1. Introduction
The capelin (
Mallotus villosus) is a small pelagic fish (SPF) with a circumpolar distribution in the northern hemisphere and is a principal prey item for many species of predatory fish, whales, and seabirds. Beyond its ecological significance, the capelin is also highly valued by the fishing industry in the North Atlantic region, where it is commercially exploited [
1]. Four genetically distinct regional groups have been identified within the circumpolar capelin population, i.e., the West Pacific, East Pacific, Newfoundland, and Northeast Atlantic/West Greenland regional groups [
2]. All capelin populations undertake extensive feeding-, overwintering- and spawning migrations, facilitating massive energy transfer from deep cold waters to warmer coastal ecosystems [
3].
Capelin populations in the Northeast Atlantic Ocean (Iceland and Barents Sea) are demersal spawners, except for beach spawning populations in local fjords [
3,
4]. On the other hand, capelin populations in the Northwest Atlantic are predominantly beach spawners [
5]. While females in beach spawning populations may spawn more than once during their lifetime, very few capelin in the large demersal spawning populations (e.g., Icelandic capelin and Barents Sea capelin) survive first spawning and are thus classified as semelparous [
4].
As capelin approach maturity, a noticeable size difference is observed between males and females. Generally, males exhibit faster growth and tend to be 1-3 cm longer than females at maturity [
3,
6]. The attainment of maturity in capelin is primarily determined by size, with a maturity threshold of approximately 14 cm in total length [
3,
7,
8,
9]. Capelin show significant spatial and temporal variation in growth rates, [
10,
11,
12] and rapid early growth has been shown to be strongly related to early sexual maturity [
13,
14].
The asymptotic length (
L∞) of capelin varies across different populations and locations. In the Northwest Atlantic Ocean, it is generally estimated to be around 20 cm [
12,
15,
16], while smaller
L∞ have been observed in Icelandic waters and the Barents Sea [
3,
17]. The underlying factors contributing to these variations, whether genetic or environmental, remain uncertain. Due to the high mortality rate after first spawning, capelin larger than 20 cm make up a very small fraction of the population, and specimens exceeding 22 cm in total length are only occasionally found [
18].
Research on SPF has predominantly been based on field studies, with relatively few controlled laboratory studies. The paucity of laboratory studies on SPF can be attributed to the practical challenges of rearing these species from hatch to adulthood. Laboratory studies have been performed on all life stages of Atlantic and Pacific herring (
Clupea harengus and
Clupea pallasii), providing valuable insights into their biology [
19]. There is also a growing number of laboratory studies on species such as the European sardine (
Sardina pilchardus) and European anchovy (
Engraulis encrasicolus) (e.g., [
20,
21]) and recently, the Japanese anchovy (
Engraulis japonicus) has been described as a promising species for use as a model organism for marine teleosts [
22].
Studies on captive capelin have a relatively long history, originating with the successful spawning of capelin in captivity in Russia in 1958 [
23]. Since then, research on capelin has been conducted in laboratory settings and outdoor enclosures, covering a wide range of topics. These include growth, development, feeding, behaviour, endocrinology, metabolism, spawning, responses to abiotic factors, and reactions to toxic substances [
4,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47].
These previous studies are, however, limited to the use of capelin of wild origin and have only been performed during egg- and larval stages, or with captive adults. There is currently a lack of research involving capelin reared beyond the larval stage. The longest study to date reared capelin larvae in large outdoor enclosures for four months, during which the larvae reached a length of up to 32 mm by feeding on natural zooplankton [
26]. Additionally, laboratory studies have employed traditional intensive cultivation methods, including the use of rotifers as initial feed, but these studies have been limited to the early larval stages [
34,
47].
This study presents the first successful cultivation of capelin from hatch to adulthood. The primary objectives of the study were to establish a foundation for future laboratory studies on all life stages of the capelin and present new insights into the growth dynamics of this species.
2. Materials and Methods
2.1. Egg Collection
Capelin eggs and sperm were collected from newly caught fish on board a commercial fishing vessel (Vikingur AK 100) at the height of the spawning season on March 6th, 2021. The fish were caught with a purse seine in Faxaflói, close to the southwest coast of Snæfellsnes (approximately 64.4°N, 23.4°W). A volume of about 1L of capelin eggs were manually stripped into two 5L plastic buckets. Sperm from about 50 males were then collected the same way and gently mixed with the eggs. The mixture was allowed to sit for approximately five minutes before the buckets were filled up with clean seawater. Shortly after contact with seawater the eggs hardened and glued together, forming egg clusters (2 - 3cm in diameter) on the bottom. The eggs were stored at 4 °C in a dark cooling room until the ship docked in Akranes on March 12th. During the time on-board, ~90% water changes were made daily. Upon landing, the eggs were transported to the MFRI Aquaculture Research Station (ARS), which is located on the Reykjanes peninsula near Grindavík, southwest Iceland.
2.2. Incubation
Upon arrival at the research station the glued egg clusters were broken down into to smaller pieces and disinfected with Pyceze™ (1 ml/10 L) for 30 minutes. During this process some of the eggs were freed, but most remained glued to other eggs in relatively small clusters (< 2 cm in diameter). After disinfection the small clusters and individual eggs were evenly distributed over the bottom of a 3.2 m3 circular black fiberglass tank (2 m diameter, 1 m height). The fertilization rate was highly variable among the small clusters, as contamination from rotting unfertilized eggs caused high or total mortality in some clusters.
Fertilized and unaffected eggs hatched over a one-week period from March 30th to April 6th, 2021 (25 to 31 days after fertilization). However, most of the eggs hatched synchronously on April 5th after the eggs were siphoned from the tank bottom through a plastic tube into buckets. During the few minutes stop in the buckets, many eggs were seen hatching and most of them hatched within a few hours after being returned to the tank. The remaining fertilized eggs hatched after the procedure was repeated on the next day (April 6th). As most of the eggs hatched on April 5th, this day will hereafter be considered the hatching day for the whole group.
