Submitted:
09 October 2024
Posted:
11 October 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
1.1. Hemagglutinin
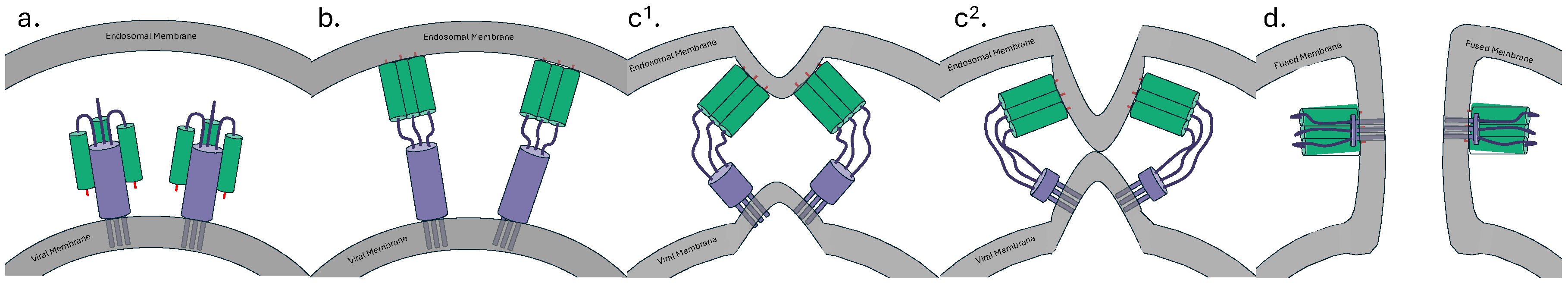
2. bnAbs against the HA Stem
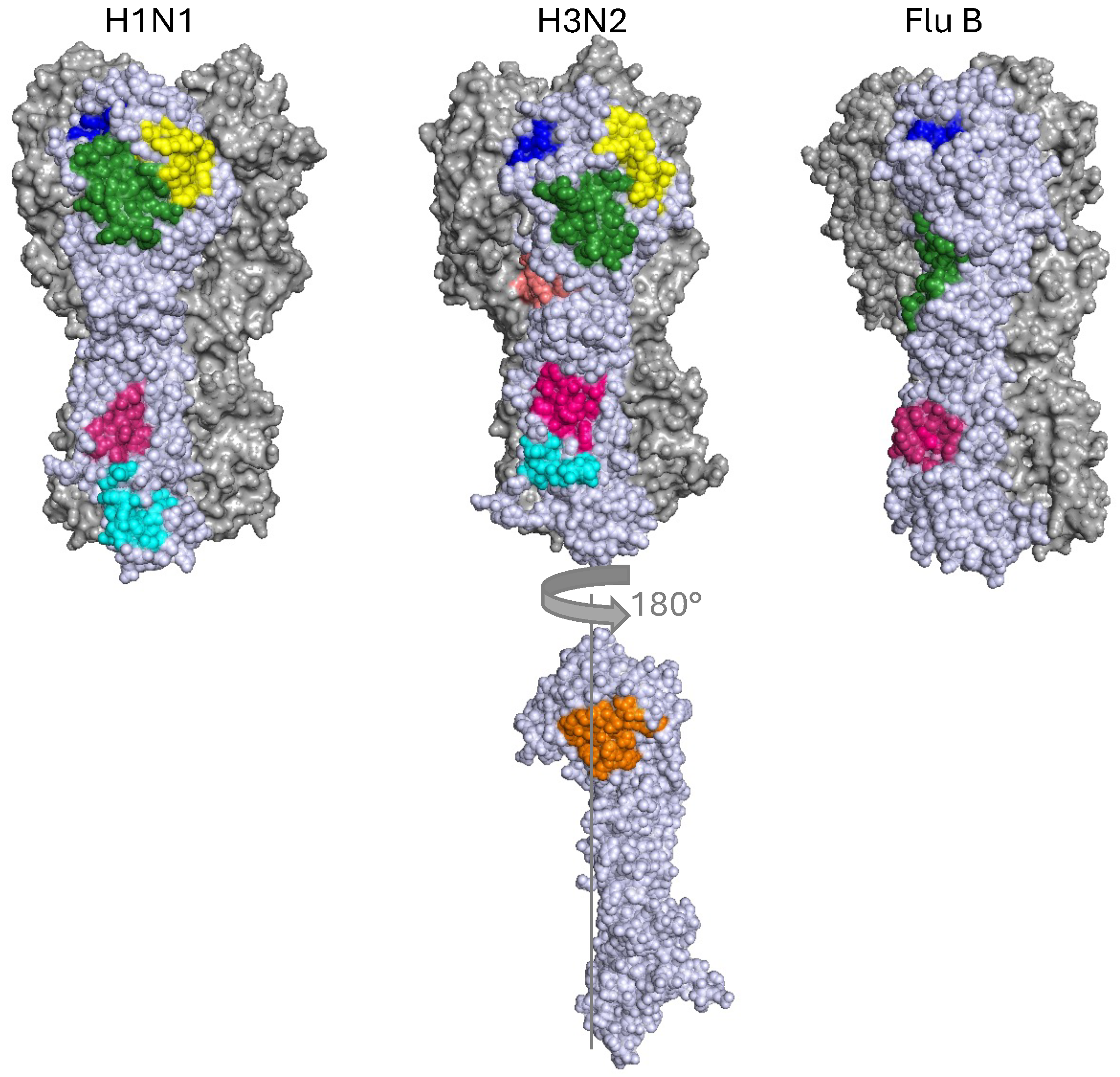
2.1. The Central Stem Epitope
2.2. The Fusion Peptide and Anchor Epitope
| Name | In vitro Binding | In vitro Neutralisation | In vivo Protection | Germline Encoded IGHV | CDR Recognition Mode | ADCC activity | Source | Escape Mutants | IgG-type in Studies | Ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Central Stem | C179 | H1, H2, H5, H6, H9 | H1, H2, H5, H6, H9 | H1, H5 | - | - | Yes | Mouse | T332K, V395E * |
IgG2a | [9,27,28] | ||||||
| 27F3 | H1, H2, H5, H6, H9, H11, H12, H13, H16, H3, H7, H10, FluB | H1, H5, H6, H3, H7, H10 | - | IGHV1–69 | CDRH2 | - | Humans | - | IgG1 | [29,30] | |||||||
| FI6 | H1-H16 | H1, H5, H3, H7 | H1, H5, H3 | IGHV3–30 |
CDRH3 CDRL1 | Yes | Humans | R62K, D239G, R240Q T333K, A388T ° | - | [23] [21,22,31,32] | |||||||
| CR6261 | H1, H2, H5, H6, H8, H9 | H1, H2, H5, H6, H8, H9 | H1, H5 | IGHV1–69 | CDRH2 | Weak | Humans | A388V | IgG1 | [18,30,33,34,35] | |||||||
| CR6323 | H1, H2, H5, H6, H8, H9 | H1, H2, H5, H6, H8, H9 | - | IGHV1–69 | HCDR2 | - | Humans | H357L/T* | IgG1 | [34] | |||||||
| 09-2A06 | H1 | H1 | - | IGHV1–69 | - | - | Humans | - | - | [36] | |||||||
| 09-3A01 | H1 | H1 | - | IGHV4–39 | - | - | Humans | - | - | ||||||||
| 05-2G02 | H1, H3, H5 | H1, H3, H5 | - | IGHV1–18 | - | - | Humans | - | - | ||||||||
| A06 | H1, H5 | H1, H5 | H1 | IGHV1–69 | - | - | Humans | - | IgG1 | [37] | |||||||
| 39.18 | H1, H2 | H1, H2 | - | IGHV1–69 | - | - | Humans | - | - | [38,39] | |||||||
| 39.29 | H1, H2, H3 | H1, H2, H3 | H1, H3 | IGHV3-30 | CDRH3 | - | Humans | G387K, D391Y/G | - | ||||||||
| 81.39 | H1, H2, H3 | H1, H2, H3 | - | IGHV3-15 | - | - | Humans | - | - | ||||||||
| 36.89 | H3 | H3 | - | IGHV1–18 | - | - | Humans | - | - | ||||||||
| FE43 | H1, H5, H6, H9 | H1, H5, H6, H9 | H1, H5, H6 | IGHV1–69 | - | - | Humans | None found | IgG1 | [40] | |||||||
| FB110 | H1, H2, H5 | H1, H2, H5 | - | IGHV3-23 | - | - | Humans | None found | IgG3 | ||||||||
| 3Е1 | H1, H5, H9, H3, H7 | H1, H5, H9, H3, H7 | H1, H5 | IGHV4-4 | Mostly Heavy Chain | - | Humans | - | IgG1 | [41] | |||||||
| CT149 | H1, H5, H9, H3, H7 | H5, H9, H3, H7 | H1, H5, H3, H7 | IGHV1–18 | CDRH3 CDRH2 |
Yes | Humans | - | IgG1 | [42] | |||||||
| 31.a.83 | H1, H2, H5, H9, H3, H7 | H1, H2, H5, H9, H3, H7 | - | IGHV3–23 | Mostly CDRH3 CDRH2 |
- | Humans | - | - | [43] | |||||||
| 56.a.09 | H1, H5, H3, H7 | H1, H5, H3, H7 | - | IGHV6–1 | Mostly CDRH3 CDRH2 |
- | Humans | - | - | ||||||||
| CR9114 | H1, H2, H5, H6, H8, H9, H12, H13, H16, H3, H4, H7, H10, H15, FluB | H1, H2, H5, H6, H8, H9, H12, H3, H4, H7, H10 | H1, H2, H3, H5, H9, FluB | IGHV1–69 | CDRH2 | Weak | Humans | R62K, D239G, R240Q, L335V, D363G, A388T ° | IgG1 | [30,31,33,44,45] | |||||||
| F10 | H1, H2, H5, H6, H8, H9, H11, H13, H16 | H1, H2, H5, H6, H8, H9, H11 | H1, H5 | IGHV1–69 | CDRH2 | Yes | Humans | N460, S123, E190D+G225D, N203VHA + E329KNA * |
IgG1 | [19,30,32,46] | |||||||
| MEDI8852 | H1-H18 | H1, H2, H5, H6, H9, H3, H7 | H1, H5, H3 | IGHV6-1 | CDRH2 CDRH3 CDRL1 |
Yes | Humans | - | IgG1 | [47,48] | |||||||
| CR9117 | Mouse homologue of CR9114, presumed to have similar neutralization capacity | - | Yes | Mouse | - | IgG2a | [33] | ||||||||||
| Anchor Domain | Polyclonal response (FISW84 / 222-1C06 were named) | H1, H2, H5 | H1, H2, H5 | H1 | IGHV3-23 IGHV3-30 IGHV3-30-3 IGHV3-48 |
CDRk3 CDRH2 CDRH3 |
No | Humans | - | IgG1 | [26] | ||||||
| Fusion Peptide | CR8020 | H3, H4, H7, H10, H14, H15 | H3, H7, H10 | H3, H7 | IGHV1–18 | CDRH1 CDRH3 |
Weak | Humans | D372N, G376E * | IgG1 | [20,25,49,50] | ||||||
| CR8043 | H3, H4, H7, H10, H14, H15 | H3, H7, H10 | H3, H7 | IGHV1–3 | CDRH1 CDRH3 |
- | Humans | R378M, Q380R/T * | IgG1 | [25,50] | |||||||
| 9H10 | H3, H9 | H3, H10 | H3 | - | - | - | Mice | R378M T385R Q387R/T G386E * |
- | [50] | |||||||
3. bnAbs against the HA Head Domain
3.1. Receptor Binding Site
3.2. Lateral Patch
3.3. Vestigial Esterase
3.4. Interface and Occluded Epitope
| Name | In vitro Binding | In vitro Neutralisation | In vivo Protection | Germline Encoded IGHV | CDR Recognition Mode | ADCC activity | Source | Escape Mutants | IgG-type in Studies | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RBS | S139/1 | H1, H2, H3, H5, H9, H13 | H1, H2, H3, H5, H9, H13, H16 | H1, H3 | - | CDRH2 | - | Mouse | K156, G158, S193, insertion at 133a * | IgG2a | [53,54,75] |
| C05 | H1, H2, H9, H12, H3 | H1, H2, H3 | H1, H3 | IGHV3-23 | CDRH3 | Weak | Human | insertion at 133a | - | [54,76] | |
| F045-092 | H1, H2, H13, H3 | H1, H2, H3, H13 | - | IGHV1–69 | CDRH3 | - | Human | 133A insertion * | - | [30,57] | |
| K03.12 | H1, H3 | - | - | IGHV1-2 | CDRH3 | - | Human | - | IgG1 | [77] | |
| 2G1 | H2 | H2 | H2 | IGHV1–69 | - | - | Human | - | - | [30,78] | |
| FE17 | H1, H9 | H1, H9 | H1, H5 | IGHV1–69 | - | - | Human | S145N* | IgG1 | [40] | |
| 12H5 | H1, H5 | H1, H5 | H1, H5 | IGHV9-1 alignment | CDRH2, CDRH3 | - | Mouse | Y98A, A137E, H141A, A142E, G143R, A144E, W153A, D190A * | IgG1 | [59] | |
| 1F1 | H1 | H1 | H1 | IGHV3-30 | CDRH3 | - | Human | D190E, D225G * | - | [79] | |
