1. Introduction
Metal contamination in aquatic ecosystems poses significant challenges to ecological health, necessitating the development and application of comprehensive assessment methods. Ecological risk assessments at a metal contaminated site typically involve three complementary approaches: prospective assessments, ongoing ecotoxicological monitoring, and bioassessment [
1,
2,
3]. Prospective assessments, utilizing chemical monitoring data, predict potential risks, while ecotoxicological monitoring evaluates the current status of aquatic ecosystems. bioassessments, conducted in the field using ecological bioindicators, are essential for confirming the actual ecological impact of metals based on causal associations [
4].
The integration of these approaches provides a more comprehensive understanding of metal contamination effects by incorporating multiple lines of evidence. This integrated framework aids in diagnosing chemical, ecotoxicological, and ecological conditions, offering a holistic view of environmental health. Moreover, it is particularly valuable in identifying key toxicants and stressors that may not be apparent through predictive models or current monitoring alone [
5].
Traditionally, ecological indices such as species diversity and richness have been widely employed as general indicators of ecological status. However, these indices assume that all species contribute equally to the ecological system based solely on their presence-absence and abundance [
6]. This assumption may lead to an oversimplification of complex ecological dynamics and potentially obscure the specific impacts of environmental stressors [
7].
To address these limitations, more sophisticated trait-based bioindicators have been developed. The Species At Risk (SPEAR) index represents a significant advancement in this field [
8,
9,
10]. SPEAR integrates trait information, such as physiological sensitivity, into community structure data, allowing for a more nuanced understanding of ecosystem health, particularly in relation to pesticide and metal contamination.
Another example of a trait-based index is the Benthic Macroinvertebrate Index (BMI), which includes measures such as the Biological Monitoring Working Party (BMWP) [
11] and the Korean Benthic macroinvertebrate Index of Biological Integrity (KB-IBI) [
12,
13]. These indices incorporate trait information on tolerance against biological oxygen demand, providing insights into organic matter pollution and low dissolved oxygen levels in aquatic ecosystems.
The incorporation of trait information in these indices represents a significant improvement over traditional metrics. By considering species-specific characteristics and sensitivities, these advanced bioindicators enable the identification of key stressors responsible for diagnosed ecological impacts [
8,
14]. This trait-based approach provides a more comprehensive and accurate assessment of ecosystem health, allowing for more effective environmental management and conservation strategies.
Despite these advancements, the practical application of metal-specific bioindicators, such as
, to field datasets has been limited [
15]. [
16] made a pioneering contribution by applying existing metal sensitivity data to field observations. Their study revealed a discrepancy between laboratory-derived physiological sensitivity and observed field effects, underscoring the complexity of natural ecosystems and the limitations of extrapolating laboratory results to real-world scenarios.
Interestingly, the study by [
16] found that predator ratios served as a more meaningful community descriptor in elucidating the effects of metal contamination in field conditions. These findings emphasize the critical need for further validation of the
index across diverse metal-contaminated regions. Such validation would help to establish the robustness and applicability of the index across different ecological contexts and contamination scenarios.
To enhance the reliability of the
index, further validation across diverse metal-contaminated regions is essential, along with the incorporation of additional sensitivity data, particularly for a wider range of taxa, with an emphasis on insect species [
16]. Expanding the sensitivity database would allow for the derivation of more refined sensitivity values at specific taxonomic levels, potentially increasing the precision and broadening the applicability of the
index in different ecological contexts.
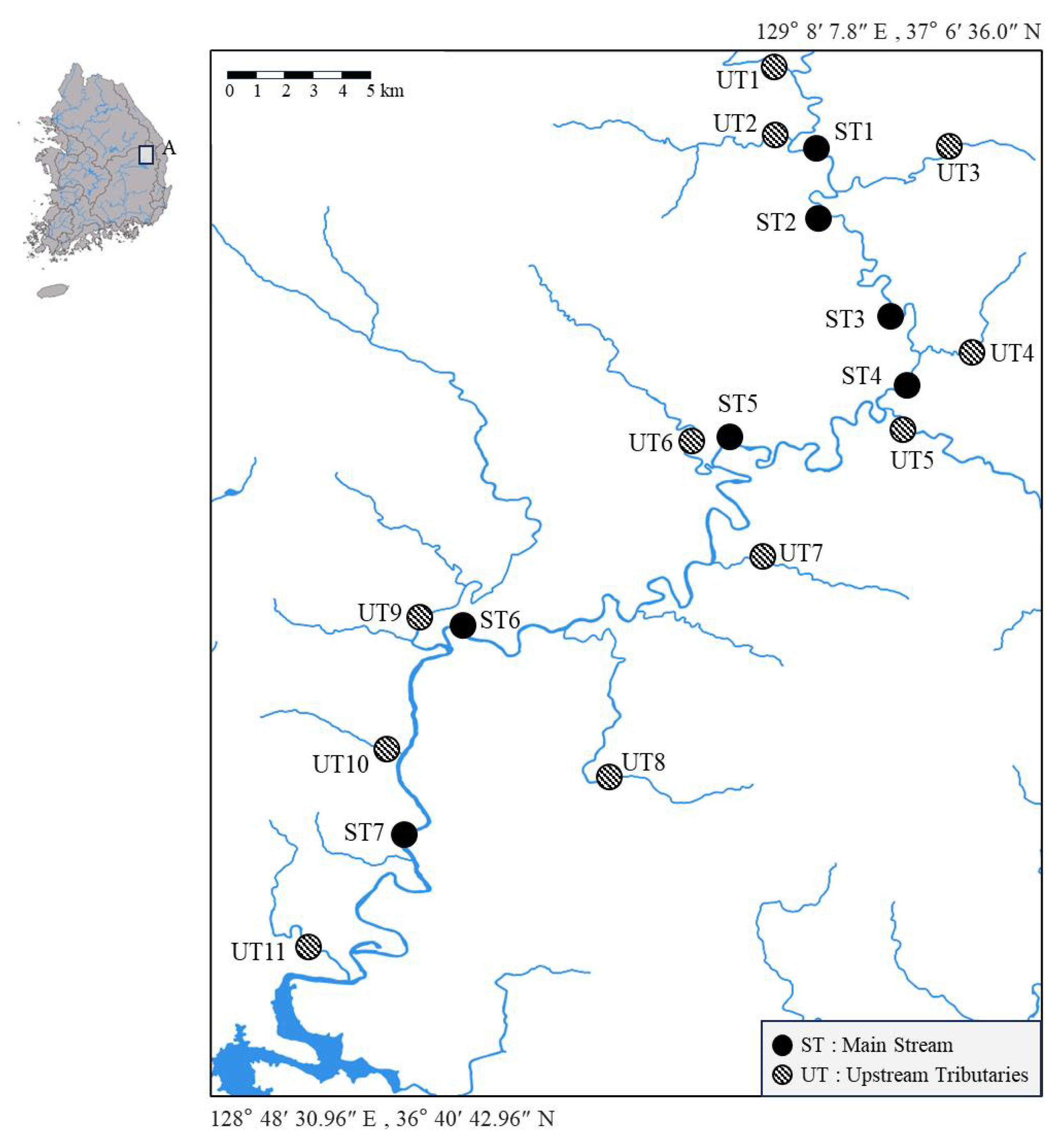
This study also calculates and compares the index across different sections of the watershed, including the main stream and tributaries. Such site-specific analyses provide a deeper understanding of the spatial variability in metal contamination impacts, potentially revealing distinct contamination patterns, identifying areas of concern, and highlighting zones of ecological resilience within the watershed. This refined trait-based approach would better bridge the gap between laboratory-derived data and field observations, ultimately leading to more effective environmental management strategies.
