can react with the proximal amino acid residues, including lysine, cysteine, arginine, and tyrosine, and results in an intra- and extra- crosslinking of the proteins [
14,
15]. This cross-linking of proteins and other macro biological molecules may mainly contribute to its chronic toxicity. It was found that chronic occupational exposure to formaldehyde expressed deleterious effects on different organs in humans, including lung, upper respiratory tract, bone marrow and brain [
16]. Chronic exposure can produce carcinogenic, neurotoxic, reproductive, allergic, immunological, genetic and respiratory toxicities.
2.2.1. Carcinogenesis
Dozens years ago, an investigation of more than 25000 formaldehyde workers in 10 plants indicated that nasopharyngeal cancer markedly increased compared with the ordinary people. Based on toxicological and epidemiological data from nasopharyngeal cancer and leukemia studies obtained in workplaces, sufficient evidence confirmed a linkage between formaldehyde exposure and nasopharyngeal cancer, nasal and paranasal cancer, and leukemias [
17]. Therefore, IARC classified formaldehyde as human carcinogen (Group Ⅰ) for nasopharyngeal cancer in 2004 and leukemia (especially myeloid leukemia) in 2012 [
17,
18]. Due to taking careful protective measures in the working places, recent epidemiological data suggest that the correlation between formaldehyde occupational exposure and the occurrence of cancer is limited [
18].
2.2.2. Genotoxicity and Mutagenicity
Formaldehyde can react with DNA and displays genotoxicity during mutation tests in in vitro and in vivo [
19]. The National Institute for Occupational Safety and Health (NIOSH), American Conference of Governmental Industrial Hygienists (ACGIH), and Occupational Safety & Health Administration (OSHA), all advised that exposure level of formaldehyde above the exposure limits exhibit adverse effects on the biomarkers of genotoxicity [
16]. These biomarkers include sister chromatid exchanges, DNA-protein cross-links, and micronucleus frequency, which play an important role in the carcinogenicity. The primary DNA alternations after formaldehyde exposure are DNA-protein crosslinks and mutation in the phosphorylation of the tumour suppressor p53 [
20]. Formaldehyde increased DNA-protein crosslinks, sister-chromatid exchanges, micronuclei frequency, and cytotoxicity in V79 Chinese hamster cells in a concentration-dependent manner [
19]. In accordance, human studies on occupational formaldehyde exposure also showed increases in DNA damage, micronucleus formation, sister chromatid exchanges and chromosome aberrations in peripheral lymphocytes and nasal mucosa [
19]. Sister-chromatid exchanges, an index of DNA replication product interchanges between sister chromatids, markedly increased in peripheral blood lymphocytes from 57 pathologists with occupational exposure to formaldehyde (55.2 μg/m
3) in comparison with the controls [
21]. Also, DNA mono-adduct and DNA–protein crosslinks are present in all tissues of rats after inhalation exposure to 1, 30, and 300 ppb formaldehyde for 28 days [
22]. These findings confirmed the genotoxic effect of formaldehyde in humans.
Formaldehyde also induces mutagenicity. Gaseous exposure showed direct, time-dependent and dose-dependent mutagenic activity in five
Salmonella typhimurium test strains (TA98, TA100, TA1535, TA102 and TA1537) [
23].
2.2.3. Respiratory and Lung Toxicity
Epidemiological studies have shown significant relationship between formaldehyde exposure levels and the incidence, as well as the severity of various respiratory diseases. Formaldehyde causes inflammation in the respiratory tract via oxidative stress, immunological activation, airway remodelling, aggravating preexisting pulmonary inflammation, and compromising lung function [
24]. Formaldehyde damages respiratory epithelial cells that leads to loss of function [
16]. For example, emphysema occurred in rabbits after formaldehyde exposure. Histological observation revealed a ciliated shedding of nasal mucosal cells, vascular congestion, subepithelial edema, cell proliferation, peribronchial lymphocyte infiltration [
25]. Formaldehyde induces dose-dependent oxidative stress, which is detrimental to respiratory tissues and has time-dependent carcinogenic effect in the upper respiratory tract. Oxidative stress occurred during occupational air-formaldehyde exposure at a level lower than 0.10 ppm, which is regulatory limit of air-formaldehyde exposure in occupational settings [
25].
2.2.4. Neurotoxicity
Epidemiological studies have shown that work-related exposure to formaldehyde results in headaches, anxiety, fatigue, sleep disorders, and cognitive disorders [
26]. In several pathological conditions, including Alzheimer’s disease, an increase in the expression of formaldehyde-generating enzymes and elevated levels of formaldehyde in brain have been reported [
27]
Formaldehyde affects neurological function through non-enzymatic condensation with neuramines, catecholamines, and indoleamines to form tetrahydroisoquinoline and tetrahydro-beta carboline (THBC), respectively. In mice, THBC has been shown to cause loss of passive avoidance retention and reduction in spontaneous motor activity [
28]. Through meta-analysis and bioinformatics analysis, formaldehyde may mediate Alzheimer’s disease, Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and brain cancer by regulating the dysregulation of folate metabolic pathways [
29]. Moreover, formaldehyde cross-links amyloid-beta (Aβ) monomer to form Aβ dimer, oligomers, and fibrils. These aggregation products induced neurotoxicity and have been regarded positively correlated with the degree of dementia in Alzheimer’s disease patients [
30].
Formaldehyde still affects memory, learning, and behavior. Hippocampus is an area related to learning and memory consolidation [
31]. It is well established that this neurotoxin affects the CA1 and CA3 regions of the hippocampus, which play crucial roles in memory consolidation [
31].
2.2.5. Reproductive and Developmental Toxicity
In male rats and mice, formaldehyde reduces sperm counts and increases rates of deformed sperm cells, leads the sperm malformation, increases the bone marrow micronucleus rate, damages testicular tissues and decreases the levels of serum testosterone [
32]. Formaldehyde induces chromosome and DNA damage, oxidative stress, altered level and/or function of enzymes, hormones and proteins, apoptosis, toxicogenomic and epigenomic effects, which all contribute to reproductive toxicities [
32].
In female mice, formaldehyde causes histological alteration in ovary and uterus, including irregular estrous cycles, damaged and smaller oocytes and fewer mitochondria and fibrosis in reproductive tissue [
32,
33]. The expression of Fas gene and the enhancement of caspase activity may be an important mechanism of formaldehyde-induced ovarian toxicity in female animals. The expression of Fas gene and caspase-8 mRNA and the activities of caspase-8 and caspase-3 in the ovarian tissue of formaldehyde-exposed animals were significantly higher than those of the control groups, and increased with the increase of dose [
34].
In humans who suffer from occupational exposure to formaldehyde, increases in menstrual disorders and infertility have been observed [
32] Formaldehyde also shows developmental toxicities, including spontaneous abortion, stillborn births, congenital malformations and other structural abnormalities, low birth weight and premature births [
32]. A significant linear trend has been found that the increasing of serum formaldehyde concentration level led to the increasing risk of miscarriage among 118 women with a diagnosed miscarriage and 191 healthy women [
35]. Maternal formaldehyde exposure during pregnancy increases the risk of spontaneous abortion, and congenital heart malformations by 24% [
33].
Moreover, formaldehyde exhibits allergic effect. Formaldehyde and its releasers (agents that release formaldehyde after usage) are usually present in cosmetics, pharmaceuticals, household detergents, and industrial applications including adhesives, paints, lacquers and metalworking fluids [
36]. They can induce contact allergy and allergic contact dermatitis. In 1950s, the incidence occurred as high as 3.9 % in Western Europe and North America and it decreased to 1.5%~2.5% at the present [
37].
Although formaldehyde exhibits various deleterious effects for humans and animals, the beneficial effects of this endogenous generation compound arouses attention in recent years. It has been found that the low concentration of formaldehyde increases human melanoma cell proliferation, MAPK pathway activation, and support the survival of one-carbon-cycle-defective cells recently [
38].
3. Formaldehyde-Derived Harmful Compounds
Apart from its own toxicity, formaldehyde can be transformed to other harmful compounds during food processing, including heterocyclic aromatic amines, methylimidazole, advanced glycation-end products, N-nitrosamines, acrolein and acrylamide.
