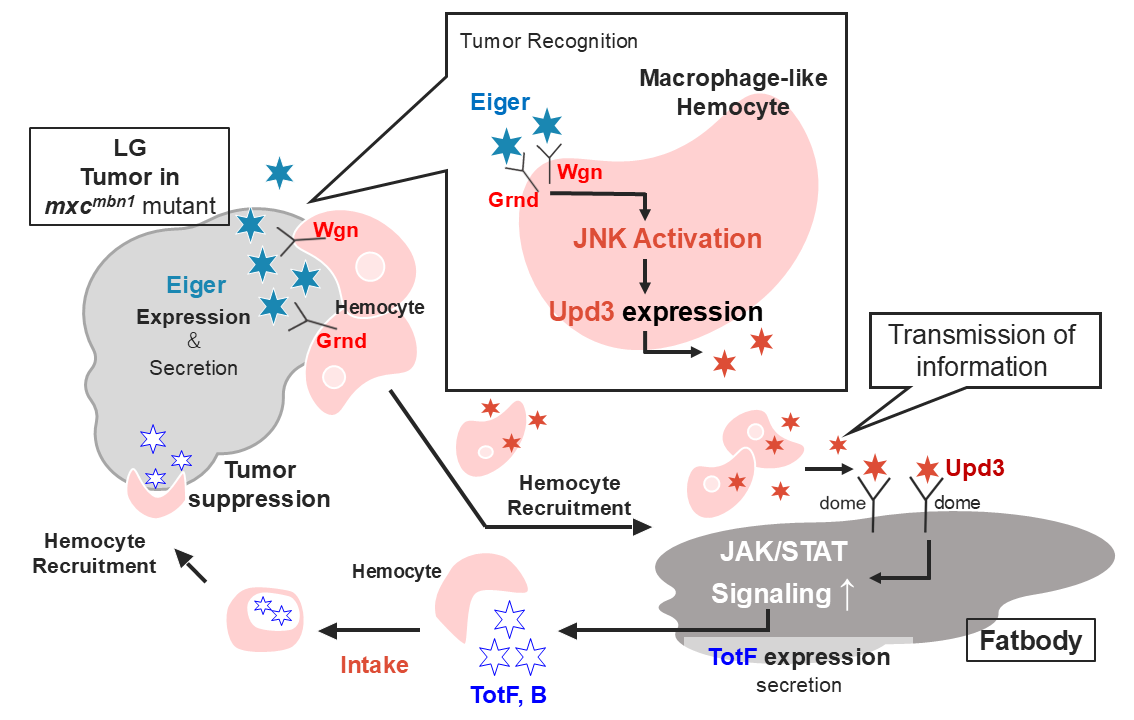
1. Introduction
Innate immunity is the first line of defense against infectious pathogens and tumor cells. Although insects, including
Drosophila, do not have an acquired immune system, they exhibit immunological responses against microbes, parasite eggs, wounds, and tumors [
1]. When microbial pathogens enter the body, humoral factors are induced. The fat body (FB) produces secreted proteins of low molecular weight, such as antimicrobial peptides (AMPs) and Turandot proteins (Tots) [
2,
3,
4]. Depending on the type of invader (fungi, gram-negative bacteria, or gram-positive bacteria), different proteins are produced in the FB. Each AMP is induced by one or both Toll- and Imd-mediated signaling pathways [
4], homologous to the mammalian Toll-like receptor (TLR) and Tumor necrosis factor (TNF) pathways, respectively [
5,
6]. Five major AMPs (Drosomycin, Defensin, Diptericin, Metchnikowin, and AttacinA) are induced in the tumor-bearing mutant larvae and have cytotoxic effects against tumors [
7,
8,
9].
In addition to these well-known innate immunity pathways, the JAK/STAT signaling pathway plays a critical role in inducing another immune-responsible protein family called the Tot after infection [
10]. When Unpaired (Upd) cytokines, such as Upd3, bind to their receptor, Domeless (Dome), the JAK/STAT pathway is activated in the cytoplasm [
11]. Consequently, the transcription of
Tot family genes is induced in FB [
6,
12]. Similar to AMPs, TotB and TotF proteins induced by the JAK/STAT pathway also have cytotoxic effects against tumors. These Tot proteins are induced in FB and incorporated into circulating hemocytes [
13]. As a result, apoptosis is observed in the lymph gland (LG) tumors in the induction of AMPs and Tots, and tumor cell proliferation is also inhibited in Tots. However, the mechanism by which the effect of Tots on LG tumors located away from the FB has not yet been characterized.
The c-Jun N-terminal kinase (JNK) pathway is also activated in response to diverse intracellular stresses including tumors [
14]. When a cytokine called Eiger, corresponding to a mammalian TNF family protein, binds to its receptors composed of Wengen and Grindelwald, they activate the JNK signaling pathway in the cytoplasm, including Hep, corresponding to JNKK, and Bsk, corresponding to JNK [
15]. Activated Bsk phosphorylates and activates the transcription factor Jra, which induces the transcription of
upd genes encoding ligands for the JAK/STAT pathway [
16]. However, the interaction between these signaling pathways in
mxcmbn1 larvae remains to be elucidated.
Hemocytes in the hemolymph, which are classified as plasmatocytes, lamellocytes, and crystal cells also play critical roles in eliminating pathogens [
17]. Plasmatocytes, the equivalent of mammalian macrophages, comprise 95% of all
Drosophila hemocytes and play essential roles in the cellular immune response such as phagocytosis [
18,
19]. During the larval stage, new hemocytes are produced in a hematopoietic tissue called the LG [
20]. In larvae hemizygous for a loss-of-function mutation in the
multi sex combs (
mxc) gene, the hemocyte precursor cells in the LG overproliferate and differentiate abnormally [
7,
21,
22,
23]. The hemocyte precursor cells in the LG of
mxcmbn1 mutant larvae become malignant and invade other tissues [
21,
24]. When mutant LG cells are transplanted into the abdominal cavity of normal flies, they continue to proliferate and infiltrate other adult tissues. Some aberrant hemocytes expressing undifferentiated markers are released into the hemolymph. These leukemia-like phenotypes are lethal to mutant larvae from the third-instar larval to the pupal stages [
7,
23,
24]. In response to LG tumors, three innate immune pathways, the Toll-mediated, Imd-mediated, and JAK/STAT pathways, are activated to induce five major AMPs and four Tots in the FB of
mxcmbn1 larvae [
7,
13]. Consequently, these AMPs stimulate apoptosis specifically in LG tumors [
7,
8,
9]. For AMPs to be induced in FB, distant from the LG tumor, the information relevant to the presence of tumor cells must be transmitted to FB. Mutant hemocytes, as well as normal hemocytes transplanted from control larvae, are preferentially associated with LG tumors in mutant larvae [
9]. However, whether hemocytes are recruited to FB efficiently in
mxcmbn1 has not been investigated. The possibility that the recruitment of hemocytes to this tissue is due to the tumor characteristics of the mutant hemocytes has not been ruled out. To clarify, we investigated whether hemocytes transplanted from normal larvae were recruited more efficiently to mutant FB than to control tissue.
The depletion of TotB and TotF compromises apoptosis and enhances tumor cell proliferation [
13]. However, the mechanism by which the JAK/STAT pathway is activated in FB has not been studied. Therefore, we aimed to clarify whether circulating hemocytes play a critical role in conveying tumor information to FB to activate the JAK/STAT pathway. Upd3, a ligand of the JAK/STAT pathway, produced by tumor cells signal the endocrine tissue to activate the JAK/STAT pathway [
11]. Our previous study also showed that hemocytes from
mxcmbn1 larvae ectopically express Upd3; consequently, this pathway is activated [
13]. Thus, we also aimed to elucidate the mechanism by which the JAK/STAT signaling pathway is activated in FB via circulating hemocytes in response to LG tumors.
In the present study, we first examined whether
upd3 expression was required to induce Tot mRNA expression in FB, that is, the activation of the JAK/STAT pathway. We further investigated the effects of ectopic expression on LG tumor growth. Second, we focused on the mechanism by which Upd3 was highly expressed in the hemocytes of
mxcmbn1 larvae. The TNF-like cytokine Eiger is expressed in the tumors of
Drosophila imaginal discs and activates the JNK pathway [
25]. Therefore, we investigated whether Eiger was highly expressed in the LG tumors of
mxcmbn1 larvae. Third, we examined whether Eiger expression in LG tumors was required to activate the JNK pathway in circulating hemocytes. We then assessed whether the JNK pathway down-stream of the Eiger receptors in circulating hemocytes was required to activate the JAK/STAT pathway in FB. Additionally, we examined their effects on the growth of LG tumors. Based on these results, we proposed a model to explain how the higher expression of Eiger in LG tumors of
mxcmbn1 larvae leads to the activation of the JAK/STAT pathway and, ultimately, induction of
Tot expression in FB. AMPs and Tots secreted from FB are taken up by plasmatocytes of mutant larvae [
7,
13]. Thus, we also determined whether Tot proteins were taken up by macrophage-like plasmatocytes and transported to the tumor. The current results will be important for the future analysis of this tumor suppression mechanism, which may involve inter-tissue communication via hematopoietic cells, in
Drosophila and other animal models.
