1. Introduction
Increased inflammation and heightened physiological stress reactivity have been associated with pathophysiology of mood disorders. Peripheral blood elevations in the proinflammatory cytokines including interleukin-6 receptor (IL-6R), glycoprotein 130 (gp130), and interleukin-10 (IL-10) are the most reliable biomarkers of increased inflammation in patients with depressive symptoms [
1]. Cytokines can cross the blood brain barrier and affect central neural function, which may promote depressive symptoms and low mood [
2,
3]. The role of proinflammatory cytokines in depressive symptoms is supported by prospective studies, which have shown that acute administration of proinflammatory cytokines can trigger depressive symptoms [
4,
5]. Proinflammatory cytokines have been shown to mediate serotonin deficiency via tryptophan degradation by indoleamine 2,3-dioxygenase, which results in decreased availability of tryptophan for cerebral serotonin synthesis [
5]. Meanwhile, animal models suggest that exposure to stressors facilitates the expression of proinflammatory cytokines and promotes depressive-like behaviour [
6]. Physiological responses to acute stressors can vary widely among individuals experiencing the same stressor [
7], and reactivity to stressors can be a predictor of depressive symptoms [
8]. A meta-analysis showed that patients with major depressive disorder show similar baseline stress cortisol levels to healthy controls, but patients with major depressive disorder have significantly higher cortisol levels during the recovery period [
9]. While an adaptive stress response is flexible and short-lived, patients with major depressive disorder seem to have blunted stress reactivity, and impaired recovery from acute stressor [
9,
10]. An exaggerated, prolonger response to acute stressors may facilitate higher increase of proinflammatory cytokines.
The underlying biological mechanisms by which proinflammatory cytokines and stress can affect neurogenesis is through alterations of the kynurenine (KYN) pathway. KYN formation from tryptophan seems to be activated by psychological or physiological stress as well as directly by inflammatory factors. Proinflammatory cytokines activate KYN pathway to influence tryptophan metabolism and secrete neurotoxins, which may either decrease serotonin production or promote serotonin reuptake [
11,
12].
Peripheral tryptophan conversion to KYN under proinflammatory and under stress conditions has been linked to neuroinflammation and may contribute to depressed mood [
13]. In humans, interferon α (IFN-α) therapy revealed that an increase in the serum KYN /kynurenic acid (KYNA) ratio is significantly associated with the severity of depression [
14].
N-3 polyunsaturated fatty acids (
n-3 PUFA) may reduce morbidity by regulating inflammation and stress-responsive systems. Higher serum
n-3 PUFA seem to buffer from an uptick in stimulated proinflammatory cytokine release during stress exposure. A randomized, controlled trial showed that daily supplementation of 2.5 grams of
n-3 PUFA (EPA and DHA) reduced overall cortisol and IL-6 throughout the stressor [
15]. It was suggested that
n-3 PUFA supplementation alters damaging effects of stress and may thus reduce the risk of depression.
N-3 PUFA have anti-inflammatory properties and are suggested to positively influence brain functioning [
16]. Numerous studies have been conducted on the effects of anti-inflammatory properties of
n-3 PUFA on mood. While there is evidence for higher plasma
n-3 PUFA being associated with improved mood [
17], a number of randomized controlled trials showed limited effects of
n-3 PUFA supplementation on mood either as a main effect or in interaction with other variables such as stress in healthy individuals [
18,
19]. Inconsistent results on the effect of
n-3 PUFA supplementation on mood may be due to the insufficient quality of evidence. Studies in this area often implemented supplementation protocols shorter than 3 months – time crucial to achieve a significant increase of one of the
n-3 PUFA, DHA, in human blood cells [
18]. A meta-analysis of twenty-five studies indicated that
n-3 PUFA supplementation lowered depressive symptomology as compared with placebo [
20]. Meanwhile, the quality of evidence from the meta-analysis is insufficient: 25% of studies included in the meta-analysis had a high risk of bias, and funnel plot inspection indicated that the results may be biased. Meanwhile, a randomised clinical trial, which tested the effects of
n-3 PUFA supplementation on late-life depression risk and mood scores, indicated that supplementation with
n-3 PUFA does not prevent depression. There was no difference in mood scores between experimental and control groups and there was a small but statistically significant increase in the risk of depression among those who supplemented
n-3 PUFA
[19].
Overall, controlled intervention trials addressing the causal nature of the effect of
n-3 PUFA on mood in healthy males are scarce, and results from the limited number of studies available are divergent. When studying biological mechanisms in depressive symptoms, it is crucial to consider possible gender differences. Inflammatory responses differ between males and females - responses in females have been shown to be more pronounced than in males [
21]. Gender differences in inflammatory responses seem to also apply to the peripheral immune system in patients with depression. One cross-sectional study found that serum levels of IL-6 and TNF-α were higher in depressed females than depressed males [
22]. This suggests that the association between proinflammatory cytokines and depressive symptoms is possibly gender-specific, and hence males and females need to be studied individually.
The aim of the present study is to evaluate the effect of n-3 PUFA supplementation on the regulation of inflammation, KYN metabolism, depressive symptoms, mood; and stress-induced changes to the KYN metabolism and mood in healthy males. Using a placebo-controlled design participants were administered to receive supplementation for 12 weeks. Serum concentrations of KYN pathway metabolites were investigated —including KYN, and KYNA, quinolinic acid, xanthurenic acid, picolinic acid, 3-hydroxykynurenine, and 3-hydroxyanthranilic acid. Assessed inflammation markers included interleukin (IL)-6R alpha, IL-10, gp130, and TNF RI/TNFRSF1A. The n-3 PUFA supplementation was controlled by investigating serum concentration levels and percentages of omega-3 PUFA derivatives.
2. Materials and Methods
2.1. Ethics Statement
The study was approved by the Bioethics Committee for Research Projects at the University of Gdańsk (protocol number 44/2020). All methods were performed in accordance with relevant guidelines and regulations. Informed consent forms were obtained from all participants.
2.2. Participants
Volunteers, 51 males were recruited in this study (
Table 1- Characteristics of participants) between the ages 25-49. The age of the participants was chosen based on biological development, which was associated with relatively stable behavioural patterns [
23,
24]. Exclusion criteria included DSM-5 – psychiatric disorders other than depression and anxiety [
25], neurological disorders, other severe chronic conditions, and supplementation of dietary supplements containing
n-3 PUFA. Out of the 51 volunteers enrolled in the study, four dropped out after the first intervention period (two from the experimental group and two from the control group).
2.3. Procedure
Using a placebo-controlled design, 51 participants were administered to receive 4000 mg/day of fish oil (EPA 2234mg; DHA 916mg) (n=27) or placebo (MCT) (n=24) for 12 weeks. Before supplementation blood samples were collected and mood was assessed using validated psychological measures: Mood Adjective Check List (UMACL) and DASS-21 (t0). After the completion of the 12-weeks supplementation, mood was assessed and blood samples were collected (t1). Following this, a stress response was induced with a validated stress manipulation test, the Trier Social Stress Test. After the stress manipulation test, mood was reassessed, and blood samples were collected at two time points: following an acute stressor (t2) and 1 hour after an acute stressor (t3).
