Submitted:
08 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Subjects
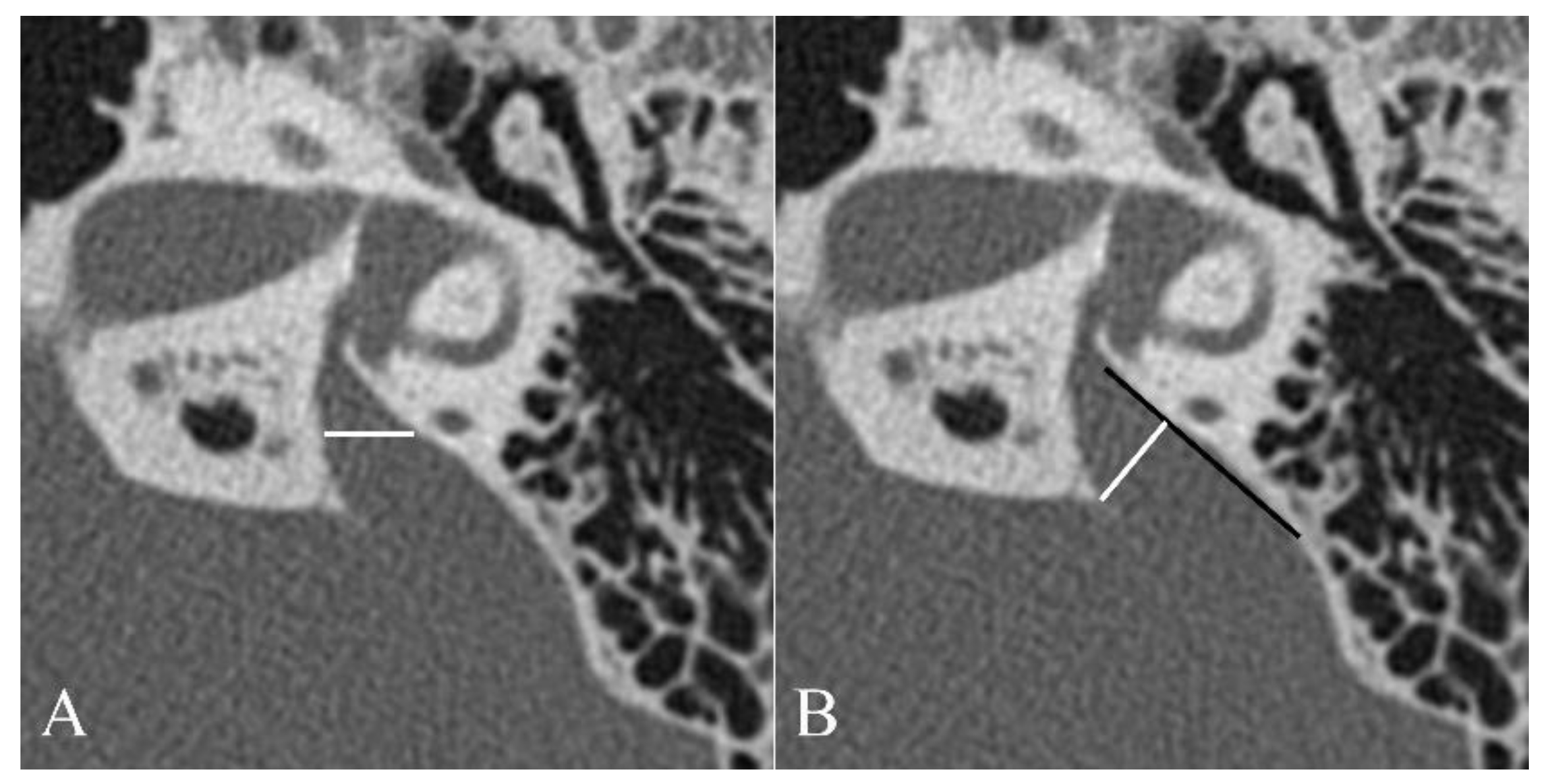
2.2. Image Evaluation
2.3. Measurement of WBT
2.4. Hearing Assesment
2.5. Statistics
3. Results
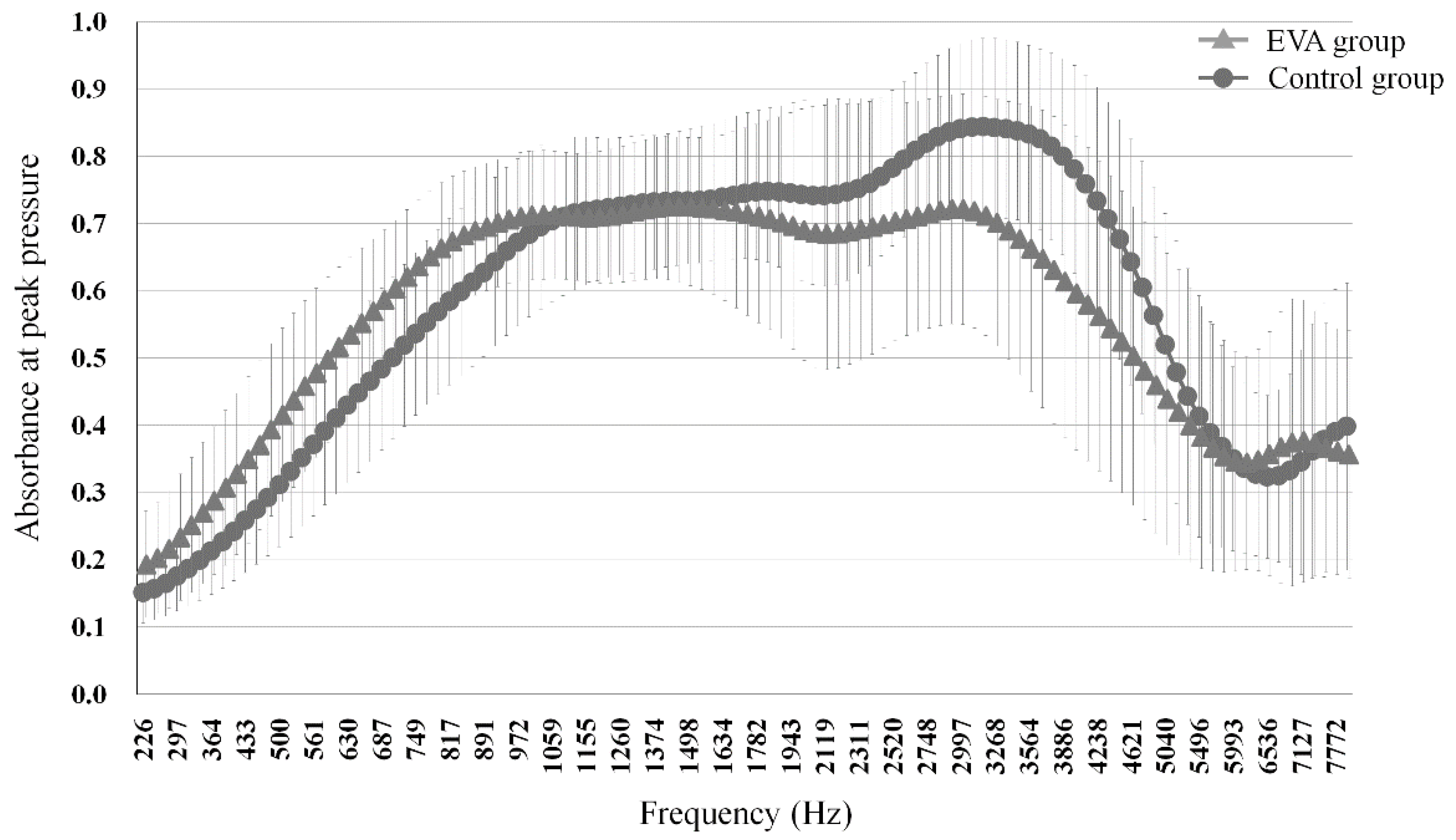
3.1. Comparison of Absorbance
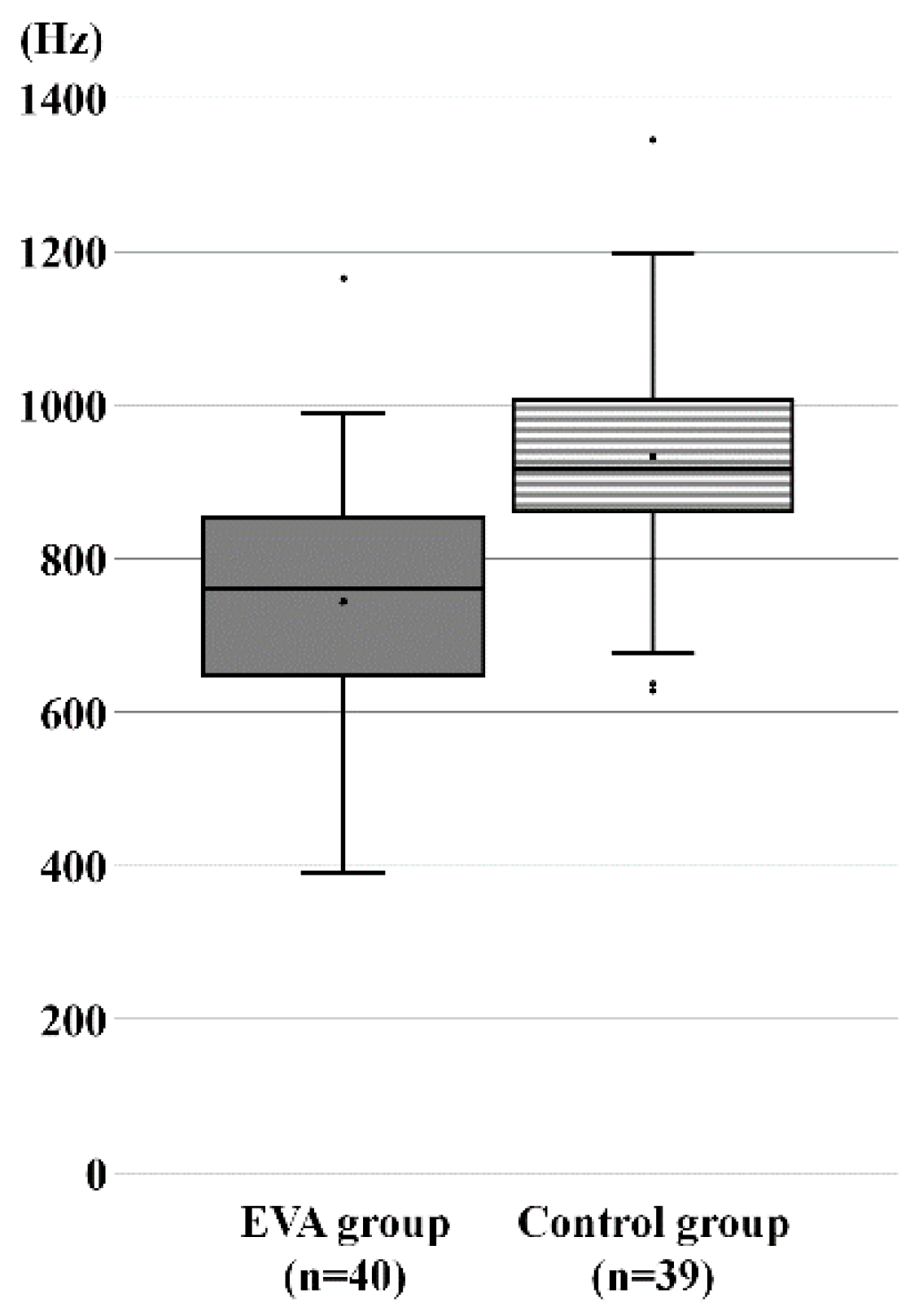
3.2. Comparison of RF
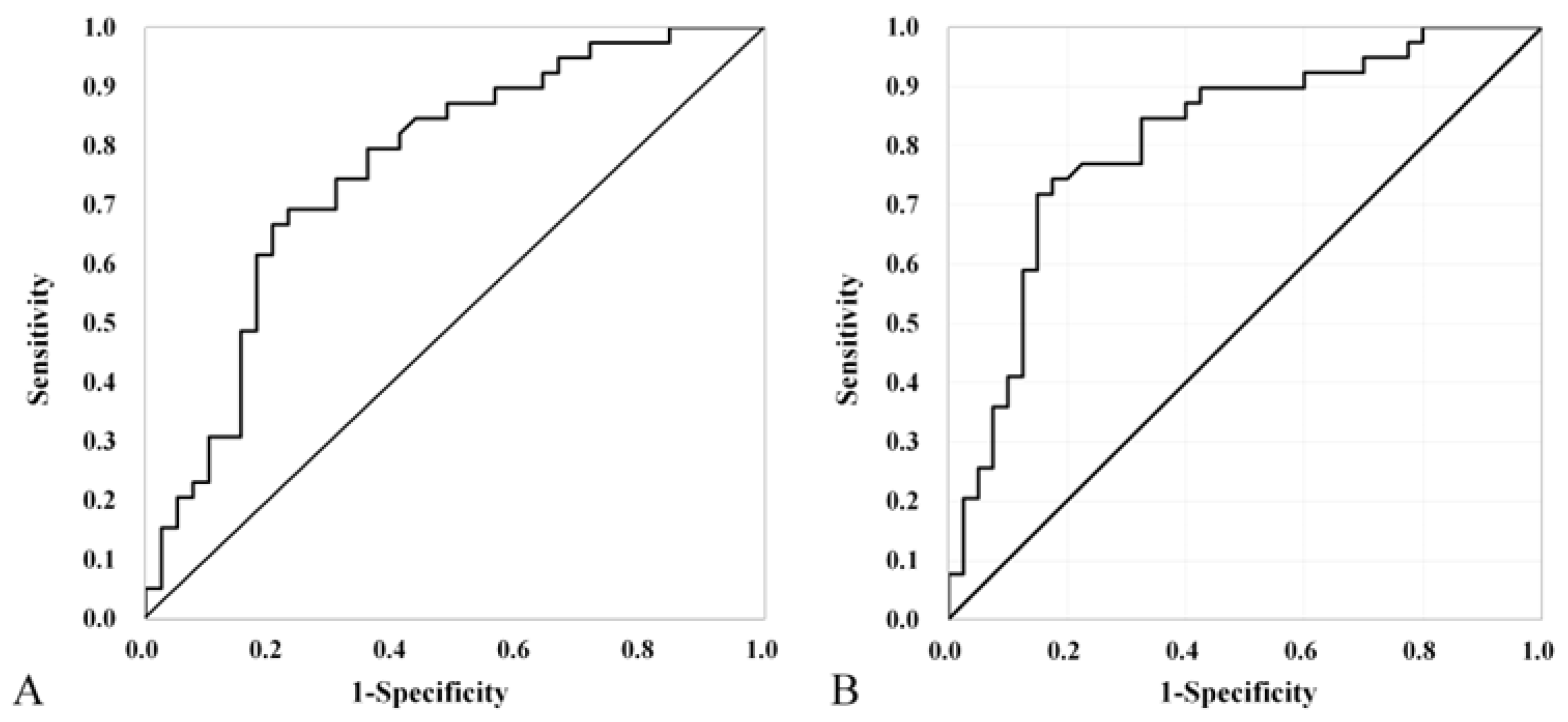
3.2. ROC analysis for absorbance and RF
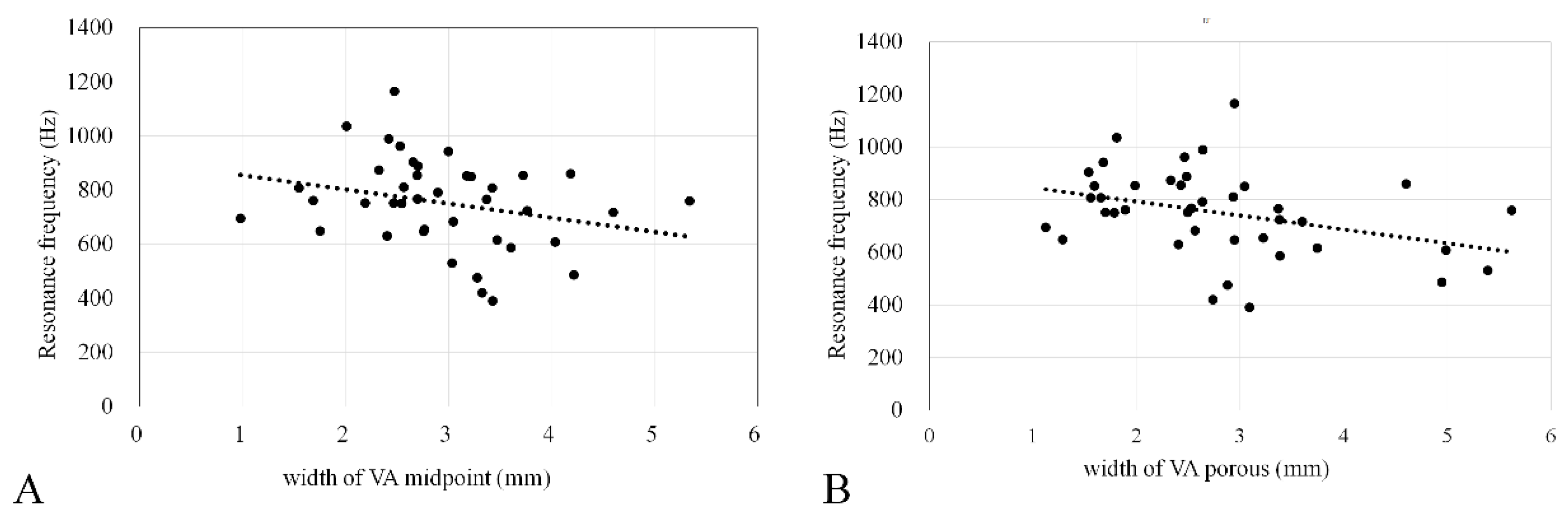
3.5. Correlation between EVA Size and RF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holte, L.; Margolis, R.H.; Cavanaugh, R.M. Jr. Developmental changes in multifrequency tympanograms. Audiology 1991, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Aithal, S.; Kei, J.; Driscoll, C. Wideband absorbance in young infants (0-6 months): a cross-sectional study. J. Am. Acad. Audiol. 2014, 25, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Carlborg, B.I.; Farmer, J.C. Jr. Transmission of cerebrospinal fluid pressure via the cochlear aqueduct and endolymphatic sac. Am. J. Otolaryngol. 1983, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Gyo, K.; Goode, R.L. Effect of increased inner ear pressure on middle ear mechanics. Otolaryngol. Head Neck Surg. 1998, 118, 703–708. [Google Scholar] [PubMed]
- Macrae, J.H. Effects of body position on the auditory system. J. Speech Hear. Res. 1972, 15, 330–339. [Google Scholar] [CrossRef]