2. Results
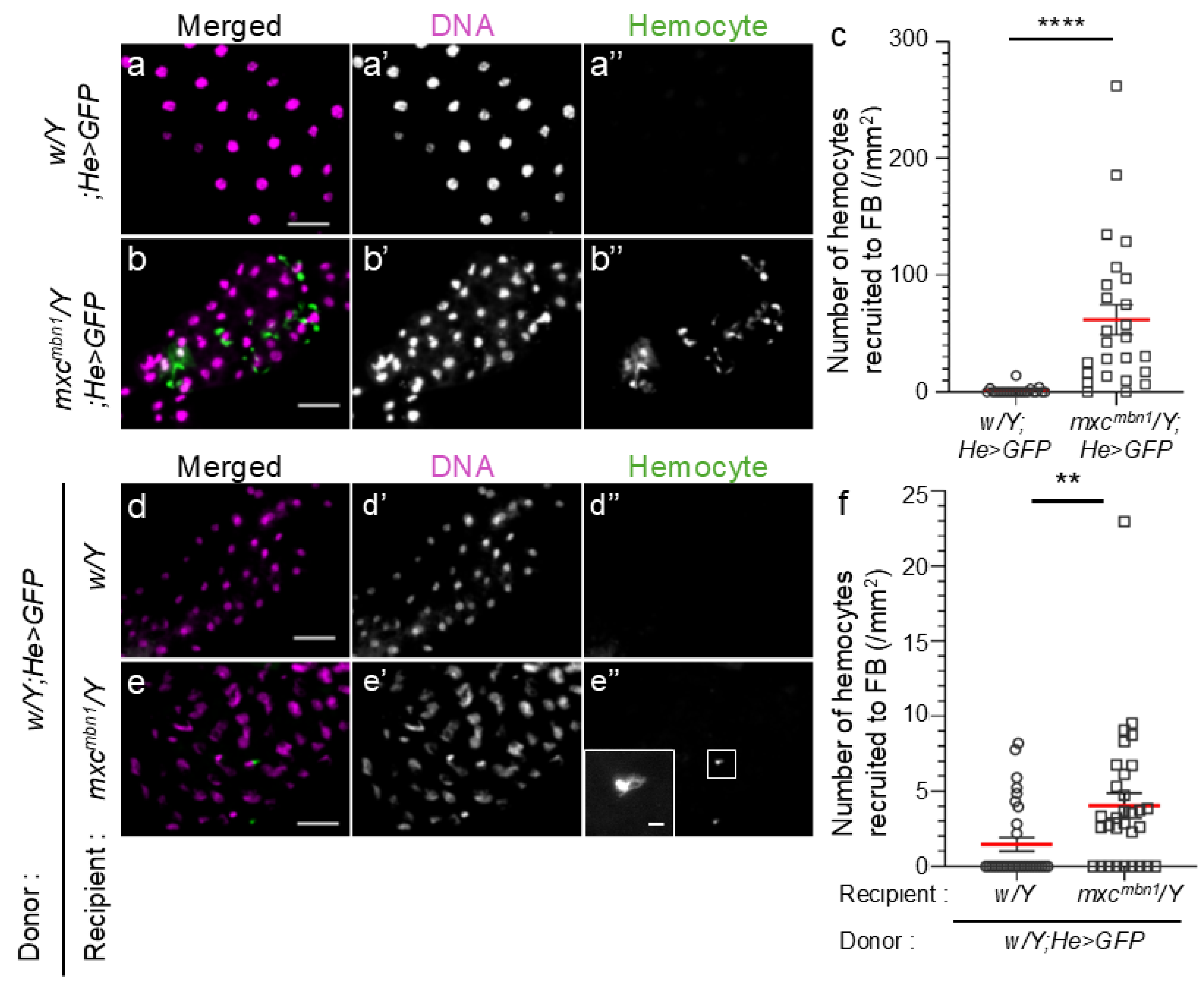
2.1. Increase in Normal Hemocytes Associated with the FB in mxcmbn1 Mutant Larvae Harboring the LG Tumor
Normal circulating hemocytes are recruited to the LG tumor more efficiently in mutant larvae than in normal larvae when the cells are transplanted into mutant larvae [
9]. To understand the mechanism by which information regarding tumor is transmitted to the FB, we first examined whether more circulating hemocytes were associated with the FB in mutant larvae. We scored the circulating hemocytes labeled with GFP on the FB in mutant
(mxcmbn1/Y; He>GFP) (
Figure 1b) and control (
w/Y; He>GFP) (
Figure 1a) larvae, and converted the average cell number in each larva to that in a 1 mm
2 of the FB area. In total, 61.9 hemocytes on average (n = 25) were observed in 1 mm
2 of the FB area in
mxcmbn1 larvae, whereas 1.1 hemocytes (n = 22) were observed in the same FB area in normal larvae (
Figure 1a, b). The differences were significant (
p <0.0001, Welch’s
t-test) (
Figure 1c). 60-fold more hemocytes were localized on the FB in mutant larvae bearing LG tumors than in control larvae. As 4-fold more hemocytes were contained in the hemolymph of mutant (7.7 ± 1.1 × 10
4 cells in 1 mL of hemolymph, n = 16) than of control (1.8 ± 0.1 × 10
4 cells in 1 mL of hemolymph, n = 12) larvae, mutant hemocytes were recruited more efficiently to the FB.
To clarify whether normal circulating hemocytes are also recruited to the FB in mutant larvae, we transplanted larval hemolymph containing GFP-labeled normal hemocytes (
w/Y;He>GFP) into control and mutant larvae at the third-instar stage. 15 h after the transplantation of hemolymph, in which 1.2 × 10
4 circulating hemocytes (
w/Y;He>GFP) were contained on average (n = 21) (
Figure 1d, e), we counted the number of transplanted GFP+ hemocytes on the FB and converted it to the number of cells per unit area (1 mm
2) of the tissues. We scored 4.0 hemocytes on average (n = 31) per FB area in
mxcmbn1 larvae (
Figure 1e, f), whereas 1.5 hemocytes (n = 31) were in the same area in normal larvae (
Figure 1d, f). The increase in mutant larvae was significant (
p < 0.01, Welch’s
t-test). Confocal microscopy observation confirmed that the transplanted normal hemocytes were associated with the surface, but not the inside, of the FB in normal and mutant larvae (see the images only for reviewing in a repository).
Figure 1.
Hemocytes localized on the fat body (FB) in control and mxcmbn1 mutant larvae, and normal hemocytes transplanted from control larvae on the FB in control and mxcmbn1 larvae.
Figure 1.
Hemocytes localized on the fat body (FB) in control and mxcmbn1 mutant larvae, and normal hemocytes transplanted from control larvae on the FB in control and mxcmbn1 larvae.
(a, b) Fluorescence images of circulating hemocytes labeled by GFP on the DAPI-stained FB in normal control (w/Y;He>GFP)(a) and mxcmbn1 (mxcmbn1/Y;He>GFP)(b) larvae. The circulating hemocytes are visualized in green in a and b (white in a” and b”). DNA is stained in magenta in a and b (white in (a’ and b’). Scale bars:100 μm. (c) Quantification of the number of hemocytes localized on the FB. The average number of hemocytes per unit area (mm2) of FB was calculated. (n = 22 (w/Y;He>GFP), n = 25 (mxcmbn1/Y;He>GFP), Welch's t-test, ****p<0.0001). The red line indicates the mean value; error bars indicate the standard error of the mean (SEM). (d, e) Fluorescence images of normal circulating hemocytes labeled by GFP (w; He>GFP), which were transplanted from control larvae on the DAPI-stained FB in (d) normal control (w/Y) and (e) mxcmbn1 mutant (mxcmbn1/Y) larvae. The transplanted normal hemocytes are visualized in green in d and e (white in d”, e”). DNA is stained in magenta in d and e (white in d’ and e’). Scale bars:100 μm. (f) Quantification of the number of hemocytes localized on the FB. The average number of hemocytes per unit area of FB was calculated (n = 31 (w/Y;He>GFP), n = 31 (mxcmbn1/Y;He>GFP). Welch's t-test, **p<0.01). The red line indicates the mean value; error bars indicate the SEM.
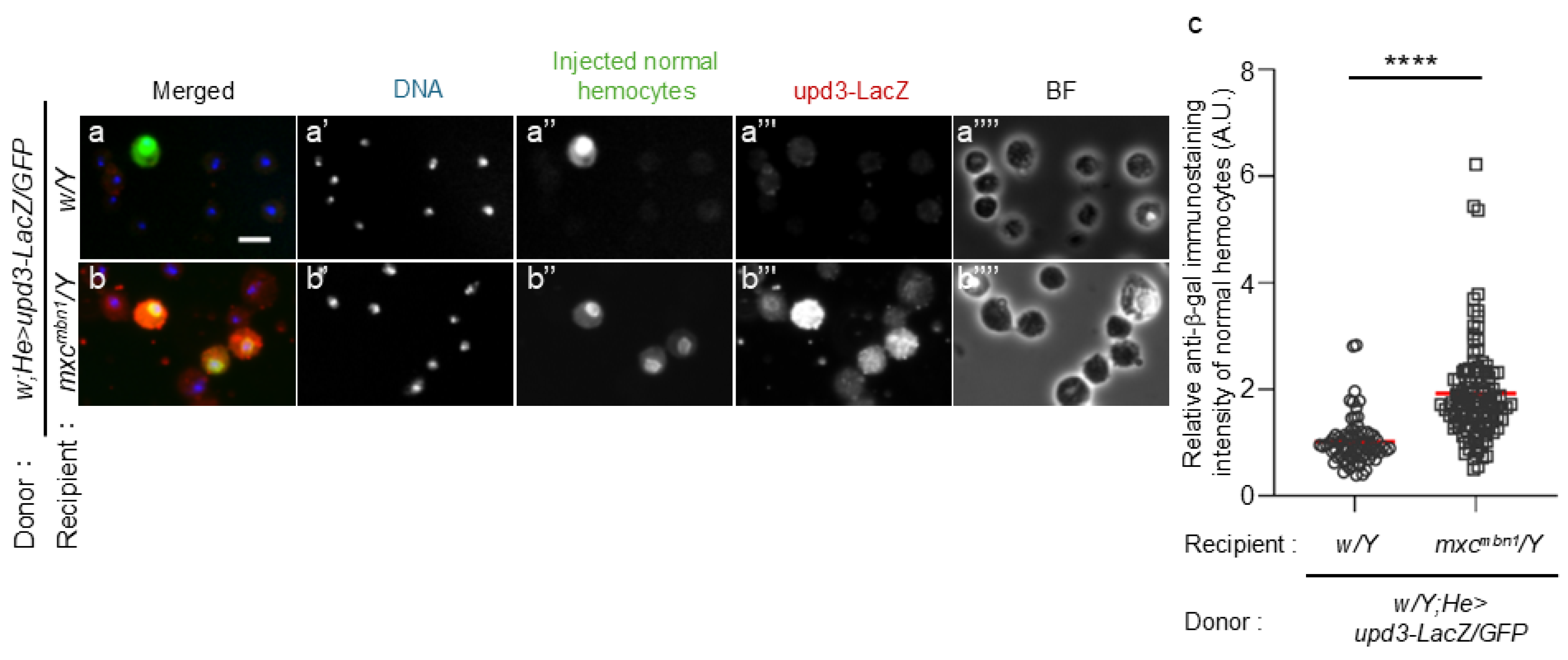
2.2. Ectopic upd3 Expression in Normal Hemocytes Transplanted into mxcmbn1 Larvae and Hemocyte-Specific Depletion of upd3 Resulted in Reduced TotF mRNA Levels and Increased LG Tumor Growth
To clarify the possibility that hemocytes activate the JAK/STAT signaling pathway in the FB via Upd3, we initially observed ectopic
upd3 expression in normal hemocytes transplanted into
mxcmbn1 larvae. We transplanted normal hemocytes harboring the
upd3-LacZ reporter that monitors gene expression (
w/Y;He>GFP/upd3-LacZ) into control and
mxcmbn1 larvae at the third-instar stage. 15 h after transplantation, we observed a few anti-β-gal immunostaining signals above the background level (n = 73 cells examined) (
Figure 2a, a”’). In contrast, we observed a robust immunostaining signal in GFP
+ normal hemocytes in
mxcmbn1 larvae (n = 108 cells examined) (
Figure 2b, b”’). The difference between control and mutant larvae was significant (
p <0.0001, Welch’s
t-test)(
Figure 2c).