3.1. Participation of Formaldehyde in the Formation of Heterocyclic Aromatic Amines
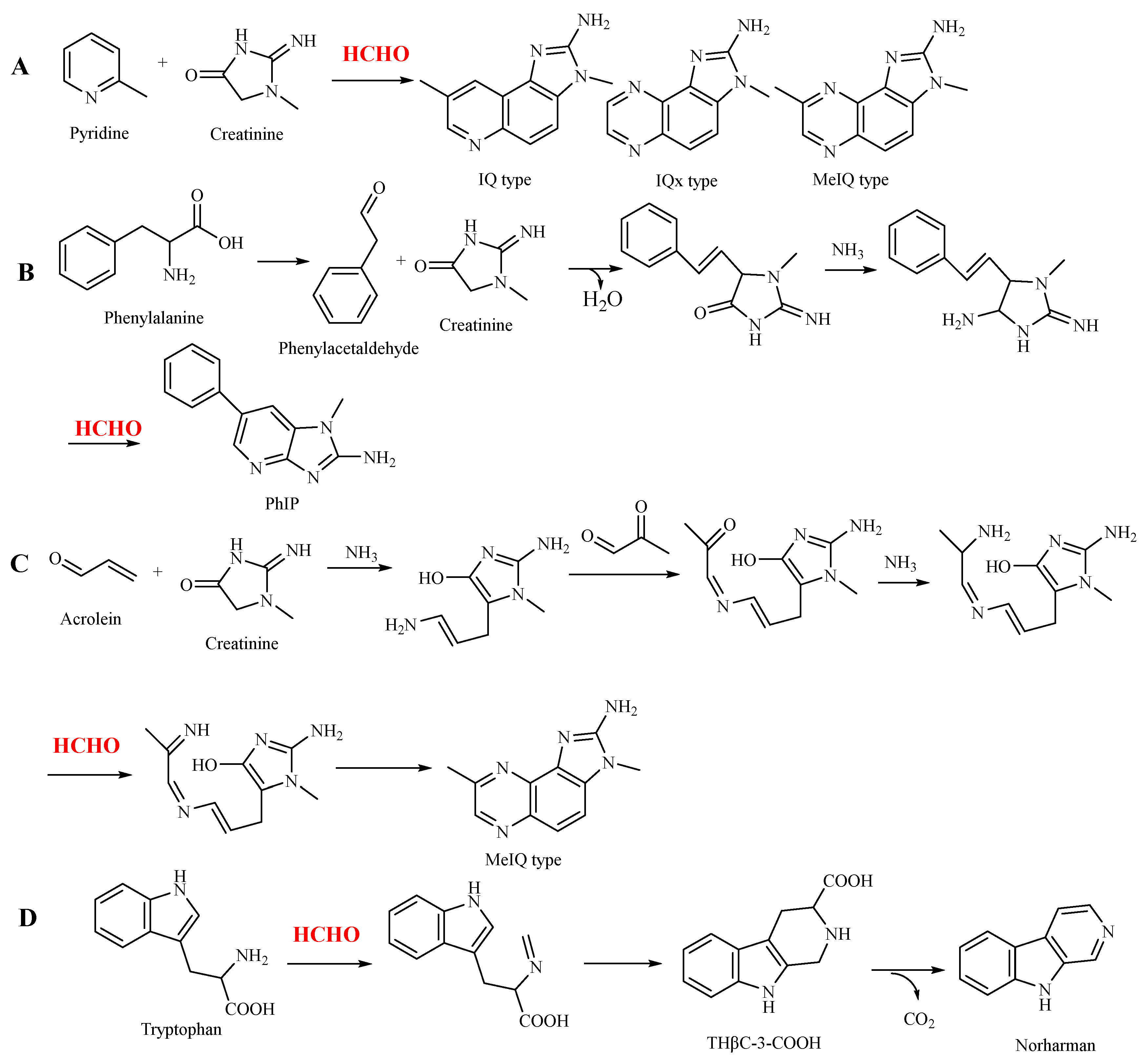
Heterocyclic aromatic amines are highly mutagenic and carcinogenic substances. They are a group of cluster compounds that have a heterocyclic structure commonly comprising three aromatic cycles with nitrogen atoms and can be divided into two groups, aminoimidazoazaarenes and amino carbolines, or five-membered amines of a heterocyclic and six-membered amines of a heterocyclic nature [
39]. Four types of heterocyclic aromatic amines are produced at a higher extent under processing and cooking conditions [
40], including 2-amino-3-methylimidazo [4,5-f]quinoline (IQ), 2-amino-3,4-dimethyl-imidazo [4,5-f]quinoline (MeIQ), 2-amino-3,8-dimethyl-imidazo [4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP). All of them have an imidazole ring and a pyridine or pyrazine ring (
Figure 1). Pyrazines are produced via Strecker degradation between α-dicarbonyl compounds and amino acids, whereas pyridines have been recently proposed by cyclization and oligomerization of short-chain reactive aldehydes with ammonia and ammonia-producing compounds [
41].
The formation of three types of heterocyclic aromatic amines requires formaldehyde (
Figure 1). IQ heterocyclic aromatic amines use creatinine (from creatine) as the precursor, which reacts with pyridine or pyrazine along with formaldehyde to generate IQ, IQx and MeIQ (
Figure 1A). Given the participation of formaldehyde, creatinine also reacts with phenylacetaldehyde (from phenylalanine) to generate PhIP-type heterocyclic aromatic amines (
Figure 1B). However, the accurate formation mechanism remains unknown. Hidalgo and Zamora [
42] proposed a more detailed formation pathway (
Figure 1C), in which other reactive carbonyl compounds are involved in their formation, including acrolein and methylglyoxal (
Figure 1C).
Moreover, formaldehyde plays a role in the generation of norharman (
Figure 1 4D). In this pathway, formaldehyde reacts with tryptophan via the Pictet–Spengler reaction to generate tetrahydro-β-carbolines (THβCs). THβCs can be formed under moderate conditions, and up to 500 mg/L THβCs has been detected in foods [
43]. Under thermal processing conditions, THβCs can be oxidized to generate norharman [
44].
