1. Introduction
Hepatitis B virus (HBV), a member of the
Hepadnaviridae family, primarily infects human hepatocytes and is a major global health concern due to its role in the development of cirrhosis and hepatocellular carcinoma (HCC) [1-3]. Over 292 million individuals worldwide are estimated to be chronic carriers of HBV, with nearly 50% of HCC cases in the Asia-Pacific region linked to chronic HBV infection [4, 5]. A functional cure for HBV is defined by the sustained loss of detectable HBsAg and the reduction of serum HBV DNA to unquantifiable levels for at least six months [
6]. Current treatment options include pegylated interferon-alpha and HBV polymerase inhibitors such as nucleos(t)ide analogs (NAs), which can effectively suppress viral replication[7, 8]. However, these therapies rarely achieve a complete cure, largely because they do not target HBV’s covalently closed circular DNA (cccDNA) [
9]. cccDNA is a stable, episomal form of the HBV genome that resides in the nuclei of infected hepatocytes. It serves as a key template for viral RNA transcription and is crucial for the persistence of HBV infection [4, 5]
. Unlike other forms of HBV DNA, cccDNA is highly resistant to current antiviral therapies, making it a central challenge in efforts to cure HBV [
10]. Even in patients who have achieved functional cure, cccDNA can remain detectable in liver tissue, contributing to the risk of viral reactivation [
11]. This persistence highlights the critical need for therapeutic strategies that specifically target cccDNA, which plays a pivotal role in maintaining chronic HBV infection.
Although much progress has been made in HBV research, a significant limitation is the lack of suitable animal models that can accurately replicate the formation and maintenance of cccDNA. Mice, despite being one of the most commonly used laboratory animals due to their well-characterized immune system and ease of genetic manipulation, are not naturally susceptible to HBV infection. Consequently, alternative methods have been developed, including the hydrodynamic injection (HDI) of HBV genomes into mice
via adeno-associated virus (AAV) vectors [
12]. However, these models often fail to establish chronic HBV infection due to difficulties in achieving stable and sustained viral replication, limiting their utility for studying cccDNA dynamics and testing potential therapeutic interventions [
13].
Previous studies have demonstrated that mice infected with recombinant AAV-HBV (rAAV-HBV) can exhibit persistent HBV infection, showing promise as a potential model for chronic hepatitis B (CHB)[14, 15]. However, reliable detection of cccDNA in these models has been challenging, limiting their utility in therapeutic research. Consequently, developing a robust mouse model that supports both the formation and maintenance of cccDNA is essential for advancing HBV research and evaluating novel therapeutic approaches aimed at curing chronic HBV infection.
In this study, we aimed to establish a mouse model that not only demonstrates persistent HBV infection but also allows for the reliable detection and quantification of cccDNA in infected hepatocytes. By using an rAAV-HBV1.3 vector in C57BL/6 mice, we sought to create a model that accurately reflects the chronic phase of HBV infection and provides a valuable tool for studying the role of cccDNA in disease progression and treatment. We hypothesize that this model will demonstrate the successful establishment of such a model and its potential use in evaluating new therapeutic strategies targeting cccDNA.