The present study aims to validate the SPEAR index as an effective tool for assessing the ecological impacts of metal contamination in aquatic ecosystems. Specifically, the refined SPEAR index is applied to the upper Nakdong River, a region significantly affected by industrial activities, including zinc smelting and mining operations. This refined index incorporates newly obtained acute toxicity data from 20 indigenous species of the Korean peninsula, thereby enhancing its ecological accuracy and regional applicability. By evaluating the index across various sections of the watershed, including the main stream and tributaries, this study offers a comprehensive analysis of metal-induced ecological impacts and identifies key stressors and pollution sources within this critical river system in South Korea.
3. Results
3.1. Updated Metal Sensitivity and SPEAR Index Calculation
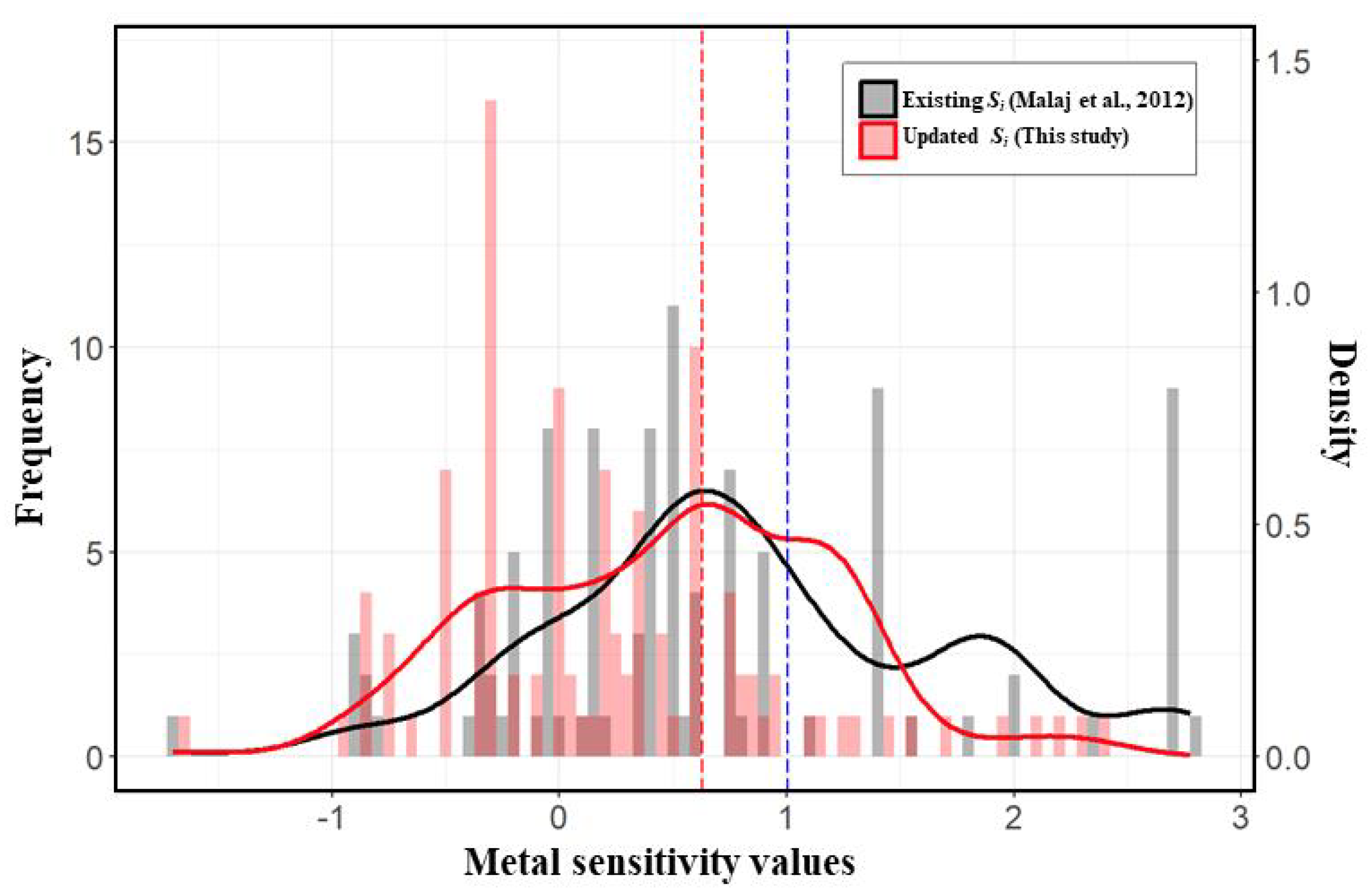
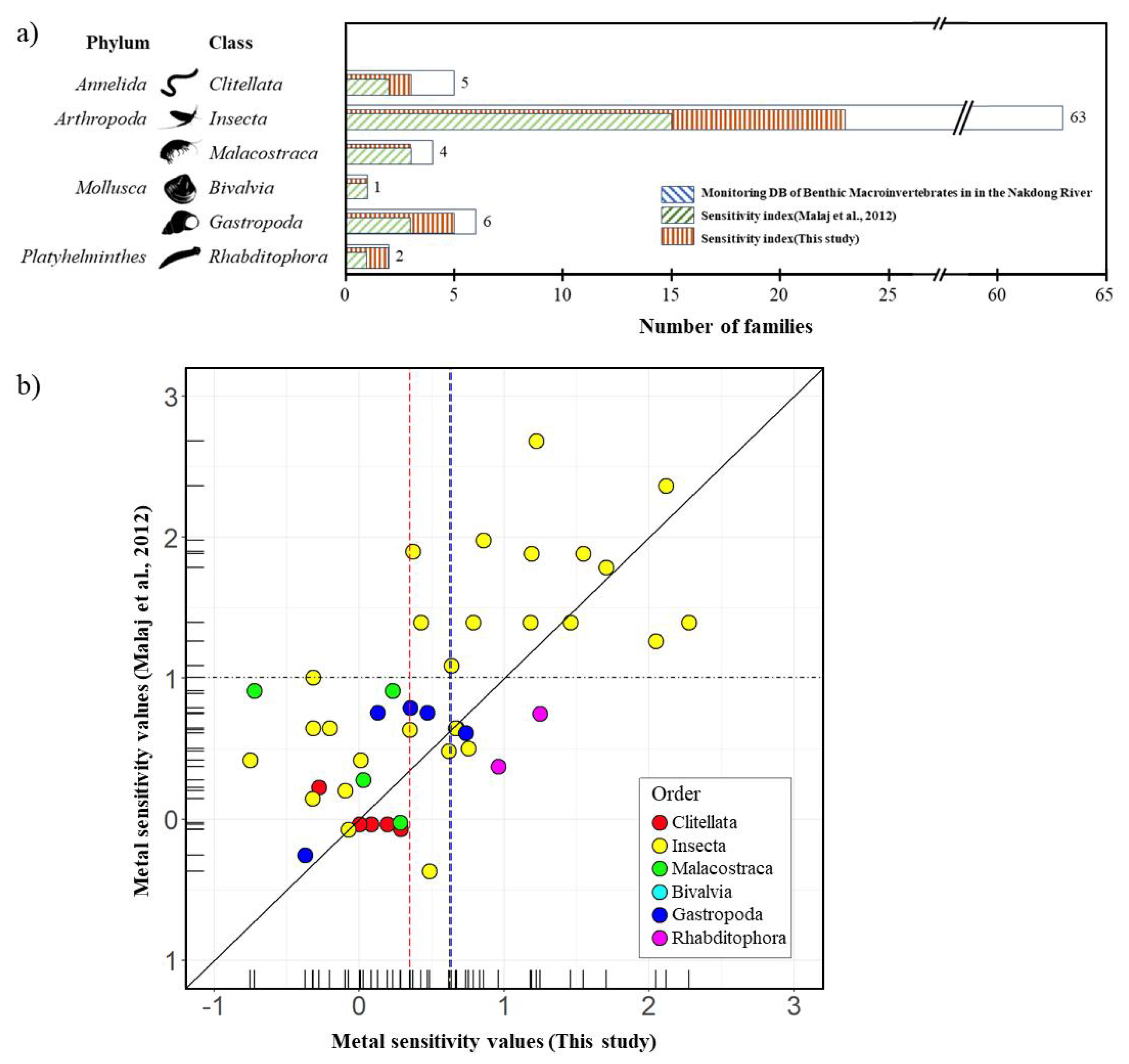
Metal sensitivity was updated using new toxicity test results for twenty indigenous species in Korea, primarily focusing on insect taxa (Table S1). The total number of families with available toxicity data increased from 122 in 2012 to 411 in the current study. The median value of metal sensitivity shifted from 1.01 in 2012 to 0.63 in the present study (
Figure 2). The LME model analysis revealed no statistically significant differences among the rankings of the 11 heavy metals examined (Figure S1 and Table S2). Consequently, an overall heavy metal sensitivity value (
) was calculated for each taxon (Table S2 and S3). The most sensitive species were identified as
Villorita cyprinoides (-2.89),
Physella gyri (-2.58), and