2.4. Mood Assessment
Mood Adjective Check List: Polish adaptation of the Mood Adjective Check List was used to assess mood [
26]
, (Polish adaptation [
27]). The questionnaire comprises a list of 29 adjectives. Participants rate the degree to which their present mood corresponds to each of the adjectives on the 1 to 4 scale. The final score is represented by the three dimensions: energetic arousal (EA), tense arousal (TA) and hedonic tone (HT). High levels of energetic arousal correspond to being restful, energetic and vigorous; high scores of tense arousal correspond to being stressed, anxious or tense; and high scores of hedonic tone are associated with being cheerful, satisfied and happy.
DASS-21: Polish adaptation of the Depression, Anxiety and Stress Scale - 21 Items (DASS-21) was used to assess mood [
28]. DASS-21 measures the emotional states of depression, anxiety and stress (for each of the subscales the minimum value is 10 and the maximum value is 42, with higher scores representing higher levels of the emotional states).
2.5. Stress Manipulation Test
The Trier Social Stress Test (TSST) was used to induce a stress response in participants [
29]. The TSST is a three-stage psychosocial stress task conducted in front of a panel of experimenters, (i) preparation; (ii) public speaking task; and (iii) mental arithmetic task. The TSST is a reliable method for inducing psychosocial stress [
30].
2.6. Sample Collection
Blood samples were taken into 4 mL sodium citrate vacutainer tubes and then centrifuged (4oC, 4000 x g for 10 min). Plasma and RBCs were collected with a disposable Pasteur pipette and transferred into separate Eppendorf probes and stored in a −80oC freezer until further analysis.
2.7. Fatty Acids Analysis - Assessment of EPA and DHA
Serum percentage share and concentration of EPA and DHA were determined using gas chromatography coupled with mass spectrometry. Briefly, total lipids were extracted from serum samples with a chloroform-methanol mixture (2:1, v/v) according to Folch et al. [
31] nd after dried under a nitrogen stream were subjected to 3 h of hydrolysis with 0.5 M KOH at 90°C. After incubation, the mixtures were acidified with 6 M HCl. 1 mL of water was added, unesterified Fatty acids were extracted thrice with 1 mL of n-hexane and the organic phase was evaporated under a nitrogen stream. The extracts were then derivatized into fatty acid methyl esters (FAME) with 10% boron trifluoride in methanol solution at 55°C for 1.5 h. Then, 1 mL of water was added and the FAME were extracted with 3×1 mL n-hexane, dried under a nitrogen stream and stored at –20°C until analysis.
The FAME were analyzed with a GC-EI-MS QP-2020 NX (Shimadzu, Kyoto, Japan) with chromatographic separation on a Zebron ZB-5MSi capillary column, 30 m × 0.25 mm i.d. × 0.25 μm film thickness, (Phenomenex, Torrance, CA, USA). The samples were injected into dichloromethane. 1 μl of sample was injected at a split mode. Column temperature was set in a range of 60 °C to 300 °C (4 °C/min), with helium as the carrier gas at the column head pressure of 60 kPa. The temperature of injection, ion source, and transfer line were 300 °C, 200 °C, and 300 °C, respectively. Electron energy used for FAME ionization was 70 eV; 19-methylarachidic acid was used as an internal standard. Full scan mode was used with mass scan range m/z 45 to 700. Accurate identification of the FA profile including EPA and DHA was possible based on FAME mixture standards (Larodan, Michigan, USA and Merck, Darmstadt, Germany).
2.8. Assessment of Tryptophan and Its Metabolites
Plasma concentrations of KYN pathway metabolites (3-HK, KYNA, QA, XA, PA, 3-HAA) were determined using high-performance liquid chromatography with tandem mass spectrometry (LC–MS/MS), with prior protein precipitation and derivatization as previously described [
32]. Acetonitrilic solution of internal standards was added to plasma followed by vortexing and centrifugation. After drying the supernatant in an air flow, methanolic solution of hydrochloric acid was added, incubation and second drying in an air flow took place. To the dry residue, aqueous formic acid solution was added followed by vortexing and injected into an ExionLC™ chromatographic system equipped with two binary pumps, degasser, column oven and PAL HTC autosampler coupled with 4500 QTrap triple quadrupole mass spectrometer.
2.9. Statistical Analysis
For every studied outcome variable, we performed a two-way mixed models ANOVA with group (O-3 / placebo), time (before/after supplementation) and group*time interaction as fixed effects and participant as random effect. Additionally, for variables measured before and after the stress test we performed a similar two-way mixed models ANOVA (with time before, after and 1 hour after stress). If one or more effects have been statistically significant, we conducted a post-hoc analysis with pairwise comparisons of estimated marginal means. We corrected for multiple comparisons with Tukey HSD correction. For all analyses, results were deemed statistically significant if p < 0.05. Data analysis was carried out in Python (v.3.11) using packages Numpy (v.1.26) [
33], Pandas (v.2.2) [
34] and Pymer4 (v.0.8) [
35]. Plots were created with Matplotlib (v.3.8) [
36] and Seaborn (v.0.13) [
37].