2. Materials and Methods
2.1. rAAV Production
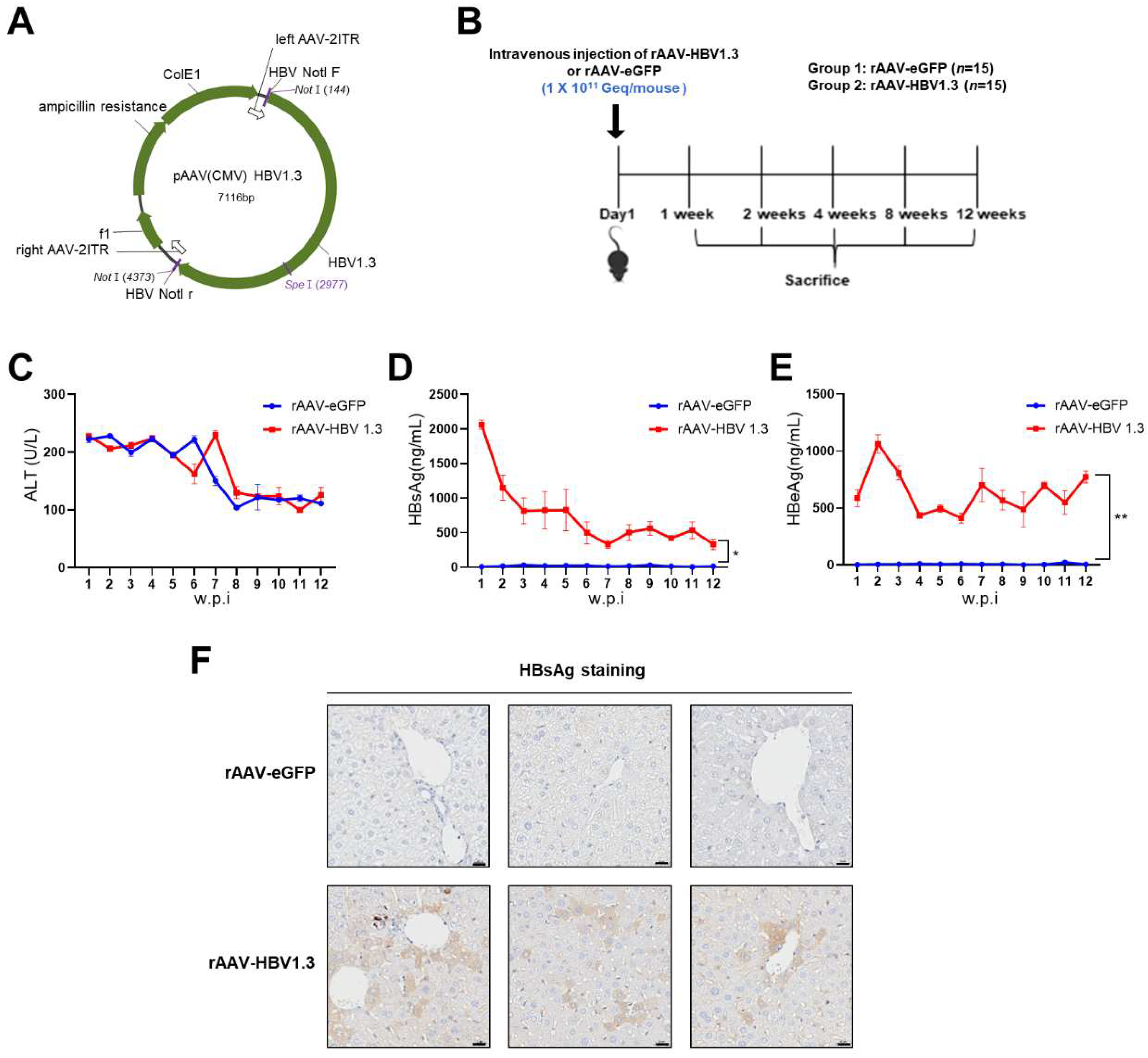
Plasmid AAV-HBV 1.3 (pAAV-HBV1.3, 1.3-fold genome) containing a greater-than-genome-length HBV genotype C fragment derived from a Korean patient (nt 1073–3215–2067; GenBank: DQ683578.1) was constructed (
Figure 1A). The rAAV8-HBV vectors carrying 1.3 copies of the HBV genome (genotype C) were produced from VectorBuilder (Korea) using pAAV-HBV1.3.
