1. Introduction
Nowadays, food is intended to offer health benefits that extend beyond their nutritional value and consumer demand for functional foods has steadily increased.
With reference to the bread making sector, in recent years the scientific research has been focused on one hand to reduce the sodium intake [
1,
2,
3] and on the other hand to produce bread enriched in fibre, protein and/or bioactive compounds [
4,
5,
6,
7,
8,
9]. Tomato (
Lycopersicon esculentum Mill.) is one of the most widely cultivated vegetable crops in Mediterranean countries. Significant amounts are consumed in the form of processed products such as tomato juice, paste, ketchup, sauce and salsa. The report published by Ismea [
10] confirms that Italy has a fundamental role within the world production, being the third producer of fresh tomatoes destined for processing (15% of world production); it is also confirmed as the leading producer and exporter of tomato derivatives. Tomato processing cycles generate a by-product, known as tomato pomace (TP), representing at the most 4% of the fresh fruit weight; this percentage increases when small sized tomatoes as cherry and date tomatoes are processed, since they have a relatively high peel to volume ratio. Industrially derived TP is generally made up of a mixture of tomato peels and seeds, whose chemical composition is distinctly different from each other; on the whole, tomato pomace contains chemically diverse valuable components such as lycopene, phenols, dietary fibre, proteins, and oil [
11,
12,
13,
14], that are reported to possess anti-inflammatory, antioxidant, and chemopreventive properties [
15]. Furthermore, tomato peels were found to contain high levels of potassium ion and relatively low amounts of Na+; this makes the Na/K ratio lower, and tomato peels a potentially useful supplement for people suffering of cardiovascular diseases [
16].
Thus, considering the potential health benefits of tomato pomace, which is usually used as a feed additive, and the increasing consumers’ trend towards functional/fortified foods, several attempts for its incorporation into different food matrices such as crackers, cookies, bread, tarhana, pasta, hamburger, and tomato paste have been made [
13,
17,
18,
19]. Whilst tomato pomace addition undoubtedly improved the nutrient (lycopene, protein, dietary fibre) content of these foods as well as their antioxidant efficacy in vitro, sometimes it worsened their textural and sensory characteristicsc [
13,
20]. Moreover, the use of the tomato pomace as ingredients perfectly fits into the “Farm to Fork” (F2F) European Commission strategy, which aims to halve food waste by 2030 and ensure a sustainable food production.
To the best of our knowledge, no study has focused on the possible use of tomato pomace from “small-sized” tomatoes as an ingredient in the preparation of durum wheat bread. All this considered, aims of the present study were: i) to characterize bioactive compounds of small-sized tomato pomace obtained by different food-grade extractions; ii) to evaluate the effects of the addition of different amounts of tomato pomace to on the durum wheat dough for bread production; iii) to characterize the functional bread obtained by addition of different amounts of tomato pomace for physical, chemical, nutritional parameters, and sensory characteristics.
2. Materials and Methods
2.1. Preparation of Tomato Pomace Flour (TPF)
Tomato pomace (TP) (a mixture of peels, seeds and residual pulp in small amounts) was industrially obtained from the processing of small tomatoes (cherry and date tomatoes) and stored at -20 °C. To obtain a homogeneous matrix as an ingredient for durum wheat bread, TP was placed in a circulating air oven (ThermoScientific, Heratherm oven, MA, USA) at 80 °C overnight, then finely grinded using a blender (La Moulinette, Moulinex, Écully, France) and passed through a sieve with a mesh size of 500 µm. The tomato pomace flour (TPF) obtained was characterised for moisture, lipid content and fatty acid profile, fibre carotenoids, total polyphenol content and profile, antioxidant activity. All the analyses were performed in triplicate.
2.1.1. TPF Characterization
Moisture content was determined by drying at 105 °C until constant weight with a circulating air oven (ThermoScientific, Heratherm oven, MA, USA).
Lipid content was estimated according to the acid hydrolysis method [
21]. Five grams of grinded sample were placed with 2 mL of ethanol (95%) and 10 mL of HCl solution (25 mL HCl 37% + 110 mL distilled water) in a glass flask and heated at 70–80 °C for 30 min with stirring. After cooling, were added 10 mL of ethanol (95%), and the sample was transferred to a separatory funnel. The glass flask was washed with 25 mL of ethyl ether, after 25 mL of petroleum ether was added and the mixture was stirred. The extraction of total lipids was repeated twice with 25 mL of ethyl and petroleum ethers (1:1). After separation the ether extracts were filtered on anhydrous Na
2SO
4 and evaporated under vacuum. The lipid residue was dried in a 100 °C oven (ThermoScientific, Herathermoven, Italy) to constant weight.
Total (TDF), soluble (SDF) and insoluble (IDF) dietary fibre was determined according to the enzymatic method AOAC 991.43 using the Total Dietary Fiber Assay Kit (K-TDFR, Megazyme, Ireland).