3.2. Participation of Formaldehyde in the Formation of Methylimidazole and Imidazole-Type Advanced Glycation End Products
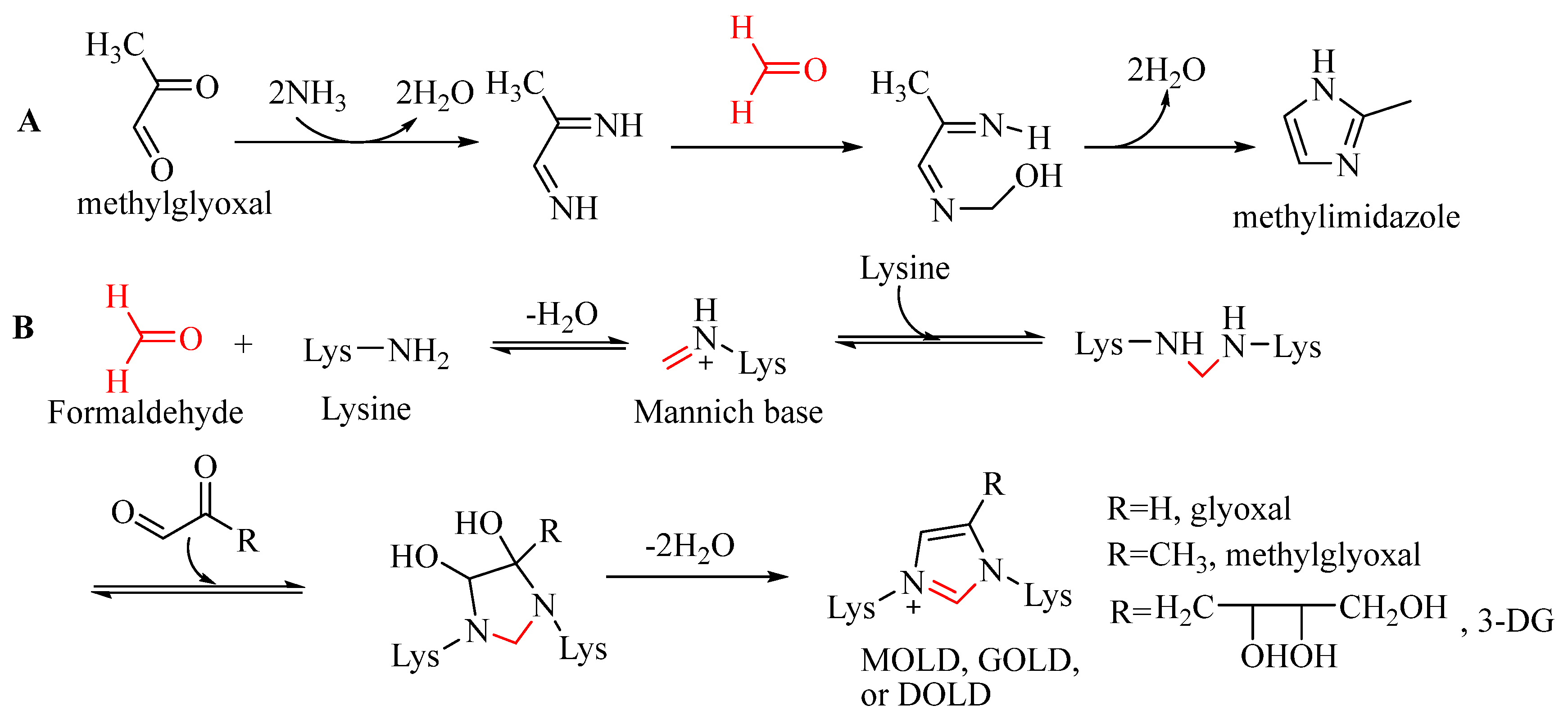
Methylimidazoles, including 2-methylimidazole and 4(5)-methylimidazoles, are produced by Maillard reaction in thermally processed foods and beverages [
45]. They are classified as ‘possibly carcinogenic to humans’ (2B) by the IARC in 2011 [
46], and proved to induce hyperexcitation, convulsions and anaemia in animals [
47]. Methylimidazoles can also decrease sperm mobility by disrupting the blood–testis barrier integrity [
48]. The precursor of methylimidazoles is methylglyoxal, which is produced from glucose degradation via the Maillard reaction and caramelization. Two formation pathways of methylimidazoles have been proposed, and formaldehyde is involved in each pathway. The first pathway is the ammonolysis reaction proposed by Moon and Shibamoto [
49]. The ammonolysis of methylglyoxal produces formamide, which subsequently reacted with 2-aminopropanal (produced from the reaction of formaldehyde with acetamide) to produce 4- or 5-methylimidazole. The second one is based on the Debus–Radziszewski imidazole synthesis (
Figure 2A), in which methylglyoxal reacts with ammonia to generate an intermediate and then with formaldehyde to generate methylimidazoles [
45,
50].
Dietary advanced glycation end products (dAGEs) are complex and heterogeneous compounds derived from nonenzymatic glycation reactions during food processing and cooking, they are closely associated with various chronic diseases like endogenous AGEs [
51]. 1,2-Dicarbonyl compounds, such as glyoxal and methylglyoxal, are key precursors of dAGEs. They react with lysine and arginine residuals to produce various AGEs, such as carboxymethyl lysine, imidazolium cross-link glyoxal lysine dimer (GOLD), carboxyethyl lysine, methylglyoxal lysine dimer (MOLD), etc. [
52]. Amongst them, GOLD, MOLD and DOLD are formed by the participation of a C 1 unit (
Figure 2B), with a structure similar to methylimidazoles. Brinkmann et al. [
53] proposed a pathway for their formation. During the reaction, one molecule of glyoxal or methylglyoxal reacts with the amino groups to generate diamine and then with another dicarbonyl compound. After oxidation to release a molecule of formic or acetic acids and then dehydration, an imidazolium end-product with a C 1 unit is formed.
Formaldehyde can be generated exogenously in foods and endogenously in humans [
6]. Considering that formaldehyde can react with amino groups easily along with dicarbonyl compounds (at a physiological temperature) to generate imidazole salts [
6,
54], here we propose an alternative way to generate GOLD, MOLD and DOLD as shown in
Figure 2B. In this pathway, ε-NH
2 of lysine residual reacts with formaldehyde to generate a Mannich base intermediate, and another lysine residual reacts with Mannich base to form an intermediate with two imino groups. Both imino groups simultaneously attack the two carbonyl carbons of dicarbonyls and then dehydrate two water molecules to generate imidazoles.
3.3. N-Nitrosamines
N-nitrosamines are a class of mutagenic and carcinogenic compounds characterized by a nitroso functional group, which is attached directly to a nitrogen atom; the amino nitrogen lone-pair electrons are delocalized into the π-electron system of double-bonded oxygen with two major contributing resonance forms. They are commonly found in drinking water, tobacco smoke, food, and household products [
55]
Nitrosation is favourable under acidic conditions. However, under neutral and basic conditions, formaldehyde can efficiently catalyse the nitrosation of secondary amines to generate N-nitrosamines [
56,
57]. In this pathway, the secondary amines react with formaldehyde to give an iminium ion intermediate, which then reacts with nitrite to yield a nitrosamine coupled with the elimination of formaldehyde (
Figure 3A). Formaldehyde markedly increased nitrosamine formation. In the presence of 0.05 M formaldehyde, nitrosamine formation from dimethylamine increased by 110% at pH 3.0, 170% at pH 3.5, 240% at pH 4.0, 1700% at pH 5.0 and 2000% at pH 6.0 after 3.5-h incubation at 37 °C [
58].
The catalytic efficiency of formaldehyde is influenced by amines. At pH 7, the presence of formaldehyde enhanced N-nitrosamine formation by a factor of 26, 29, 10, 152 and 5 from dimethylamine, methylethylamine, diethylamine, pyrrolidine and morpholine, respectively [
59].
Moreover, formaldehyde can directly react with amino acids, such as cysteine, serine and threonine, to generate N-nitrosamines. During their formation, formaldehyde reacts with the amino group to produce a Mannich base, which reacts with the SH group in cysteine or the OH group in serine (Ser) or threonine (Thr) to generate heterocyclic carboxylic acids [
60]. The heterocyclic carboxylic acids are readily nitrosatable under acidic conditions, and they react with nitrite to produce various N-nitroso compounds [
61]. In stead of catalysis, formaldehyde is involved in the molecules of N-nitrosamines in this formation mechanism (
Figure 3B). Under neutral or basic conditions, we hypothesized that the imino groups in the heterocyclic carboxylic acids can further react with a formaldehyde molecule to form iminium ion, which then reacts with nitrite to generate N-nitrosamines (
Figure 3B). Considering that formaldehyde can greatly increase the formation of N-nitrosamines [
58,
59], this mechanism may induce the production of a large quantify of N-nitrosamines from the above-mentioned amino acids during thermal processing of foods. Moreover, 1,2-dicarbonyl compounds have been reported to generate formaldehyde, especially under basic conditions [
6]. Therefore, typical dicarbonyl compounds, such as glyoxal, methylglyoxal and 1-deoxyosone ubiquitously existing in foods, may also promote N-nitrosamine formation in the presence of nitrite.