2.2. Animals
C57BL/6 mice (six-weeks-old; male) were purchased from Orient Bio Inc. (Seongnam, Korea). The rAAV-HBV1.3 mouse model was generated by administering vector genome equivalents of rAAV-HBV1.3 (1x1011 vg diluted in 200 µL PBS) via intravenous injection. The control group was administered intravenous injection of rAAV-eGFP (1x1011 vg diluted in 200 uL PBS). Blood was collected weekly using the Retro-orbital Sinus method, and the collected blood was centrifuged at 13,000 rpm for 15 min at 4°C to separate the serum. All animal care and experimental procedures followed the guidelines of the Institutional Animal Care and Use Committee and were approved by the Center for Medical Science of the Catholic University of Korea (2023-0153-01).
2.3. Serum Analyses
Serum HBsAg and HBeAg levels were analyzed using ELISA kits (Wantai, Biofarm). The serum was measured using a 100-fold dilution with PBS. Serum ALT levels were measured using a chemical analyzer according to the manufacturer’s protocol (Catalyst One Chemistry Analyzer; IDEXX Laboratories, Westbrook, ME, USA) and analyzed using commercial quantitative assays.
2.4. Quantification of Total HBV DNA and cccDNA Copy Numbers by qRT-PCR
Total HBV DNA and cccDNA levels in liver tissues were measured by qRT-PCR, as previously described [
16]. Total DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen, USA). A TaqMan probe analysis was performed to detect HBV DNA expression. Total DNA was quantified at a concentration of 500 ng, and a total volume of 20 µL was used, which included the probe and master mix (Roche, USA). Target DNA levels were determined by TaqMan gene expression assays (Applied Biosystems, Foster City, CA, USA). The assay IDs for each gene were Actb (Mm02519580_g1; Thermo Fisher Scientific) and HBV DNA (Vi03453405_s1; Thermo Fisher Scientific). This process was performed using a Light Cycler 480 II (Roche Diagnostics). To detect cccDNA, total DNA was treated with PSD (Plasmid-Safe ATP-Dependent DNase, LGC Biosearch Technologies, United Kingdom). A total of 500 ng of DNA from mouse tissue was used under the following conditions and ingredients: 10U PSD, 8 µl of 25 mM ATP, 20 µl of 10X buffer, and ultra-pure water for a total volume of 50 µl; incubation at 37°C for 1 h, followed by heat inactivation at 70°C for 30 min. cccDNA levels in the liver tissues were assessed using TaqMan gene expression analysis. The cccDNA was quantified using the mouse mitochondrial gene ND2 (Mm04225288_s1) for episomal DNA detection. cccDNA was detected using a forward primer (CCGTGTGCACTTCGCTTCA), reverse primer (GCACAGCTTGGAGGCTTGA), and probe ([6FAM] CATGGAGACCACCGTGAACGCCC [BBQ].
2.5. Immunohistochemistry
Liver tissues were fixed in 4% paraformaldehyde for one day and embedded in paraffin. After deparaffinization, the microwave (RHS-1, Milestone, Italy) incubated the specimens in citrate buffer (0.01M, pH 6.0) at 121°C for 20 min. To prevent endogenous peroxide activity, sections were incubated in methanol containing 3% H2O2 for 30 min. The sections were stained with anti-HBsAg (Invitrogen, Cat# MA5-13059) and incubated overnight at 4°C. Subsequently, the slides were washed three times with PBS-T (Phosphate-Buffered Saline with Tween 20, pH 7.4) and incubated with EnVision Detection Systems Peroxidase/DAB, Rabbit/Mouse (#Cat K5007, Dako, USA) at 4°C for 45 min. The DAB chromogen was added for 30 s, followed by counterstaining with hematoxylin.
2.6. Statistical Analysis
Statistical analyses were performed using the unpaired t-test via the GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). The significance level was set at *p < 0.05, **p < 0.01.