| 5J8 | H1 | H1 | H1 | IGHV4-b | - | - | Human | R(133A)I, K(133A)Q, A137T, D199H, K222Q * | - | [58] | |
| CH65 | H1 | H1 | - | IGHV1-2 | CDRH3 | - | Human | G200D, K/R insertion at 133A * | IgG1 | [56,80] | |
| CH67 | H1 | H1 | - | IGHV1-2 | CDRH3 | - | Human | likely as CH65 | IgG1 | [56,80] | |
| 3D11 | H1 | H1 | H1 | - | - | - | Mouse | K153E, D200E * | IgG1 | [81] | |
| 8M2 | H2 | H2 | H2 | IGHV1–69 | - | - | Human | G142D * | - | [30,78] | |
| 8F8 | H2 | H2 | H2 | IGHV3-33 | - | - | Human | R144Q/M/K, T134K * | - | [78] | |
| A2.91.3 | H3 | H3 | - | - | CDRH3 | - | Mouse | K189N, F193S/K, L194P, Y195A * | IgG1 | [82] | |
| AVFluIgG03 | H5 | H5 | H5 | IGHV3-23 | CDRH3 | - | Human | S159I, R193M/W * | IgG1 | [83,84] | |
| FLD21.140 | H5 | H5 | H5 | IGHV4-31 | CDRH3 | - | Human | S159I, R193M/W * | IgG1 | [84] | |
| 13D4 | H5 | H5 | H5 | Mouse IGHV1-9 | CDRH3 | - | Mouse | K/R193N * | - | [83] | |
| Hab21 | H5 | H5 | - | - | - | - | Mouse | H136A, D197A, A198G, A199G, E200A, N207A, P208A, P225A, N258A | [85] | ||
| H5.3 | H5 | H5 | - | - | CDRH3 | - | Human | - | - | [86] | |
| CR8033 | FluB | FluB | FluB | IGHV3-9 | CDRH Dominant | - | Human | P161Q | - | [55] | |
| VE | H3v-47 | H3 | H3 | H3 | IGHV1–69 | CDRH2, CDRH3, CDRL1, CDRL3 | Yes | Human | None found | IgG1 | [64] |
| F005-126 | H3 | H3 | - | - | CDRH3 | - | Human | N285Y * | IgG1 | [74] | |
| H5M9 | H5 | H5 | H5 | - | CDRH1-3, CDRL1-2 | - | Mouse | D53A/N, E78K, E83aA/K, Y274A * | IgG1 | [87] | |
| 9F4 | H5 | H5 | H5 | - | - | Yes | Mouse | R62G * | IgG2b | [88,89] | |
| 100F4 | H5 | H5 | H5 | - | - | Yes | Human | D72A, E116Q/L * | - | [90,91] | |
| 4F5 | H5 | H5 | H5 | IGHV3-43 | - | Yes | Human | W70, L71, L72, G73, N74, P75 * | - | [92,93] | |
| 1H5 | H7 | - | H7 | - | - | Yes | Mouse | R58K * | IgG2a | [94] | |
| 1H10 | H7 | - | H7 | - | - | Yes | Mouse | R58K * | IgG2a | [94] | |
| 46B8 | FluB | FluB | FluB | - | - | Yes | Human | S301F | IgG1 | [95] | |
| CR8071 | FluB | FluB | FluB | IGHV1-18 | - | Yes | Human | None found | - | [55,95] | |
| Lateral Patch | CL6649 | H1 | H1 | - | IGHV4-39 | CDRH3, CDRL1, CDRL3 |
- | Human | K176Q S175N+K176Q * |
- | [96] |
| H7.HK1 | H7, H10, H15 | H7 | H7 | IGHV4-59 | CDRH1-3, CDRL1, CDRL2 |
- | Human | R57K * | IgG1 | [97,98] | |
| 07-5F01 | H7 | H7 | H7 | IGHV4-31 | - | - | Human | R57K * | IgG2a | [97,98] | |
| HA Multimerization Interface and Occluded Site | FluA-20 | H1-H12, H14, H15 | - | H1, H3, H5, H7 | IGHV4-61 | CDRH3, CDRL2 | Weak | Human | In H1: P103G, R230A, P231G, V233G, R239A * | - | [71] |
| 8H10 | H3, H4 | H3 | H3 | IGHV5-9-1 | CDRH1-3, CDRL1, CDRL3 |
Yes | Human | - | IgG2a, IgG1 | [73] | |
| S5V2-29 | H1, H2, H3, H4, H7, H9, H14 | - | H1, H3 | IGHV4-61 | - | Yes in IgG2c but not IgG1 | Human | - | IgG1 and IgG2c | [72] | |
| H2214 | H1, H2, H3, H4, H14 | - | H1, H3 | IGHV3-23 | - | Yes in IgG2c but not IgG1 | Human | - | IgG1 and IgG2c | [72] | |
| H7-200 | H7, H15 | - | H7 | - | CDRH Dominant, CDRL3 | - | Human | - | - | [99] | |
| H7.5 | H7 | H7 | - | - | CDRH2, CDRL3 | - | Human | - | - | [99,100] |
4. bnAbs in Clinical Trials
| Name | Type and Target | Dosage/ Infection Model | Result | Trial Registry ID/ Reference |
|---|---|---|---|---|
| CT-P27 | CT-120 & CT-149 mAb’s targeting the stem region of group 1 and group 2 influenza hemagglutinin | 10 mg/kg CT-P27, 20 mg/kg CT-P27, or placebo in an influenza challenge model | Reduction of AUC of Viral Load, as measured by Quantitative PCR of Nasopharyngeal Swab for patients who received CT-P27 | NCT02071914, [105] |
| 90 mg/kg CT-P27, 45 mg/kg CT-P27, or placebo | NCT03511066 was terminated due to CT-P27 inactivation | NCT03511066. | ||
| MEDI8852 | Human IgG1 kappa monoclonal antibody (MAb) targeting H1N1 and H3N2 viruses, as well as subtypes such as H2, H5, H6, H7, and H9 via the stem region | 750 mg or 3000mg MEDI8852 given with oseltamivir or 3000 mg MEDI8852 on its own to patients with acute, uncomplicated influenza caused by Type A strains. | MEDI8852 provided no statistically significant improvement over oseltamivir alone, potentially worsened disease in combination compared to oseltamivir alone | NCT02603952,[116] |
| Low dose and high dose of MEDI8852 and oseltamivir in comparison to oseltamivir and placebo | Withdrawn due to company decision | NCT03028909 | ||
| VIS410 | Human immunoglobulin IgG1 monoclonal antibody engineered to bind to the stem region of group 1 and 2 influenza A hemagglutinins | Influenza challenge with H1N1 followed by a single administration of VIS410 or placebo | No results posted | NCT02468115, [117] |
| 2000mg or 4000mg VIS410 was given to patients with uncomplicated influenza A infection and compared to a placebo | Statistically significant improvement in signs and symptoms of influenza infection on day 3 and 4 with VIS410 compared to placebo. Statistically significant reduction in time to resolution of peak viral load when patients were given VIS410. | NCT02989194, [118] | ||
| 3600 mg or 8400 mg VIS410 combined with oral oseltamivir or placebo with oseltamivir in patients hospitalised with influenza A infection | No statistically significant reduction in time to cessation of oxygen, or reduction of viral load in nasopharyngeal samples | NCT03040141 | ||
