Submitted:
08 October 2024
Posted:
09 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Analytical Methods for the Simultaneous Determination of EDCs
2.1.1. Bisphenols
2.1.2. Parabens
2.1.3. PAHs
2.1.4. Glyphosate and Its Metabolites
2.1.5. Phthalates (Diesters and Corresponding Monoesters)
2.1.6. Pyrethroids and Chlorpyrifos
2.1.7. Perfluoroalkyl Substance (PFAS)
2.2. Control of Contamination
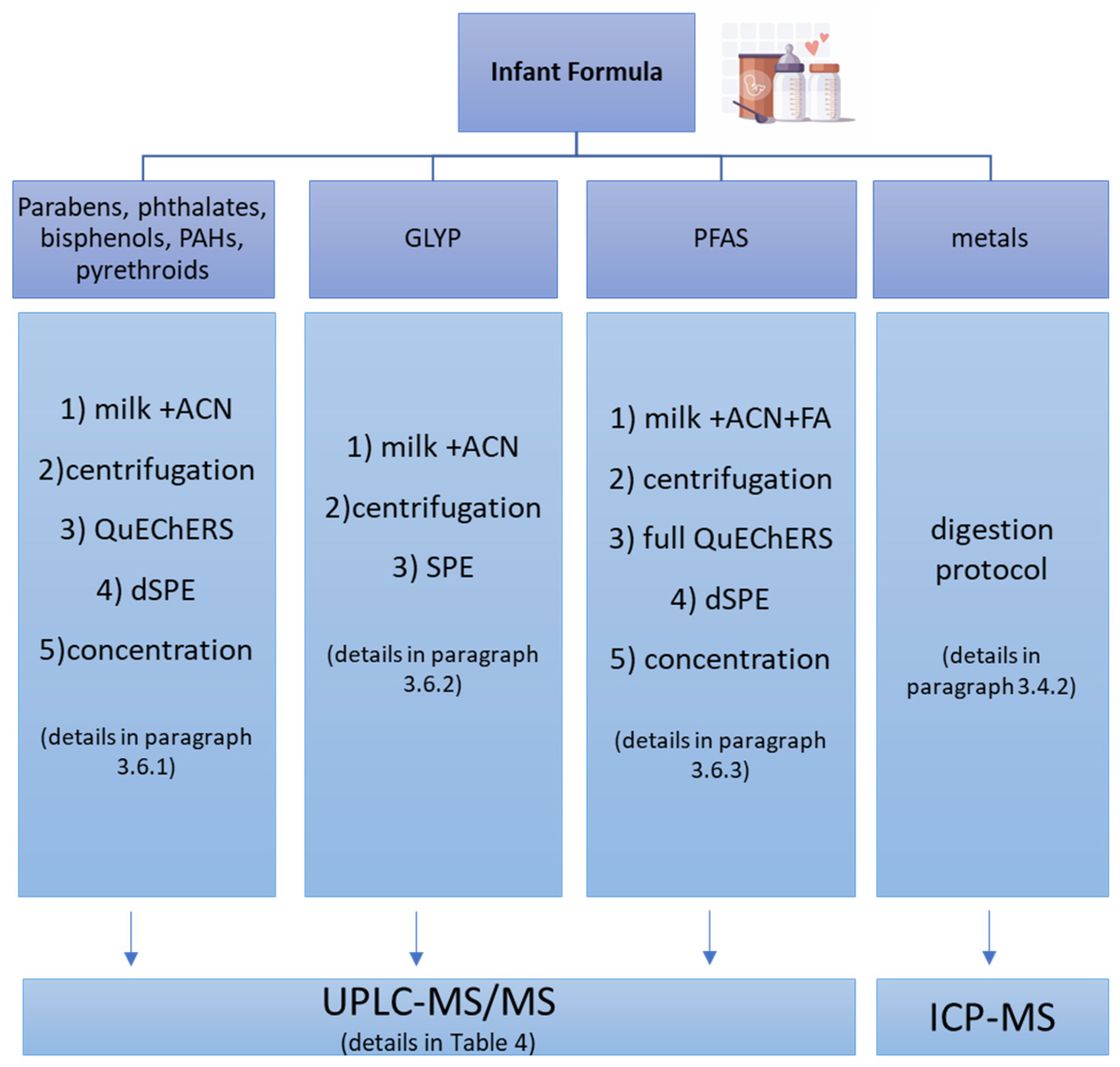
2.3. Sample Pre-Treatment
2.4. Quantification of Assessed and Suspected EDCs in Baby Bottles
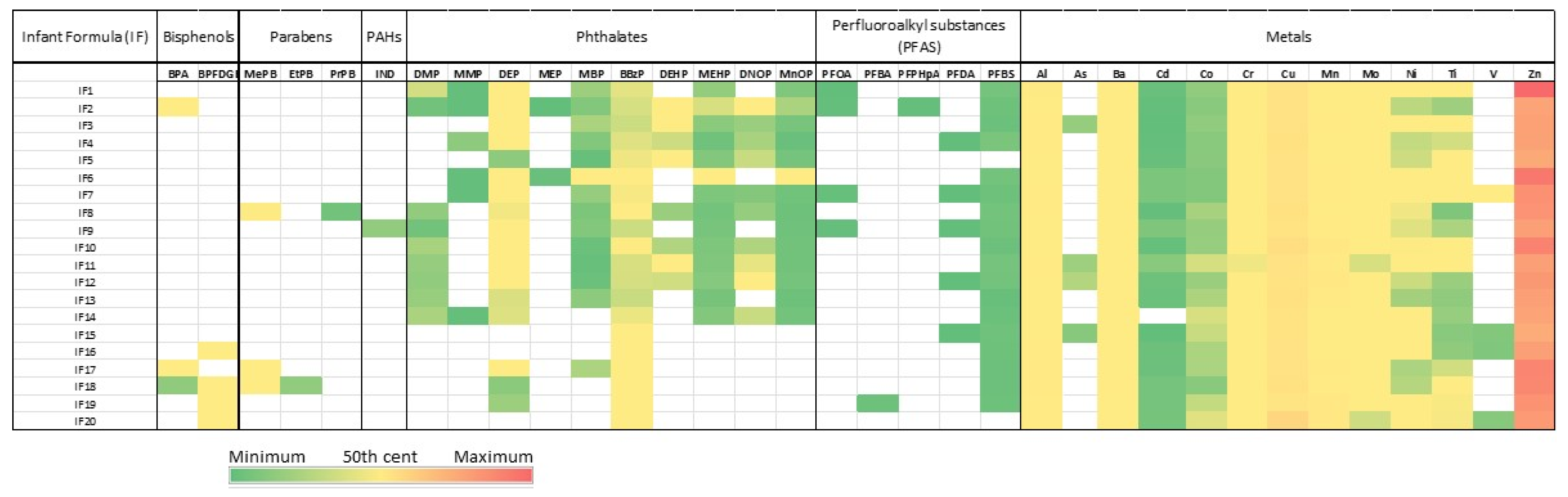
2.5. Quantification of Assessed and Suspected EDCs in Infant Formula
2.6. Estimated Dietary Exposure in Infants at 1 Month of Age
3. Conclusions
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Preparation of Standard Solutions.