Leptodea leptodon (-2.00), with families Mycetopodidae (-1.88) and Hyalellidae (-0.97) also showing high sensitivity. Among insect orders, Coleoptera (-0.31) and Ephemeroptera (-0.009) were most sensitive, while Odonata (1.82) were relatively tolerant. Other taxa such as Plecoptera (1.18) and Trichoptera (1.19) exhibited intermediate sensitivity.
In the upper Nakdong River, 81 families across 6 classes of species were recorded, with Insecta representing the highest abundance at 94%. The most abundant orders within Insecta were Ephemeroptera (50%), Diptera (17%), and Trichoptera (8.1%). The taxon-specific coverage of the metal sensitivity index in this study accounted for 46% of the recorded taxa, compared to 31% in the previous sensitivity index reported by [
15]. Insecta had the lowest coverage, with 37% of the 63 families covered in this study, while the previous research reported only 24% coverage. Other taxa in this study showed coverage rates exceeding 60% (
Figure 2).
Figure 3 illustrates the impact of the updated ecotoxicity database on taxon coverage and metal sensitivity. The percentage of families with available toxicity data increased from 44% to 63% of the total number of families recorded in the study area (
Figure 3-a).
Figure 3-b demonstrates the correlation between metal sensitivities derived from the existing and updated ecotoxicity databases.
The analysis of sensitivity indices revealed distinct patterns across different datasets and stream types. For the whole dataset, the median value of -2024t exceeded that of -2012, indicating an overall increase in sensitivity with the updated index. In the main stream, -2024t demonstrated a higher median value compared to -2024s. However, this difference was not observed in the tributaries, where the median values of -2024t and -2024s were comparable.