3. Results
3.1. Fatty Acids Profile
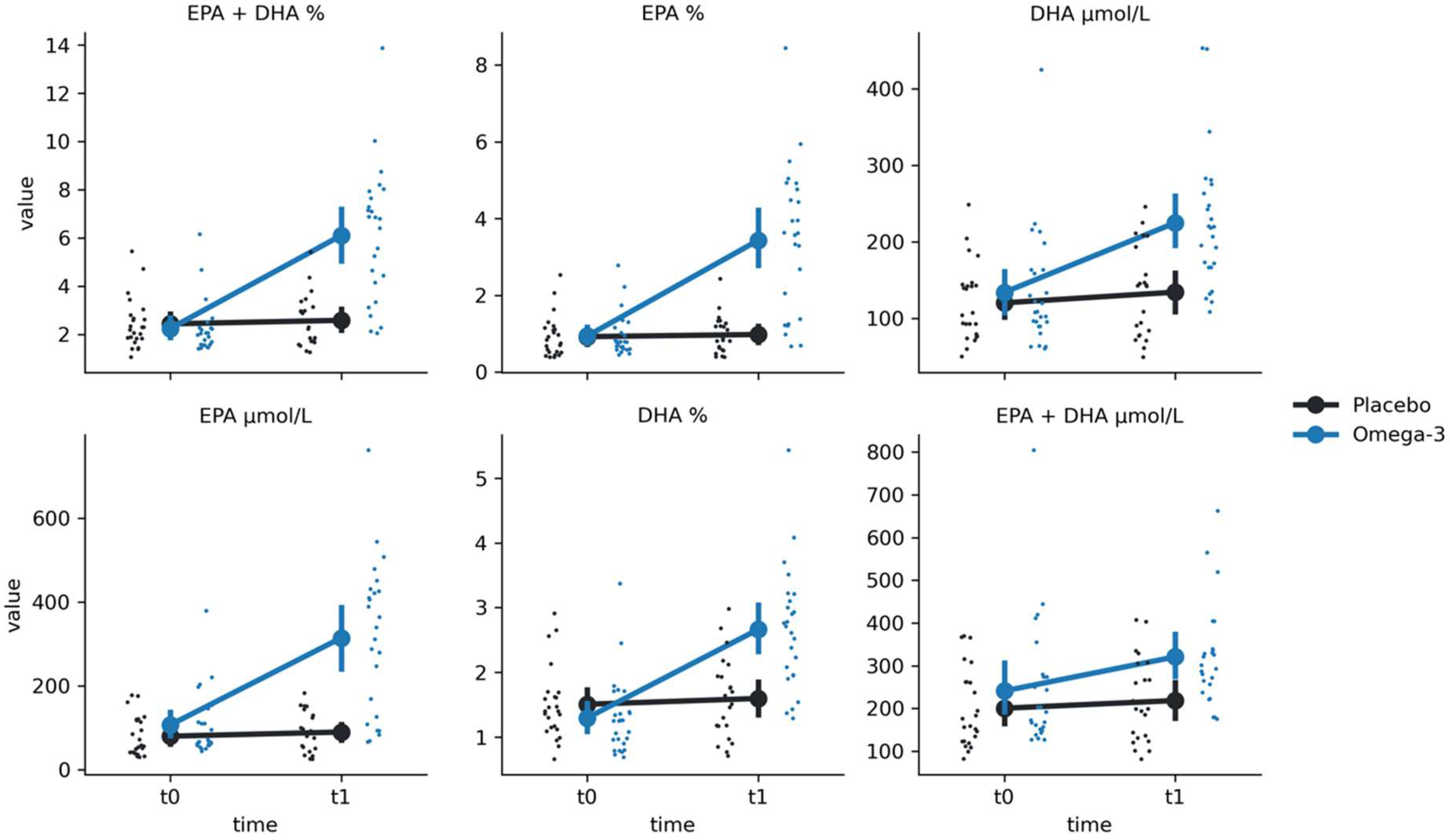
For EPA %, we have found significant group-by-time interaction (F(1, 49.29) = 34.94, p < 0.001) (
Figure 1;
Table 2). Post hoc comparisons revealed a significantly higher EPA % values at t1 in comparison to t0 for O-3 group (Mt0 = 0.94 ± 0.55, Mt1 = 3.44 ± 1.93, t = -8.76, p < 0.001) but not for the placebo group (Mt0 = 0.92 ± 0.54, Mt1 = 0.98 ± 0.5, t = -0.17, p = 0.86). Similarly, we found a group-by-time interaction for EPA μmol/L (F(1, 47.12) = 36.84, p < 0.001). Post hoc comparisons revealed a significantly higher EPA μmol/L values at t1 in comparison to t0 for O-3 group (Mt0 = 107.37 ± 76.18, Mt1 = 314.83 ± 180.14, t = -7.23, p < 0.001) but not for the placebo group (Mt0 = 80.49 ± 47.72, Mt1 = 90.4 ± 47.43, t = -0.17, p = 0.86).
Similarly a group-by-time interaction was found for DHA % (F(1, 43.46) = 10.62, p = 0.002). Post hoc comparisons revealed a significantly higher DHA μmol/L values at t1 in comparison to t0 for O-3 group (Mt0 = 133.74 ± 76.82, Mt1 = 224.74 ± 90.09, t = -5.50, p < 0.001) but not for the placebo group (Mt0 = 120.1 ± 50.09, Mt1 = 134.04 ± 60.03, t = -0.74, p = 0.46). Again, the same interaction was found for DHA μmol/L (F(1, 43.46) = 10.62, p = 0.002). Post hoc comparisons revealed a significantly higher DHA μmol/L values at t1 in comparison to t0 for O-3 group (Mt0 = 133.74 ± 76.82, Mt1 = 224.74 ± 90.09, t = -5.50, p < 0.001) but not for the placebo group (Mt0 = 120.1 ± 50.09, Mt1 = 134.04 ± 60.03, t = -0.74, p = 0.46).
As expected, EPA + DHA % results also revealed a significant group-by-time interaction (F(1, 49.89) = 19.63, p < 0.001), with O-3 group having higher values at t1 than t0 (Mt0 = 2.24 ± 1.09, Mt1 = 6.1 ± 2.79, t = -9.30, p < 0.001) and no differences in the placebo group (Mt0 = 2.43 ± 1.04, Mt1 = 2.58 ± 1.07 ± 50.09, t = -0.29, p = 0.77). Finally, the same pattern was found for EPA + DHA μmol/L, with a significant interaction (F(1, 48.49) = 5.77, p = 0.02), time differences in the O-3 group (Mt0 = 241.11 ± 149.37, Mt1 = 320.85 ± 120.01, t = -5.19, p < 0.001) but not for the placebo group (Mt0 = 200.58 ± 91.96, Mt1 = 218.58 ± 98.76, t = -0.72, p = 0.48).