Carotenoids determination was conducted according to Jaeger de Carvalho et al. [
22]. 15 g of sample were extracted with 25 ml of acetone and filtered under vacuum. The extraction was conducted three or more time until the TP became colourless. Successively, the acetone extract was transferred in a separatory funnel, an aliquot of petroleum ether was added, and the acetone was removed by the addition of distilled water. The water was discarded, and the procedure was repeated four times. The ether fraction was passed on anhydrous sodium sulphate, transferred in a volumetric flask and the volume was made up by petroleum ether. The absorbance was read at 450 nm, using a Perkin Elmer lambda 25 UV-VIS spectrometer.
2.1.2. TPF Fatty ACID Profiles
Fatty acid methyl esters (FAMEs) were prepared according to Regulation (EU) 2022/2105 [
23]. In a screw-top test tube 0.1 g of the extracted oil was mixed with 2 mL of heptane. The methanolic solution of potassium hydroxide (2 M; 0.2 mL) was then added, the cap fitted with a PTFE joint was screwed on and shaken vigorously for 30 seconds. After stratification the upper phase becomes clear and containing the FAMEs. The separation of FAMEs was carried out using a Shimadzu gas chromatograph (Shimadzu GC-17A) equipped with a flame ionization detector (FID) and a capillary column (DB-wax; Agilent Technologies, Wilmington, DE; 30 m length ×0.25 mm i.d. and 0.25 μm of film thickness). As a carrier gas was used helium (flow rate of 1.5 ml/min). The initial oven temperature was kept at 165°C for 15 min and then programmed to rise at 5 °C/min up to 200 °C, maintained for 2 min, and followed by a second gradient of 5 °C/min to a final temperature of 260 °C, which was held for 5 min. The injector and detector temperatures were 260 and 300 °C, respectively. Hydrogen and compressed air were used for the flame detector. The injection volume was 1 μL with a split ratio of 50. Identification of each fatty acid was performed by a comparison the retention times of the corresponding peak with that of reference standards. Results were expressed as percentages of total fatty acids.
2.1.3. Extraction of TPF Polyphenols
An aliquot (about 150 g) of TPF described above was suspended in 400 mL of three different solvents/solvent mixtures: distilled Water (W); distilled Water:Ethanol 75:25v/v (WE) and distilled Water:Ethanol:formic Acid 70:29:1 v/v (WEF). Extractions were carried out overnight at 4 °C in the dark; after that, they were filtered using a Whatman® qualitative filter paper, Grade 1 (Merck KGaA, Darmstadt, Germany) with the help of a vacuum pump. After removing the alcohol present in WE and WEF samples at reduced pressure (Rotavapor®, BUCHI) the resulting aqueous solutions (WE, WEF and W) were frozen at -20 °C and then lyophilized with a yield of 3.45 ± 0.07%.
2.1.4. Total Polyphenol Content and Antioxidant Activity of TPF Extracts
TPF extracts (W, WE, WEF) obtained as described in the previous paragraph were characterized for total polyphenol content (TPC) and antioxidant activity (AA). An aliquot of the lyophilized samples (1 g) was resuspended in distilled water (8 ml) and filtered through 0.45 μm Albet filters (Hahnemühle FineArt GmbH, Dassel, Germany). The TPC of W, WE and WEF TPF extracts was evaluated following the assay of Folin-Ciocolateau (FC) [
24] using gallic acid as a standard. An aliquot of each extract (100 µL) was mixed with the FC reagent (750 μL; 1:10) and allowed to react for 5 min in the dark; then 750 μl di Na
2CO
3 (60 g/L, w/vol) were added, the mixture was kept in the dark for 90 min. The absorbance was measured at 725 nm (Perkin Elmer lambda 25 UV-VIS spectrometer) and results were expressed as µg of gallic acid equivalents (GAE)/g of lyophilized tomato extract.
Antioxidant capacity of the TPF extracts, resuspended in distilled water, was determined by the DPPH assay, following the method reported by Brand-Williams et al. [
25], with some modifications. An aliquot (3 ml) of the DPPH solution (3.94 mg/mL of methanol) was mixed with 50 µL of extract, homogenized and incubated in the dark for 60 min at 25 °C.
