1. Introduction
Microsatellite instability (MSI) is a genetic phenomenon characterized by alterations in the length of repetitive DNA sequences known as microsatellites. These microsatellites consist of short DNA sequences in which a nucleotide unit (such as adenine [A], thymine [T], cytosine [C], or guanine [G]) is repeated multiple times in tandem. MSI can arise from errors during DNA replication, deficiencies in error repair mechanisms, and various genetic mutations. In the context of colon cancer, the relevance of MSI is particularly pronounced. While most colon cancers are sporadic and not linked to an inherited genetic predisposition, approximately 15-20% of colon cancer cases exhibit MSI, often associated with an inherited genetic predisposition or specific gene mutations [
1,
2,
3].
The presence of MSI in colon cancer is due to the inactivation of DNA repair systems. These error repair systems are essential for correcting mutations that occur during normal cellular replication. When these systems fail, errors in the lengths of the microsatellite repeats accumulate, leading to the activation of oncogenes or the inactivation of tumor suppressor genes, which can contribute to cancer development [
4].
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is an inherited condition linked to microsatellite instability in colon cancer. Individuals with Lynch syndrome are at a heightened risk of developing colon cancer and other malignancies due to mutations in specific DNA repair genes.
The detection of MSI in colon cancer tumors holds significant clinical implications. Tumors exhibiting MSI may respond differently to specific treatments and often demonstrate a better prognosis than those without this feature [
5].
Given this context, the determination of MMR protein status by immunohistochemistry is ideally performed routinely in all cases of colon and rectal cancer, utilizing a panel of four antibodies [
1]. However, the application of such resources may be constrained globally due to logistical, economic, and access-related challenges, especially considering that just over 80% of cases are expected to yield normal test results. Consequently, an abbreviated approach for establishing the status of MMR proteins is desirable, optimizing costs, turnaround times, and workload. This has been successfully demonstrated in stomach cancer and corroborated by a recent systematic review focused on colon cancer [
6,
7].
Since MLH1 and MSH2 proteins represent the primary components of the DNA repair system responsible for detecting and correcting errors, while PMS2 and MSH6 are additional components expressed only when MLH1 and MSH2 are present, respectively [
8], we hypothesize that a diagnostic panel utilizing only these two antibodies will possess a diagnostic performance comparable to that of a four-antibody panel. A reduced panel with similar diagnostic accuracy to a larger panel can minimize costs, workload, and turnaround times, making it particularly advantageous for laboratories or health systems with limited resources or for countries with transitioning or developing economies.
2. Materials and Methods