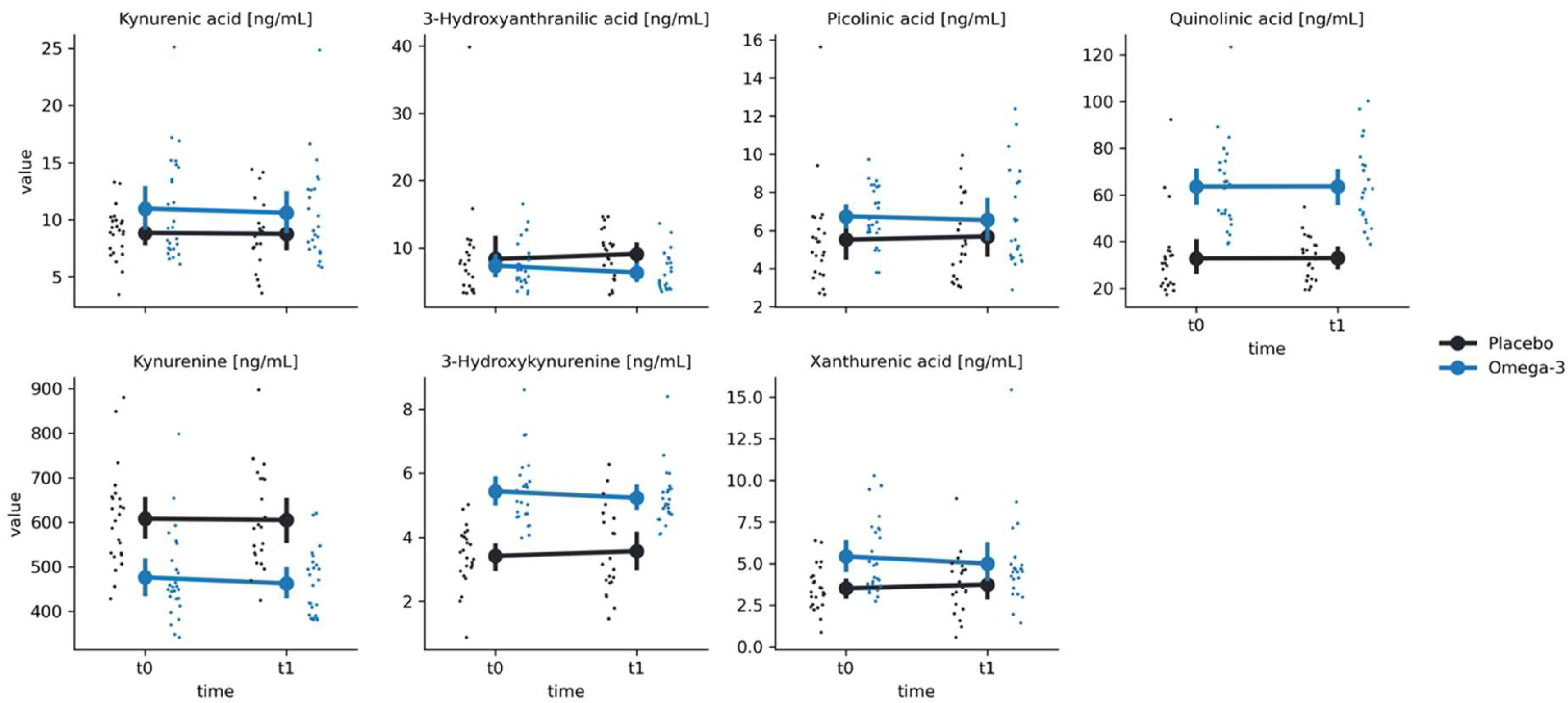
3.2. Kynurenine Pathway
For all kynurenine metabolites, there were significant group effects (all p-values < 0.01), however we have not found any significant time effects nor group-by-time interactions (all p-values > 0.05) (
Figure 2 and
Figure 3;
Table 2).
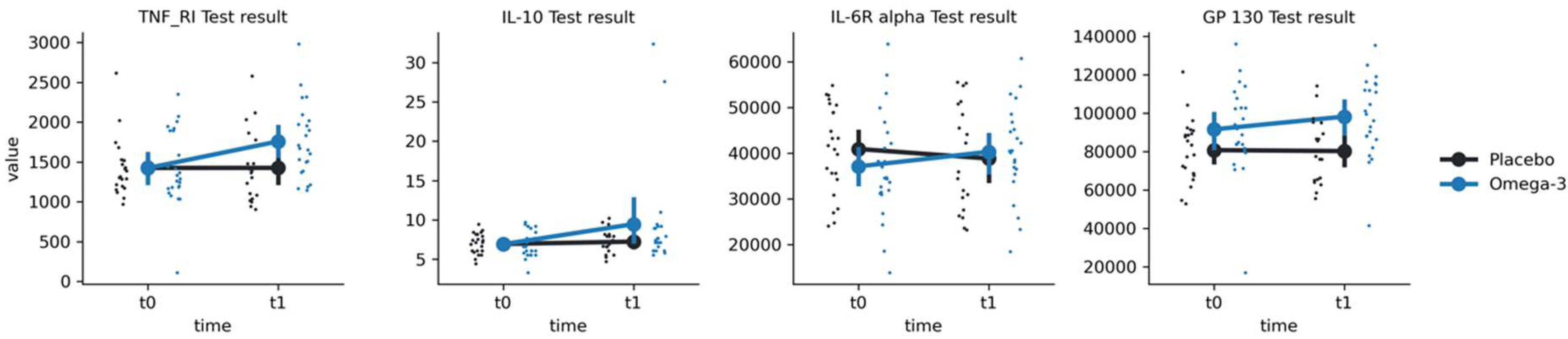
3.3. Inflammatory Markers
For IL-10, we have found a significant main effect of time (F(1, 28.3) = 4.30, p = 0.047) but not group or group-by-time interaction (p-values > 0.05) (
Figure 4;
Table 2). Post-hoc comparisons revealed significantly higher IL-10 at t1 in comparison to t0 (Mt0 = 6.89 ± 1.61, Mt1 = 9.44 ± 6.66, t = -2.72, p = 0.011) in the O-3 group but not in placebo group (Mt0 = 6.92 ± 1.3, Mt1 = 7.22 ± 1.53, t = -0.302, p > 0.05). For GP 130 we have found both a main effect of group (F(1, 46.2) = 6.57, p = 0.014) and a group-by-time interaction (F(1, 39.4) = 7.07, p = 0.011). Post-hoc comparisons revealed significantly higher GP 130 results at t1 in comparison to t0 in the O-3 group (Mt0 = 91537 ± 22774,Mt1 = 98177 ± 20706, t = -3.28, p = 0.002) but not in the placebo group (Mt0 = 80718 ± 16218, Mt1 = 80225 ± 17079, t = 0.58, p = 0.56). For IL-6R alpha, we have found a significant group-by-time interaction (F(1, 39.5) = 10.33, p = 0.003), with post-hoc tests showing an increase in the O-3 group (Mt0 = 37097 ± 11295, Mt1 = 40293 ± 10220, t = 3.54, p = 0.001) but not in the placebo group (Mt0 = 40901 ± 9695, Mt1 = 38815 ± 11586, t = 1.09, p = 0.28). For TNF_RI, we found both a main effect of time (F(1, 42.4) = 7.88, p = 0.007) and a group-by-time interaction (F(1, 42.4) = 10.92, p = 0.002). Post-hoc tests revealed an increase in TNF_RI in the O-3 group (Mt0 = 1424 ± 464, Mt1 = 1759 ± 465, t = -4.49, p < 0.001) but not in the placebo group (Mt0 = 1426 ± 351, Mt1 = 1426 ± 458, t = 0.34, p = 0.74).
