Submitted:
08 October 2024
Posted:
09 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
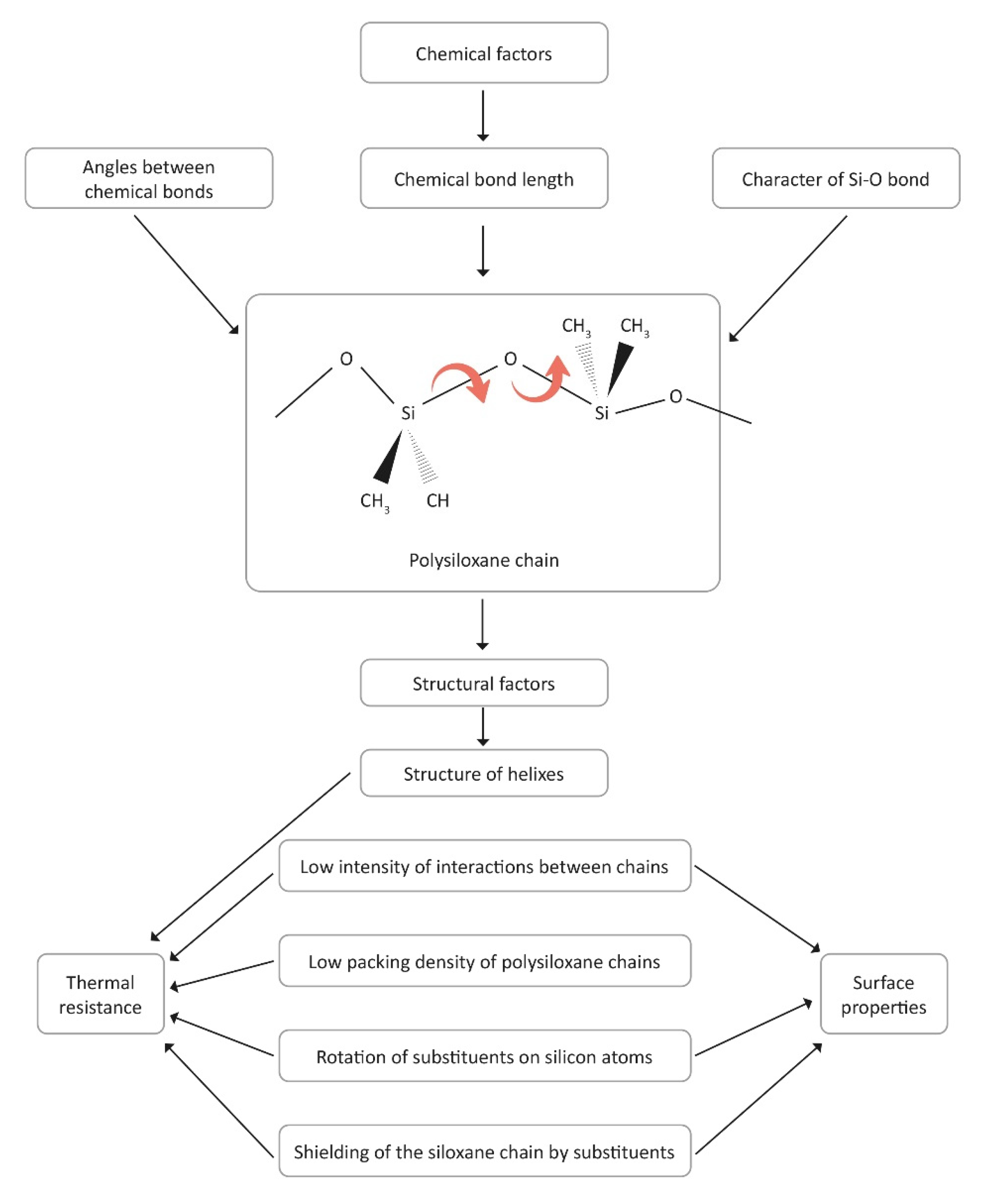
3. Factors Related to the Structure of Organosilicon Polymers
4. Factors Related to Micro and Nano Additives
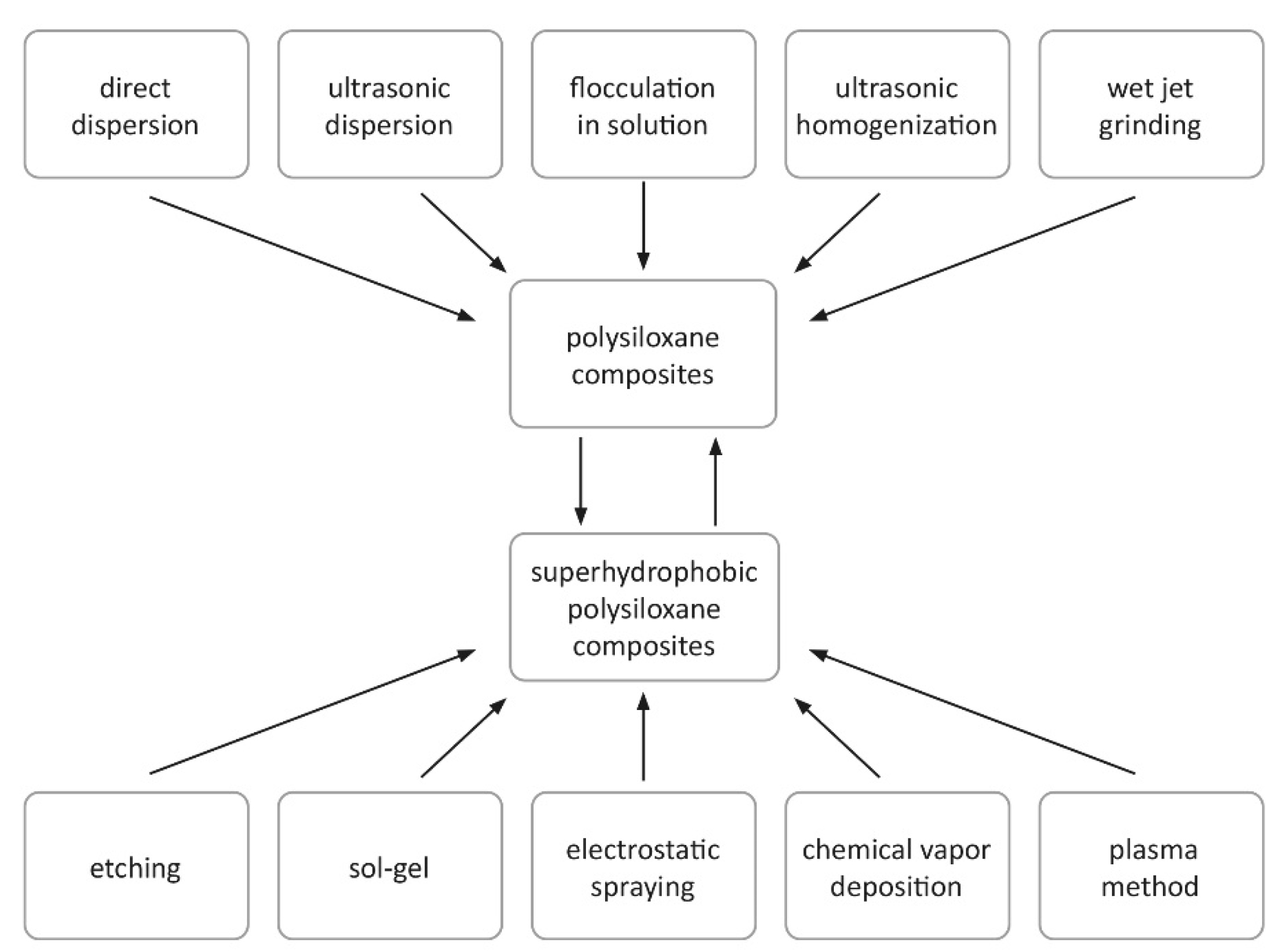
5. Factors Related to Composite Manufacturing Methods
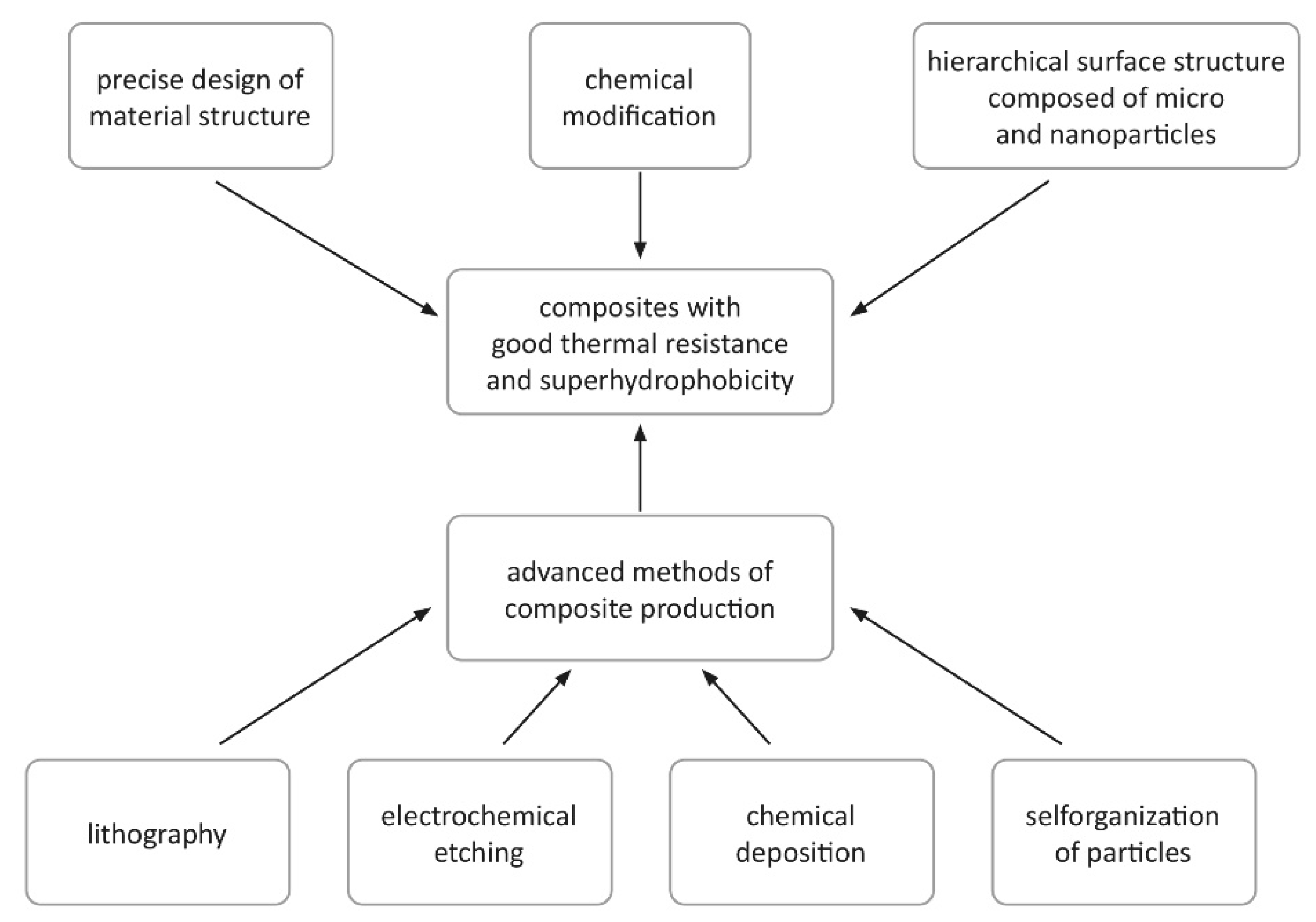
6. Analysis of Methods for Achieving Simultaneous Thermal Stability and Surface Superhydrophobicity in Silicone-Containing Composites
7. Conclusions
- Structure of organosilicon polymers: One of the key factors determining the unique properties of these composites is the structure of the organosilicon polymer. The thermal properties are due to the presence of Si-O bonds, which are characterized by high thermal stability. The rotation of substituents around Si-C bonds along the polysiloxane chain limits the interactions between adjacent segments, which also affects the thermal resistance of the material.
- Micro- and nanoparticle additives: Another important factor influencing the properties of composites is the selection of appropriate microfillers and nanoparticles. Additives such as aluminium oxide (Al₂O₃) or boron nanoparticles significantly affect thermal conductivity and mechanical properties, which allows the use of composites in more demanding applications.