After incubation, the absorbance of each sample was spectrophotometrically read at 515 nm, using a Perkin Elmer lambda 25 UV-VIS spectrometer. The radical scavenging activity percentage (RSA %) was calculated according to the following formula:
2.1.5. HPLC/DAD and HPLC/ESI/MS Analyses of TPF Extracts
Variable aliquots of the freeze-dried TPF extracts (see paragraph 2.1.4) were redissolved in 80% aqueous methanol, further filtered, and sent to analytical determinations. HPLC/DAD analyses were carried out on a Ultimate3000 “UHPLC focussed” instrument equipped with a binary high-pressure pump, a Photodiode Array detector, a Thermostatted Column Compartment and an Automated Sample Injector (Thermo Scientific, Italy). Collected data were processed through a Chromeleon Chromatography Information Management System v. 6.80. Chromatographic runs were all performed using a method developed by Siracusa et al. [
26]. A series of HPLC/ESI/MS analyses were also performed on the extracts to unambiguously identify chromatographic peaks; the HPLC apparatus used was the same described above, whilst ESI mass spectra were acquired by a Thermo Scientific Exactive Plu Orbitra MS (Thermo Fisher Scientific, Inc., Milan, Italy), using a heated electrospray ionization (HESI II) interface. Mass spectra were recorded operating in positive and negative ion mode in the m/z range 120-1500 at a resolving power of 25000 (full-width-at-half-maximum, at m/z 200, RFWHM), resulting in a scan rate of > 1.5 scans/sec when using automatic gain control target of 1.0 × 106 and a C-trap inject time of 250 ms. under the following conditions: capillary temperature 300 °C, nebulizer gas (nitrogen) with a flow rate of 60 arbitrary units; auxiliary gas flow rate of 10 arbitrary units; source voltage 3 kV; capillary voltage 82.5 V; tube lens voltage 85 V. The Orbitrap MS system was tuned and calibrated in positive modes, by infusion of solutions of a standard mixture of sodium dodecyl sulfate (Mr 265.17 Da), sodium taurocholate (Mr 514.42 Da) and Ultramark (Mr 1621 Da). Data acquisition and analyses were performed using the Excalibur software. Results are reported in microgram (μg) of compound per gram (g) of extract.
2.2. Preparation of Functional Bread Samples Using TPF as Partial Wheat FLOUR substitute
Bread samples were prepared with durum wheat semolina, TPF (10 and 20 %, w/w), tap water, compressed yeast, NaCl and extra virgin olive oil (EVO), following the experimental scheme reported in
Table 1. All the ingredients apart from TPF were bought in a local supermarket.
The doughs were leavened and baked in a bread maker machine (Moulinex OW240E, Écully, France). Bread samples were left to cool up to 20 °C and first on the loaf were determined colour parameters, after samples were grounded in a home grinder (La Moulinette, Moulinex, Écully, France) before chemical analyses. Two independent baking tests were run, and all the analyses were performed in triplicate Thus, the reported result of each analytical determination is the average of 6 values (2 sample replicates × 3 analytical replicates) unless otherwise stated.
2.2.1. Physico-Chemical Analyses of Functional BREAD samples
Moisture content was determined by gravimetric analysis using an oven (ThermoScientific, Herathermoven, Italy) at 105 °C for 4 h. Total carotenoids content was determined according to the methodology reported in the
Section 2.1.1 pH values and the total titratable acidity (TTA) were determined, using a pH meter (Mettler Toledo, MP 220, Milano, Italy), as follow: 10 g of grounded sample was mixed with 90 mL of distilled water. First the pH value was read, then the suspension was titrated with 0.1 M NaOH to a pH of 8.5. TTA was expressed on dry matter as ml of NaOH needed for titration [
27].
TPC and AA were determined according to the methods described in the
Section 2.1.4 on WE extract obtained as follow: 1 g of grounded bread sample was added with 8 mL of WE and after 10 min of stirring, the solution was centrifuged at 10 °C for 15 min at 8000 rpm (ALC 4128, Italy).
Bread colour evaluation was performed by a colorimeter Konica Minolta CM-2500d (Bremen, Germany), with the illuminant D65. The CIE L*a*b* and C and h were determined on ten different point both on the crust and crumb. For the colour evaluation of the crumb, bread loaves were cutted in slices of 2 cm. The colour differences among Control bread and samples with different amounts of TPF were expressed as E, calculated using the following equation:
where subscript “x” indicates the bread formulated with 10 or 20% TPF and the subscript “0” indicates the Control.
2.2.2. Sensory Evaluation of Functional Bread Samples
The sensory evaluation of bread was carried out using descriptive analysis, performed in the Sensory laboratory of the Department of Agricoltura, Alimentazione e Ambiente (Di3A), University of Catania (Italy) designed according to the UNI EN ISO 8589:2014 guidance [
28]. The sensory profile of the bread samples was determined according to the UNI EN ISO 13299:2016 method [
29] and was carried out by 12 trained panellists with several years of tasting experience, often used in our previous studies on bread. The panellists agreed to take part in the research and signed an informed consent form in accordance with the principles of the Declaration of Helsinki, as our institution does not have an ethics committee for sensory and food quality evaluation studies.
A list of descriptors was generated by the panellists using handmade bread for the sensory evaluation (
Table 2) [
7,
9].