- Demir, E.; Afacan, N.N.; Celiker, M.; Celiker, F.B.; İnecikli, M.F.; Terzi, S.; Dursun, E. Can wideband tympanometry be used as a screening test for superior semicircular canal dehiscence? Clin. Exp. Otorhinolaryngol. 2019, 12, 249–254. [Google Scholar] [CrossRef]
- Ishizu, K.; Tamae, A.; Kubo, K.; Yoshida, T.; Matsumoto, N.; Yasui, T.; Nakagawa, T. Diagnosis and following up of Ménière’s disease using multifrequency tympanometry-Cutoff values and temporal changes in measurements. Auris Nasus Larynx 2018, 45, 81–87. [Google Scholar] [CrossRef]
- Nakashima, T.; Ueda, H.; Furuhashi, A.; Sato, E.; Asahi, K.; Naganawa, S.; Beppu, R. Air-bone gap and resonant frequency in large vestibular aqueduct syndrome. Am. J. Otol. 2000, 21, 671–674. [Google Scholar]
- Ota, I.; Sakagami, M.; Kitahara, T. The third mobile window effects in otology/ neurotology. J. Int. Adv. Otol. 2021, 17, 156–161. [Google Scholar] [CrossRef]
- Emmett, J.R. The large vestibular aqueduct syndrome. Am. J. Otol. 1985, 6, 387–415. [Google Scholar]
- Govaerts, P.J.; Casselman, J.; Daemers, K.; De Ceulaer, G.; Somers, T.; Offeciers, F.E. Audiological findings in large vestibular aqueduct syndrome. Int. J. Pediatr. Otorhinolaryngol. 