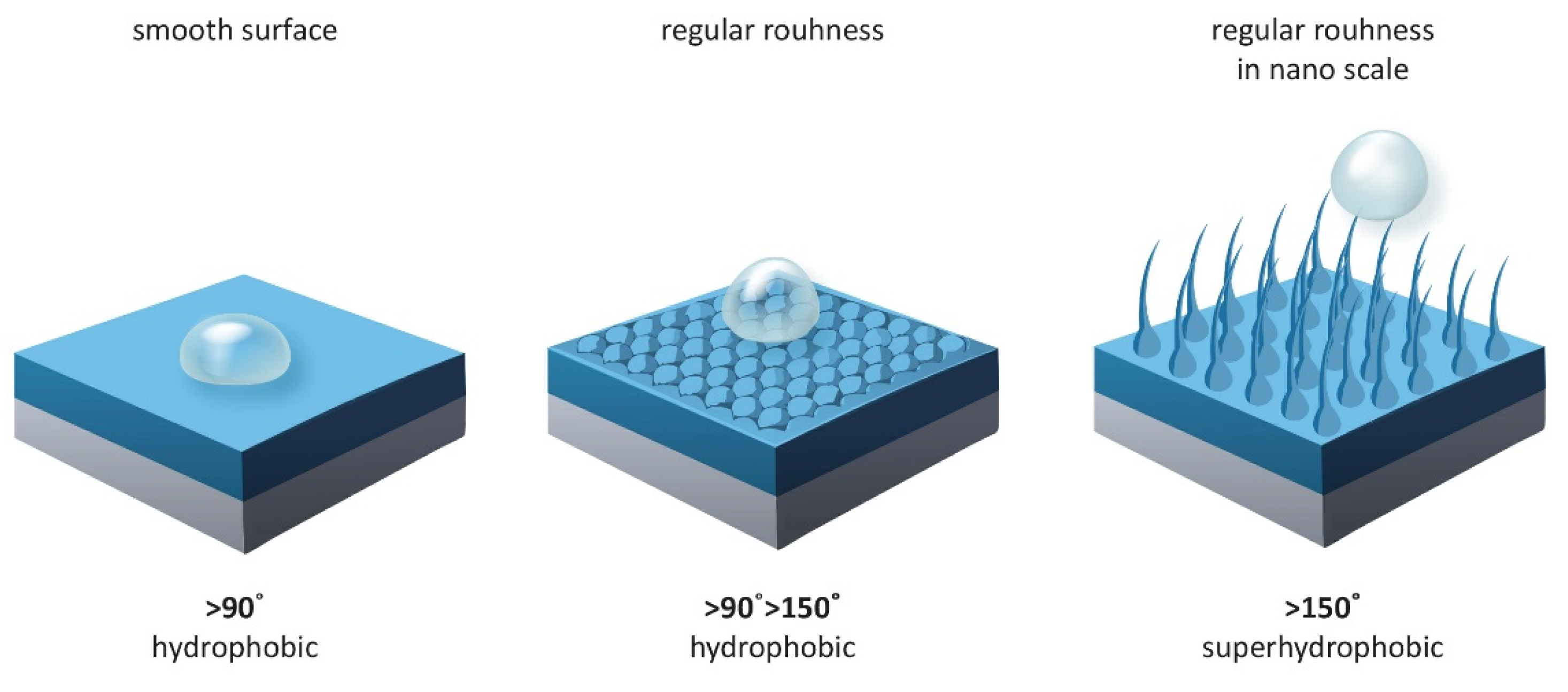
- Surface modification: Achieving simultaneous superhydrophobicity and thermal stability requires precise surface modification and appropriate selection of manufacturing methods. The use of methods such as sol-gel technique, chemical deposition or lithography allows obtaining surface structure on micro- and nano-scale, which helps to minimize contact with water.
- Integration of manufacturing methods: The best results in the production of composites with exceptional properties are achieved with integrated manufacturing techniques that allow for controlled deposition of successive layers of materials or independent assembly of polymers. An example is layered epoxy-silicone composites, which exhibit excellent protective properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BN | Boron Nitride |
| BN-MG | Boron Nitride Nanoplatelet-Multilayer Graphene |
| CAGR | The Compound Annual Growth Rate |
| CDs | Carbon Dots |
| GO | Graphene Oxide |
| HSN | Hollow Silica Nanospheres |
| MPSi | Modified Precipitated Silica |
| MQ | a type of silicone resin with a unique structure |
| ND | Nanodiamond |
| PDDA | Poly(dimethyldiallylammonium chloride) |
| PDMS | Poly(dimethylsiloxane) |
| POSS | Polyhedral Oligomeric Silsesquioxane |
| PSi | Precipitated Silica |
| REACH | Registration, Evaluation, Authorisation and Restriction of Chemicals |
| RGO | Reduced Graphene Oxide |
| RoHS Directive | The Restriction of Hazardous Substances Directive |
| SC | Silicon Carbide |
| SCNR | Slightly Crosslinked Natural Rubber |
| SR | Silicone Rubber |
| Tg temperature | the glass transition temperature |
| TGA | Thermogravimetric Analysis |
| UiO-66-NH2 | 2-aminoterephthalate; oxygen (2-); zirconium (4+); tetrahydroxide |
| VOCs | Volatile Organic Compounds |
References
- Verma, G.; Sheshkar, N.; Pandey, C.; Gupta, A. Recent trends of silicon elastomer-based nanocomposites and their sensing applications. J. Polym. Res. 2022, 29, 195–221. [CrossRef]
- Nazir, M.T.; Phung, B.T.; Yeoh, G.H.; Yasin, G.; Akram, S.; Bhutta, M.; Mehmood, M.; Hussain, S.; Yu, S.; Kabir, I. Enhanced dielectric and thermal performance by fabricating coalesced network of alumina trihydrate/boron nitride in silicone rubber for electrical insulation. Bull. Mater. Sci. 2020, 43, 1–5. [CrossRef]