The bread samples were cut into 15 mm thick slices 10 min before tasting. The first and last bread slices were discarded, and the bread slices were served on a plastic plate coded with three-digit numbers. The order of presentation of the samples was balanced using all the presentation combinations possible. Samples were judged without repetition. Judges were provided with water to rinse their mouths between samples. The judges rated the intensity of the selected sensory attributes on a frequency scale from 1 (no sensation/extremely weak) to 9 (extremely intense) (
Table 2) (Smart Sensory Solutions S.r.L., Sassari, Italy). Data were expressed as mean ± standard deviation.
2.3. Statistical Analysis
One-way analysis of variance (ANOVA) of experimental data was performed using Minitab19 statistical software (Minitab, State College, PA, USA). When significant differences were assessed by ANOVA (p < 0.05), Fisher's Least Significant Difference (LSD) test was calculated at the 95% confidence level.
3. Results
3.1. Physico-Chemical, Compositional and Functional Characterization of TPF and TPF Extracts
Characterization of TPF was carried out and the data are presented in
Table 3. The TP was dried to a residual moisture of about 3.53%. TPF was characterized by a lipid content of 12.3%, higher than the amount reported by Azabou et al. [
11]. The distribution of fatty acids showed that the two most important were linoleic (49.90%) and oleic (20.67%), followed by palmitic (18.45%) and stearic (5.94%) (
Table 3). The polyunsaturated fatty acid (PUFA) content of TPF lipid was 52.01% and the polyunsaturated fatty acid/saturated fatty acid (PUFA/SFA) index was 2.01, suggesting a beneficial effect on cardiovascular health [