| MHAA4549A | Human monoclonal antibody, IgG1, targeting the influenza A virus hemagglutinin stem across multiple subtypes | Influenza challenge with H3N2 influenza virus followed by a dose of 400 mg, 1200 mg or 3600 mg | Statistically significant reduction in AUC of virus in nasopharyngeal samples was seen at 3600mg compared to placebo. Influenza symptom scores, mucus weight, and inflammatory biomarkers were also reduced. | NCT01980966 |
| 3600mg or 8400mg given either on its own or with oseltamivir to patients hospitalised with severe influenza infection | MHAA4549A did not improve clinical outcomes over OTV alone. MHAA4549A+OTV did not further reduce viral load versus placebo+OTV.MHAA4549A did not alleviate symptoms quicker than a placebo. | NCT02293863, [119] | ||
| 3600mg or 8400mg given to patients with uncomplicated seasonal influenza A infection | 3600mg dose was able to statistically reduce the number of days to alleviate symptoms compared to the control | NCT02623322 | ||
| CR8020 | A mAb targeting the stem region of group 2 influenza A hemagglutinin |
15 mg/kg CR8020 given before challenge with a H3N2 influenza virus. | No results | NCT01938352 |
| CR6261 | mAb that targets the stem region of group 1 and group 2 influenza hemagglutinin | 50 mg/kg administered one day after challenge with H1N1 | Statistically reduced percentage of participants who experienced influenza symptoms. No statistically significant reduction in AUC or viral shedding. | NCT02371668, [120] |
| CR8020/ CR6261 | Withdrawn due to preliminary efficacy results from an influenza challenge trial | NCT01992276 |
5. Broadly Protective Vaccines in Clinical Trials
| Phase | Name of vaccine | Target/ Type of vaccine | Dosage/ Infection model | Results | Trial Registry ID/ Reference |
|---|---|---|---|---|---|
| Recruiting | fH1/DSP-0546LP | Post-fusion hemagglutinin antigen | Combination of 2 dose levels of fH1 (2 and 8 μg), 3 dose levels of DSP-0546LP (2.5, 5, and 10 μg), and placebo. Each dose level of fH1 will be combined with the low, medium, and high dose level of DSP-0546LP to assess safety, tolerability, and immunogenicity | Active | NCT06460064, [124] |
| Phase 1 | EBS-UFV-001 |
Induction of antibodies against conserved stem antigens across group 1 and 2 via a hemagglutinin stabilized stem nanoparticle vaccine | Testing the safety, tolerability and immunogenicity of 20 µg or 60 µg of UFluA as single dose or as two dose | No results posted | NCT05155319, [126] |
| H1ssF | HA stem domain from Influenza A/New Caledonia/20/1999 (H1N1) genetically fused to the ferritin protein from H. pylori. | 20 mcg was given to group 1, group 2 received 60 mcg on a prime boost schedule. | All regimes generated an increased IC80 concentration when tested in a pseudoviral neutralization assay against the homologous H1N1 A/New Caledonia/20/99 virus | NCT03814720 | |
| GSK3816302A | Chimeric vaccines of D-SUIV cH8/1 N1, D-SUIV cH5/1 N1, and D-SUIV cH11/1 N1 to induce cross reactive stem targeting antibodies against H1 stem | Chimeric H5, H8 and H11 with and without adjuvants AS03 or AS01 were tested for their reactogenicity, safety and immunogenicity. H8 and H5 were given with a placebo second dose, or all three were given. | An increase in anti H1 stem antibodies, as measured by ELISA and MN assay, was seen across all dose schedules with adjuvant AS03 providing a statistically significant increase in humoral immune response for anti-H1 stem antibody by ELISA at Day 29 and Day 85. Increases in antibody titres against H2 and H18 were also identified. | NCT03275389, [123] | |
| Phase 1/2 | G1 mHA | Mini-hemagglutinin stem-derived protein vaccine antigen | Single dose of influenza G1 mHA with or without Al(OH)3 adjuvant at two dose levels to evaluate safety, reactogenicity and immunogenicity | Active | NCT05901636, [121,122] |
| Phase 3 | (M-001) | A recombinant 45 kDa protein produced in Escherichia coli. consisting of three repetitions of nine linear, conserved influenza A and B epitopes to form a single recombinant protein. Epitopes were derived from: M1 matrix protein, NP and HA | Vaccination with 1mg dose of M-001 twice: Once at Day 0, and once at Day 21 then followed for 2 years | No statistical difference in prevention of influenza infection. Did not statistically reduce the number of patients with influenza like symptoms, or a reduction of severity of either qRT-PCR or culture-confirmed influenza illness | NCT03450915, [125] |
6. bnAbs in Current and Future Directions
6.1. Escape Mutations
6.2. Immunogenicity of bnAbs
6.3. Antibody-Dependent Enhancement
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K; Doherty, P.C.; Palese, P; Shaw, M.L; Treanor, J.; Wesbter, R.G.; et al., Influenza. Nature Reviews Disease Primers, 2018. 4(1): p. 3. [CrossRef]
- Collins, J.P., Campbell, A.P.; Openo, K.; Farley, M.; Cummings, C.N.; Hill, M.; Schaffner, W.; Lindegren; M.L.; Thomas, A.; Billing, L.; et al., Outcomes of Immunocompromised Adults Hospitalized With Laboratory-confirmed Influenza in the United States, 2011-2015. Clin Infect Dis, 2020. 70(10): p. 2121-2130. [CrossRef]
- WHO. Influenza seasonal. 2024 [cited 2024 28.08.2024]; Available from: https://www.who.int/health-topics/influenza-seasonal#tab=tab_1.