Apart from the participation of the above-mentioned harmful compounds; formaldehyde can be converted to acrolein; a highly toxic agent that promotes the occurrence and development of various diseases; including cardiovascular disease; alcoholic liver disease; Alzheimer’s disease; diabetes; aging and chronic obstructive pulmonary disease [
62,
63]. The cross-condensation of formaldehyde and acetaldehyde yields acrolein; which easily occurred and was the first commercial synthesis method of acrolein discovered in 1942 [
64]. In thermally processed foods; acrolein can be converted to acrylic acid and then generate acrylamide. Acrylamide was classified as a priority control contaminant by the US Environmental Protection Agency; as a group 2A carcinogen by IARC. It caused neurotoxicity; reproductive, developmental toxicity and carcinogenicity [
65,
66]
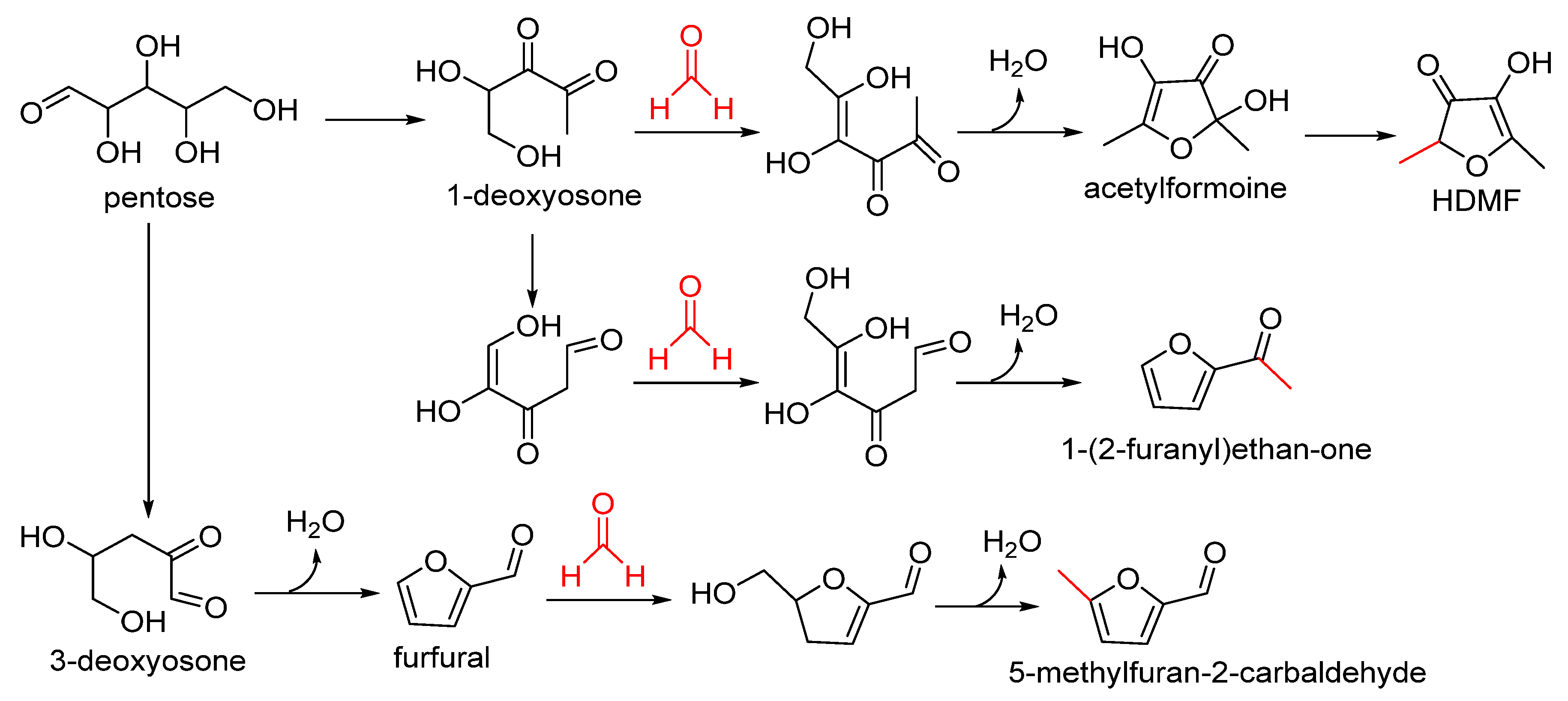
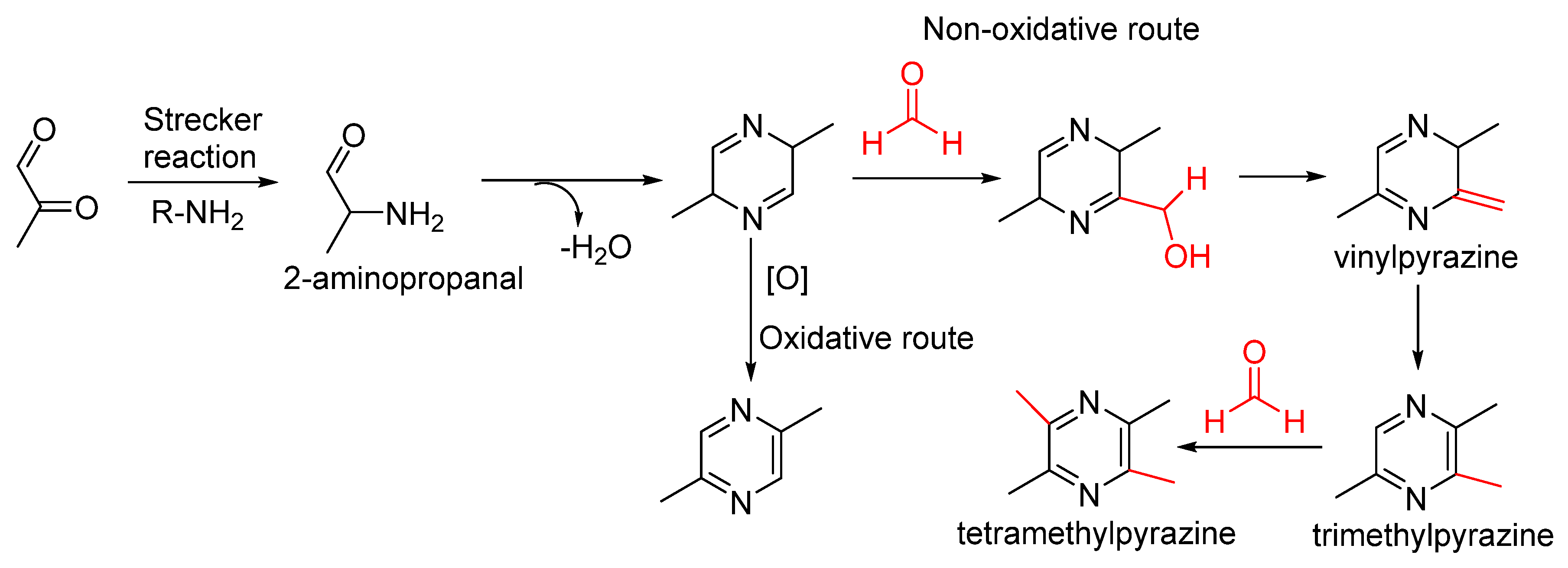
4. Flavour Formation in Foods
Although formaldehyde is a toxic contaminant and produces derivative harmful compounds in foods, it still plays a role in flavour formation, especially heterocyclic aromas, including oxygen-, sulphur- and nitrogen-containing ones [
67]. Here, we take furanones, pyrazines and thiazolidines as the sample of formaldehyde formation of oxygen-, sulphur- and nitrogen-containing heterocyclic aromas.
2.3. Thiazoles
Thiazoles are five-membered aromatic rings containing sulphur along with nitrogen. The most important types of thiazoles are alkylthiazoles, acetylthiazoles and hydroxyethylthiazoles. Alkylthiazole, such as 2,4,5-trimethylthiazole, can be found in meat, potato, coffee, cooked beef, heated lamb and cooked chicken. It has a chocolaty, nutty, coffee-like aroma, with a meaty flavour [
67].