1999, 51, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Sato, E.; Sugiura, M.; Yoshino, T.; Naganawa, S.; Nakashima, T. Hearing loss in patients with enlarged vestibular aqueduct: air-bone gap and audiological Bing test. Int. J. Audiol. 2005, 44, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Grais, E.M.; Li, Y.; Zhao, F. Three-dimensional wideband absorbance immittance findings in young adults with large vestibular aqueduct syndrome. Laryngoscope Investig. Otolaryngol. 2023, 8, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Du, H.; Gao, J.; Xu, Y.; Zhao, N.; Gao, S.; Xing, Y.; Qian, X.; Gao, X.; Yang, Y. Characteristics of large vestibular aqueduct syndrome in wideband acoustic immittance. Front. Neurosci. 2023, 17, 1185033. [Google Scholar] [CrossRef]
- BSA. Recommended procedure tympanometry. Br Soc. Audiol. 2013, 1–20. [Google Scholar]
- Jerger, J.; Jerger, S.; Mauldin, L. Studies in impedance audiometry. I. Normal and sensorineural ears. Arch. Otolaryngol. 1972, 96, 513–523. [Google Scholar] [CrossRef]
- Valvassori, G.E.; Clemis, J.D. The large vestibular aqueduct syndrome. Laryngoscope 1978, 88, 723–728. [Google Scholar] [CrossRef]
- Vijayasekaran, S.; Halsted, M.J.; Boston, M.; Meinzen-Derr, J.; Bardo, D.M.; Greinwald, J.; Benton, C. When is the vestibular aqueduct enlarged? A statistical analysis of the normative distribution of vestibular aqueduct size. A.J.N.R. Am. J. Neuroradiol. 2007, 28, 1133–1138. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, J.; Choi, J.Y. Correlation of vestibular aqueduct size with air-bone gap in enlarged vestibular aqueduct syndrome. Laryngoscope 2016, 126, 1633–1638. [Google Scholar] [CrossRef]