4.3. Sampling Collection
4.4. Instrumentation
4.4.1. General Procedure UPLC-MS/MS Analysis
4.4.2. ICP-AES Analysis
4.5. Analytical procedures
4.6. Formula Milk Samples Pre-Treatment
4.6.1. QuEChERS for Phthalates, Bisphenols, PAHs, Parabens, and Pyrethroid Extractions
4.6.2. Milk Sample Filtration for Glyphosate and Its Metabolite Extractions
4.6.3. QuEChERS for PFAS
4.7. Overall Dietary Exposure to Single EDCs in the Infants
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Work Group on Breastfeeding. Breastfeeding and the Use of Human Milk. Pediatrics 1997, 100, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Section on Breastfeeding; Breastfeeding and the Use of Human Milk. Pediatrics 2005, 115, 496–506. [CrossRef] [PubMed]
- LaKind, J.S.; Wilkins, A.A.; Berlin, C.M. Environmental Chemicals in Human Milk: A Review of Levels, Infant Exposures and Health, and Guidance for Future Research. Toxicology and Applied Pharmacology 2004, 198, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Grønnestad, R.; Karimi, M.; Lie, E.; Manyilizu, W.B.; Mdegela, R.H.; Mokiti, F.; Murtadha, M.; et al. Prenatal Exposure to Persistent Organic Pollutants in Northern Tanzania and Their Distribution between Breast Milk, Maternal Blood, Placenta and Cord Blood. Environmental Research 2019, 170, 433–442. [Google Scholar] [CrossRef]
- Martín-Carrasco, I.; Carbonero-Aguilar, P.; Dahiri, B.; Moreno, I.M.; Hinojosa, M. Comparison between Pollutants Found in Breast Milk and Infant Formula in the Last Decade: A Review. Science of The Total Environment 2023, 875, 162461. [Google Scholar] [CrossRef]
- European Chemical Agency (ECHA) and European Food Safety Authority (EFSA) with the technical support of the Joint Research Centre (JRC); Andersson, N.; Arena, M.; Auteri, D.; Barmaz, S.; Grignard, E.; Kienzler, A.; Lepper, P.; Lostia, A.M.; Munn, S.; et al. Guidance for the Identification of Endocrine Disruptors in the Context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018, 16, 5311. [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews 2009, 30, 293–342. [Google Scholar] [CrossRef]
- De Mendonça Pereira, B.F.; De Almeida, C.C.; Leandro, K.C.; Da Costa, M.P.; Conte-Junior, C.A.; Spisso, B.F. Occurrence, Sources, and Pathways of Chemical Contaminants in Infant Formulas. Compr. Rev. Food Sci. Food Safe 2020, 19, 1378–1396. [Google Scholar] [CrossRef]
- De Almeida, C.C.; Baião, D.D.S.; Rodrigues, P.D.A.; Saint’Pierre, T.D.; Hauser-Davis, R.A.; Leandro, K.C.; Paschoalin, V.M.F.; Da Costa, M.P.; Conte-Junior, C.A. Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk. Int. J. Environ. Res. Public Health 2022, 19, 11178. [Google Scholar] [CrossRef]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global Standard for the Composition of Infant Formula: Recommendations of an ESPGHAN Coordinated International Expert Group. Journal of Pediatric Gastroenterology & Nutrition 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Davidowski, L.; Grosser, Z.; Sarojam, P. Perkin Elmer Application Note. The Analysis of Baby Foods and Juices for Metals to Protect a Sensitive Population; Food Safety; Shelton, CT, USA, 2009. https://resources.perkinelmer.com/corporate/pdfs/downloads/app_analysis_of_baby_foods_juices_for_metal.pdf. (accessed on 03 October 2024).
- Burrell, S.-A.M.; Exley, C. There Is (Still) Too Much Aluminium in Infant Formulas. BMC Pediatr. 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Chuchu, N.; Patel, B.; Sebastian, B.; Exley, C. The Aluminium Content of Infant Formulas Remains Too High. BMC Pediatr. 2013, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, J.; Rodriguez, I.; Mahadevan-Bava, S.; Exley, C. Prescription Infant Formulas Are Contaminated with Aluminium. Int. J. Environ. Res. Public Health 2019, 16, 899. [Google Scholar] [CrossRef]
- Weisstaub, G.; Uauy, R. Non-Breast Milk Feeding in Developing Countries: Challenge from Microbial and Chemical Contaminants. Ann. Nutr. Metab. 2012, 60, 215–219. [Google Scholar] [CrossRef]
- Oldring, P.K.T.; Castle, L.; O’Mahony, C.; Dixon, J. Estimates of Dietary Exposure to Bisphenol A (BPA) from Light Metal Packaging Using Food Consumption and Packaging Usage Data: A Refined Deterministic Approach and a Fully Probabilistic (FACET) Approach. Food Additives & Contaminants: Part A 2014, 31, 466–489. [Google Scholar] [CrossRef]
- Commission Directive 2011/8/EU of 28 January 2011. Amending Directive 2002/72/EC as Regards the Restriction of Use of Bisphenol A in Plastic Infant Feeding Bottles. Official Journal of the European Union 2011, L 26, 11-14. http://data.europa.eu/eli/dir/2011/8/oj.