2.3. Rearing Conditions
The capelin were reared indoors in a flow-through system. The station's seawater is obtained from 50 m deep boreholes. It is naturally filtered through lava bedrock and has a relatively stable temperature (6.7 - 7.9 °C), salinity (31 ppt) and pH (7.7), all-year-round. During the embryonic and larval stages, the waterflow was kept at 5 L/min and was gradually increased as the fish developed, so that the oxygen saturation was maintained above 90% without adding pure oxygen. During the first and second year of the study the mean temperatures were 7.4 ± 0.01 and 7.2 ± 0.01°C, respectively. The fish were always fed in moderate excess.
Three days post hatch (3 dph), live rotifers (Reed Mariculture Inc, Campbell, CA, USA) were introduced into the tank as a potential prey item for the capelin larvae. The rotifers were fed (S.parkle Selco®, INVE Ltd), enriched for 6 - 8 hours (Larviva Multigain, BioMar A/S, Aarhus, Denmark), and added to the nursery tank two times per day (9:00 and 15:30) along with 30 ml of microalgal paste (Rotigreen ® omega, Reed Mariculture Inc, Campbell, California, USA). During the first 10 dph the larvae were fed small rotifers (Brachionus rotundiformis, S-type, 85 - 150 μm), before a larger strain of rotifers (Brachionus plicatilis, L-type, ~210 μm) was introduced in a 1:1 ratio to the S-type on 11 dph. The S-type was taken off the menu at 38 dph and at the same time newly hatched artemia nauplii (Artemia salina, AF artemia, INVE Ltd, Salt Lake City, Utah, USA) were introduced as prey. The artemia feeding schedule was the same as for the rotifers. Feeding with rotifers ceased at 45 dph when weaning onto dry feed was initiated (Larviva Prostart, Biomar AS, initial particle size 200 μm). During the first week, the larvae were handfed first thing in the morning when prey levels were low. Thereafter, continuous feeding (24 hours) was implemented using an automatic belt feeder (FIAP GmbH). From 60 dph and until the weaning was completed at 99 dph, the artemia nauplii were replaced with EG artemia (INVE Ltd), enriched for ~20 h with Easy DHA Selco® (INVE Ltd).
Until 255 dph, the young capelin were kept in the 3.2 m3 circular black hatchery tank under simulated annual variation in day length. At this point, approximately a quarter of the fish (n = 267) were randomly selected and moved to a 4 m3 green rectangular fiberglass tank (measuring 2 m x 2 m x 1 m). From that day onwards these fish were reared under continuous light.
Only the fish that were transferred to the green tank on day 255 were subjected to regular measurement. This group will henceforth be referred to as group A. The remaining fish were split between three black hatchery tanks. The cultivation of the fish in one of those tanks (group B) is briefly described in section 2.4.1.
At 527 dph, the fish in group A were transferred back to a 3.2 m3 circular black hatchery tank for cultivation until the end of the study. The transfers between tanks were conducted carefully using a soft and fine-meshed fishnet (Laguna Pond Net, HAGEN group®, Montreal, Canada). After being netted, the fish were released into 20L buckets, moved a 12 m distance, and gently poured into the new tanks.
2.4. Measurements and Data Analysis
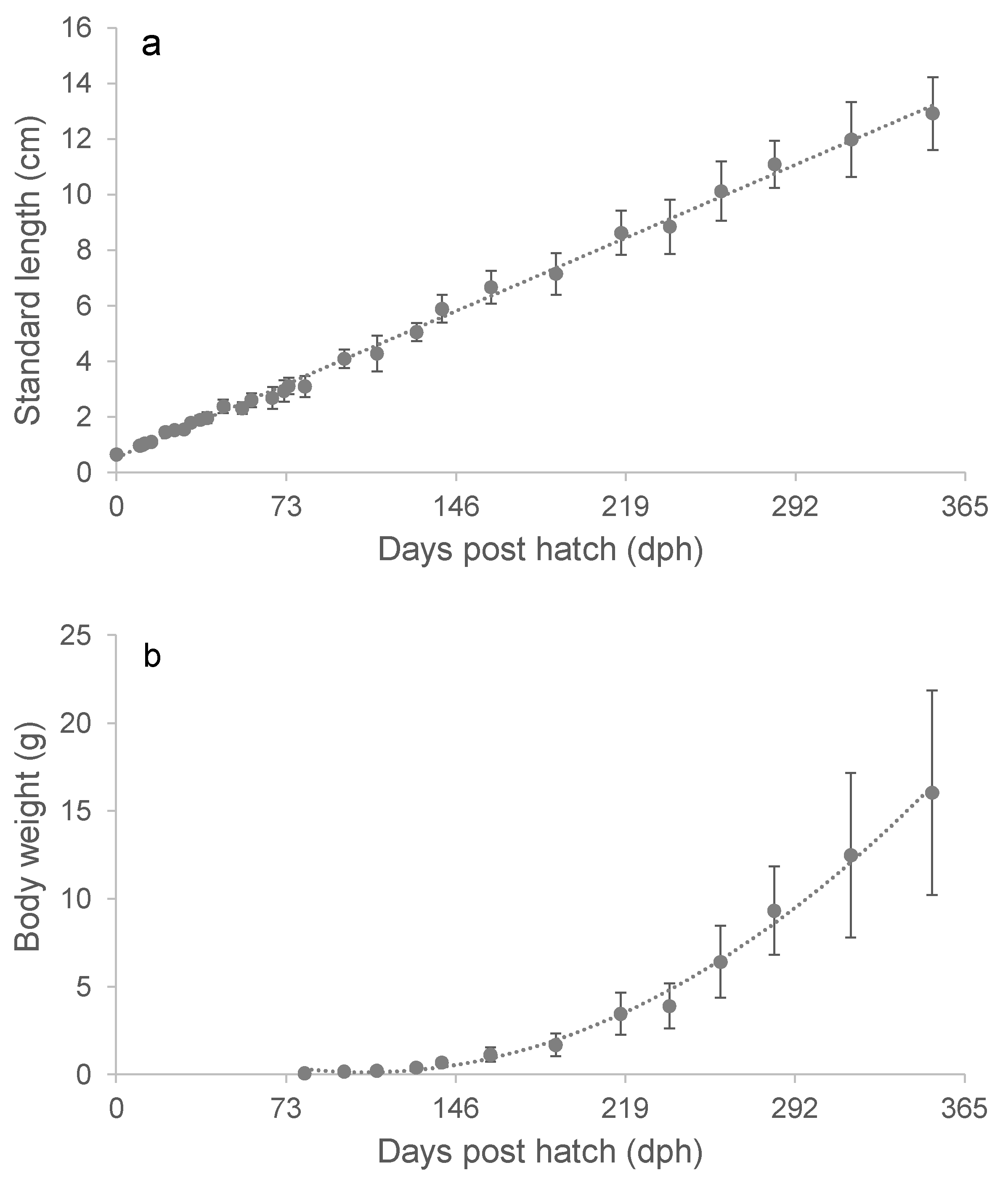
Temperature and oxygen of the rearing water were measured daily throughout the study. Dead fish were collected and counted daily from day 255 and until the end of the study. Over the course of the nearly two-year study, the mean body size of the fish in group A was estimated 34 times. A random sample of 5 - 35 fish was collected for each measurement but all the remaining fish were measured on the last day of the study. The sample sizes and intervals between measurements (1 - 100 days) increased with the size of the fish.
