Submitted:
10 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mathematical Model Formulation
- The susceptible prey grows logistically in the absence of infected prey and predator and prey infected with both strains neither contribute to the reproduction nor to the carrying capacity thus we have,
- Infection of both strains within the prey population occurs horizontally via direct contact following the mass action incidence i.e.where are the rate of transmission of strain j infection.
-
Only susceptible prey participates in the herding group where the rate of herd shape is
- if the herd shape is circle or square,
- if the herd shape is cube or sphere, .
- The infected prey dies out either due to disease by strain 1 or 2 or naturally ( denotes the total death rate)and upon recovery( denotes the recovery rates) from the infection they re-enter the susceptible population.
- Predators attack the susceptible prey according to the Holling-II interaction functional type created by S.Djilali [30] given asand the prey infected with strains 1 and 2 according to the Holling type-I functional response given as
- Predators attack the boundary of the herd and the prey on the outer herd can injure the predators leading to their death given as .
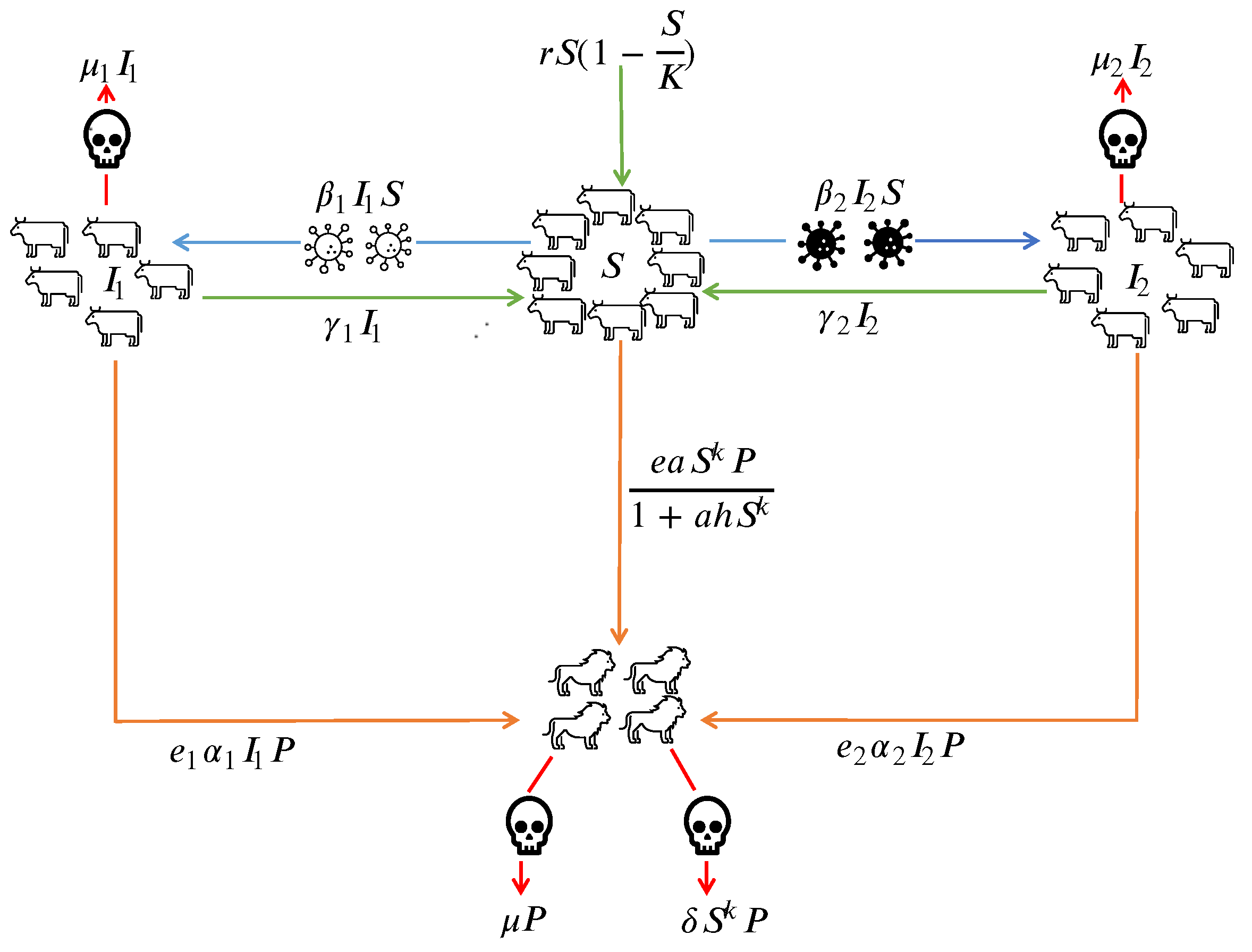
3. Description of Parameters
| Parameters | Description |
| S | Susceptible Prey |
| Prey Infected with strain 1 | |
| Prey Infected with strain 2 | |
| P | Predator |
| r | Intrinsic growth rate of susceptible prey |
| K | carrying capacity of susceptible prey |
| rate of transmission of strain 1 infection | |
| rate of transmission of strain 2 infection | |
| recovery rate from strain 1 | |
| recovery rate from strain 2 | |
| a | rate of predation on susceptible prey |