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Official Journal of the European Union 2011, L 12, 1-89. http://data.europa.eu/eli/reg/2011/10/oj.
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA Journal 2023, 21, 6857. [CrossRef]
- Chen, H.-C.; Chang, J.-W.; Sun, Y.-C.; Chang, W.-T.; Huang, P.-C. Determination of Parabens, Bisphenol A and Its Analogs, Triclosan, and Benzophenone-3 Levels in Human Urine by Isotope-Dilution-UPLC-MS/MS Method Followed by Supported Liquid Extraction. Toxics 2022, 10, 21. [Google Scholar] [CrossRef]
- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Para Hydroxybenzoates (E 214-219). EFSA Journal 2004, 2, 83. [CrossRef]
- Khansari, N.; Adib, N.; Alikhani, A.; Babaee, T.; Khosrokhavar, R. Development and Validation of a New Method for Determination of Methylparaben in Iran Market Infant Formulae by HPLC. J. Environ. Health Sci. Engineer. 2021, 19, 565–572. [Google Scholar] [CrossRef]
- Dualde, P.; Pardo, O.; F. Fernández, S.; Pastor, A.; Yusà, V. Determination of Four Parabens and Bisphenols A, F and S in Human Breast Milk Using QuEChERS and Liquid Chromatography Coupled to Mass Spectrometry. Journal of Chromatography B 2019, 1114–1115, 154–166. [CrossRef]
- Kishikawa, N.; Wada, M.; Kuroda, N.; Akiyama, S.; Nakashima, K. Determination of Polycyclic Aromatic Hydrocarbons in Milk Samples by High-Performance Liquid Chromatography with Fluorescence Detection. Journal of Chromatography B 2003, 789, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Edeme, J.N.; Tesi, G.O.; Bassey, F.I.; Martincigh, B.S.; Nwajei, G.E. Polycyclic Aromatic Hydrocarbon Concentrations in Commercially Available Infant Formulae in Nigeria: Estimation of Dietary Intakes and Risk Assessment. Food and Chemical Toxicology 2014, 72, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 208/2005 of 4 February 2005 Amending Regulation (EC) No 466/2001 as Regards Polycyclic Aromatic hydrocarbons. Official Journal of the European Union 2005, L 34, 3-5. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:034:0003:0005:EN:PDF. (accessed 03 October 2024).
- Santilio, A.; Girolimetti, S.; Picardo, V. Rapid, Sensitive and Selective Detection of Glyphosate in Wheat and Rice Flour Using a Polar Stationary Phase by LC–MS/MS. Journal of Environmental Science and Health, Part B 2022, 57, 184–191. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and Diet: A Review of the Food Monitoring and Epidemiology Data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Smerieri, A.; Testa, C.; Lazzeroni, P.; Nuti, F.; Grossi, E.; Cesari, S.; Montanini, L.; Latini, G.; Bernasconi, S.; Papini, A.M.; Street, M.E. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One. 2015, 10(2), e0117831. [Google Scholar] [CrossRef]
- Testa, C.; Nuti, F.; Hayek, J.; De Felice, C.; Chelli, M.; Rovero, P.; Latini, G.; Papini, A.M. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012, 4(4), 223–229. [Google Scholar] [CrossRef]
- Cao, X. Phthalate Esters in Foods: Sources, Occurrence, and Analytical Methods. Comp Rev. Food Sci. Food Safe 2010, 9, 21–43. [Google Scholar] [CrossRef]
- Nuti, F.; Hildenbrand, S.; Chelli, M.; Wodarz, R.; Papini, A.M. Synthesis of DEHP metabolites as biomarkers for GC-MS evaluation of phthalates as endocrine disrupters. Bioorg Med Chem. 2005, 13(10), 3461–3465. [Google Scholar] [CrossRef]
- Van Der Meer, T.P.; Van Faassen, M.; Frederiksen, H.; Van Beek, A.P.; Wolffenbuttel, B.H.R.; Kema, I.P.; Van Vliet-Ostaptchouk, J.V. Development and Interlaboratory Validation of Two Fast UPLC–MS-MS Methods Determining Urinary Bisphenols, Parabens and Phthalates. Journal of Analytical Toxicology 2019, 43, 452–464. [Google Scholar] [CrossRef]
- Tran, K.; Twohig, M.; Young, M.; Aubin, A.; Meruva, N.; Fujimoto, G.; Stevens, R.; Roush, J.; Hudalla, C.J. Determination of the Oregon Pesticide List in Cannabis Using a Simple Extraction Procedure With dSPE Cleanup and UPLC-MS/MS. Waters Application Note 2018, APNT134998466. https://www.waters.com/webassets/cms/library/docs/720006373en.pdf. (accessed 03 October 2024).
- Bokkers, B.G.H.; van de Ven, B.; Janssen, P.; Bil, W.; van Broekhuizen, F.; Zeilmaker, M.; Oomen, A.G. Per- and Polyfluoroalkyl Substances (PFASs) in Food Contact Materials. Rijksinstituut voor Volksgezondheid en Milieu, Letter Report 2018-0181, 1-108. [CrossRef]
- Zeilmaker, M.J.; Fragki, S.; Verbruggen, E.M.J.; Bokkers, B.G.H.; Lijzen, J.P.A. Mixture Exposure to PFAS: A Relative Potency Factor Approach. Rijksinstituut voor Volksgezondheid en Milieu, Letter Report 2018-0070. [CrossRef]
- European Food Safety Authority (EFSA) Perfluorooctane Sulfonate (PFOS), Perfluorooctanoic Acid (PFOA) and Their Salts: Scientific Opinion of the Panel on Contaminants in the Food Chain. The EFSA Journal 2008, 653, 1–131. [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Scientific Opinion on the Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA Journal 2020, 18, 6223. [CrossRef]
- Di Nisio, A.; Rocca, M.S.; De Toni, L.; Sabovic, I.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; De Filippis, V.; Plebani, M.; Foresta, C. Endocrine Disruption of Vitamin D Activity by Perfluoro-Octanoic Acid (PFOA). Sci. Rep. 2020, 10, 16789. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K. Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism. Int. J. Mol. Sci. 2021, 22, 2148. [Google Scholar] [CrossRef]
- Pípal, M.; Wiklund, L.; Caccia, S.; Beronius, A. Assessment of Endocrine Disruptive Properties of PFOS: EFSA/ECHA Guidance Case Study Utilising AOP Networks and Alternative Methods. EFSA Journal 2022, 20(S1):e200418. [CrossRef]
- Commission Recommendation (EU) 2022/1431 of 24 August 2022 on the Monitoring of Perfluoroalkyl Substances in Food. Official Journal of the European Union 2022, L 221, 105-109. http://data.europa.eu/eli/reco/2022/1431/oj.