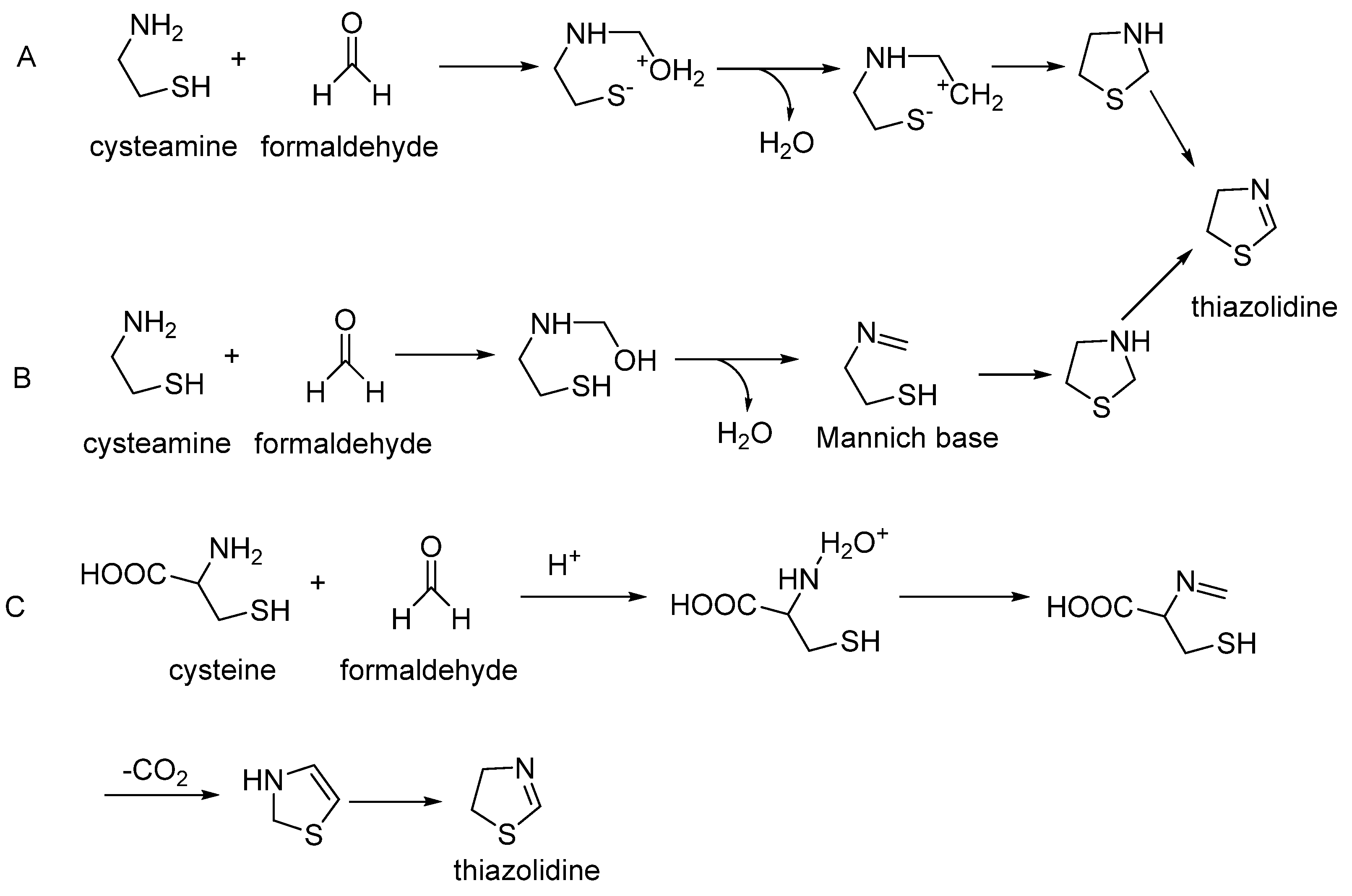
Sakaguchi and Shibamoto found that thiazolidine was detected in the reaction mixture of D-glucose and cysteamine [
88], which is generated from the reaction of glyoxal with cysteine [
89]. Amine on cysteamine attacks the carbonyl group on formaldehyde; the protonation of the hydroxyl group leads to the presence of a water molecule and carbocation. Another nucleophilic attack of the thiolate on carbocationic carbon leads to the formation of a substituted thiazolidine (
Figure 6A). The amine of cysteamine can react with formaldehyde to produce a Mannich intermediate, which attacks the SH group and results in a five-atom cycle, and then can dehydrogenate to form thiazolidine (
Figure 6B). Du found that cysteine can directly react with formaldehyde to generate thiazolidine [
90]. They proposed that the amine of cysteine undergoes nucleophilic reaction with formaldehyde following a similar pathway to cysteamine. After cycling of the Mannich base, this intermediate undergoes decarboxylation and then oxidizes (dehydrogenation) to produce thiazolidine (
Figure 6C).
In addition to thiazolidine, formaldehyde participates the formation of other sulfur-containing cyclic aromas, including thiophene, thiazoles and polysulfides. For example, 5-methyl-2-thiophene, 3-(methylsulfanyl) thiophene, 2-methylthiazole, 2,5-dimethyl-thiazole are derived from formaldehyde [
91,
92]. Futhermore, formaldehyde easily reacts with hydrogen sulfide to generate various polysulfides, including 1, 2, 5, 6-tetrathiacyclooctane, 3, 5-dimethyl-1, 2, 4-trithiolane, 1, 2, 5-trithiepane, 4, 6-dimethyl-1, 2, 3-trithiane, and 1, 2, 3-trithiolane [
93]. This type of sulfur-containing compounds has low aroma threshold and contribute significantly to flavor [
93].
5. Perspective
Formaldehyde is a toxic aldehyde that is related to various deiseases, including cancer. However, food scientists seldom take concern about formaldehyde in foods because of its very low level in processed foods, especially thermally processed ones. Considering that formaldehyde is scarcely detected in heat-treated foods prepared from food materials with a high content of formaldehyde (fish, shiitake mushroom, etc.), this reactive aldehyde is supposed to be be converted to other compounds. Therefore, future research should focus on three aspects. Firstly, elucidate its formation mechanism and estimate its potential or actual formation level in thermally processed foods. Secondly, conduct in-depth investigation on the transformation mechanism of formaldehyde to other compounds, namely, its elimination pathway during food processing. Thirdly, strategies to inhibit its formation must be taken, and take measures to divert the formation pathway of harmful compounds to beneficial ones.
Author Contributions
Conceptualization, X.Y.-S., and C. M. -Y.; writing—original draft preparation, X.Y.-S., W.Y.-Z. and Y. X. -L; writing—review and editing, J.-Z., and J.Y.-O; supervision, S.Y.-O.; funding acquisition, S.Y.-O., J.-Z., and J.Y.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Education Department of Guangdong Province (2024GCZX011), and the National Natural Science Foundation of China (grant number 32372450, 32102097, 32472457).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Food Safety Authority. Endogenous Formaldehyde Turnover in Humans Compared with Exogenous Contribution from Food Sources. EFSA J. 2014, 12. [Google Scholar]
- Xiao, R.; He, R. Metabolism of Formaldehyde In Vivo. In Formaldehyde and Cognition; Springer Netherlands: Dordrecht, 2017; pp 21–46. [Google Scholar]
- Toh, J.D.W.; Crossley, S.W.M.; Bruemmer, K.J.; Ge, E.J.; He, D.; Iovan, D.A.; Chang, C.J. Distinct RNA N-Demethylation Pathways Catalyzed by Nonheme Iron Alkbh5 and FTO Enzymes Enable Regulation of Formaldehyde Release Rates. Proc. Natl. Acad. Sci. 2020, 117, 25284–25292. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in Plant Life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef]
- Reingruber, H.; Pontel, L.B. Formaldehyde Metabolism and Its Impact on Human Health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- 6 Li, Y.; Ou, J.; Huang, C.; Liu, F.; Ou, S.; Zheng, J. Chemistry of Formation and Elimination of Formaldehyde in Foods. Trends Food Sci. Technol. 2023, 139, 104134. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Tang, Y.; & Li, Z. The Toxicity of Continuous Long-Term Low-Dose Formaldehyde Inhalation in Mice. Immunopharmacol Immunotoxicol. 2016, 38, 495–501. [Google Scholar] [CrossRef]
- Fappiano, L.; Carriera, F.; Iannone, A.; Notardonato, I.; & Avino, P. A Review on Recent Sensing Methods for Determining Formaldehyde in Agri-Food Chain: A Comparison with the Conventional Analytical Approaches. Foods, 2022, 11, 1351. [Google Scholar] [CrossRef]
- Dhareshwar, S.S.; Stella, V.J. Your Prodrug Releases Formaldehyde: Should You Be Concerned? No! J. Pharm. Sci. 2008, 97, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Hagos, S.; Gebeya, D.; & Teklay, A. Effects of Formalin Toxicity among Preclinical I Medical Students, Adigrat University, Ethiopia. Cough, 2018, 42, 43–8. [Google Scholar] [CrossRef]
- Augenreich, M.; Stickford, J.; Stute, N.; Koontz, L.; Cope, J.; Bennett, C.; & Ratchford, S.M. Vascular Dysfunction and Oxidative Stress Caused by Acute Formaldehyde Exposure in Female Adults. Am J Physiol Heart Circ Physiol. 2020, 319, H1369–H1379. [Google Scholar] [CrossRef]
- Arici, S.; Karaman, S.; Dogru, S.; Cayli, S.; Arici, A.; Suren, M.; Karaman, T.; & Kaya, Z. Central Nervous System Toxicity after Acute Oral Formaldehyde Exposure in Rabbits: An experimental study. Hum Exp Toxicol 2014, 33(11),1141-1149.