3.4. Depressive Symptoms and Mood Measures
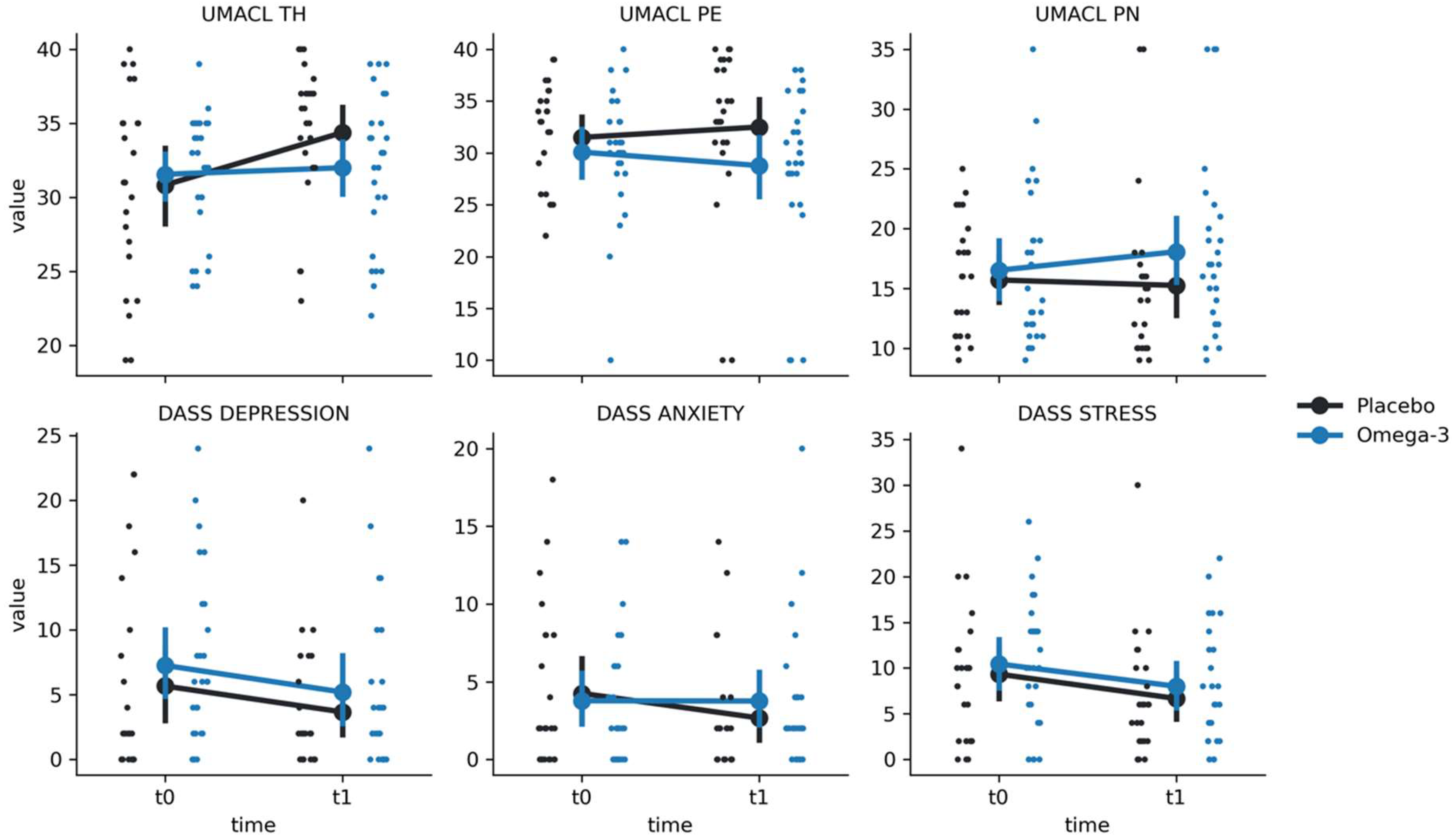
For DASS, we have found significant effects of time in the STRESS scale (F(1, 47.9) = 6.93, p = 0.011) and in the DEPRESSION scale (F(1, 47.7) = 5.20, p = 0.027). However, there were no significant group-by-time interactions (all p-values > 0.05), indicating that there was no effect of O3 supplementation on DASS outcomes (
Figure 5;
Table 2). For UMACL TH, we found a significant time effect (F(1, 49) = 10.77, p = 0.002) and a significant group-by-time interaction (F(1, 49) = 6.50, p = 0.014). Post-hoc tests indicated that there was an increase in scores from t0 to t1 in the placebo group (Mt0 = 30.83 ± 6.38, Mt1 = 34.38 ± 4.69; t = -4.00; p < 0.001) but not in the O-3 group (Mt0 = 31.56 ± 4.25, Mt1 = 32.0 ± 4.93; t = -0.53, p = 0.60). No significant differences were found for other UMACL subscales (p > 0.05).
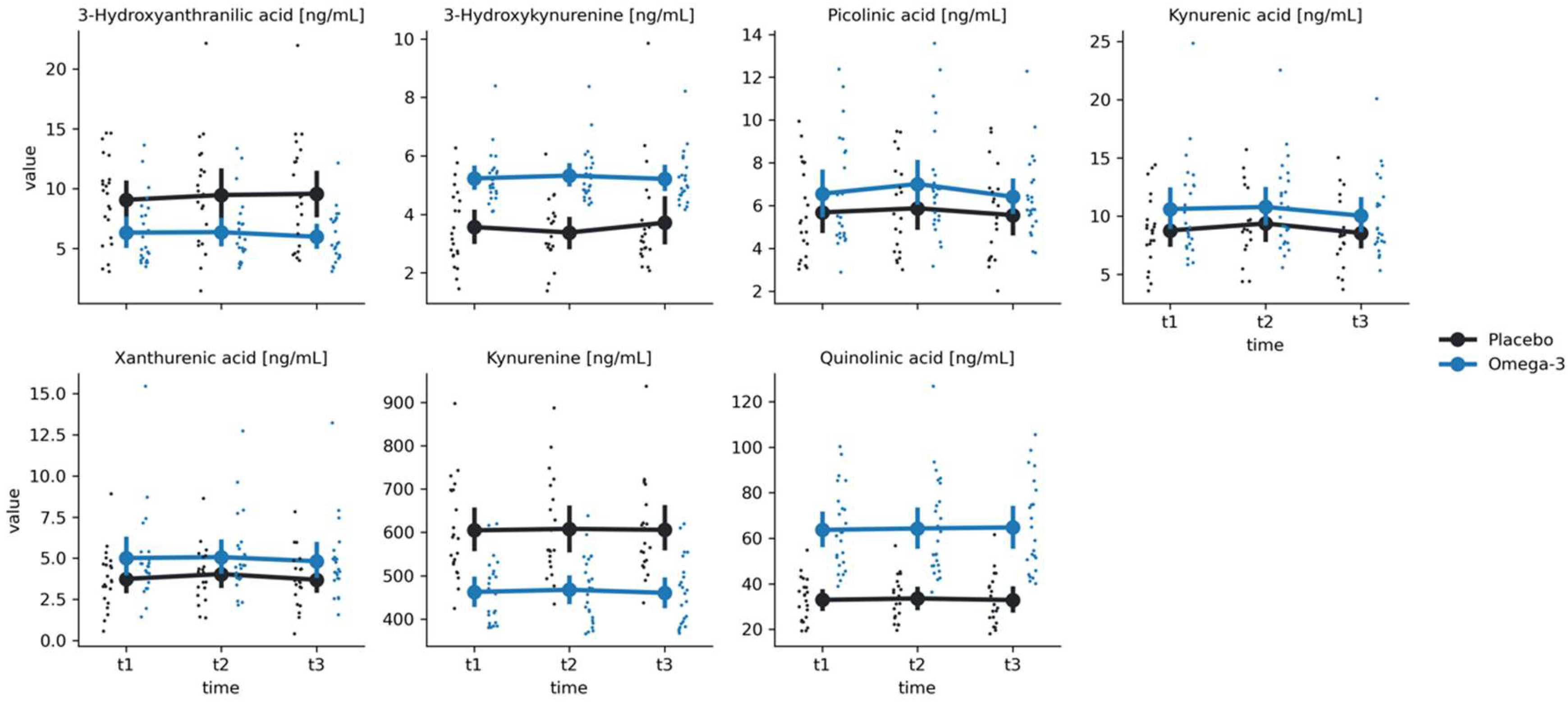
3.5. Stress Induction
For KYN metabolites, we have found significant group effects for 3-Hydroxykynurenine (F(1,45.7) = 32.83, p < 0.001), Kynurenine (F(1, 45.04) = 26.94, p < 0.001), Quinolinic acid (F(1,45.12) = 46.82, p < 0.001), Xanthurenic acid (F(1, 45.01), p = 0.046) and 3-Hyrdroxyanthranilic acid (F(1,44.96) = 10.32, p = 0.002) (
Table 2). Additionally, we have found a significant time effect for kynurenic acid (F(2, 84.1) = 10.30, p < 0.001). Post-hoc comparisons revealed significant lowering of kynurenic acid levels between t2 and t3 for both the O-3 group (Mt2 = 10.78 ± 3.78, Mt3 = 10.03 ± 3.43, t = 3.38, p = 0.003) and the placebo group (Mt2 = 9.35 ± 3.05, Mt3 = 8.54 ± 2.91, t = 2.96, p = 0.011). Additionally, for the O-3 group kynurenic acid levels were significantly lower in t3 in comparison to t1 (Mt1 = 10.6 ± 4.29, Mt2 = 10.03 ± 3.43, t = 3.08, p = 0.008). However, we have not found any significant group-by-time interactions for any of the KYN metabolites (all p values > 0.05).