| k | rate of prey herd shape |
| h | handling time spent by a predator on healthy prey |
| rate of predation on prey infected with strain 1 | |
| rate of predation on prey infected with strain 2 | |
| e | conversion rate of susceptible prey to predator |
| conversion rate of prey infected with strain 1 to predator | |
| conversion rate of prey infected with strain 2 to predator | |
| mortality rate of a predator due to prey herd | |
| natural death rate of predator | |
| total death rate of prey infected with strain 1 | |
| total death rate of prey infected with strain 2 |
4. Well-Posedness of the Formulated Model
5. Equilibrium Points
-
The basic reproduction number at the disease-free equilibrium point iswhere
-
where are solutions of equations:andFor positive solutions, the following conditions should hold:and
-
where
-
where
-
where ,
-
where ,
-
where
6. Stability Conditions
-
is a saddle point because the eigenvalues of variational matrix are
-
is locally asymptotically stable ifand and
-
for the variational matrix iswhere, ,, , ,, , ,,The characteristic polynomial of matrix J iswhereAccording to Routh-Hurwitz criteria is locally asymptotically stable if and only if
7. Numerical Simulation
| Parameters | Numerical Values | Source |
| r | 0.15 | Assumed |
| K | 25 | Assumed |
| 0.01 | [6] | |
| 0.02 | [6] | |
| 0.03 | [6] | |
| 0.01 | Assumed | |
| a | 0.5 | [7] |
| k | 0.55 | [7] |
| h | 2 | [7] |
| 0.15625 | Assumed | |
| 0.8 | Assumed | |
| e | 0.85 | [7] |
| 0.17 | [7] | |
| 0.17 | [7] | |
| 0.0145 | [7] | |
| 0.5 | [7] | |
| 0.08 | Assumed | |
| 0.05 | [7] |
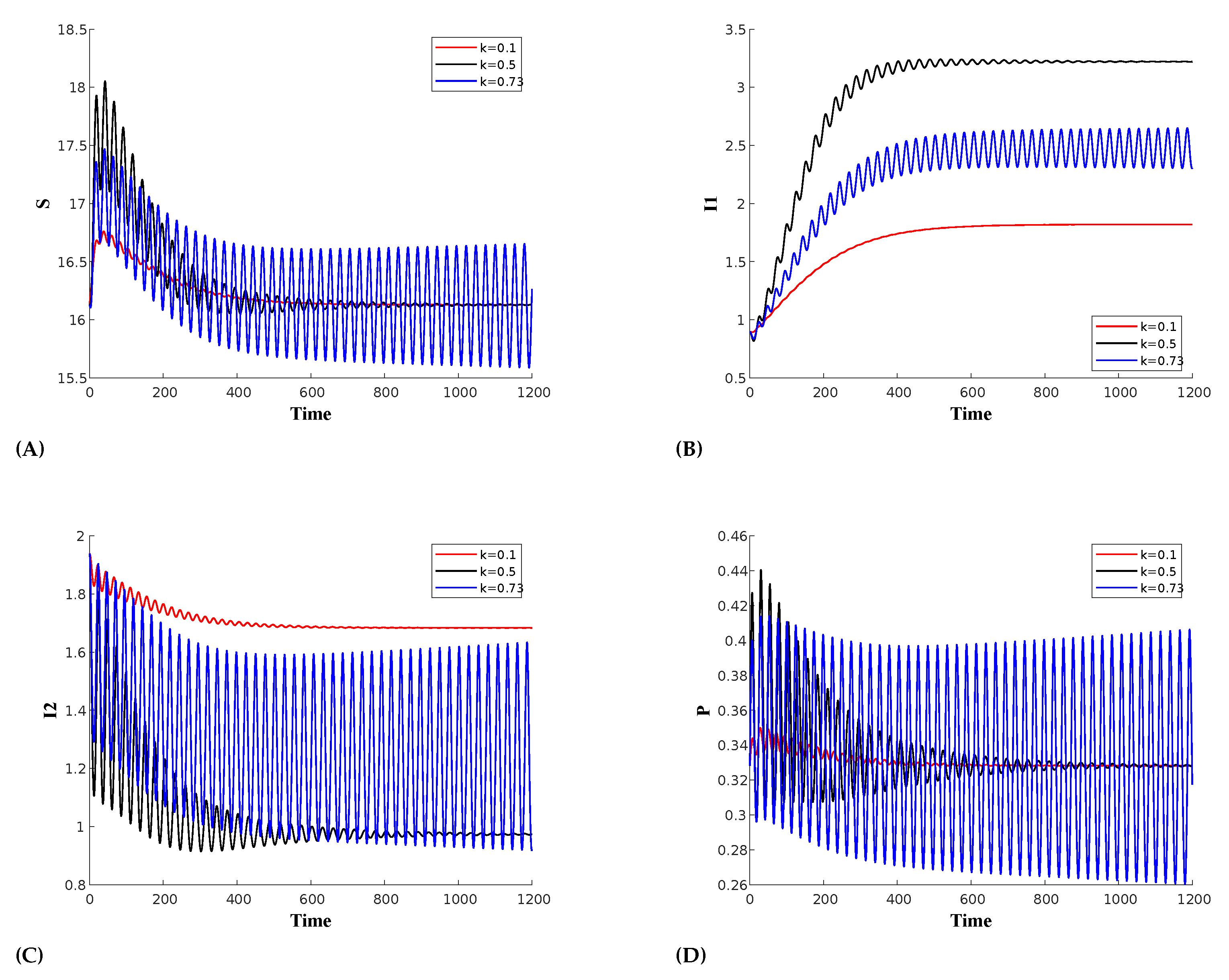
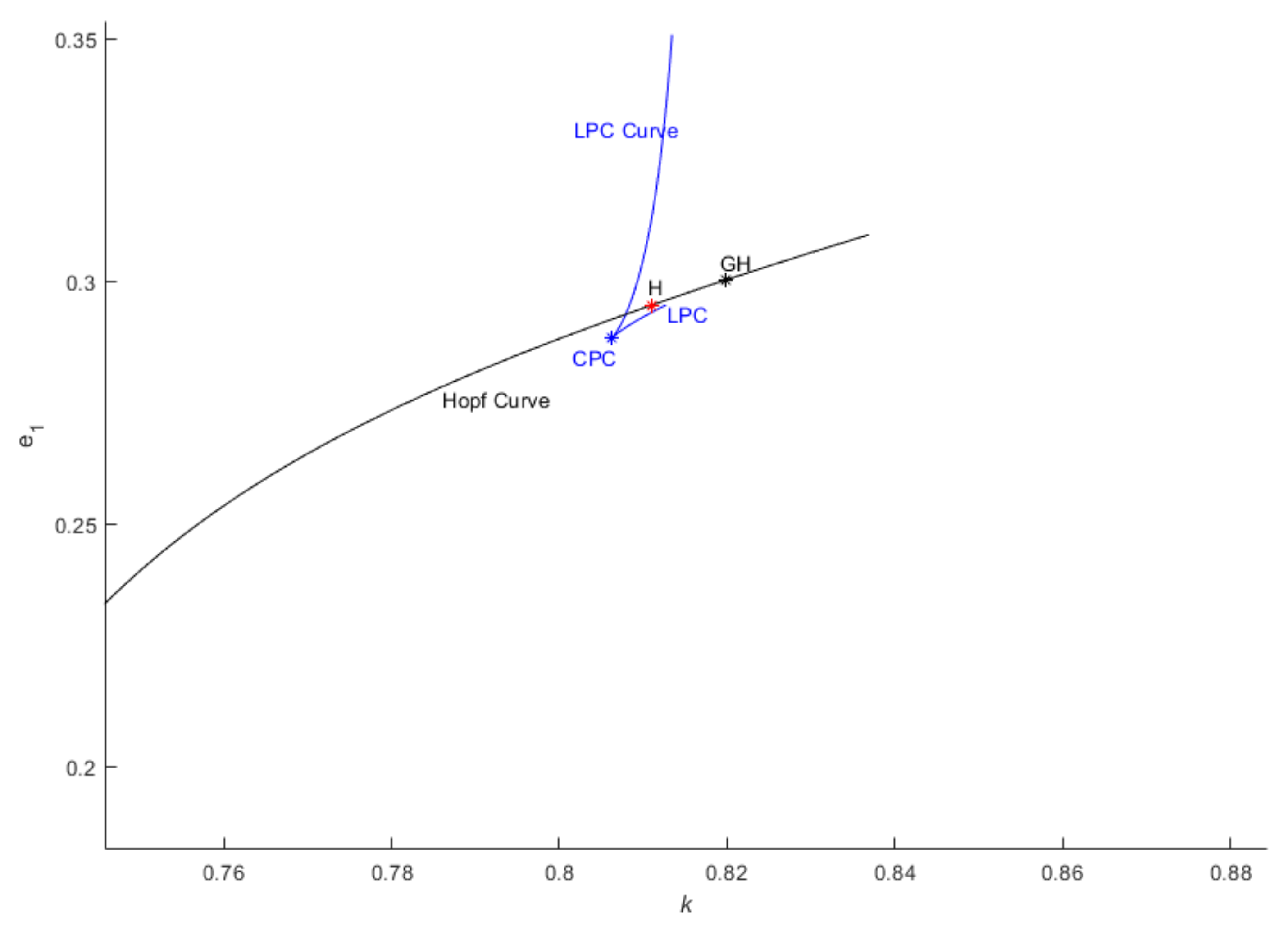
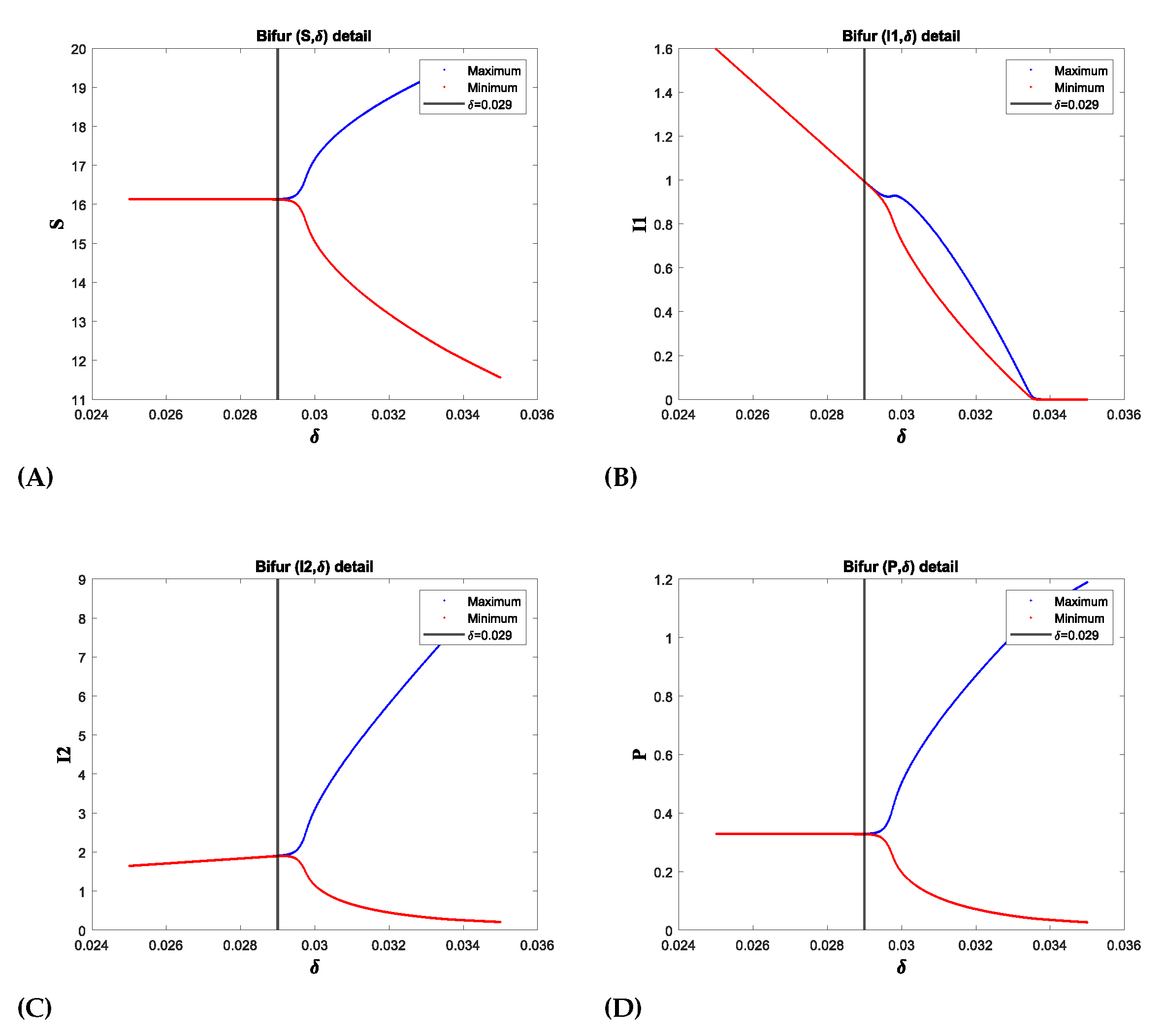