(a, b) Anti-β-gal immunostaining of the circulating hemocytes transplanted from normal larvae at mature third instar stage in normal (w/Y) (a) and mxcmbn1 (b) larvae (mxcmbn1/Y). Scale bar: 10 µm; blue, DNA (white in a’, b’); green, transplanted hemocytes labeled by GFP fluorescence (white in a”, b”) (w/Y;He>GFP/upd3-LacZ); red, anti-β-gal immunostaining to monitor the upd3 expression (white in a”’, b”’). BF, Brightfield microscopy image in a””, b””. (c) The relative fluorescence intensity of anti-β-gal immunostaining. The fluorescence intensity of each transplanted hemocyte was quantified and displayed on the y-axis relative to the fluorescence intensity of the normal control set at 1. X-axis from left to right: normal control (w/Y) (n = 73), mxcmbn1 (mxcmbn1/Y) (n = 108), The average fluorescence intensity is shown as a red line. (Welch's t test, ****p < 0.0001). Error bars indicate standard error of the mean (SEM).
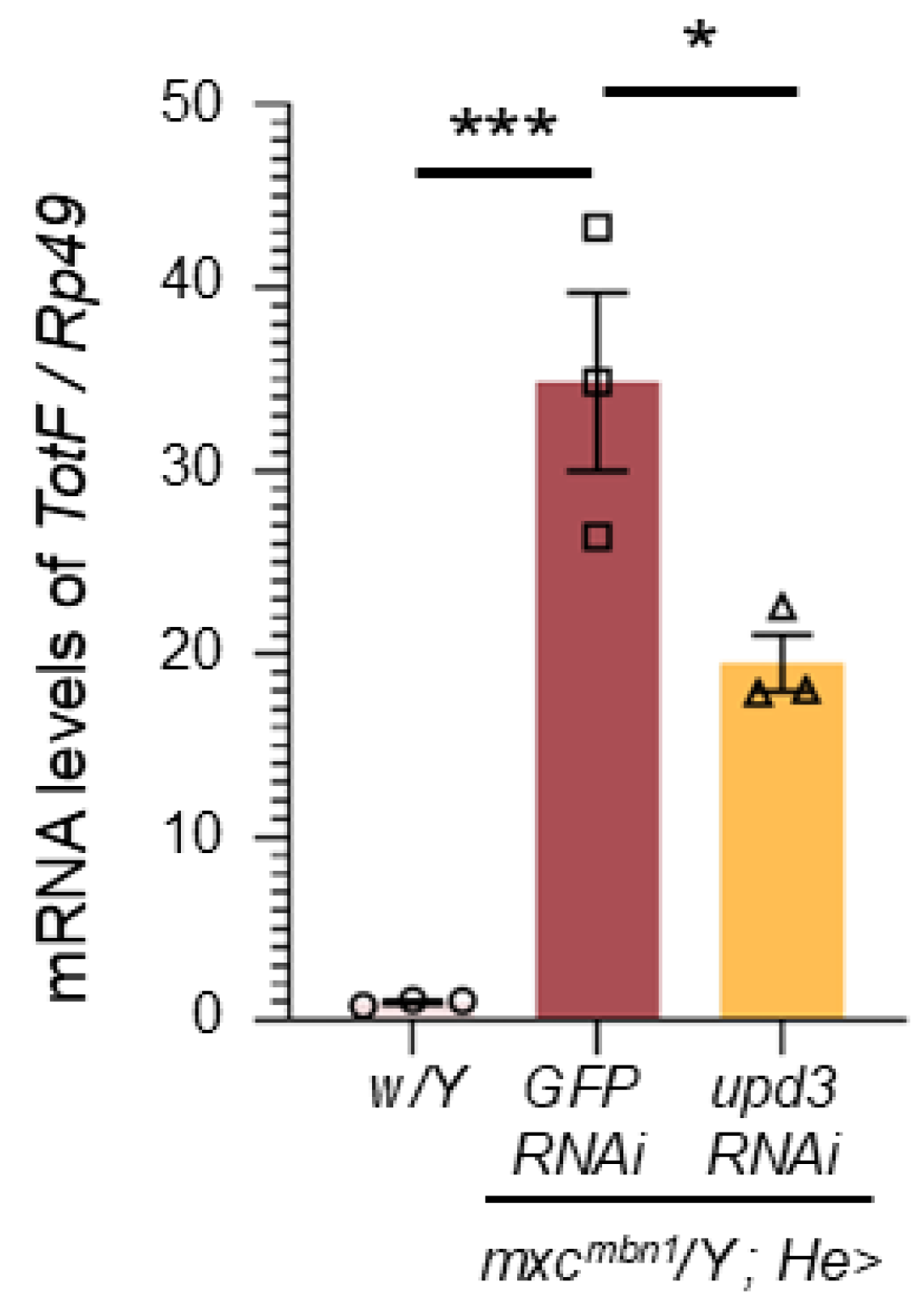
We investigated whether depletion of
upd3 affected
TotF expression in the FB of
mxcmbn1 larvae (
Figure 3). We quantified
TotF mRNA levels by qRT-PCR using RNAs from normal control larvae (
w/Y),
mxcmbn1 larvae expressing hemocyte-specific dsRNA against
GFP mRNA (
mxcmbn1/Y;He>GFPRNAi) as a control for depletion, and
mxcmbn1 larvae expressing hemocyte-specific
upd3 depletion (
mxcmbn1/Y;He>upd3RNAi). The
TotF level in
mxcmbn1 larvae with hemocyte-specific
upd3 depletion was reduced to 55.9 % of that in
mxcmbn1 larvae without the depletion (
Figure 3). This difference was significant (
p < 0.05, one-way analysis of variance (ANOVA) with Bonferroni correction).
Quantification of mRNA levels of TotF in the FB of mature third-instar larvae by qRT-PCR. X-axis from left to right: normal control (w/Y), hemocyte-specific expression of dsRNA against GFP mRNA in mxcmbn1 mutant larvae (mxcmbn1/Y;He>GFPRNAi), mxcmbn1 with hemocyte-specific depletion of upd3 (mxcmbn1/Y;He>upd3RNAi); Y-axis shows mRNA levels of the target gene (TotF) relative to the endogenous control gene (Rp49). (One-way ANOVA with Bonferroni correction. *p < 0.05, ***p < 0.001 n = 3). Bars indicate mean mRNA levels of the target gene (mean of three quantification data) and error bars indicate the SEM.
We further investigated whether
upd3 depletion in hemocytes influences the hyperplasia of LG tumors in
mxcmbn1 larvae. The average LG sizes of the normal control,
mxcmbn1 larvae (
mxcmbn1/Y;He>GFPRNAi), and
mxcmbn1 larvae harboring the
upd3 depletion (
mxcmbn1/Y;He>upd3RNAi) were 0.04 mm
2 (n = 20), 0.36 mm
2 (n = 20), and 0.53 mm
2 (n = 17), respectively (
Figure S1d). The LG size of
mxcmbn1 with
upd3 depletion (
mxcmbn1/Y;He>upd3RNAi) was more than 1.4-fold higher than that of
mxcmbn1 (
mxcmbn1/Y;He>GFPRNAi). This difference was significant (
p < 0.0001, one-way ANOVA with Bonferroni correction).
2.3. Hyper-Activation of JNK in Normal Hemocytes Transplanted into mxcmbn1 Larvae and Its Role in Inducing TotF Expression and Suppressing Tumor Growth
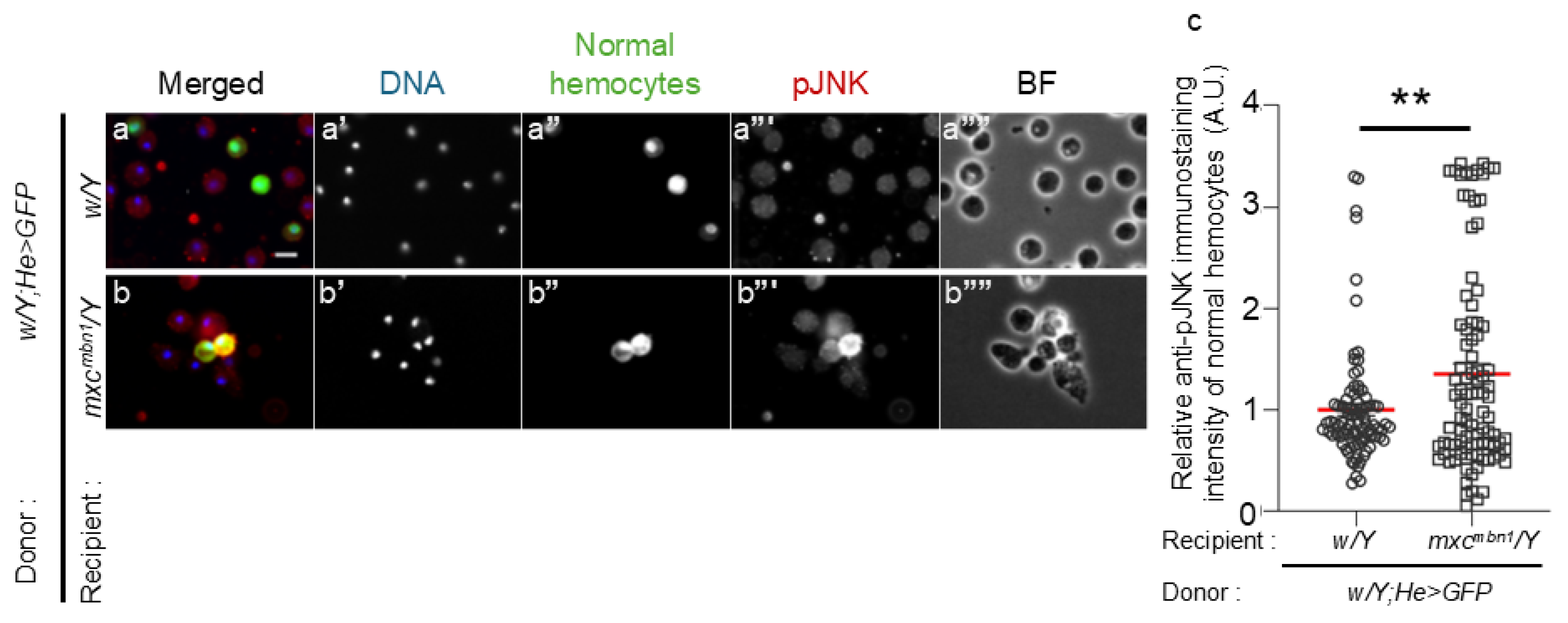
We next investigated whether the JNK pathway was activated in normal hemocytes transplanted into
mxcmbn1 larvae. 15 h after the transplantation of the normal hemocytes (1.2 × 10
4 cells) (
w/Y;He>GFP) into normal larvae (
w/Y), we detected only a few immunostaining signals with the anti-phosphorylated JNK antibody over the background level in GFP+ transplanted cells (n = 88 cells examined) (
Figure 4a’’’). In contrast, we observed a strong immunostaining signal in GFP+ normal hemocytes in the hemolymph of
mxcmbn1 larvae (n = 96 cells) (
Figure 4b”’). The differences in the signal intensity corresponding to the extent of the JNK activation between the control and mutant larvae were significant (
Figure 4c). Moreover, MMP1, another downstream target of the JNK pathway, was also induced in normal hemocytes transplanted into mutant larvae (
Figure S2).
