Submitted:
08 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
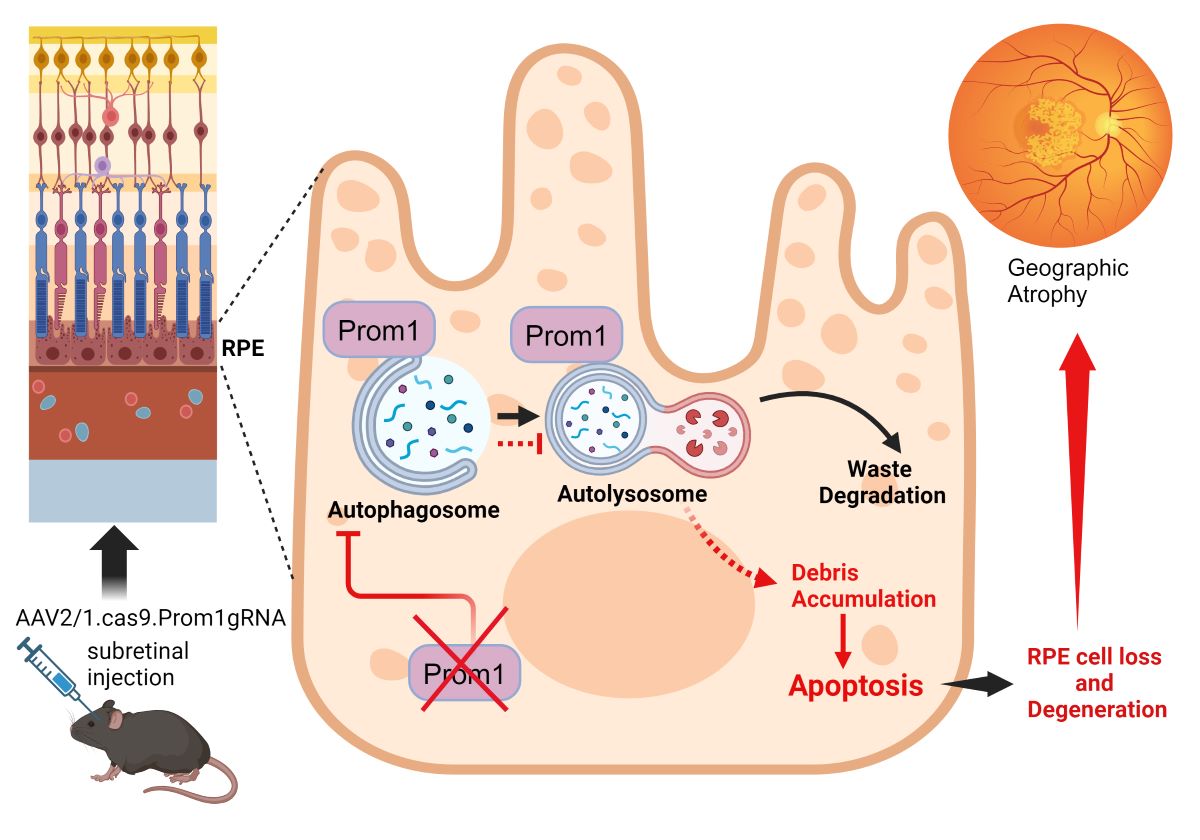
Introduction
Materials and Methods
Materials
Mice and Colony Management
Subretinal Injections
Electroretinogram (ERG) in Mice
Chromogenic and Fluorescent Prom1 RNAscope Assays in Mouse Retina Sections
H&E Staining and Histology
Immunohistochemistry
Transmission Electron Microscopy (TEM)
Analysis of Single-Cell RNA-Sequencing RPE and Retina Datasets
Statistical Analysis
Results
Expression of Prom1 in Mouse RPE in Situ
AAV2/1-Mediated Prom1-Knockdown (KD) in Vivo Using CRISPR/Cas9-gRNA
RPE-Specific Prom1-KD in a Mouse Model Causes RPE Cell Death
Prom1 Gene Expression in Human and Mouse Single-Cell RNA-Seq Datasets
Discussion
Limitations
Data Availability Statement
Acknowledgments
Conflict of Interest
References
- Miraglia, S.; Godfrey, W.; Yin, A.H.; Atkins, K.; Warnke, R.; Holden, J.T.; Bray, R.A.; Waller, E.K.; Buck, D.W. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 1997, 90, 5013-5021.