3. Results
3.1. rAAV-HBV1.3 Induces a Chronic HBV Mouse Model
In this study, we developed a chronic HBV mouse model using rAAV-HBV1.3, which was administered
via intravenous injection to C57BL/6 mice. The mice were observed over a 12-week period to monitor HBV infection and liver damage (
Figure 1B). To assess liver injury, serum ALT levels were measured at different time points. ALT levels in both the rAAV-HBV1.3 and rAAV-eGFP groups were elevated until week 8 but returned to normal levels starting from week 9 (
Figure 1C). The early increase in ALT levels is likely due to the acute inflammatory response induced by the intravenous injection rather than the viral infection itself. This transient rise in ALT levels subsided by week 9, indicating a resolution of the initial acute liver stress.
HBsAg and HBeAg levels in the serum were also measured. HBsAg levels peaked at week 1 and then gradually decreased but remained detectable until week 12 (
Figure 1D), suggesting sustained viral protein expression and ongoing HBV infection. HBeAg levels peaked at week 2 and were similarly maintained at considerable levels until week 12 (
Figure 1E), reinforcing the idea that viral replication persisted during this period. Immunohistochemistry confirmed the presence of HBsAg in hepatocytes up to week 12 (
Figure 1F), supporting the chronicity of the infection.
3.2. Detection of HBV DNA and cccDNA in the rAAV-HBV1.3 Mouse Model
To further characterize HBV infection in the rAAV-HBV1.3 mouse model, we quantified intrahepatic HBV DNA levels over a 12-week period using qRT-PCR. As shown in
Figure 2A, HBV DNA levels peaked at week 1 (approximately 3.5x10^11 copies/μL) and remained detectable until week 12, indicating persistent viral replication. Crucially, we detected cccDNA in liver tissues from rAAV-HBV1.3 mice.
Figure 2B shows cccDNA levels peaked at week 2 (about 2x10^8 copies/μL) and maintained detectable levels (approximately 5x10^7 copies/μL) throughout the 12-week period. This persistence of cccDNA mirrors the clinical scenario of chronic HBV infection. The detection of cccDNA is particularly important because it directly links our model to the pathophysiology of chronic HBV infection. The peak of cccDNA at week 2 likely reflects the early phase of HBV replication when cccDNA formation is most active. Its subsequent persistence indicates the stability of this episomal DNA, which remains a major therapeutic challenge in curing chronic HBV infection. Although there are individual differences among mice, all mice injected with rAAV-HBV1.3 exhibited the expression of HBV DNA and cccDNA.
4. Discussion
Chronic hepatitis B (CHB) remains a significant global health challenge due to the persistence of covalently closed circular DNA (cccDNA), a key viral reservoir that sustains infection despite antiviral therapy. Achieving a complete cure, which requires the clearance of HBsAg and the elimination of all forms of HBV DNA, including cccDNA, is extremely difficult. Current treatment goals focus on functional cure, defined by the clearance of HBsAg and undetectable serum HBV DNA levels [17, 18]. However, even in patients who achieve functional cure, inactive cccDNA or integrated HBV DNA can remain in hepatocytes, posing a risk of viral reactivation, especially in cases of immune suppression [
19].
The persistence of cccDNA underscores its importance as a therapeutic target for CHB management. However, the development of robust animal models for cccDNA research has been hampered by the narrow species specificity of HBV, limiting the availability of suitable models. Although mice are widely used in research due to their convenience, they cannot be naturally infected with HBV because murine NTCP, the receptor critical for HBV entry, does not facilitate the internalization of the virus. [13, 20].
The persistence of cccDNA underscores its importance as a therapeutic target for CHB management. However, the development of robust animal models for cccDNA research has been hampered by the narrow species specificity of HBV, limiting the availability of suitable models. Although mice are widely used in research due to their convenience, they cannot be naturally infected with HBV because murine NTCP, the receptor critical for HBV entry, does not facilitate the internalization of the virus. This lack of a fully susceptible mouse model has been a major obstacle in studying the full HBV life cycle, particularly the formation and persistence of cccDNA.
