Submitted:
08 October 2024
Posted:
09 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
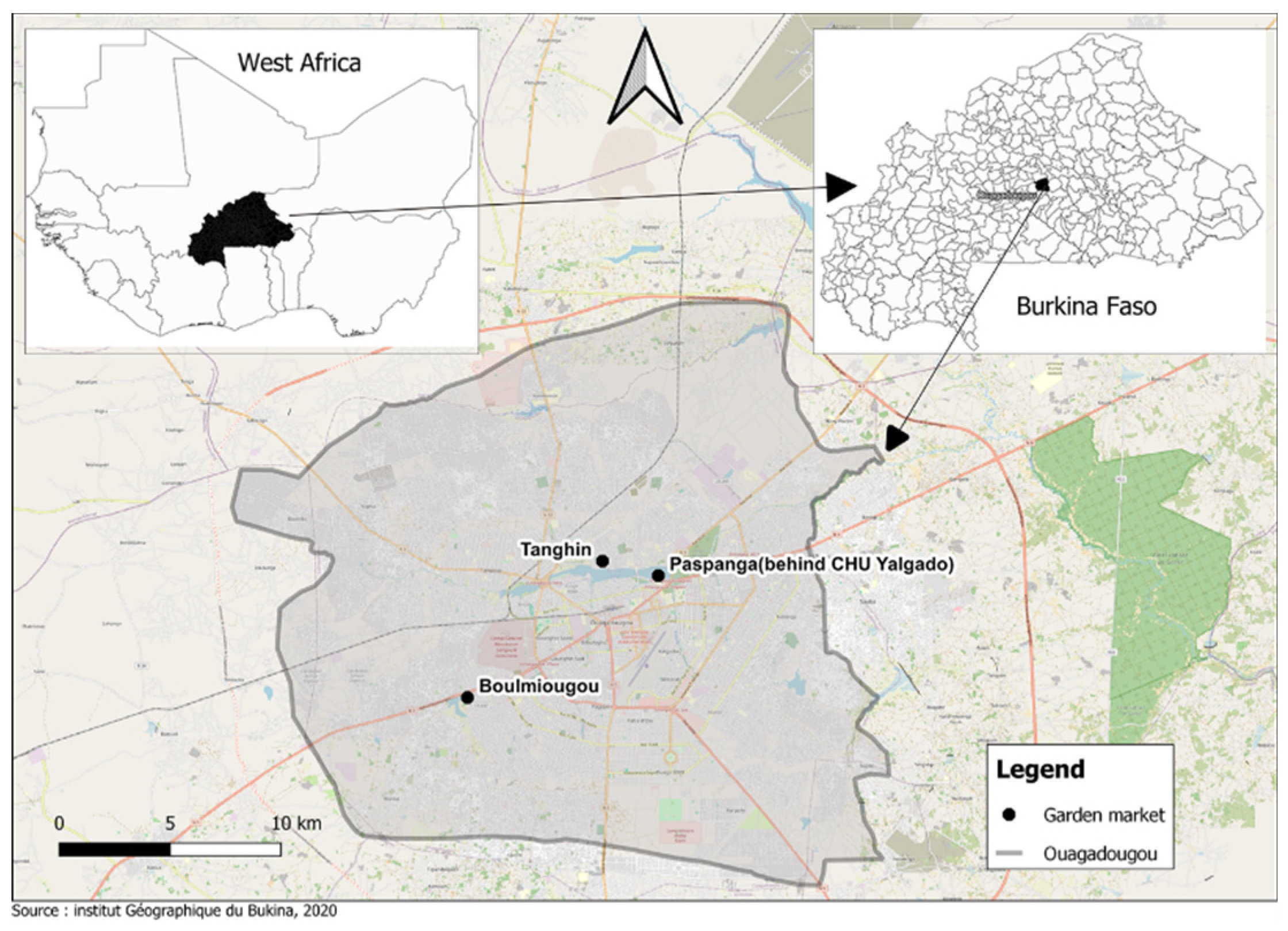
2.1. Sites and Period of the Study
2.2. Sampling
2.3. Bacterial Isolation and Identification
2.4. Phenotypic Confirmation of ESBL Production
2.5. Antimicrobial Susceptibility Testing
2.6. Detection of Carbapenemase Production
3. Results
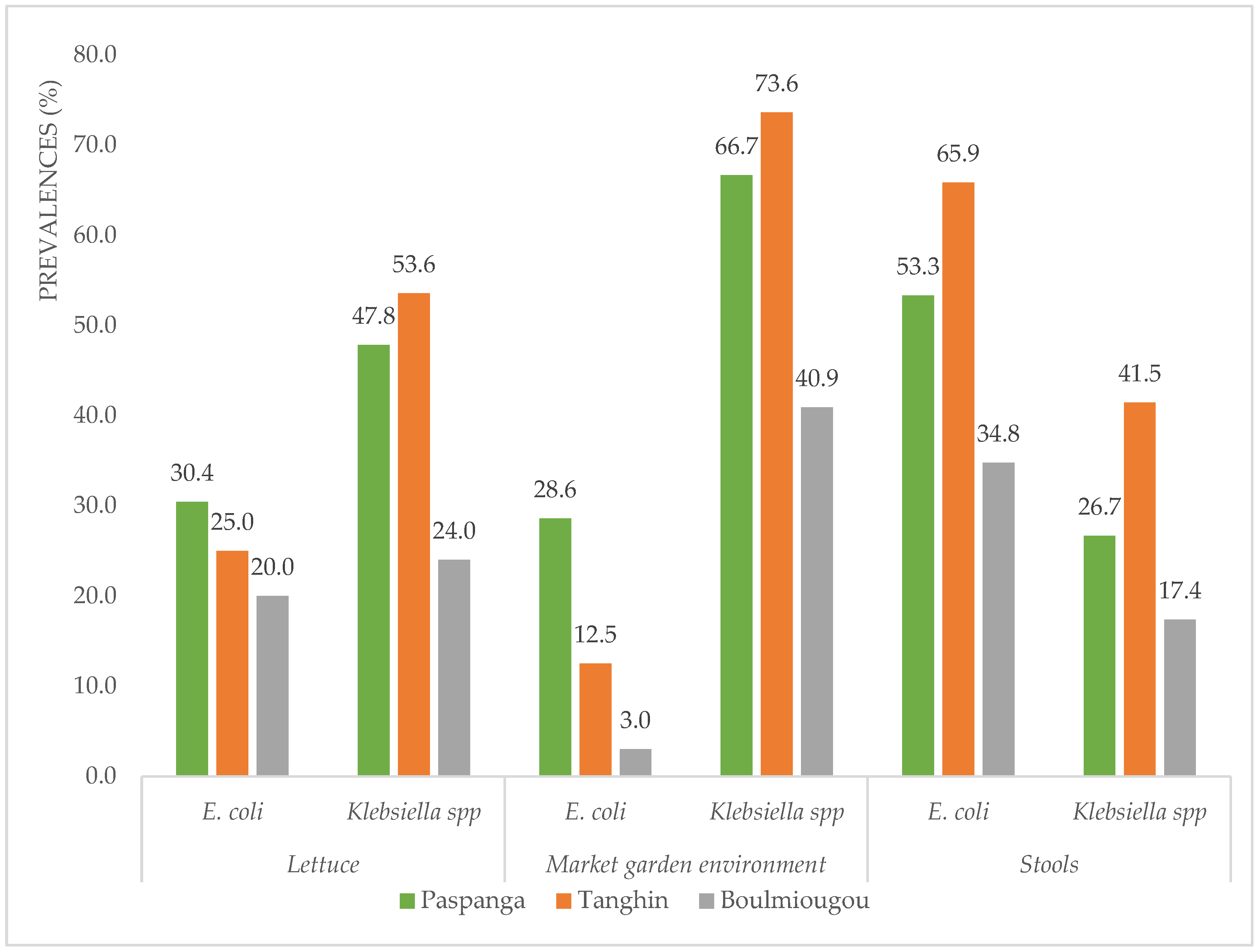
3.1. Prevalence of ESBL-Ec and ESBL-K in Different Sample Types
3.2. Prevalence of ESBL-Ec and ESBL-K at Different Sites
3.3. Antibiotic Resistance
3.4. Prevalence of AmpC-ESBL and Carbapenemase Producers at the Market Gardens Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators Global Burden of Bacterial Antimicrobial Resistance in 2019 : A Systematic Analysis. Lancet 2022, 399, 629–655. [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: a narrative review. Pharmaceuticals 2023, 16, 1–54. [Google Scholar] [CrossRef] [PubMed]
- World Bank Drug-Resistant Infections ; Washington, DC, USA. 2017.