- Weigmann, A.; Corbeil, D.; Hellwig, A.; Huttner, W.B. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A 1997, 94, 12425-12430. [CrossRef]
- Chen, H.; Luo, Z.; Dong, L.; Tan, Y.; Yang, J.; Feng, G.; Wu, M.; Li, Z.; Wang, H. CD133/prominin-1-mediated autophagy and glucose uptake beneficial for hepatoma cell survival. PLoS One 2013, 8, e56878. [CrossRef]
- Florek, M.; Haase, M.; Marzesco, A.M.; Freund, D.; Ehninger, G.; Huttner, W.B.; Corbeil, D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res 2005, 319, 15-26. [CrossRef]
- Pleskac, P.; Fargeas, C.A.; Veselska, R.; Corbeil, D.; Skoda, J. Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease. Cell Mol Biol Lett 2024, 29, 41. [CrossRef]
- Ragi, S.D.; Lima de Carvalho, J.R., Jr.; Tanaka, A.J.; Park, K.S.; Mahajan, V.B.; Maumenee, I.H.; Tsang, S.H. Compound heterozygous novel frameshift variants in the PROM1 gene result in Leber congenital amaurosis. Cold Spring Harb Mol Case Stud 2019, 5. [CrossRef]
- Maw, M.A.; Corbeil, D.; Koch, J.; Hellwig, A.; Wilson-Wheeler, J.C.; Bridges, R.J.; Kumaramanickavel, G.; John, S.; Nancarrow, D.; Roper, K.; et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet 2000, 9, 27-34. [CrossRef]
- Yang, Z.; Chen, Y.; Lillo, C.; Chien, J.; Yu, Z.; Michaelides, M.; Klein, M.; Howes, K.A.; Li, Y.; Kaminoh, Y.; et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest 2008, 118, 2908-2916. [CrossRef]
- Zhang, Q.; Zulfiqar, F.; Xiao, X.; Riazuddin, S.A.; Ahmad, Z.; Caruso, R.; MacDonald, I.; Sieving, P.; Riazuddin, S.; Hejtmancik, J.F. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum Genet 2007, 122, 293-299. [CrossRef]
- Pras, E.; Abu, A.; Rotenstreich, Y.; Avni, I.; Reish, O.; Morad, Y.; Reznik-Wolf, H.; Pras, E. Cone-rod dystrophy and a frameshift mutation in the PROM1 gene. Mol Vis 2009, 15, 1709-1716.
- Eidinger, O.; Leibu, R.; Newman, H.; Rizel, L.; Perlman, I.; Ben-Yosef, T. An intronic deletion in the PROM1 gene leads to autosomal recessive cone-rod dystrophy. Mol Vis 2015, 21, 1295-1306.
- Khan, A.O.; Bolz, H.J. Pediatric Cone-Rod Dystrophy with High Myopia and Nystagmus Suggests Recessive PROM1 Mutations. Ophthalmic Genet 2015, 36, 349-352. [CrossRef]
- Imani, S.; Cheng, J.; Shasaltaneh, M.D.; Wei, C.; Yang, L.; Fu, S.; Zou, H.; Khan, M.A.; Zhang, X.; Chen, H.; et al. Genetic identification and molecular modeling characterization reveal a novel PROM1 mutation in Stargardt4-like macular dystrophy. Oncotarget 2018, 9, 122-141. [CrossRef]
- Strauss, R.W.; Munoz, B.; Ahmed, M.I.; Bittencourt, M.; Schonbach, E.M.; Michaelides, M.; Birch, D.; Zrenner, E.; Ervin, A.M.; Charbel Issa, P.; et al. The Progression of the Stargardt Disease Type 4 (ProgStar-4) Study: Design and Baseline Characteristics (ProgStar-4 Report No. 1). Ophthalmic Res 2018, 60, 185-194. [CrossRef]
- Kniazeva, M.; Chiang, M.F.; Morgan, B.; Anduze, A.L.; Zack, D.J.; Han, M.; Zhang, K. A new locus for autosomal dominant stargardt-like disease maps to chromosome 4. Am J Hum Genet 1999, 64, 1394-1399. [CrossRef]
- Abalem, M.F.; Omari, A.A.; Schlegel, D.; Khan, N.W.; Jayasundera, T. Macular hyperpigmentary changes in ABCA4-Stargardt disease. Int J Retina Vitreous 2019, 5, 9. [CrossRef]