- Wang, G.; Li, A.; Zhao, W.; Xu, Z.; Ma, Y.; Zhang, F.; Zhang, Y.; Zhou, J.; He, Q. A Review on Fabrication Methods and Research Progress of Superhydrophobic Silicone Rubber Materials. Adv. Mater. Interfaces 2020, 2001460. [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Klapsa,W.; Dziechciarz, A. Self-Cleaning Coatings and Surfaces of Modern Building Materials for the Removal of Some Air Pollutants. Materials 2021, 14, 2161. [CrossRef]
- Teisala, H.; Baumli, P.; Weber, S.A.L; Vollmer, D.; Butt, H-J. Grafting Silicone at Room Temperaturea Transparent, Scratch-resistant Nonstick Molecular Coating. Langmuir 2020, 36, 4416−4431. [CrossRef]
- Haghshenas, N.; Nejat, A.; Seyedmehdi, S. A.; Ou, J.; Amirfazli, A; Chini, S.F. Adhesion Aspects of Silicone Rubber Coatings for High Voltage Insulators: A Critical Review. Rev. Adhesion Adhesives, 2021, 9, 434-480. [CrossRef]
- Lee, J.-Y.; Oh, M.-H.; Park, J.-H.; Kang, S.-H.; Kang, S.-K. Three-Dimensionally Printed Expandable Structural Electronics via Multi-Material Printing Room-Temperature-Vulcanizing (RTV) Silicone/Silver Flake Composite and RTV. Polymers 2023, 15, 2003. [CrossRef]
- Lamont, C.; Grego, T.; Nanbakhsh, K.; Shah Idil, A.; Giagka, V.; Vanhoestenberghe, A.; Cogan, S.; Donaldson, N. Silicone encapsulation of thin-film SiOx, SiOxNy and SiC for modern electronic medical implants: a comparative long-term ageing studyJ. Neural Eng. 2021, 18 055003. [CrossRef]
- Syafiq, A.; Balakrishnan, V.; Ali, M.S.; Dhoble, S.J.; Rahim, N.A.; Omar, A.; Bakar, A.H.A. Application of transparent self-cleaning coating for photovoltaic panel: a review, Current Opinion in Chemical Engineering, 2022, 36, 100801. [CrossRef]
- Tayel, S.A.; Abu El-Maaty, A.E.; Mostafa, E.M.; Elsaadawo, Y.F. Enhance the performance of photovoltaic solar panels by a self-cleaning and hydrophobic nanocoating. Sci Rep 2022, 12, 21236. [CrossRef]
- Syafiq, A.; Pandey, A.K.; Adzman, N.N.; Rahim, N.A. Advances in approaches and methods for self-cleaning of solar photovoltaic panels. Solar Energy 2018, 162, 597-619. [CrossRef]
- Global Silicone Coating Market—Industry Trends and Forecast to 2029. Data Bridge Market Researc, June 2022, 350 pp.
- Silicone Coating Market Size & Share Analysis—Growth Trends & Forecasts (2024—2029), Mordor Intelligence Research & Advisory, November 2023.