- Hunter, L.L.; Shahnaz, N. Acoustic Immittance Measures: Basic and Advanced Practice; Plural Publishing: San Diego, (CA), USA, 2014. [Google Scholar]
- Minor, L.B.; Solomon, D.; Zinreich, J.S.; Zee, D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 249–258. [Google Scholar] [CrossRef]
- Tonndorf, J.; Tabor, J.R. Closure of the cochlear windows: its effect upon air- and bone-conduction. Ann. Otol. Rhinol. Laryngol. 1962, 71, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Gopen, Q.; Rosowski, J.J.; Merchant, S.N. Anatomy of the normal human cochlear aqueduct with functional implications. Hear. Res. 1997, 107, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Levenson, M.J.; Parisier, S.C.; Jacobs, M.; Edelstein, D.R. The large vestibular aqueduct syndrome in children. A review of 12 cases and the description of a new clinical entity. Arch. Otolaryngol. Head Neck Surg. 1989, 115, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; Rosowski, J.J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol. Neurotol. 2008, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.H.; Rosowski, J.J.; Shahnaz, N.; Voss, S.E. Assessment of ear disorders using power reflectance. Ear Hear. 2013, 34, 48S–53S. [Google Scholar] [CrossRef]
- Rosowski, J.J.; Stenfelt, S.; Lilly, D. An overview of wideband immittance measurements techniques and terminology: you say absorbance, I say reflectance. Ear Hear. 2013, 34, 9S–16S. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshida, T.; Sugimoto, S.; Shimono, M.; Teranishi, M.; Naganawa, S.; Sone, M. Effects of endolymphatic hydrops on acoustic energy absorbance. Acta Otolaryngol. 2020, 140, 626–631. [Google Scholar] [CrossRef]
- Sone, M.; Yoshida, T.; Morimoto, K.; Teranishi, M.; Nakashima, T.; Naganawa, S. Endolymphatic hydrops in superior canal dehiscence and large vestibular aqueduct syndromes. Laryngoscope 2016, 126, 1446–1450. [Google Scholar] [CrossRef]
- Sato, E.; Nakashima, T.; Lilly, D.J.; Fausti, S.A.; Ueda, H.; Misawa, H.; Uchida, Y.; Furuhashi, A.; Asahi, K.; Naganawa, S. Tympanometric findings in patients with enlarged vestibular aqueducts. Laryngoscope 2002, 112, 1642–1646. [Google Scholar] [CrossRef]
- Sundgaard, J.V.; Värendh, M.; Nordström, F.; Kamide, Y.; Tanaka, C.; Harte, J.; Paulsen, R.R.; Christensen, A.N.; Bray, P.; Laugesen, S. Inter-rater reliability of the diagnosis of otitis media based on otoscopic images and wideband tympanometry measurements. Int. J. Pediatr. Otorhinolaryngol. 2022, 153, 111034. [Google Scholar] [CrossRef]