- Bhattacharya, S.; Yin, J.; Winborn, C.S.; Zhang, Q.; Yue, J.; Chaum, E. Prominin-1 Is a Novel Regulator of Autophagy in the Human Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci 2017, 58, 2366-2387. [CrossRef]
- Bhattacharya, S.; Yin, J.; Huo, W.; Chaum, E. Loss of Prom1 impairs autophagy and promotes epithelial-mesenchymal transition in mouse retinal pigment epithelial cells. J Cell Physiol 2023. [CrossRef]
- Cehajic-Kapetanovic, J.; Birtel, J.; McClements, M.E.; Shanks, M.E.; Clouston, P.; Downes, S.M.; Charbel Issa, P.; MacLaren, R.E. Clinical and Molecular Characterization of PROM1-Related Retinal Degeneration. JAMA Netw Open 2019, 2, e195752. [CrossRef]
- Lee, W.; Paavo, M.; Zernant, J.; Stong, N.; Laurente, Z.; Bearelly, S.; Nagasaki, T.; Tsang, S.H.; Goldstein, D.B.; Allikmets, R. Modification of the PROM1 disease phenotype by a mutation in ABCA4. Ophthalmic Genet 2019, 40, 369-375. [CrossRef]
- Permanyer, J.; Navarro, R.; Friedman, J.; Pomares, E.; Castro-Navarro, J.; Marfany, G.; Swaroop, A.; Gonzalez-Duarte, R. Autosomal recessive retinitis pigmentosa with early macular affectation caused by premature truncation in PROM1. Invest Ophthalmol Vis Sci 2010, 51, 2656-2663. [CrossRef]
- Hwang, S.; Kang, S.W.; Jang, J.H.; Kim, S.J. Genetic and clinical characteristics of PROM1-related retinal degeneration in Korean. Sci Rep 2023, 13, 21877. [CrossRef]
- Paavo, M.; Lee, W.; Parmann, R.; Lima de Carvalho, J.R., Jr.; Zernant, J.; Tsang, S.H.; Allikmets, R.; Sparrow, J.R. Insights Into PROM1-Macular Disease Using Multimodal Imaging. Invest Ophthalmol Vis Sci 2023, 64, 27. [CrossRef]
- Ricca, A.M.; Han, I.C.; Hoffmann, J.; Stone, E.M.; Sohn, E.H. Macular Atrophy and Phenotypic Variability in Autosomal Dominant Stargardt-Like Macular Dystrophy Due to Prom1 Mutation. Retina 2023, 43, 1165-1173. [CrossRef]
- Muhlfriedel, R.; Michalakis, S.; Garcia Garrido, M.; Biel, M.; Seeliger, M.W. Optimized technique for subretinal injections in mice. Methods Mol Biol 2013, 935, 343-349. [CrossRef]
- Naguib, S.; Backstrom, J.R.; Gil, M.; Calkins, D.J.; Rex, T.S. Retinal oxidative stress activates the NRF2/ARE pathway: An early endogenous protective response to ocular hypertension. Redox Biol 2021, 42, 101883. [CrossRef]
- Petralia, R.S.; Wang, Y.X. Review of Post-embedding Immunogold Methods for the Study of Neuronal Structures. Front Neuroanat 2021, 15, 763427. [CrossRef]
- Voigt, A.P.; Whitmore, S.S.; Lessing, N.D.; DeLuca, A.P.; Tucker, B.A.; Stone, E.M.; Mullins, R.F.; Scheetz, T.E. Spectacle: An interactive resource for ocular single-cell RNA sequencing data analysis. Exp Eye Res 2020, 200, 108204. [CrossRef]
- Hori, A.; Nishide, K.; Yasukuni, Y.; Haga, K.; Kakuta, W.; Ishikawa, Y.; Hayes, M.J.; Ohnuma, S.I.; Kiyonari, H.; Kimura, K.; et al. Prominin-1 Modulates Rho/ROCK-Mediated Membrane Morphology and Calcium-Dependent Intracellular Chloride Flux. Sci Rep 2019, 9, 15911. [CrossRef]
- Chan, S.; Filezac de L’Etang, A.; Rangell, L.; Caplazi, P.; Lowe, J.B.; Romeo, V. A method for manual and automated multiplex RNAscope in situ hybridization and immunocytochemistry on cytospin samples. PLoS One 2018, 13, e0207619. [CrossRef]