30]. TPF is an important source of dietary fibre with a total fibre content of approximately 52.3%, of which 5.3% was soluble and 47% was insoluble. These values were similar to those reported by Azabou et al. [
11], but lower than those reported by Navarro-González et al. [
14] due to the genetic diversity and climatic variations of tomato fruits. The insoluble/soluble fibre ratio was about 8.9, as tomato pomace and seeds are usually rich in cellulose, hemicellulose and lignin [
11]. In addition, TPF is a potential source of natural antioxidants and contains remarkable residual levels of both total carotenoids and polyphenols (
Table 3).
Data in the
Table 4 show that both the amount and the distribution of the polyphenols strongly depend on the solvent used. In fact, the water-ethanol-formic acid mixture (WEF) extracted the highest amount of polyphenol, bringing the total amount from 1500 µg/g of total polyphenols in water (W) and water-ethanol systems (WE) to 7900 µg of total polyphenols/g of TPF extract. The highest antioxidant activity is measured in the WE extract (69.4%) the lowest in the water one (6.3%). Moreover, these data also show that the antioxidant activity, measured after DPPH method, is not directly correlated to the total polyphenols, as demonstrated by data related to W and WE systems, showing almost the same level of polyphenols but the widest difference in DPPH value. On the other hand, the WEF extraction system, although showed the highest polyphenols value, had a lower antioxidant activity respect to WE system.
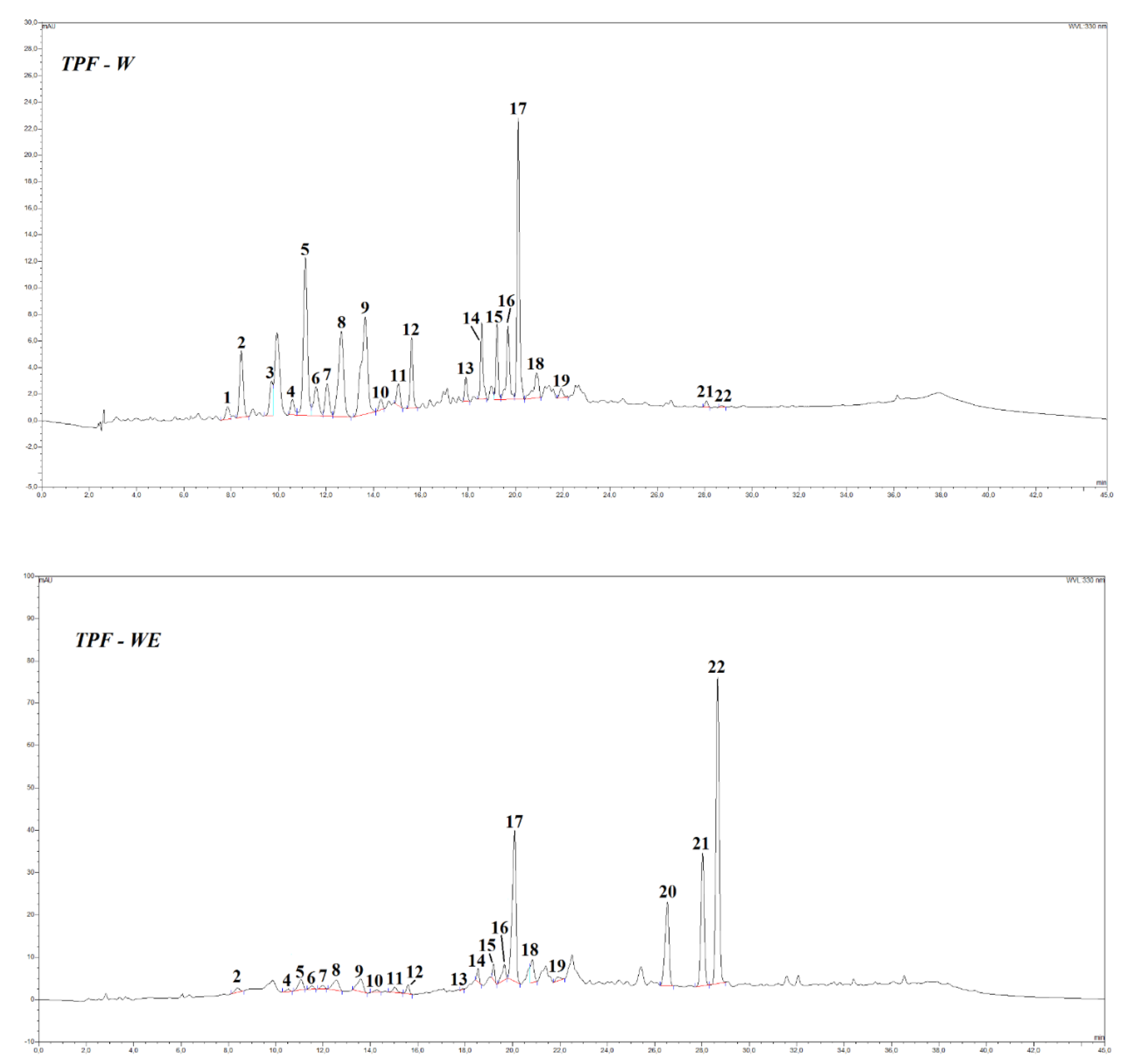
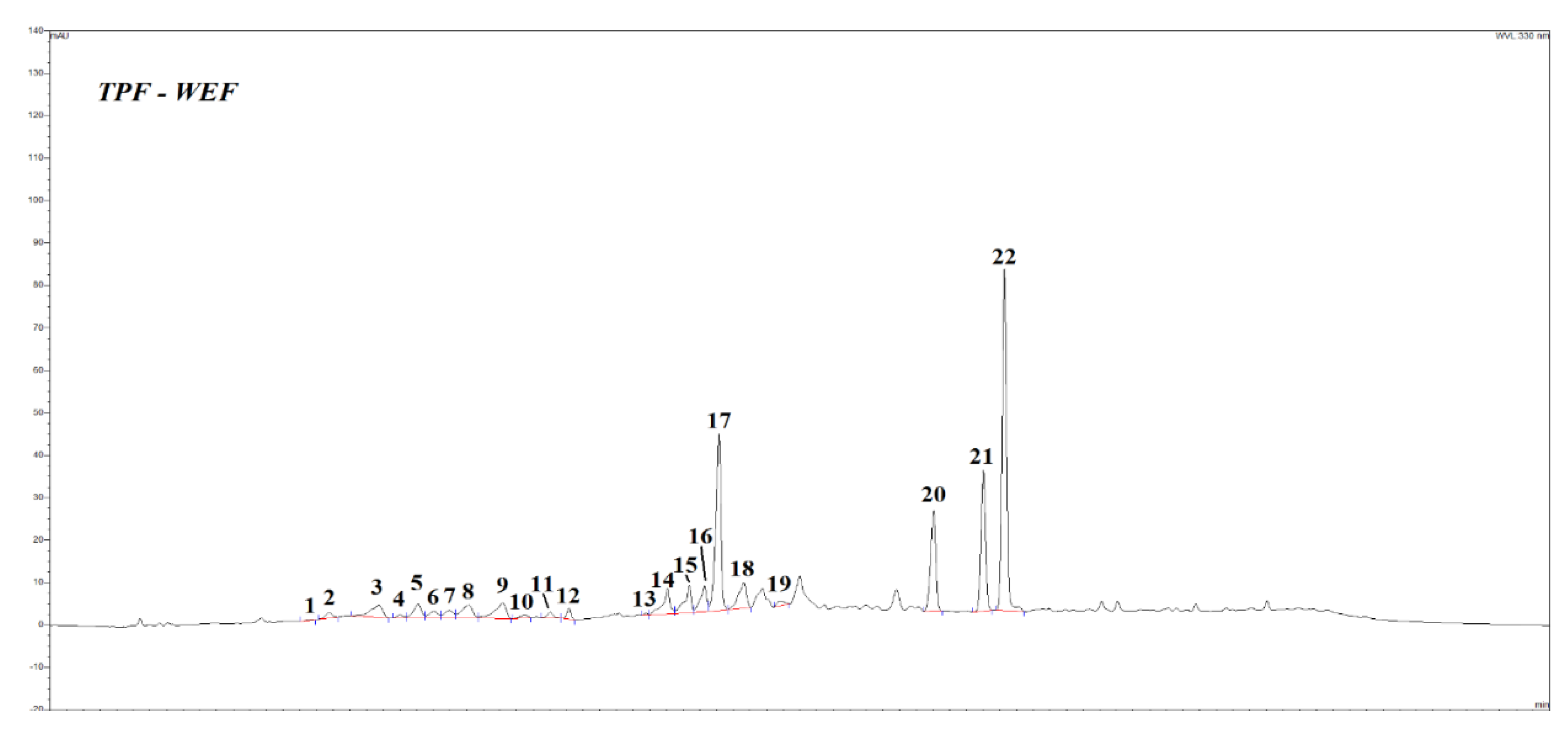
Figure 1 showed the HPLC profile of the TPF extracts, peak numbers refer to
Table 4. Similarly to what found for TPC and antioxidant activity values, these extracts are characterized by different profiles depending on the extraction solvent used.