We have not found any effects of stress induction on UMACL outcomes (all p-values > 0.05).
4. Discussion
Changes in the fatty acids profile after 3-months of supplementation with omega-3 PUFA indicate that n-3 PUFA supplementation raised plasma DHA and EPA concentrations. However, no significant treatment effects were found for any of the measures of KYN pathway, depressive symptoms, and mood. Hence, it can be concluded that the study found no evidence that elevating n-3 PUFA status in healthy males using n-3 PUFA supplements has any benefit to kynurenine metabolism, mood, depressive symptoms, or stress responsivity KYN pathway.
4.1. n-3 PUFA and Kynurenine Pathway
The main observation from this placebo-controlled experiment is that elevating
n-3 PUFA levels in healthy males had no effect on none of KYN metabolites. One potential explanation for the null effect of omega-3 PUFA supplementation on KYN metabolites is a potential role of a moderating effect of physical activity (PA) on the relationship between KYN pathway and the fatty acids profile. Results from a randomised controlled trial suggest that
n-3 PUFA supplementation accompanied by endurance training leads to increased plasma concentrations of neurotoxic 3
-hydroxykynurenine and its formation of neuroprotective metabolite - picolinic acid.
N-3 PUFAs did not impact KYN pathway among a group of non-active participants [
32].
When drawing conclusions on kynurenine pathway, it is crucial to consider group differences, which occurred for all kynurenine metabolites. Despite group differences, the distributions had similar variance and therefore it seemed appropriate to compare the two groups. Furthermore, the study tested for interaction effect, which can be detected independently of group effects. This allows comparing two groups regardless of differences between the groups.
4.2. n-3 PUFA, Depressive Symptoms and Mood
In the current study no significant differences were observed when comparing
n-3 PUFA with placebo groups in longitudinal mood scores except for the subscale ‘hedonic tone’. An increase in ‘hedonic tone’ scores after 12 weeks of
n-3 PUFA supplementation in the placebo group but not in the omega-3 group is an unexpected finding. Heonic tone is the trait underlying one’s characteristic ability to feel pleasure and lower scores represent a reduced capacity to experience pleasure [
27]. Hence, it can be concluded that placebo group improved their mood on that one dimension. Considering a relatively large number of variables, it however cannot be excluded that the finding was due to chance.
While results from the current study contrast some evidence in support of
n-3 PUFA to improve mood, they are consistent with results from a meta-analysis that showed no benefit of
n-3 PUFA supplementation on depressive symptoms in community-based samples of adults without clinical depression at baseline [
38]. A possible explanation for null effects of
n-3 PUFA supplementation on mood could be the sample of participants recruited in the current study. None of the participants in the study was screened for major depressive disorder, and majority of participants scored normal at DASS-21. Considering that at baseline participants scored low on all subscales of DASS-21 and all subscales of Mood Adjective Check List, the effects of
n-3 PUFA supplementation could have been very subtle and not detected. Our findings are consistent with the results from the largest randomised clinical trial (N=18,353), which indicate that
n-3 PUFA does not prevent depressive symptoms or improve mood [
19]. In this randomised trial participants without clinically relevant depressive symptoms at baseline were randomised for
n-3 PUFA supplementation of omega-3/placebo over a 5-year treatment period.
When drawing conclusions from the study, it is crucial to consider that the study sample consisted of a general adult population without clinically relevant depressive symptoms at baseline. The aim of this study was to investigate the effect of
n-3 PUFA supplementation on mood, including depressive symptoms. Hence, the conclusions from this study can only be applied to individuals with no mental health problems. It cannot be extended to the clinical population of patients with clinically diagnosed major depressive disorder. It is possible that a sample of patients with major depressive disorder could have yielded different results. To support this, a meta-analysis of the effects of
n-3 PUFA on depressed mood indicated that individuals with diagnosed depressive disorder might to some extent benefit from
n-3 PUFA supplementation but no evidence of any benefits in individuals without a diagnosis of depressive disorder was found. In contrast, there is some evidence for the effectiveness of
n-3 PUFA on mood in the general population. For example, the study conducted on a subset of data from “The Hordaland Health Study ‘97–‘99”, population based cross-sectional health survey from Norway, showed that regular use of cod liver oil is negatively associated with high levels of depressive symptoms in the general population [
39]. This study has further indicated that risk of symptoms of depression in relation to duration of cod liver oil supplementation decreases with time, i.e.
, participants who have been supplementing liver cod oil for 9-12 months had less symptoms of depression compared with those who supplemented liver cod oil for 1-4 months. It therefore seems that duration of
n-3 PUFA supplementation might be crucial when investigating mood. While 12 weeks’ supplementation period is justified in terms of adequate time crucial to achieve a significant increase of one of the
n-3 PUFA, DHA, in human blood cells; it seems that more time might be needed to detect changes in mood.
4.3. n-3 PUFA and Inflammation
n-3 PUFA supplementation was successful in elevating neuroprotective factors including TNF_RI, IL-6R and gp130. Significantly higher levels of IL-10 were found after the supplementation period in Omega-3 group. When drawing conclusions this effect it is crucial to consider that this finding needs to be taken with caution. Visual inspection of individual cases showed that n-3 PUFA group included two outliers, which might have driven the interaction effect that has been observed.