- Zhang, L.; Li, Y.; Wang, L.; Zhang, S.; Zhang, G.; Zuo, M. . & Cong, B. A Fatal Case of Accidental Oral Formaldehyde Poisoning and Its Pathomorphological Characteristics. Int J Legal Med. 2022, 136, 1303–1307. [Google Scholar]
- Metz, B.; Kersten, G.F.; Hoogerhout, P.; Brugghe, H.F.; Timmermans, H.A.; De Jong, A.D. . & Jiskoot, W. Identification of Formaldehyde-Induced Modifications in Proteins: Reactions With Model Peptides. J Biol Chem. 2004, 279, 6235–6243. [Google Scholar] [PubMed]
- Tayri-Wilk, T.; Slavin, M.; Zamel, J.; Blass, A.; Cohen, S.; Motzik, A. . & Kalisman, N. Mass Spectrometry Reveals the Chemistry of Formaldehyde Cross-Linking in Structured Proteins. Nat Commun. 2020, 11, 3128. [Google Scholar]
- Bernardini, L.; Barbosa, E.; Charao, M.F.; & Brucker, N. Formaldehyde Toxicity Reports from In Vitro and In Vivo Studies: A Review and Updated Data. Drug Chem Toxicol. 2022, 45, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jahan, S.A.; & Lee, J.T. T. (2011). Exposure to Formaldehyde and Its Potential Human Health Hazards. J Environ Sci Health. C. 2011, 29, 277–299. [Google Scholar] [CrossRef]
- Protano, C.; Buomprisco, G.; Cammalleri, V.; Pocino, R.N.; Marotta, D.; Simonazzi, S. . & Vitali, M. The Carcinogenic Effects of Formaldehyde Occupational Exposure: A Systematic Review. Cancers, 2021, 14, 165. [Google Scholar]
- Kang, D.S.; Kim, H.S.; Jung, J.H.; Lee, C.M.; Ahn, Y.S.; & Seo, Y.R. R. Formaldehyde Exposure and Leukemia Risk: A Comprehensive Review and Network-Based Toxicogenomic Approach. Genes and Environ. 2021, 43, 1–10. [Google Scholar] [CrossRef]
- Umansky, C.; Morellato, A.E.; Rieckher, M.; Scheidegger, M.A.; Martinefski, M.R.; Fernández, G.A. . & Pontel, L.B. Endogenous Formaldehyde Scavenges Cellular Glutathione Resulting in Redox Disruption and Cytotoxicity. Nat commun. 2022, 13, 745. [Google Scholar] [PubMed]
- Ghelli, F.; Cocchi, E.; Bellisario, V.; Buglisi, M.; Squillacioti, G.; Santovito, A. Bono. R. The Formation of SCEs as an Effect of Occupational Exposure to Formaldehyde. Arch Toxikol. 2022, 96, 1101–1108. [Google Scholar] [CrossRef]
- Leng, J.; Liu, C.W.; Hartwell, H.J.; Yu, R.; Lai, Y.; Bodnar, W.M. . & Swenberg, J.A. Evaluation of Inhaled Low-Dose Formaldehyde-Induced DNA Adducts and DNA–Protein Cross-Links by Liquid Chromatography–Tandem Mass Spectrometry. Arch Toxikol. 2019, 93, 763–773. [Google Scholar]
- Chen, P.W.; Kuo, T.C.; Liu, Z.S.; & Lu, H.F. F. Assessment of the Mutagenicity of Two Common Indoor Air Pollutants, Formaldehyde and Toluene. Indoor Air. 2021, 31, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Afzal, M.; Goyal, A.; Gupta, G.; Thapa, R.; Kazmi, I. ;... & Dua, K. The Impact of Formaldehyde Exposure on Lung Inflammatory Disorders: Insights Into Asthma, Bronchitis, and Pulmonary Fibrosis. Chem Biol Interact, 2024; 111002. [Google Scholar]
- Squillacioti, G.; Bellisario, V.; Ghelli, F.; & Bono, R. Occupational Exposure to Formaldehyde and Oxidative Stress in Italian Workers. Eur J Public Health. 2021, 31, 584. [Google Scholar] [CrossRef]
- [26] Huang, J.W.; Lu, Y.; Zhang, B.; Yang, S.P.; Zhang, Q.; Cui, H.Y.; Lu, X.X.; Zhao, Y.; Yang, X.; & Li, R. Antagonistic Effffect of Epigallocatechin-3-Gallate on Neurotoxicity Induced by Formaldehyde. Toxicol. 2019, 412, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tulpule, K.; & Dringen, R. Formaldehyde in Brain: an Overlooked Player in Neurodegeneration? J Neurochem. 2013, 127, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, B.; Ismail, N.; Smith, K.; Li, T.; Dai, R.; & Deng, Y. Neurotoxicity and Underlying Mechanisms of Endogenous Neurotoxins. Int J Mol Sci. 2021, 22, 12805. [Google Scholar] [CrossRef]
- Rana, I.; Rieswijk, L.; Steinmaus, C.; & Zhang, L.P. P. Formaldehyde and Brain Disorders: A Meta Analysis and Bioinformatics Approach. Neurotox Res. 2021, 39, 924–948. [Google Scholar] [CrossRef]
- Kou, Y.; Zhao, H.; Cui, D. , Han, H.; & Tong, Z. Formaldehyde Toxicity in Age-Related Neurological Dementia. Ageing Res Rev. 2022, 73, 101512. [Google Scholar]
- Aydin, D.D.; Taşkınalp, O.; Uluçam, E.; Karadağ, H.; Özfidan, G.K.; Topuz, R.; & Ersoy, O. The Effect of Acute and Chronic Formaldehyde Exposure on Learning and Memory in Male and Female Rats. Int J Neurosci. 2023, 1–9. [Google Scholar] [CrossRef]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; & Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A Systematic Review. Mutat Res Rev Mutat Res. 2011, 728, 118–138. [Google Scholar] [CrossRef]
- Treesh, S.A.; Aburawi, S.M.; Elghedamsi, M.T.; Jaafari, H.A.E.; Alzowam, R.; Shibani, N.; & Khair, N.S. Protective Role of Vitamin C on Histopathological Effect of Formaldehyde on Reproductive System in Female Albino Mice (Histological Study). Int J Adv Res. 2019, 7, 529–540. [Google Scholar] [CrossRef]
- Peng, G.; Zhong, C.; Zhang, Q.; Xie, Y.; & Gong, F. Effect of Formaldehyde on E xpressions of Fas Apoptosis Pathway-Related Genes of Ovary Tissues in Female Rats. Journal of Central South University Medical Sciences, 2010, 35, 341–345. [Google Scholar] [PubMed]
- Xu, W.; Zhang, W.; Zhang, X.; Dong, T.; Zeng, H.; & Fan, Q. Association between Formaldehyde Exposure and Miscarriage in Chinese Women. Medicine, 96, e7146.