(a, b) Fluorescence images of circulating hemocytes transplanted from normal larvae (w/Y;He>GFP) in normal (w/Y) (a) and mxcmbn1 larvae (mxcmbn1/Y) (b). Scale bar: 10 µm; blue, DNA (white in a’, b’); green, transplanted hemocytes (white in a”, b”); red, the hemocytes harboring the activated JNK (pJNK) (white in a”’, b”’). BF, Brightfield microscopy image in a””, b””. (c) The relative fluorescence intensity of anti-pJNK immunostaining. The fluorescence intensity of each transplanted hemocyte was quantified and displayed on the y-axis relative to the fluorescence intensity of the normal control set at 1. X-axis from left to right: normal control (w/Y) (n = 88), mxcmbn1 (mxcmbn1/Y) (n = 96) larvae, The average fluorescence intensity is shown as a red line. (Welch's t test, **p < 0.01). Error bars indicate the SEM.
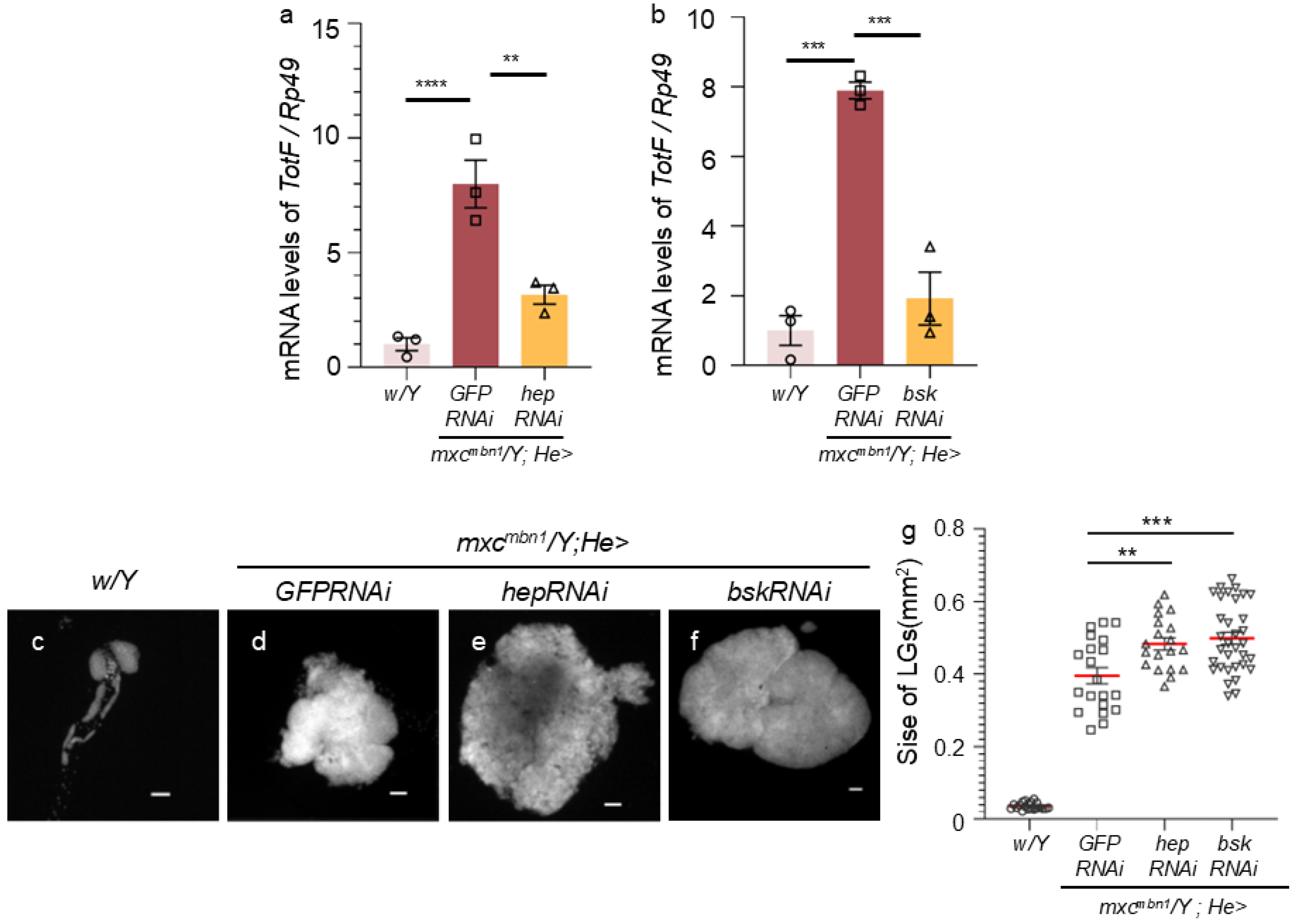
As the JNK pathway can induce the expression of Upd3 during development [
26], we next investigated whether the induction of
TotF expression in the FB depended on the JNK pathway in the hemocytes of
mxcmbn1 larvae. First, we examined whether the JNK pathway was activated to induce
TotF expression in the FB of normal control larvae (
w/Y) and
mxcmbn1 larvae expressing control dsRNA against
GFP mRNA (
mxcmbn1/Y;He>GFPRNAi), the mutant larvae harboring the hemocyte-specific
hep depletion (
mxcmbn1/Y;He>hepRNAi) and those harboring
bsk depletion (
mxcmbn1/Y;He>bskRNAi). The
TotF mRNA levels in
mxcmbn1 with the hemocyte-specific depletion of
hep and
bsk were reduced to an average of 39.6% and 24.3% of those in
mxcmbn1, respectively (
Figure 5a, b). This difference was significant (
p < 0.01,
Figure 5a;
p < 0.001,
Figure 5b; one-way ANOVA with Bonferroni correction).
We further investigated whether the depletion of JNK in the hemocytes of
mxcmbn1 larvae enhanced the hyperplasia of LG tumors (
Figure 5c-g). The average LG size of normal control (
w/Y) and
mxcmbn1 (
mxcmbn1/Y; He>GFPRNAi) larvae at the third instar stage was 0.04 mm
2 (n = 20) and 0.39 mm
2 (n = 20), respectively. The LGs of
mxcmbn1 were more than 12-fold higher on average than those of the controls (
Figure 5g). LG sizes further increased in
mxcmbn1 larvae harboring the hemocyte-specific depletion
hep (
mxcmbn1/Y; He>hepRNAi) and those harboring the depletion of
bsk (
mxcmbn1/Y; He>bskRNAi) to 0.48 mm
2 (n = 19) and 0.50 mm
2 (n = 33), respectively (
Figure 5e, f). These differences from LG sizes of
mxcmbn1 larvae without these depletions were significant (
p < 0.01,
p < 0.001, one-way ANOVA with Bonferroni correction) (
Figure 5g).
(a, b) Quantification of TotF mRNA levels in the FB of mature third instar larvae by qRT-PCR. X axis from left to right: normal control (w/Y), mxcmbn1 expressing control dsRNA against GFPdsRNAs specifically in hemocytes (mxcmbn1/Y; He>GFPRNAi), and hemocyte-specific depletion of JNKK (hep) in mxcmbn1 (mxcmbn1/Y;He>hepRNAi) (a) or hemocyte-specific depletion of JNK (bsk) in mxcmbn1 (mxcmbn1/Y;He>bskRNAi) (b). The y-axis shows the mRNA levels of the target gene (TotF) relative to the endogenous control gene (Rp49). One-way ANOVA with Bonferroni correction, **p < 0.01, ***p < 0.001, ****p < 0.0001 n = 3. Bars indicate relative mRNA levels of the target gene (mean of three quantification data), and error bars indicate the SEM. (c-f) DAPI-stained images of LGs from mature third instar larvae. (c) normal control (w/Y); (d) mxcmbn1 mutant control (mxcmbn1/Y;He>GFPRNAi); (e) mxcmbn1 mutant larvae with hemocyte-specific hep depletion (mxcmbn1/Y;He>hepRNAi); (f) mxcmbn1 mutant larvae with hemocyte-specific bsk depletion (mxcmbn1/Y;He>bskRNAi) Scale bar is 100 µm. (g) Quantification of the LG sizes. From left to right: normal control (w/Y), mxcmbn1 expressing hemocyte-specific dsRNA against GFP mRNA (mxcmbn1/Y;He>GFPRNAi), mxcmbn1 harboring hemocyte-specific hep depletion (mxcmbn1/Y;He>hepRNAi) and mxcmbn1 harboring hemocyte-specific bsk depletion (mxcmbn1/Y;He>bskRNAi). One-way ANOVA with Bonferroni correction, **p < 0.01, ***p < 0.001, n = 20 (w/Y), n = 20 (mxcmbn1/Y;He>GFPRNAi), n = 19 (mxcmbn1/Y;He>hepRNAi), n = 33 (mxcmbn1/Y;He>bskRNAi). Red lines indicate means and error bars indicate the SEM.