Up until day 504, the sex of the measured fish was not determined but in subsequent measurements, the gender was identified through visual inspection of external appearances. Based on a visual inspection, the presence of skeletal deformities was documented for each fish at the last day of the study. The deformities were broadly classified based on their location as either head or trunk deformities.
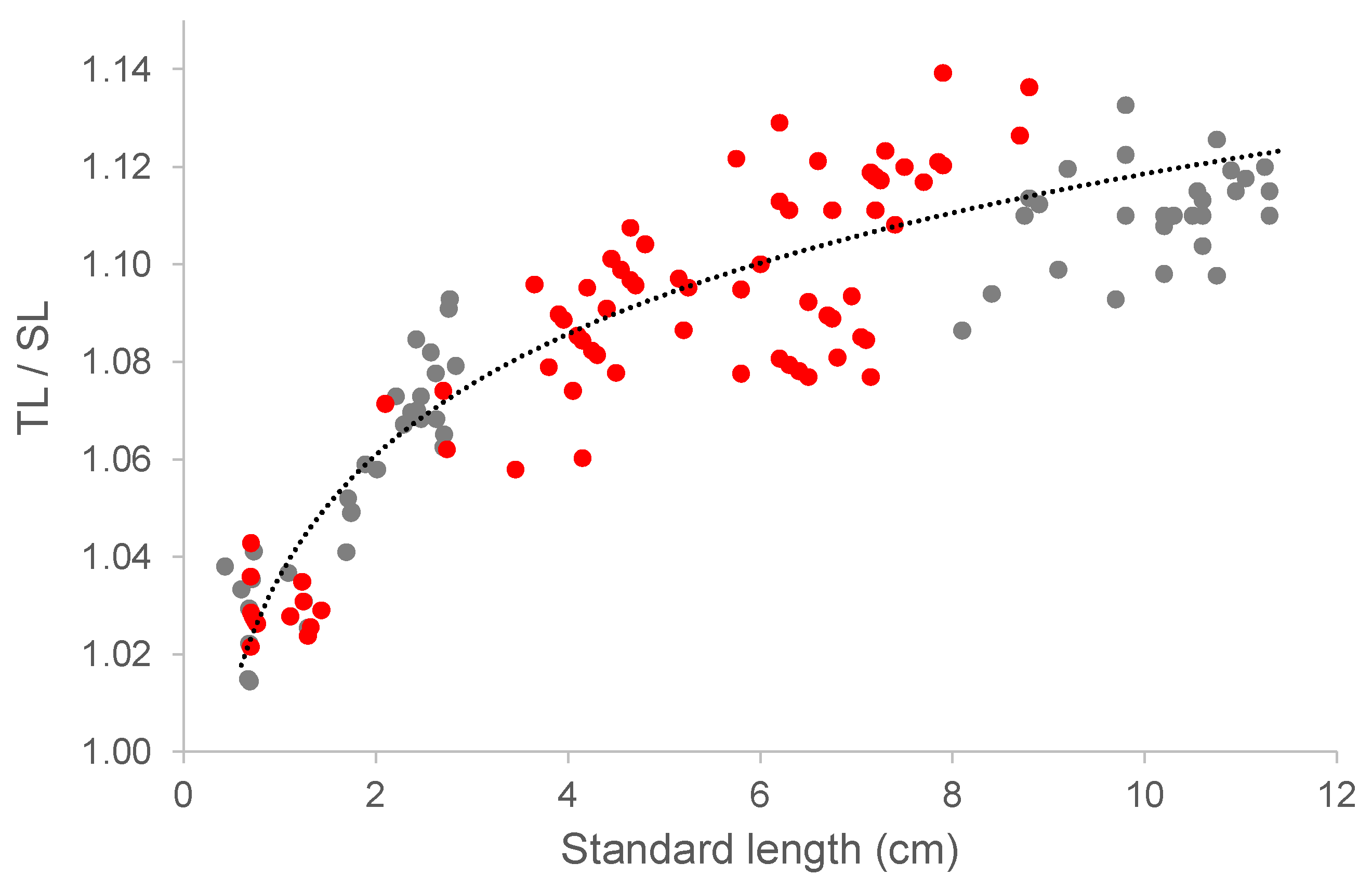
Body length was measured as standard length (SL) up to 260 dph, with only 48 larvae measured for both SL and total length (TL). After day 260 all fish were measured for both SL and TL. Before the age of 260 dph SL was converted to TL using TL
/SL ratios of larvae and juveniles measured for both SL and TL below the age of 260 dph. This was done using the following equation:
The dataset used to establish this formula was comprised of 154 fish with SL ranging from 0.68 to 9.8 cm. Of these fish, 87 fish were sourced from unpublished research conducted at the ARS (
Figure 1).