In particular, presence of ethanol in the extraction solvent mixture (samples WE and WEF) apparently promotes the extraction of flavonoid aglycones (naringenin and quercetin), whilst water used as the sole solvent (sample W) brings into solution preferably a series of hydroxycinnamic acid derivatives (Fig. 1 and
Table 4). This phenomenon is broadly known in literature, as reported also by our group for a similar matrix [
31]. Tomato is indeed widely reported to contain a plethora of specialized metabolites, including vitamins, flavonoids (flavonols and flavanones), hydroxycinnamic acids, and their derivatives with glucose and/or quinic acid [
32]. In our previous studies, we found out small-sized tomatoes accumulate remarkable amounts of these molecules, especially in long-shelf-life ecotypes [
26,
33].
3.2. Characterization of Bread with TPF
The results presented in
Table 5 and
Table 6 suggest that the addition of TPF to the bread formulation affects several properties. During the baking process, water evaporation affects the weight of the bread, the addition of TPF increases the weight of the bread samples, suggesting that TPF can reduce the baking loss, probably due to its ability to retain water. The height of the bread is consistent within the samples, indicating that the addition of TPF doesn't significantly affect this parameter (
Table 5).
The addition of different levels of TPF induced significant differences in the colour parameters both between the fortified bread samples and the control bread. The control bread showed the highest L* value in both crust and crumb, while increasing the percentage of TPF decreased the L* value. The a* and b* parameters showed significant differences between the samples, highlighting the lowest values in the control bread, and increasing the levels of TPF increased the a* and b* parameters (
Table 5).
Table 6 shows the data on moisture, pH, acidity, fibre, carotenoids, total polyphenols and DPPH activity for the bread samples.
Bread samples with TPF had the highest moisture content, confirming the ability of TPF to reduce water losses during baking. The pH value shows a significant decrease as the percentage of TPF increases. The titratable acidity also increases with the addition of TPF: the 20% TPF bread sample has the highest titratable acidity, while the control has the lowest. Even without considering the contribution of organic acids from fermentation processes to the distinctive aroma of bread, the titratable acidity in bread can have a significant impact on other aspects of the bread flavour profile. The higher the titratable acidity, the tangier the bread will taste. The presence of organic acids can impart a pleasant sour or pungent flavour, enhancing the overall flavour profile of bread. The fibre content of the bread increases as the percentage of TPF increases due to the high fibre level of TPF (
Table 3). The sample with 20% TPF has the highest fibre content (about 9.6%). Both fortified TPF breads could use the following nutrition and health claims on label according to Regulation (EC) 1924/2006: “High Fibre” [
34].
The control bread had the lowest level of carotenoids (0.6 mg/kg), while the samples with TPF show an increased amount of carotenoids, with the highest concentration at 20% TPF (5.0 mg/kg). These data indicate that the contribution to carotenoids is totally and proportionally related to the amount of TPF added. Similarly to the carotenoids, the control has the lowest polyphenol content (292.6 mg/kg) and the TPF added samples showed higher polyphenol levels, with the highest concentration at 20% TPF (708.9 mg/kg). The DPPH percentage represents the ability of the samples to scavenge DPPH radicals, with a higher percentage indicating greater antioxidant activity. The TPF bread samples showed the highest antioxidant activity, with the highest DPPH percentage observed in the bread sample with 20% TPF (16.9%). The use of TPF increases the levels of carotenoids and polyphenols, and potentially enhances antioxidant activity.
Table 7 shows the mean intensity values perceived in the different bread samples for the sensory attributes of appearance, odour, flavour, taste and texture in the different bread samples.