Samples. The pathology files of our Institution were searched for cases of colon cancer treated consecutively in the year 2017. The patients had a histopathological diagnosis of adenocarcinoma and determination of the MMR by immunohistochemistry with the four proteins and PCR.
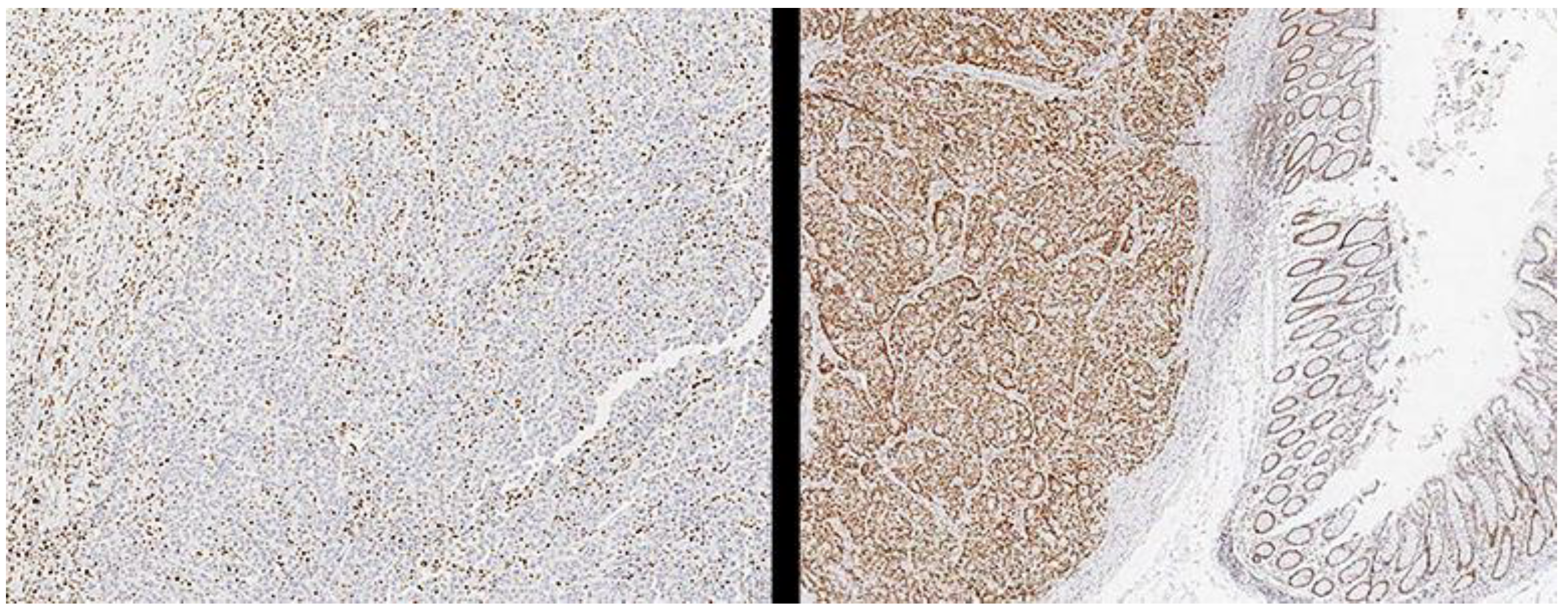
Methods. Monoclonal antibodies were used against MLH1 (clone G168-728, Cell Marque, Rocklin, California, USA), PMS2 (clone MRQ-28, Cell Marque, Rocklin, California, USA), MSH2 (clone G219- 1129, Cell Marque, Rocklin, California, USA) and MSH6 (Clone SP93, Cell Marque, Rocklin, California, USA) were performed automatically on Benchmark Ultra II equipment (Roche Diagnostics, Rotkreuz, Switzerland) following the instructions of the supplier and the results were classified. Cases such as pMMR or dMMR are by the guidelines of the College of American Pathologists [
9] (
Figure 1). Once the status of the MMR system was determined, the cases were reclassified as dMMR or pMMR using only the evaluation of PMS2 and MSH6, considering one case as dMMR when it showed negative immunoreaction against one of the two markers, and classifying it as pMMR. When both were absent. The evaluation was performed by a pathologist experienced in gastrointestinal pathology or pathology oncology (> ten years of experience, > 50 publications in the last five years). The pathologists reviewed the slides again to minimize the performance bias (interpretation bias). With this information, a comparison of the diagnostic accuracy of both tests was made using the PCR test as the reference standard, which was performed using the QIAamp C.A.A mini kit (Qiagen, Valencia, CA, USA) and MMR was determined in the tumor DNA. using EasyPGX
® readyMSI, including mononucleotide repeats: BAT25, BAT26, NR21, NR22, NR24, NR27, CAT25 and MONO27.
For all cases, the relevant clinical and pathological data were obtained from the clinical history and the pathology file, including the following data: sex, age, tumor site, tumor size, histological type, histological grade, vascular invasion, perineural invasion, depth of invasion, lymph node status, and clinical stage.
Statistics. Descriptive statistics were performed with a summary of categorical data with count and percentage, and for the numerical variables by median and interquartile range in the case of non-parametric distribution and by mean and standard deviation otherwise. Said determination was made with the Kolmogorov-Smirnov test. To calculate diagnostic accuracy and compare both trials, sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy were calculated, and the areas under the curve were compared with a chi-square test with a receiver operating characteristic curve (ROC) as described previously [
10]. SPSS version 29.0 software (IBM, Armonk, NY, USA) was used for all analyses, and statistical significance was considered for p-values less than 0.05.3.
Results
We identified 202 cases, of which the average age was 57.9 + 15.2 years (range 20 to 90 years), 119 (58.9%) were women, and 83 were men. A family history of cancer was reported in 48 (23.8%) cases and a personal history in thirteen (6.4%) cases. There was a history of colonic polyps in 23.8% (48 cases).