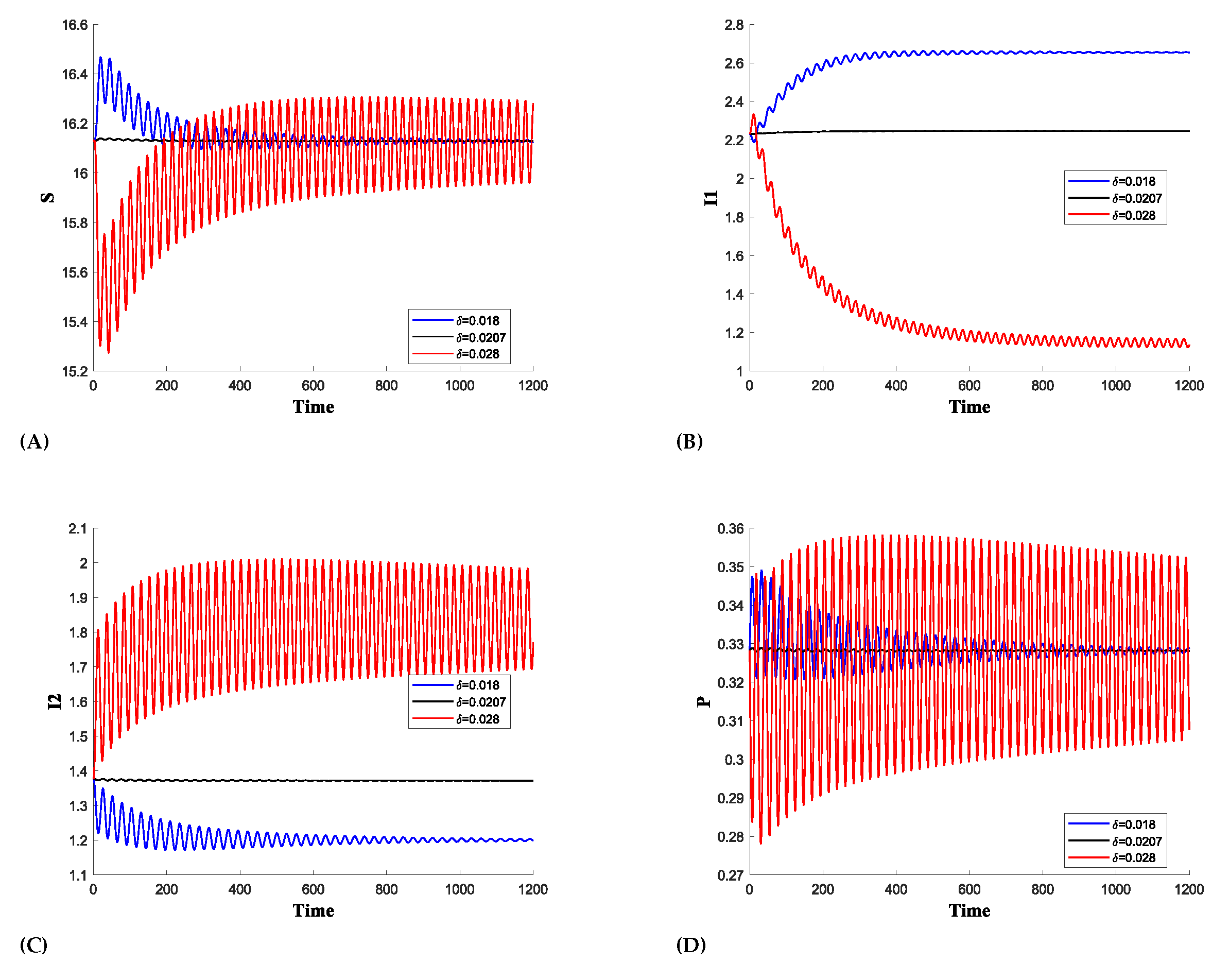
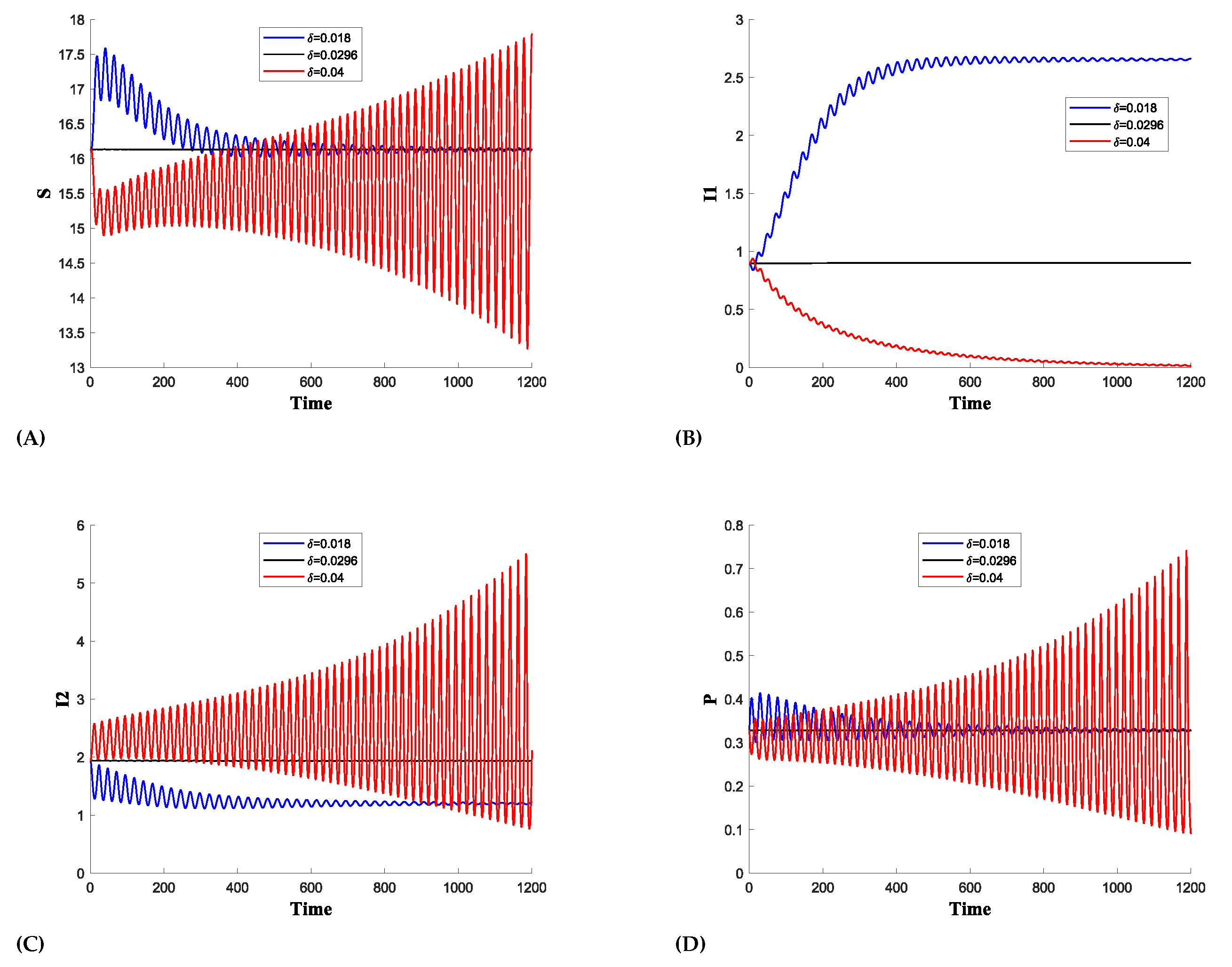
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Upadhyay, R.K.; Iyengar, S.R. K.; Rai, V. Chaos: an ecological reality? International Journal of Bifurcation and Chaos 1998, 08, 1325–1333. [Google Scholar] [CrossRef]
- Aziz-Alaoui, M. Study of a Leslie–Gower-type tritrophic population model. Chaos Solitons & Fractals 2022, 14, 1275–1293. [Google Scholar] [CrossRef]
- Verma, H.; Antwi-Fordjour, K.; Hossain, M.; Pal, N.; Parshad, R.D.; Mathur, P. A “Double” fear effect in a tri-trophic food chain model. The European Physical Journal Plus 2021, 136. [Google Scholar] [CrossRef]
- Verma, H.; Mathur, P. Analysis of chaotic situation in three species food chain model. Ganita 2019, 69, 57–65. [Google Scholar]
- Garai, S.; Hossain, M.; Karmakar, S.; Pal, N. Chaos, periodic structures, and multistability: Complex dynamical behaviors of an eco-epidemiological model in parameter planes. Chaos an Interdisciplinary Journal of Nonlinear Science 2023, 33. [Google Scholar] [CrossRef]
- Jana, S.; Kar, T. Modeling and analysis of a prey–predator system with disease in the prey. Chaos Solitons & Fractals 2013, 47, 42–53. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Srinivasu, P.; Bairagi, N. Pelicans at risk in Salton sea — an eco-epidemiological model. Ecological Modelling 2001, 136, 103–112. [Google Scholar] [CrossRef]
- Gupta, A.; Dubey, B. Bifurcations and multi-stability in an eco-epidemic model with additional food. The European Physical Journal Plus 2022, 137. [Google Scholar] [CrossRef]
- Upadhyay, R. K.; Roy, P. Spread of a disease and its effect on population dynamics in an eco-epidemiological system. Communications in Nonlinear Science and Numerical Simulation 2014, 19, 4170–4184. [Google Scholar] [CrossRef]