- Keeping, J. Conformal Coatings. Capter 13. In Lead-free Soldering Process Development and Reliability, Bath J. (Ed.). Wiley Online Books Online 2020 ISBN: 9781119482093, Hoboken, NJ 07030, USA . [CrossRef]
- Maske, V.A.; More, A.P. Conformal coatings for lithium-ion batteries: A comprehensive review. Progress in Organic Coatings 2024, 188, 108252. [CrossRef]
- Zou, L.; Descamps, P. Method to Predict Performances of PCB Silicone Conformal Coating under Thermal Aging. Appl. Sci. 2022, 12, 11268. [CrossRef]
- Owen, M.J. Silicone Surface Fundamentals Macromol. Rapid Commun. 2021, 42, 2000360. [CrossRef]
- Vidal, K.; Gómez, E.; Goitandia, A.M.; Angulo-Ibáñez, A.; Aranzabe, E. The Synthesis of a Superhydrophobic and Thermal Stable Silica Coating via Sol-Gel Process. Coatings 2019, 9, 627. [CrossRef]
- Li, D.; Ma, L.; Zhang, B; Chen S. Large-scale fabrication of a durable and self-healing super-hydrophobic coating with high thermal stability and long-term corrosion resistance. Nanoscale, 2021,13, 7810-7821. [CrossRef]
- Chojnowski, J.; Cypryk, M. Synthesis of linear polysiloxanes in Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications Jones, R.G.; Ando, W.; Chojnowski, J.Eds.; Springer Science & Business Media, 2013, pp. 3-41. [CrossRef]
- Apeloig, Y. Theoretical Aspects of Organosilicon Compounds in Organic Silicon Compounds Patai, S.; Rappoport Z. Eds.; John Wiley & Sons, Ltd. 1989, 1, pp. 57-225. ISBN: 0-471-91993-4 . [CrossRef]
- Weinhold, F.; West, R. The Nature of the SiliconOxygen Bond. Organometallics 2011, 30, 5815–5824. [CrossRef]
- Dvornic, P.R. Thermal properties of polysiloxanes in Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications Jones, R.G.; Ando, W.; Chojnowski, J.Eds.; Springer Science & Business Media, 2013, pp 185-212. [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Cygańczuk, K.; PastuszkaŁ.; Jurecki, L. Silicone Resin-Based Intumescent Paints, Materials 2020, 13, 4785; [CrossRef]
- Tong, Y.; Liu, H.; Chen, A.; Guan, H.; Kong, J.; Liu, S.; He, C. Effect of surface chemistry and morphology of silica on the thermal and mechanical properties of silicone elastomers. J. Appl. Polym. Sci. 2018, 135. 46646. [CrossRef]
- Chen, J.; Jian Liu, J.; Zhilong Peng, Z.; Yao, Y. Shaohua Chen: The microscopic mechanism of size effect in silica-particle reinforced silicone rubber composites. Engineering Fracture Mechanics 2021, 255, 107945. [CrossRef]
- Zhou, W.; Qi, S.; Tu, C.; Zhao, H.; Wang, C.; Kou, J. Effect of the Particle Size of Al2O3 on the Properties of Filled Heat-Conductive Silicone Rubber. Journal of Applied Polymer Science, 2007, 104, 1312–1318. [CrossRef]
- Wang, Y.; Wu, J.; Yin, Y. Effect of Micro and Nano-Size Boron Nitride and Silicon Carbide on Thermal Properties and Partial Discharge Resistance of Silicone Elastomer Composite. IEEE Transactions on Dielectrics and Electrical Insulation 2020, 27, 377-385. [CrossRef]
- Kong, S.M.; Mariatti, M.; Busfield, J.J.C. Effects of types of fillers and filler loading on the properties of silicone rubber composites, Journal of Reinforced Plastics and Composites 2015, 30, 1087–1096. [CrossRef]
- Guchait, A.; Saxena, A.; Chattopadhyay, S.; Mondal, T.; Influence of Nanofillers on Adhesion Properties of Polymeric Composites. ACS Omega 2022, 7, 5, 3844–3859. [CrossRef]
- Kong, K.T.S.; Mariatti, M.; Rashid, A.A.; Busfield J.J.C. Effect of processing methods and functional groups on the properties of multi-walled carbon nanotube filled poly(dimethyl siloxane) composite. Polym. Bull. 2012, 69, 937–953. [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; · Khan, F.U.; · Ullah R.; Iqbal, M.; Ali, A.; Ullah B. Recent Progress in Silane Coupling Agent with Its Emerging Applications. J Polym Environ 2021, 29, 3427–3443. [CrossRef]
- Jin, G.; Lu, Y.; Yu P.; Zhang, L. Simple method to prepare fluorescent silicon rubber by melt-compounding with crude carbon dots fluid, Materials Today Communications, 2021, 27, 102413. [CrossRef]
- Han, R.; Li, Y.; Zhu,Q.; Niu K., Research on the preparation and thermal stability of silicone rubber composites: A review. Composites Part C: Open Access, 2022, 8, 100249. [CrossRef]
- Dow Silicones Corp. Fluorosilicone Elastomers Comprising Yellow Iron Oxide U.S. Patent US20170267829, 21 September 2017.
- Dow Silicones Corp. I. Fluorosilicone Elastomers for High Temperature Performance: U.S. Patent US 2010/0166996, 1 July 2020.