- COMMISSION STAFF WORKING DOCUMENT Poly- and Perfluoroalkyl Substances (PFAS) Accompanying the Document COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS Chemicals Strategy for Sustainability Towards a Toxic-Free Environment. 2020. EUR-Lex - 52020SC0249. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=SWD:2020:249:FIN. (accessed on 03 October 2024).
- Van Beijsterveldt, I.A.L.P.; Van Zelst, B.D.; De Fluiter, K.S.; Van Den Berg, S.A.A.; Van Der Steen, M.; Hokken-Koelega, A.C.S. Poly- and Perfluoroalkyl Substances (PFAS) Exposure through Infant Feeding in Early Life. Environment International 2022, 164, 107274. [Google Scholar] [CrossRef]
- Barbarossa, A.; Gazzotti, T.; Zironi, E.; Serraino, A.; Pagliuca, G. Short Communication: Monitoring the Presence of Perfluoroalkyl Substances in Italian Cow Milk. Journal of Dairy Science 2014, 97, 3339–3343. [Google Scholar] [CrossRef]
- LaKind, J.S.; Naiman, J.; Verner, M.-A.; Lévêque, L.; Fenton, S. Per- and Polyfluoroalkyl Substances (PFAS) in Breast Milk and Infant Formula: A Global Issue. Environmental Research 2023, 219, 115042. [Google Scholar] [CrossRef]
- Organtini, K.L.; Hird, S.; Adams, S. QuEChERS Extraction of Per- and Polyfluoroalkyl Substances (PFAS) from Edible Produce with Sensitive Analysis on Xevo TQ-XS. Waters Application Note 2023. https://www.waters.com/content/dam/waters/en/app-notes/2021/720007333/720007333-en.pdf. (accessed 03 October 2024).
- Muscat, M.; Sinagra, E.; Lia, F. Presence of Phthalate Esters Used as Common Plasticisers in Maltese Shoreline Sand. Environments 2023, 10, 94. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, R.; Jiménez-Díaz, I.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A. A Multiresidue Method for the Determination of Selected Endocrine Disrupting Chemicals in Human Breast Milk Based on a Simple Extraction Procedure. Talanta 2014, 130, 561–570. [Google Scholar] [CrossRef]
- Siddique, S.; Zhang, G.; Coleman, K.; Kubwabo, C. Investigation of the Migration of Bisphenols from Baby Bottles and Sippy Cups. Current Research in Food Science 2021, 4, 619–626. [Google Scholar] [CrossRef]
- Cirillo, T.; Latini, G.; Castaldi, M.A.; Dipaola, L.; Fasano, E.; Esposito, F.; Scognamiglio, G.; Francesco, F.D.; Cobellis, L. Exposure to Di-2-Ethylhexyl Phthalate, Di- N -Butyl Phthalate and Bisphenol A through Infant Formulas. J. Agric. Food Chem. 2015, 63, 3303–3310. [Google Scholar] [CrossRef]
- Ferrer, E.; Santoni, E.; Vittori, S.; Font, G.; Mañes, J.; Sagratini, G. Simultaneous Determination of Bisphenol A, Octylphenol, and Nonylphenol by Pressurised Liquid Extraction and Liquid Chromatography–Tandem Mass Spectrometry in Powdered Milk and Infant Formulas. Food Chemistry 2011, 126, 360–367. [Google Scholar] [CrossRef]
- Santonicola, S.; De Felice, A.; Cobellis, L.; Passariello, N.; Peluso, A.; Murru, N.; Ferrante, M.C.; Mercogliano, R. Comparative Study on the Occurrence of Polycyclic Aromatic Hydrocarbons in Breast Milk and Infant Formula and Risk Assessment. Chemosphere 2017, 175, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Ancillotti, C.; Checchini, L.; Fibbi, D.; Rossini, D.; Ciofi, L.; Rivoira, L.; Profeti, C.; Orlandini, S.; Furlanetto, S. Determination of Phthalate Diesters and Monoesters in Human Milk and Infant Formula by Fat Extraction, Size-Exclusion Chromatography Clean-up and Gas Chromatography-Mass Spectrometry Detection. Journal of Pharmaceutical and Biomedical Analysis 2018, 148, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Jin, Q.; He, H.; Bian, T.; Wang, S.; Fan, J. Determination of 17 Phthalate Esters in Infant Milk Powder and Dairy Products by GC–MS with 16 Internal Standards. Chromatographia 2016, 79, 903–910. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Current Biology 2011, 21, R877–R883. [Google Scholar] [CrossRef]
- Olivares, M.; Uauy, R. Copper as an Essential Nutrient. The American Journal of Clinical Nutrition 1996, 63, 791S–796S. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environmental Management 2020, 65, 131–159. [Google Scholar] [CrossRef]
- Shih, J.-H.; Zeng, B.-Y.; Lin, P.-Y.; Chen, T.-Y.; Chen, Y.-W.; Wu, C.-K.; Tseng, P.-T.; Wu, M.-K. Association between Peripheral Manganese Levels and Attention-Deficit/Hyperactivity Disorder: A Preliminary Meta-Analysis. NDT 2018, Volume 14, 1831–1842. [Google Scholar] [CrossRef]
- De Onis, Mercedes, World Health Organization. Dept. of Nutrition for Health and Development, World Health Organization. WHO child growth standards: growth velocity based on weight, length and head circumference: methods and development. World Health Organization Eds. Publisher: Geneva, Switzerland: World Health Organization. 2009, 1-242. ISBN: 978-92-4-154763-5. https://www.who.int/publications/i/item/9789241547635. (accessed 03 October 2024).