One hundred and seven (53%) cases were presented in the sigmoid, followed by the descending colon in 53 (26.2) cases, the right colon in 34 (16.8%), and 8 (4%) cases in the transverse colon. The predominant clinical stage was stage II in 60.9%, followed by stage III (19.3%), stage IV (12.4%) and stage I (7.4%). In 75.2% of the cases (n=152), the histological type was an adenocarcinoma without other specification (NOS); 12.4% were mucinous, and the remaining 12.4% represented different histological subtypes. The predominant histological grade was G2 (59.9%), followed by G3 (34.2%) and G1 (5.9%). Lymphatic permeation was observed in 38 (18.8%) cases, venous invasion in 21 (10.4%), and perineural charge in 24 (11.9%).
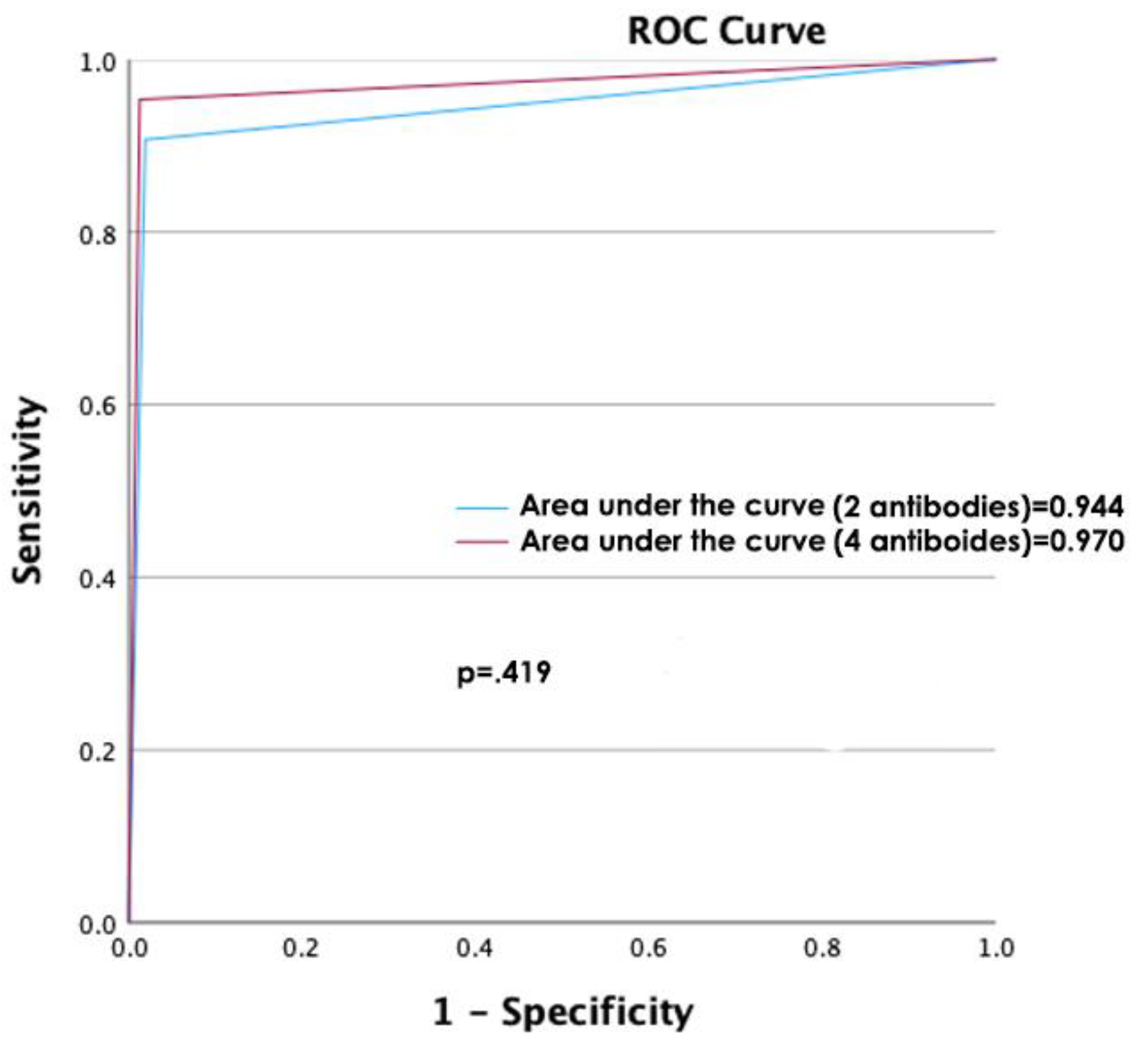
The diagnostic accuracy of determining the MMR protein status using the analysis of the four antibodies was compared against the analysis using only two antibodies (
Table 1,
Figure 2), which were very similar when comparing the areas under the curve (p=.782).