In a study by Zaichao et al., cccDNA formed during the transduction of AAV-HBV1.04 was not derived from rcDNA but shared the same sequence as that observed in the sequencing data of wild-type HBV cccDNA [
14]. Additionally, in rAAV-HBV1.2 and rAAV-HBV1.3 mouse models, viral proteins can be generated from both the viral episome and cccDNA [15, 21]. These models have proven valuable for studying chronic HBV infection and cccDNA dynamics.
Our study builds on this approach by successfully establishing a chronic mouse model of HBV infection using a recombinant AAV carrying the HBV genome, rAAV-HBV1.3. This model supports sustained HBV replication and cccDNA formation, mimicking key aspects of chronic infection in humans. We observed that HBV DNA levels peaked early, at week 1, and remained stable for 12 weeks, indicating continuous viral replication. Crucially, cccDNA levels peaked at week 2 and persisted throughout the study period, confirming the long-term presence of this viral reservoir. The persistence of cccDNA in our model is particularly significant, as it mirrors the clinical scenario where cccDNA remains detectable in patients even after serum HBV DNA becomes undetectable. This makes our model particularly relevant for studying therapeutic strategies targeting cccDNA.
The genotype-specific nature of HBV also plays a crucial role in disease progression and response to therapy. Genotype C, the most prevalent genotype in South Korea[
22], is associated with more severe disease progression compared to other genotypes such as B. Our model, using rAAV-HBV1.3 with genotype C, thus provides a valuable platform for studying the pathophysiology of HBV in the Korean context. Previous studies have demonstrated cccDNA expression in mice infected with rAAV-HBV1.3 using Southern blot analysis, which aligns with our findings showing detectable cccDNA in liver tissues of infected mice [
21].
Our results are distinct from previous HDI-based HBV models that injected plasmid, where cccDNA was not reliably detected, and HBsAg was maintained at lower levels [
23]. In contrast, our rAAV-HBV1.3 mouse model demonstrated sustained high levels of HBsAg in serum and detectable cccDNA throughout the 12-week period. This suggests that our model more closely replicates the chronic nature of HBV infection, making it a valuable tool for long-term studies of CHB pathogenesis and for evaluating new antiviral therapies aimed at eradicating cccDNA.
Although we were unable to distinguish whether the signals detected in the liver tissue represented false positives for HBV DNA or cccDNA, no signals were observed in the negative control group, reinforcing the validity of our findings. However, further studies using more sensitive and specific techniques such as Southern blotting or in situ hybridization may be warranted to confirm the exclusive detection of cccDNA. Additionally, our model demonstrated normal serum ALT levels by week 12, suggesting minimal liver damage at later stages of infection, despite the persistence of viral markers such as HBsAg and HBeAg. Both HBsAg and HBeAg are associated with cccDNA expression and can serve as surrogate markers for ongoing viral activity[
24].
In conclusion, we successfully established a CHB mouse model using rAAV-HBV1.3, which supports persistent HBV infection and cccDNA expression over an extended period. This model provides a valuable platform for studying the dynamics of cccDNA in chronic HBV infection and for evaluating novel therapeutic strategies aimed at eradicating this critical viral reservoir. Future studies should focus on refining detection techniques and exploring therapeutic interventions targeting cccDNA to move closer to a complete cure for CHB.