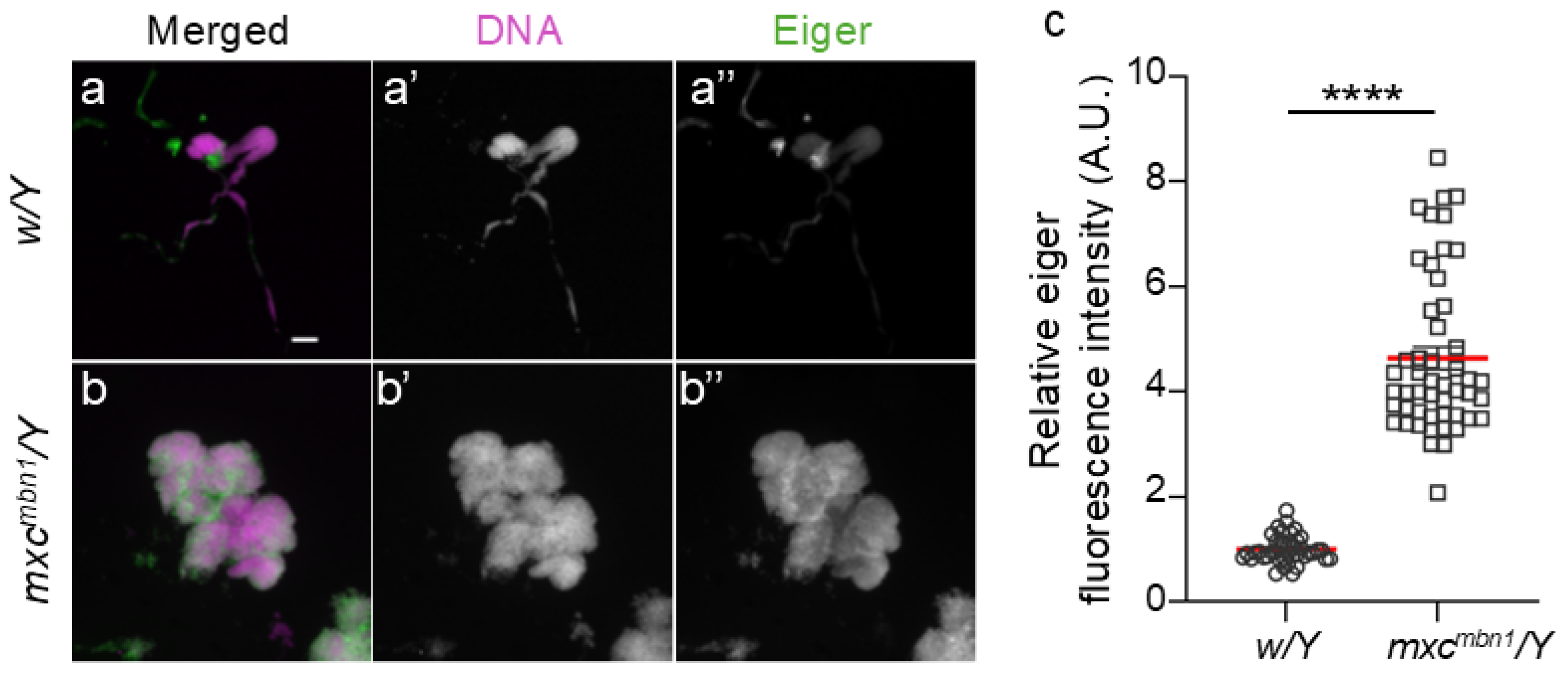
2.4. Ectopic Expression of Eiger, a TNF Superfamily Ligand, in LG Tumors of mxcmbn1 Mutant Larvae
To understand the mechanism by which the activation of the JNK pathway occurs in hemocytes, leading to
TotF induction in the FB, we investigated whether Eiger is involved in JNK activation in
mxcmbn1 larvae. We examined Eiger expression in the LG tumors of the mutant larvae by immunostaining with an anti-Eiger antibody (
Figure 6a, b). The average fluorescence intensity of whole lobe regions of LGs was calculated from immunostaining images of LGs from control (
w/Y) and
mxcmbn1 (
mxcmbn1/Y) larvae at the third-instar stage. The average fluorescence intensity was 5-fold higher in mutant LGs (n = 50) than in normal control LGs (n = 42). This difference was significant (
p <0.0001, Welch’s
t-test) (
Figure 6c). These results indicated that Eiger expression was considerably higher in LG tumors of
mxcmbn1 than in control LGs.
(a, b) Fluorescence images of the LGs from mature third instar larvae immunostained with anti-Eiger antibody. a represents normal control (w/Y) and b represents LG of mxcmbn1 larvae (mxcmbn1/Y). Scale bar is 100 µm; magenta indicates DNA staining (white in a’, b’) and green indicates antibody staining signal (white in a”, b”). (c) Quantification of fluorescence intensity in anti-Eiger immunostaining of LG in control and mxcmbn1 larvae. Relative values are shown on the Y-axis with the mean fluorescence intensity of the normal control as 1. From left to right, the X-axis shows the fluorescence intensity in LGs of normal control (w/Y), mxcmbn1 larvae (mxcmbn1/Y). Welch's t-test, ****p < 0.0001, n = 42 (w/Y), n = 50 (mxcmbn1/Y). Error bars indicate the SEM.
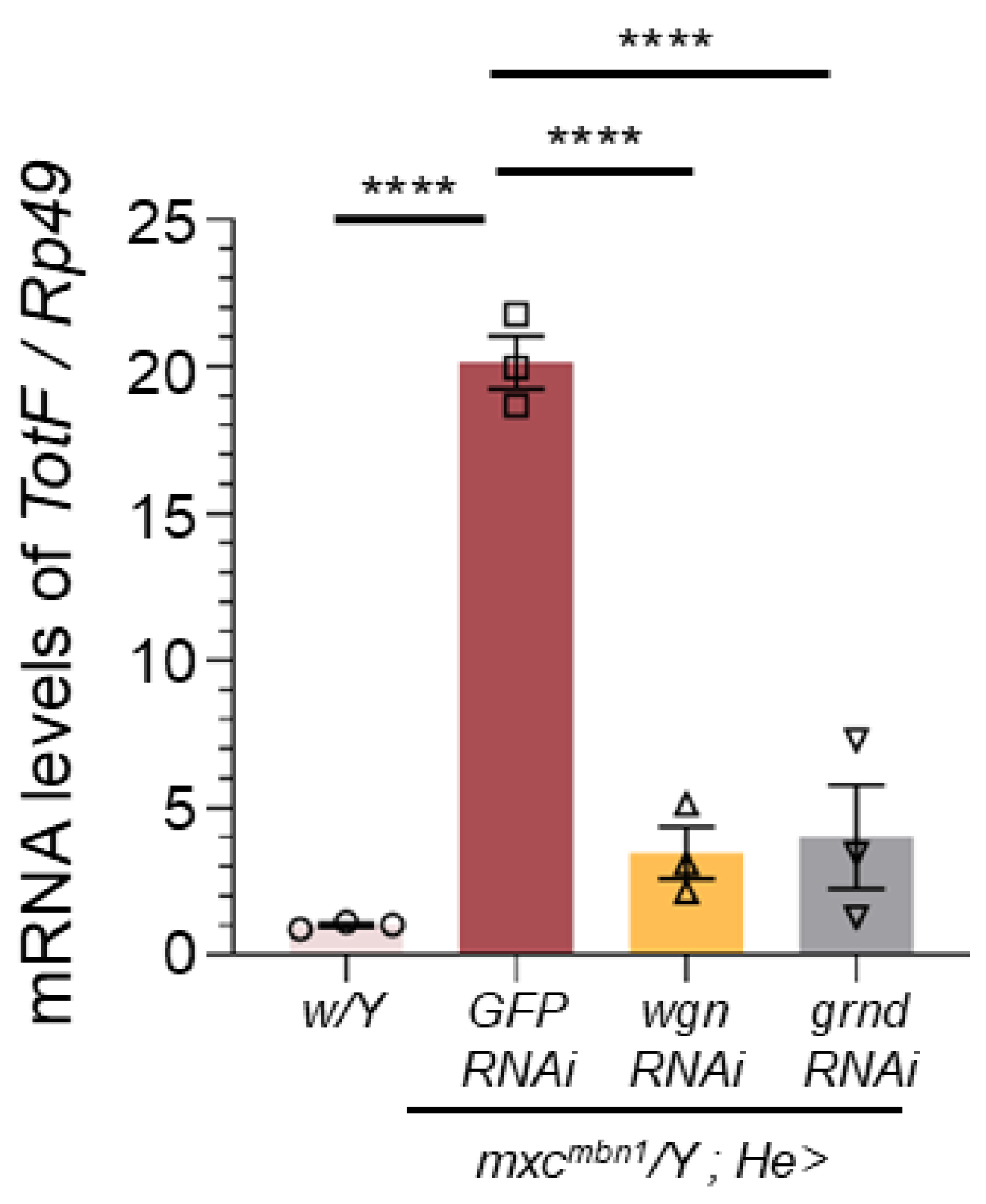
2.5. Depletion of the Eiger Receptors in Circulating Hemocytes Resulted in the Inhibition of TotF Induction in the FB and Enhancement of LG Tumor Growth in mxcmbn1
To address the mechanism by which the JNK pathway is activated in hemocytes, we investigated whether Eiger receptors on hemocytes are required for
TotF induction in the FB of mutant larvae. Eiger binds to two types of receptors encoded by
wgn and
grnd, which commonly activate the JNK pathway to induce
upd3 transcription. We quantified the
TotF mRNA levels in
mxcmbn1 larvae harboring hemocyte-specific depletion of
wgn (
mxcmbn1/Y;He>wgnRNAi) or
grnd (
mxcmbn1/Y;He>grndRNAi). We performed qRT-PCR using total RNA prepared from normal,
mxcmbn1, and mutant larvae harboring these depletions. The hemocyte-specific depletion of
wgn and
grnd in
mxcmbn1 resulted in reduced mRNA levels of 17.1% and 20.0% of those in
mxcmbn1 larvae, respectively (
Figure 7). These differences were significant (
p <0.0001, one-way ANOVA with Bonferroni correction).
Quantification of the TotF mRNA levels in the FB of mature third instar larvae with circulating hemocytes harboring depletion of receptor components (Wgn and Grnd) by qRT-PCR. X-axis from left to right: normal control (w/Y), mxcmbn1 larvae hemocyte-specific expression of dsRNA against GFP mRNA (mxcmbn1/Y;He>GFPRNAi), and mxcmbn1 larvae with hemocyte-specific depletion of wgn (mxcmbn1/Y;He>wgnRNAi), mxcmbn1 with the depletion of grnd (mxcmbn1/Y;He>grndRNAi). The y-axis shows the mRNA levels of TotF relative to the endogenous control gene (Rp49). Bars indicate relative mRNA levels of the target gene (mean of triplicate quantification data), and error bars indicate the SEM. ****p <0.0001, One-way ANOVA with Bonferroni correction.