- Ghosh, M.; Li, X.Z. Mathematical modelling of prey-predator interaction with disease in prey. International Journal of Computing Science and Mathematics 2016, 7, 443. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Arino, O. A predator-prey model with disease in the prey. Nonlinear Analysis 1999, 36, 747–766. [Google Scholar] [CrossRef]
- Gupta, A.; Dubey, B. Bifurcation and chaos in a delayed eco-epidemic model induced by prey configuration. Chaos Solitons & Fractals 2022, 165, 112785. [Google Scholar] [CrossRef]
- Lazebnik, T.; Bunimovich-Mendrazitsky, S. Generic approach for mathematical model of multi-strain pandemics. PloS One 2022, 17, e0260683. [Google Scholar] [CrossRef]
- Alexi, A.; Rosenfeld, A.; Lazebnik, T. Multi-species prey–predator dynamics during a multi-strain pandemic. Chaos an Interdisciplinary Journal of Nonlinear Science 2023, 33. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Singh, S. The analysis of global stability boundary and multistability in the nonlinear dynamical system of an advanced heavy water reactor. Nuclear Science and Engineering 2017, 188, 187–197. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Bifurcation analysis of the simplified models of boiling water reactor and identification of global stability boundary. Nuclear Engineering and Design 2017, 315, 93–103. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Bifurcation analysis of density wave oscillations in natural circulation loop. International Journal of Thermal Sciences 2017, 120, 446–458. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Detailed bifurcation analysis with a simplified model for advance heavy water reactor system. Communications in Nonlinear Science and Numerical Simulation 2015, 20, 186–198. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Characterization of stability limits of Ledinegg instability and density wave oscillations for two-phase flow in natural circulation loops. Chemical Engineering Science 2017, 168, 204–224. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Bifurcations emerging from a double Hopf bifurcation for a BWR. Progress in Nuclear Energy 2019, 117, 103049. [Google Scholar] [CrossRef]
- Djilali, S.; Cattani, C.; Guin, L. N. Delayed predator–prey model with prey social behavior. the European Physical Journal Plus 2021, 136. [Google Scholar] [CrossRef]
- Ajraldi, V.; Pittavino, M.; Venturino, E. Modeling herd behavior in population systems. Nonlinear Analysis Real World Applications 2011, 12, 2319–2338. [Google Scholar] [CrossRef]
- Venturino, E.; Petrovskii, S. Spatiotemporal behavior of a prey–predator system with a group defense for prey. Ecological Complexity 2013, 14, 37–47. [Google Scholar] [CrossRef]
- Elena, E.; Grammauro, M.; Venturino, E.; Simos, T. E.; Psihoyios, G.; Tsitouras, C.; Anastassi, Z. Ecoepidemics with Two Strains: Diseased Prey. In Proceedings of the AIP Conference, 14 September 2011. [Google Scholar] [CrossRef]
- Elena, E.; Grammauro, M.; Venturino, E. Predator’s alternative food sources do not support ecoepidemics with twostrains-diseased prey. Network Biology 2013, 3, 29–44. [Google Scholar]
- Roman, F.; Rossotto, F.; Venturino, E. Ecoepidemics with two strains: diseased predators. WSEAS Transactions on Biology and Biomedicine 2011, 8, 73–85. [Google Scholar]
- Bosica, C.; De Rossi, A.; Fatibene, N.L.; Sciarra, M.; Venturino, E. Two-strain ecoepidemic systems: the obligated mutualism case. Applied Mathematics & Information Sciences 2015, 09, 1677–1685. [Google Scholar]