For instance, in the Plecoptera order, the previous study assigned a uniform sensitivity value (1.39) to all lower taxa, whereas our study calculated different sensitivity values for each family: Chloroperlidae (0.78), Nemouridae (0.42), Perlodidae (1.45), and Perlidae (2.27). Conversely, Capniidae, Leuctridae, and Taeniopterygidae were assigned the sensitivity value of the higher taxonomic group (1.18). The median sensitivity value for species in the upper Nakdong River was 0.63 in our study, compared to 1.01 derived from the previous sensitivity index.
3.2. Association between Metal Concentrations and SPEAR Index
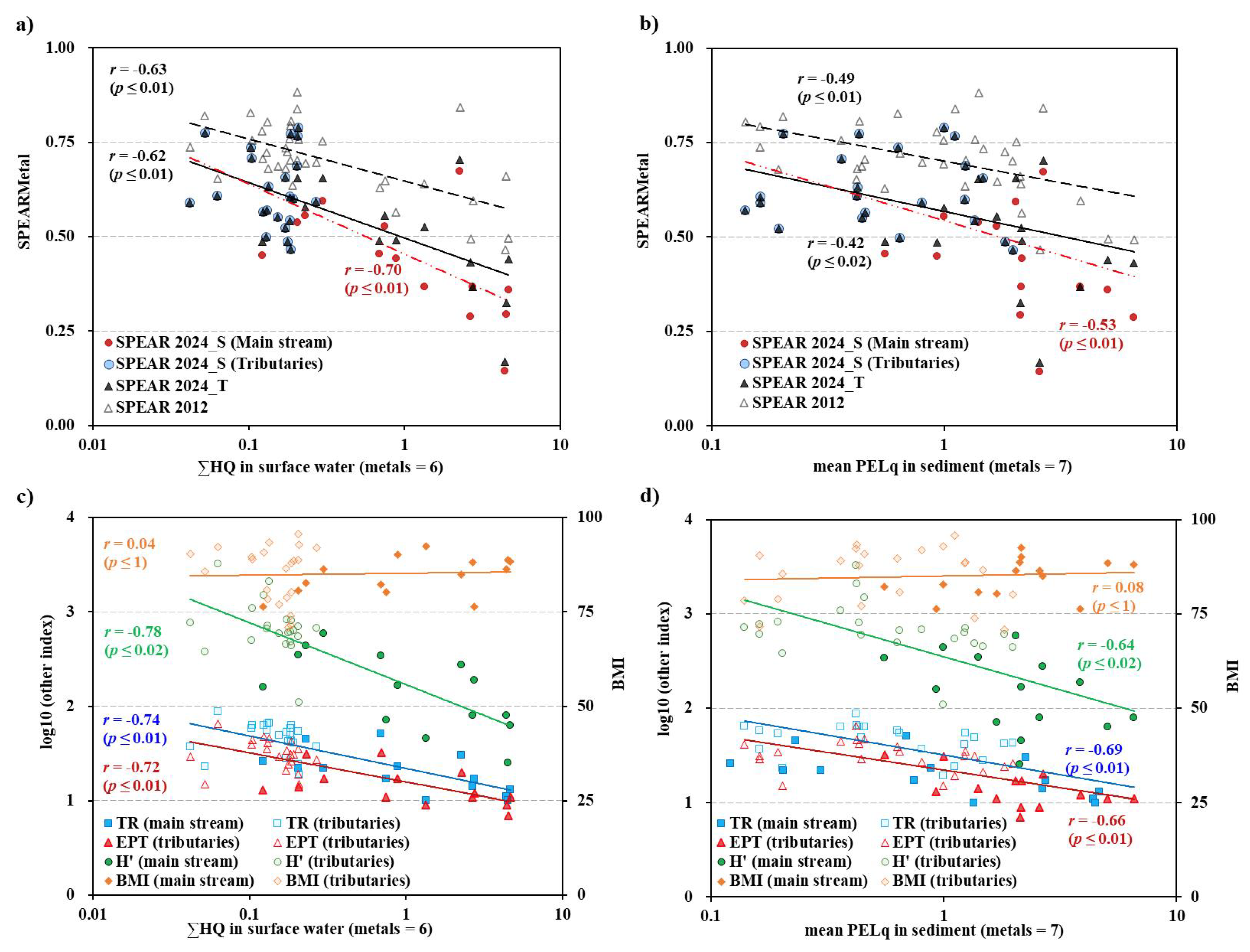
The updated SPEAR index values for whole dataset (SPEAR-2024t) based on the new metal sensitivity data were lower than those derived from the previous version (SPEAR-2012) (
Figure 4-a). The median values were 0.70 for SPEAR-2012 and 0.56 for SPEAR-2024t, with a mean difference of 0.14±0.06(mean±SD). While most SPEAR-2012 values exceeded 0.5, the majority of SPEAR-2024 values in the main stream fell below the severe level of SPEAR index (0.5) [
10,
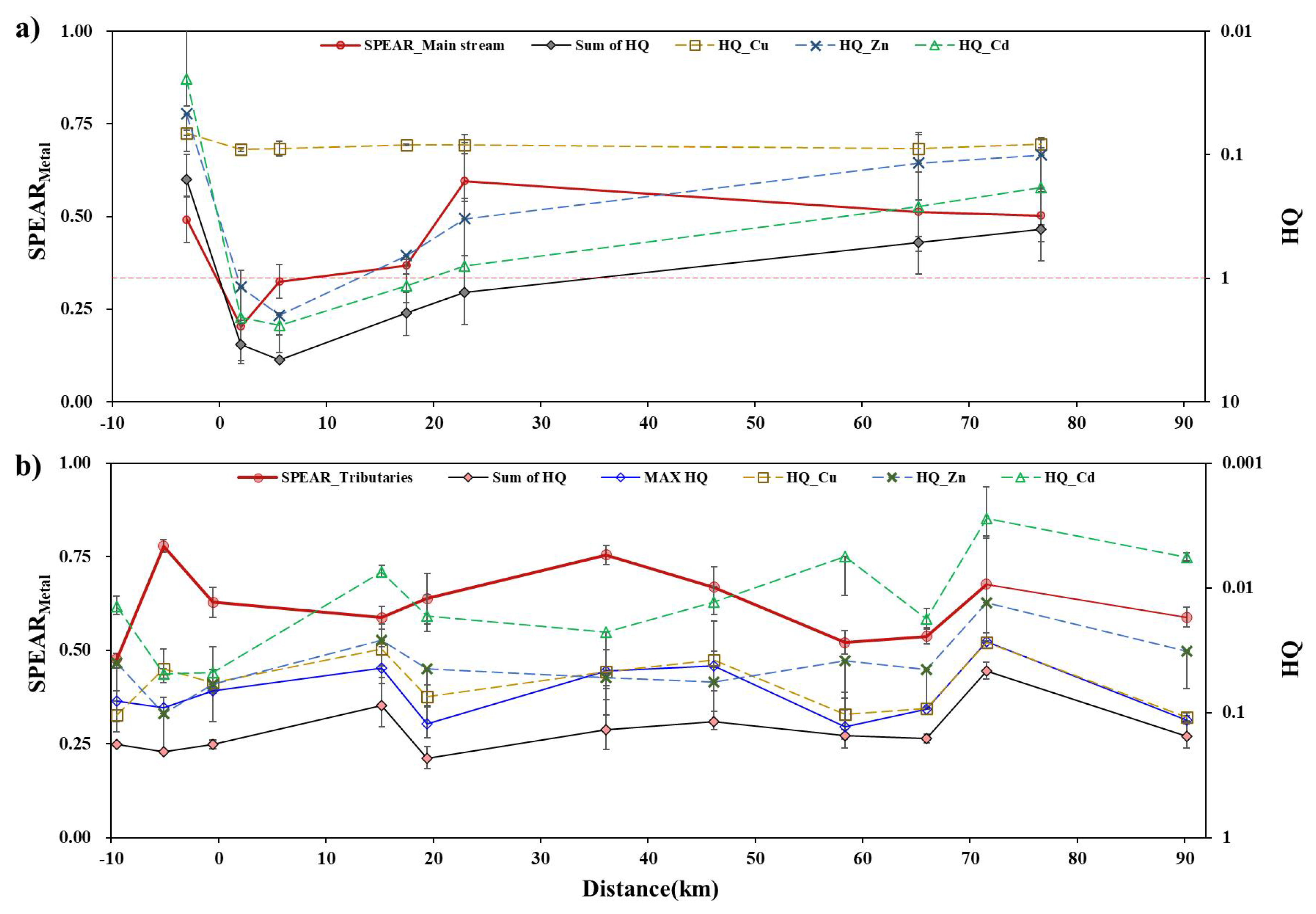