We quantified the LG size in normal (
w/Y),
mxcmbn1 larvae (
mxcmbn1/Y;He>GFPRNAi), and those harboring depletion of
wgn or
grnd genes encoding one of the Eiger receptors (
Figure S3a-e). The average LG size of normal and
mxcmbn1 larvae was 0.04 mm
2 (n = 20) and 0.39 mm
2 (n = 20), respectively. In contrast, the average LG sizes of
mxcmbn1 larvae harboring the hemocyte-specific depletion of
wgn and those harboring the
grnd depletion were 0.51 mm
2 (n = 19) and 0.45 mm
2 (n = 42), respectively (
Figure S3f). Their LG sizes were 1.3- and 1.1-fold larger than those of
mxcmbn1 without RNAi, respectively. These differences were significant (
p < 0.001 and
p < 0.05, one-way ANOVA with Bonferroni correction).
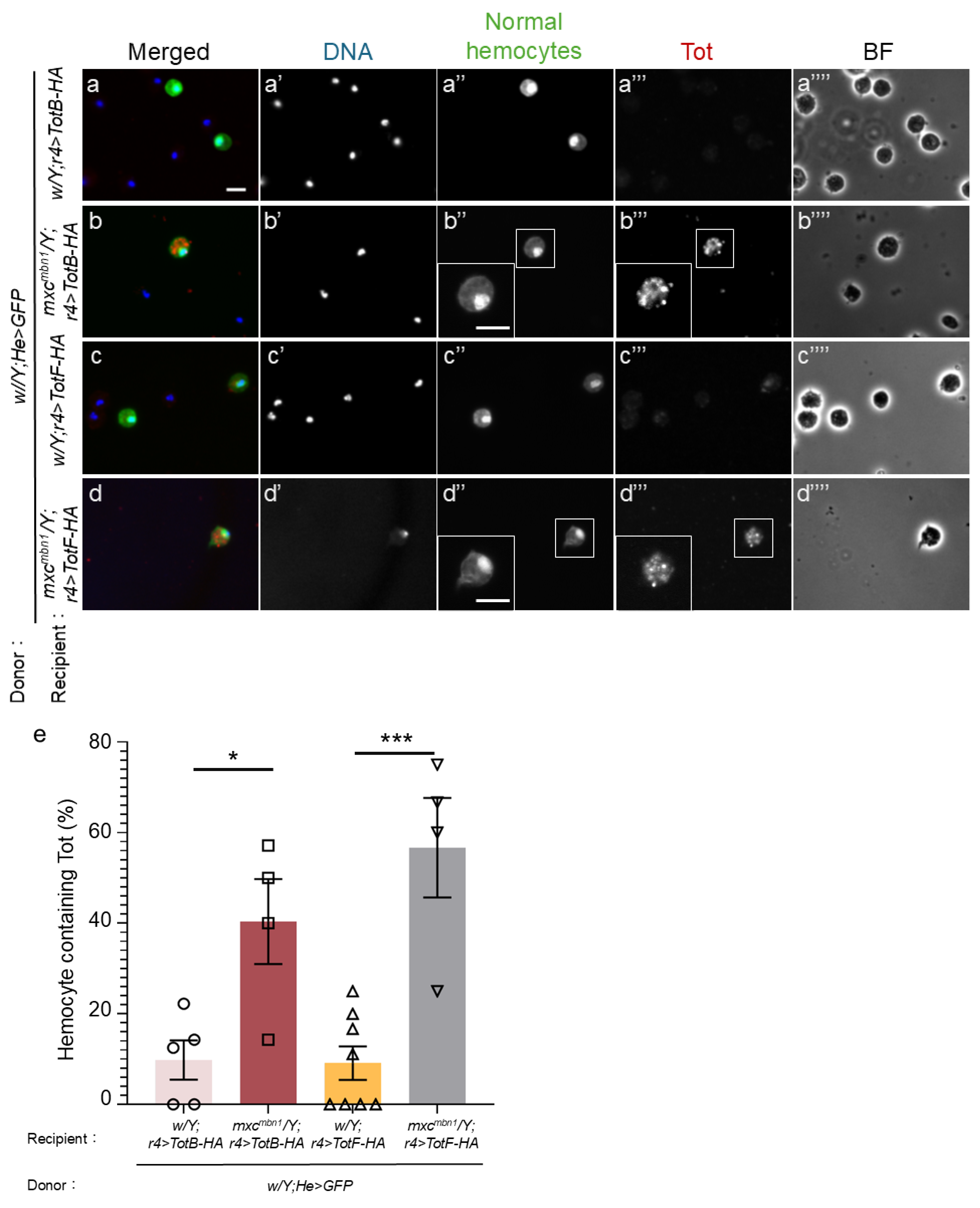
2.6. TotB and F Proteins Induced in the FB were Incorporated into Transplanted Normal Hemocytes in mxcmbn1 Larvae But Not in Those in Control Larvae
The Tot proteins produced in the FB have an antitumor effect on LG tumors in
mxcmbn1 larvae [
13]. Thus, we next investigated the mechanisms by which Tot proteins exert tumor-specific effects. TotB and TotF proteins are incorporated into circulating hemocytes in mutant larvae. To exclude the possibility that these phenotypes may reflect the characteristics of mutant tumor cells, we confirmed whether normal hemocytes transplanted from control larvae incorporated these Tot proteins. We transplanted normal circulating hemocytes (1.2 × 10
4 hemocytes on average) expressing GFP from control larvae (
w/Y;He>GFP) into mutant larvae in which HA-tagged TotB (
mxcmbn1/Y;r4>TotB-HA) or TotF (
mxcmbn1/Y;r4>TotF-HA) were expressed in their FB. 15 h after transplantation, we performed anti-HA immunostaining of circulating hemocytes in
mxcmbn1 larvae. We observed a distinctive immunostaining signal for TotB in the cytoplasm of 40.4% of GFP+ hemocytes (
w/Y;He>GFP) in
mxcmbn1 larvae (n = 9 cells out of 23 cells) (
Figure 8b’’, 8e), whereas we found fewer hemocytes showing TotB signal, whose intensity was slightly above the background level (9.8% of GFP+ hemocytes) in control larvae (
w/Y; r4>TotB-3HA) (n = 39 cells) (
Figure 8a’’, 8e). The difference was statistically significant (
p < 0.05,
Figure 8e). Consistently, 56.7% of circulating hemocytes in
mxcmbn1 larvae showed a distinctive anti-HA immunostaining signal for TotF (n = 9 cells out of 16 cells) (
Figure 8d’’, 8e). The difference was statistically significant (
p < 0.001,
Figure 8e).
(a-d) Anti-HA immunostaining of circulating hemocytes to detect HA-tagged TotB (a, b) and TotF (c, d), induced in the FB in normal control larvae (w/Y; r4>TotB-HA) (a), (w/Y; r4>TotF-HA) (c), and mxcmbn1 larvae (mxcmbn1/Y; r4>TotB-HA) (b), and (mxcmbn1/Y; r4>TotF-HA) (d). The same hemolymph volume containing circulating hemocytes from control larvae (w; He>GFP) was transplanted into the mutant larvae. The transplanted normal hemocytes labeled by GFP fluorescence are colored in green in a-d (white in (a”–d”). Anti-HA immunostaining signal of the hemocytes is in red in a–d (white in (a’’’–d’’’). DNA is blue in a–d (white in a’–d’). Magnified images of the hemocytes are presented in the insets in b”, b”’, and d”, d”’. All scale bars represent 10 μm. (e) Percentages of normal hemocytes containing TotB or TotF proteins induced in the FB in control and mxcmbn1 mutant larvae. X-axis from left to right: normal control larvae in which HA-tagged TotB was ectopically expressed in the FB of w/Y; r4>HA-TotB, mxcmbn1 /Y; r4>HA-TotB, w/Y; r4>HA-TotF, and mxcmbn1 /Y; r4>HA-TotF larvae. Bars indicate the average frequencies of GFP+ hemocytes containing TotB or TotF, and error bars indicate SEM. *p <0.05, ***p <0.001, One-way ANOVA with Bonferroni correction.