The sample with 20% TPF had the highest crumb colour intensity due to the presence of TPF rich in carotenoids. Regarding the uniformity of alveolation, no differences were observed between the control bread and the bread with TPF, but the samples with 10% TPF had the most consistent and uniform alveolation.
Increasing the level of TPF in bread decreased the typical odour of bread and increased the odour of fresh and dried tomatoes. Both attributes were perceived in bread with TPF with a mean intensity that increased as the percentage of TPF increased. The addition of 20% TPF increased the intensity of the odour of dried tomatoes more than that of fresh tomatoes.
Among the flavour attributes, "bread" and "yeasty" were perceived less as the TPF content increased. On the contrary, the dried tomato flavour showed a significant increase as the percentage of TPF in the bread formulation increased, suggesting that the use of TPF influenced the typical taste and odour of this baked product. The fresh tomato flavour attribute showed more intensity in the bread with TPF compared to the control bread, but this attribute seems to be less influenced by the levels of TPF in the bread. The intensity of the taste attributes including sweet, salty, sour, bitter and astringency were similar between the bread samples except for sour. The intensity of sourness perceived by panellists increased significantly only in bread with 20% TPF, in accordance with the trend observed for titratable acidity (
Table 6). Regarding textural attributes, the use of TPF significantly increases the perceived surface moistness, according to the observed trend of moisture determined in bread samples (
Table 6), thus confirming a greater capacity of TPF to retain water during the cooking process. On the contrary, the use of TPF makes the bread less soft and obviously increases the intensity of the grittiness due to the high fibre content. Finally, the "overall" attribute shows similar values in the three experimental bread samples, suggesting that the overall quality of the bread samples remains relatively constant, regardless of the percentage of TPF.
4. Conclusions
In this study for the first time was proposed the use of tomato pomace flour (TPF) deriving from the processing of cherry and date tomatoes as an ingredient to improve the nutritional value of durum wheat bread. TPF is a rich source of polyunsaturated and monounsaturated fatty acids, dietary fibre and polyphenols, and its use, even at a level of 10% in the formulation of durum wheat bread, allows the production of a bread high in fibre with a considerable content of bioactive compounds. In addition, the use of TPF reduces the weight loss of the bread during baking, allowing to produce a product with a maximum moisture content in accordance with current legislation. This technological advantage could encourage the food industry to use this low-cost waste.
From a sensory point of view, TPF-fortified breads showed an overall acceptance similar to that of the control bread, although the addition of TPF increases the intensity of the typical tomato colour, flavour and odour as well as the acidity. No effect seems to be induced on texture characteristics such as softness, cohesiveness, dryness and chewiness, which could negatively affect consumer acceptance.
Furthermore, the proposed use of TP is in line with the EU's "Farm to Fork" strategy, both from a health and environmental point of view, providing an opportunity for the food industry to adopt sustainable practices and stimulate product reformulation to improve nutrient profiles using production waste.
Author Contributions
Conceptualization, C.R. and E.A.; methodology, L.R., E.A. and C.R.; validation, L.R. and E.A.; formal analysis, S.B., L.P., L.S. and M.V.F..; investigation, S.B., L.P. M.V.F.; data curation, S.B., L.P., L.S. and E.A.; writing—original draft preparation, L.S., E.A. and C.R..; funding acquisition, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by 7th Framework Programme (7°PQ) of the European Commission (agreement No. 618127 ERANET ARIMNET2 “Coordination of research in Mediterranean area”) title “VIPACFood-Valorization of Industrial fruits by Products and algae biomass waste: Development of Active Coatings to extend Food shelf life and reduce food losses.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arena, E.; Brighina, S.; Mazzaglia, A.; Spina, A.; Muccilli, S.; Giannone, V.; Fallico, B. Use of a natural low Na salt in durum wheat bread. Ital. J. Food Sci. 2015, 77–81. [Google Scholar]
- Arena, E.; Muccilli, S.; Mazzaglia, A.; Giannone, V.; Brighina, S.; Rapisarda, P.; Fallico, B.; Allegra, M.; Spina, A. Development of Durum Wheat Breads Low in Sodium Using a Natural Low-Sodium Sea Salt. Foods 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Rapisarda, P.; Fallico, B.; Arena, E. Partial Replacement of NaCl in Bread from Durum Wheat (Triticum turgidum L subsp. durum Desf.) with KCl and Yeast Extract: Evaluation of Quality Parameters During Long Storage. Food Bioprocess Technol. 2015a, 8, 1089–1101. [CrossRef]
- Brighina, S.; Arena, E.; Mazzaglia, A.; Spina, A.; Muccilli, S.; Giannone, V.; Fabroni, S.; Rapisarda, P.; Fallico, B. Quality parameters of wholegrain durum wheat bread enriched with citrus fibre. Ital. J. Food Sci. 2015, 67–71. [Google Scholar]
- Johnston, C.; Leong, S.Y.; Teape, C.; Liesaputra, V.; Oey, I. In vitro digestion properties and use of automatic image analysis to assess the quality of wheat bread enriched with whole faba bean (Vicia faba L.) flour and its protein-rich fraction. Food Res. Int. 2023, 174, 113630. [Google Scholar] [CrossRef] [PubMed]
- Nouska, C.; Irakli, M.; Palakas, P.; Lytou, A.E.; Bouloumpasi, E.; Biliaderis, C.G.; Lazaridou, A. Influence of sesame cake on physicochemical, antioxidant and sensorial characteristics of fortified wheat breads. Food Res. Int. 2024, 178, 113980. [Google Scholar] [CrossRef]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of prickly pear peel flour as bioactive and functional ingredient in bread preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef]
- Spina, A.; Muccilli, S.; Arena, E.; Brighina, S.; Fallico, B.; Giannone, V.; Rapisarda, P. Durum wheat breads enriched with citrus fruits pectin and flavonoids. Ital. J. Food Sci. 2015b, 72–76.