- Sennaroglu, L. Histopathology of inner ear malformations: do we have enough evidence to explain pathophysiology? Cochlear Implants Int. 2016, 17, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, C.; Kirkim, G.; Kirazli, T. Middle ear impedance measurements in large vestibular aqueduct syndrome. Auris Nasus Larynx 2009, 36, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Jackler, R.K.; De La Cruz, A. The large vestibular aqueduct syndrome. Laryngoscope 1989, 99, 1238–1242. [Google Scholar] [CrossRef]
- Berrettini, S.; Forli, F.; Bogazzi, F.; Neri, E.; Salvatori, L.; Casani, A.P.; Franceschini, S.S. Large vestibular aqueduct syndrome: audiological, radiological, clinical, and genetic features. Am. J. Otolaryngol. 2005, 26, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Ideura, M.; Nishio, S.Y.; Moteki, H.; Takumi, Y.; Miyagawa, M.; Sato, T.; Kobayashi, Y.; Ohyama, K.; Oda, K.; Matsui, T.; Ito, T.; Suzumura, H.; Nagai, K.; Izumi, S.; Nishiyama, N.; Komori, M.; Kumakawa, K.; Takeda, H.; Kishimoto, Y.; Iwasaki, S.; Furutate, S.; Ishikawa, K.; Fujioka, M.; Nakanishi, H.; Nakayama, J.; Horie, R.; Ohta, Y.; Naito, Y.; Kakudo, M.; Sakaguchi, H.; Kataoka, Y.; Sugahara, K.; Hato, N.; Nakagawa, T.; Tsuchihashi, N.; Kanda, Y.; Kihara, C.; Tono, T.; Miyanohara, I.; Ganaha, A.; Usami, S.I. Comprehensive analysis of syndromic hearing loss patients in Japan. Sci. Rep. 2019, 9, 11976. [Google Scholar] [CrossRef]