Author Contributions
Conceptualization, W.H., S.B. and P.S.S.; methodology, D.H.S ; validation P.S.S and S.B. ; resources, S.B. and W.H. ; writing—original draft preparation, D.H.S.; writing—review and editing, W.H. and P.S.S.; visualization, D.H.S and J.W.H.; supervision, J.W.H., S.K.Y. and P.S.S ; project administration, S.B. and S.K.Y. ; funding acquisition, S.B. and W.H. ; All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by intramural funds (grant numbers 2022-NG-002-01) from the Korea National Institute of Health.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee and was approved by the Center for Medical Science of the Catholic University of Korea (2023-0153-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsukuda, S., and K. Watashi. "Hepatitis B Virus Biology and Life Cycle." Antiviral Res 182 (2020): 104925. [CrossRef]
- Esser, K., X. Cheng, J. M. Wettengel, J. Lucifora, L. Hansen-Palmus, K. Austen, A. A. Roca Suarez, S. Heintz, B. Testoni, F. Nebioglu, M. T. Pham, S. Yang, A. Zernecke, D. Wohlleber, M. Ringelhan, M. Broxtermann, D. Hartmann, N. Huser, J. Mergner, A. Pichlmair, W. E. Thasler, M. Heikenwalder, G. Gasteiger, A. Blutke, A. Walch, P. A. Knolle, R. Bartenschlager, and U. Protzer. "Hepatitis B Virus Targets Lipid Transport Pathways to Infect Hepatocytes." Cell Mol Gastroenterol Hepatol 16, no. 2 (2023): 201-21. [CrossRef]
- Sung, P. S., D. J. Park, J. H. Kim, J. W. Han, E. B. Lee, G. W. Lee, H. C. Nam, J. W. Jang, S. H. Bae, J. Y. Choi, E. C. Shin, S. H. Park, and S. K. Yoon. "Ex Vivo Detection and Characterization of Hepatitis B Virus-Specific Cd8(+) T Cells in Patients Considered Immune Tolerant." Front Immunol 10 (2019): 1319. [CrossRef]
- Jacobson, I. M., R. S. Brown, Jr., B. J. McMahon, R. P. Perrillo, and R. Gish. "An Evidence-Based Practical Guide to Vaccination for Hepatitis B Virus." J Clin Gastroenterol 56, no. 6 (2022): 478-92. [CrossRef]
- Lin, C. L., and J. H. Kao. "Development of Hepatocellular Carcinoma in Treated and Untreated Patients with Chronic Hepatitis B Virus Infection." Clin Mol Hepatol 29, no. 3 (2023): 605-22. [CrossRef]
- Wong, G. L. H., E. Gane, and A. S. F. Lok. "How to Achieve Functional Cure of Hbv: Stopping Nucs, Adding Interferon or New Drug Development?" Journal of Hepatology 76, no. 6 (2022): 1249-62.
- Wang, X., X. Liu, Z. Dang, L. Yu, Y. Jiang, X. Wang, and Z. Yan. "Nucleos(T)Ide Analogues for Reducing Hepatocellular Carcinoma in Chronic Hepatitis B Patients: A Systematic Review and Meta-Analysis." Gut Liver 14, no. 2 (2020): 232-47.
- Li, Q., B. Sun, Y. Zhuo, Z. Jiang, R. Li, C. Lin, Y. Jin, Y. Gao, and D. Wang. "Interferon and Interferon-Stimulated Genes in Hbv Treatment." Front Immunol 13 (2022): 1034968. [CrossRef]
- Wei, L., and A. Ploss. "Hepatitis B Virus Cccdna Is Formed through Distinct Repair Processes of Each Strand." Nature Communications 12, no. 1 (2021). [CrossRef]
- Bhat, S. A., and S. N. Kazim. "Hbv Cccdna-a Culprit and Stumbling Block for the Hepatitis B Virus Infection: Its Presence in Hepatocytes Perplexed the Possible Mission for a Functional Cure." ACS Omega 7, no. 28 (2022): 24066-81.