- Cavoretto, R.; Collino, S.; Giardino, B.; Venturino, E. A two-strain ecoepidemic competition model. Theoretical Ecology 2014, 8, 37–52. [Google Scholar] [CrossRef]
- Banerjee, M.; Kooi, B.; Venturino, E. An Ecoepidemic Model with Prey Herd Behavior and Predator Feeding Saturation Response on Both Healthy and Diseased Prey. Mathematical Modelling of Natural Phenomena 2017, 12, 133–161. [Google Scholar] [CrossRef]
- Djilali, S. Impact of prey herd shape on the predator-prey interaction. Chaos Solitons & Fractals 2019, 120, 139–148. [Google Scholar] [CrossRef]
- Antwi-Fordjour, K.; Westmoreland, S.P.; Bearden, K.H. Dual fear phenomenon in an eco-epidemiological model with prey aggregation. The European Physical Journal Plus 2024, 139. [Google Scholar] [CrossRef]
- Kooi, B.W.; Venturino, E. Ecoepidemic predator–prey model with feeding satiation, prey herd behavior and abandoned infected prey. Mathematical Biosciences 2016, 274, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Xiao, D.; Beier, J.C. On the Delayed Ross–Macdonald Model for Malaria Transmission. Bulletin of Mathematical Biology 2008, 70, 1098–1114. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Samanta, G.P. Analysis of a predator–prey model with herd behavior and disease in prey incorporating prey refuge. International Journal of Biomathematics 2019, 12, 1950007. [Google Scholar] [CrossRef]
- Venturino, E. The usefulness of mathematics in agriculture, for the environment and in contrasting diseases: insights from a wide range of simple models. Communications in Applied and Industrial Mathematics 2024, 15, 27–49. [Google Scholar] [CrossRef]