- Knobler, S.L.; Mack, A.; Mahmoud, A.; Lemon, S.M. Story of Influenza. 2005, Institute of Medicine (US): Forum on Microbial Threats.
- Fierer, J., D. Looney, and J.-C. Pechère, 2 - Nature and Pathogenicity of Micro-organisms, in Infectious Diseases (Fourth Edition), J. Cohen, W.G. Powderly, and S.M. Opal, Editors. 2017, Elsevier. p. 4-25.e1. [CrossRef]
- Ryu, W.-S., Chapter 15 - Influenza Viruses, in Molecular Virology of Human Pathogenic Viruses, W.-S. Ryu, Editor. 2017, Academic Press: Boston. p. 195-211. [CrossRef]
- Fujimura, S.F., Purple Death: The Great Flu of 1918, in Perspectives in Health. 2003: Pan American Health Organization.
- Gerhard, W.; Yedwell, J.; Frankel, M.E.; Webster, R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature, 1981. 290(5808): p. 713-717. [CrossRef]
- Okuno, Y.; Isegawa, F.; Sasao, F.; Ueda, S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol, 1993. 67(5): p. 2552-8. [CrossRef]
- Lousa, D.; C.M. Soares, Molecular mechanisms of the influenza fusion peptide: insights from experimental and simulation studies. FEBS Open Bio, 2021. 11(12): p. 3253-3261. [CrossRef]
- Cheung, C.S.-F.; Gorman, J.; Andrews, S.F.; Rawi, R.; Reveiz, M.; Shen, C.-H.; Wang, Y.; Harris, D.R.; Nazzari, A.F.; Olia, A.S.; et al., Structure of an influenza group 2-neutralizing antibody targeting the hemagglutinin stem supersite. Structure, 2022. 30(7): p. 993-1003.e6.
- Throsby, M., van den Brink, E.; Jongeneelen, M.; Poon, L.L.M; Alard, Ph; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al., Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One, 2008. 3(12): p. e3942. [CrossRef]
- Nath Neerukonda, S., R. Vassell, C.D. Weiss, Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin. Vaccines (Basel), 2020. 8(3). [CrossRef]
- Sun, X., Ling, Z.; Yang, Z.; Sun, B. Broad neutralizing antibody-based strategies to tackle influenza. Curr. Opi. Virol. 2022. 53: p. 101207.
- Tan, H.X., Jegaskanda, S.; Juno, J.A.; Esterbauer, R.; Wong, J.; Kelly, H.G.; Liu, Y.; Tilmanis, D.; Hurt, A.C.; Yedwell, J.W.; e al. Subdominance and poor intrinsic immunogenicity limit humoral immunity targeting influenza HA stem. J Clin Invest, 2019. 129(2): p. 850-862. [CrossRef]
- Eggink, D., P.H. Goff, P. Palese, Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J Virol, 2014. 88(1): p. 699-704. [CrossRef]
- Jiao, C., Wang, B; Chen, P.; Jian, Y.; Liu; J. Analysis of the conserved protective epitopes of hemagglutinin on influenza A viruses. Frontiers in Immunology, 2023. 14. [CrossRef]
- Ekiert, D.C., Bhabha, G.; Elsliger, M.-A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science, 2009. 324(5924): p. 246-51. [CrossRef]
- Sui, J., Hwang, W.C.; Pérez, S .; Wei, G.; Aird, D.; Chen, L.-.m; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al., Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009. 16(3): p. 265-273. [CrossRef]
- Tharakaraman, K.; Subramanian, V.; Cain, D.; Sasisekharan, V.; Sasisekharan, R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell Host Microbe, 2014. 15(5): p. 644-51. [CrossRef]
- Corti, D., Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al., A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science, 2011. 333(6044): p. 850-6. [CrossRef]
- Limberis, M.P., Adam, V.S.; Wong, G.; Gren, J.; Kobasa, D.; Ross, T.M.; Kobinger, G.P.; Tretiakova, A.; Wilson, J.M. Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci Transl Med, 2013. 5(187): p. 187ra72. [CrossRef]
- Morgan, S.B., Holzer, B.; Hemmink, J.D.; Salguero, F.J.; Schwartz, J.C.; Agatic, G.; Cameroni, E.; Guarino, B.; Porter, E.; Rijal, P.; et al., Therapeutic Administration of Broadly Neutralizing FI6 Antibody Reveals Lack of Interaction Between Human IgG1 and Pig Fc Receptors. Front Immunol, 2018. 9: p. 865. [CrossRef]
- Ekiert, D.C., Friesen, R.H.E.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.W.M.; Brandenburg, B.; et al., A highly conserved neutralizing epitope on group 2 influenza A viruses. Science, 2011. 333(6044): p. 843-50. [CrossRef]
- Friesen, R.H., Lee, P.S.; Stoop, E.J.M.; Hoffman, R.M.B.; Ekiert, D.C.; Bhabha, G.; Yu, W.; Juraszek, J.; Koudstaal, W.; Jongeneelen, M.; et al., A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci U S A, 2014. 111(1): p. 445-50. [CrossRef]
- Guthmiller, J.J., Han, J.; Utset, H.A.; Li, L.; Lan, L.Y.-L.; Henry, C.; Stamper, C.T.; McMahon, M.; O'Dell, G.; Fernández-Quintero, M.L.; et al., Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature, 2022. 602(7896): p. 314-320. [CrossRef]
- McCraw, D.M., Myers, M.L.; Gulati, N.M.; Prabhakaran, M.; Brand, J.; Andrews, S.; Gallagher, J.R.; Maldonado-Puga, S.; Kim, A.J.; Torian, U.; et al., Designed nanoparticles elicit cross-reactive antibody responses to conserved influenza virus hemagglutinin stem epitopes. PLoS Pathog, 2023. 19(8): p. e1011514. [CrossRef]
- Sakabe, S., Iwatsuki-Horimoto, K.; Horimoto, T.; Nidom, .C.A; Le, M.t.Q.; Takano, R.; Kubota-Koketsu, R.; Okuno, Y.; Ozawa, M.; Kawaoka, Y. A cross-reactive neutralizing monoclonal antibody protects mice from H5N1 and pandemic (H1N1) 2009 virus infection. Antiviral Res, 2010. 88(3): p. 249-55. [CrossRef]
- Lang, S., Xie, J.; Zhu, X.; Wu, N.C., Lerner, R.A.; Wilson, I.A. Antibody 27F3 Broadly Targets Influenza A Group 1 and 2 Hemagglutinins through a Further Variation in V(H)1-69 Antibody Orientation on the HA Stem. Cell Rep, 2017. 20(12): p. 2935-2943. [CrossRef]
- Chen, F., Tzarum, N.; Wilson, I.A.; Law, M. V(H)1-69 antiviral broadly neutralizing antibodies: genetics, structures, and relevance to rational vaccine design. Curr Opin Virol, 2019. 34: p. 149-159. [CrossRef]
- Roubidoux, E.K., Carreño, J.M; McMahon, M.; Jiang, K.; van Bakel, H.; Wilson, P.; Krammer, F. Mutations in the Hemagglutinin Stalk Domain Do Not Permit Escape from a Protective, Stalk-Based Vaccine-Induced Immune Response in the Mouse Model. mBio, 2021. 12(1). [CrossRef]
- Muralidharan, A., Gravel, C.; Harris, G.; Hashem, A.M.; Zhang, W.; Safronetz, D.; Van Domselaar, G.; Krammer,, F.; Sauve, S.; Rosu-Myles, M.; et al., Universal antibody targeting the highly conserved fusion peptide provides cross-protection in mice. Hum Vaccin Immunother, 2022. 18(5): p. 2083428. [CrossRef]
- Sutton, T.C., Lamirande, E.W.; Bock, K.W.; Moore, I.N.; Koudstaal, W.; Rehman, M.; Weverling, G.J.; Goudsmit, J.; Subbarao, K. In Vitro Neutralization Is Not Predictive of Prophylactic Efficacy of Broadly Neutralizing Monoclonal Antibodies CR6261 and CR9114 against Lethal H2 Influenza Virus Challenge in Mice. J Virol, 2017. 91(24). [CrossRef]
- Throsby, M., van den Brink, E.; Jongeneelen, M.; Poon, L.L.M; Alard, Ph; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al., Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One, 2008. 3(12): p. e3942. [CrossRef]
- Park, J.K., Xiao, Y.; Ramuta, M.D.; Rosas, L.A.; Fong, S.; Matthews, A.M.; Freeman, A.D.; Gouzoulis, M.A.; Batchenkova, N.A.; Yang, X.; Pre-existing immunity to influenza virus hemagglutinin stalk might drive selection for antibody-escape mutant viruses in a human challenge model. Nat Med, 2020. 26(8): p. 1240-1246. [CrossRef]
- Li, G.M., Chiu, C.; Wrammert, J.; McCausland, M.; Andrews, S.F.; Zheng, N.-Y.; Lee, J.-H.; Huang, M.; Qu, X.; Edupuganti, S.; et al., Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A, 2012. 109(23): p. 9047-52. [CrossRef]
- Kashyap, A.K., Steel, J.; Rubrum, A.; Estelles, A.; Briante, R.; Ilyushina, N.A.; Xu, L.; Swale, R.E.; Faynboym, A.M.; Foreman, P.K.; et al., Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS Pathog, 2010. 6(7): p. e1000990. [CrossRef]
- Nakamura, G., Chai, N.; Park, S.; Chiang, N.; Lin, Z.; Chiu, H.; Fong, R.; Yan, D.; Kim, J.; Zhang, J.; et al., An In Vivo Human-Plasmablast Enrichment Technique Allows Rapid Identification of Therapeutic Influenza A Antibodies. Cell Host Microbe, 2013. 14(1): p. 93-103.