The findings on elevated levels of TNF_RI, IL-6R and gp130 are in line with previous research. IL-6R has a direct binding with membrane-bound gp130, which is a protein receptor with neuroprotective abilities. IL-6R through interaction with protein gp130 play a critical role in mediating anti-inflammatory response [
40,
41]. While IL-6 may have a negative effect on mood, gp130 may counteract depressive symptoms. There is in vitro and clinical evidence that Omega-3 PUFAs protect against inflammation through production of LOX and CYP450 lipid mediators [
42]. These lipid mediators seem to underpin the antidepressant, anti-inflammatory and neuroprotective effects of PUFAs.
4.4. Omega-3 and Stress-Responsivity Kynurenine Metabolism and Mood
The experiment found no differences between the experimental and control groups in stress responsivity kynurenine metabolism. While studies on the effect of acute stress on kynurenine metabolites conducted on humans are scarce, an animal model suggests a potential role of a moderating effect of physical activity in this relationship [
43]. Based on the animal model it seems that skeletal muscle PGC-1α1 modulates KYN pathway and mediates resilience to stress-induced depression. There is evidence for a differential effect of omega-3 PUFA on KYN pathway in physically active and physically inactive males. In a randomised clinical trial
n-3 PUFAs did not impact KYN pathway in non-active participants [
32]. Considering a possible moderating effect of physical activity on kynurenine pathway, future research should investigate physical activity in the relationship between acute stress and kynurenine pathway. This could possibly extend animal models.
4.5. Strengths of the Study
Our study is the first placebo-controlled trial to investigate the effect of omega-3 PUFA supplementation on kynurenine pathway. This placebo-controlled trial’s multiple strengths include the 12-week supplementation period – time crucial to achieve a significant increase of one of the omega-3 fatty acids, DHA, in human blood cells. To ensure construct validity, participants were administered Omega-3 PUFA supplements certified by the International Fish Oil Standards™ (IFOS™) Program. This is particularly important because IFOS verifies the contents of individual omega-3 fatty acids against manufacturer’s declarations, which ensures construct validity. In the current study MCT was used as placebo - both MCT and n-3 PUFA were administered in the form of similar looking capsules. Plasma EPA and DHA concentrations were also measured to investigate whether Omega-3 PUFA supplementation was successful. In addition, all psychological tools applied in the following study are valid and reliable.
4.6. Limitations of the Study
Some limitations of the study included methodological limitations of the materials used. While Tier Social Stress Test is a validated tool to induce stress in participants, the standardised procedure of stress induction does not allow to change the tasks when inducing stress at different time points. Hence, stress was only induced at one time point. Stress induction with Tier Social Stress Test at two different time points (i.e.
, before and after the intervention) would require applying the same procedures, and hence the stressor could have been perceived less stressful during the second time point [
44]. For this reason, each participant completed the acute laboratory stressor only after the intervention. We could not therefore adjust for baseline reactivity to account for potential interindividual variability. The decision to induce stress only at one time point was based on the familiarity of the laboratory stressor. Nevertheless, there might have been individual differences in experiencing stress among the participants. To control for individual differences in experiencing stress, it could have served useful to investigate subjective feelings towards the stress induction test. To measure mood, Mood Adjective Check List was applied. This tool only measures some dimensions of mood – i.e.
, energetic arousal (EA), tense arousal (TA) and hedonic tone (HT).
Although Mood Adjective Check List can be used as a repeated measure, it may not be sensitive to minor mood changes [
27]. Furthermore, the study implemented a 12-weeks protocol, which was enough time to achieve a significant increase of one of the omega-3 fatty acids, DHA, in human blood cells. However, it is likely that in order to see a change in depressive symptoms, a longer protocol should have been implemented [
39].
4.7. Implications
The Academy of Nutrition and Dietetics recommends at least 500 mg/day of EPA and DHA for adults [
45]. Health benefits promoted include mental health. Our findings suggest that daily Omega-3 PUFA supplementation alone may not have any clinical usefulness to improve kynurenine pathway and mood in a non-clinical group of males without a diagnosis of major depressive disorder. Yet, it is successful in improving inflammation markers, which can be beneficial for overall health.
5. Conclusions
Combining experimental and longitudinal methodologies, this study found no evidence that elevating n-3 PUFA status in healthy males using n-3 PUFA supplements has any benefit to kynurenine pathway, mood, depressive symptoms or stress responsivity kynurenine metabolism. The clinical usefulness of n-3 PUFA supplementation in healthy males to improve kynurenine pathway and mood is therefore questionable. When drawing conclusions from the study, it is crucial to consider that the study group consisted of healthy males rather than males with major depressive disorder. Future research on the effect of n-3 PUFA on kynurenine pathway and mood should extend animal models by further investigating clinical samples of patients with major depressive disorder in this relationship.
Author Contributions
Monika Bidzan-Wiącek and Jędrzej Antosiewicz contributed to the conceptualization. Monika Bidzan-Wiącek, Maja Tomczyk, Magdalena Błażek and Jędrzej Antosiewicz contributed to the methodology. Monika Bidzan-Wiącek contributed to the writing of the original draft preparation. Monika Bidzan-Wiącek, Maja Tomczyk, Magdalena Błażek, Adriana Mika and Jędrzej Antosiewicz contributed to writing—review and editing. Monika Bidzan-Wiącek, Maja Tomczyk and Adriana Mika contributed to the investigation. Monika Bidzan-Wiącek contributed to the project administration. Monika Bidzan-Wiącek and Jędrzej Antosiewicz contributed to the funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Research supported by: Narodowe Centrum Nauki, (110.13039/501100004281) * 2019/35/N/N77/03757.
Institutional Review Board Statement
In this section, please add the Institutional Review Board Statement and approval number for studies involving humans or animals. Please note that the Editorial Office might ask you for further information. Please add “The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” OR “Ethical review and approval were waived for this study, due to REASON (please provide a detailed justification).” OR “Not applicable.” for studies not involving humans or animals. You might also choose to exclude this statement if the study did not involve humans or animals.