- Ouedraogo, A.; Pierre, H.; …, A.B.-M. et S.; 2017, undefined Émergence et diffusion de la résistance aux antibiotiques en afrique de l’Ouest: facteurs favorisants et évaluation de la menace. Documentation.Ird.Fr 2017, 27, 147–154. [Google Scholar]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of antimicrobial resistance in Low- and Middle-Income Countries : a scattered picture. Antimicrob. Resist. Infect. Control 2021, 10, 1–19. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An overview of antimicrobial stewardship optimization: the use of antibiotics in humans and animals to prevent resistance. Antibiotics 2022, 11, 1–31. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Richter, L.; Du Plessis, E.M.; Duvenage, S.; Korsten, L. Prevalence of extended-spectrum β-Lactamase producing Enterobacterales in Africa’s water-plant-food interface: A meta-analysis (2010–2022). Front. Sustain. Food Syst. 2023, 7. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and global health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- B: Bacterial Priority Pathogens List, 2024: Bacterial pathogens of public health importance to guide research, development andsStrategies to prevent and control antimicrobial resistance; Geneva, 2024.
- Caliskan-Aydogan, O.; Alocilja, E.C. A Review of carbapenem resistance in enterobacterales and its detection techniques. Microorganisms 2023, 11, 1–26. [Google Scholar] [CrossRef]
- Baeza, L.L.; Pfennigwerth, N.; Greissl, C.; Göttig, S.; Saleh, A.; Stelzer, Y.; Gatermann, S.G.; Hamprecht, A. Comparison of five methods for detection of carbapenemases in Enterobacterales with proposal of a new algorithm. Clin. Microbiol. Infect. 2019, 25, 1286.e9–1286.e15. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections caused by carbapenem-resistant Enterobacteriaceae: An update on therapeutic options. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Mó, I.; da Silva, G.J. Tackling carbapenem resistance and the imperative for One Health strategies—insights from the portuguese perspective. Antibiotics 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, B.; Ouédraogo, H.S.; Zongo, O.; Ouédraogo, G.A.; Tapsoba, F.; Bougma, S.; Zongo, K.J.; Zeba, B.; Traoré, Y.; Sanou, I.; et al. Emergence of New Delhi Metallo- β -Lactamase (NDM) genes detected from clinical strains of Escherichia coli isolated in Ouagadougou, Burkina Faso. Int. J. Microbiol. 2023, 2023. [Google Scholar] [CrossRef]
- Manenzhe, R.I.; Zar, H.J.; Nicol, M.P.; Kaba, M. The Spread of carbapenemase-producing bacteria in Africa: A systematic review. J. Antimicrob. Chemother. 2015, 70, 23–40. [Google Scholar] [CrossRef]
- Garba, Z.; Bonkoungou, I.O.J.; Millogo, N.O.; Natama, H.M.; Vokouma, P.A.P.; Bonko, M. dit A.; Karama, I.; Tiendrebeogo, L.A.W.; Haukka, K.; Tinto, H.; et al. Wastewater from healthcare centers in Burkina Faso is a source of ESBL, AmpC-β-Lactamase and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae. BMC Microbiol. 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Garba, Z.; Kaboré, B.; Bonkoungou, I.J.O.; Natama, M.H.; Rouamba, T.; Haukka, K.; Kirveskari, J.P.; Tinto, H.; Sangaré, L.; Barro, N.; et al. Phenotypic detection of carbapenemase and AmpC-β-Lactamase production among extended spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella Spp. isolated from clinical specimens. Antibiotics 2024, 13, 1–11. [Google Scholar] [CrossRef]
- Ben Said, L.; Jouini, A.; Klibi, N.; Dziri, R.; Alonso, C.A.; Boudabous, A.; Ben Slama, K.; Torres, C. Detection of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in vegetables, soil and water of the farm environment in Tunisia. Int. J. Food Microbiol. 2015, 203, 86–92. [Google Scholar] [CrossRef]