34]. SPEAR-2024s (median value: 0.64) calculated separately for main stream and tributaries, yielded lower values than SPEAR-2024t (0.56), with a mean difference of 0.13±0.15. A signicifant difference was observed between SPEAR-2024t (0.56) and SPEAR-2024s (0.45) in the main stream (mean difference: 0.11±0.0085), but not in the tributaries (0.04±0.0042). These results mean that the separting calculation of SPEAR index is more sensitive to metal contamination.
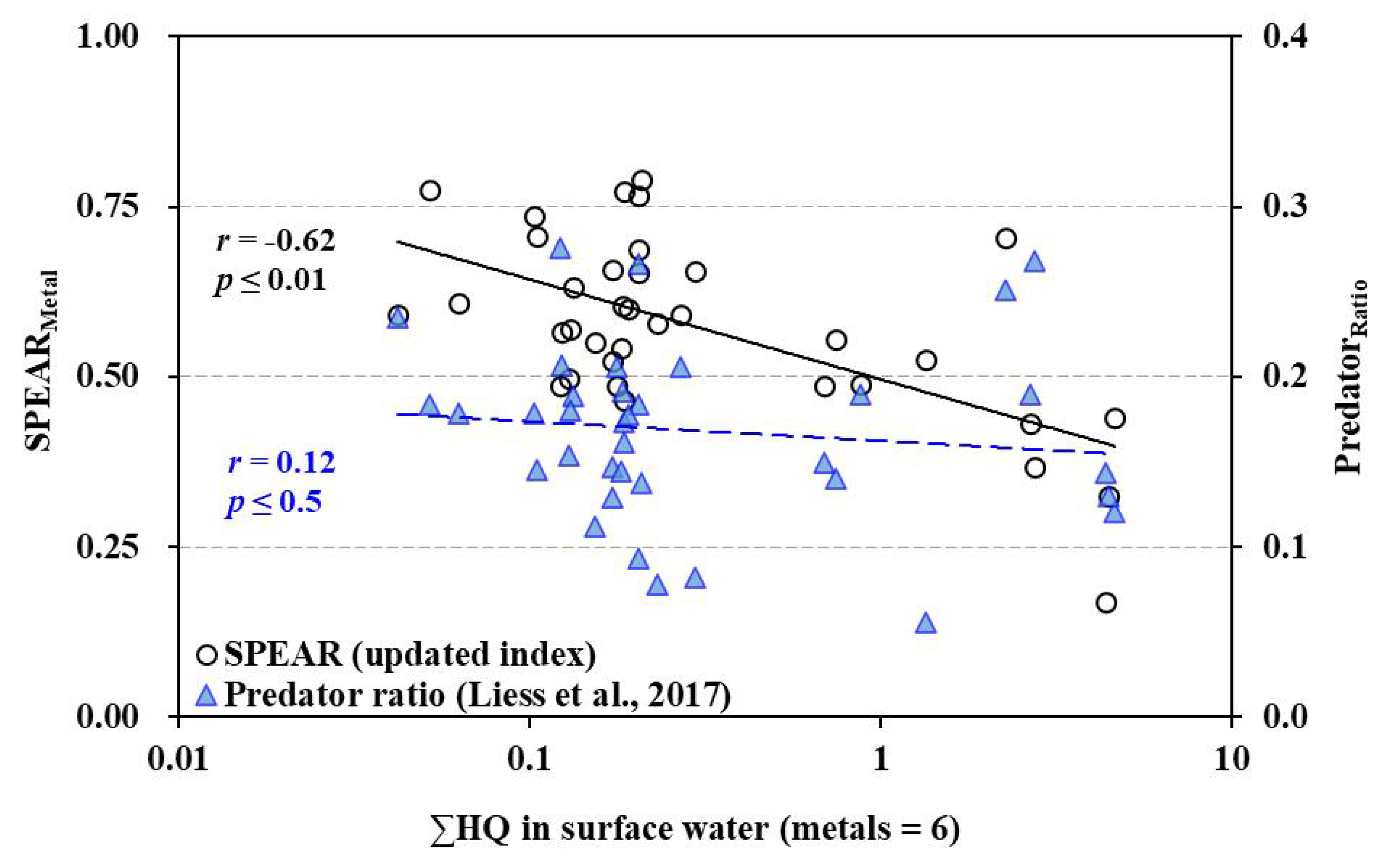
The correlation coefficients (
r) between SPEAR indexs and the sum of HQ in surface water were -0.63 (SPEAR-2012), -0.62 (SPEAR-2024t), and -0.70 (SPEAR-2024s). The refined SPEARs showed a significant correlation with both the sum of HQ for six metals in water and mPELq for seven metals in sediment (
Figure 4-b). The correlation coefficients (
r) between SPEAR indexs and mPELq in sediment were -0.49 (SPEAR-2012), -0.42 (SPEAR-2024t), and -0.53 (SPEAR-2024s). The correlation coefficients (
r) between SPEAR-2024s were greater than thos of SPEAR-2012 and SPEAR-2024t. These results mean that the separting calculation of SPEAR index is more specific to metal contamination.