- Ghany, M. G., and A. S. Lok. "Functional Cure of Hepatitis B Requires Silencing Covalently Closed Circular and Integrated Hepatitis B Virus DNA." J Clin Invest 132, no. 18 (2022). [CrossRef]
- Yang, D., L. Liu, D. Zhu, H. Peng, L. Su, Y. X. Fu, and L. Zhang. "A Mouse Model for Hbv Immunotolerance and Immunotherapy." Cell Mol Immunol 11, no. 1 (2014): 71-8. [CrossRef]
- Wettengel, J. M., and B. J. Burwitz. "Innovative Hbv Animal Models Based on the Entry Receptor Ntcp." Viruses 12, no. 8 (2020). [CrossRef]
- Xu, Z., L. Zhao, Y. Zhong, C. Zhu, K. Zhao, Y. Teng, X. Cheng, Q. Chen, and Y. Xia. "A Novel Mouse Model Harboring Hepatitis B Virus Covalently Closed Circular DNA." Cell Mol Gastroenterol Hepatol 13, no. 4 (2022): 1001-17. [CrossRef]
- Lucifora, J., A. Salvetti, X. Marniquet, L. Mailly, B. Testoni, F. Fusil, A. Inchauspe, M. Michelet, M. L. Michel, M. Levrero, P. Cortez, T. F. Baumert, F. L. Cosset, C. Challier, F. Zoulim, and D. Durantel. "Detection of the Hepatitis B Virus (Hbv) Covalently-Closed-Circular DNA (Cccdna) in Mice Transduced with a Recombinant Aav-Hbv Vector." Antiviral Res 145 (2017): 14-19. [CrossRef]
- Allweiss, L., B. Testoni, M. Yu, J. Lucifora, C. Ko, B. Qu, M. Lutgehetmann, H. Guo, S. Urban, S. P. Fletcher, U. Protzer, M. Levrero, F. Zoulim, and M. Dandri. "Quantification of the Hepatitis B Virus Cccdna: Evidence-Based Guidelines for Monitoring the Key Obstacle of Hbv Cure." Gut 72, no. 5 (2023): 972-83. [CrossRef]
- Zoulim, F., and B. Testoni. "Eliminating Cccdna to Cure Hepatitis B Virus Infection." Journal of Hepatology 78, no. 4 (2023): 677-80. [CrossRef]
- Xia, Y., and T. J. Liang. "Development of Direct-Acting Antiviral and Host-Targeting Agents for Treatment of Hepatitis B Virus Infection." Gastroenterology 156, no. 2 (2019): 311-24. [CrossRef]
- Wang, Y., Y. Li, W. Zai, K. Hu, Y. Zhu, Q. Deng, M. Wu, Y. Li, J. Chen, and Z. Yuan. "Hbv Covalently Closed Circular DNA Minichromosomes in Distinct Epigenetic Transcriptional States Differ in Their Vulnerability to Damage." Hepatology 75, no. 5 (2022): 1275-88. [CrossRef]
- Zhang, Z., Q. Zhang, Y. Zhang, Y. Lou, L. Ge, W. Zhang, W. Zhang, F. Song, and P. Huang. "Role of Sodium Taurocholate Cotransporting Polypeptide (Ntcp) in Hbv-Induced Hepatitis: Opportunities for Developing Novel Therapeutics." Biochem Pharmacol 219 (2024): 115956. [CrossRef]
- Ko, C., J. Su, J. Festag, R. Bester, A. D. Kosinska, and U. Protzer. "Intramolecular Recombination Enables the Formation of Hepatitis B Virus (Hbv) Cccdna in Mice after Hbv Genome Transfer Using Recombinant Aav Vectors." Antiviral Res 194 (2021): 105140. [CrossRef]
- Liu, Z., Y. Zhang, M. Xu, X. Li, and Z. Zhang. "Distribution of Hepatitis B Virus Genotypes and Subgenotypes: A Meta-Analysis." Medicine (Baltimore) 100, no. 50 (2021): e27941.
- Du, Y., R. Broering, X. Li, X. Zhang, J. Liu, D. Yang, and M. Lu. "In Vivo Mouse Models for Hepatitis B Virus Infection and Their Application." Front Immunol 12 (2021): 766534. [CrossRef]
- Jin, Y., S. Wang, S. Xu, S. Zhao, X. Xu, V. Poongavanam, L. Menendez-Arias, P. Zhan, and X. Liu. "Targeting Hepatitis B Virus Cccdna Levels: Recent Progress in Seeking Small Molecule Drug Candidates." Drug Discov Today 28, no. 7 (2023): 103617. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).