- Pignato, S.; Coniglio, M.A.; Faro, G.; Weill, F.X.; Giammanco, G. Plasmid-mediated multiple antibiotic resistance of Escherichia coli in crude and treated wastewater used in agriculture. J. Water Health 2009, 7, 251–258. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: A Review. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef]
- Sahota, P. Contaminated irrigation water : A source of human pathogens on growing vegetables. Int. J. Cell Sci. Mol. Biol. 2018, 3, 5–7. [Google Scholar] [CrossRef]
- Gekenidis, M.T.; Walsh, F.; Drissner, D. Tracing antibiotic resistance genes along the irrigation water chain to Chive: Does tap or surface water make a difference? Antibiotics 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H.; Cho, G.S.; Franz, C.M.A.P. Multidrug-resistant extended spectrum β-lactamase (ESBL)-producing Escherichia coli from farm produce and agricultural environments in Edo State, Nigeria. PLoS One 2023, 18, 1–20. [Google Scholar] [CrossRef]
- Anokyewaa Appau, A.A.; Ofori, L.A. Antibiotic Resistance Profile of E. coli isolates from lettuce, poultry manure, irrigation water, and soil in Kumasi, Ghana. Int. J. Microbiol. 2024, 2024. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Zhang, S.; Zhang, J.; Yang, G. Antibiotic-resistant extended spectrum ß-lactamase- and Enterobacteriaceae isolated from retail food products and the pearl river in Guangzhou, China. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Couturier, J.; Rabate, M.; Nesa, D.; Adam, M.; Prat, L.; Jolivet, S. Évaluation de la dissémination des bactéries à partir des toilettes : étude expérimentale. Hygiènes 2022, 30(1), 29–35.
- Mittal, G.; Gaind, R.; Kumar, D.; Kaushik, G.; Gupta, K.B.; Verma, P.K.; Deb, M. Risk factors for fecal carriage of carbapenemase producing Enterobacteriaceae among intensive care unit patients from a tertiary care center in India. BMC Microbiol. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Health, O. WHO integrated global surveillance on ESBL-producing E. coli using a “One Health” approach: Implementation andopportunities. 2021. [Google Scholar]
- Soré, S.; Sanou, S.; Sawadogo, Y.; Béogo, S.; Dakouo, S.N.P.; Djamalladine, M.D.; Lboudo, K.S.; Ouoba, B.; Zoungrana, J.; Poda, A.; et al. Faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in healthy volunteers and hospitalized patients in Ouagadougou, Burkina Faso: Prevalence, resistance profile, and associated risk factors. African J. Clin. Exp. Microbiol. 2021, 22, 157–163. [Google Scholar] [CrossRef]
- Ouédraogo, A.S.; Sanou, S.; Kissou, A.; Poda, A.; Aberkane, S.; Bouzinbi, N.; Nacro, B.; Ouédraogo, R.; Van De Perre, P.; Carriere, C.; et al. Fecal carriage of Enterobacteriaceae producing extended-spectrum beta-lactamases in hospitalized patients and healthy community volunteers in Burkina Faso. Microb. Drug Resist. 2017, 23, 63–70. [Google Scholar] [CrossRef]
- Soré, S.; Diarra, F.B.J.; Bonkoungou, J.I.; Sawadogo, C.; Bationo, B.G.; Ky, H.; Madingar, P.D.-M.; Ouédraogo, A.S.; Sanou, I. Prevalence and characterization of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolated from poultry in Ouagadougou, Burkina Faso. African J. Microbiol. Res. 2021, 15, 447–453. [Google Scholar] [CrossRef]
- Sanou, S.; Ouedraogo, A.S.; Lounnas, M.; Zougmore, A.; Poda, A.; Zoungrana, J.; Ouedraogo, G.A.; Traore/Ouedraogo, R.; Ouchar, O.; Carriere, C.; et al. Epidemiology and molecular characterization of Enterobacteriaceae producing extended spectrum β-lactamase in intensive and extensive breeding animals in Burkina Faso. 2019, 1–16. [CrossRef]
- Kagambèga, A.B.; Dembélé, R.; Traoré, O.; Wane, A.A.; Mohamed, A.H.; Coulibaly, H.; Fall, C.; Bientz, L.; M’Zali, F.; Mayonnove, L.; et al. Isolation and characterization of environmental extended spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae from Ouagadougou, Burkina Faso. Pharmaceuticals 2024, 17, 1–13. [Google Scholar] [CrossRef]
- WHO, Global Tricycle Surveillance ESBL E. coli; 2021; ISBN 9789240021402.