- Søgaard, R.; Poulsen, P.B.; Gelardi, R.M.; Geschke, S.; Schwensen, J.F.B.; & Johansen, J.D. Hidden Formaldehyde in Cosmetic Products. Contact Dermatitis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Goossens, A.; & Aerts, O. Contact Allergy to and Allergic Contact Dermatitis from Formaldehyde and Formaldehyde Releasers: A Clinical Review and Update. Contact Dermatitis, 2022, 87, 20–27. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, G.; Jing, M.; Han, Y.; Li, J.; Zhao, J. . & Chen, P.R. Genetically Encoded Formaldehyde Sensors Inspired by A Protein Intra-Helical Crosslinking Reaction. Nat Commun. 2021, 12, 581. [Google Scholar]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N. . & Aadil, R.M. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods, 2021, 10, 1466. [Google Scholar]
- Iammarino, M.; Marino, R.; Nardelli, V.; Ingegno, M.; & Albenzio, M. Red Meat Heating Processes, Toxic Compounds Production and Nutritional Parameters Changes: What about Risk–Benefit? Foods, 2024, 13, 445. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Formation of Heterocyclic Aromatic Amines with the Structure of Aminoimidazoazarenes in Food Products. Food Chem. 2020, 313, 126128. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Carbonyl Chemistry and the Formation of Heterocyclic Aromatic Amines with the Structure of Aminoimidazoazaarene. J. Agric. Food Chem. 2022, 70, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Peña, A.; Salgado, A. Identification, Formation, and Occurrence of Perlolyrine: A β-Carboline Alkaloid with a Furan Moiety in Foods. J. Agric. Food Chem. 2023, 71, 13451–13461. [Google Scholar] [CrossRef]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent Advances in Heterocyclic Aromatic Amines: An Update on Food Safety and Hazardous Control from Food Processing to Dietary Intake. Compr. Rev. Food Sci. Food Saf. 2020, 19, 124–148. [Google Scholar] [CrossRef]
- Hengel, M.; Shibamoto, T. Carcinogenic 4-Methylimidazole Found in Beverages, Sauces, and Caramel Colors: Chemical Properties, Analysis, and Biological Activities. J. Agric. Food Chem. 2013, 61, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Raters, M.; Elsinghorst, P.W.; Goetze, S.; Dingel, A.; Matissek, R. Determination of 2-Methylimidazole, 4-Methylimidazole, and 2-Acetyl-4-(1,2,3,4-Tetrahydroxybutyl)Imidazole in Licorice Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry Stable-Isotope Dilution Analysis. J. Agric. Food Chem. 2015, 63, 5930–5934. [Google Scholar] [CrossRef]
- Akbari, N.; Shafaroodi, H.; Jahanbakhsh, M.; Sabah, S.; Molaee- Aghaee, E.; Sadighara, P. 4-Methylimidazole, a Carcinogenic Component in Food, Amount, Methods Used for Measurement; a Systematic Review. Food Chem. X 2023, 18, 100739. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, W.; Tang, H.; Wu, X.; Yang, X.; Sun, F. 4-Methylimidazole Exposure Impairs Sperm Mobility by Reducing the Expression of Blood-Testis Barrier Junction Protein in Mouse Testes. Reprod. Biol. 2024, 24, 100928. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Formation of Carcinogenic 4-Methylimidazole in Maillard Reaction Systems. J. Agric. Food Chem. 2011, 59, 615–618. [Google Scholar] [CrossRef]
- Jang, H.W.; Jiang, Y.; Hengel, M.; Shibamoto, T. Formation of 4-Methylimidazole and Its Precursors, α-Dicarbonyl Compounds, in Maillard Model Systems. J. Agric. Food Chem. 2013, 61, 6865–6872. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Fu, L. Dietary Advanced Glycation End-products: Perspectives Linking Food Processing with Health Implications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2559–2587. [Google Scholar] [CrossRef] [PubMed]
- Eggen, M.D.; Glomb, M.A. Analysis of Glyoxal- and Methylglyoxal-Derived Advanced Glycation End Products during Grilling of Porcine Meat. J. Agric. Food Chem. 2021, 69, 15374–15383. [Google Scholar] [CrossRef]
- Brinkmann, E.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. ChemInform Abstract: Characterization of an Imidazolium Compound Formed by Reaction of Methylglyoxal and Nα-Hippuryllysine. ChemInform 1996, 27, chin–199610265. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, K.; Huang, C.; Zheng, J.; Zhou, H.; Ou, J.; Ou, S. Glycine and Serine Markedly Eliminate Methylglyoxal in the Presence of Formaldehyde via the Formation of Imidazole Salts. Food Chem. 2022, 369, 130952. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Wang, Q.; Chen, J.; Xia, W.; & Liao, E. Effects of Inoculating Autochthonous Starter Cultures on Changes of N-Nitrosamines and Their Precursors in Chinese Traditional Fermented Fish during In Vitro Human Digestion. Foods, 2024, 13, 2021. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, I.W.; Dirat, O.; Teasdale, A.; Whiting, M. Potential for the Formation of N -Nitrosamines during the Manufacture of Active Pharmaceutical Ingredients: An Assessment of the Risk Posed by Trace Nitrite in Water. Org. Process Res. Dev. 2020, 24, 1629–1646. [Google Scholar] [CrossRef]
- Eisenbrand, G.; Baum, M.; Cartus, A.T.; Diel, P.; Engel, K.-H.; Engeli, B. .. & Steinberg, P. Salivary Nitrate/Nitrite and Acetaldehyde in Humans: Potential Combination Effects in the Upper Gastrointestinal Tract and Possible Consequences for the in Vivo Formation of N-Nitroso Compounds—a Hypothesis. Arch. Toxicol. 2022, 96, 1905–1914. [Google Scholar]
- Kurechi, T.; Kikugawa, K.; Ozawa, M. Effect of Malondialdehyde on Nitrosamine Formation. Food Cosmet. Toxicol. 1980, 18, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breider, F.; Barrios, B.; Minakata, D.; Deng, H.; Von Gunten, U. Role of Carbonyl Compounds for N -Nitrosamine Formation during Nitrosation: Kinetics and Mechanisms. Environ. Sci. Technol. 2024, 58, 4792–4801. [Google Scholar] [CrossRef] [PubMed]
- Kamps, J.J.A.G.; Hopkinson, R.J.; Schofield, C.J.; Claridge, T.D.W. How Formaldehyde Reacts with Amino Acids. Commun. Chem. 2019, 2, 126. [Google Scholar] [CrossRef]
- Tricker, A.R.; Kubacki, S.J. Review of the Occurrence and Formation of Non-volatile N -nitroso Compounds in Foods†. Food Addit. Contam. 1992, 9, 39–69. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Liu, F.; Zheng, J.; Ou, J.; Zhao, D.; Ou, S. Origin and Fate of Acrolein in Foods. Foods 2022, 11, 1976. [Google Scholar] [CrossRef]
- Hong, J.; Song, Y.; Xie, J.; Xie, J.; Chen, Y.; Li, P. . & Yu, Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis Elegans. Foods, 2022, 11, 1590. [Google Scholar]
- Folliard, V.; Postole, G.; Devaux, J.F.; Dubois, J.L.; Marra, L.; & Auroux, A. Oxidative Coupling of A Mixture of Bio-Alcohols to Produce A More Sustainable Acrolein: an in Depth Look in the Mechanism Implying Aldehydes Co-Adsorption and Acid/Base Sites. Appl Catal B. 2020, 268, 118421.
- Govindaraju, I.; Sana, M.; Chakraborty, I.; Rahman, M.H.; Biswas, R.; & Mazumder, N. Dietary acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods.
- Pérez-Lucas, G.; Navarro, G.; & Navarro, S. Understanding How Chemical Pollutants Arise and Evolve in the Brewing Supply Chain: A Scoping Review. Foods, 2024, 13, 1709. [Google Scholar] [CrossRef] [PubMed]
- Chemistry and Technology of Flavors and Fragrances, 1st ed.; Rowe, D.J., Ed.; Wiley, 2004.