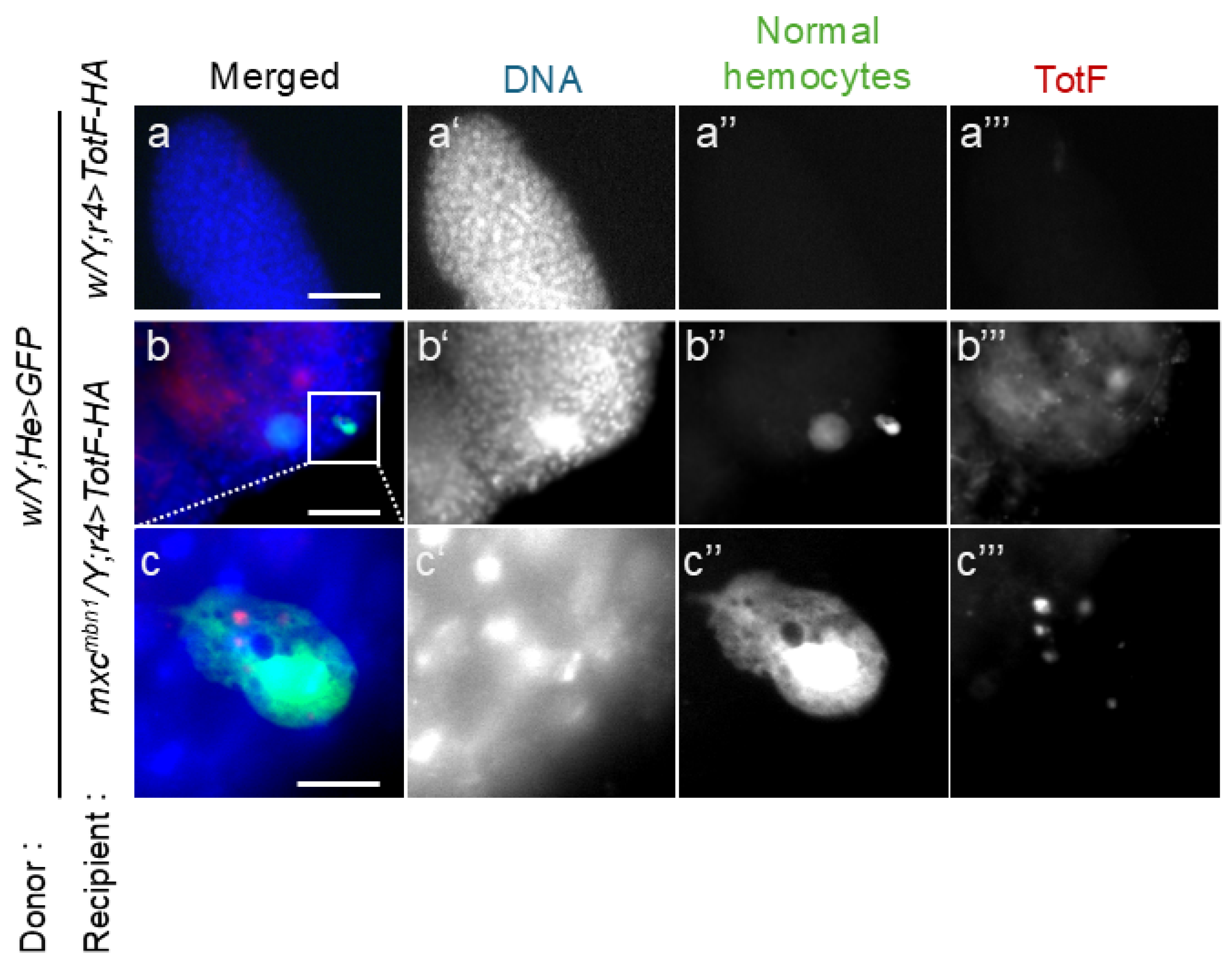
2.7. Normal Hemocytes Containing TotF were Closely Associated with the LGs in mxcmbn1 Larvae But Not in Normal Larvae
To clarify the hypothesis that hemocytes incorporating Tot proteins secreted from the FB would be recruited and release the antitumor proteins toward the LG tumor, we investigated whether TotF was incorporated into transplanted normal hemocytes and associated with the LG tumor in
mxcmbn1 larvae. We transplanted normal hemocytes (1.4 × 10
4 cells of
w/Y;He>GFP) in mutant larvae expressing either of the HA-tagged Tot proteins in their FB
(mxcmbn1/Y;r4>TotB-HA, or
mxcmbn1/Y;
r4>TotF-HA). 15 h after transplantation, we collected the mutant LGs and examined whether the hemocytes closely associated with LG tumors contained Tots by anti-HA immunostaining. Among transplanted normal hemocytes, 22.2 % of the GFP+ hemocytes contained Tot proteins in the cytoplasm (
Figure 9b’’’, c’’’) . In contrast, we did not find any hemocytes containing Tot proteins on the LGs in the control larvae after transplanting the same hemolymph volume from the control larvae (
Figure 9a’’’).
(a-c) Anti-HA immunostaining of normal circulating hemocytes expressing GFP transplanted from normal larvae (w/Y;He>GFP) on the LG lobes to detect HA-tagged TotF in control (a) and mxcmbn1 (b, c) larvae. A transplanted hemocyte enclosed by a square in (b) is magnified in (c ). The transplanted hemocytes are in green in a-c (white in a”–c”). Anti-HA immunostaining signal (Tot) corresponding the TotF signal is in red in a-c (white in a’’’-c’’’). The PC cells have a property that incorporates the Tot-HA proteins. DNA is colored in blue in a-d (white in (a’-c’)). The weak blue signal around the hemocyte in c represents DNA staining of the LG cells on which the hemocyte was localized. Scale bars in a, c and b represent 50 μm and 10 μm, respectively.
3. Discussion
In
mxcmbn1 mutant larvae, the hemocyte precursor cells in the LG show malignant tumor phenotypes. In response to the tumor, the JAK/STAT pathway is activated in the FB, thereby inducing Tot family proteins with antitumor activity. However, the mechanism by which this induction occurs remained unclear prior to this study. We found that many hemocytes were recruited to the LG tumor and FB in the mutant larvae. Transplanting normal hemocytes into mutant larvae also resulted in efficient recruitment. Thus, we hypothesized that hemocytes recruited to the FB convey tumor information from the LG tumor. The induction of TotA, B, C, and F in mutant FB depends on the activation of the JAK/STAT pathway [
13]. We addressed the mechanism of pathway activation in the FB separated from the LG tumor. Upd3, a functional ortholog of the mammalian IL-6 cytokine [
27], was ectopically expressed in the hemocytes of
mxcmbn1 larvae. Depletion of
upd3 in hemocytes decreased
TotF mRNA levels in the FB of mutant larvae, which eventually resulted in the enhancement of LG tumor growth. These genetic data indicate that the ectopic
upd3 expression in hemocytes is required for
TotF induction in FB and the LG tumor suppression. Second, we examined the mechanism by which
upd3 was induced in hemocytes. Eiger, which corresponds to the mammalian TNF cytokines [
28], was highly expressed in LG tumors. We depleted mRNAs encoding Eiger receptors and signaling factors that constitute the JNK pathway in hemocytes. In every case,
TotF mRNA levels in the FB were reduced and tumor growth was increased, indicating that the activation of the JNK pathway in hemocytes is required to suppress LG tumor in
mxcmbn1 larvae. Third, we also examined how Tots selectively exhibit antitumor effects. TotB and TotF were incorporated into circulating hemocytes, and the cells containing the Tots were recruited to the tumors.
3.1. Signal Transfer from the LG Tumor toward the FB via Circulating Hemocytes Expressing Upd3
In
mxcmbn1 larvae carrying the LG tumor, the JAK/STAT pathway in the FB is activated to induce tumor suppressor
Tot genes [
13]. However, to activate the pathway in FB distant from the tumor, information relevant to the tumor must be conveyed to the FB in which antitumor proteins are produced. Transplanted normal hemocytes are efficiently recruited to LG tumors in
mxcmbn1 larvae [
9]. Moreover, the present study showed that many mutant and normal hemocytes were recruited to the FB of
mxcmbn1 larvae. The binding of Upd3 to its receptor domain activates the JAK/STAT signaling pathway [
11]. Based on these findings, hemocytes on the tumor may have been recruited to the FB through the hemolymph after tumor recognition. Therefore, the information is transmitted to the FB. Consequently, the JAK/STAT pathway may be activated in FB. Similarly, Upd3 is ectopically expressed in the larvae bearing malignant tumors related to the loss of cell polarity [
29]. These data suggest that this cytokine is involved in the activation of the innate immune pathway in the FB.
We confirmed that the ectopic expression of Upd3 in normal hemocytes transplanted into
mxcmbn1 larvae required for
TotF induction in the FB. Hemocytes may be recruited to the FB while secreting Upd3 cytokines through the hemolymph to the receptor on the FB surface. This activates the JAK/STAT pathway and promotes
TotF expression. The pro-inflammatory cytokine IL-6 is also secreted primarily by lymphocytes and macrophages, which is consistent with findings in
Drosophila [
30]. IL-6 exerts a tumor-suppression effect on cancer cells derived from mammalian breast cancer [
31]. These findings support the hypothesis that macrophage-like blood cells are involved in cancer-related signaling via cytokines, the expression of which is induced. Circulating hemocytes also accumulate on imaginal disc tumors in
Drosophila dlg mutant larvae, and the Toll-mediated innate immune pathway is activated in the FB of mutant larvae [
8]. This previous finding is consistent with our model that Upd3 in hemocytes is required to signal the FB in LG tumor-bearing larvae.
3.2. Macrophage-like Plasmatocytes May Recognize the Eiger Cytokine Secreted from LG Tumors and Convey Information on Tumor Cells to the FB
Our current data suggested that Upd3, which is ectopically induced and secreted from hemocytes, may promote the activation of the JAK/STAT pathway in the FB, whereas little Upd3 expression was observed in the normal hemocytes of tumor-free larvae. Consistently, high Upd3 expression has been reported in the hemocytes of mutants with imaginal disc tumors [
32]. Based on these findings, the Upd3 induced in hemocytes may be used as a signal to notify the FB cells of the presence of tumor cells.
We next investigated why hemocytes could recognize LG tumors. Eiger, a member of the TNF family in
Drosophila, is highly expressed in LG tumors. Furthermore, the Eiger receptor and JNK signaling factors downstream of the receptor in hemocytes are required for
TotF expression in the FB. Eiger binds to its receptors Wgn and Grnd and activates the JNK pathway [
15]. Downstream of the JNK pathway, one of the targets, Upd3, is produced [
16]. The recognition of LG tumor cells expressing Eiger via its receptors on the surface of hemocytes may promote Upd3 expression. Subsequently, these hemocytes are recruited to the FB while expressing Upd3. This induces
TotF expression in FB. JNK is activated in the hemocytes of
mxcmbn1 mutant larvae [
9,
24]. Our results confirm that the ectopic expression of Upd3 and activation of the downstream JNK pathway in hemocytes are required to induce
TotF expression in the FB. Based on these results, plasmatocytes that accept Eiger via its receptors activate the intracellular JNK pathway. This may promote the expression of Upd3, and eventually TotF, in FB.
In cultured cells derived from mammalian breast cancer, TNF-α is a potent activator of immune cells via the induction of inflammatory cytokines such as IP-10 [
33]. Increased IP-10 levels are associated with the pathology of various inflammatory disorders, including cancer. Therefore, a similar regulatory mechanism may be present in
Drosophila, in which a TNF ortholog acts as a cytokine that mediates communication between tissues. If our model is correct, the downregulation of Eiger in LG tumors would inhibit the activation of JNK in hemocytes. This must be verified in future experiments by transplanting normal hemocytes into mutant larvae. The mechanism by which hemocytes are recruited to LG tumors also remains unclear; the induction of Eiger expression may activate the JNK pathway, which in turn leads to cell death [
34]. The activation of JNK and induction of its target, Mmp1, have already been reported in LG tumors of
mxc mutant larvae, resulting in the disassembly of the basement membrane of LG cells [
9,
24]. The resulting cellular fragments may be phagocytosed by macrophage-like plasmatocytes and recognized as a sign of a tissue damage. Which chemokine(s) recruit plasmatocytes to LG tumors needs to be clarified in the future.