During the first 58 dph, the larval length was measured using ImageJ software and photographs taken with an iPhone 8 (Apple, Cupertino, California, USA) attached to the eyepiece of a Wild M3Z stereomicroscope (Wild Heerbrugg AG, Gais, Switzerland) using a NexYZ DX smartphone adapter kit (Celestron®, Torrance, California, USA). From 67 dph the length was visually measured to the nearest mm with a ruler, and from 81 dph, individual body weights (W) were measured to the nearest 0.01 g. Before 81 dph, the larvae were euthanized before being photographed or measured, but thereafter, all measurements were performed under tricaine methane sulfonate anesthesia (0.05 g/L, Finquel®, Intervet International B.V. Boxmeer, Netherlands) and the fish were returned to the tank after measurements. During the final measurement, all fish were humanely killed with an overdose of tricaine methane sulfonate and measured as described before.
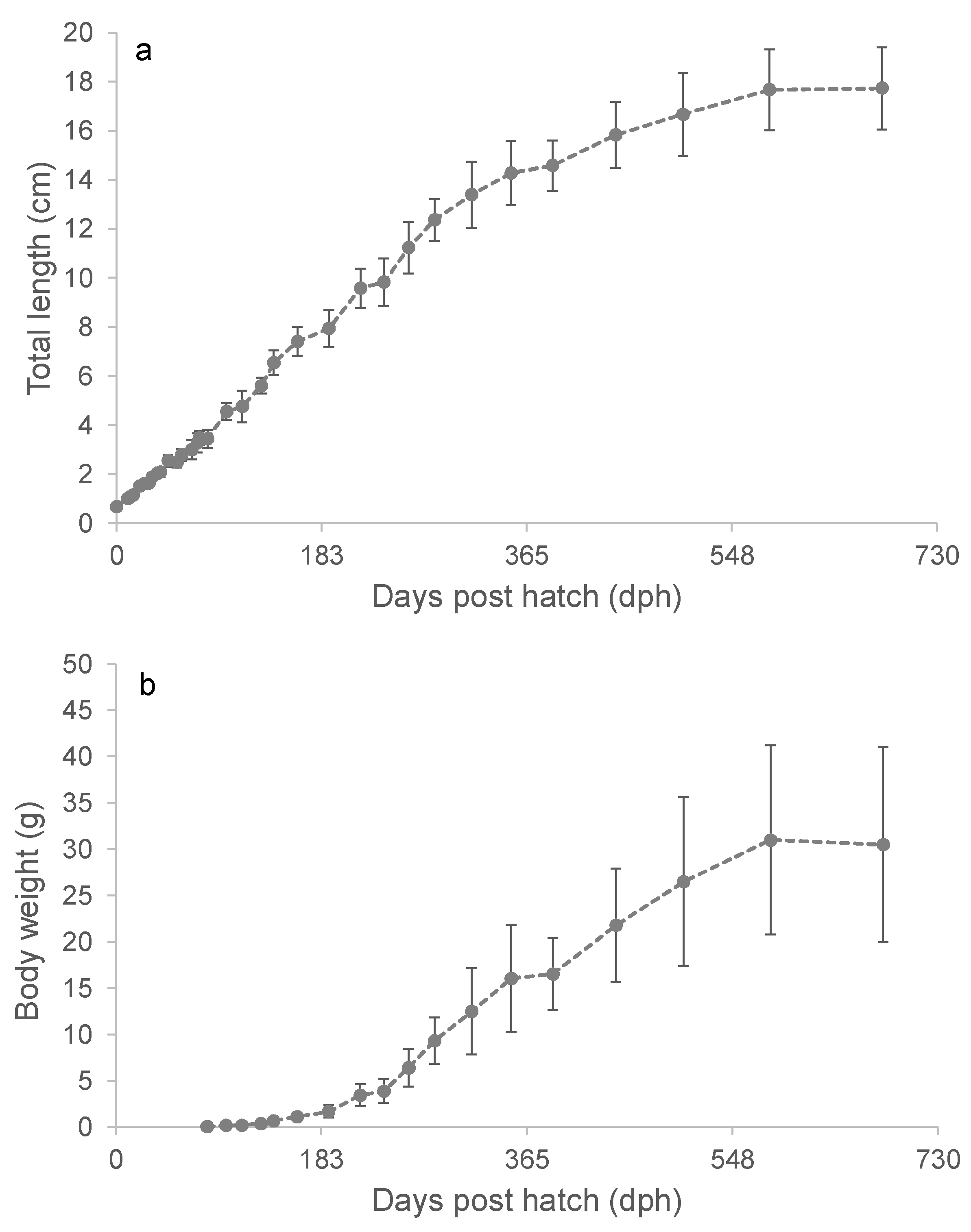
The growth performance of the capelin during the first 351 days of the study was assessed based on their SL. The absolute daily incremental growth rate during this period is expressed as mm/day, as determined through a linear regression analysis. The growth performance over the entire 681-day study period was evaluated using TL.