- Cao, X.-L.; Dufresne, G.; Clement, G.; Bélisle, S.; Robichaud, A.; Beraldin, F. Levels of Bisphenol A Diglycidyl Ether (BADGE) and Bisphenol F Diglycidyl Ether (BFDGE) in Canned Liquid Infant Formula Products in Canada and Dietary Intake Estimates. Journal of AOAC International 2009, 92, 1780–1789. [Google Scholar] [CrossRef]
- Nobile, M.; Arioli, F.; Pavlovic, R.; Ceriani, F.; Lin, S.-K.; Panseri, S.; Villa, R.; Chiesa, L.M. Presence of Emerging Contaminants in Baby Food. Food Additives & Contaminants: Part A 2020, 37, 131–142. [Google Scholar]
- European Medicines Agency. Sep 8, 2015. European Public MRL Assessment Report (EPMAR) Propyl 4-Hydroxybenzoate and Its Sodium Salt (All Food Producing Species). EMA/CVMP/632934/2014.9p. Available online: https://www.ema.europa.eu/en/documents/mrl-report/propyl-4-hydroxybenzoate-its-sodium-salt-all-food-producing-species-european-public-mrl-assessment_en.pdf. (accessed on 03 October 2024).
- European Commision European Commission. 2015. Commission Implementing Regulation (EU) No 2015/1080, O.J. L 175, of 03 July 2015 Amending Regulation (EU) No 37/2010 as Regards the Substance Propyl 4-Hydroxybenzoate and Its Sodium Salt. Official Journal of the European Union 2015, L 175, 11-13. http://data.europa.eu/eli/reg_impl/2015/1080/oj. (accessed on 03 October 2024).
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Update of the Risk Assessment of Di-butylphthalate (DBP), Butyl-benzyl-phthalate (BBP), Bis(2-ethylhexyl)Phthalate (DEHP), Di-isononylphthalate (DINP) and Di-isodecylphthalate (DIDP) for Use in Food Contact Materials. EFSA Journal 2019, 17, 5838. [CrossRef]
- Evaluation of Certain Food Additives and Contaminants: 33. Report of the Joint FAO WHO Expert Committee on Food Additives; Joint Expert Committee on Food Additives, Weltgesundheitsorganisation, Eds.; Technical report series / World Health Organization; Geneva, 1989; ISBN 978-92-4-120776-8.
- Toxicological Profile for Aluminum; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles; Agency for Toxic Substances and Disease Registry (US): Atlanta (GA), 2008. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34. (accessed on 03 October 2024).
- United States Environmental Protection Agency (U.S. EPA). Regional Screening Level (RSL) Subchronic Toxicity Supporting. 2022. https://semspub.epa.gov/work/HQ/404463.pdf. (accessed on 03 October 2024).
- European Food Safety Authority; Cadmium Dietary Exposure in the European Population. EFSA Journal 2012, 10, 2551. [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on the Risks to Public Health Related to the Presence of Chromium in Food and Drinking Water. EFSA Journal 2014, 12, 3595. [CrossRef]
- Vanek, V.W.; Borum, P.; Buchman, A.; Fessler, T.A.; Howard, L.; Jeejeebhoy, K.; Kochevar, M.; Shenkin, A.; Valentine, C.J.; Novel Nutrient Task Force, Parenteral Multi-Vitamin and Multi–Trace Element Working Group; et al. A.S.P.E.N. Position Paper: Recommendations for Changes in Commercially Available Parenteral Multivitamin and Multi–Trace Element Products. Nutrition in Clinical Practice 2012, 27, 440–491. [CrossRef]
- Astolfi, M.L.; Marotta, D.; Cammalleri, V.; Marconi, E.; Antonucci, A.; Avino, P.; Canepari, S.; Vitali, M.; Protano, C. Determination of 40 Elements in Powdered Infant Formulas and Related Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 5073. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 Supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-on Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding. Official Journal of the European Union 2015, L 25, 1-29. https://eur-lex.europa.eu/eli/reg_del/2016/127/oj. (accessed on 03 October 2024).
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2015. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA Journal 2015, 13, 4002. [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on Dietary Reference Values for zinc. EFSA Journal 2014, 12, 3844. [CrossRef]
- Yan, X.; Calafat, A.; Lashley, S.; Smulian, J.; Ananth, C.; Barr, D.; Silva, M.; Ledoux, T.; Hore, P.; Robson, M.G. Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Human and Ecological Risk Assessment: An International Journal 2009, 15, 565–578. [Google Scholar] [CrossRef]
- Mortensen, G.K.; Main, K.M.; Andersson, A.-M.; Leffers, H.; Skakkebæk, N.E. Determination of Phthalate Monoesters in Human Milk, Consumer Milk, and Infant Formula by Tandem Mass Spectrometry (LC–MS–MS). Anal. Bioanal. Chem. 2005, 382, 1084–1092. [Google Scholar] [CrossRef]
- COMMUNICATION FROM THE COMMISSION TO THE COUNCIL 252 The Combination Effects of Chemicals: Chemical mixtures. EUR-Lex-52012DC0252. 2012. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:52012DC0252. (accessed on 03 October 2024).