4. Discussion
In this study of 202 cases of colon cancer with known microsatellite instability status, we compared the determination of said status by immunohistochemistry with a traditional panel of 4 antibodies against a panel of 2 antibodies without finding statistically significant differences in their diagnostic capacity (sensitivity 95.35% vs. 90.7%; specificity 98.74% vs. 98.11%, positive predictive value 95.35% vs 92.86%; negative predictive value 98.74% vs 97.50% and area under the curve 0.970 vs 0.944, respectively, p=0.419).
Detection of microsatellite instability has become an essential tool in colon cancer diagnosis and patient stratification [
11]. Tumors with MSI often have distinct clinical and pathologic features, including location in the right colon, a specific histologic pattern (cribriform or medullary), and lymphocytic infiltration. Identification of MSI in tumors can help guide treatment and prognostic decisions. Colon cancer patients with MSI tend to respond better to specific treatments. The high immunogenicity of MSI tumors may lead to increased T-lymphocyte infiltration and increased sensitivity to immunotherapy, such as immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors) [
12]. Also, some studies suggest that MSI tumors may be less resistant to certain types of chemotherapy, such as 5-fluorouracil (5-FU) [
13,
14]. MSI is also linked to specific inherited syndromes, such as Lynch syndrome. Identifying MSI in patients with a family history of cancer can lead to early identification of individuals at risk and implementation of prevention and early detection strategies [
15]. Understanding the importance of MSI in colon cancer is critical to developing more personalized therapeutic approaches and effective early detection strategies.
On the other hand, saving and making the pathology laboratory more efficient is a relevant topic in medicine and biomedical research. Pathology laboratories play a crucial role in diagnosing and treating various diseases, providing detailed information about tissue and cell samples obtained from patients [
16]. However, the pathology analysis process can be costly regarding time, human resources, and finances. Therefore, finding ways to save and improve efficiencies in these laboratories can positively impact healthcare and research. Performing a diagnostic test with immunohistochemistry against two antibodies instead of four reduces the labor and financial burden on pathology laboratories, patients, and health systems [
17].
This has already been done in colon cancer. A recent systematic review [
7] shows that the evaluation of a test with two antibodies is very similar to the traditional one with four, specifically showing a weighted percentage of 1.1% of reported cases with non-dimeric loss that would not have been identified using a two -antibody approach. Considering the overall low rate of cases with non-dimeric loss, (<0.5%), the implementation of the two-antibody test algorithm seems appropriate for both screenings to identify patients at increased risk of Lynch syndrome, as well as identifying patients who may benefit from checkpoint inhibition therapy. Using this two-antibody testing algorithm will substantially reduce costs by 50% in all MSI cases while only causing a minimal delay in MMR status assignment in MSI cases. This systematic review has limitations, most related to heterogeneity between studies.
Our study attempted to reduce bias by using a reference standard as a control, performing immunohistochemistry in a standardized and automated manner, and evaluated by pathologists with experience in gastrointestinal pathology, with objective cut-off points. In addition, to reduce selection and performance biases, the cases correspond to cases consecutively treated and studied over one year.
When evaluating a diagnostic test, it is essential to consider several biases that can influence the interpretation of the results and the precision of the test. The limitations of our study are that there may be a subtle selection bias as it is a national referral hospital and that the evaluation of immunohistochemistry in cases with heterogeneous expression in large surgical specimens and years after being performed may differ from the initial evaluation.
5. Conclusions
In conclusion, our results demonstrate that a two-antibody approach is as effective as a four-antibody approach in determining the status of MMR proteins to establish the same. However, if the purpose of the study is to screen for Lynch syndrome, we think that the approach with the four antibodies should continue to be used.
Author Contributions
“Conceptualization, all authors.; methodology, L.S.L.S..; software, L.S.L.S.; validation, L.S.L.S, L.F.R.M, M.S.C.; formal analysis, L.S.L.S.; investigation, all authors; resources, C.Z.N, L.S.L.S.; data curation, all authors.; writing—original draft preparation, all authors; writing—review and editing, all authors; visualization, all authors; supervision, L.S.L.S.; project administration, L.S.L.S.; funding acquisition, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board and Ethics Committee of Instituto Nacional de Cancerología, Ciudad de México (register number No. 2023/126 on September 19, 2023).