- Chai, N., Swem, L.R.; Reichelt, M.; Chen-Harris, H.; Luis, E.; Park, S.; Fouts, A.; Lupardus, P.; Wu, T.D.; Li, O.; et al., Two Escape Mechanisms of Influenza A Virus to a Broadly Neutralizing Stalk-Binding Antibody. PLoS Pathog, 2016. 12(6): p. e1005702. [CrossRef]
- Corti, D., Suguitan Jr.; A.L.; Pinna, D.; Silacci, C.; Fernandez-Rodriguez, B.M.; Vanzetta, F.; Santos, C.; Luke, C.J.; Torres-Velez, F.J.; Temperton, N.J.; et al., Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest, 2010. 120(5): p. 1663-73. [CrossRef]
- Wang, W., Sun, X.; Li, Y.; Su, J.; Ling, Z.; Zhang, T.; Wang, F.; Zhang, H.; Chen, H.; Ding, J.; et al., Human antibody 3E1 targets the HA stem region of H1N1 and H5N6 influenza A viruses. Nature Communications, 2016. 7(1): p. 13577. [CrossRef]
- Wu, Y.; Cho, M.; Shore, D.; Song, M.; Choi, J.; Jiang, T.; Deng, Y.-Q.; Bourgeois, M.; Almli, L.; Yang, H.; et al., A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nature Communications, 2015. 6(1): p. 7708. [CrossRef]
- Joyce, M.G., Wheatley, A.K.; Thomas, P.V.; Chuang, G.-Y.; Soto, C.; Bailer, R.T.; Druz, A.; Georgiev, I.S.; Gillespie, R.A.; Kanekiyo, M.; et al., Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell, 2016. 166(3): p. 609-623. [CrossRef]
- Beukenhorst, A.L., Frallicciardi, J.; Koch, C.M.; Klap, J.M.; Phillips, A.; Desai, M.M.; Wichapong, K.; Nicolaes, G.A.F.; Koudstaal, W.; Alter, G.; et al., Corrigendum: The influenza hemagglutinin stem antibody CR9114: Evidence for a narrow evolutionary path towards universal protection. Frontiers in Virology, 2023. 3. [CrossRef]
- Beukenhorst, A.L.; Frallicciardi, J.; Rice, K.L.; Koldijk, M.H.; Moreira de Mello, J.C.; Klap, J.M.; Hadjichrysanthou, C.; Koch, C.M.; da Costa, K.A.S.; Temperton, N.; et al., A pan-influenza monoclonal antibody neutralizes H5 strains and prophylactically protects through intranasal administration. Sci. Rep., 2024. 14(1): p. 3818. [CrossRef]
- Prachanronarong, K.L., Canale, A.S.; Liu, P.; Somasundaran, M.; Hou, S.; Poh, Y.-P.; Han, T.; Zhu, Q.; Renzette, N.; Zeldovich, K.B.; et al., Mutations in Influenza A Virus Neuraminidase and Hemagglutinin Confer Resistance against a Broadly Neutralizing Hemagglutinin Stem Antibody. J Virol, 2019. 93(2). [CrossRef]
- Ali, S.O.; Takas, T.; Nyborg, A.; Shoemaker, K.; Kallewaard, N.L.; Chiong, R.; Dubovsky, F.; Mallory, R.M. Evaluation of MEDI8852, an Anti-Influenza A Monoclonal Antibody, in Treating Acute Uncomplicated Influenza. Antimicrob Agents Chemother, 2018. 62(11). [CrossRef]
- Paules, C.I., Lakdawala, S.; McAuliffe, J.M.; Paskel, M.; Vogel, L.; Kallewaard, N.L.; Zhu, Q.; Subbarao, K. The Hemagglutinin A Stem Antibody MEDI8852 Prevents and Controls Disease and Limits Transmission of Pandemic Influenza Viruses. J Infect Dis, 2017. 216(3): p. 356-365. [CrossRef]
- Mark Throsby, R.H., Edward Friesen, Theodorus Hendrikus, Jacobus Kwaks, Mandy Antonia, Catharina Jongeneelen, Human binding molecules capable of neutralizing influenza virus H3N2 and uses thereof. 2010: United States.
- Tan, G.S., Lee, P.S.; Hoffman, R.M.B.; Mazel-Sanchez, B.; Krammer, F.; Leon, P.E.; Ward, A.B.; Wilson, I.A.; Palese, P. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. J Virol, 2014. 88(23): p. 13580-92. [CrossRef]
- Myers, M.L.; Gallagher, J.R.; Kim, A.J.; Payne, W.H.; Maldonado-Puga, S.; Assimakopoulos, H.; Bock, K.W.; Torian, U.; Moore, I.N.; Harris, A.K. Commercial influenza vaccines vary in HA-complex structure and in induction of cross-reactive HA antibodies. Nature Communications, 2023. 14(1): p. 1763. [CrossRef]
- Yu, X., Tsibane, T.; McGraw, P.A.; House, F.S.; Keefer, C.J.; Hicar, M.D.; Tumpey, T.M.; Pappas, C.; Perrone, L.A.; Martinez, O.; et al, Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature, 2008. 455(7212): p. 532-6. [CrossRef]
- Yoshida, R., Igarashi, M.; Ozaki, H.; Kishida, N.; Tomabechi, D.; Kida, H.; Ito, K.; Takada, A. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog, 2009. 5(3): p. e1000350. [CrossRef]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O'Neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature, 2012. 489(7417): p. 526-32. [CrossRef]
- Dreyfus, C., Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongneelen, M.; et al. Highly conserved protective epitopes on influenza B viruses. Science, 2012. 337(6100): p. 1343-8. [CrossRef]
- Whittle, J.R.R., Zhang, R.; Khurana, S.; King, L.R.; Manischewitz, J.; Golding, H.; Dormitzer, P.R.; Haynes, B.F.; Walter, E.B.; Moody, M.A.; et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proceedings of the National Academy of Sciences, 2011. 108(34): p. 14216-14221. [CrossRef]
- Lee, P.S., Ohshima, N.; Stanfield, R.L.; Yu, W.; Iba, Y.; Okuno, Y.; Kurosawa, Y.; Wilson, I.A. Receptor mimicry by antibody F045–092 facilitates universal binding to the H3 subtype of influenza virus. Nat. Commun. 2014. 5(1): p. 3614. [CrossRef]
- Krause, J.C., Tsibane, T.; Tumpey, T.M.; Huffman, C.J.; Basler, C.F.; Crowe Jr, J.E. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J Virol, 2011. 85(20): p. 10905-8. [CrossRef]
- Li, T., Chen, J.; Zheng, Q.; Xue, W.; Zhang, L.; Rong, R.; Zhang, S.; Wang, Q.; Hong, M.; Zhang, Y.; et al. Identification of a cross-neutralizing antibody that targets the receptor binding site of H1N1 and H5N1 influenza viruses. Nature Commun, 2022. 13(1): p. 5182. [CrossRef]
- Guthmiller, J.J., Han, J.; Li, L.; Freyn, A.W.; Liu, S.T.H.; Stovicek, O.; Stamper, C.T.; Dugan, H.L.; Tepora, M.E.; Utset, H.A.; et al. First exposure to the pandemic H1N1 virus induced broadly neutralizing antibodies targeting hemagglutinin head epitopes. Sci Transl Med, 2021. 13(596). [CrossRef]
- Raymond, D.D., Bajic, G.; Ferdman, J.; Suphaphiphat, P.; Settembre, E.C.; Moody, M.A.; Schmidt, A.G.; Harrison, S.C. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc Natl Acad Sci U S A, 2018. 115(1): p. 168-173. [CrossRef]
- Jia, M.; Zhao, H.; Morano, N.C.; Lu, H.; Lui, Y.-M.; Du, H.; Becker, J.E.; Yuen, K.-Y.; Ho, D.D; Kwong, P.D.; et al. Human neutralizing antibodies target a conserved lateral patch on H7N9 hemagglutinin head. Nature Communications, 2024. 15(1): p. 4505. [CrossRef]
- Zheng, Z., Paul, S.S.; Mo, X.; Yuan, Y.-R.; Tan, Y.J. The Vestigial Esterase Domain of Haemagglutinin of H5N1 Avian Influenza A Virus: Antigenicity and Contribution to Viral Pathogenesis. Vaccines (Basel), 2018. 6(3). [CrossRef]
- Bangaru, S., Zhang, H.; Gilchuk, I.M.; Voss, T.G.; Irving, R.P.; Gilchuk, P.; Matta, P.; Zhu, X.; Lang, S.; Nieusma, T.; et al. A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA. Nature Communications, 2018. 9(1): p. 2669. [CrossRef]
- Crowe, J.E., Antibody Determinants of Influenza Immunity. J Infect Dis, 2019. 219(Suppl_1): p. S21-s29. [CrossRef]
- Gao, R.; Sheng, Z.; Sreenivasan, C.C.; Wang, D.; Li, F. Influenza A Virus Antibodies with Antibody-Dependent Cellular Cytotoxicity Function. Viruses, 2020. 12(3): p. 276.