- Nilsson, O.R.; Kari, L.; Rosenke, R.; Steele-Mortimer, O. Protocol for RNA fluorescence in situ hybridization in mouse meningeal whole mounts. STAR Protoc 2022, 3, 101256. [CrossRef]
- Bennett, J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther 2003, 10, 977-982. [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849-860. [CrossRef]
- Seo, S.J.; Krebs, M.P.; Mao, H.; Jones, K.; Conners, M.; Lewin, A.S. Pathological consequences of long-term mitochondrial oxidative stress in the mouse retinal pigment epithelium. Exp Eye Res 2012, 101, 60-71. [CrossRef]
- Auricchio, A.; Kobinger, G.; Anand, V.; Hildinger, M.; O’Connor, E.; Maguire, A.M.; Wilson, J.M.; Bennett, J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 2001, 10, 3075-3081. [CrossRef]
- Kaneko, H.; Dridi, S.; Tarallo, V.; Gelfand, B.D.; Fowler, B.J.; Cho, W.G.; Kleinman, M.E.; Ponicsan, S.L.; Hauswirth, W.W.; Chiodo, V.A.; et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011, 471, 325-330. [CrossRef]
- Gao, J.; Cui, J.Z.; To, E.; Cao, S.; Matsubara, J.A. Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J Neuroinflammation 2018, 15, 15. [CrossRef]
- Mullin, N.K.; Voigt, A.P.; Boese, E.A.; Liu, X.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Transcriptomic and Chromatin Accessibility Analysis of the Human Macular and Peripheral Retinal Pigment Epithelium at the Single-Cell Level. Am J Pathol 2023, 193, 1750-1761. [CrossRef]
- Senabouth, A.; Daniszewski, M.; Lidgerwood, G.E.; Liang, H.H.; Hernandez, D.; Mirzaei, M.; Keenan, S.N.; Zhang, R.; Han, X.; Neavin, D.; et al. Transcriptomic and proteomic retinal pigment epithelium signatures of age-related macular degeneration. Nat Commun 2022, 13, 4233. [CrossRef]
- Galloway, C.A.; Dalvi, S.; Hung, S.S.C.; MacDonald, L.A.; Latchney, L.R.; Wong, R.C.B.; Guymer, R.H.; Mackey, D.A.; Williams, D.S.; Chung, M.M.; et al. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. Proc Natl Acad Sci U S A 2017, 114, E8214-E8223. [CrossRef]
- Hallam, D.; Collin, J.; Bojic, S.; Chichagova, V.; Buskin, A.; Xu, Y.; Lafage, L.; Otten, E.G.; Anyfantis, G.; Mellough, C.; et al. An Induced Pluripotent Stem Cell Patient Specific Model of Complement Factor H (Y402H) Polymorphism Displays Characteristic Features of Age-Related Macular Degeneration and Indicates a Beneficial Role for UV Light Exposure. Stem Cells 2017, 35, 2305-2320. [CrossRef]
- Schmitz-Valckenberg, S.; Fleckenstein, M.; Zouache, M.A.; Pfau, M.; Pappas, C.; Hageman, J.L.; Agron, E.; Malley, C.; Keenan, T.D.L.; Chew, E.Y.; et al. Progression of Age-Related Macular Degeneration Among Individuals Homozygous for Risk Alleles on Chromosome 1 (CFH-CFHR5) or Chromosome 10 (ARMS2/HTRA1) or Both. JAMA Ophthalmol 2022, 140, 252-260. [CrossRef]
- Karg, M.M.; Moorefield, M.; Hoffmann, E.; Philipose, H.; Krasniqi, D.; Hoppe, C.; Shu, D.Y.; Shirahama, S.; Ksander, B.R.; Saint-Geniez, M. Microglia preserve visual function loss in the aging retina by supporting retinal pigment epithelial health. Immun Ageing 2023, 20, 53. [CrossRef]