- Zhang, Y.; Zeng, X.; Lai, X.; Li, H.; Zhou, Q.; Huang, X.; Suppression Effect and Mechanism of Amine-Containing MQ Silicone Resin on the Tracking and Erosion Resistance of Silicone Rubber. ACS Omega 2017, 2, 8, 5111–5121. [CrossRef]
- 38. Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Reduced Graphene Oxide Embedded with MQ Silicone Resin Nano-Aggregates for Silicone Rubber Composites with Enhanced Thermal Conductivity and Mechanical Performance. Polymers 2018, 10, 1254. [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced Thermal Conductivity of Epoxy–Graphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [CrossRef]
- Deng, B.; Shi, Y.; Zhang, X.; Ma, W.; Liu, H.; Gong, C. Thermally Conductive and Electrically Insulated Silicone Rubber Composites Incorporated with Boron Nitride−Multilayer Graphene Hybrid Nanofiller. Nanomaterials 2022, 12, 2335. [CrossRef]
- Yu, S.; Zuo, H. Xu, X.; Ning, N.; B. Yu, B. Zhang, L.; Tian, M. Self-Healable Silicone Elastomer Based on the Synergistic Effect of the Coordination and Ionic Bonds. ACS Appl. Polym. Mater. 2021, 3, 5, 2667–2677. [CrossRef]
- Xu, S.; Gao, Q.; Zhou, C.; Li, J.; Shen, L. Hongjun Lin, Improved thermal stability and heat-aging resistance of silicone rubber via incorporation of UiO-66-NH2, Materials Chemistry and Physics, 2021, 274, 125182. [CrossRef]
- Ma, X.; Zhang, J.; Ma, X.; Zhang, Z.; Shen, S.; Wang,J.; Wang, S.; Peidong Xu, Yang, S.; Wei, L. Tetrafunctional vinyl polysilsesquioxane and its covalently cross-linked vinyl liquid silicone rubber for resistance to high temperature oxidation combustion and ablative behavior, Corrosion Science, 2023, 221, 111315. [CrossRef]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W. Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites. Materials 2018, 11, 1298. [CrossRef]
- Thompson, D.B.; Fawcett, A.S.; Michael A. Brook M.A. Simple Strategies to Manipulate Hydrophilic Domains in Silicones in Silicon Based Polymers: Advances in Synthesis and Supramolecular Organization, Ganachaud, F.; Boileau, S.; Boury B. (Eds.), Springer, Dordrecht, Germany, 2008, pp.29-39. [CrossRef]
- Gorodov, V. V.; Milenin, S. A.; Demchenko, N. V.; Muzafarov, A. M. Carboxyl-Containing Polydimethylsiloxanes: Synthesis and Properties. INEOS OPEN, 2020, 3, 43–54. [CrossRef]
- Song, Y.; Yu, J.; Yu, L.; Alam, F.; Dai, W.; Li, C.; Jiang, N. Enhancing the thermal, electrical, and mechanical properties of silicone rubber by addition of graphene nanoplatelets. Mater. Des. 2015, 88, 950–957. [CrossRef]
- Zhang, Z.; Chen, P.; Nie, W.; Xu, Y.; Zhou, Y. Enhanced mechanical, thermal and solvent resistance of silicone rubber reinforced by organosilica nanoparticles modified graphene oxide. Polymer 2020; 203, 122772. [CrossRef]
- Yan, J.; Hu, C.; Chen, K.; Lin, Q. Release of graphene from graphene-polyethylene composite films into food simulants. Food Packag. Shelf Life. 2019; 20, 100310. [CrossRef]
- Li, D.; Dong, L.; Chen, Y.; Luo, C.; Zhou, J.; Liu, G.; Ren, H. Thermally Conductive and Antistatic Properties of Silicone Rubber Reinforced by the Modified Graphene Oxide. Polymers (Basel). 2022, 14, 4703. [CrossRef]
- Zhu, Q.; Wang, Z.; Zeng, H.; Yang, T.; Wang, X. Effects of graphene on various properties and applications of silicone rubber and silicone resin. Compos. Part A Appl. Sci. Manuf. 2021, 142, 106240. [CrossRef]
- Hu, Z.; Wu, W.; Chen, X.; Chen, Y.; Chen, J.; Hao, Z. Flexible thermal conductive Al2O3@siloxane composite with rapid self-healing property based on carboxyl-amine dynamic reversible bonds. RSC Adv., 2022, 12, 6649-6658. [CrossRef]
- Li, Z.; Wang, X.; Bai, H.; Cao, M. Advances in Bioinspired Superhydrophobic Surfaces Made from Silicones: Fabrication and Application. Polymers 2023, 15, 543. [CrossRef]