The condition factor (CF) was calculated as follows:
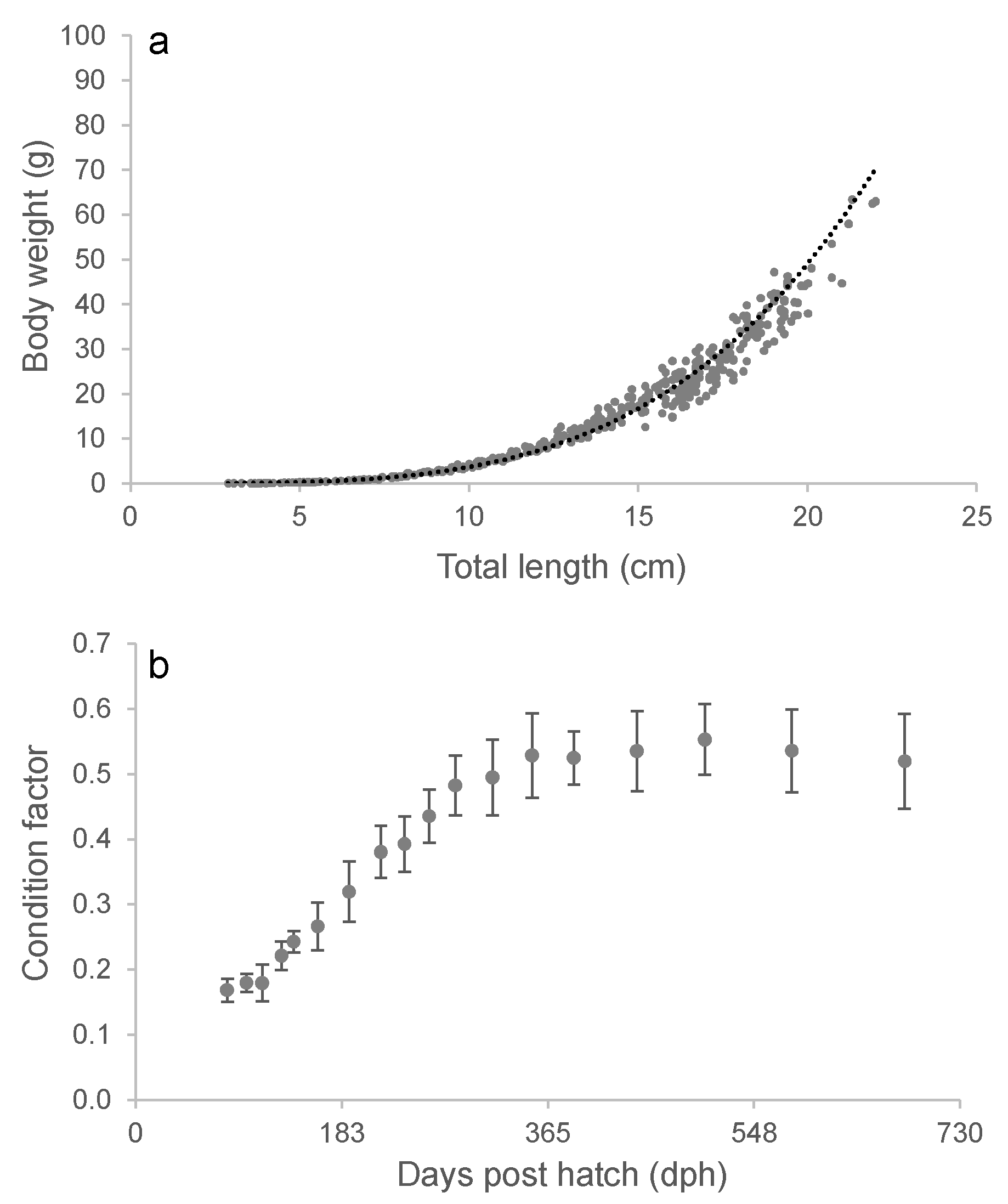
The standard allometric equation W = aTLb was used to establish the relationship between W and TL. Females with CF greater than 0.7 and external features suggesting the retention of ovulated eggs were excluded from the weight-length relationship.
2.4.1. Group B
After transferring the experimental fish to the 4 m3 green tank on day 255, the remaining fish were divided among three 3.2 m3 black tanks. One of these tanks contained approximately 300 fish (group B), which were cultivated under similar conditions as the experimental group (7°C and LD 24:0) until 942 dph. Group B was mainly used to provide eggs and larvae for subsequent hatching experiments and was therefore mostly left undisturbed from measurements. After being separated from group A on day 255, group B was however subjected to measurements at three time points: at 526 dph (27 fish), and subsequently at 806 and 942 dph, where all remaining fish (126 and 43, respectively) were measured. Since group B was cultivated some nine months longer than group A, the measured TL of the fish in group B was incorporated into dataset to improve the accuracy of the growth parameter estimates in the von Bertalanffy growth analysis for the cultivated capelin (see next section).
2.4.2. Growth comparison between cultivated and wild Icelandic capelin
The long-term growth performances of the hatchery reared capelin were compared with growth data for wild Icelandic capelin from year-classes 1981 – 2018. This comparison was carried out utilizing von Bertalanffy growth curves (VB) which were fitted to length-at-age data for both the cultivated capelin and each year-class of wild capelin using non-linear least squares regression as follows:
where
La is total length (cm) at age
a (years),
L∞ is asymptotic length,
K is a growth coefficient expressing the rate at which
L∞ is approached, and
t0 is theoretical age at which length is zero. For all VB model estimates, the initial values for the model fitting process were
t0 = 0, while
L∞ and
K were estimated via the concept of the Ford-Walford plot [
48].
Only metamorphosed capelin (TL> 8 cm) [
49,
50] were included in the VB analysis. Growth data for wild capelin were extracted from MFRI database containing all available individual capelin measurements from samples collected 1981-2023 during assessment surveys, other research surveys and from fisheries. Inclusion criteria were that each individual had to have a valid estimate of TL, W, and age, while obvious errors were removed. Acoustic and pelagic trawl assessment surveys have in general taken place biannually, i.e., autumn surveys in September-November and winter surveys mainly in January-February. Capelin fisheries have historically taken place in summer (Jun-Aug), autumn (Sep-Nov) and winter (Jan-Mar), while in most of the recent years the catches have mainly been taken during winter. Catches have been taken in varying proportions by seine and pelagic trawl, which may suggest that there is usually not continuous sampling from all seasons for each year-class. However, to get as coherent evaluation of the growth pattern of each year-class as possible, all available survey and fisheries measurements were used. This resulted in a total of more than 582 thousand individual measurements from the 1981-2018 year-classes.