- Collino, S.; Venturino, E.; Ferreri, L.; Bertolotti, L.; Rosati, S.; Giacobini, M. Models for two strains of the Caprine Arthritis Encephalitis Virus Disease. In Biomat 2015, Mondaini, R.P.,Eds.; 2016, 297–318. [CrossRef]
- Fournié, G.; Walker, P.; Porphyre, T.; Métras, R.; Pfeiffer, D. Mathematical Models of infectious diseases in livestock: Concepts and application to the spread of highly pathogenic Avian influenza virus strain type H5N1. In Springer eBooks; 2011; pp. 183–205. [Google Scholar] [CrossRef]
- Doeschl-Wilson, A.; Knap, P.; Opriessnig, T.; More, S. Review: Livestock disease resilience: from individual to herd level. Animal 2021, 15, 100286. [Google Scholar] [CrossRef]
- Mandal, D.S.; Samanta, S.; Alzahrani, A.K.; Chattopadhyay, J. STUDY OF a PREDATOR–PREY MODEL WITH PEST MANAGEMENT PERSPECTIVE. Journal of Biological Systems 2019, 27, 309–336. [Google Scholar] [CrossRef]
- Verma, H.; Mishra, V.N.; Mathur, P. Effectiveness of lock down to curtail the spread of corona virus: A mathematical model. ISA Transactions 2022, 124, 124–134. [Google Scholar] [CrossRef]
- Mikheev, V.N.; Pasternak, A.F. Defense behavior of fish against predators and parasites. Journal of Ichthyology 2006, 46, S173–S179. [Google Scholar] [CrossRef]
- Demandt, N.; Praetz, M.; Kurvers, R.H.J.M.; Krause, J.; Kurtz, J.; Scharsack, J.P. Parasite infection disrupts escape behaviours in fish shoals. In Proceedings of the Royal Society B Biological Sciences, 4 November 2020. [Google Scholar] [CrossRef]
- Allan, B.J.M.; Illing, B.; Fakan, E.P.; Narvaez, P.; Grutter, A.S.; Sikkel, P.C.; McClure, E.C.; Rummer, J.L.; McCormick, M.I. Parasite infection directly impacts escape response and stress levels in fish. Journal of Experimental Biology 2020. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Zhang, X. Dynamics of a multi-strain malaria model with diffusion in a periodic environment. Journal of Biological Dynamics 2022, 16, 766–815. [Google Scholar] [CrossRef]