| Group | Chemical compound | Abbreviations | CAS number |
|---|---|---|---|
| Bisphenols | Bisphenol A | BPA | 80-05-7 |
| 4,4′-(1-Methylethylidene)bisphenol | |||
| Bisphenol S | BPS | 80-09-1 | |
| 4,4′-Sulfonylbisphenol | |||
| Bisphenol F | BPF | 620-92-8 | |
| 4,4′-Methylenebisphenol | |||
| Bisphenol F Bis(3-chloro-2-hydroxypropyl)ether | BPFDGE | 374772-79-9 | |
| 1-chloro-3-[4-[4-(3-chloro-2-hydroxypropoxy)phenyl]methyl]phenoxy]propan-2-ol | |||
| Parabens | Methylparaben | MePB | 99-76-3 |
| Methyl 4-hydroxybenzoate | |||
| Ethylparaben | EtPB | 120-47-8 | |
| Ethyl 4-hydroxybenzoate | |||
| n-Propylparaben | PrPB | 94-13-3 | |
| Propyl 4-hydroxybenzoate | |||
| iso-Propylparaben | iPrPB | 4191-73-5 | |
| Propan-2-yl 4-hydroxybenzoate | |||
| n-Butylparaben | BuPB | 94-26-8 | |
| Butyl 4-hydroxybenzoate | |||
| iso-Butylparaben | iBuPB | 4247-02-3 | |
| 2-Methylpropyl 4-hydroxybenzoate | |||
| Benzylparaben | BzPB | 94-18-8 | |
| Propyl 4-hydroxybenzoate | |||
| Polycyclic aromatic hydrocarbons (PAHs) | Anthracene | ANTHR | 120-12-7 |
| Anthracene | |||
| Pyrene | PYR | 129-00-0 | |
| Pyrene | |||
| Phenanthrene | PHEN | 85-02-8 | |
| Phenanthrene | |||
| Chrysene | CHRY | 218-02-9 | |
| Chrysene | |||
| Benz[a]antracene | BAA | 56-55-3 | |
| Tetraphene | |||
| Benzo[b]fluoranthene | BKF | 205-99-2 | |
| Benzo[e]acephenanthrylene | |||
| Benzo[k]fluoranthene | BBF | 207-08-9 | |
| Benzo[a]pyrene | BAP | 50-32-8 | |
| Benzo[pqr]tetraphene | |||
| Benzo[ghi]perylene | BGHIP | 191-24-2 | |
| Benzo[ghi]perylene | |||
| Dibenz[a,h]anthracene | DAA | 53-70-3 | |
| Benzo[k]tetraphene | |||
| Indeno [1,2,3-cd]pyrene | IND | 193-39-5 | |
| Indeno [1,2,3-cd]pyrene | |||
| Polar pesticides | Glyphosate | GLY | 1071-83-6 |
| 2-(Phosphonomethylamino)acetic acid | |||
| Glufosinate | GLUF | 51276-47-2 | |
| 2-Amino-4-[hydroxy(methyl)phosphoryl]butanoic acid | |||
| AMPA | AMPA | 1066-51-9 | |
| (Aminomethyl)phosphonic acid | |||
| Pyrethroids and chlorpyrifos | Cypermethrin | CP | 52315-07-8 |
| [cyano-(3-phenoxyphenyl)methyl] 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate | |||
| Cyfluthrin | CYFL | 68359-37-5 | |
| [cyano-(4-fluoro-3-phenoxyphenyl)methyl] 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate | |||
| Chlorpyrifos | CPS | 2921-88-2 | |
| Diethoxy-sulfanylidene-(3,5,6-trichloropyridin-2-yl)oxy-λ5-phosphane | |||
| Phthalates | Dimethyl phthalate | DMP | 131-11-3 |
| Dimethyl benzene-1,2-dicarboxylate | |||
| * Monomethyl phthalate | MMP | 4376-18-5 | |
| 2-(Methoxycarbonyl)benzoic acid | |||
| Diethyl phthalate | DEP | 84-66-2 | |
| Diethyl benzene-1,2-dicarboxylate | |||
| * Monoethyl phthalate | MEP | 2306-33-4 | |
| 2-(Ethoxycarbonyl)benzoic acid | |||
| Dibutyl phthalate | DBP | 84-74-2 | |
| Dibutyl benzene-1,2-dicarboxylate | |||
| # Mono-n-butyl phthalate | MBP | 131-70-4 | |
| 2-(Butoxycarbonyl)benzoic acid | |||
| Butylbenzyl phthalate | BBP | 85-68-7 | |
| 2-O-benzyl 1-O-butyl benzene-1,2-dicarboxylate | |||
| * Monobenzyl phthalate | MBzP | 2528-16-7 | |
| 2-[(Benzyloxy)carbonyl]benzoic acid | |||
| Di(2-ethylexyl) phthalate | DEHP | 117-81-7 | |
| Di(2-ethylhexyl)benzene-1,2-dicarboxylate | |||
| * Mono(2-ethylexyl) phthalate | MEHP | 4376-20-9 | |
| 2-(2-Ethylhexoxycarbonyl)benzoic acid | |||
| * Mono(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | 40321-99-1 | |
| 2-(2-Ethyl-5-hydroxyhexoxycarbonyl)benzoic acid | |||
| * Mono(2-ethyl-5-oxohexyl) phthalate | MEOHP | 40321-98-0 | |
| 2-(2-Ethyl-5-oxohexoxycarbonyl)benzoic acid | |||
| Di-n-octyl phthalate | DNOP | 117-84-0 | |
| Dioctyl benzene-1,2-dicarboxylate | |||
| * Monooctyl phthalate | MnOP | 5393-19-1 | |
| 2-(Octoxycarbonyl)benzoic acid | |||
| Perfluoroalkyl substances (PFAS) | 2,2,3,3,4,4,4-heptafluorobutanoic acid Perfluoro-n-butanoic acid |
PFBA | 375-22-4 |
| 2,2,3,3,4,4,5,5,5-nonafluoropentanoic acid Perfluoro-n-pentanoic acid |
PFPeA | 2706-90-3 | |
| 2,2,3,3,4,4,5,5,6,6,6-undecafluorohexanoic acid Perfluoro-n-hexanoic acid |
PFHxA | 307-24-4 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptanoic acid Perfluoro-n-heptanoic acid |
PFPHpA | 375-85-9 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctanoic acid Perfluoro-n-octanoic acid |