2.6. Statistical Analysis
For the cultivated capelin in group A, data for TL, W, and CF are presented as means ± SD. The significant deviation of the sex ratio from 1:1 was assessed using the χ² goodness-of-fit test. Shapiro-Wilk tests were conducted to evaluate whether TL, W, and CF for males and females followed a normal distribution. Levene’s tests were applied to assess the homogeneity of variances between sexes. Subsequently, Welch’s t-tests were used to examine gender differences in TL, W, and CF at a significance level of p = 0.05.
Additionally, Shapiro-Wilk tests were used to evaluate whether the VB parameters of the wild year-classes deviated from normality [
51]. The VB parameters of the cultivated capelin were then compared with those of the wild populations. For
t0 and
L∞, one-sample t-tests were used, while the
K parameter, due to its non-normal distribution, was analyzed using the Wilcoxon signed-rank test. All statistical and VB analyses were conducted using R version 4.3.0 (R Core Team, 2023).
4. Discussion
In this study, the growth and development of capelin reared under laboratory conditions is described from hatch to adulthood for the first time. The study has helped elucidate the growth dynamics of capelin and provides information about the husbandry practices required to rear this delicate species in a laboratory.
The experimental fish used in this study originated from artificially fertilized eggs collected aboard a commercial fishing vessel. The larvae hatched over a one-week period, with the majority hatching immediately after being subjected to mechanical stress from being siphoned from the nursery tank into a bucket. This phenomenon is in line with observations made by Frank and Leggett [
52], who noted that the emergence of capelin larvae from beach gravel is triggered by the mechanical actions of wind and waves.
One of the main challenges in intensive cultivation of marine teleosts is the development of first feeding strategies that can provide the larvae with feed of the right size and necessary nutrients for growth and development [
53]. Wild first feeding capelin larvae eat a variety of prey but seem to base their foraging on small organisms such as
Acartia spp and
Temora longicornis [
54]. The prey size for first-feeding capelin in a large outdoor enclosure ranges from 40–300 μm in length [
26]. However, based on Frank and Leggett [
29] the optimal prey size for growth and survival of first feeding capelin larvae is at the lower end of this range, or between 40 to 51 μm. In the present study, enriched S-type rotifers (
Brachionus rotundiformis, 85 - 150 μm) were used as first feed for the capelin larvae. While the prey sizes were within the range reported by Moksness [
26], Frank and Leggett [
29] suggest that first feeding capelin may prefer even smaller prey. However, in this study, most larvae had started ingesting the S-type rotifers on 3 dph at 7 °C, which is consistent with Morgan et al. [
34] and about one day earlier than reported by Moksness [
26]. This indicates that prey size in the current study was sufficently small.
The early onset of exogenous feeding and high growth rates during the early developmental stages in the present study may be largely attributed to the light characteristics in the nursery tank. As most fish larvae are visual feeders, the conspicuousness of the prey is vital for successful foraging. The addition of live algae or algae concentrate to nursery tanks (green water technique) is widely used in the intensive farming of marine fish. In addition to increasing growth and survival by improving visual contrast for foraging, the green water technique can provide direct nutrition through active ingestion, and indirectly by the enrichment of live prey in the nursery tank [
55,
56]. While the nutritional benefits of adding phytoplankton to the nursery tank in the present study are unknown, the green water technique clearly influenced behavior, as the larvae were observed to swim down from the surface and away from the tank walls directly after the algae were added to the tank water. Similar behavior was seen in black 150 L cylindrical tanks (unpublished results). However, in an otherwise similar environment, this behavior was not observed among capelin larvae reared in white 0.5 m
3 tanks. Furthermore, in these white tanks the larvae did not ingest rotifers and died from starvation (unpublished results). This underlines the importance of light conditions during the early development and suggests that dark colored tanks may be more suitable than light colored tanks for the intensive cultivation of capelin. Similarly, prey ingestion, larval growth and survival of other species have been found to be influenced by tank color due to background contrast against live feed [
57,
58,
59], or due to phototaxis to light colored tank walls [
60].
In the present study, 38.6% of the capelin in group A survived from day 255 to day 681. The mortality observed during measurements indicated a significant impact of handling stress, as 17-70% of randomly selected fish died within a day after being measured. It was established that mechanical stress was not the cause of mortality, as no skin injuries were observed. However, the capelin displayed signs of lethargy, such as swimming upside down, when temporarily placed in a bucket containing approximately 7 liters of seawater prior to measurement. It can therefore be hypothesized that the mortality after the measurements were to some extent caused by high levels of physiological stress. Other factors, such as exposure to air during length measurements and the absence of a starvation period prior to handling, may also contribute to the observed high mortality rates. Overall, the study highlights the delicate nature of capelin and the challenges it may pose in future laboratory studies. Additionally, the present study is relevant to fisheries management, as the small and fragile capelin may be more likely to suffer escape mortality from trawling than larger and more robust species. Previous studies have shown that escape mortalities of SPF can be substantial and may be caused by stress, exhaustion, and contact with netting [
61,
62]. Given the observed handling-related mortalities, studies of capelin survivability after contact with fishing gear is warranted.
During the first year in the present study, the hatchery-reared capelin exhibited remarkable linear growth, with a mean SL increment of 0.36 mm per day. This growth rate by far surpasses the growth observed in previous studies where capelin larvae were fed cultured rotifers, which reported either negligible or slightly positive growth rates [
34,
47]. In a series of mesocosm experiments conducted by Ivarjord et al. [
40], it was observed that capelin larvae feeding on natural zooplankton exhibited a mean SL increment of 0.25 mm over periods ranging from 35 to 79 days. Under similar conditions Frank and Leggett [
29] reported larval growth of 0.23 mm per day. These experiments were conducted under comparable or higher temperature conditions compared with the present study. In another study, Moksness [
26] reported a mean SL increment of 0.29 mm per day at approximately 8°C for capelin larvae released into a large basin with self-sustaining natural populations of phyto- and zooplankton, with a mean prey density ranging from approximately 11 to 16 organisms per liter. Moksness [
26] also mentioned growth rates in a similar experiment with prey density ten times higher. In that experiment, daily increments of 0.31 and 0.44 mm were observed over 15 and 26 days from hatching, respectively. In comparison with wild capelin larvae, the daily growth rates in the present study were within the range of calculated values for Icelandic larvae (0.3 - 0.4 mm/day) [
63], as well as values documented in Canadian waters (0.11 - 0.49 mm/day) [
64,
65] and the Barents Sea (0.33 mm/day) [
66]. Taken together, the growth comparisons mentioned above strongly suggest that the nutrition and rearing conditions in the current study were indeed adequate.