- Drieux, L.; Brossier, F.; Sougakoff, W.; Jarlier, V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: Review and bench guide. Clin. Microbiol. Infect. 2008, 14, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- CLSI, CLSI M100-ED31 : 2021 Performance standards for antimicrobial susceptibility testing. 2021.
- Bush, K.; Bradford, P.A. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Meddicine 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Solimini, A.G.; Pantanella, F.; De Giusti, M.; Cummins, E. Human exposure to antibiotic resistant-Escherichia coli through irrigated lettuce. Environ. Int. 2019, 122, 270–280. [Google Scholar] [CrossRef]
- Markkanen, M.A.; Haukka, K.; Pärnänen, K.M.M.; Dougnon, V.T.; Bonkoungou, I.J.O.; Garba, Z.; Tinto, H.; Sarekoski, A.; Karkman, A.; Kantele, A.; et al. Metagenomic analysis of the abundance and composition of antibiotic resistance genes in hospital wastewater in Benin, Burkina Faso, and Finland. mSphere 2023, 8, 1–17. [Google Scholar] [CrossRef]
- Ramatla, T.; Mafokwane, T.; Lekota, K.; Monyama, M.; Khasapane, G.; Serage, N.; Nkhebenyane, J.; Bezuidenhout, C.; Thekisoe, O. “One Health” Perspective on Prevalence of co-existing extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae: A comprehensive systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 1–17. [Google Scholar] [CrossRef]
- Soré, S.; Sawadogo, Y.; Bonkoungou, J.I.; Kaboré, S.P.; Béogo, S.; Sawadogo, C.; Bationo, B.G.; Ky, H.; Madingar, P.D.M.; Ouédraogo, A.S.; et al. Detection, identification and characterization of extended-spectrum beta-lactamases producing enterobacteriaceae in wastewater and salads marketed in Ouagadougou, Burkina Faso. Int. J. Biol. Chem. Sci. 2020, 14, 2746–2757. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Zhu, L.; Wang, J. Field-based evidence for enrichment of antibiotic resistance genes and mobile genetic elements in manure-amended vegetable soils. Sci. Total Environ. 2019, 654, 906–913. [Google Scholar] [CrossRef]
- Gudda, F.O.; Waigi, M.G.; Odinga, E.S.; Yang, B.; Carter, L.; Gao, Y. Antibiotic-contaminated wastewater irrigated vegetables pose resistance selection risks to the gut microbiome. Environ. Pollut. 2020, 264, 114752. [Google Scholar] [CrossRef]
- Schwaiger, K.; Helmke, K.; Hölzel, C.S.; Bauer, J. Antibiotic resistance in bacteria isolated from vegetables with regards to the marketing stage (Farm vs. Supermarket). Int. J. Food Microbiol. 2011, 148, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Meijs, A.P.; Rozwandowicz, M.; Hengeveld, P.D.; Dierikx, C.M.; de Greeff, S.C.; van Duijkeren, E.; van Dissel, J.T. Human carriage of ESBL/PAmpC-producing Escherichia coli and Klebsiella pneumoniae in relation to the consumption of raw or undercooked vegetables, fruits, and vresh herbs. Microbiol. Spectr. 2024, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ouchar Mahamat, O.; Tidjani, A.; Lounnas, M.; Hide, M.; Benavides, J.; Somasse, C.; Ouedraogo, A.S.; Sanou, S.; Carrière, C.; Bañuls, A.L.; et al. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in hospital and community settings in Chad. Antimicrob. Resist. Infect. Control 2019, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Paterson, D.L.; Harris, P.N.A.; Furuya-Kanamori, L.; Edwards, F.; Laupland, K.B. Mortality, Hospital length of stay, and recurrent bloodstream infections associated with extended-spectrum beta-lactamase-producing Escherichia coli in a Low prevalence region: A 20-year population-based large cohort study. Int. J. Infect. Dis. 2024, 138, 84–90. [Google Scholar] [CrossRef]
- Richter, L.; DuPlessis, E.; Duvenage, S.; Korsten, L. Occurrence, identification, and antimicrobial resistance profiles of extended-spectrum and AmpC β-lactamase-producing Enterobacteriaceae from fresh vegetables retailed in Gauteng province, South Africa. Foodborne Pathog. Dis. 2019, 16. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An african perspective. Antimicrob. Resist. Infect. Control 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Zurfluh, K.; Nüesch-Inderbinen, M.; Morach, M.; Berner, A.Z.; Hächler, H.; Stephan, R. Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl. Environ. Microbiol. 2015, 81, 3115–3120. [Google Scholar] [CrossRef]
- Kagambèga, A.B.; Dembélé, R.; Bientz, L.; M’Zali, F.; Mayonnove, L.; Mohamed, A.H.; Coulibaly, H.; Barro, N.; Dubois, V. Detection and characterization of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae from hospital effluents of Ouagadougou, Burkina Faso. Antibiotics 2023, 12. [Google Scholar] [CrossRef]
- Ragheb, S.M.; Govinden, U.; Osei Sekyere, J. Genetic support of carbapenemases: A One Health systematic review and meta-analysis of current trends in Africa. Ann. N. Y. Acad. Sci. 2022, 1509, 50–73. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
| Samples | N | ESBL-Ec (%) | ESBL-K (%) | |
|---|---|---|---|---|
| Fresh produce | Lettuce | 76 | 19 (25.0) | 33 (43.4) |
| Market garden environment | Soil | 76 | 8 (10.5) | 47 (61.8) |
| Manure | 62 | 13 (21.0) | 34 (54.8) | |
| Water | 63 | 11 (17.5) | 43 (68.3) | |
| Human | Stools | 79 | 42 (53.2) | 24 (30.4) |
| Total | 356 | 93 (26.1) | 181 (50.8) | |
| Antibiotic (μg) | Resistant ESBL-Ec (%) | Resistant ESBL-K (%) |
|---|---|---|
| Amoxicillin+ clavulanic acid (30) | 45/93 (48.4) | 116/181 (64.1) |
| Meropenem (10) | 0/93(0.0) | 4/181 (2.2) |
| Gentamycin (10) | 17/93 (18.3) | 40/181 (22.1) |
| Amikacin (30) | 2/93 (2.2) | 6/181 (3.3) |
| Ciprofloxacin (5) | 32/93 (34.4) | 46/181 (25.4) |
| Ofloxacin (5) | 30/93 (32.3) | 30/181 (16.6) |
| Nalidixic acid (30) | 41/93 (44.1) | 59/181 (32.6) |
| Tetracycline (30) | 80/93 (86.0) | 161/181 (89.0) |
| Sulfametoxazole+ trimethoprim (25) | 77/93 (82.8) | 158/181 (87.3) |
| Chloramphenicol (30) | 5/93 (5.38) | 42/181 (23.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