- Perry, J.; Sher, E.; Kawai, K.; Redfield, S.; Sun, T.; Kenna, M. Newborn hearing screening results in patients with enlarged vestibular aqueduct. Laryngoscope 2023, 133, 2786–2791. [Google Scholar] [CrossRef]
- Shahnaz, N.; Feeney, M.P.; Schairer, K.S. Wideband acoustic immittance normative data: ethnicity, gender, aging, and instrumentation. Ear Hear. 2013, 34, 27S–35S. [Google Scholar] [CrossRef]
| Control group | |
| 1 | No history of acquired ear diseases |
| 2 | No acute or chronic upper respiratory inflammation |
| 3 | Normal findings on pretest otoscopic examination |
| 4 | Type-A tympanometry |
| 5 | Normal hearing level (≤25 dBHL from 500 to 2000Hz, air-bone gap ≤10 dB from 500 to 2000 Hz) |
| Enlarged vestibular aqueduct (EVA) group | |
| 1 | Patients who meet criteria 1–4 in the control group |
| 2 | VA with a diameter of >1.5 mm at the midpoint |
| EVA group | Control group | p-value | ||
| Number of subjects | 25 | 28 | ||
| Number of ears | 40 | 39 | ||
| Sex | ||||
| Male | 21 (52.5%) | 23 (59%) | 0.361 | |
| Female | 19 (47.5%) | 16 (41%) | ||
| Side of WBT | ||||
| Right | 19 (%) | 21 (%) | 0.651 | |
| Left | 21 (%) | 18 (%) | ||
| Age at WBT (years) | 14 (4–49) | 12 (4–48) | 0.822 | |
| Hearing level (dB) | 80 ± 22.2 | 9.0 ± 4.0 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