Studies have demonstrated that fast growing capelin tend to mature earlier than slower growing capelin [
14]. In capelin, the onset of spawning is highly size dependent and for fisheries management of capelin in the Barents Sea, all capelin >14 cm are assumed to be maturing [
8]. Given the high and steady growth rate of the hatchery-reared capelin in the present study, it is not surprising that some of them developed secondary sex characteristics and mature gonads as early as one year post hatch, or two years earlier than most of their wild counterparts in Icelandic waters [
3]. Under the constant light and temperature in the present study, the timing of maturation was asynchronous, and capelin displaying secondary sex characteristics and mature gonads were observed among immature fish during the period from one year after hatch until the termination of the study. This made it possible to collect eggs and sperm from the cultivated capelin at any time of the year. In May 2022, the first attempt to produce larvae from 14-month old, cultivated capelin broodstock proved to be successful.
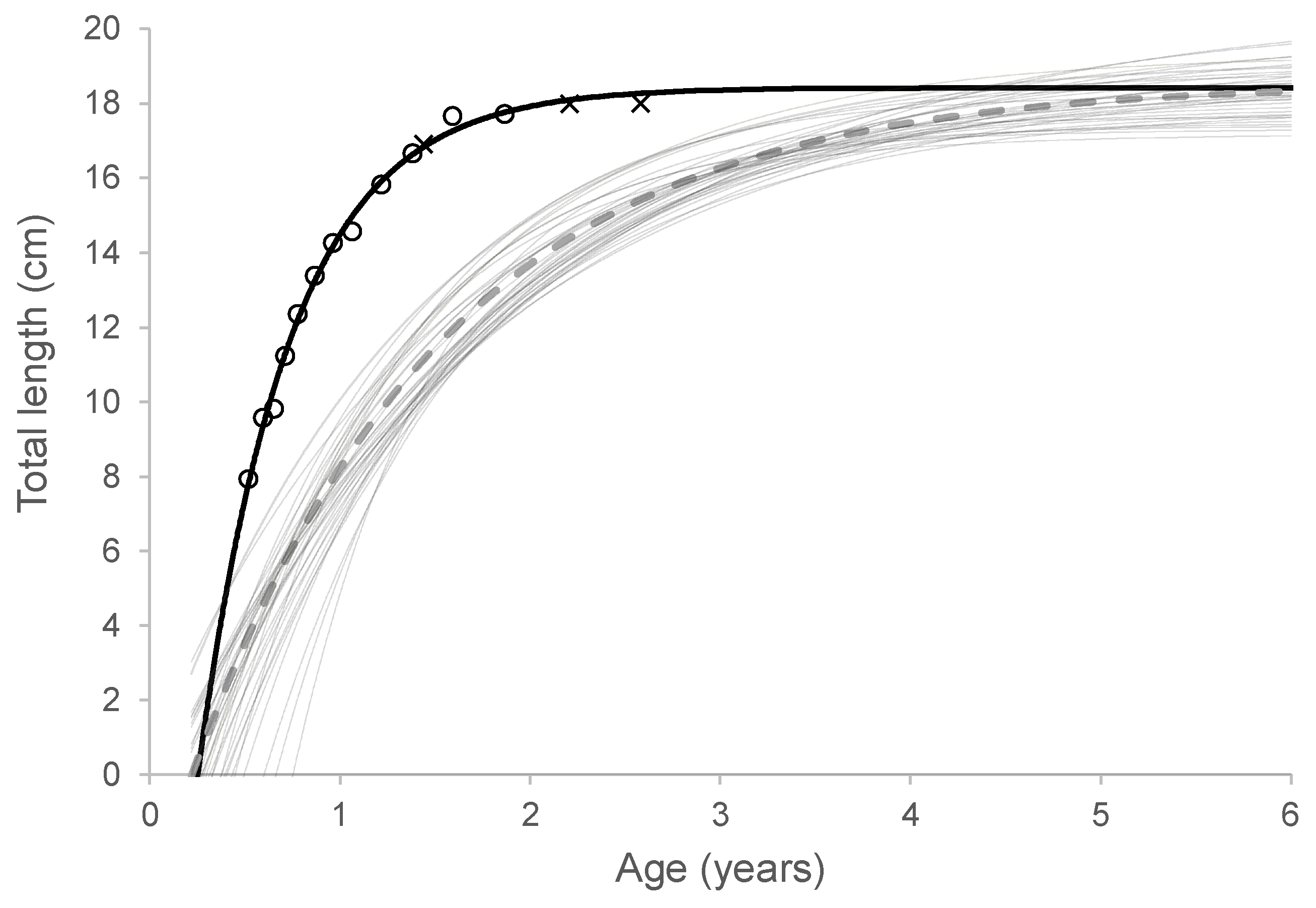
Under the stable environmental conditions provided in the present study, the growth trajectory of metamorphosed capelin (> 8 cm) closely followed the growth trajectory predicted by the VB equation. Over the course of the study, it was observed that the growth of the cultivated capelin in group A slowed down in the second year after hatching, suggesting that the fish were nearing their threshold L∞. This observation was further supported by the growth trajectory of group B, which exhibited a similar pattern. Between 806 and 942 dph, group B showed no growth in length, and at these time points, it only exhibited a slightly higher mean TL (18.0 cm) compared to group A (17.7 cm) at day 681.