- Chai, N., Swem, L.R.; Park, S.; Nakamura, G.; Chiang, N.; Estevez, A.; Fong, R.; Kamen, L.; Kho, E.; Reichelt, M.; et al. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat Commun, 2017. 8: p. 14234. [CrossRef]
- Yan, L., Sun, L.; Guo, C.; Li, L.; Sun, J.; Huang, X.; Zhao, P.; Xie, X.; Hu, J. Neutralizing antibody PR8-23 targets the footprint of the sialoglycan receptor binding site of H1N1 hemagglutinin. J Med Virol, 2021. 93(6): p. 3508-3515. [CrossRef]
- Li, J., Wang, Y.; Liang, Y.; Ni, B.; Wan, Y.; Liao, Z.; Chan, K.-h.; Yuen, K.-y.; Fu, X.; Shang, X.; et al. Fine antigenic variation within H5N1 influenza virus hemagglutinin's antigenic sites defined by yeast cell surface display. Eur J Immunol, 2009. 39(12): p. 3498-510. [CrossRef]
- Yewdell, J.W., Taylor A.; Yellen, A.; Caton, A.; Gerhard, W.; Bächi, T. Mutations in or near the fusion peptide of the influenza virus hemagglutinin affect an antigenic site in the globular region. J Virol, 1993. 67(2): p. 933-42. [CrossRef]
- Bangaru, S., Lang, S.; Schotsaert, M.; Vanderven, H.A.; Zhu, X.; Kose, N.; Bombardi, R.; Finn, J.A.; Kent, S.J.; Gilchuk, P.; et al. A Site of Vulnerability on the Influenza Virus Hemagglutinin Head Domain Trimer Interface. Cell, 2019. 177(5): p. 1136-1152.e18. [CrossRef]
- Watanabe, A., McCarthy, K.R.; Kuraoka, M.; Schmidt, A.G.; Adachi, Y.; Onodera, T.; Tonouchi, K.; Caradonna, T.M.; Bajic, G.; Song, S.; et al. Antibodies to a Conserved Influenza Head Interface Epitope Protect by an IgG Subtype-Dependent Mechanism. Cell, 2019. 177(5): p. 1124-1135.e16. [CrossRef]
- Bajic, G., Maron, M.J.; Adachi, Y.; Onodera, T.; McCarthy, K.R.; McGee, C.E.; Sempowski, G.D.; Takahasi, Y.; Kelsoe, G.; Kuraoka, M.; et al. Influenza Antigen Engineering Focuses Immune Responses to a Subdominant but Broadly Protective Viral Epitope. Cell Host Microbe, 2019. 25(6): p. 827-835.e6. [CrossRef]
- Iba, Y., Fujii, Y.; Ohshima, N.; Sumida, T.; Kubota-Koketsu, R.; Ikeda, M.; Wakiyama, M.; Shirouzu, M.; Okada, J.; Okuno, Y.; et al. Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses. J Virol, 2014. 88(13): p. 7130-44. [CrossRef]
- Lee, P.S.; Yoshida, R.; Ekiert, D.C.; Sakai, N.; Suzuki, Y.; Takada, A.; Wilson, I.A. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci U S A, 2012. 109(42): p. 17040-5. [CrossRef]
- He, W.; Tan, G.S.; Mullarkey, C.E.; Lee, A.J.; Lam, M.M.W.; Krammer, F.; Henry, C.; Wilson, P.C.; Ashkar, A.A.; Palese, P. et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A, 2016. 113(42): p. 11931-11936. [CrossRef]
- McCarthy, K.R.; Watanabe, A.; Kuraoka, M.; Do, K.T.; McGee, C.E.; Sempowski, G.D.; Kepler, T.B.; Schmidt, A.G.; Kelsoe, G.; Harrison, S.C. Memory B Cells that Cross-React with Group 1 and Group 2 Influenza A Viruses Are Abundant in Adult Human Repertoires. Immunity, 2018. 48(1): p. 174-184.e9. [CrossRef]
- Krause, J.C.; Tsibane, T.; Tumpey, T.M.; Huffman, C.J.; Albrecht, R.; Blum, D.L.; Ramos, I:; Fernandez-Sesma, A.; Edwards, K.M.; García-Sastre, A.; et al. Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. J Virol, 2012. 86(11): p. 6334-40. [CrossRef]
- Tsibane, T.; Ekiert, D.C.; Krause, J.C.; Martinez, O.; Crowe Jr., J.E.; Wilson, I.A.; Basler, C.F. Influenza human monoclonal antibody 1F1 interacts with three major antigenic sites and residues mediating human receptor specificity in H1N1 viruses. PLoS Pathog, 2012. 8(12): p. e1003067. [CrossRef]
- Schmidt, A.G.; Therkelsen, M.D.; Stewart, S.; Kepler, T.B.; Liao, H.-X.; Moody, M.A.; Haynes, B.F.; Harrison, S.C. Viral receptor-binding site antibodies with diverse germline origins. Cell, 2015. 161(5): p. 1026-1034. [CrossRef]
- Yang, F.; Yan, S.; Zhu, L.; Wang, F.X.C.; Liu, F.; Cheng, L.; Yao, H.; Wu, N.; Lu, R.; Wu, H. Evaluation of panel of neutralising murine monoclonal antibodies and a humanised bispecific antibody against influenza A(H1N1)pdm09 virus infection in a mouse model. Antiviral Res, 2022. 208: p. 105462.
- Portnoff, A.D.; Patel, N.; Massare, M.J.; Zhou, H.; Tian, J.-H.; Zhou, B.; Shinde, V.; Glenn, G.M.; Smith, G. Influenza Hemagglutinin Nanoparticle Vaccine Elicits Broadly Neutralizing Antibodies against Structurally Distinct Domains of H3N2 HA. Vaccines (Basel), 2020. 8(1). [CrossRef]
- Lin, Q.; Li, T.; Chen, Y.; Lau, S.-Y.; Wei, M.; Zhang, Y.; Zhang, Z.; Yao, Q.; Li, J.; Li, Z.; et al. Structural Basis for the Broad, Antibody-Mediated Neutralization of H5N1 Influenza Virus. J Virol, 2018. 92(17). [CrossRef]
- Zuo, Y., Wang, P.; Sun, J.; Guo, S.; Wang, G.; Zuo, T.; Fan, S.; Zhou, P.; Liang, M.; Shi, X.; et al. Complementary recognition of the receptor-binding site of highly pathogenic H5N1 influenza viruses by two human neutralizing antibodies. J Biol Chem, 2018. 293(42): p. 16503-16517. [CrossRef]
- Wu, R.; Li, X.; Leung, H.-C.; Cao, Z.; Qiu, Z.; Zhou, Y.; Zheng, B.-J.; He, Y A novel neutralizing antibody against diverse clades of H5N1 influenza virus and its mutants capable of airborne transmission. Antiviral Res 2014. 106: p. 13-23.