PFOA | 335-67-1 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,9-heptadecafluorononanoic acid Perfluoro-n-nonanoic acid |
PFNA | 375-95-1 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-nonadecafluorodecanoic acid Perfluoro-n-decanoic acid |
PFDA | 335-76-2 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-henicosafluoroundecanoic acid Perfluoro-n-undecanoic acid | PFUnDA | 2058-94-8 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-tricosafluorododecanoic acid Perfluoro-n-dodecanoic acid | PFDoDA | 307-55-1 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,13-pentacosafluorotridecanoic acid Perfluoro-n-tridecanoic acid |
PFTrDA | 72629-94-8 | |
| 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-heptacosafluorotetradecanoic acid Perfluoro-n-tetradecanoic acid |
PFTreDA | 376-06-7 | |
| 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonic acid Perfluoro-1 butanesulphonamide |
PFBS | 375-73-5 | |
| 1,1,2,2,3,3,4,4,5,5,5-undecafluoropentane-1-sulfonic acid Perfluoropentanesulphonic acid |
PFPeS | 2706-91-4 | |
| 1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonic acid Perfluorohexanesulphonic acid |
PFHxS | 355-46-4 | |
| 1,1,2,2,3,3,4,4,5,5,6,6,7,7,7-pentadecafluoroheptane-1-sulfonic acid Perfluoroheptanesulphonic acid |
PFHpS | 375-92-8 | |
| 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctane-1-sulfonic acid Perfluorooctanesulphonic acid |
PFOS | 1763-23-1 | |
| 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,9-nonadecafluorononane-1-sulfonic acid Perfluorononanesulfonic acid |
PFNS | 68259- | |
| 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-pentacosafluorododecane-1-sulfonic acid Perfluorododecanenesulfonic acid |
PFDS | 79780-39-5 | |
| 2,3,3,3-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)propanoic acid 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid |
GenX (HFPO-DA) | 13252-13-6 | |
| 2,2,3-trifluoro-3-[1,1,2,2,3,3-hexafluoro-3-(trifluoromethoxy)propoxy]propanoic acid 4,8-Dioxa-3H-perfluorononanoic acid |
ADONA | 919005-14-4 | |
| 2-(6-chloro-1,1,2,2,3,3,4,4,5,5,6,6-dodecafluorohexoxy)-1,1,2,2-tetrafluoroethanesulfonic acid Perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid) |
9Cl-PF3ONS | 756426-58-1 | |
| 2-(8-chloro-1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8-hexadecafluorooctoxy)-1,1,2,2-tetrafluoroethanesulfonic acid 11-chloroeicosafluoro-3-oxaundecane-1-sulfonic acid |
11Cl-PF3OUdS | 763051-92-9 | |
| 1H,1H,2H,2H-Perfluorohexanesulphonic acid 3,3,4,4,5,5,6,6,6-nonafluorohexane-1-sulfonic acid |
4 :2 FTS | 757124-72-4 | |
| 1H,1H,2H,2H-perfluorooctanesulfonic acid 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctane-1-sulfonic acid |
6:2 FTS | 27619-97-2 | |
| 31H,1H,2H,2H-Perfluorodecanesulfonic acid 3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecane-1-sulfonic acid |
8 :2 FTS | 39108-34-4 | |
| 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonamide Perfluorobutane Sulfonamide |
FBSA | 30334-69-1 | |
| Perfluorooctanesulfonamide 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctane-1-sulfonamide |
FOSA | 754-91-6 | |
| Metals | Aluminum | Al | 7429-90-5 |
| Arsenic | As | 7440-38-2 | |
| Barium | Ba | 7440-39-3 | |
| Bismuth | Bi | 7440-69-9 | |
| Cadmium | Cd | 7440-43-9 | |
| Cobalt | Co | 7440-48-4 | |
| Chromium | Cr | 7440-47-3 | |
| Copper | Cu | 7440-50-8 | |
| Manganese | Mn | 7439-96-5 | |
| Molybdenum | Mo | 7439-98-7 | |
| Nickel | Ni | 7440-02-0 | |
| Lead | Pb | 7439-92-1 | |
| Titanium | Ti | 7440-32-6 | |
| Thallium | Tl | 7440-28-0 | |
| Vanadium | V | 7440-62-2 | |
| Zinc | Zn | 7440-66-6 |
| EDC group | Compound | Frequency (%) | Mean±sd | Median | Min-Max |
|---|---|---|---|---|---|
| Bisphenol | BPA | 3/20 (15) | 1.76±1.28 | 2.25 | 0.31-2.73 |
| BPFDGE | 4/20 (20) | 5.43±1.97 | 6.35 | 2.48-6.54 | |
| Parabens | MePB | 3/20 (15) | 2.9±2.95 | 1.36 | 1.04-6.29 |
| EtPB | 1/20 (5) | 0.32 | 0.32 | ||
| PrPB | 1/20 (5) | 0.12 | 0.12 | ||
|
Polycyclic aromatic hydrocarbons (PAHs) |
IND | 1/20 (5) | 0.32 | 0.32 | 0.32–0.32 |
| Phthalates | DMP | 9/20 (45) | 0.37±0.19 | 0.34 | 0.11-0.75 |
| MMP | 6/20 (30) | 0.07±0.11 | 0.03 | 0.02-0.29 | |
| DEP | 17/20 (85) | 1.51±1.56 | 1.27 | 0.28-7.21 | |
| MEP | 2/20 (10) | 0.03±0.03 | 0.03 | 0.01-0.05 | |