3.3. Association between Metal Contamination and Generic Ecological Indices
The relationship between metal contamination and various ecological indices was examined to assess the specificity of observed impacts. Generic ecological indices, including TR, EPT, H’, showed significant negative correlations with both
and mPELq (
Figure 4c and 4d). Specifically, sites with higher metal concentrations exhibited reduced species richness (
r = -0.74,
p < 0.01), a lower proportion of EPT taxa (
r = -0.72,
p < 0.01), and decreased H’ values (
r = -0.78,
p = 0.02) compared to uncontaminated sites.
These correlations suggest a selective disappearance of metal-sensitive species from contaminated areas. In contrast, the Benthic Macroinvertebrate Index (BMI), which is primarily sensitive to organic pollution, showed no significant correlation with metal contamination levels (r = 0.11, p > 0.05). This lack of association between BMI and metal contamination indicators ( and mPELq) suggests that organic matter pollution was not a significant stressor in the studied system, further emphasizing the specificity of the observed ecological impacts to metal contamination.
3.4. Relationship between Individual Metal Concentration and SPEAR Index
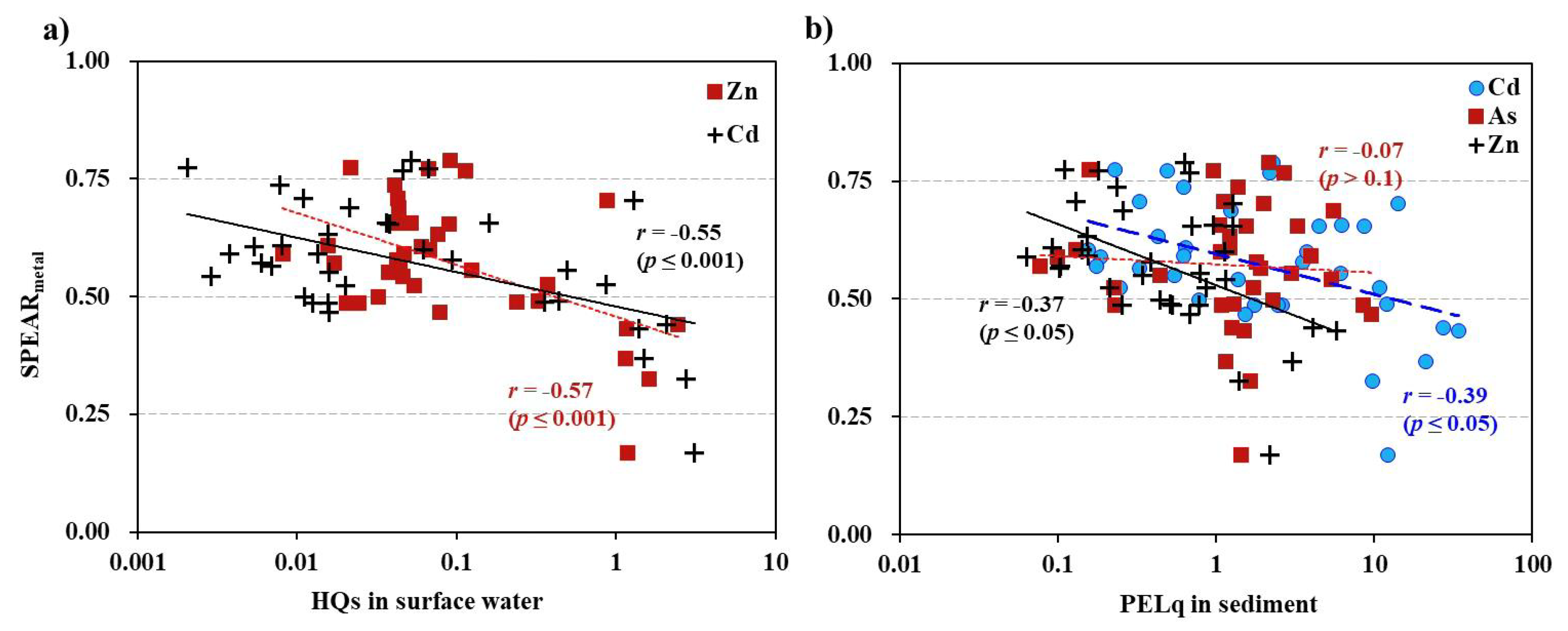
In the main stream samples, Cd and Zn were identified as potential key toxicants in surface water through HQ analysis (Chung et a., 2024). SPEAR-2024s values for metals in the main stream and whole dataset also showed significant correlations with HQ values of Cd and Zn, with HQ ranges from 0.02 to 3.1 (
Figure 5-a). Other metals did not show significant relationships with SPEAR-2024s. In sediment samples, while both Cd and As were previously identified as key toxicants contributing to sediment toxicity, Cd and Zn displayed a significant relationship with the
(
r = 0.39,
p < 0.05 for Cd;
r = 0.37,
p < 0.05 for Zn). As did not show a significant correlation. Notably, Cd PELq values exceeded 1 at several sites, while As PELq values remained below 1. Zn, despite PELq values below 1, demonstrated a significant relationship with the SPEAR index (
r = 0.08,
p > 0.1).