- Yu, C.; Lad, E.M.; Mathew, R.; Shiraki, N.; Littleton, S.; Chen, Y.; Hou, J.; Schlepckow, K.; Degan, S.; Chew, L.; et al. Microglia at sites of atrophy restrict the progression of retinal degeneration via galectin-3 and Trem2. J Exp Med 2024, 221. [CrossRef]
- Ronning, K.E.; Karlen, S.J.; Miller, E.B.; Burns, M.E. Molecular profiling of resident and infiltrating mononuclear phagocytes during rapid adult retinal degeneration using single-cell RNA sequencing. Sci Rep 2019, 9, 4858. [CrossRef]
- O’Koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.W.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723-737 e727. [CrossRef]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The major cell populations of the mouse retina. J Neurosci 1998, 18, 8936-8946. [CrossRef]
- Corbeil, D.; Karbanova, J.; Fargeas, C.A.; Jaszai, J. Prominin-1 (CD133): Molecular and Cellular Features Across Species. Adv Exp Med Biol 2013, 777, 3-24. [CrossRef]
- Mak, A.B.; Nixon, A.M.; Kittanakom, S.; Stewart, J.M.; Chen, G.I.; Curak, J.; Gingras, A.C.; Mazitschek, R.; Neel, B.G.; Stagljar, I.; et al. Regulation of CD133 by HDAC6 promotes beta-catenin signaling to suppress cancer cell differentiation. Cell Rep 2012, 2, 951-963. [CrossRef]
- Dellett, M.; Sasai, N.; Nishide, K.; Becker, S.; Papadaki, V.; Limb, G.A.; Moore, A.T.; Kondo, T.; Ohnuma, S. Genetic background and light-dependent progression of photoreceptor cell degeneration in Prominin-1 knockout mice. Invest Ophthalmol Vis Sci 2014, 56, 164-176. [CrossRef]
- Puertas-Neyra, K.; Coco-Martin, R.M.; Hernandez-Rodriguez, L.A.; Gobelli, D.; Garcia-Ferrer, Y.; Palma-Vecino, R.; Telleria, J.J.; Simarro, M.; de la Fuente, M.A.; Fernandez-Bueno, I. Clinical exome analysis and targeted gene repair of the c.1354dupT variant in iPSC lines from patients with PROM1-related retinopathies exhibiting diverse phenotypes. Stem Cell Res Ther 2024, 15, 192. [CrossRef]
- Fujinami, K.; Oishi, A.; Yang, L.; Arno, G.; Pontikos, N.; Yoshitake, K.; Fujinami-Yokokawa, Y.; Liu, X.; Hayashi, T.; Katagiri, S.; et al. Clinical and genetic characteristics of 10 Japanese patients with PROM1-associated retinal disorder: A report of the phenotype spectrum and a literature review in the Japanese population. Am J Med Genet C Semin Med Genet 2020, 184, 656-674. [CrossRef]
- Liang, J.; She, X.; Chen, J.; Zhai, Y.; Liu, Y.; Zheng, K.; Gong, Y.; Zhu, H.; Luo, X.; Sun, X. Identification of novel PROM1 mutations responsible for autosomal recessive maculopathy with rod-cone dystrophy. Graefes Arch Clin Exp Ophthalmol 2019, 257, 619-628. [CrossRef]
- Wang, Y.; Wang, P.; Li, S.; Ouyang, J.; Jia, X.; Xiao, X.; Yang, J.; Li, X.; Sun, W.; Zhang, Q. Characterization of PROM1 p.Arg373Cys Variant in a Cohort of Chinese Patients: Macular Dystrophy Plus Peripheral Bone-Spicule Degeneration. Invest Ophthalmol Vis Sci 2021, 62, 19. [CrossRef]
- Clark, B.S.; Stein-O’Brien, G.L.; Shiau, F.; Cannon, G.H.; Davis-Marcisak, E.; Sherman, T.; Santiago, C.P.; Hoang, T.V.; Rajaii, F.; James-Esposito, R.E.; et al. Single-Cell RNA-Seq Analysis of Retinal Development Identifies NFI Factors as Regulating Mitotic Exit and Late-Born Cell Specification. Neuron 2019, 102, 1111-1126 e1115. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