To provide additional context to the results of this study, the growth performance of the cultivated capelin was compared to the measured growth data of 38 year-classes of wild Icelandic capelin (year-classes 1981 to 2018). Based on the VB growth model, the cultivated capelin in this study exhibited an
L∞ of 18.4 cm, which aligned with the median
L∞ of 18.6 cm of the wild capelin cohorts. However, in the absence of seasonal variation in temperature and daylength experienced by wild capelin, the cultivated capelin showed a
K of 2.06 year
-1, or almost three times higher than the mean
K of the wild Icelandic capelin (
K = 0.75). As a result of the higher growth rate, the cultivated capelin were projected to reach their
L∞ in approximately 2.6 years, whereas the wild capelin would take about 6 years to reach their
L∞. This suggests a correspondingly shorter lifespan and generation time for the cultivated capelin compared to their wild counterparts. These findings align with previous studies showing that capelin inhabiting colder waters tend to reach sexual maturity and achieve maximum length at a later stage in their life cycle, resulting in an extended lifespan [
12,
15].
In the current study the cultivated capelin had essentially the same
L∞ and
t0 when compared with the median
L∞ and
t0 of the 38 wild Icelandic cohorts. Among the wild Icelandic cohorts, there was a distinct variation in the VB parameters. While it is beyond the scope of this study to elucidate these observed variations, it is worth noting that previous studies suggested regional differences in
L∞ of capelin. Populations in the West Atlantic Ocean have usually been reported to have
L∞ at around 20 cm [
12,
15,
16]. In contrast, the mean
L∞ of 16 year-classes of Barents Sea capelin was found to be 17.0 cm [
17], and 18.6 cm for the 38 year-classes of the Icelandic capelin presented in this study. These regional differences may suggest that
L∞ could be influenced by environmental factors, either through slow evolutionary divergence and/or through phenotypic plasticity. To further understand the impact of environmental variations on capelin growth dynamics, future studies could be conducted under controlled laboratory conditions.
While the
L∞ of the cultivated capelin was predicted to be 18.4 cm, there was considerable variation in body size among the fish at the final measurement. In group A, the smallest fish measured approximately 14 cm, while the largest male reached 22.0 cm in length and weighed 63 g. It is worth noting that even larger fish were found in group B at 806 dph, with the largest male measuring 22.7 cm and weighing 89 g. These sizes, although remarkable, fall short of the longest recorded capelin, which was a 10-year-old female measuring 25.2 cm [
18].
Although the laboratory-reared capelin in the present study exhibited good appetite and rapid growth, the occurrence of skeletal malformations, particularly in the head region, raises questions about the husbandry practices used. All of the 103 fish examined during the last measurement exhibited craniofacial deformities, including compressed, or swayed snout, protruded lower jaw, and lower jaw papilloma. Previous studies have shown that repeated contact or collisions with tank walls can induce jaw injuries among captive adult capelin and other fish species [
35,
67,
68]. Therefore, it is likely that the craniofacial malformations in the present study were caused by mechanical injury during the daily cleaning process, as the fish became agitated and swam erratically in all directions.
To address this issue and improve the welfare of adult capelin, the use of large tanks (>3 m
3) and/or tanks with self-cleaning ability is recommended. Tank size has been shown to influence wall collisions and schooling behavior in capelin. For instance, captive adult capelin kept in a 7 m
3 tank collided less frequently with the walls and formed a tight school, whereas no schooling behavior and frequent wall collisions were observed in a 0.5 m
3 tank [
35].
While previous studies have shown that wild-caught adult capelin display courtship behavior and spawning when kept in aquariums and tanks (e.g., [
4,
23,
25,
37]), the hatchery reared capelin in the present study did not spawn in the tanks. Further investigation is needed to determine the reason for this, but it is possible that stable environmental conditions during the maturation process and the lack of suitable substrate, such as sand or gravel, may have contributed to the absence of spawning.
It has generally been assumed that wild male capelin have a 100% post-spawning mortality rate, while females in some populations may survive to spawn again the following year [
3,
69]. However, Christiansen et al. [
4] found that a small proportion of captive male capelin can survive spawning and resume growth after a period of growth depression. In the present study, it was observed that 28% and 33% of males exhibited prominent secondary sex characteristics on days 581 and 681 post hatch, respectively. Despite this, the mortality rate among mature males during this period was low. These findings show that, in the absence of the mechanical stress associated with copulation and spawning, a significant proportion of mature males survive maturity. It is also noteworthy that in group B, males with distinct spawning ridges were alive, approximately one year after they developed secondary sex characteristics (observed in June 2023).
In the present study, the length-weight relationship over the entire studied size range (from 2.6 – 22.0 cm) was strongly allometric, with a weight exponent (
b-value) of 3.75. A more detailed examination revealed the presence of at least two distinct growth phases, roughly corresponding to the first and second year of the study period. The first one started at approximately 5 cm in TL (112 dph) and is reflected as a steady increase in CF. This coincides with profound morphological changes observed in wild capelin between 5 and 8 cm in TL. These changes include a rapid thickening of the body musculature and a transition from a translucent juvenile morphology to the adult form [
50]. After the fish had completed metamorphosis to the adult form, the CF continued to rise towards the end of the first year. This may largely be attributed to the accumulation of adipose tissue as the capelin approached maturation. This phenomenon has been previously observed in wild capelin during the feeding months as they prepare for spawning the following year [
3]. Thus, the CF of the capelin increased linearly with age during the first year, until it approached a plateau at a value of approximately 0.53 when the mean TL was about 14 cm. The observed plateau in body condition during the second year of the study may suggest isometric growth following the initial period of rapid growth. However, capelin in group B demonstrated a notable increase in appetite as they entered their third year of life. At 806 days post-hatch (dph), group B exhibited mean CF of 0.61 ± 0.07, TL of 18.0 ± 1.72 cm, and W of 37.9 ± 12 g. These measurements indicate that the fish in group A would likely have also gained weight if the study had been extended beyond day 681.