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Fabroni, S.; Fallico, B.; Rapisarda, P.; Arena, E. Wholegrain durum wheat bread fortified with citrus fibres: Evaluation of quality parameters during long storage. Front. Nutr. 2019, 6, 422996. [Google Scholar] [CrossRef]
- ISMEA, Report n.1/2022. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/12391 (December 2022).
- Azabou, S.; Louati, I.; Ben Taheur, F.; Nasri, M.; Mechichi, T. Towards sustainable management of tomato pomace through the recovery of valuable compounds and sequential production of low-cost biosorbent. Environ. Sci. Pollut. Res. 2020, 27, 39402–39412. [Google Scholar] [CrossRef]
- Farinon, B.; Felli, M.; Sulli, M.; Diretto, G.; Savatin, D.V.; Mazzucato, A.; Merendino, N.; Costantini, L. Tomato pomace food waste from different variants as a high antioxidant potential resource. Food Chem. 2024, 452, 139509. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Jesús Periago, M. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral valorisation of tomato by-products towards bioactive compounds recovery: Human health benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef] [PubMed]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Betrouche, A.; Estivi, L.; Colombo, D.; Pasini, G.; Benatallah, L.; Brandolini, A.; Hidalgo, A. Antioxidant Properties of Gluten-Free Pasta Enriched with Vegetable By-Products. Molecules 2022, 27, 8993. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Prasad, P.; Sangwan, R.S.; Yadav, S.K. Tomato processing byproduct valorization in bread and muffin: Improvement in physicochemical properties and shelf life stability. J. Food Sci. Technol. 2018, 55, 2560–2568. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Estivi, L.; Bertuglia, K.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J.; Hidalgo, A. Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants 2022, 11, 2087. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Jaeger de Carvalho, L. M.; Barros Gomes, P.; Luiz de Oliveira Godoy, R.; Pacheco, S.; Fernandes do Monte, P. H.; Nutti Regini, M.; Ramalho Ramos S., R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- European Parliament & Council of the European Union. Regulation (EC) No. 2105/2022 of the European Parliament and of the Council of 4th november 2022 on rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive oil. Off. J. Eur. Off. J. Eur. Union (OJEU) 2022, L284, 23–48. [Google Scholar]
- Singleton, V.L.; Rossi Jr, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Siracusa, L.; Patané, C.; Rizzo, V.; Cosentino, S.L.; Ruberto, G. Targeted secondary metabolic and physico-chemical traits analysis to assess genetic variability within a germplasm collection of “long storage” tomatoes. Food Chem. 2018, 244, 275–283. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC Determination of Sugars, Organic Acids and Ethanol in Sourdough Process. LWT Food Sci. Technol. 2002, 35, 407–414. [Google Scholar] [CrossRef]
- UNI EN ISO 8589. Analisi Sensoriale—Guida Generale per la Progettazione di Locali di Prova. Ente Italiano di Normazione; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- UNI EN ISO 13299. Analisi Sensoriale—Metodologia—Guida Generale per la Definizione del Profilo Sensoriale. Ente Italiano di Normazione; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- Manzo, N.; Pizzolongo, F.; Meca, G.; Aiello, A.; Marchetti, N.; Romano, R. Comparative Chemical Compositions of Fresh and Stored Vesuvian PDO “Pomodorino Del Piennolo” Tomato and the Ciliegino Variety. Molecules 2018, 23, 2871. [Google Scholar] [CrossRef]
- Siracusa, L.; Patanè, C.; Avola, G.; Ruberto, G. Polyphenols as chemotaxonomic markers in Italian “long storage” tomato genotypes. J. Agric. Food Chem. 2012, 60, 309–314. [Google Scholar] [CrossRef]
- European Parliament & Council of the European Union. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union (OJEU) 2006, L404, 9–25. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).