- Winarski, K.L.; Thornburg, N.J.; Yu, Y.; Sapparapu, G.; Crowe Jr., J.E.; Spiller, B.W. Vaccine-elicited antibody that neutralizes H5N1 influenza and variants binds the receptor site and polymorphic sites. Proceedings of the National Academy of Sciences, 2015. 112(30): p. 9346-9351. [CrossRef]
- Zhu, X.; Guo, Y.-H.; Jiang, T.; Wang, Y.-D.; Chan, K.-H.; Li, X.-F., Yu, W.; McBride, R.; Paulson, J.C.; Yuen, K.-Y., et al. A unique and conserved neutralization epitope in H5N1 influenza viruses identified by an antibody against the A/Goose/Guangdong/1/96 hemagglutinin. J Virol, 2013. 87(23): p. 12619-35. [CrossRef]
- Paul, S.S.; Mok, C.-K.; Mak, T.-M.; Ng, O.-W.;.Aboagye, J.O.; Wohlbold, T.J.; Krammer, F.; Tan, Y.-J. A cross-clade H5N1 influenza A virus neutralizing monoclonal antibody binds to a novel epitope within the vestigial esterase domain of hemagglutinin. Antiviral Res, 2017. 144: p. 299-310.
- Zheng, Z.; Teo, S.H.C.; Arularasu, S.C.; Liu, Z.; Mohd-Ismail, N.K.; Mok, C.K.; Ong, C.B.; Chu, J.J.-h.; Tan, Y.-J. Contribution of Fc-dependent cell-mediated activity of a vestigial esterase-targeting antibody against H5N6 virus infection. Emerg Microbes Infect, 2020. 9: p. 95-110. [CrossRef]
- Qian, M.; Hu, H.; Zuo, T.; Wang, G.; Zhang, L.; Zhou, P. Unraveling of a neutralization mechanism by two human antibodies against conserved epitopes in the globular head of H5 hemagglutinin. J Virol, 2013. 87(6): p. 3571-7. [CrossRef]
- Wang, S.; Ren, H.; Jiang, W.; Chen, H.; Hu, H.; Chen, Z.; Zhou, P. Divergent Requirement of Fc-Fcγ Receptor Interactions for In Vivo Protection against Influenza Viruses by Two Pan-H5 Hemagglutinin Antibodies. J Virol, 2017. 91(11). [CrossRef]
- Zhang, X.; Qi, X.; Zhang, Q.; Zeng, X.; Shi, Z.; Jin, Q:; Zhan, F.; Xu, Y.; Liu, Z.; Feng, Z.; Jiao, Y. Human 4F5 single-chain Fv antibody recognizing a conserved HA1 epitope has broad neutralizing potency against H5N1 influenza A viruses of different clades. Antiviral Res 2013. 99(2): p. 91-99.
- Jin, Q.; Yao, Z.; Liu, F.; Di, Y.; Gao, J.; Zhang, X. The protective effect of a combination of human intracellular and extracellular antibodies against the highly pathogenic avian influenza H5N1 virus. Hum Vaccin Immunother, 2022. 18(1): p. 2035118. [CrossRef]
- Tan, G.S., et al., Broadly-Reactive Neutralizing and Non-neutralizing Antibodies Directed against the H7 Influenza Virus Hemagglutinin Reveal Divergent Mechanisms of Protection. PLOS Pathogens, 2016. 12(4): p. e1005578. [CrossRef]
- Chai, N.; Swem, L.R.; Park, S.; Nakamura, G.; Chiang, N.; Estevez, A.; Fong, R.; Kamen, L.; Kho, E.; Reichelt, M.; et al. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat Commun, 2017. 8: p. 14234. [CrossRef]
- Raymond, D.D., Bajic, G.; Ferdman, J.; Suphaphiphat, P.; Settembre, E.C.; Moody, M.A.; Schmidt, A.G.; Harrison, S.C. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc Natl Acad Sci U S A, 2018. 115(1): p. 168-173. [CrossRef]
- Henry Dunand, C.J.; Leon, P.E.; Huang, M.; Choi, A.; Chromikova, V.; Ho, I.Y.; Tan, G.S.; Cruz, J.; Hirsh, A.; Zheng, N.-Y.; et al. Both Neutralizing and Non-Neutralizing Human H7N9 Influenza Vaccine-Induced Monoclonal Antibodies Confer Protection. Cell Host Microbe, 2016. 19(6): p. 800-13. [CrossRef]
- Jia, M.; Zhao, H.; Morano, N.C.; Lu, H.; Lui, Y.-M.; Du, H.; Becker, J.E.; Yuen, K.-Y.; Ho, D.D; Kwong, P.D.; et al. Human neutralizing antibodies target a conserved lateral patch on H7N9 hemagglutinin head. Nature Communications, 2024. 15(1): p. 4505. [CrossRef]
- Dong, J.; Gilchuk, I.; Li, S.; Irving, R.; Goff, M.T.; Turner, H.L.; Ward, A.B.; Carnahan, R.H.; Anti-influenza H7 human antibody targets antigenic site in hemagglutinin head domain interface. J Clin Invest, 2020. 130(9): p. 4734-4739. [CrossRef]
- Turner, H.L.; Pallesen, J.; Lang, S.; Bangaru, S.; Urata, S.; Li; S.; Cottrell, C.A.; Bowman, C.A.; Crowe, Jr., J.E.; Wilson, I.A.; et al. Potent anti-influenza H7 human monoclonal antibody induces separation of hemagglutinin receptor-binding head domains. PLoS Biol, 2019. 17(2): p. e3000139. [CrossRef]
- Kohler, G.; C. Milstein. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 1975. 256(5517): p. 495-7. [CrossRef]
- Vidarsson, G.; G. Dekkers, T.; Rispens. IgG Subclasses and Allotypes: From Structure to Effector Functions. Frontiers in Immunology, 2014. 5. [CrossRef]
- Brezski, R.J.; G. Georgiou. Immunoglobulin isotype knowledge and application to Fc engineering. Curr Opin Immunol, 2016. 40: p. 62-9. [CrossRef]
- Tharakaraman, K.; Subramanian, V.; Cain, D.; Sasisekharan, V.; Sasisekharan, R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell Host Microbe, 2014. 15(5): p. 644-51. [CrossRef]
- Yi, K.S.; Choi, J.-A.; Kim, P.; Ryu, D.-K.; Yang, E.; Son, D.; Shin, J.; Park, H.; Lee, S.; Lee, H.; et al. Broader neutralization of CT-P27 against influenza A subtypes by combining two human monoclonal antibodies. PLOS ONE, 2020. 15(7): p. e0236172. [CrossRef]
- Kallewaard, N.L.; Corti, D.; Collins, P.J.; Neu, U.; McAuliffe, J.M.; Benjamin, E.; Wachter-Rosati, L.; Palmer-Hill, F.J.; Yuan, A.Q.; Walker, P.A.; et al. Structure and Function Analysis of an Antibody Recognizing All Influenza A Subtypes. Cell, 2016. 166(3): p. 596-608. [CrossRef]
- Tharakaraman, K.; Subramanian, V.; Viswanathan, K.; Sloan, S.; Yen, H.-L.; Barnard, D.L.; Leung, Y.H.C.; Szretter, K.J.; Koch, T.J.; Delaney, J.C.; et al. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proceedings of the National Academy of Sciences, 2015. 112(35): p. 10890-10895. [CrossRef]
- Izadi, A.; Hailu, A.; Godzwon, M.; Wrighton, S.; Olofsson, B.; Schmidt, T.; Söderlund-Strand, A.; Elder, E.; Appelberg, S.; Valsjö, M.; et al. Subclass-switched anti-spike IgG3 oligoclonal cocktails strongly enhance Fc-mediated opsonization. Proceedings of the National Academy of Sciences, 2023. 120(15). [CrossRef]
- Bolton, M.J.; Arevalo, C.P.; Griesman, T.;Li; S.H.; Bates, P.; Wilson, P.C.; Hensley, S.E. IgG3 subclass antibodies recognize antigenically drifted influenza viruses and SARS-CoV-2 variants through efficient bivalent binding. Proc Natl Acad Sci U S A, 2023. 120(35): p. e2216521120. [CrossRef]
- Bowles, J.A.; Wang, S.-Y.; Link, B.K; Allan, B.; Beuerlein, G.; Campbell, M.-A.; Marquis, D.; Ondek, B.; Wooldridge, J.E.; Smith, B.J.; et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood, 2006. 108(8): p. 2648-2654. [CrossRef]