- Roldan, J.A.M.; Otranto, D. Zoonotic parasites associated with predation by dogs and cats. Parasites & Vectors 2023, 16. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; Zowalaty, M.E.E.; Ramadan, H.; Morgan, M.; Helmy, Y.A. Salmonellosis: An overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Siddik, S.B.M.; Abdullah, F.A.; Ismail, A.I.M. Mathematical Model of Dengue Virus with Predator-Prey Interactions. Sains Malaysiana 2020, 49, 1191–1200. [Google Scholar] [CrossRef]
- De Araújo, R.G.; Jorge, D.C.; Dorn, R.C.; Cruz-Pacheco, G.; Esteva, M.L.M.; Pinho, S.T. Applying a multi-strain dengue model to epidemics data. Mathematical Biosciences 2023, 360, 109013. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, S.T.; Adekunle, A.I.; McBryde, E.S.; Meehan, M.T. Modelling the ecological dynamics of mosquito populations with multiple co-circulating Wolbachia strains. Scientific Reports 2022, 12. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, H.; Sun, W.; Scoglio, C. Transmission dynamics of multi-strain dengue virus with cross-immunity. Applied Mathematics and Computation 2021, 392, 125742. [Google Scholar] [CrossRef]
- Martcheva, M. Evolutionary consequences of predation for pathogens in prey. Bulletin of Mathematical Biology 2009, 71, 819–844. [Google Scholar] [CrossRef]
- Dhooge, A.; Govaerts, W.; Kuznetsov, Y.A. MATCONT. ACM Transactions on Mathematical Software 2003, 29, 141–164. [Google Scholar] [CrossRef]
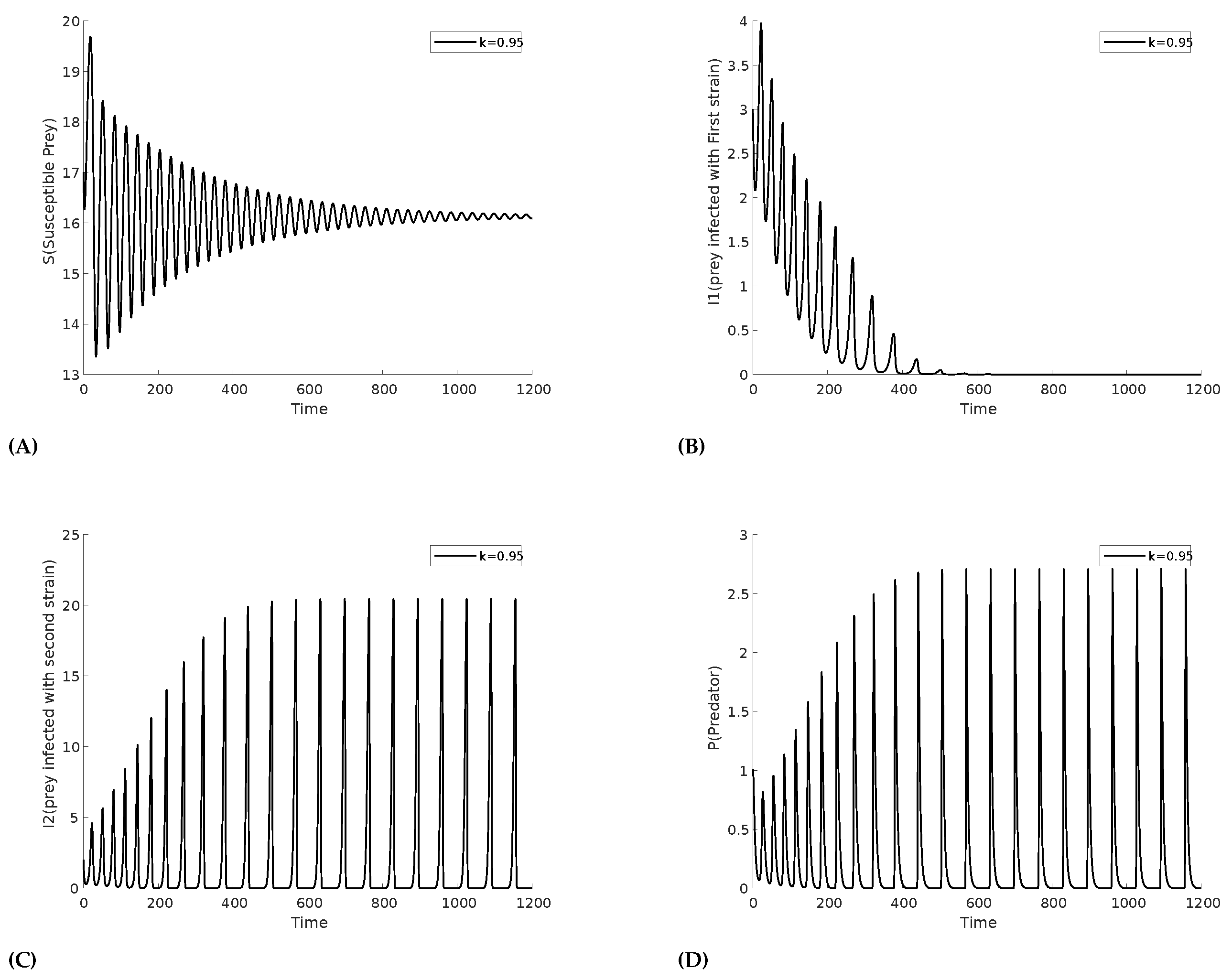
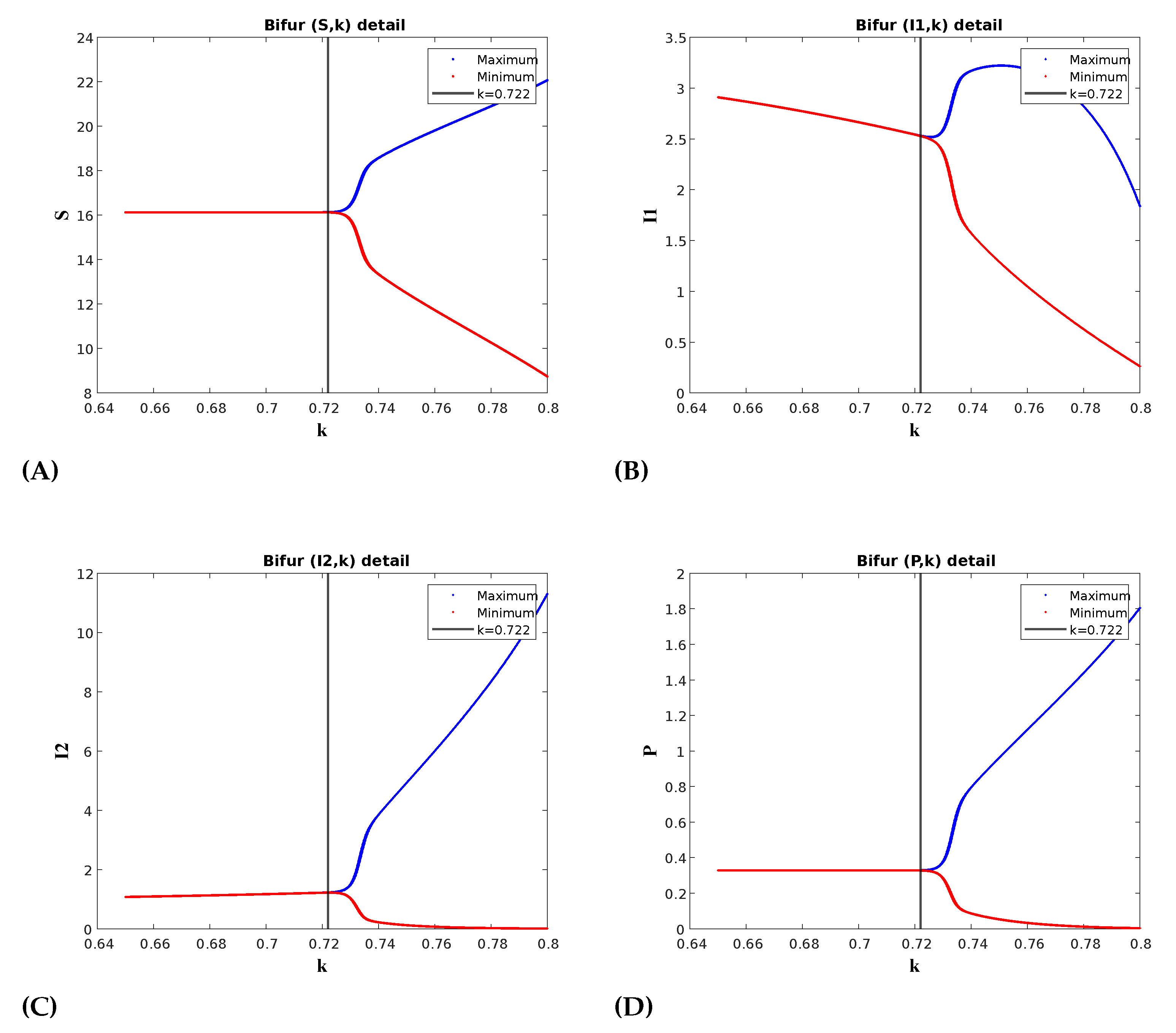
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