- Jeevahan , J.; Chandrasekaran, M.; Joseph, G.B.; Durairaj, R. B.; Mageshwaran, G. Superhydrophobic surfaces: a review on fundamentals, applications, and challenges. J. Coat. Technol. Res., 2018, 15, 231–250. [CrossRef]
- Marmur, A. Wetting of hydrophobic rough surfaces: To be heterogeneous or not to be. Langmuir 2003, 19, 8343–8348. [CrossRef]
- Liu, S.; Liu, S.; Wang, Q.; Zuo, Z.; Wei, L.; Chen, Z.; Liang, X. Improving surface performance of silicone rubber for composite insulators by multifunctional Nano-coating. Chemical Engineering Journal 2023, 451, Part 2, 138679. [CrossRef]
- Balabanava, N.; Wierzbicki, R.; Zielecka, M.; Rymuza, Z. Effect of roughness on adhesion of polymeric coatings used for microgrippers. Microelectronic Engineering 2007, 84, 1227–1230. [CrossRef]
- Demir, E.; Güler, Ö. Production and properties of epoxy matrix composite reinforced with hollow silica nanospheres (HSN): mechanical, thermal insulation, and sound insulation properties. J Polym Res 2022, 29, 477 . [CrossRef]
- Bertling, J.; Blömer, J.; Kümmel, R. Hollow microsperes. Chemical Engineering & Technology: Industrial Chemistry-Plant Equipment-Process Engineering-Biotechnology, 2004, 27, 829-837. [CrossRef]
- Yung, K.C.; Zhu, B.; Yue, T.M.; Xie, C. Preparation and properties of hollow glass microsphere-filled epoxy-matrix composites. Compos Sci Technol 2009, 69, 260–264. [CrossRef]
- Zhao, Y.; Zhang, J.; Xu, Q.; Mi, H-Y.; Zhang, Y.; Li, T.; Sun, H.; Han, J.; Liu, C.; Shen, C. Ultrastable and Durable Silicone Coating on Polycarbonate Surface Realized by Nanoscale Interfacial Engineering. ACS Appl. Mater. Interfaces 2020, 12, 11, 13296–13304. [CrossRef]
- Zhang, Q.; Liu, H.; Zhao, S.; Dong, W. Hydrophobic and optical properties of silica antireflective coating prepared via sol-gel methodMater. Res. Express 2021, 8, 046403. [CrossRef]
- Lv, C.; Wang, F.; He, C.; Kang, J.; He, X.; Li, Z. Preparation of silicone-PCL composite particles with hierarchical structure and the super-hydrophobic fabrics via directly electrostatic spraying, Surface and Coatings Technology, 2022, 449, 128933. [CrossRef]
- Shen, Z.; Hou, C.; Liu, S.; Guan, Z. Micro-nanostructured silicone-carbon composite coatings with superhydrophobicity and photoluminescence prepared by oxidative chemical vapor deposition J. Appl. Polym. Sci. 2014, 131, 40400. [CrossRef]
- Liu, X.; Zhou, Z.; Chen, M.; Liu, Z.; Jiang, S.; Wang, L. Preparation of Durable Superhydrophobic Coatings Based on Discrete Adhesives. Coatings 2024, 14, 463. [CrossRef]
- Kumar, A.; Mishra, V.; Negi, S.; Kar, S. A systematic review on polymer-based superhydrophobic coating for preventing biofouling menace. J Coat Technol Res 2023, 20, 1499–1512. [CrossRef]
- Cao, C.; Yi, B.; Zhang, J.; Hou, C.; Wang, Z.; Lu, G.; Huang, X.; Yao, X.;Sprayable superhydrophobic coating with high processibility and rapid damage-healing nature. Chemical Engineering Journal, 2020, 392, 124834. [CrossRef]
- Huang, W.; Jiang, X.; Zhang, Y.; Tang, Z.; Sun, Z.; Liu, Z.; Zhao, L.; Liu Y., Robust superhydrophobic silicone/epoxy functional coating with excellent chemical stability and self-cleaning ability. Nanoscale, 2023, 15, 17793-17807. [CrossRef]
- Beaugendre, A.; Lemesle, C.; S. Bellayer, S.; S. Degoutin, S.; S. Duquesne, S.; Casetta, M.; Pierlot, C.; Jaime, F.; Kim, T.; Jimenez, m. Flame retardant and weathering resistant self-layering epoxy-silicone coatings for plastics. Progress in Organic Coatings 2019, 136, 105269. [CrossRef]
- Cordoba, A.; Cauich-Rodríguez, J.V.; Vargas-Coronado, R.F.; Velázquez-Castillo, R.; Esquivel, K. A Novel In Situ Sol-Gel Synthesis Method for PDMS Composites Reinforced with Silica Nanoparticles. Polymers 2024, 16, 1125. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