Informed Consent Statement
Patient consent was waived due to this is a retrospective study without any risk and the data and material used correspond to archived material and clinical files review.
Data Availability Statement
The data from this study are available by mailing the corresponding author upon a reasonable request
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eikenboom EL, van der Werf-‘t Lam AS, Rodríguez-Girondo M, Van Asperen CJ, Dinjens WNM, Hofstra RMW, Van Leerdam ME, Morreau H, Spaander MCW, Wagner A, Nielsen M. Universal Immunohistochemistry for Lynch Syndrome: A Systematic Review and Meta-analysis of 58,580 Colorectal Carcinomas. Clin Gastroenterol Hepatol 2022, 20, e496–e507. [Google Scholar] [CrossRef] [PubMed]
- Cervantes B, André T, Cohen R. Deficient mismatch repair/microsatellite unstable colorectal cancer: therapeutic advances and questions. Ther Adv Med Oncol 2024, 16, 17588359231170473. [Google Scholar] [CrossRef] [PubMed]
- Mendonça Gorgulho C, Krishnamurthy A, Lanzi A, Galon J, Housseau F, Kaneno R, Lotze MT. Gutting it Out: Developing Effective Immunotherapies for Patients With Colorectal Cancer. J Immunother 2021, 44, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Samowitz WS, Curtin K, Lin HH, Robertson MA, Schaffer D, Nichols M, Gruenthal K, Leppert MF, Slattery ML. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology 2001, 121, 830–8. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, NN. Colorectal Cancer: Preoperative Evaluation and Staging. Surg Oncol Clin N Am 2022, 31, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Haron NH, Mohamad Hanif EA, Abdul Manaf MR, Yaakub JA, Harun R, Mohamed R, Mohamed Rose I. Microsatellite Instability and Altered Expressions of MLH1 and MSH2 in Gastric Cancer. Asian Pac J Cancer Prev 2019, 20, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Aiyer KTS, Doeleman T, Ryan NA, Nielsen M, Crosbie EJ, Smit VTHBM, Morreau H, Goeman JJ, Bosse T. Validity of a two-antibody testing algorithm for mismatch repair deficiency testing in cancer; a systematic literature review and meta-analysis. Mod Pathol 2022, 35, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, Miller R, Riaz N, Douillard JY, Andre F, Scarpa A. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Colon and Rectum Biomarker Reporting, College of American Pathologists. Available at: https://documents.cap.org/protocols/ColoRectal.Bmk_1.3.0.0.REL_CAPCP.pdf?_gl=1*20ehoq*_ga*MjEzMzc5MzcxMi4xNjkyODk0MDIy*_ga_97ZFJSQQ0X*MTY5Mjg5NDAyMS4xLjAuMTY5Mjg5NDAyOS4wLjAuMA. Access verified august 2023.
- Hanley JA, McNeil BJ. The meaning and use of the area under a Receiver Operating Characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Eso Y, Shimizu T, Takeda H, Takai A, Marusawa H. Microsatellite instability and immune checkpoint inhibitors: Toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020, 55, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lee CT, Chow NH, Chen YL, Ho CL, Yeh YM, Lin SC, Lin PC, Lin BW, Chu CA, Tsai HW, Lee JC. Clinicopathological features of mismatch repair protein expression patterns in colorectal cancer. Pathol Res Pract 2021, 217, 153288. [Google Scholar] [CrossRef] [PubMed]
- Chen L, Chen G, Zheng X, Chen Y. Expression status of four mismatch repair proteins in patients with colorectal cancer: clinical significance in 1238 cases. Int J Clin Exp Pathol 2019, 12, 3685–3699. [Google Scholar]
- Battaglin F, Naseem M, Lenz HJ, Salem ME. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol 2018, 16, 735–745. [Google Scholar]
- Martínez-Roca A, Giner-Calabuig M, Murcia O, Castillejo A, Soto JL, García-Heredia A, Jover R. Lynch-like Syndrome: Potential Mechanisms and Management. Cancers (Basel) 2022, 14, 1115. [Google Scholar] [CrossRef] [PubMed]
- Cheah PL, Looi LM, Horton S. Cost Analysis of Operating an Anatomic Pathology Laboratory in a Middle-Income Country. Am J Clin Pathol 2017, 149, 1–7. [Google Scholar]
- Neil A, Pfeffer S, Burnett L, BiPAC. Benchmarking in pathology: developing a benchmarking complexity unit and associated key performance indicators. Pathology 2013, 45, 66–70. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).