A clear gradient of decreasing SPEAR index values was observed along the main stream, correlating with increasing distance from the Zn smelter. Near the smelter, HQ values exceeded 1, with both the sum and maximum HQ values following a similar pattern. Specifically, HQ values for Zn and Cd were greater than 1 in proximity to the smelter. In contrast, SPEAR values in the tributaries showed no discernible pattern, and the sum of HQ values remained below 1 (Figure A2).
In conclusion, Cd and Zn were identified as the primary stressors responsible for the ecological impacts of metal contamination in the upper Nakdong River near and downstream of the Zn smelter.
3.5. Multivariate Statistical Analysis to Analyze the Associtat Between Environmental Factors and Community Indices
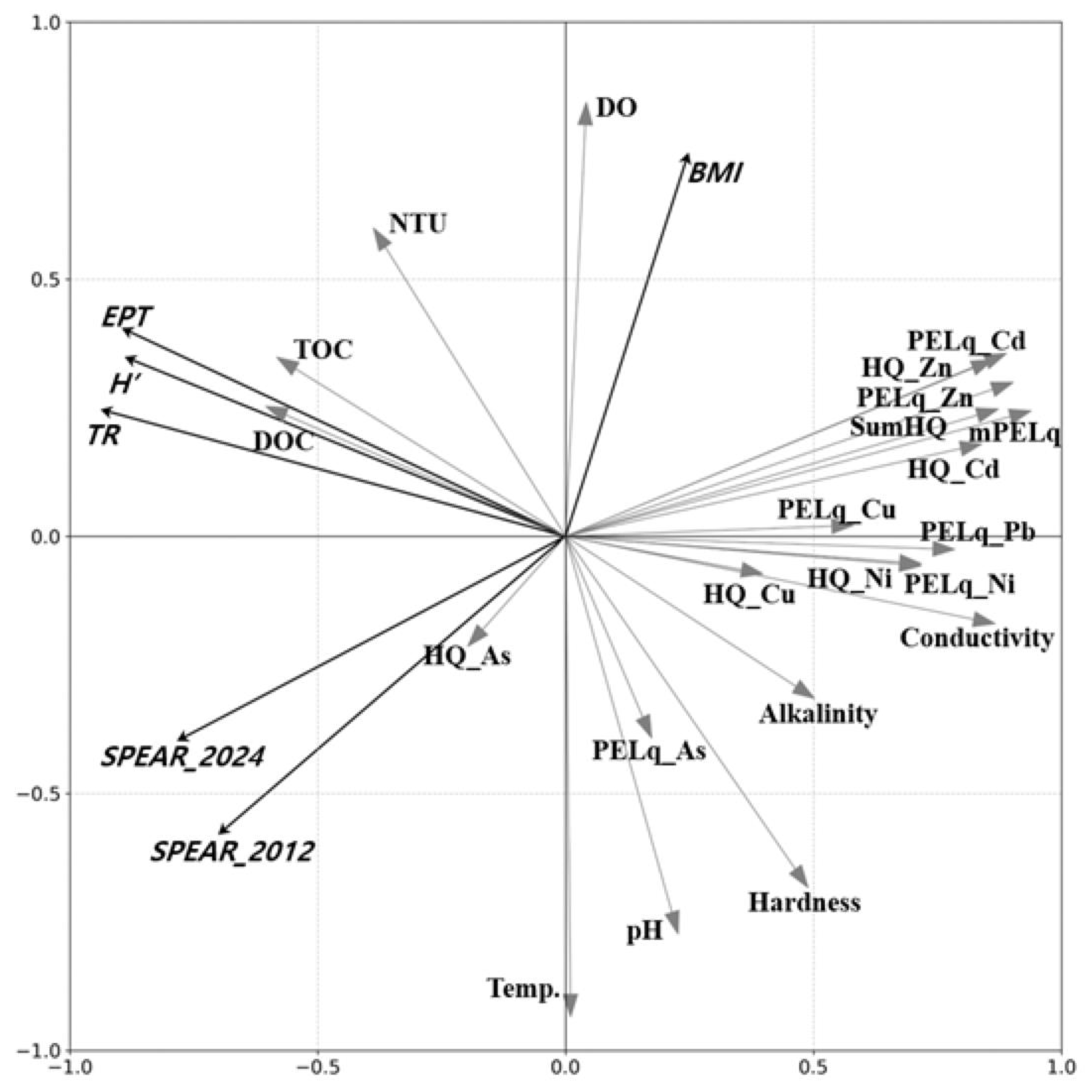
Principal Component Analysis (PCA) was employed to elucidate the associations between environmental factors and community indices.
Figure 6 presents the ordination plots from the PCA, illustrating the relationships between ecological indices and environmental factors for the total samples from the main stream and tributaries citeliess2005analyzing. This visual representation aids in the interpretation of the complex interactions between various environmental parameters and community indices in the studied ecosystem. These findings highlight the value of using multiple indices, including both generic and stressor-specific indicators like SPEAR, in comprehensively assessing the ecological impacts of metal contamination in aquatic ecosystems.
The first principal component (PC1) demonstrated strong negative associations with most community indices, except for BMI. PC1 also showed negative correlations with metal contamination indicators, with the exception of As, and with conductivity. Notably, BMI did not show a significant association with PC1, suggesting its independence from metal contamination effects.
The second principal component (PC2) exhibited a strong positive association with BMI and weak positive associations with other community indices. Interestingly, generic community indices (TR, EPT, and H’) showed weak positive associations with PC2, while SPEAR indices demonstrated weak negative relationships. PC2 was positively correlated with dissolved oxygen (DO) and turbidity (NTU), and weakly associated with total organic carbon (TOC), dissolved organic carbon (DOC), Cd (both HQ-Cd and PELq-Cd), Zn (both HQ-Zn and PELq-Zn), HQ, and mPELq. Conversely, PC2 showed negative relationships with temperature, pH, and water hardness.