3.3. Possible Role of Circulating Hemocytes as a Vector to Transport Secreted Antitumor Proteins, Tots, to the LG Tumor
Many circulating hemocytes and hemocytes recruited to the LG tumor internalized TotF secreted from the FB. This suggests that hemocytes act as a vector that allows Tot proteins to be selectively supplied to the tumor. Normal hemocytes transplanted into mutant larvae are also recruited to LG tumors [
9] and FB (this study). TotF and TotB proteins are incorporated into the cytoplasm of circulating hemocytes and immunostaining signals for Tots are present in small vesicles in the cytoplasm of hemocytes [
13]. Other AMPs, such as Drosomycin and Defensin, also exhibit antitumor activity by being incorporated into hemocytes and recruited to the LG tumor [
7]. Mammalian tumor-associated macrophages (TAMs) facilitate disease progression by promoting tumor cell growth and suppressing adaptive immune responses [
35]. The extent of TAM recruitment to tumors tends to correlate with poor disease prognosis [
36]. Some TAMs secrete cytokines such as globule-EGF factor 8 and IL-6, which lead to the tumorigenicity of cancer stem cells [
37]. Other mammalian cytokines are also consistently incorporated into the cytoplasm in vesicle-like forms [
38]. Similarly, Eiger induces cell death via Rac1-dependent endocytosis [
39]. Based on these observations, we speculate that
Drosophila hemocytes may also take up Tot proteins secreted from the FB, probably via endocytosis. Subsequently, hemocytes are recruited to the tumor, where they release Tots, probably via exocytosis, for example, as observed in cytotoxic granule discharge from natural killer cells and T lymphocytes [
40]. Alternatively,
Drosophila plasmatocytes, which are equivalent to mammalian macrophages, have phagocytic functions, which may be involved in the incorporation of Tots. In the near future, identifying the factors involved in Tot dynamics will be necessary. Additionally, the mechanisms underlying the uptake and release of Tot antitumor proteins need to be clarified.
4. Materials and Methods
4.1. Drosophila Stocks
w1118, abbreviated as
w, was used as a normal control stock . The recessive lethal allele of
mxc,
mxcmbn1 (#6360, Bloomington Drosophila Stock Center (BDSC, Indiana University, Bloomington, USA) was used [
7,
22]. As the
mxc is a X-linked gene, the mutant male (
mxcmbn1/Y) and control male (
w/Y) were used for experiments. For the depletion of JNK factors and several other proteins, we used the following
UAS-RNAi stocks;
P{w+mC=UAS-GFP.dsRNA.R}142 (
UAS-GFPRNAi) (BDSC, #BL9330) [
13],
P{KK110348}VIE-260B (
UAS-upd3RNAi) (VDRC, #v106869) [
41],
P{y+t7.7 v+t1.8=TRiP.HMC03962}attP40 (
UAS-wgnRNAi) (BDSC, #BL55275) [
42],
P{KK109939}VIE-260B (
UAS-grndRNAi) (VDRC, #104538) [
43],
P{TRiP.HMC03539}attP2 (
UAS-bskRNAi) (#53310) [
44], and
P{y+t7.7 v+t1.8=TRiP.GL00089}attP2 (
UAS-hepRNAi) (BDSC, #BL35210) [
45].
P{w[+mC]=upd3-lacZ.Z}5F (
upd3-LacZ) (BDSC, #BL-98418) was used to monitor the
upd3 gene expression. To induce expression of HA-tagged TotB and TotF, M{UAS-TotB.ORF.3xHA.GW}ZH-86Fb (FlyORF, Zurich, Switzerland, #002780)) and M{UAS-TotF.ORF.3 xHA.GW}ZH-86Fb (FlyORF, #00351) were used [
13].Following the GAL4-driver stocks were used;
P{w+mC=He-Gal4.Z}(BDSC, #BL8699) for induction of gene expression in circulating hemocytes [
23],
P{upd3-GAL4} (provided by N. Perrimon, Harvard Medical School) to induce gene expression in the medulla zone of the LG [
10],
P{w+mC=r4-GAL4}3(BDSC, #BL33832) for FB (FB)-specific gene expression [
7,
13]. All
Drosophila stocks were maintained on standard cornmeal food at 25℃ as previously described [
7,
46]. For an efficient induction of GAL4-dependent gene expression, individuals carrying the
GAL4 driver gene and
UAS transgenes were raised at 28℃. The fly food was prepared according to a previous procedure [
46].
4.2. Sample Preparation and Immunostaining of LGs in Larvae
As
mxcmbn1 was maintained in the stock balanced by
FM7a, P{w[+mC]=sChFP}1 carrying the marker gene RFP, mature larvae hemizygous for mxcmbn1 at the third instar stage were selected based on the absence of RFP fluorescence. Normal control males (
w/Y) pupated at 6 d (28°C) and 7 d (25°C) after egg laying (AEL), whereas the
mxcmbn1 males remained in 3rd instar larval stage at 8 d (28°C) and 10 d (25°C) AEL. A comparison between the control and
mxcmbn1 larvae was performed on the same day that the wandering larvae at the third-instar stage appeared to minimize a delay that might allow hyperplastic tissue to grow. To obtain the larvae, five
pairs of flies were maintained in culture tubes and allowed to
lay eggs for 24 h on food. To compare the sizes of the LGs, a pair of anterior lobes of the LG from mature larvae at the third-instar stage was collected and fixed in paraformaldehyde. After staining with DAPI solution (#5748, FUJIFILM Wako Pure Chemical, Osaka, Japan), the fixed LG samples embedded in Vector Shield (#H-1000, Vector Laboratories, Newark, CA, USA) were gently flattened under a covered glass to prepare specimens of a constant thickness [
7]. Specimens were observed under a fluorescence microscope (I×81; Olympus Co., Tokyo, Japan) equipped with a CCD camera (ORCA-R2; Hamamatsu Photonics, Hamamatsu, Japan). MetaMorph (version 7.8 13.0, Molecular Devices Inc., San Jose, CA, USA) was used for image processing. The lobe areas of all LGs were measured from the fluorescence images of DAPI-stained samples using ImageJ (version 1.47, National Institutes of Health, Bethesda, MD, USA).
After the fixed LGs were blocked in 10% NGS in PBST (PBS supplemented with 0.1% Triton X-100), the specimens were incubated with anti-Eiger antibody ([
25], a gift from M. Miura, Tokyo University) overnight at 4°C. Alexa Fluor 488-conjugated anti-rabbit and anti-mouse IgG antibodies (#A11008 and #A1100, Thermo Fisher Scientific, Oregon, USA) were used to detect the primary antibody. Fluorescence images of the immunostained samples were acquired as described above.
4.3. Preparation of Hemocytes in Drosophila Larval Hemolymph and Immunostaining of the Hemocytes
Mature third instar larvae were dissected in a Drosophila Ringer solution (3 mM CaCl2-2H2O, 182 mM KCl, 46 mM NaCl, 10 mM Tris-base) containing 0.02 µg/mL 1-phenyl-2-thioureic acid (#166-13702, FUJIFILM Wako Pure Chemical, Osaka, Japan). The cells in the hemolymph were allowed to adhere to a glass slide and fixed in 4% paraformaldehyde solution. After staining with DAPI, the specimens were observed under a fluorescence microscope controlled by MetaMorph (version 7.8 13.0; Molecular Devices Inc., San Jose, CA, USA), as described above.
Hemocytes collected and fixed as described above were blocked with 10% NGS in PBS and incubated with anti-JNK/SAPK antibody (#559304, Merck, Darmstadt, Germany) to detect phosphorylated JNK expression, anti-β-galactosidase antibody (#02150039, MP Biomedicals, Irvine, CA, USA) to monitor the LacZ reporter expression, or anti-Mmp1 antibody (3A6B4, 3B8D12 and 5H7B11, DSHB, IA, USA) [
47]overnight at 4°C. To detect the HA-TotF incorporated in the hemocytes localized on the LGs, anti-HA antibody (#2367, Cell Signaling Technology, Danvers, MA, USA) was used. To detect each primary antibody that reacted with the samples, Alexa Fluor 488- or 594-conjugated anti-rabbit or anti-mouse IgG antibodies (#A11008, Thermo Fisher, Oregon, USA) were used according to the animal species in which the primary antibodies were created. Fluorescence images of the samples were acquired as previously described.
To detect the HA-TotF protein incorporated in the hemocytes closely associated with the LGs, the tissues were collected and rinsed with PBS several times before fixation. The LGs, together with associated hemocytes, were fixed with 3.7% paraformaldehyde and blocked with 10% NGS in PBS. Anti-HA immunostaining was performed as described above.
4.4. Transplantation of Hemocytes in Drosophila Larvae
A constant volume of larval hemolymph containing circulating hemocytes was injected into a recipient third-instar larva using glass needles, as previously described [
9]. The needles were prepared from G1.2 (Narishige Co., Tokyo, Japan) using a grass puller (PN-31, Narishige Co., Tokyo, Japan) and used after the tip was sharpened. The hemolymph was injected within 5 m of dissection of the recipient larvae to avoid melanization and clogging. After injection, the larvae were placed on wet blocking papers for 1 h to recover from the damage and raised on standard food for 15 h before observation.
4.5. Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from larval FB at the third instar stage using TRIzol reagent (Invitrogen, Waltham, MA, USA). cDNA was synthesized from total RNA using a PrimeScript High Fidelity RT-PCR Kit (TaKaRa, Clontech Laboratories, Shiga, Japan) with oligo dT primers. Real-time PCR was performed using the following qPCR primers: RP49-Fw, 5’-TTCCTGGTGCACAACGTG-3’ and RP49-Rv, 5’-TCTCCTTGCGCTTCTTGG-3’; TotF-Fw, 5’- AGGCACGTCAAATGCTCGC-3’ and TotF-Rv, 5’-TGTTGGTTGTTGTGTGCCCG-3’. The PCR reaction was carried out using a cycling program consisting of an initial denaturation at 95℃ for 5 m, followed by 40 cycles at 95℃ for 5 s and 60℃ for 30 s. The temperature was increased from 60℃ to 95℃ at a rate of 0.1℃/s. Real-time PCR was performed on a Thermal Cycler Dice® Real Time System III (TaKaRa bio., Shiga, Japan) using TB Green Premix Ex Taq II (#RR820A, TaKaRa Bio, Shiga, Japan). Each sample was analyzed in triplicate on a PCR plate, and the final results were obtained by averaging three biological replicates. For quantification, the ∆∆Ct method was used to determine the differences between target gene expression and that of the reference gene, Rp49.
4.6. Statistical Analysis
Scatter plots were created using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) or Microsoft Office Excel 2016 (Microsoft, Redmond, WA, USA) to determine the number of hemocytes in the LGs and FB. The fluorescence intensity of LGs or circulating hemocytes was quantified using ImageJ (Ver.1.54h, National Institutes of Health, Bethesda, MD, USA). Each dataset was assessed using Welch’s
t-test as previously described [
8,
23,
47]. Before then, an F-test was performed to determine equal or unequal variances. Welch’s
t-test was performed when the value was less than 0.05 (unequal variance), whereas Student's
t-test was performed when the value was greater than 0.05 (with equal variance). One-way analysis of variance (ANOVA) was used to analyze differences among groups. A
p-value of 0.05 or less was considered statistically significant.