| MBP | 14/20 (70) | 0.48±0.93 | 0.22 | 0.04-3.65 | |
| BBzP | 20/20 (100) | 1.27±0.91 | 0.94 | 0.68-3.45 | |
| DEHP | 8/20 (40) | 1.09±0.76 | 0.89 | 0.38-2.79 | |
| MEHP | 14/20 (70) | 0.40±0.61 | 0.21 | 0.09-2.40 | |
| DNOP | 10/20 (50) | 0.68±0.40 | 0.60 | 0.23-1.52 | |
| MnOP | 14/20 (70) | 0.24±0.35 | 0.12 | 0.05-1.46 | |
| Perfluoroalkyl substances (PFAS) | PFOA | 4/20 (20) | 0.02±0.01 | 0.03 | 0.01-0.03 |
| PFBA | 1/20 (5) | 0.09 | 0.09 | ||
| PFPHpA | 1/20 (5) | 0.02 | 0.02 | ||
| PFDA | 5/20 (25) | 0.01±0.001 | 0.01 | 0.009-0.011 | |
| PFBS | 18/20 (90) | 0.096±0.03 | 0.09 | 0.04-0.13 | |
| Metals | Al | 20/20(100) | 546±254 | 528 | 192-1005 |
| As | 4/20(20) | 11±3.7 | 11 | 7.3-16.3 | |
| Ba | 20/20(100) | 178±101.8 | 156.5 | 60.6-458.2 | |
| Cd | 19/20(95) | 3±2.1 | 2 | 0.4-7.7 | |
| Co | 20/20(100) | 13±5.9 | 10.8 | 6.5-24.7 | |
| Cr | 20/20(100) | 81±47.3 | 71.1 | 28-252 | |
| Cu | 20/20(100) | 4168±1172 | 3953 | 3085-8649 | |
| Mn | 20/20(100) | 768±538 | 571 | 173-1820 | |
| Mo | 20/20(100) | 116±72.1 | 100.8 | 21.3-269.3 | |
| Ni | 20/20(100) | 32±15.1 | 30.6 | 13.1-65.9 | |
| Ti | 20/20(100) | 35±31.8 | 25.8 | 5.8-104.0 | |
| V | 4/20(20) | 13±13.3 | 6.9 | 6-33.3 | |
| Zn | 20/20(100) | 34740±6942.4 | 32042.7 | 25883.2-52680.8 |
| EDCs | Chemical | Frequency (%) | Mean±sd | Median | Min–Max | EDI for infant’s weight (kg) at the 50th percentile (average for age) | TDI values reported in the literature |
|---|---|---|---|---|---|---|---|
| Bisphenols | BPA | 1/11 (9.1) | 2.73a | 2.73 | 2.73–2.73 | 59.74 | 0.2 (ng/Kg/b.w./day) [19] |
| 0.3±0.86b | 0.02 | 0.49-59.74 | 6.46 | ||||
| 0.25±0.82c | 0 | 0-59.74 | 5.43 | ||||
| BPFDGE | 1/11 (9.1) | 6.39 | 6.39 | 6.39–6.39 | 139.84 | Maximum PDI of BPFDGE < 3.4 µg/kg body weight/day [61] | |
| Parabens | MePB | 1/11 (9.1) | 1.04 | 1.04 | 1.04–1.04 | 22.7 | Σ of parabens in food for EFSA 0-10 mg/kg b.w. [21] |
| PrPB | 1/11 (9.1) | 0.12 | 0.12 | 0.12–0.12 | 2.62 | ADI for PrPB at 1.25 mg /Kg/b.w./day) [62,63,64]. | |
| Phthalates | DMP | 7/11 (64) | 0.41±0.43 | 0.36 | 0.11-0.75 | 8.92 | 0.9–7.2 and 1.6–11.7 μg/kg b.w. for DBP, BBP, 50 μg/kg b.w. per day for DEHP [65] |
| MMP | 5/11 (45) | 0.08±0.09 | 0.03 | 0.02-0.29 | 1.73 | ||
| DEP | 9/11 (82) | 1.91±2.02 | 1.27 | 0.8-7.21 | 42.23 | ||
| MEP | 2/11 (18) | 0.03±0.02 | 0.03 | 0.01-0.05 | 1.11 | ||
| MBP | 9/11 (82) | 0.57±1.15 | 0.22 | 0.04-3.65 | 12.64 | ||
| BBzP | 11/11 (100) | 1.09±0.46 | 0.91 | 0.68-2.32 | 24.14 | ||
| DEHP | 5/11 (45) | 1.15±0.89 | 0.71 | 0.38-2.79 | 25.54 | ||
| MEHP | 9/11 (82) | 0.50±0.74 | 0.9 | 0.09-2.44 | 11.16 | ||
| DNOP | 6/11 (62) | 0.67±0.4 | 0.6 | 0.34-1.12 | 14.81 | ||
| MnOP | 9/11 (82) | 0.3±0.45 | 0.12 | 0.05-1.46 | 6.65 | ||
| PFAS | PFOA | 2/11(18) | 0.07 | 0.03-0.11 | 0.3 | ||
| PFPHpA | 1/11 (9.1) | 0.102 | 0.102 | 0.102 | 0.02 | TWI: Σ of PFAS for EFSA 4.4 ng/Kg body weight/week [38] | |
| PFDA | 2/11(18) | 0.05 | 0.04-0.06 | 0.01 | |||
| PFBS | 11/11 (100) | 0.46 | 0.17-0.74 | 0.07 | |||
| Metals | Al | 11/11(100) | 493±252 | 390 | 193-851 | 10778 | PTWI of 7 mg/kg for FAO Expert Committee [66] Sub-chronic oral Reference Dose of 1mg/kg per day for ATSDR [67,68] |
| As | 2/11(18) | 10±3 | 10 | 7-12 | 209 | PTWI of 0,015 mg/kg of body weight Sub-chronic oral Reference Dose of 0.0003 mg/kg per day for [68] |
|
| Ba | 11/11(100) | 151±99 | 133 | 61-405 | 3315 | ||
| Cd | 10/11(91) | 2±2 | 1 | 1-8 | 52 | PTWI of 2.5 μg kg−1 b.w. by [69] Sub-chronic oral Reference Dose of 0.001 mg/kg per day by [68] |
|
| Co | 11/11(100) | 15±7 | 14 | 7-25 | 320 | ||
| Cr | 11/11(100) | 64±26 | 59 | 28-131 | 1399 | 300 mg/kg b.w. PTDI [70] |
|
| Cu | 11/11(100) | 4510±1490 | 4006 | 3319-8649 | 98679 | 0.20 mg/day for infant 0-6 months old [71] |
|
| Mn | 11/11(100) | 741±561 | 597 | 185-1774 | 16212 | 0.0504 and 5.04 μg g−1 in formula as minimum and maximum content [72] upon [73] | |
| Mo | 11/11(100) | 95±68 | 86 | 21-244 | 2072 | ||
| Ni | 11/11(100) | 32±15 | 31 | 13-65 | 707 | 2.8 mg/kg b.w./ day PTDI [74] | |
| Ti | 11/11(100) | 31±32 | 22 | 6-104 | 686 | ||
| V | 2/11(18) | 7±1 | 7 | 6-7 | 152 | ||
| Zn | 11/11(100) | 35252±8504 | 31194 | 25883-52681 | 771407 | a Tolerable Upper Limit of 7 mg/day for SCF [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