- Xiao, Q.; Huang, Q.; Ho, C.-T. Occurrence, Formation, Stability, and Interaction of 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone. ACS Food Sci. Technol. 2021, 1, 292–303. [Google Scholar] [CrossRef]
- Blank, I.; Fay, L.B. Formation of 4-Hydroxy-2,5-Dimethyl-3(2 H )-Furanone and 4-Hydroxy-2(or 5)-Ethyl-5(or 2)-Methyl-3(2 H )-Furanone through Maillard Reaction Based on Pentose Sugars. J. Agric. Food Chem. 1996, 44, 531–536. [Google Scholar] [CrossRef]
- Wang, Y.; Rodolfo Juliani, H.; Simon, J.E.; Ho, C.-T. Amino Acid-Dependent Formation Pathways of 2-Acetylfuran and 2,5-Dimethyl-4-Hydroxy-3[2H]-Furanone in the Maillard Reaction. Food Chem. 2009, 115, 233–237. [Google Scholar] [CrossRef]
- Hwang, Young-Sook; Kim, Sang-Hyun; Sin, Tae-Seon; Cho, Jun-Hyun; Lee, In-Seok; Oh, Kwang-Soo. Volatile Flavor Constituents of Cooked Oyster Sauce Prepared from Individually Quick-Frozen Oyster Crassostrea Gigas Extract. Korean J. Fish. Aquat. Sci. 2015, 48, 668–673. [Google Scholar]
- Lu, J.-W.; Lin, C.-Y.; Fang, M. Roasted Fish Reaction Flavor by Plant-Based Ingredients. Food Chem. 2024, 460, 140492. [Google Scholar] [CrossRef]
- Peralta, R.R.; Shimoda, M.; Osajima, Y. Further Identification of Volatile Compounds in Fish Sauce. J. Agric. Food Chem. 1996, 44, 3606–3610. [Google Scholar] [CrossRef]
- Sha, K.; Zhang, Z.-J.; Sun, B.-Z.; Li, H.-P.; Song, H.-L.; Lang, Y.-M.; Lei, Y.-H.; Li, H.-D.; Zhang, Y. Investigation of Physicochemical and Textural Characteristics and Volatile Compounds of Kazakh Dry-Cured Beef. Food Sci. Technol. Res. 2017, 23, 375–383. [Google Scholar] [CrossRef]
- Zhan, H.; Cui, H.; Yu, J.; Hayat, K.; Wu, X.; Zhang, X.; Ho, C.-T. Characteristic Flavor Formation of Thermally Processed N-(1-Deoxy-α-d-Ribulos-1-Yl)-Glycine: Decisive Role of Additional Amino Acids and Promotional Effect of Glyoxal. Food Chem. 2022, 371, 131137. [Google Scholar] [CrossRef]
- Cai, W.; Feng, T.; Yao, L.; Sun, M.; Song, S.; Wang, H.; Yu, C.; Liu, Q. Characterisation of Differential Aroma Markers in Roasted Coffee Powder Samples by GC×GC- TOF- MS and Multivariate Statistical Analysis. Food Biosci. 2024, 59, 104207. [Google Scholar] [CrossRef]
- Fayek, N.M.; Xiao, J.; Farag, M.A. A Multifunctional Study of Naturally Occurring Pyrazines in Biological Systems; Formation Mechanisms, Metabolism, Food Applications and Functional Properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 5322–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Cao, H.; & Xiao, J. Pyrazines in Food. Handbook of Dietary Phytochemicals, 2020, 1-25.
- Ren, A.; Zhang, Y.; Bian, Y.; Liu, Y.; Zhang, Y.; Ren, C.; Zhou, Y.; Zhang, T.; Feng, X. Pyrazines in Food Samples: Recent Update on Occurrence, Formation, Sampling, Pretreatment and Analysis Methods. Food Chem. 2024, 430, 137086. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Schmalzried, F.; Davidek, T.; Blank, I.; Kerler, J. Study on the Role of Precursors in Coffee Flavor Formation Using In-Bean Experiments. J. Agric. Food Chem. 2009, 57, 9923–9931. [Google Scholar] [CrossRef]
- Xing, H.; Yaylayan, V. Mechanochemistry of Strecker Degradation: Interaction of Glyoxal with Amino Acids. Food Chem. 2024, 439, 138071. [Google Scholar] [CrossRef]
- Shu, C.-K.; Lawrence, B.M. Formation of 2-(1-Hydroxyalkyl)-3-Oxazolines from the Reaction of Acyloins and Ammonia Precursors under Mild Conditions. J. Agric. Food Chem. 1995, 43, 2922–2924. [Google Scholar] [CrossRef]
- Rizzacasa, M.; Ricca, M. Chemistry and Biology of Acyloin Natural Products. Synthesis 2023, 55, 2273–2284. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Wang, X.-Y.; Zhu, B.-W.; Du, M.; Dong, L.; Dong, X.-P.; Xu, X.-B. Model Studies on the Formation of 2-Vinylpyrazine and 2-Vinyl-6-Methylpyrazine in Maillard-Type Reactions. Food Chem. 2022, 374, 131652. [Google Scholar] [CrossRef]
- Low, M.Y.; Parker, J.K.; Mottram, D.S. Mechanisms of Alkylpyrazine Formation in a Potato Model System Containing Added Glycine. J. Agric. Food Chem. 2007, 55, 4087–4094. [Google Scholar] [CrossRef]
- Parker, J.K.; Balagiannis, D.P.; Desforges, N.; Mottram, D.S. Flavor Development in a Meat-Based Petfood Containing Added Glucose and Glycine. In ACS Symposium Series; Mottram, D.S., Taylor, A.J., Eds.; American Chemical Society: Washington, DC, 2010; Vol. 1042, pp 85–93. [Google Scholar]
- Jiang, W.; Wang, X.; Ma, Y.; Du, M.; Wu, C.; Xu, X. Mechanism of Carbon Skeleton Formation of 2,3,5-Trimethylpyrazine via a Conversion Reaction between Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2023, 71, 5337–5344. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Shibamoto, T. Formation of Heterocyclic Compounds from the Reaction of Cysteamine and D-Glucose, Acetaldehyde, or Glyoxal. J. Agric. Food Chem. 1978, 26, 1179–1183. [Google Scholar] [CrossRef]
- Liu, M.; Yu, J.; Zhou, T.; Xu, H.; Hayat, K.; Zhang, X.; Ho, C.-T. Formation Priority of Pyrazines and 2-Acetylthiazole Dependent on the Added Cysteine and Fragments of Deoxyosones during the Thermal Process of the Glycine–Ribose Amadori Compound. J. Agric. Food Chem. 2022, 70, 11643–11651. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, Y.; Ma, Q.; Li, Y.; Wang, B.; Bai, S.; Fan, B.; Wang, F. The Number and Position of Unsaturated Bonds in Aliphatic Aldehydes Affect Meat Flavorings System: Insights on Initial Maillard Reaction Stage and Meat Flavor Formation from Thiazolidine Derivatives. Curr. Res. Food Sci. 2024, 8, 100719. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Rakotondrabe, T.F.; Chen, G.; & Guo, M. Advances in Microbial Analysis: Based on Volatile Organic Compounds of Microorganisms in Food. Food Chem. 2023, 418, 135950. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Xia, X.; Deng, S.; Cui, H.; Hayat, K.; Zhang, X.; & Ho, C.T. Reduced Asynchronism Between Regenerative Cysteine and Fragments of Deoxyosones Promoting Formation of Sulfur-Containing Compounds Through Extra-Added Xylose and Elevated Temperature During Thermal Processing of 2 Threityl-Thiazolidine-4-Carboxylic Acid. Food Chem. 2023, 404, 134420. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Y.; Xie, Y.T.; Yan, W.Y.; Ma, Y.K.; Hu, S.; & Yu, A.N. The Volatile Organic Compounds Generated from the Maillard Reaction Between L-Ascorbic Acid And L-Cysteine in Hot Compressed Water. J Sci Food Agric. 2024, 104, 5764–5775. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).