Informed Consent Statement
Any research article describing a study involving humans should contain this statement. Please add “Informed consent was obtained from all subjects involved in the study.” OR “Patient consent was waived due to REASON (please provide a detailed justification).” OR “Not applicable.” for studies not involving humans. You might also choose to exclude this statement if the study did not involve humans. Written informed consent for publication must be obtained from participating patients who can be identified (including by the patients themselves). Please state “Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
Data will be shared upon reasonable request. To request data from the study please email the principal investigator: monika.bidzan@gumed.edu.pl.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
References
- Haroon E, Raison CL, & Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology; 2012;37(1):137-162. [CrossRef]
- Beurel E, Toups M, & Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 107; 2020:234-256. [CrossRef]
- Bhatt S, Devadoss T, Jha NK, Baidya M, Gupta G, Chellappan DK, et al. Targeting inflammation: A potential approach for the treatment of depression. Metabolic brain disease. 2023;38(1):45-59. [CrossRef]
- Dantzer R, & Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, behavior, and immunity. 2007;21(2):153-160. [CrossRef]
- Felger JC, & Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199-229. [CrossRef]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS & Devries AC. Stress and IL-1β contribute to the development of depressive-like behavior following peripheral nerve injury. Molecular psychiatry. 2010;15(4):404-414. [CrossRef]
- Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, et al. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology. 2020;114:104599. [CrossRef]
- Felsten G. Stress reactivity and vulnerability to depressed mood in college students. Personality and Individual Differences. 2004;36(4):789-800. [CrossRef]
- Burke HM, Davis MC, Otte C, & Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846-856 . [CrossRef]
- Geurts SA & Sonnentag S. Recovery as an explanatory mechanism in the relation between acute stress reactions and chronic health impairment. Scandinavian journal of work, environment & health. 2006;32(6):482-492. [CrossRef]
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, & Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11(3):198-209. [CrossRef]
- Tanaka T, Mori M, Sekino M, Higashijima U, Takaki M, Yamashita Y, et al. Impact of plasma 5-hydroxyindoleacetic acid, a serotonin metabolite, on clinical outcome in septic shock, and its effect on vascular permeability. Scientific Reports. 2021;11(1):14146. [CrossRef]
- Schwarcz R, Bruno JP, Muchowski PJ & Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature Reviews Neuroscience. 2012;13(7):465-477. [CrossRef]
- Wichers MC, Koek, GH, Robaeys G, Verkerk R, Scharpe S, & Maes MJ. IDO and interferon-α-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Molecular psychiatry. 2005;10(6):538-544. [CrossRef]
- Madison AA, Belury MA, Andridge R, Renna ME, Rosie Shrout M, Malarkey WB, et al. Omega-3 supplementation and stress reactivity of cellular aging biomarkers: an ancillary substudy of a randomized, controlled trial in midlife adults. Molecular psychiatry; 2021;26(7):3034-3042. [CrossRef]
- Thesing CS, Bot M, Milaneschi Y, Giltay EJ, & Penninx BW. Omega-3 polyunsaturated fatty acid levels and dysregulations in biological stress systems. Psychoneuroendocrinology. 2018; 97: 206-215. [CrossRef]
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M., et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Translational psychiatry. 2019;9(1):190. [CrossRef]
- Giles GE., Mahoney CR, Urry HL, Brunyé TT, Taylor HA, & Kanarek RB. Omega-3 fatty acids and stress-induced changes to mood and cognition in healthy individuals. Pharmacology Biochemistry and Behavior. 2015;132:10-19. [CrossRef]
- Okereke OI, Vyas CM, Mischoulon D, Chang G, Cook NR, Weinberg A, et al. Effect of long-term supplementation with marine omega-3 fatty acids vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. Jama. 2021;326(23):2385-2394. [CrossRef]
- Wolters M, von der Haar A, Baalmann AK, Wellbrock M, Heise TL & Rach S. Effects of N-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—a Systematic Review and Meta-analysis. Nutrients. 2021;13(4):1070. [CrossRef]
- Klein SL, & Flanagan KL. Sex differences in immune responses. Nature Reviews Immunology. 2016;16(10):626-638. [CrossRef]
- Birur B, Amrock EM, Shelton RC & Li L. Sex differences in the peripheral immune system in patients with depression. Frontiers in psychiatry. 2017; 8:108. [CrossRef]
- Dever GA. Epidemiology in health services management. 1984. Jones & Bartlett Learning.
- Watson D, Maître B, Whelan CT & Russell H. Technical Paper on the Measurement of Multidimensional Quality of Life in Ireland. Social Inclusion Technical Paper No. 7, Dublin: Department of Social Protection. 2016.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., text rev.). 2022. [CrossRef]
- Matthews G, Jones DM, Chamberlain AG. Refining the measurement of mood: The UWIST Mood Adjective Checklist. British Journal of Psychology. 1990;81:17–42. [CrossRef]
- Goryńska E. Przymiotnikowa Skala Nastroju UMACL Geralda Matthewsa, A. Grahama Chamberlaina, Dylana M. Jonesa: Podręcznik. 2005. Warszawa: Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego.
- Makara-Studzinska M, Tyburski E, Załuski M, Adamczyk K, Mesterhazy J & Mesterhazy A. Depression Anxiety Stress Scales-21--Polish Version (DASS-21) [Database record]. APA PsycTests. 2022. [CrossRef]
- Kirschbaum C, Pirke KM, & Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1-2): 76-81. [CrossRef]
- Giles GE, Mahoney CR, Brunyé TT, Taylor HA, & Kanarek RB. Stress effects on mood, HPA axis, and autonomic response: comparison of three psychosocial stress paradigms. PloS one. 2014;9(12):e113618. [CrossRef]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497-509.
- Tomczyk M, Bidzan-Wiącek M, Kortas JA, Kochanowicz M, Jost Z, Fisk HL, et al. Omega-3 fatty acid supplementation affects tryptophan metabolism during a 12-week endurance training in amateur runners: a randomized controlled trial. Scientific Reports. 2024;14(1):4102. [CrossRef]
- Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with NumPy. Nature. 2020. 585:357–362. [CrossRef]
- Pandas development team. pandas-dev/pandas: Pandas (v2.2.2). Zenodo. 2024. [CrossRef]
- Jolly E. Pymer4: Connecting R and Python for Linear Mixed Modeling. Journal of Open Source Software. 2018;3(31): 862. [CrossRef]
- Hunter JD. Matplotlib: A 2D graphics environment. Computing in science & engineering. 2007;9(03):90-95. [CrossRef]
- Waskom ML. seaborn: statistical data visualization. Journal of Open Source Software. 2021;6(60): 3021. [CrossRef]
- Appleton KM, Rogers PJ, & Ness AR. Updated systematic review and meta-analysis of the effects of n− 3 long-chain polyunsaturated fatty acids on depressed mood. The American journal of clinical nutrition, 91; 2010:757-770. [CrossRef]
- Raeder MB, Steen VM, Vollset SE & Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. Journal of affective disorders. 2007;101(1-3):245-249. [CrossRef]
- Rose-John S. Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochimica Polonica. 2003;50(3):603-611. [CrossRef]
- Rose-John S, Scheller J, Elson G, & Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. Journal of leukocyte biology. 2006;80(2):227-236. [CrossRef]
- Borsini A, Nicolaou A, Camacho-Muñoz D, Kendall AC, Di Benedetto MG, Giacobbe J, et al. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Molecular psychiatry. 2021;26(11):6773-6788. [CrossRef]
- Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014; 159:33-45. [CrossRef]
- Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, & Clarke G. The trier social stress test: principles and practice. Neurobiology of stress. 2017; 6:113-126. [CrossRef]
- Vannice G, & Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. Journal of the Academy of Nutrition and Dietetics. 2014;114(1):136-153. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).