The PCA results also revealed that metal contamination, particularly by Cd and Zn, had weak negative relationships with SPEAR indices but weak positive relationships with other community indices. This differential response highlights the specificity of the SPEAR indices in detecting metal contamination effects.
In summary, the multivariate analysis yielded the following key findings: (1) BMI did not showed any correlation with metal contamination, but positive correlations with NTU and DO, weak positive associations with DOC and TOC, and negative correlations with temperature, pH, and water hardness. (2) Other community indices (TR, EPT, and H’) were associated with metal contamination as well as conductivity and alkalinity. (3) SPEAR indices demonstrated strong associations with metal contamination, particularly with Cd and Zn. The main stressors, such as Cd and Zn, showed stronger associations with SPEAR indices compared to generic community indices, underscoring the specificity of SPEAR in detecting metal contamination effects.
3.6. Discussion
This study provides a comparative analysis of two fundamental approaches to assessing ecological risks from metal contamination: toxicological methods and eco-epidemiological techniques. Each method offers distinct advantages that, when integrated, allow for a more comprehensive understanding of how metal pollution affects aquatic ecosystems [
35,
36].
Toxicological approaches, such as the calculation of HQ based on controlled experiments, offer predictive insights into ecological impacts through dose-response relationships. While these methods are powerful for identifying potential risks, they may not fully account for the complex, real-world interactions among multiple stressors [
35,
37].
Eco-epidemiological methods, on the other hand, provide a retrospective perspective by focusing on the observed ecological state of ecosystems affected by contamination. These approaches, particularly through the use of bioindicators, can directly reflect the cumulative impact of stressors like metals on community structure and function [
15,
16,
28,
38].
Trait-based bioindicators, such as SPEAR index, add significant value to eco-epidemiological methods by targeting species traits sensitive to specific stressors. The SPEAR index has been shown to offer a more precise and focused diagnosis of metal-induced effects in aquatic ecosystems. However, limitations exist, particularly the potential for confounding factors, such as the presence of other pollutants or environmental variables, that could obscure the association between metal exposure and ecological impact [
15,
16].
The application of the SPEAR index for metals in the upper Nakdong River watershed provided significant insights into ecosystem responses to metal contamination. Both existing and updated SPEAR
indices showed robust associations with metal contamination levels in water (HQ) and sediment (mPELq) (
Figure 4). Notably, this study is the first to demonstrate a correlation between SPEAR
and metal contamination in water and sediment, expanding the index’s utility beyond water column assessments. This relationship suggests that
is sensitive to integrated metal exposure effects from both water and sediment compartments, reflecting long-term exposure and bioaccumulation processes.
These findings underscore the robustness of SPEAR as an indicator of metal contamination across different environmental compartments, enhancing its ecological relevance and potential for holistic ecosystem health assessment. The index’s ability to bridge the gap between chemical monitoring data and ecological effects offers a more integrative approach to assessing metal impacts in aquatic ecosystems. Further research is needed to explore the mechanistic basis of the relationship between SPEAR and sediment contamination, and to validate these findings across diverse aquatic ecosystems with varying sediment characteristics and metal contamination profiles.
The updated metal sensitivity database, encompassing a substantial portion of families in the study area, enhanced the index’s sensitivity (
Figure 4). This improvement allowed for the detection of subtle shifts in community structure, which is crucial for early warning in environmental monitoring programs. A key finding was that calculating the SPEAR index separately for main streams and tributaries increased its sensitivity and diagnostic power. This result underscores the importance of considering habitat-specific variations when applying ecological indices, aligning with research advocating for the subdivision of study areas based on ecological or hydrological characteristics [
34].
To elucidate the complex interactions between various environmental factors and metal contamination, Principal Component Analysis (PCA) was employed. This multivariate approach revealed intricate relationships between metal contamination and other environmental variables, providing a more comprehensive understanding of how multiple factors shape aquatic community structures [
39,
40].
The study highlights the importance of incorporating uncertainty analysis in the application of the SPEAR index. Reporting the percentage of family coverage in the watershed would provide transparency and improve decision-making regarding the index’s reliability in different ecological contexts [
15].
Interestingly, the predator ratio, previously proposed as a diagnostic tool for metal contamination, did not correlate with either the SPEAR index or the sum of hazard quotients. This discrepancy suggests that different bioindicators may respond differently depending on the ecosystem and stressor types, reinforcing the need for multiple lines of evidence in ecological assessments [
2,
41].
While the SPEAR index performed well in this study, the homogeneity of water chemistry across the watershed may have limited the role of metal bioavailability. This observation suggests the need for further validation in watersheds with more varied physicochemical conditions [
42].
Future research directions should focus on validating the SPEAR index across watersheds with diverse habitat conditions, water chemistry profiles, and baseline community structures. Investigating the relationship between metal bioavailability and SPEAR index performance in different chemical environments, such as those with varying pH or dissolved organic carbon levels, would offer valuable insights. Furthermore, integrating the SPEAR index with other ecological indicators could yield a more holistic assessment of ecosystem health, potentially leading to the development of multi-metric indices that are more adaptable to specific environmental contexts.
3.7. Conclusions
This study demonstrates that the SPEAR index for metals is an effective and sensitive tool for evaluating the ecological impacts of metal contamination in aquatic ecosystems. The index’s strong correlation with metal exposure concentrations and its ability to detect subtle changes in community structure make it a valuable bioindicator for environmental monitoring, particularly in watersheds impacted by multiple pollution sources such as zinc smelting and mining activities. While the SPEAR index has shown significant potential, further research is needed to validate its applicability across diverse environmental conditions. The findings underscore the importance of refining the index for use in varied ecological contexts and integrating it with other ecological indicators to create a more comprehensive tool for ecological risk assessment. Ultimately, the development and application of such refined bioindicators will enhance our ability to monitor, assess, and manage metal contamination in aquatic ecosystems more effectively.