- Forero-Torres, A.; de Vos, S.; Pohlman, B.L.; Pashkevich, M.; Cronier, D.M.; Dang, N.H.; Carpenter, S.P.; Allan, B.W.; Nelson, J.G.; Slapak, C.A.; et al. Results of a Phase 1 Study of AME-133v (LY2469298), an Fc-Engineered Humanized Monoclonal Anti-CD20 Antibody, in FcγRIIIa-Genotyped Patients with Previously Treated Follicular Lymphoma. Clinical Cancer Res, 2012. 18(5): p. 1395-1403. [CrossRef]
- Van Der Horst, H.J.; Nijhof, I.S.; Mutis, T.; Chamuleau, M.E.D. Fc-Engineered Antibodies with Enhanced Fc-Effector Function for the Treatment of B-Cell Malignancies. Cancers, 2020. 12(10): p. 3041. [CrossRef]
- Ko, S.; Park, S.; Sohn, M.H.; Jo, M.; Ko, B.J.; Na, J.-H.; Yoo, H.; Jeong, A.L.; Ha, K.; Woo, J.R.; et al. An Fc variant with two mutations confers prolonged serum half-life and enhanced effector functions on IgG antibodies. Exp. Mol. Med., 2022. 54(11): p. 1850-1861. [CrossRef]
- Natsume, A.; In, M.; Takamura, H.; Nakagawa, T.; Shimizu, Y.; Kitajima, K.; Wakitani, M.; Ohta, S.; Satoh, M.; Shitara, K.; et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res, 2008. 68(10): p. 3863-72. [CrossRef]
- Chu, T.H.; E.F. Patz; M.E. Ackerman. Coming together at the hinges: Therapeutic prospects of IgG3. mAbs, 2021. 13(1): p. 1882028. [CrossRef]
- Ali, S.O., et al., Takas, T.; Nyborg, A.; Shoemaker, K.; Kallewaard, N.L.; Chiong, R.; Dubovsky, F.; Mallory, R.M. Evaluation of MEDI8852, an Anti-Influenza A Monoclonal Antibody, in Treating Acute Uncomplicated Influenza. Antimicrob Agents Chemother, 2018. 62(11). [CrossRef]
- Sloan, S.E.; Szretter, K.J.; Sundaresh, B.; Narayan, K.M.; Smith, P.F.; Skurnik, D.; Bedard, S.; Trevejo, J.M.; Oldach, D.; Shriver, Z. Clinical and virological responses to a broad-spectrum human monoclonal antibody in an influenza virus challenge study. Antiviral Res, 2020. 184: p. 104763. [CrossRef]
- Hershberger, E.; Sloan, S.; Narayan, K.; Hay, C.A.; Smith, P.; Engler, F.; Jeeninga, R.; Smits, S.; Trevejo, J.; Shriver, Z.; et al. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study. EBioMedicine, 2019. 40: p. 574-582. [CrossRef]
- Lim, J.J.; Nilsson, A.C.; Silverman, M.; Assy, N.; Kulkarni, P.; McBride, J.M.; Deng, R.; Li, C.; Yang, X.; Nguyen, A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of MHAA4549A, a Monoclonal Antibody, plus Oseltamivir in Patients Hospitalized with Severe Influenza A Virus Infection. Antimicrob Agents Chemother, 2020. 64(7). [CrossRef]
- Han, A.; Czajkowski, L; Rosas, L.A.; Cervantes-Medina, A.; Xiao, Y.; Gouzoulis, M.; Lumbard, K.; Hunsberger, S.; Reed, S.; Athota, R.; et al. Safety and Efficacy of CR6261 in an Influenza A H1N1 Healthy Human Challenge Model. Clinical Infectious Diseases, 2021. 73(11): p. e4260-e4268. [CrossRef]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.B.; van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science, 2015. 349(6254): p. 1301-1306. [CrossRef]
- Van Der Lubbe, J.E.M.; Huizingh, J.; Verspuij, J.W.A.; Tettero, L.; Schmit-Tillemans, S.P.R.; Mooij, P.; Mortier, D.; Koopman, G.; Bogers, W.M.J.M.; et al., Mini-hemagglutinin vaccination induces cross-reactive antibodies in pre-exposed NHP that protect mice against lethal influenza challenge. NPJ Vaccines, 2018. 3(1). [CrossRef]
- Folschweiller, N.; Abeele, C.V.; Chu, L.; Van Damme, P.; García-Sastre, A.; Krammer, F.; Nachbagauer, R.; Palese, P; Solórzano, A.; Bi, D.; et al. Reactogenicity, safety, and immunogenicity of chimeric haemagglutinin influenza split-virion vaccines, adjuvanted with AS01 or AS03 or non-adjuvanted: a phase 1-2 randomised controlled trial. Lancet Infect Dis, 2022. 22(7): p. 1062-1075. [CrossRef]
- Nishiyama, A.; Adachi, Y.; Tonouchi, K.; Moriyama, S.; Sun, L.; Aoki, M.; Asanuma, H.; Shirakura, M.; Fukushima, A.; Yamamoto, T.; et al. Post-fusion influenza vaccine adjuvanted with SA-2 confers heterologous protection via Th1-polarized, non-neutralizing antibody responses. Vaccine, 2023. 41(31): p. 4525-4533. [CrossRef]
- Atmar, R.L.; Bernstein, D.I.; Winokur, P.; Frey, S.E.; Angelo, L.S.; Bryant, C.; Ben-Yedida-T.; Roberts, P.C.; El Sahly, H.M.; Keitel, W.A. Safety and immunogenicity of Multimeric-001 (M-001) followed by seasonal quadrivalent inactivated influenza vaccine in young adults - A randomized clinical trial. Vaccine, 2023. 41(16): p. 2716-2722. [CrossRef]
- Corbett, K.S.; Moin, S.M.; Yassine, H.M.; Cagigi, A.; Kanekiyo, M.; Boyoglu-Barnum, S.; Myers, S.I.; Tsybovsky, Y.; Wheatley, A.K.; Schramm, C.A.; et al. Design of Nanoparticulate Group 2 Influenza Virus Hemagglutinin Stem Antigens That Activate Unmutated Ancestor B Cell Receptors of Broadly Neutralizing Antibody Lineages. mBio, 2019. 10(1). [CrossRef]
- Lee, P.S.; Yoshida, R.; Ekiert, D.C.; Sakai, N.; Suzuki, Y.; Takada, A.; Wilson, I.A. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci U S A, 2012. 109(42): p. 17040-5. [CrossRef]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O'Neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature, 2012. 489(7417): p. 526-32. [CrossRef]
- Sarah F. Andrews, Y.H., Kaval Kaur, Lyubov I. Popova, Irvin Y. Ho, Noel T. Pauli, Carole J. Henry Dunand, William M Taylor, Samuel Lim, Min Huang, Xinyan Qu, Jane-Hwei Lee, Marlene Salgado-Ferrer, Florian Krammer, Peter Palese, Jens Wrammert, Rafi Ahmed, and Patrick C. Wilson. Immune history profoundly affects broadly protective B cell responses to influenza. Science Translational Medicine, 2015. 7(316): p. 316ra192.
- Bajic, G.; van der Poel, C.E.; Kuraoka, M.; Schmidt, A.G.; Carroll, M.C.; Kelsoe, G.; Harrison, S.C. Autoreactivity profiles of influenza hemagglutinin broadly neutralizing antibodies. Scientific Reports, 2019. 9(1). [CrossRef]
- Willey, S.; Aasa-Chapman, M.M.I.; O'Farrell, S.; Pellegrino, P.; Williams, I.; Weiss, R.A.; Neil, S.J.D. Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection. Retrovirology, 2011. 8(1): p. 16. [CrossRef]
- Monsalvo, A.C.; Batalle, J.P.; Lopez, M.F.; Krause, J.C.; Klemenc, J.; Hernandez, J.Z.; Maskin, B.; Bugna, J.; Rubinstein, C.; Aguilar, L.; et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nature Medicine, 2011. 17(2): p. 195-199. [CrossRef]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol., 2020. 5(10): p. 1185-1191. [CrossRef]
- Rao, G.K.; Prell, R.A.; Laing, S.T.; Burleson, S.C.M.; Nguyen, A.; McBride, J.M.; Zhang, C.; Sheinson, D.; Halpern, W.G. In Vivo Assessment of Antibody-Dependent Enhancement of Influenza B Infection. Toxicol Sci, 2019. 169(2): p. 409-421. [CrossRef]
- Winarski, K.L.; Tang, J.; Klenow, L.; Lee, J.; Coyle, E.M.; Manischewitz, J.; Turner, H.L.; Takeda, K.; Ward, A.B.; Golding, H.; et al. Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc Natl Acad Sci U S A, 2019. 116(30): p. 15194-15199. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




